Abstract
Advances in the neuroscientific understanding of bodily autonomic awareness, or interoception, have led to the hypothesis that human trait anxiety sensitivity (AS)—the fear of bodily autonomic arousal—is primarily mediated by the anterior insular cortex. Despite broad appeal, few experimental studies have comprehensively addressed this hypothesis. We recruited 55 individuals exhibiting a range of AS and assessed them with functional magnetic resonance imaging (fMRI) during aversive fear conditioning. For each participant, three primary measures of interest were derived: a trait Anxiety Sensitivity Index score; an in‐scanner rating of elevated bodily anxiety sensations during fear conditioning; and a corresponding estimate of whole‐brain functional activation to the conditioned versus nonconditioned stimuli. Using a voxel‐wise mediation analysis framework, we formally tested for ‘neural mediators’ of the predicted association between trait AS score and in‐scanner anxiety sensations during fear conditioning. Contrary to the anterior insular hypothesis, no evidence of significant mediation was observed for this brain region, which was instead linked to perceived anxiety sensations independently from AS. Evidence for significant mediation was obtained for the dorsal anterior cingulate cortex—a finding that we argue is more consistent with the hypothesized role of human cingulofrontal cortex in conscious threat appraisal processes, including threat‐overestimation. This study offers an important neurobiological validation of the AS construct and identifies a specific neural substrate that may underlie high AS clinical phenotypes, including but not limited to panic disorder. Hum Brain Mapp 36:3950–3958, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: anxiety sensitivity, interoception, fear, autonomic, insular cortex, cingulate cortex
INTRODUCTION
Anxiety sensitivity (AS) is a widely studied dispositional trait that contributes to individual differences in fearfulness and risk of anxiety disorders [Reiss, 1991; Taylor, 1995]. It refers to the specific fear of arousal‐related bodily sensations—“the fear of fear”—linked to the belief that such sensations may have harmful physical, psychological, or social consequences. AS has been described as an anxiety amplifier—when individuals high in AS feel anxious they become alarmed about accompanying arousal sensations, which further intensifies their anxiety [Reiss, 1991]. Experimentally, AS has been shown to predict self‐reported anxiety in response to biological challenges (e.g., carbon dioxide inhalation) independently from actual physiological arousal changes [Asmundson et al., 1994; Forsyth et al., 1999; Melzig et al., 2011; Zvolensky and Eifert, 2001]. In other words, it is the cognitive appraisal of such bodily sensations that underpins AS. Recent advances in the neuroscientific understanding of bodily autonomic awareness have led to the hypothesis that AS may be underpinned by a key component of the brain's interoceptive system—the anterior insular cortex (AIC).
This ‘insular theory of anxiety’ [Paulus and Stein, 2006], which draws upon an influential model of interoception [Craig, 2011], proposes that subjective feelings of anxiety arise through misrepresentations of bodily arousal states integrated within the AIC. Within this framework, individuals high in AS experience an augmented AIC “anticipatory prediction error signal” that is responsible for triggering subsequent appraisals of anxiety. However, a reasonable alternative hypothesis derived from neuroimaging studies of human fear conditioning is that AS may in fact be more closely aligned with putative functions of the dorsal anterior cingulate cortex (dACC). Based on such evidence, this region—together with adjacent dorsomedial prefrontal cortex (dmPFC)—has been argued to be critically involved in the conscious appraisal of threating stimuli [Kalisch and Gerlicher, 2014], in addition to its well‐characterized role in the expression of sympathetic autonomic fear responses [Milad et al., 2007].
Though to date few neuroimaging studies have directly examined AS, current evidence seems to favor the AIC mediation hypothesis. Most notably, Stein et al. [2007] and Killgore et al. [2011] reported significant positive correlations between AS scores and functional activation of the AIC in the context of emotional face processing tasks. No such correlations were reported for the dACC, although of note, it was not consistently activated by these tasks at the group level. Neither were these tasks demonstrated to evoke significant increases in participants' arousal state, a critical caveat, as it is the fear of such arousal sensations that is the fundamental contextual basis for AS.
This study was designed to test these competing hypotheses: is it the AIC or dACC that primarily mediates human AS? To do so, we combined fMRI with Pavlovian fear conditioning designed to provoke increased sympathetic autonomic arousal, self‐reported anxiety sensations and corresponding neural activation of the AIC, dACC, and extended ‘fear network.’ These measures were then integrated in a brain‐based mediation analysis to formally characterize ‘neural mediators’ of the experimental manifestation of AS—that is, brain regions driving the predicted relationship between an individual's AS level and their actual experience of bodily anxiety sensations during fMRI.
