Abstract
The pathological hallmark of Parkinson's disease is the degeneration of dopaminergic nigrostriatal neurons, leading to depletion of striatal dopamine. Recent neuroanatomical work has identified pathways for communication across striatal subdivisions, suggesting that the striatum provides a platform for integration of information across parallel corticostriatal circuits. The aim of this study was to investigate whether dopaminergic dysfunction in Parkinson's disease was associated with impairments in functional connectivity across striatal subdivisions, which could potentially reflect reduced integration across corticostriatal circuits. Utilizing resting‐state functional magnetic resonance imaging (fMRI), we analyzed functional connectivity in 39 patients with Parkinson's disease, both “on” and “off” their regular dopaminergic medications, along with 40 age‐matched healthy controls. Our results demonstrate widespread impairments in connectivity across subdivisions of the striatum in patients with Parkinson's disease in the “off” state. The administration of dopaminergic medication significantly improved connectivity across striatal subdivisions in Parkinson's disease, implicating dopaminergic deficits in the pathogenesis of impaired striatal interconnectivity. In addition, impaired striatal interconnectivity in the Parkinson's disease “off” state was associated with pathological decoupling of the striatum from the thalamic and sensorimotor (SM) networks. Specifically, we found that although the strength of striatal interconnectivity was positively correlated with both (i) the strength of internal thalamic connectivity, and (ii) the strength of internal SM connectivity, in both healthy controls and the Parkinson's disease “on” state, these relationships were absent in Parkinson's disease when in the “off” state. Taken together our findings emphasize the central role of dopamine in integrated striatal function and the pathological consequences of striatal dopamine denervation in Parkinson's disease. Hum Brain Mapp 36:1278–1291, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: connectivity, dopamine, network, thalamus, Parkinson's disease, resting‐state, striatum, subcortex
Abbreviations
- BOLD
Blood Oxygen Level Dependent
- CBM
Cerebellar Network
- DC
Dorsal Caudate
- DCP
Dorsal Caudal Putamen
- DMN
Default Mode Network
- DRP
Dorsal Rostral Putamen
- LFPN
Left Frontoparietal Network
- MNI
Montreal Neurological Institute stereotactic atlas
- MRI
Magnetic Resonance Imaging
- PCP
Postcommissural Putamen
- ROI
Region of Interest
- RPFN
Right Frontoparietal Network
- SM
Sensorimotor Network
- VAN
Ventral Attention Network
- VIS
Visual Network
- VRP
Ventral Rostral Putamen
- VSi
Inferior Ventral Striatum
- VSs
Superior Ventral Striatum
INTRODUCTION
The human basal ganglia are fundamental for integrated brain function. The principal input structure of the basal ganglia, the striatum, receives topographically organized input from the cerebral cortex, which is then channeled via pallidal and thalamic nuclei before projecting back to the cortex, forming parallel anatomical corticostriatal “loops” (Alexander et al., 1986). Previous work has demonstrated that the striatal nuclei are subdivided into specialized territories associated with specific functions related to their cortical connections: the ventral striatum is implicated in motivation and reward (Schultz et al., 1997; Tanaka et al., 2004), the head of the caudate nucleus in cognition (Grahn et al., 2008; Levy et al., 1997), and the putamen in motor control (Alexander and DeLong, 1985). Although corticostriatal macrocircuits have long been thought to comprise parallel and functionally segregated channels (Alexander et al., 1990; Middleton and Strick, 2000), an emerging body of neuroanatomical data has identified pathways for communication across subdivisions of the striatum (Belin and Everitt, 2008; Bevan et al., 1997; Draganski et al., 2008; Haber et al., 2000; Haber et al., 2006; Kolomiets et al., 2001; McFarland and Haber, 2002; Mena‐Segovia et al., 2005; Tziortzi et al., 2014), suggesting that the striatum may be a subcortical nexus for the integration of neural signals across specialized corticostriatal circuits (Bar‐Gad et al., 2003; Haber, 2008; Pennartz et al., 2009). Such findings have led to a reconceptualization of the basal ganglia as a complex dual organizational system, supporting both parallel and integrative processing across corticostriatal channels (Haber, 2003). Despite growing interest in the dual organizational properties of the striatum, it is not yet known how neurodegenerative disease might perturb this system.
Ascending dopaminergic nigrostriatal neurons provide rich innervation across the entire striatal complex (Haber et al., 2000; Hedreen and DeLong, 1991), exerting neuromodulatory control over information processing across the entire striatum. Parkinson's disease, therefore, provides a unique model to study the functional importance of striatal dopamine and the consequences of dopaminergic pathology, as degeneration of nigrostriatal neurons leads to severe depletion of dopamine across the striatum (Bruck et al., 2006; Fearnley and Lees, 1991; Kish et al., 1988). The loss of striatal dopamine in Parkinson's disease has been implicated in pathological neuronal activity within basal ganglia nuclei (Hammond et al., 2007), and is assumed to underlie the development of an array of complex and pervasive symptoms spanning affective, cognitive, and motoric domains (Chaudhuri and Schapira, 2009). Many of the motor and nonmotor symptoms of Parkinson's disease are modulated by dopamine‐replacement therapy, further suggesting that physiological striatal function is dependent on dopaminergic neuromodulation (Surmeier et al., 2010). Although previous functional neuroimaging studies have demonstrated abnormal activity within specific corticostriatal circuits in Parkinson's disease (Hacker et al., 2012; Helmich et al., 2010; Kwak et al., 2010; Sharman et al., 2013), little is known about whether the dopaminergic insult in Parkinson's disease might impair integration across parallel corticostriatal macrocircuits.
Resting‐state fMRI provides a noninvasive tool for the interrogation of functional connectivity independent of task‐based confounds, allowing for the investigation of neural network function in both healthy and clinical populations (Fox and Greicius, 2010; Zhang and Raichle, 2010). The primary aim of this study was to investigate whether the dopaminergic impairments in Parkinson's disease were associated with deficits in functional connectivity across subdivisions of the striatum. To investigate this question, we utilized resting‐state fMRI to calculate functional connectivity in 39 patients with Parkinson's disease, both “on” and “off” dopaminergic medication, and in 40 healthy, age‐matched controls. We hypothesized that, when withdrawn from dopaminergic medication, patients with Parkinson's disease would demonstrate reduced connectivity across subdivisions of the striatum compared to healthy controls, potentially reflecting impaired integration across parallel corticostriatal circuits in the “off” state. Furthermore, we predicted that the administration of dopaminergic therapy would ameliorate striatal connectivity deficits observed in Parkinson's disease, implicating dopamine in the modulation of striatal interconnectivity. Finally, we predicated that dopaminergic impairments in striatal connectivity would be associated with dysfunction within extra‐striatal networks in Parkinson's disease, reflecting the importance of striatal dopamine in large‐scale network function.
