Abstract
Trait markers of schizophrenia aid the dissection of the heterogeneous phenotypes into distinct subtypes and facilitate the genetic underpinning of the disease. The microstructural integrity of the white matter tracts could serve as a trait marker of schizophrenia, and tractography‐based analysis (TBA) is the current method of choice. Manual tractography is time‐consuming and limits the analysis to preselected fiber tracts. Here, we sought to identify a trait marker of schizophrenia from among 74 fiber tracts across the whole brain using a novel automatic TBA method. Thirty‐one patients with schizophrenia, 31 unaffected siblings and 31 healthy controls were recruited to undergo diffusion spectrum magnetic resonance imaging at 3T. Generalized fractional anisotropy (GFA), an index reflecting tract integrity, was computed for each tract and compared among the three groups. Ten tracts were found to exhibit significant differences between the groups with a linear, stepwise order from controls to siblings to patients; they included the right arcuate fasciculus, bilateral fornices, bilateral auditory tracts, left optic radiation, the genu of the corpus callosum, and the corpus callosum to the bilateral dorsolateral prefrontal cortices, bilateral temporal poles, and bilateral hippocampi. Posthoc between‐group analyses revealed that the GFA of the right arcuate fasciculus was significantly decreased in both the patients and unaffected siblings compared to the controls. Furthermore, the GFA of the right arcuate fasciculus exhibited a trend toward positive symptom scores. In conclusion, the right arcuate fasciculus may be a candidate trait marker and deserves further study to verify any genetic association. Hum Brain Mapp 36:1065–1076, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: schizophrenia, endophenotype, trait marker, diffusion spectrum imaging, tractography
INTRODUCTION
Schizophrenia is a highly heterogeneous and heritable psychiatric disorder. Although a number of candidate genes have been implicated in the illness, the relationships between genotype and phenotype remain mostly unclear [Craddock et al., 2006; Turner et al., 2006]. The concept of endophenotype was proposed to further the understanding of the genetic basis of psychopathology [Gottesman and Gould 2003; Gottesman and Shields 1973]. An endophenotype is a measurable inherent characteristic that can be obtained from neuropsychology, neurophysiology, or neuroimaging and associated with candidate gene(s) [Bertisch et al., 2010; Bramon et al., 2008; Shaikh et al., 2011]
To emphasize the causal link between a gene and a disease formed by an endophenotype, the original criteria included heritability, association with the disease, stability over time, independence of clinical state, and cosegregation within the patients' families [Gottesman and Gould, 2003; Lenzenweger, 2013]. Later, the more flexible term “trait marker” was adopted to refer to a stable, heritable characteristic that indicates vulnerability to the disease but does not necessarily entail evidence for the casual link [Beedie et al., 2011; Mathalon et al., 2000; Pflueger et al., 2007]. The identification of endophenotypes aids the classification of heterogeneous phenotypes into biologically distinct subtypes of individuals and facilitates understanding of the genetic underpinning and early detection of the disease [Greenwood et al., 2007; Leboyer et al., 1998].
Schizophrenia is a syndrome that is characterized by complex symptomatic manifestations and cognitive dysfunctions in multiple domains [Dickson et al., 2012; Glahn et al., 2007; Tamminga and Holcomb, 2005]. These features have been attributed to the disruption of the underlying structures of neural networks [Ellison‐Wright and Bullmore, 2009; Kubicki et al., 2007]. Diffusion MRI is a technique that is capable of detecting the microstructural integrities of the axonal fibers of the white matter [Basser et al., 2000; Nucifora et al., 2007] and has revealed disruptions of these networks in vivo [Kanaan et al., 2005]. Numerous diffusion MRI studies have reported abnormal white matter regions in patients with schizophrenia and also in first‐episode, drug‐naïve, and high‐risk patients [Lee et al., 2013; Peters et al., 2008; Samartzis et al., 2014]. Evidence suggests that the structural integrity of the white matter is highly heritable [Skudlarski et al., 2013], which supports its potential as a trait marker for the study of genetic vulnerability to schizophrenia.
Because patients with schizophrenia have typically been ill for an extended period of time, it is unclear whether their exhibited neurobiological abnormalities are trait markers [Gouzoulis‐Mayfrank et al., 2003] or consequences of the disease state [Loberg et al., 2004]. One useful paradigm for identifying biological measures as trait markers is the evaluation of individuals, such as unaffected siblings of patients, who are at a higher risk than the general population [Allen et al., 2009; Chen et al., 2006; Leboyer et al., 1998]. Unaffected siblings have genetic backgrounds and early‐life environments that are similar to those of patients and are subject to a nearly ninefold greater risk of developing schizophrenia [Sadock and Sadock, 2007]. Although unaffected siblings of patients do not develop schizophrenia, they share 50% of their genes with the patients [Brunelin, et al., 2008] and may have similar but less severe impairments [Karlsgodt, et al., 2007]. Consequently, the estimated values of trait markers in unaffected siblings should be similar to those of patients and intermediate between those of controls and patients [Knochel, et al., 2012; Oertel, et al., 2010]. For instance, Brunelin et al. [2007] and Marcelis et al. [2004] studied metabolite elevation in response to stress, and found that siblings showed a response which was intermediate between patients and controls.