MATERIALS AND METHODS
Participants
Fifty‐five healthy participants were recruited for this study (Table 1). All underwent the MINI International Neuropsychiatric Interview conducted by either an experienced psychiatrist (EV) or clinical psychologist (MF) to exclude any Axis I psychiatric disorders [Sheehan et al., 1998]. None had a personal history of neurological or psychiatric illness. On the scanning day, participants completed Spanish versions of the Anxiety Sensitivity Index (ASI)−3 [Taylor et al., 2007] and State‐Trait Anxiety Inventory [STAI; Spielberger, 1983]. ASI‐3 and STAI scores are provided in Table 1. All participants had normal or corrected‐to‐normal vision and provided written informed consent, following a complete description of the study protocol, which was approved by the Institutional Review Board of the University Hospitals of Bellvitge and Del Mar, Barcelona, Spain.
Table 1.
Participant characteristics (N = 55)
| Ratio | |
|---|---|
| Gender | 17 male, 38 female |
| Mean (standard deviation), range | |
| Age, years | 21.7 (4.2), 18–40 |
| Education level, years | 14.3 (2.1), 12–20 |
| ASI‐3, total score | 12.7 (8.5), 1–32 |
| STAI, state anxiety score | 13.2 (6.9), 1–33 |
| STAI, trait anxiety score | 17.5 (8.5), 1–42 |
ASI‐3 = Anxiety Sensitivity Index‐3; STAI = State‐Trait Anxiety Inventory.
Experimental Design
All participants completed two experimental fear conditioning sessions: a primary session devoted to fMRI (Week 1) and a supplementary session devoted to psychophysiological validation (Week 2). For the fMRI session, we modified the fear conditioning task reported in Reinhardt et al. [2010] with our analyses focusing solely on the characterization of subjective, autonomic, and neural responses during the fear conditioning or acquisition task phase. Briefly, during scanning, an unconditioned stimulus (US; aversive auditory noise burst; 100 ms) was paired with one of two conditioned stimuli (a blue or yellow sphere), thus forming a conditioning stimulus (CS+) and nonconditioned stimulus (CS−). The CS–US pairing was counterbalanced across subjects and occurred with a partial reinforcement rate of 50%, thus enabling the classification of CS+nonpaired and CS+paired trials and the subsequent analysis of autonomic and neural responses (CS+nonpaired > CS− trials) without US confounding. Each CS was presented 32 times (presentation time: 2 s, with a variable intertrial interval of 4.785–7.250 s). In CS–US paired trials, the presentation of the US occurred 1.9 s after the onset of the CS+ and coterminated. The US volume was individually set between 95 and 110 dB based on a pretask calibration with background scanning noise and participants' ratings of unpleasantness (minimum rating of 7 on a 11‐point Likert scale). Upon conclusion of scanning, the US was reconfirmed to be moderately‐to‐highly unpleasant (11‐point scale, mean/standard deviation (SD) = 7.65/1.56, range 3–10). Whereas in Reinhardt et al. [2010], the US was presented in an initial familiarization–habituation phase, it was removed here in order to enhance aversiveness during conditioning trials.
Immediately after the fear‐conditioning phase (i.e., while in the scanner; as a natural continuation of the task phase), participants were asked to rate their experience of bodily anxiety sensations to the CS+ and CS− on a five‐point Likert scale Self‐Assessment Manikin [SAM; Bradley and Lang, 1994], with responses ranging from 1 = “not anxious” to 5 = “very anxious.” Participants were familiarized with this rating prior to scanning and were instructed specifically to focus on their experience of physical (bodily/somatic) anxiety sensations, as emphasized by the SAM cartoon (Fig. 1a) and the accompanying question: “How anxious did the blue/yellow sphere make you feel?” During training, general examples were given such as feeling anxious sensations in the stomach or chest, but participants were not instructed to focus on any particular bodily sensations. Participants also made emotional valence ratings of the CS+ and CS− on an equivalent five‐point SAM (Fig. 1b), with responses ranging from 1 = “very unpleasant” to 5 = “very pleasant.” Specifically, participants responded to the question “how pleasant did you find the blue/yellow sphere? Although the anxiety ratings were of primary interest as an outcome measure, emotional valence ratings were important to confirm the aversiveness of the CS+. The task was programmed in Presentation® (Neurobehavioral Systems) and was delivered using magnetic resonance imaging (MRI)‐compatible high‐resolution goggles and headphones (VisuaStim Digital, Resonance Technology). SAM responses were made using a hand‐held optical‐fiber response recording device, which participants were familiarized with prior to scanning.
Figure 1.
(a) The self‐assessment manikin (SAM) used to assess bodily anxiety sensations, (b) emotional valence. Left‐to‐right, the manikins correspond to Likert scale ratings of 1–5.