MATERIALS AND METHODS
Participants
The 39 patients with Parkinson's disease included in this study were recruited from the Parkinson's Disease Research Clinic at the Brain and Mind Research Institute. All patients satisfied the United Kingdom Parkinson's Disease Society Brain Bank criteria and were not demented (Martinez‐Martin et al., 2011). All 39 patients were scanned twice (with an average time of 2.4 weeks between sessions): once in their clinically defined “off” state, having been withdrawn from dopaminergic medication overnight (Parkinson's disease “off”), and once in their clinically defined “on” state having received their usual dopaminergic medication (Parkinson's disease “on”). Patients with Parkinson's disease were randomly assigned to complete the assessment either in the “on” or “off” state first. The 40 healthy control subjects were also recruited from the Brain and Mind Research Institute at the University of Sydney. Healthy control participants were screened for a history of neurological or psychiatric disorders as well as the concurrent use of any psychoactive medications. Parkinson's disease patients and healthy controls were matched for age, sex, and scores on the mini‐mental state examination (MMSE). In addition, patients with Parkinson's disease were assessed on the Hoehn and Yahr Scale and the motor section of the unified Parkinson's disease rating scale (UPDRS‐III) in their clinically defined “off” state. Table 1 shows the demographic and clinical details of the participants in the study. Dopaminergic dose equivalence scores were also calculated for each patient. Specifically, 18 patients were on a combination of l‐dopa plus a dopaminergic agonist and adjuvant therapy; 13 patients were on l‐dopa monotherapy; and 8 patients were on l‐dopa plus adjuvant therapy (either a dopamine agonist, entacapone or a monoamine oxidase inhibitor). No patients in the cohort were taking antipsychotic medication or cholinesterase inhibitors. Permission for the study was obtained from the University of Sydney's Human Research Ethics Committee and all patients gave written informed consent.
Table 1.
Demographics and clinical variables
| Parkinson's disease | Healthy controls | |
|---|---|---|
| N | 39 | 40 |
| Male/Female | 33/6 | 32/8 |
| Age | 65.8 (7.6) | 62.1 (6.5) |
| MMSE | 28.2 (1.1) | 29.1 (1.2) |
| Disease duration (years) | 7.4 (5.0) | — |
| DDE | 906.9 (591.0) | — |
| UPDRS‐III Off | 34.4 (15.1) | — |
| H&Y | 2.3 (1.3) | — |
Mean and standard deviation (SD) reported for patient demographics and clinical variables. MMSE reported for patients with Parkinson's disease in the ‘off’ state. Abbreviations: DDE, Dopamine Dose Equivalent; H&Y, Hoehn & Yahr Scale; MMSE, Mini‐Mental State Examination; UPDRS‐III, Unified Parkinson's Disease Rating Scale motor section III.
Image Acquisition
Images were acquired on a General Electric 3 Tesla MRI (General Electric, Milwaukee). T2*‐weighted echo planar functional images were acquired in interleaved order with repetition time (TR) = 3 s, echo time (TE) = 32 ms, flip angle 90°, 32 axial slices covering the whole brain, field of view = 220 mm, interslice gap = 0.4 mm, and raw voxel size = 3.9 mm by 3.9 mm by 4 mm thick. Each resting‐state scan lasted 7 min (140 TRs). During the resting‐state scan, patients were instructed to lie awake with their eyes closed and to let their minds wander freely.
Image Pre‐Processing
Preprocessing and analyses were conducted using Statistical parametric mapping software version 21 (SPM8, Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/). Images were preprocessed according to a standard pipeline, as described previously (Shine et al., 2013a). Scans were first slice‐time corrected to the median slice in each TR, then realigned to create a mean realigned image, with measures of 6° of rigid head movements calculated for later use in the correction of minor head movements. For quality assurance, each trial was analyzed using ArtRepair (Mazaika et al., 2009) and trials with a large amount of global drift or scan‐to‐scan head movements >1 mm were corrected using interpolation. None of the subjects included in this study demonstrated scan‐to‐scan head movements >3 mm (<1 voxels' breadth). Images were normalized to the Echo Planar Image template, resampled to 3 mm isotropic voxels and then subsequently smoothed using a 4 mm full‐width half‐maximum isotropic Gaussian kernel.
Definition of Striatal Regions of Interest
A total of 14 regions‐of‐interest (ROIs; radius 2.5 mm), seven per hemisphere, were defined within the striatum (see Supporting Information Table S1 for MNI co‐ordinates). Twelve of these striatal ROIs were defined in accordance with those described by Di Martino et al. (2008). These striatal ROIs have previously been shown to correspond to dissociable functional systems (Di Martino et al., 2008; Kelly et al., 2009) and enable broad coverage of the striatum, sampling the bilateral: inferior ventral striatum (VSi), superior ventral striatum (VSs), dorsal caudate (DC), dorsal caudal putamen (DCP), dorsal rostral putamen (DRP), and ventral rostral putamen (VRP). As previous work has shown that dopaminergic depletion is most severe in postcommissural putamen (PCP) (Bruck et al., 2006; Kish et al., 1988), we also defined an additional two ROIs (radius 2.5 mm), one per hemisphere, within the PCP. Importantly, to minimize the potential for “bleeding” of signals across individual ROIs, we ensured that all striatal ROIs were separated by a Euclidean distance of at least 9 mm.
Definition of Thalamic ROIs
Given that the thalamus comprises the major output nuclei of the striatum, and is an important relay station for traversing corticostriatal macrocircuits, we also explored how connectivity across subdivisions of the thalamus might be affected in Parkinson's disease. To calculate connectivity across subdivisions of the thalamus, we defined twelve thalamic ROIs (radius 2.5 mm), six per hemisphere, evenly dispersed across the thalamus (see Supporting Information Table S1 for MNI coordinates). These 12 thalamic ROIs enabled broad coverage of the thalamus, sampling the bilateral: superior, anterior, anteriomedial, lateral, posteriomedial, and posterior thalamus. Importantly, these regions were not defined according to putative functional boundaries, but rather to sample the broad connectivity within the thalamus as a whole. To minimize the potential for “bleeding” of signals across individual ROIs, we ensured that all thalamic ROIs were separated by a Euclidean distance of at least 9 mm.
Definition of ROIs Within Large‐Scale Networks
In this study, we investigated the strength of connectivity within large‐scale resting‐state networks (i.e., internal network connectivity). This was achieved using a two‐step approach. First, we conducted a spatial independent component analysis to define the topography of the major large‐scale resting‐state networks and identify key nodes within these networks. Second, we then conducted a seed‐based functional connectivity analysis using these nodes to calculate the strength of internal connectivity within each of the large‐scale resting‐state networks. The specific details of these analyses are outlined in further detail below.
To define large‐scale neuronal networks, preprocessed images were subjected to a group‐level spatial independent component analysis using the GIFT toolbox (Calhoun et al. 2001) in SPM8. Spatial independent component analysis is a data‐driven approach that searches for maximally independent clusters of voxels within the brain that covary together in reliable temporal relationships. Using the GIFT toolbox, this process involves data reduction using principal component analysis, followed by a spatial independent component analysis and finally, a back projection step to recreate individual subject maps for each component (Calhoun et al., 2001). In this study, each of the three groups (Healthy Controls, Parkinson's disease “on”, Parkinson's disease “off”) were subjected to group‐level independent component analysis separately, using the InfoMax algorithm to extract 20 maximally independent components for each group.
For each of the three groups, the initial sample of 20 network components was inspected visually, and those that contained noise within the white matter, edges of the brain and/or the cerebrospinal fluid were discarded (eight components for each group). As we were principally interested in exploring the consequences of Parkinson's disease on commonly reported large‐scale networks, we included a total of seven large‐scale networks of interest that are commonly reported in the literature (Laird et al., 2011).