Parallel versions of the task facilitated their repeated presentation during the Week 2 psychophysiology assessment. At both time‐points, assignment of the CS+ and CS− was counter‐balanced and pseudorandomly made across participants. The main purpose of this assessment was to confirm that the fear conditioning task indeed evoked significant changes in sympathetic autonomic arousal, as previously demonstrated [Kircher et al., 2013; Lueken et al., 2013, 2014; Reinhardt et al., 2010]. The Week 2 assessment also allowed us to examine the intrasubject consistency of the hypothesized link between trait AS and self‐reported anxiety sensations during fear conditioning/expression. To facilitate high‐quality recordings of sympathetic autonomic arousal (skin conductance response; SCR) intertrial intervals in this version of the task were extended to between 12 and 14 s, but with all other task attributes remaining equivalent. Skin conductance was recorded from the distal phalanges of the index and the middle left‐hand fingers by means of two Ag/AgCl electrodes filled with electrolyte and using a Biopac 150 polygraph (Biopac Systems). The signal was amplified and sampled at 125 Hz. We compared SCRs (square root transformed) associated with the presentation of the CS+nonpaired and CS− across the fear‐conditioning phase. Skin conductance magnitudes in microsiemens (µS) were computed by the maximum of the SCR signal between 1 and 5 s after stimulus onset. This value was subtracted from a baseline, defined by the mean of the SCR in a 1‐s time interval before the onset of the stimulus to account for baseline fluctuations [Reinhardt et al., 2010].
Participants' ratings of arousal and valence for the CS+ and CS− as well as SCR data were analyzed via repeated measures analysis of variance in IBM SPSS Statistics v22. Linear associations between these measures as well as ASI‐3 and STAI‐TA scores were estimated via Pearson's linear correlations.
Image Acquisition and Preprocessing
A 1.5 Tesla Signa Excite system (General Electric, Milwaukee, WI) equipped with an 8‐channel phased‐array head coil and single‐shot echoplanar imaging (EPI) software was used. The functional sequence consisted of gradient recalled acquisition in the steady state (time of repetition, 2,000 ms; time of echo, 50 ms; pulse angle, 90°) within a field of view of 24 cm, with a 64 × 64‐pixel matrix, and with a slice thickness of 4 mm (interslice gap, 1 mm). Twenty‐two interleaved slices, parallel to the anterior–posterior commissure line, were acquired to generate 570 whole‐brain volumes, excluding four initial dummy volumes. Imaging data were transferred and processed on a Linux platform running MATLAB version 8.2 (The MathWorks, Natick, Mass). Preprocessing was performed with Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, UK) and involved motion correction, spatial normalization and smoothing using a Gaussian filter (full‐width, half‐maximum, 8 mm). For all participants included here, their inspected head motion realignment parameters (translation and rotation estimates) were less than 2 mm and 2°, respectively, in each plane. These motion parameters were included as first‐level noise covariates in the time‐series analyses described below. Data were normalized to the standard SPM‐EPI template and resliced to 2 mm isotropic resolution in Montreal Neurological Institute (MNI) co‐ordinate space.
Mapping Fear‐Conditioned Neural Responses
Each participant's preprocessed time‐series was included in SPM first‐level general linear model analyses. Each event‐type (CS−, CS+nonpaired, and CS+paired) was individually coded by specifying the onset of each stimulus presentation as a delta (i.e., stick) function. A high‐pass filter (1/128 s) accounted for low‐frequency noise, while temporal autocorrelations were estimated using a first‐order autoregressive model [AR(1)]. Realignment parameters of each participant were included in the model. Regression coefficient estimates (betas) were calculated using a Restricted Maximum Likelihood approach and a contrast image corresponding to the primary task effect of interest (differential fear conditioning; CS+nonpaired > CS−) was estimated for each participant. These contrast images were then carried forward to the second level using the summary statistics approach to random‐effects group analyses. A one‐sample t‐test was used to estimate significant within‐group activation thresholded at P < 0.05 false discovery rate (FDR)‐corrected [Genovese et al. 2002] across the whole‐brain volume.
Mapping Neural Mediators of AS
To characterize the primary brain region(s) mediating the experimental manifestation of AS—that is, the predicted relationship between trait ASI score and self‐reported anxiety sensations—we implemented a formal brain‐based mediation analysis termed “Mediation Effect Parametric Mapping” [MEPM; Wager et al., 2008]; see additional applications in Atlas et al. [2010], Denny et al. [2013]; Lopez‐Sola et al. [2014], and Wager et al. [2009a, 2009b]. MEPM allowed us to identify, on a voxelwise basis, any brain regions activated during fear conditioning that also satisfied formal criteria for mediators in a standard three‐variable path‐modeling framework. In our model, Path a signifies the relationship between AS (X) and brain response (M)—the “brain‐trait” pathway. Path b signifies the relationship between brain response (M) and increased self‐reported anxiety sensations (Y) controlling for X—the “brain‐state” pathway. Path a*b signifies the test of mediation, that is, whether the direct X→Y relationship is significantly reduced by including M in the path model.
MEPM analyses proceeded in two stages: a primary region‐of‐interest (ROI) analysis focusing a priori on the AIC and dACC, followed by a secondary examination of other potential mediating regions. For the ROI analysis, AIC and dACC anatomical locations were defined a priori on the basis of two previously published studies. For the AIC, we defined a 10‐mm radial sphere at the peak location reported in Stein et al. [2007], which represented a significant positive correlation between right AIC activation (x,y,z = 27,22,3) and ASI total score. For the dACC, we defined a 10‐mm radial sphere at the peak location reported in Milad et al. [2007], which corresponded to greater dACC activation (x,y,z = 1,21,27) during successful fear conditioning. ROI co‐ordinates were transformed from Talairach to MNI space (SPM) using a nonlinear registration method [Lacadie et al., 2008]. For these regions to be considered significant mediators, we required that they reach statistical significance in each of the three tests comprising the path model (Paths a, b, a*b). The false‐positive rate was controlled using a voxelwise FDR [Genovese et al., 2002] at q < 0.05 across all estimated effects in the path model [a, b, a*b; Wager et al. 2008], which corresponded to minimum P = 0.01.
To examine if other nonhypothesized regions emerged as significant mediators, we conducted a secondary analysis of path effects across all voxels identified as significant from the initial whole‐brain task analysis: that is, including regions identified as significantly activated during fear conditioning. Regional path effects from this analysis are reported as significant if surviving P uncorrected <0.001. For both analysis approaches, statistical significance of the peak voxel path effects were computed via a bootstrap test (10,000 permutations), as described in Wager et al. [2008].
RESULTS
Fear‐Conditioned Subjective and Autonomic Responses
For the Week 1 (fMRI) assessment, participants' in‐scanner ratings indicated successful acquisition (i.e., learning and subsequent anticipation) of the CS+. Significant within‐group effects were apparent for both self‐reported anxiety (F 1,54 = 148.5, P < 0.0001) and emotional valence ratings (F 1,54 = 114.2, P < 0.0001), thus confirming the differential aversiveness of the CS+ versus CS−; see Supporting Information Table S1.
The pattern of subjective responses observed during Week 1 were replicated at Week 2 (anxiety, F 1,54 = 191.6, P < 0.0001; valence, F 1,54 = 145.7, P < 0.0001); see also Table S1 (Supporting Information). Computed differential scores for both self‐reported anxiety and emotional valence ratings (e.g., CS+ CS− rating) were also significantly positively correlated between the repeated assessments (anxiety, r = 0.27, P = 0.02; valence r = 0.26, P = 0.03).
From the Week 2 psychophysiology assessment, it was confirmed that the task elicited significant increases in sympathetic autonomic arousal in the form of differential SCRs to the CS+ versus CS− (F 1,54 = 51.4, P < 0.0001). Consistent with AS theory and prior experimental studies [Asmundson et al., 1994; Forsyth et al., 1999; Melzig et al., 2011; Zvolensky and Eifert, 2001], there was no significant correlation between participants' ASI‐3 scores and actual physiological arousal changes (differential SCR) during fear conditioning (r = 0.14 P = 0.30). Neither did we observe a significant correlation between differential SCR and self‐reported anxiety sensations at Week 2 (r = 0.11, P = 0.42).
As anticipated, a significant positive correlation was observed between ASI‐3 scores and self‐reported anxiety sensations evoked during fear conditioning (Week 1; r = 0.26, P < 0.03; Week 2; r = 0.38, P < 0.002). Considering historical debate regarding the AS versus “trait anxiety” constructs [Reiss, 1997], we also examined correlations between STAI trait anxiety scores [Spielberger, 1983] and differential self‐reported anxiety sensations at both time‐points. No significant positive correlations were observed with STAI trait anxiety (Week 1: r = 0.15, P = 0.27; Week 2: r = 0.19, P = 0.17).
We also investigated the influence of age and gender on the primary variables of interest (reported above), as well as the functional activation of the AIC and dACC (reported below) and identified no significant relationships. These results are presented in Supporting Information Table S3.
Fear‐Conditioned Neural Responses
The initial fMRI analysis indicated that successful fear conditioning was associated with robust group‐level activation of the extended “fear‐arousal network,” including the AIC and dACC (P < 0.05 FDR; whole‐brain). Figure 2 illustrates the pattern of coactivation of these regions together with participants' corresponding Week 1 self‐reported anxiety sensations (Panel 2a) and Week 2 SCRs (Panel 2b). All significant brain regional activations during fear conditioning are reported in Supporting Information Table S2. It can be noted that the AIC and dACC/extended anterior medial wall were among the most robustly activated in terms of their estimated significance. Confirmatory analysis based on the a priori defined ROIs indicated somewhat stronger activation of the right AIC (peak Z = 6.48) compared to dACC region (peak Z = 3.28).