Recent evidence from the functional neuroimaging literature suggests that Parkinson's disease is characterized by complex and poorly understood changes in functional connectivity that occur both within, but also beyond, the classical sensorimotor‐striatal circuitry (Baggio et al., [Link]; Dubbelink et al., 2014; Göttlich et al., 2013). In addition, recent work has revealed that the striatum is intimately linked to multiple large‐scale resting‐state networks, including sensorimotor, limbic, and heteromodal networks (such as the dorsal attention, ventral attention, default mode, and frontoparietal networks) (Choi et al., 2012). Based on these observations, it is conceivable that the striatal pathology of Parkinson's disease may contribute to connectivity impairments in multiple large‐scale cerebral networks (Shine et al., 2013b; Shine et al., 2013c). Therefore, although the primary focus of this study was to investigate striatal connectivity impairments, we also explore how the dopaminergic pathology of Parkinson's disease might perturb the connectional properties of extra‐striatal resting‐state networks.
To objectively define the components representing large‐scale functional networks, we spatially sorted the seven components of interest using a series of seeds known to comprise important regions within each network. Specifically, we used a posterior cingulate cortex seed (8mm sphere centered on 0 −52 35) to extract the default mode network (DMN); bilateral dorsolateral prefrontal cortex masks (8 mm spheres centered around −45 11 34 and 45 11 34) to extract a frontoparietal network (FPN) for the left (LFPN) and right hemisphere (RFPN); a right anterior insula mask (8 mm sphere centered around 32 20 −2) to extract the ventral attention network (VAN); a right occipital cortex seed (8 mm sphere centered on 11 −92 −2) to extract the visual network (VIS); a midline precentral gyrus mask (8 mm sphere centered around 0 −31 67) to extract the sensorimotor network (SM); and a cerebellar seed (8 mm sphere centered around 31 −64 −42) to extract the cerebellar network (CBM). As the basal ganglia and thalamus were sampled using predefined ROIs (as described above), the spatial components associated with the basal ganglia and thalamus were discarded.
Each of the large‐scale network components included in the study was then subjected to a random effects analysis across each group, after which a stringent statistical threshold of p < 0.001 (false discovery rate [FDR]: p < 0.05, cluster size >50) was applied to strictly define the spatial topography of each network component. ROIs were subsequently defined, five per network, such that they recapitulated key regions within each network component as previously reported in the literature (Laird et al., 2011). Importantly, all the defined ROIs for each of the individual networks of interest fell within network topography that was common across all three groups (threshold: p < 0.001, FDR: p < 0.05). Spherical ROIs of radius 4 mm were centered on each of these coordinates (see Supporting Information Table S2 for MNI coordinates).
Calculation of Functional Connectivity
Smoothed images were imported into the Functional Connectivity toolbox (http://www.nitrc.org/projects/conn) in SPM8. A temporal low band pass filter was applied retaining frequencies between 0.009–0.08 Hz. Spurious variance was reduced by regression of nuisance waveforms derived from six variable head motion parameters (and their first temporal derivative), mean whole‐brain signal, and the signal extracted from 4 mm radius masks placed in the white matter (27 −21 28) and cerebrospinal fluid (CSF) (−21 −36 20). The BOLD time‐course was extracted from each of the nuisance‐corrected source ROIs and then Pearson's correlation coefficients were calculated for each pair‐wise connection across ROIs spanning the striatum, thalamus and other large‐scale networks. These values were then normalized using a Fisher's r‐to‐Z transformation.
Connectivity Across Subdivisions of the Striatum
To investigate connectivity across the subdivisions of the striatum, we calculated the strength of connectivity across each possible connection between the 14 (seven per hemisphere) striatal ROIs, and compared the strength of these individual striatal ROI‐to‐ROI connections between healthy controls, Parkinson's disease “on” and Parkinson's disease “off” using a series of t‐tests (independent samples for comparison of healthy controls and Parkinson's disease and a paired‐samples t‐test for comparison across medication states). A false detection rate of p < 0.05 was administered to correct for multiple comparisons after testing between the three groups for this specific analysis (Benjamini and Hochberg, 2000).
To calculate global patterns of connectivity across striatal subdivisions, we also obtained the mean connectivity across the entire striatum by calculating the mean Fisher's r‐to‐Z score for all connections between the 14 striatal ROIs. In addition, we also calculated the mean connectivity across subdivisions of the anterior striatum, by calculating the mean Fisher's r‐to‐Z score for connections between the bilateral VSi, VSs, and DC; and of the posterior striatum, by calculating the mean Fisher's r‐to‐Z score for connections between the bilateral DCP, DRP, VRP, and PCP. These global measures of striatal interconnectivity were subsequently compared between the three groups.
Connectivity Within Large‐Scale and Thalamic Networks
In addition, we calculated the strength of internal network connectivity for each large‐scale cortical network and for the thalamic network. This measure was calculated by taking the mean Fisher's r‐to‐Z score for all connections between ROIs belonging to a given network. We subsequently used this score to compare the strength of within‐network connectivity between the three groups.
Relationship Between Striatal Interconnectivity and Large‐Scale Network Connectivity
To explore whether the strength of striatal interconnectivity was related to the strength of internal connectivity within large‐scale neuronal networks, we correlated the mean striatal interconnectivity measure (calculated as the mean Fisher's r‐to‐Z score for connections across all subdivisions of the striatum) for each individual subject, against their internal network connectivity for each of the large‐scale networks. Correlations between striatal interconnectivity and large‐scale network connectivity were estimated using Pearson's product‐moment correlations and were compared statistically using the Dunn and Clark statistic (Dunn and Clark, 1974). In addition, we performed an analysis to determine whether the internal connectivity of any given extra‐striatal large‐scale network might relate to the internal connectivity within other networks. To achieve this, the strength of internal connectivity within each extra‐striatal large‐scale network was correlated with the strength of internal connectivity within each remaining extra‐striatal network, separately. A false detection rate of p < 0.05 was administered to correct for multiple comparisons after comparing the three groups (Benjamini and Hochberg, 2000).
Group‐Level Results are Robust to the Effects of Head Movement
To ensure that group‐level results were not influenced by spurious correlations as a result of head movement in the scanner, we conducted two further analyses. First, we calculated the mean framewise displacement, defined as the sum of the absolute values of the temporal derivative of the six head motion parameters for each individual subject (Power et al., 2012), and then compared this value across each of the three groups statistically using t‐tests. Second, to ensure that all significant group‐level results in this study were robust to the effects of head motion, we performed additional partial correlation analyses for each of the significant correlations observed in this study, using the mean framewise displacement as a covariate (see “Results: Group‐level results are robust to the effects of head movement”).
Statistical Analysis
An independent samples t‐test was used for the comparison of healthy controls and Parkinson's disease and a paired‐samples t‐test was used for the comparison across medication states in the Parkinson's disease cohort. For each test utilized, multiple comparisons were corrected for using a Bonferroni correction at the group level. In addition, we also assessed for a potential relationship between our primary imaging findings and clinical motor impairment in Parkinson's disease by correlating both mean striatal interconnectivity (anterior, posterior, and total striatum, respectively) and mean thalamic interconnectivity against the severity of motor impairment (as assessed using the UPDRS‐III) for each individual subject in the “off” state.