Figure 2.
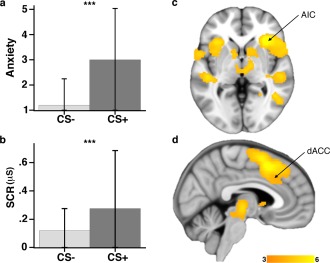
(a) In‐scanner ratings of self‐reported anxiety sensations—scale ranging 1 = “not anxious” to 5 = “very anxious.” (b) Offline skin conductance responses during fear conditioning (SCR; µS) acquired at Week 2. Mean and standard deviations are displayed. (c) Whole‐brain FDR‐corrected activation during fear conditioning emphasizing responses in the right AIC; and (d) dACC. Color‐bar activation magnitude corresponds to SPM T values. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Neural Mediators of AS
To reiterate, in our model, Path a signifies the relationship between AS (X) and brain response (M)—the “brain‐trait” pathway. Path b signifies the relationship between brain response (M) and increased self‐reported anxiety sensations (Y) controlling for X—the “brain‐state” pathway. Path a*b signifies the test of mediation, that is, whether the direct X→Y relationship is significantly reduced by including M in the path model.
From the primary ROI analysis, evidence of significant mediation was obtained for the dACC but not the right AIC (Fig. 3). The dACC displayed positive path coefficients for both Path a (r = 0.49) and b effects (r = 0.38), suggesting that higher AS was associated with greater dACC activation during fear conditioning, which predicted increased self‐reported anxiety sensations (a = 0.03 (0.01), Z = 4.07; b = 0.92 (0.25), Z = 3.71; a*b = 0.03 (0.01), Z = 3.62, q < 0.05 FDR).
Figure 3.
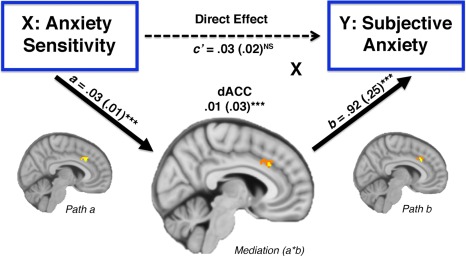
Brain mediation effect parametric mapping: the dACC cluster demonstrating significant positive path a, b and a*b effects. Mean standardized path coefficients are shown with their standard errors (in parentheses). MNI co‐ordinates (x, y, z): path a (8, 22, 34); b (8, 22, 34); a*b (6, 24, 32). Voxels emphasized yellow correspond to q < 0.05 (FDR) with those in orange representing subthreshold contiguous voxels (P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The right AIC, conversely, was found to demonstrate a significant “brain‐state” (Path b) effect (b = 1.15 (0.32), Z = 3.32), indicating that greater AIC activation predicted increased self‐reported anxiety sensations when controlling for AS (Fig. 4). There was no evidence of a significant “brain‐trait” pathway for this region.
Figure 4.
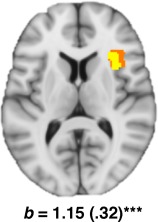
Right AIC cluster identified as a significant path b effect from the primary ROI analysis. MNI co‐ordinates (x, y, z): path b (32, 22, 8). Voxels emphasized yellow correspond to q < 0.05 (FDR) with those in orange representing subthreshold contiguous voxels (P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
From the broader exploratory analysis, no additional regions demonstrated evidence for significant mediation, although other “brain‐trait” (Path a) and “brain‐state” (Path b) effects were identified. For the former, higher AS predicted greater dorsal caudate nucleus activation during fear conditioning (a = 0.03 (0.01), Z = 2.96). For the latter, dorsal anterior midbrain (b = 1.88 (0.42), Z = 3.70) and left AIC activation (b = 1.79 (0.49), Z = 3.00) predicted increased self‐reported anxiety sensations, adjusted for AS. These additional “nonmediating” regional effects are shown in Figure 5.
Figure 5.