RESULTS
Connectivity Across Subdivisions of the Striatum
Our data demonstrate widespread deficits in functional connectivity between subdivisions of the striatum in patients with Parkinson's disease compared to healthy controls (see Fig. 2 for between‐group differences between individual striatal region‐to‐region connections). Specifically, we found that connectivity is most severely impaired in patients with Parkinson's disease in the “off” state, demonstrating impaired connectivity across the anterior striatum (t = 3.06, p = 0.003), posterior striatum (t = 4.10, p < 0.001), and the striatum as a whole (t = 3.86, p < 0.001; Fig. 1b), compared to healthy controls. In the “on” state, patients with Parkinson's disease revealed increased connectivity across the posterior striatum (t = 2.99, p < 0.003) and total striatum (t = 2.89, p < 0.005), compared to the “off” state, however, differences were not significant for the anterior striatum (t = 1.49, p = 0.138). When compared to healthy controls, patients in the “on” state demonstrated reduced connectivity for the posterior striatum (t = 2.78, p < 0.005), but not for the anterior (t = 1.58, p = 0.119) or the striatum as a whole (t = 1.13, p = 0.263). Between‐group differences were also present when connectivity across striatal subdivisions was calculated for the left and right hemispheres individually.
Figure 2.
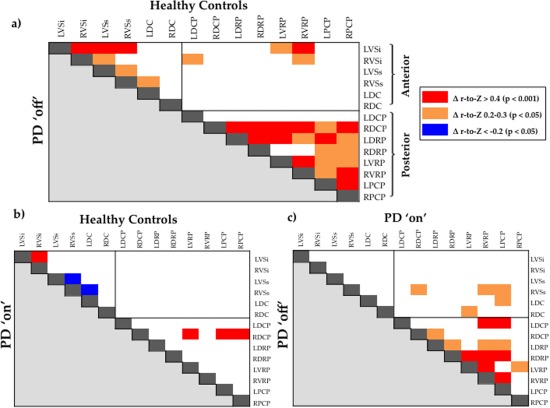
Matrices demonstrating significant between‐group differences in functional connectivity for each striatum‐to‐striatum connection. (a) Significant differences in functional connectivity for individual striatum‐to‐striatum connections for healthy controls versus Parkinson's disease “off”. (b) Significant differences in functional connectivity for individual striatum‐to‐striatum connections for healthy controls versus Parkinson's disease “on”. (c) Significant differences in functional connectivity for individual striatum‐to‐striatum connections for Parkinson's disease “on” versus Parkinson's disease “off”. Abbreviations—DC, Dorsal caudate; DCP, Dorsal caudal putamen; DRP, Dorsal rostral putamen; HC, Healthy Controls; L, Left hemisphere; PCP, Postcommissural putamen; PD, Parkinson's disease; R, Right hemisphere; VRP, Ventral rostral putamen; VSi, Ventral striatum inferior; VSs, Ventral striatum superior. Δ r‐to‐Z, difference in mean r‐to‐Z score between the two groups (group labelled above the matrix vs. group labelled left to the matrix); Red—mean r‐to‐Z score significantly greater for the group labelled above the matrix than the group labelled to the left of the matrix (p < 0.001). Orange—mean r‐to‐Z score significantly greater for the group labelled above the matrix than the group labelled to the left of the matrix (p < 0.05). Blue—mean r‐to‐Z score significantly reduced for the group labelled above the matrix than the group labelled to the left of the matrix (p < 0.05). Correction for multiple comparisons using an FDR of p = 0.05. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 1.
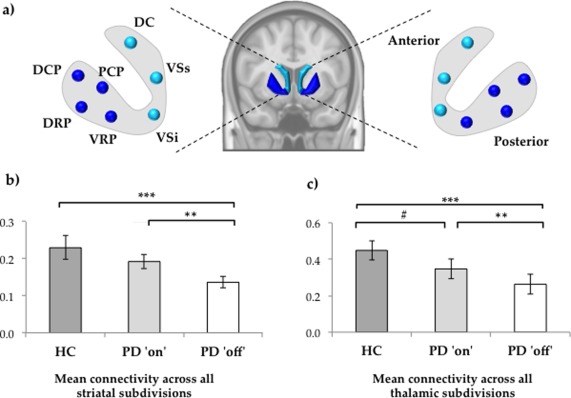
(a) Schematic diagram illustrating ROIs spanning functional subdivisions of the striatum. ROIs sampling the caudate and ventral striatum were considered to comprise the anterior striatum, whereas ROIs sampling the putamen were considered to comprise the posterior striatum. (b) Mean functional connectivity across the global striatum calculated as the mean Fisher's r‐to‐Z score for all connections between each striatal ROI. (c) Mean functional connectivity across the subdivisions of the thalamus calculated as the mean Fisher's r‐to‐Z score for all connections between each thalamic ROI. Statistical significance—*** p < 0.001; ** p < 0.005; # p < 0.01 (corrected for multiple comparisons using Bonferroni correction). Abbreviations—DC, Dorsal caudate; DCP, Dorsal caudal putamen; DRP, Dorsal rostral putamen; HC, Healthy Controls; PCP, Postcommissural putamen; PD, Parkinson's disease; VRP, Ventral rostral putamen; VSi, Ventral striatum inferior; VSs, Ventral striatum superior. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Connectivity Within Large‐Scale and Thalamic Networks
We observed deficits in functional connectivity between subdivisions of the thalamus in Parkinson's disease compared to healthy controls. Specifically, we found that global connectivity across all subdivisions of the thalamus was reduced in Parkinson's disease in the “off” state, compared to both healthy controls (t = 4.56, p < 0.001) and the Parkinson's disease “on” state (t = 2.83, p = 0.007; Fig. 1c). In addition, we also observed impaired connectivity within the thalamus in the Parkinson's disease “on” state when compared to healthy controls (t = 2.31, p = 0.023), however, this finding did not survive strict correction for multiple comparisons.
Furthermore, we observed that patients with Parkinson's disease in the “off” state demonstrate reduced connectivity within the CBM compared to healthy controls (t = 2.69, p = 0.008) and also to patients “on” medication (t = 2.93, p = 0.006), however, there were no significant differences between patients in the “on” state and healthy controls (t = 0.21, p = 0.826). Compared to healthy controls, patients with Parkinson's disease in the “off” state revealed significantly reduced connectivity within the SM (t = 2.87, p = 0.007), however, there were no differences between the Parkinson's disease “on” state, when compared to “off” state (t = 1.21, p = 0.233) and when compared to healthy controls (t = 1.95, p = 0.055). Patients in the “off” state also showed significantly reduced connectivity within the LFPN network compared to healthy controls (t = 2.87, p = 0.007), however, there were no significant differences between patients in the “on” state compared to healthy controls (t = 1.68, p = 0.099) or the “off” state (t = 1.79, p = 0.077). No other networks displayed within‐network connectivity differences between the three groups.
Decoupling of the Striatal Network in Parkinson's Disease
In healthy controls, the strength of connectivity across the striatum was strongly correlated with the strength of internal coherence within large‐scale networks of the brain (Fig. 3a). Specifically, we observed that striatal interconnectivity was strongly related to the strength of internal network connectivity for the SM (r = 0.470, p = 0.002), CBM (r = 0.403, p = 0.010), DMN (r = 0.318, p = 0.046), and thalamic (r = 0.532, p < 0.001) networks. There was also a trend toward significant positive correlation between the strength of striatal interconnectivity and the strength of internal network connectivity for the remaining large‐scale networks: LFPN (r = 0.295, p = 0.063), RFPN (r = 0.205, p = 0.102), VAN (r = 0.220, p = 0.086), and VIS (r = 0.252, p = 0.058). These relationships were also present when connectivity across striatal subdivisions was calculated specifically for the anterior and posterior striatum, and when connectivity was calculated for the left and right striatal hemispheres separately.
Figure 3.