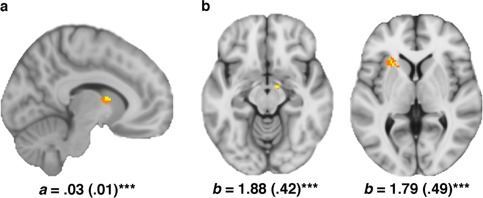
(a) Caudate nucleus cluster identified as a significant path a effect from the secondary extended ‘fear network’ analysis. MNI co‐ordinates (x, y, z): path a (10, 4, 10). (b) Dorsal anterior midbrain (∼substantia nigra) and left AIC regions that demonstrated a significant path b effects from the secondary analysis. MNI co‐ordinates (x, y, z): midbrain path b (10, −8, −12); AIC path b (−26, 20, 4). Voxels emphasized yellow correspond to q < 0.05 (FDR) with those in orange representing subthreshold contiguous voxels (P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To supplement the primary mediation results shown in Figure 2, we also present results obtained from SPM linear regression analyses between fear‐conditioned neural responses and participants' ASI‐3 and differential self‐reported anxiety sensations, respectively. In both instances, significant positive correlations survived FDR small volume correction (at whole‐brain P < 0.001 uncorrected) for the previously defined ROI (dACC‐ASI‐3 correlation; MNI peak co‐ordinate: x,y,z = 8,26,32; Z = 4.14, P = 0.005 FDR, 58 voxels; dACC‐anxiety correlation; MNI peak co‐ordinate: x,y,z = 6,22,34; Z = 3.47; P = 0.02 FDR; 25 voxels). These results are presented in Supporting Information Figure S1.
DISCUSSION
Our findings directly challenge the idea that human trait AS—the “fear of fear”—is primarily mediated by the AIC as part of a larger‐scale brain system supporting interoceptive and emotional awareness [Craig, 2011; Paulus and Stein, 2006]. While such accounts recognize a complementary role for the dACC in facilitating autonomic threat responses, the AIC is held to be the critical neural substrate through which these signals become anxious appraisals in people with higher levels of AS. Instead, we have identified the AIC, together with dorsal midbrain, as components of a “brain‐state” pathway linking fear‐evoked brain activation and self‐reported anxiety sensations independently from AS. Thus, while both the AIC and dACC were linked to self‐reported bodily anxiety sensations, likely via a joint contribution to basic interoceptive awareness [Medford and Critchley, 2010], the dACC appears more specifically involved in the conscious appraisal of the aversiveness of these sensations as a function of trait AS.
Despite considerable evidence linking dACC function to anxiety processes, experimentally and clinically [Devinsky et al., 1995; Etkin et al., 2011; Greenberg et al., 2010; Pujol et al., 2002], the mechanism by which it contributes to conscious aspects of anxiety, such as catastrophizing and worry, is not well understood. In part, this is because it has been difficult in functional neuroimaging studies to parse anxious cognitions from autonomic fear responses also represented within dACC [Milad et al., 2007]. However, in a study by Kalisch et al. [2006], it was demonstrated that when participants' ability to appraise threating stimuli was restricted by a dual task challenge, activation of the rostral dACC/dmPFC was considerably downregulated despite accompanying increases in autonomic output and self‐reported anxiety to the dual‐task demands [Kalisch et al., 2006]. Several other studies recently marshaled by these authors [Kalisch and Gerlicher, 2014] also provide evidence consistent with the view that the dACC/dmPFC region may specifically contribute to conscious threat appraisal processes, including threat‐overestimation, as represented by the construct of AS.
While we consider our findings to be consistent with this view, there is an important neuroanatomical distinction to make. Compared to these aforementioned studies [Kalisch et al., 2006], the dACC region that we have identified is located somewhat more posteriorly within “cingulofrontal” transition cortex—a region that abridges true limbic dACC ventrally and isocortex (dmPFC) dorsally [Devinsky et al., 1995]. In other studies, this region has been more consistently linked to both cognitive and autonomic components of anxious arousal [Critchley et al., 2001; Wager et al., 2009a] consistent with the idea that it underlies “psychosocially mediated visceromotor responses” [Wager et al., 2009a]. Thus, unlike the dACC/dmPFC region identified by Kalisch et al. [2006] as mostly nonoverlapping with central autonomic fear responses, the caudal dACC region identified here appears more specifically involved in the cognitive appraisal of autonomic responses to anticipated threats. Although further studies will be needed to clarify how putative appraisal processes may differentially manifest between these two areas, the idea that human cingulofrontal cortex contributes to primary schematic and stimulus‐driven forms of “proto” threat evaluation (“this is bad,” “I don't like this”) remains a compelling general hypothesis to test [Kalisch et al., 2006; Mechias et al., 2010].