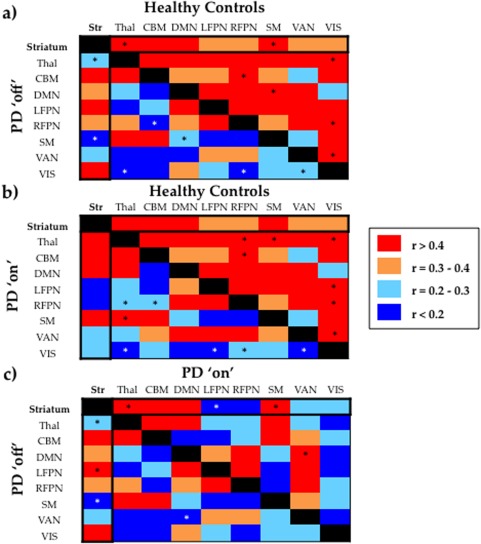
(a) Top right half of the asymmetrical matrix reveals how internal connectivity within each large‐scale network correlates with internal connectivity with the other large‐scale networks in the healthy control group. Bottom left half of the matrix reveals how internal connectivity within each network correlates with internal connectivity with the other networks in the Parkinson's disease group in the “off” state. (b) Top right half of the matrix reveals how internal connectivity within each network correlates with internal connectivity with the other networks in the healthy control group. Bottom left half of the matrix reveals how internal connectivity within each large‐scale network correlates with internal connectivity with the other large‐scale networks in the Parkinson's disease group in the “on” state. (c) Top right half of the matrix reveals how internal connectivity within each network correlates with internal connectivity with the other networks in the Parkinson's disease group in the “on” state. Bottom left half of the matrix reveals how internal connectivity within each large‐scale network correlates with internal connectivity with the other large‐scale networks in the Parkinson's disease group in the “off” state. * Denotes correlations that were significantly different between the two comparison groups (statistical significance p < 0.05, corrected for multiple comparisons using an FDR of p = 0.05). Abbreviations—CBM, Cerebellar Network; DMN, Default Mode Network; LFPN, Left Frontoparietal Network; RFPN, Right Frontoparietal Network; SM, Sensorimotor Network; Str, Striatum; Thal, Thalamus; VAN, Ventral Attention Network; VIS, Visual Network. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Importantly, the correlations between striatal interconnectivity with internal thalamic and internal SM connectivity observed in the healthy control group, were absent in Parkinson's disease in the “off” state (Fig. 3a). Specifically, we found that the correlation between the strength of connectivity across striatal subdivisions and the strength of internal connectivity within the thalamus was significantly reduced in patients with Parkinson's disease in the “off” state (r = 0.088, p = 0.594), compared to healthy controls (r = 0.532, p < 0.001; ZI* = 2.17, p = 0.05) and also compared to the “on” state (r = 0.472, p < 0.001; ZI* = 1.82, p = 0.034). Similarly, the correlation between the strength of connectivity across striatal subdivisions and strength of connectivity within the SM was reduced in patients with Parkinson's disease in the “off” state (r = 0.023, p = 0.889), compared to healthy controls (r = 0.470, p = 0.002; ZI* = 2.09, p = 0.018) and also compared to the “on” state (r = 0.470, p = 0.003; ZI* = 2.09, p = 0.018). Finally, the correlation between the strength of connectivity across striatal subdivisions with the strength of internal LFPN connectivity was significantly stronger in the Parkinson's disease “off” state (r = 0.429, p = 0.003) compared to the “on” state (r = 0.007, p = 0.483; ZI* = 1.9, p = 0.045). Importantly, all the significant results at the group level remained after partial correlation for clinical variables, such as age, general cognition (as measured by MMSE), and level of education.
Decoupling of Extra‐Striatal Large‐Scale Networks in Parkinson's Disease
Although the primary focus of this article was to characterize the effects of striatal dysfunction on the functional architecture of the brain in Parkinson's disease, we were also secondarily interested in exploring whether this disease cohort expressed impaired connectivity relationships across extra‐striatal networks. We found that, in the healthy control group, the strength of internal connectivity within many of the extra‐striatal large‐scale networks was correlated with internal connectivity within many other networks (Fig. 3a). This suggests that, in general, the strength of internal connectivity of one network correlates with the strength of internal connectivity of many other networks in the healthy brain at the group level. Remarkably, however, these inter‐relationships between extra‐striatal networks are diminished, and in many cases absent, in Parkinson's disease (Fig. 3a). These results suggest that, in Parkinson's disease, the breakdown of inter‐relationships in internal connectivity across large‐scale networks is a phenomenon that is not exclusive to the striatum, but rather, may extend to relationships between extra‐striatal networks.
Group‐Level Results are Robust to the Effects of Head Movement
When comparing the mean framewise displacement between the three groups we found no significant between‐group differences (Controls vs. Parkinson's disease “off”, p = 0.413; Controls vs. Parkinson's disease “on”, p = 0.687; Parkinson's disease “off” vs. Parkinson's disease “on”, p = 0.217), suggesting that head motion did not differentially alter connectivity across groups. Additionally, to ensure that all group‐level correlations observed in this study were robust to the effects of head motion, we performed partial correlation analyses for each of the significant results observed, using the mean framewise displacement as a covariate. Each of the significant results reported above remained significant, demonstrating that correlations observed at the group level are robust to the effects of head motion. There were two specific instances where significant results were not present after covarying for mean framewise displacement using partial correlation analyses. Specifically, in the Parkinson's disease “off” state, correlations between striatal interconnectivity with internal network connectivity within the LFPN and DMN did not reach significance after covarying for mean framewise displacement. Given that these specific results may be related to head movement, we have not reported their statistics in the results section of this study. However, all other reported results were robust to head movement displacement.
Functional Connectivity and Clinical Variables
We observed a negative correlation between clinical motor impairment (as assessed by UPDRS‐III) and mean posterior striatal interconnectivity in the “off” state (r = −0.430, p < 0.01), however, this result did not survive correction for multiple comparisons.
DISCUSSION
Given that dopaminergic denervation of the striatum represents the core pathophysiological insult in the evolution of Parkinson's disease (Dauer and Przedborski, 2003), we investigated whether functional connectivity across subdivisions of the striatum was compromised by the dopaminergic pathology of Parkinson's disease. The results of this investigation suggest an essential role of dopamine in integrated striatal function and demonstrate the pathological consequences of striatal denervation in Parkinson's disease.
In this study, we have demonstrated widespread impairments in functional connectivity across striatal subdivisions in patients with Parkinson's disease when withdrawn from dopaminergic medication (Fig. 1b), particularly between posterior regions of the striatum (Fig. 2a). In addition, we have shown that the administration of dopaminergic medication significantly improves connectivity between posterior subdivisions of the striatum in Parkinson's disease, although not to a level observed in the healthy control group (Fig. 2b,c). These results are in keeping with the known spatiotemporal progression of nigrostriatal dopaminergic cell loss in Parkinson's disease, in which the most severe dopamine deficit occurs within the posterior striatum, while anterior striatal regions are relatively less affected (Bruck et al., 2006; Fearnley and Lees, 1991; Kish et al., 1988). Together, these results suggest that impairments in striatal connectivity may be related to the underlying severity of dopamine depletion across the topography of the striatum. In addition, we also found that, when “on” dopaminergic medication, patients with Parkinson's disease display increased connectivity between specific connections in the anterior striatum compared to healthy controls (Fig. 2b). This finding is consistent with an extensive literature suggesting that the administration of dopaminergic medication may impair specific cognitive functions reliant on anterior striatal nuclei by “overdosing” the relatively intact anterior striatum in Parkinson's disease (Cools, 2006). One interpretation of this finding is that dopaminergic “overdose” in the anterior striatum induces pathological hyper‐connectivity between specific anterior striatal connections, impairing anterior striatal function in medicated Parkinson's disease. To our knowledge, these results provide the first comprehensive descriptions of functional connectivity across subdivisions of the striatum in the brain, and further, demonstrate that these patterns are sensitive to the dopaminergic state of the striatum.