One of the basic predictions that this sought to address, consistent with AS theory, was that individuals scoring higher in AS individuals should experience/report more bodily anxiety sensations during conditions of threat—which we established here via Pavlovian fear conditioning. This basic prediction was confirmed: trait AS scores successfully predicted the magnitude of in‐scanner evoked bodily anxiety sensations. Nevertheless, despite using a task that has been shown to evoke robust anticipatory anxiety responses in the cognitive and autonomic domains, and which consistently activates brain regions thought to underlie such responses, our experimental task would not have provoked the same magnitude or range of anxiety sensations as in previous laboratory‐based (nonimaging) studies of AS [Asmundson et al., 1994; Forsyth et al., 1999; Melzig et al., 2011; Zvolensky and Eifert, 2001]. To provoke AS experimentally, these studies almost exclusively used respiratory anxiety challenges, such as controlled CO2 inhalation, to induce a pattern of symptoms more consistent with full‐blown panic (e.g., dyspnea, dizziness, chest pain, tachycardia). By comparison, the current task would have evoked only mild anxiety sensations even in most AS‐prone participants. This limitation considered, it remains generally unfeasible to perform respiratory challenge studies in the fMRI environment due to the biophysical confounds of acute arterial CO2 variation on BOLD signal measurement [Birn et al., 2008]. For the same reasons, it is also undesirable to induce pronounced anxiety symptoms in people undergoing fMRI. Thus, we consider our current approach to be optimally designed with regards to studying the experimental manifestation of AS with fMRI.
A second limitation of our study is that we recorded autonomic fear responses (SCRs) separately from the fMRI session. SCR responses were not, however, a primary outcome measure given the confirmed prediction that AS manifests independently from actual changes in physiological arousal levels [Asmundson et al., 1994; Forsyth et al., 1999; Melzig et al., 2011; Zvolensky and Eifert, 2001]. Recording SCRs was useful to confirm that the task evoked significant changes in autonomic arousal (CS+ > CS−), even after a repeated assessment. The repeated assessment was also useful in demonstrating reliability in our primary associations of interest; namely, that significant changes in bodily anxiety sensations were elicited across both assessments and consistently within‐subject. Nevertheless, there remains the possibility that statistically controlling for actual changes in physiological arousal state (electrodermal and/or cardiorespiratory) may have enhanced the characterization of brain‐trait, state, and/or mediation effects. Further, although we were able to achieve good whole‐brain signal coverage imaging by scanning at 1.5 T with the current resolution, higher field fMRI with increased resolution and sensitivity is likely to improve the level of anatomical description achieved in this study.
In summary, our study offers an alternative view of the neurobiological basis of trait AS—endorsing a more fundamental role for human cingulofrontal cortex versus insular cortex in the conscious appraisal of bodily anxiety sensations. More generally, this study provides an important neurobiological validation of the AS construct—the idea that for many people, misinterpretations of threat at the mind‐–body interface can readily become a self‐fulfilling prophecy toward anxious suffering. Because AS is best known as a dimensional predictor of clinical anxiety disorders, including but not limited to panic disorder [Olatunji and Wolitzky‐Taylor, 2009], the current findings may prove translationally relevant in terms of identifying of a core domain of neural function/dysfunction that is broadly characteristic of the anxiety disorders spectrum.
Supporting information
Supporting Information Figure S1
Supporting Information Table S1
Supporting Information Table S2
Supporting Information Table S3
Conflicts of interest: None to declare
REFERENCES
- Asmundson GJ, Norton GR, Wilson KG, Sandler LS (1994): Subjective symptoms and cardiac reactivity to brief hyperventilation in individuals with high anxiety sensitivity. Behav Res Ther 32:237–241. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD (2010): Brain mediators of predictive cue effects on perceived pain. J Neurosci 30:12964–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Smith MA, Jones TB, Bandettini PA (2008): The respiration response function: The temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage 40:644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ (1994): Measuring emotion: The Self‐Assessment Manikin and the semantic differential. J Behav Ther Exp Psychiatry 25:49–59. [DOI] [PubMed] [Google Scholar]
- Craig AD (2011): Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci 1225:72–82. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2001): Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron 29:537–545. [DOI] [PubMed] [Google Scholar]