Taken together, our results provide novel evidence to suggest impaired integration across striatal subdivisions in Parkinson's disease, and moreover, implicate dopaminergic pathology in the breakdown of mechanisms that enable communication across subdivisions of the striatum. These findings may reflect impaired communication across parallel corticostriatal circuits in the dopamine‐deprived striatum in Parkinson's disease. A recent body of neuroanatomical data has revealed the existence of striato‐nigro‐striatal circuitry, comprised of spiraling neuronal connections between the striatum and the dopaminergic midbrain (substantia nigra/ventral tegmental area) (Haber, 2003; Haber et al., 2000), which have been shown to enable transfer of information across functional subdivisions of the striatum (Belin and Everitt, 2008; Everitt and Robbins, 2005; Porrino et al., 2007; Volkow et al., 2006). Although fMRI is unable to disentangle the underlying cellular mechanisms by which dopaminergic pathology compromises connectivity across striatal subdivisions in Parkinson's disease, impaired dopaminergic neurotransmission throughout striato‐nigro‐striatal circuitry represents an important candidate mechanism.
Given that our findings support a dopaminergic basis for impairments in functional connectivity across subdivisions of the striatum in Parkinson's disease (Figs. 1 and 2), we subsequently explored the impact of striatal connectivity impairments in large‐scale network function. In the healthy control group, we found a striking relationship between striatal interconnectivity and large‐scale network connectivity. Specifically we found that, in the healthy control group, the strength of connectivity across the subdivisions of the striatum was strongly correlated with the strength of internal network connectivity for the sensorimotor, thalamic, cerebellar, and default mode networks. On the contrary, in patients with Parkinson's disease “off” dopaminergic medication, striatal interconnectivity did not correlate with functional connectivity within the sensorimotor or thalamic networks (Fig. 3a). Interestingly, the administration of dopaminergic medication restored the relationship between striatal interconnectivity and connectivity within thalamic and sensorimotor networks (SMs) in Parkinson's disease (Fig. 3). Together our results suggest that, when in the “off” state, striatal dysfunction (Figs. 1 and 2) is associated with pathological decoupling of the striatum from the thalamic and sensorimotor networks in Parkinson's disease (Fig. 3), whereby communication across subdivisions of the striatum becomes unrelated to activity within thalamic and sensorimotor systems.
Previous resting‐state studies in Parkinson's disease have revealed aberrant connectivity within the SM (Esposito et al., 2013; Wu et al., 2011), suggesting that these deficits may contribute to motor symptoms of the disease. In this study we have shown that, in the face of striatal dysfunction, there is impaired connectivity within the SM accompanied by pathological dissociation of striatum from the sensorimotor system in the Parkinson's disease “off” state. Importantly, administration of dopamine ameliorates these connectivity impairments, suggesting a central role of striatal dopamine in coordinated striatal‐sensorimotor dynamics. These results complement data from work using positron emission tomography, which have revealed that the severity of dopamine depletion within the posterior striatum is related to clinical motor impairments in Parkinson's disease (Benamer et al., 2000; Seibyl et al., 1995). These findings suggest that depletion of striatal dopamine perturbs cross‐talk across parallel corticostriatal macrocircuits, which may underlie aberrant striatal‐sensorimotor dynamics in Parkinson's disease. This conclusion is supported by previous work that has proposed that integration across limbic, associative and motoric corticostriatal macrocircuits underpins the execution of coordinated behaviors (Haber, 2003; Haber and Calzavara, 2009; Haber et al., 2000; Haber et al., 2006).
The human striatum and thalamus are extensively interconnected via dense anatomical projections. In addition to their contribution to cortiostriatal circuits (Alexander et al., 1990), thalamic nuclei also provide extensive afferent input to the striatum via thalamostriatal projection fibers (Galvan and Smith, 2011), suggesting an intimate relationship between these interconnected subcortical structures. In this study, we have shown that the dopaminergic insult of Parkinson's disease is associated with impaired connectivity at the level of both the striatum (Figs. 1b and 2) and the thalamus (Fig. 1c), accompanied by a pathological dissociation of these two systems (Fig. 3a). Once again, connectional deficits within the thalamus were improved with the administration of dopamine in the Parkinson's disease cohort (Fig. 1c and 3). Previous work has shown that abnormal striatal output from the dopamine‐deprived striatum in Parkinson's disease results in tonic inhibition of the thalamus via the pallidal nuclei (Weinberger and Dostrovsky, 2011). One potential mechanistic explanation for our findings is that increased inhibition of the thalamus in the “off” state results in disordered connectivity across subdivisions of the thalamus and, moreover, hinders effective communication between striatal and thalamic systems in the Parkinson's disease “off” state. Although future studies are needed to elucidate mechanisms underlying the abnormal connectivity across striatal and thalamic systems in unmedicated Parkinson's disease, it is clear that dopaminergic deficits impair processing at multiple levels of the corticostriatal loop, with impairments being observable at the level of the striatum and thalamus.
Interestingly, pathological decoupling of the striatum from thalamic and sensorimotor networks observed in the “off” state, was accompanied by a corresponding increase in coupling between striatal and left frontoparietal systems, when compared to the Parkinson's disease “on” state (Fig. 3c). The observation that striatal interconnectivity was more strongly aligned with that of the left FPN in the “off” state might reflect compensatory recruitment of cognitive control resources, classically linked to the head of the caudate nucleus (Choi et al., 2012; Di Martino et al., 2008; Grahn et al., 2008; Levy et al., 1997), to overcome the severe dopaminergic deficit in the posterior striatum (Bruck et al., 2006; Kish et al., 1988). Of note, previous work has provided preliminary evidence to suggest that there may be compensatory rewiring of striatal‐cortical connections in Parkinson's disease (Helmich et al., 2010), however, this remains a poorly understood phenomenon and presents an important avenue for future research.
In keeping with recent accounts of pathological changes within the architecture of the cerebellum in Parkinson's disease (see Wu and Hallett, 2013 for review), we found that connectivity within the cerebellum was reduced in “off” state compared to healthy controls. Interestingly, administration of dopaminergic therapy restored connectivity within the cerebellum to a level equal to that of controls. These results are in line with results from a recent preliminary report of resting‐state fMRI in Parkinson's disease using graph theoretic analysis, revealing normalization of cerebellar activity following administration of dopaminergic therapy (Jech et al., 2013). Given the increasingly recognized capacity of the cerebellum in the execution of complex actions across multiple behavioral domains (Ramnani, 2006), the role of dopaminergic therapy in normalizing the functional architecture within the cerebellum may represent an important mechanism by which dopaminergic medication contributes to the clinical improvement observed in motor and nonmotor symptoms.
Inspired by our novel finding that interconnectivity across striatal subdivisions was correlated with the strength of internal network connectivity within a number of large‐scale networks in the healthy brain, we also explored whether such inter‐relationships were present across extra‐striatal networks. We found that in the healthy control group, large‐scale networks were related to one another by the strength of their internal connectivity (Fig. 3a), which is perhaps to be expected in the healthy brain. In contrast, we found that, in a similar manner to the striatal network, the inter‐relationships between many extra‐striatal networks breakdown in Parkinson's disease, suggesting that pathological decoupling of large‐scale networks may be a more global phenomenon than originally predicted. Interestingly, however, decoupling of the striatal network appears to be the most sensitive to the dopaminergic impairment of Parkinson's disease (Fig. 3c). Although these results warrant further exploration in future studies, the primary goal of this study was to characterize striatal impairments in Parkinson's disease, and thus, we have primarily focused on interpreting the striatal connectivity results. However, it should emphasized that striatal dysfunction is only part of the overall connectivity impairment in Parkinson's disease (Fig. 3), and that the extra‐striatal neuropathology of Parkinson's disease (Bohnen et al., 2014; Braak et al., 2004; Halliday et al., 2008) likely contributes to the whole‐brain connectivity impairments observed in this, and other work (Dubbelink et al., 2014; Göttlich et al., 2013).
Limitations and Future Directions
Although fMRI provides a powerful tool for investigating temporal correlations across regions of the brain (Smith et al., 2013), functional connectivity as measured by fMRI is not currently able to disambiguate direct monosynaptic anatomical connections from polysynaptic connections (Buckner et al., 2013). Given this limitation, it should be recognized that although a number of mechanisms have been proposed for functional integration across subdivisions of the striatum (Belin and Everitt, 2008; Bevan et al., 1997; Draganski et al., 2008; Haber et al., 2000; Haber et al., 2006; Kolomiets et al., 2001; McFarland and Haber, 2002; Mena‐Segovia et al., 2005; Tziortzi et al., 2014), fMRI is unable to disentangle the precise neuronal circuitry that supports integration across striatal subdivisions. Clarifying the neuronal circuitry that enables communication across subdivisions of the striatum, and the mechanisms by which such circuitry might be compromised by the dopaminergic pathology of Parkinson's disease, remains and important question for future research.
Of note, we did not find a significant linear relationship between striatal interconnectivity and clinical motor impairment after correction for multiple comparisons. However, it is becoming increasingly evident that the relationship between striatal dopamine and behavior is complex and most likely nonlinear (Cools, 2006; Cools and D'Esposito, 2011). In addition, recent work has demonstrated nonlinear relationships between dopamine and patterns of basal ganglia functional connectivity by pharmacologically manipulating the dopaminergic system in healthy adults (Cole et al., 2013). Although not the primary focus of this study, future work may help to clarify both linear and nonlinear relationships between striatal interconnectivity and clinical phenomena in Parkinson's disease using sophisticated statistical models and collecting a more comprehensive battery of clinical data.
CONCLUSION
The results of this investigation support an essential role of dopamine in integrated striatal function and demonstrate the pathological consequences of striatal dopamine denervation in Parkinson's disease. Our findings suggest that, in Parkinson's disease, the dopamine‐deprived striatum is associated with impaired communication across specialized territories of the striatum. Furthermore, we have shown that dopaminergic deficits in striatal connectivity are associated with dysfunction within thalamic and sensorimotor networks in Parkinson's disease. These findings lend themselves to testable predictions about the role of the striatum in integrating information across parallel corticostriatal circuits in health, and the breakdown of such integrative mechanisms in Parkinson's disease.
Supporting information
Supplementary Information Tables.
ACKNOWLEDGMENTS
We would like to thank Professor Russell A. Poldrack for his insightful comments on the manuscript.
Funding: There is no funding to report for this study.
REFERENCES
- Alexander GE, DeLong MR (1985): Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol 53:1417–1430. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Delong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR (1990): Basal ganglia‐thalamocortical circuits: Parallel substrates for motor, oculomotor, "prefrontal" and "limbic" functions. Prog Brain Res 85:119–146. [PubMed] [Google Scholar]
- Baggio H‐C, Sala‐Llonch R, Segura B, Marti M‐J, Valldeoriola F, Compta Y, Tolosa E, Junqué C: Functional brain networks and cognitive deficits in Parkinson's disease (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐Gad I, Morris G, Bergman H (2003): Information processing, dimensionality reduction and reinforcement learning in the basal ganglia. Prog Neurobiol 71:439–473. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ (2008): Cocaine seeking habits depend upon dopamine‐dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57:432–441. [DOI] [PubMed] [Google Scholar]
- Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG (2000): Correlation of Parkinson's disease severity and duration with 123I‐FP‐CIT SPECT striatal uptake. Mov Disord 15:692–698. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (2000): On the adaptive control of the false discovery fate in multiple testing with independent statistics. J Educ Behav Stat 25:60–83. [Google Scholar]
- Bevan MD, Clarke NP, Bolam JP (1997): Synaptic integration of functionally diverse pallidal information in the entopeduncular nucleus and subthalamic nucleus in the rat. J Neurosci 17:308–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Frey KA, Studenski S, Kotagal V, Koeppe RA, Constantine GM, Scott PJ, Albin RL, Muller ML (2014): Extra‐nigral pathological conditions are common in Parkinson's disease with freezing of gait: An in vivo positron emission tomography study. Mov Disord 29:1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004): Stages in the development of Parkinson's disease‐related pathology. Cell Tissue Res 318:121–134. [DOI] [PubMed] [Google Scholar]
- Bruck A, Aalto S, Nurmi E, Vahlberg T, Bergman J, Rinne JO (2006): Striatal subregional 6‐[18F]fluoro‐L‐dopa uptake in early Parkinson's disease: A two‐year follow‐up study. Mov Disord 21:958–963. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT (2013): Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci 16:832–837. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AHV (2009): Non‐motor symptoms of Parkinson's disease: Dopaminergic pathophysiology and treatment. Lancet Neurol 8:464–474. [DOI] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL (2012): The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108:2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Oei NY, Both S, van Gerven JM, Rombouts SA (2013): Differential and distributed effects of dopamine neuromodulations on resting‐state network connectivity. Neuroimage 78:59–67. [DOI] [PubMed] [Google Scholar]
- Cools R (2006): Dopaminergic modulation of cognitive function‐implications for L‐DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev 30:1–23. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M (2011): Inverted‐U‐shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry, 69:e113–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S (2003): Parkinson's disease: Mechanisms and models. Neuron 39:889–909. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP (2008): Functional connectivity of human striatum: A resting state fMRI study. Cereb Cortex 18:2735–2747. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Kloeppel S, Cook PA, Alexander DC, Parker GJM, Deichmann R, Ashburner J, Frackowiak RSJ (2008): Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28:7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbelink K, Hillebrand A, Stoffers D, Deijen JB, Twisk JWR, Stam CJ, Berendse HW (2014): Disrupted brain network topology in Parkinson's disease: A longitudinal magnetoencephalography study. Brain 137:197–207. [DOI] [PubMed] [Google Scholar]
- Dunn OJ, Clark V (1974): Applied Statistics: Analysis of Variance and Regression. New York: Wiley. [Google Scholar]