- Denny BT, Ochsner KN, Weber J, Wager TD (2013): Anticipatory brain activity predicts the success or failure of subsequent emotion regulation. Soc Cogn Affect Neurosci 9:403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA (1995): Contributions of anterior cingulate cortex to behaviour. Brain 118:279–306. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011): Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth JP, Palav A, Duff K (1999): The absence of relation between anxiety sensitivity and fear conditioning using 20% versus 13% CO2‐enriched air as unconditioned stimuli. Behav Res Ther 37:143–153. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Rauch SL, Haber SN (2010): Invasive circuitry‐based neurotherapeutics: Stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology 35:317–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Gerlicher AM (2014): Making a mountain out of a molehill: On the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neurosci Biobehav Rev 42:1–8. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Wiech K, Critchley HD, Dolan RJ (2006): Levels of appraisal: A medial prefrontal role in high‐level appraisal of emotional material. Neuroimage 30:1458–1466. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Britton JC, Price LM, Gold AL, Deckersbach T, Rauch SL (2011): Neural correlates of anxiety sensitivity during masked presentation of affective faces. Depress Anxiety 28:243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher T, Arolt V, Jansen A, Pyka M, Reinhardt I, Kellermann T, Konrad C, Lueken U, Gloster AT, Gerlach AL, Ströhle A, Wittmann A, Pfleiderer B, Wittchen HU, Straube B (2013): Effect of cognitive‐behavioral therapy on neural correlates of fear conditioning in panic disorder. Biol Psychiatry 73:93–101. [DOI] [PubMed] [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X (2008): More accurate Talairach coordinates for neuroimaging using non‐linear registration. Neuroimage 42:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López‐Solá M, Pujol J, Wager TD, Garcia‐Fontanals A, Blanco‐Hinojo L, Garcia‐Blanco S, Poca‐Dias V, Harrison BJ, Contreras‐Rodríguez O, Monfort J, Garcia‐Fructuoso F, Deus J (2014): Altered fMRI responses to non‐painful sensory stimulation in fibromyalgia patients. Arthritis Rheumatol 66:3200–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U, Straube B, Konrad C, Wittchen HU, Ströhle A, Wittmann A, Pfleiderer B, Uhlmann C, Arolt V, Jansen A, Kircher T (2013): Neural substrates of treatment response to cognitive‐behavioral therapy in panic disorder with agoraphobia. Am J Psychiatry 170:1345–1355. [DOI] [PubMed] [Google Scholar]
- Lueken U, Straube B, Reinhardt I, Maslowski NI, Wittchen HU, Ströhle A, Wittmann A, Pfleiderer B, Konrad C, Ewert A, Uhlmann C, Arolt V, Jansen A, Kircher T (2014): Altered top‐down and bottom‐up processing of fear conditioning in panic disorder with agoraphobia. Psychol Med 44:381–394. [DOI] [PubMed] [Google Scholar]
- Mechias ML, Etkin A, Kalisch R (2010): A meta‐analysis of instructed fear studies: Implications for conscious appraisal of threat. Neuroimage 49:1760–1768. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD (2010): Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Struct Funct 214:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzig CA, Holtz K, Michalowski JM, Hamm AO (2011): Interoceptive threat leads to defensive mobilization in highly anxiety sensitive persons. Psychophysiology 48:745–754. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL (2007): A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry 62:1191–1194. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Wolitzky‐Taylor KB (2009): Anxiety sensitivity and the anxiety disorders: A meta‐analytic review and synthesis. Psychol Bull 135:974–999. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB (2006): An insular view of anxiety. Biol Psychiatry 60:383–387. [DOI] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T (2002): Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage 15:847–855. [DOI] [PubMed] [Google Scholar]
- Reinhardt I, Jansen A, Kellermann T, Schuppen A, Kohn N, Gerlach AL, Kircher T (2010): Neural correlates of aversive conditioning: Development of a functional imaging paradigm for the investigation of anxiety disorders. Eur Arch Psychiatry Clin Neurosci 260:443–453. [DOI] [PubMed] [Google Scholar]
- Reiss S (1991): Expectancy theory of fear, anxiety and panic. Clin Psychol Rev 11:141–153. [Google Scholar]
- Reiss S (1997): Trait anxiety: It's not what you think it is. J Anxiety Disord 11:201–214. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59:22–33. quiz 34‐57. [PubMed] [Google Scholar]
- Spielberger CD. 1983. Manual for State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP (2007): Increased amygdala and insula activation during emotion processing in anxiety‐prone subjects. Am J Psychiatry 164:318–327. [DOI] [PubMed] [Google Scholar]
- Taylor S (1995): Anxiety sensitivity: Theoretical perspectives and recent findings. Behav Res Ther 33:243–258. [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Abramowitz JS, Holaway RM, Sandin B, Stewart SH, Coles M, Eng W, Daly ES, Arrindell WA, Bouvard M, Cardenas SJ (2007): Robust dimensions of anxiety sensitivity: Development and initial validation of the Anxiety Sensitivity Index‐3. Psychol Assess 19:176–188. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008): Prefrontal‐subcortical pathways mediating successful emotion regulation. Neuron 59:1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, van Ast VA, Hughes BL, Davidson ML, Lindquist MA, Ochsner KN (2009a): Brain mediators of cardiovascular responses to social threat, part II: Prefrontal‐subcortical pathways and relationship with anxiety. Neuroimage 47:836–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Waugh CE, Lindquist M, Noll DC, Fredrickson BL, Taylor SF (2009b): Brain mediators of cardiovascular responses to social threat: Part I: Reciprocal dorsal and ventral sub‐regions of the medial prefrontal cortex and heart‐rate reactivity. Neuroimage 47:821–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Eifert GH (2001): A review of psychological factors/processes affecting anxious responding during voluntary hyperventilation and inhalations of carbon dioxide‐enriched air. Clin Psychol Rev 21:375–400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1
Supporting Information Table S1
Supporting Information Table S2
Supporting Information Table S3