- Esposito F, Tessitore A, Giordano A, De Micco R, Paccone A, Conforti R, Pignataro G, Annunziato L, Tedeschi G (2013): Rhythm‐specific modulation of the sensorimotor network in drug‐naive patients with Parkinson's disease by levodopa. Brain 136:710–725. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW (2005): Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci 8:1481–1489. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ (1991): Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain 114:2283–2301. [DOI] [PubMed] [Google Scholar]
- Fox MD, Greicius M (2010): Clinical applications of resting state functional connectivity. Front Syst Neurosci 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Smith Y (2011). The primate thalamostriatal systems: Anatomical organization, functional roles and possible involvement in Parkinson's disease. Basal Ganglia 1:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlich M, Münte TF, Heldmann M, Kasten M, Hagenah J, Krämer UM (2013): Altered resting state brain networks in Parkinson's disease. PLoS ONE 8:e77336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM (2008): The cognitive functions of the caudate nucleus. Prog Neurobiol 86:141–155. [DOI] [PubMed] [Google Scholar]
- Haber S (2008): Parallel and integrative processing through the Basal Ganglia reward circuit: Lessons from addiction. Biol Psychiatry 64:173–174. [DOI] [PubMed] [Google Scholar]
- Haber SN (2003): The primate basal ganglia: Parallel and integrative networks. J Chem Neuroanat 26:317–330. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R (2009): The cortico‐basal ganglia integrative network: The role of the thalamus. Brain Res Bull 78:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Fudge JL, McFarland NR (2000). Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 20:2369–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R (2006): Reward‐related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive‐based learning. J Neurosci 26:8368–8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ (2012): Resting state functional connectivity of the striatum in Parkinson's disease. Brain 135:3699–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday G, Hely M, Reid W, Morris J (2008): The progression of pathology in longitudinally followed patients with Parkinson's disease. Acta Neuropathol 115:409–415. [DOI] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P (2007): Pathological synchronization in Parkinson's disease: Networks, models and treatments. Trends Neurosci 30:357–364. [DOI] [PubMed] [Google Scholar]
- Hedreen JC, DeLong MR (1991): Organization of striatopallidal, striatonigral, and nigrostriatal projections in the macaque. J Comp Neurol 304:569–595. [DOI] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I (2010): Spatial remapping of cortico‐striatal connectivity in Parkinson's disease. Cereb Cortex 20:1175–1186. [DOI] [PubMed] [Google Scholar]
- Jech R, Mueller K, Schroeter ML, Ruzicka E (2013): Levodopa increases functional connectivity in the cerebellum and brainstem in Parkinson's disease. Brain 136:e234. [DOI] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K (2009): L‐Dopa modulates functional connectivity in striatal cognitive and motor networks: A double‐blind placebo‐controlled study. J Neurosci 29:7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O (1988): Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. N Engl J Med 318:876–880. [DOI] [PubMed] [Google Scholar]
- Kolomiets BP, Deniau JM, Mailly P, Menetrey A, Glowinski J, Thierry AM (2001): Segregation and convergence of information flow through the cortico‐subthalamic pathways. J Neurosci 21:5764–5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak Y, Peltier S, Bohnen NI, Muller MLTM, Dayalu P, Seidler RD (2010): Altered resting state cortico‐striatal connectivity in mild to moderate stage Parkinson's disease. Front Syst Neurosci 4:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT (2011): Behavioral interpretations of intrinsic connectivity networks. J Cogn Neurosci 23:4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman‐Rakic PS (1997): Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci 17:3870–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez‐Martin P, Falup‐Pecurariu C, Rodriguez‐Blazquez C, Serrano‐Duenas M, Carod Artal FJ, Rojo Abuin JM, Aarsland D (2011). Dementia associated with Parkinson's disease: Applying the Movement Disorder Society Task Force criteria. Parkinsonism Relat Disord 17:621–624. [DOI] [PubMed] [Google Scholar]
- Mazaika PK, Hoeft F, Glovera GH, Reiss AL (2009): Methods and software for fMRI analysis for clinical subjects. [Google Scholar]
- McFarland NR, Haber SN (2002): Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci 22:8117–8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena‐Segovia J, Ross H, Magill P, Bolam JP (2005): The pedunculopontine nucleus In: Bolam JP, Ingham C, Magill P, editors. The Basal Ganglia VIII. Springer, New York City, NY, USA; pp 533–544. [Google Scholar]
- Middleton FA, Strick PL (2000). Basal ganglia and cerebellar loops: Motor and cognitive circuits. Brain Res Brain Res Rev 31:236–250. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Berke JD, Graybiel AM, Ito R, Lansink CS, van der Meer M, Redish AD, Smith KS, Voorn P (2009): Corticostriatal interactions during learning, memory processing, and decision making. J Neurosci 29:12831–12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ (2007). The effects of cocaine: A shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry 31:1593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012): Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N (2006): The primate cortico‐cerebellar system: Anatomy and function. Nat Rev Neurosci 7:511–22. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997): A neural substrate of prediction and reward. Science 275:1593–1599. [DOI] [PubMed] [Google Scholar]
- Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, Zea‐Ponce Y, Baldwin RM, Fussell B, Smith EO, Charney DS, Hoffer PB, Innis RB (1995): Decreased single‐photon emission computed tomographic [123I]beta‐CIT striatal uptake correlates with symptom severity in Parkinson's disease. Ann Neurol 38:589–598. [DOI] [PubMed] [Google Scholar]
- Sharman M, Valabregue R, Perlbarg V, Marrakchi‐Kacem L, Vidailhet M, Benali H, Brice A, Lehericy S (2013): Parkinson's disease patients show reduced cortical‐subcortical sensorimotor connectivity. Mov Disord 28:447–454. [DOI] [PubMed] [Google Scholar]
- Shine JM, Halliday GM, Gilat M, Matar E, Bolitho SJ, Carlos M, Naismith SL, Lewis SJ (2013a): The role of dysfunctional attentional control networks in visual misperceptions in Parkinson's disease. Hum Brain Mapp 13:22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Matar E, Ward PB, Bolitho SJ, Gilat M, Pearson M, Naismith SL, Lewis SJ (2013b): Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson's disease. Brain 136:1204–1215. [DOI] [PubMed] [Google Scholar]
- Shine JM, Matar E, Ward PB, Frank MJ, Moustafa AA, Pearson M, Naismith SL, Lewis SJ (2013c): Freezing of gait in Parkinson's disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain 136:3671–3681. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, Nichols TE, Robinson EC, Salimi‐Khorshidi G, Woolrich MW, Barch DM, U urbil K, Van Essen DC (2013): Functional connectomics from resting‐state fMRI. Trends Cogn Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, Plotkin JL (2010): The role of dopamine in modulating the structure and function of striatal circuits. Prog Brain Res 183:149–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S (2004): Prediction of immediate and future rewards differentially recruits cortico‐basal ganglia loops. Nat Neurosci 7:887–893. [DOI] [PubMed] [Google Scholar]
- Tziortzi AC, Haber SN, Searle GE, Tsoumpas C, Long CJ, Shotbolt P, Douaud G, Jbabdi S, Behrens TE, Rabiner EA, Jenkinson M, Gunn RN (2014): Connectivity‐based functional analysis of dopamine release in the striatum using diffusion‐weighted MRI and positron emission tomography. Cereb Cortex 24:1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C (2006): Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J Neurosci 26:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger M, Dostrovsky JO (2011): A basis for the pathological oscillations in basal ganglia: The crucial role of dopamine. Neuroreport 22:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Hallett M (2013). The cerebellum in Parkinson's disease. Brain 136:696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Long XY, Wang L, Hallett M, Zang YF, Li KC, Chan P (2011). Functional connectivity of cortical motor areas in the resting state in Parkinson's disease. Hum Brain Mapp 32:1443–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Raichle ME (2010): Disease and the brain's dark energy. Nat Rev Neurol 6:15–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Tables.