Previous diffusion MRI studies of siblings have analyzed the tract integrities of selected regions of interest (ROIs) or the whole white matter on a voxel‐by‐voxel basis. Decreases in fractional anisotropy (FA), which is a diffusion tensor imaging (DTI) index that indicates tract integrity, have been found in the medial frontal region [Camchong et al., 2009], hippocampus [Hao et al., 2009], cingulum and angular areas [Hoptman et al., 2008]. However, recent reviews commented that voxel‐based and ROI analyses have yet to provide consistent findings [Kanaan et al., 2005] and might not be optimal for the study of white matter integrity in patients with schizophrenia [Melonakos et al., 2011]. The inconsistency of results also signifies the heterogeneity of the disease [Boos et al., 2007; Ellison‐Wright and Bullmore, 2009].
Knochel et al. [2012] used tract‐based spatial statistics, a new method that performs voxel‐based analysis only on the voxels of the “tract skeleton” [Smith et al., 2006], in a sibling study. These authors reported that both patients and siblings exhibited decreases in white matter integrity in the commissural fibers and association fibers. Because these studies found localized regions with abnormal white matter integrities, the abnormal regions lacking tract identity can only be inferred based on existing atlases [Jones and Cercignani, 2010]. To preserve the tract identity, Kanaan et al. [2005] suggested a tractography‐based analysis (TBA) method [Kunimatsu et al., 2012; Wakana et al., 2004] that analyzes the integrity of a specific tract bundle based on diffusion tractography [Mori and van Zijl, 2002]. However, because the TBA approach invokes tractography, it is operator‐dependent and prone to poor reproducibility when less efficient fiber tracking algorithm is used [Tensaouti et al., 2011]. Although TBA is considered to be the current method of choice [Maddah et al., 2008; Melonakos et al., 2011], it is time consuming when many fiber tracts are to be reconstructed and analyzed [Zhang et al., 2010]. Due to these limitations, prior studies of siblings have focused only on several selected tracts and either revealed no abnormalities in the FA [Kubicki et al., 2013] or equivocal findings [Booset al., 2013].
In this study, we propose an automatic analysis of the microstructural properties of major tract bundles of the brain. The proposed tractography‐based automatic analysis (TBAA) method uses two pieces of information; that is, a high‐quality diffusion spectrum imaging (DSI) template and the coordinates of the cerebral white matter tracts reconstructed on the DSI template. The rationale of the method is to establish a transformation map between the DSI template and the subject's DSI data such that the tract coordinates in the template can be transformed to the native space of the individual's DSI data. The microstructural properties of a specific tract bundle can then be sampled from the subject's DSI data in a voxel‐by‐voxel manner along the tract coordinates.
With this TBAA method, we measured the tract integrities of most of the known major white matter tracts over the entire brain in patients with schizophrenia, unaffected siblings and healthy controls. Our goal was to identify the tracts that may represent trait markers of schizophrenia. We hypothesized that eligible trait markers would exhibit abnormalities in both the patients and siblings and that these abnormalities would exhibit a gradation of differences from patients to siblings to controls. To relate these differences to clinical phenotypes, correlation analyses were also performed between the trait markers and the clinical symptoms of schizophrenia.
METHODS
Participants
A total of 93 subjects were recruited; 31 patients diagnosed with schizophrenia (17 male, 14 female), 31 siblings of individuals with schizophrenia (18 males, 13 female) and 31 healthy controls (15 male, 16 female). All patients were outpatients from the Department of Psychiatry of the National Taiwan University Hospital. Patients were diagnosed after a personal interview by experienced psychiatrists according to the DSM‐IV‐TR criteria for schizophrenia. Patients exhibiting comorbidity with schizoaffective disorder and bipolar affective disorder were excluded. Siblings received a diagnostic interview with the Diagnostic Interview for Genetic Studies (DIGS) [Nurnberger et al., 1994] by research assistants who had received standardized training. The Chinese version of the DIGS and its reliability has been described previously [Chen et al., 1998]. Siblings with the following disorders were excluded: (1) schizophrenia, schizoaffective disorder, other psychotic disorder, (2) bipolar affective disorder or major depressive disorder, (3) substance use disorder, (4) mental retardation, (5) major neurological disorders such as seizure, stroke, major head injury, and so forth, and (6) significant medical diseases such as autoimmune disease, major cardiac or renal disease, and so forth. Patients and siblings were recruited from a total of 43 families. In the 31 siblings, 19 were related to the patients under investigation and 12 were not. Healthy controls were recruited via internet advertisement posted throughout the community and received the same diagnostic procedure as described for siblings by trained research assistants. They had to fulfill the same criteria for siblings and two additional criteria: (1) no significant anxiety disorder, obsessive compulsive disorder, or post‐traumatic stress disorder and so forth, (2) no first‐ or second‐degree relatives having psychotic illness. Participants with the evidence of cerebral pathology such as leukoaraiosis or brain tumors on T2‐weighted images were excluded. Handedness was measured with the Edinburgh Handedness Inventory [Oldfield, 1971], and clinical symptoms of schizophrenia were assessed using the positive and negative syndrome scale (PANSS) [Kay et al., 1987]. The PANSS raters received rating training annually, and the reliability was acceptable (the intraclass correlations ranged from 0.66 to 0.89 for the PANSS items). The hospital institutional review board approved this study, and subjects provided informed consent prior to participation.
MRI Data Acquisition
All images were acquired on a 3T MRI system (Trio, Siemens, Erlangen, Germany) with a 32‐channel phased array head coil. The slice orientations of the axial images were defined as the orientations parallel to the line between the anterior and posterior commissures, which was defined on the sagittal localizer. A fast spin echo sequence was used to acquire T2‐weighted images with 35 contiguous axial slices that covered the whole brain with the following parameters: repetition time (TR) = 5,920 ms; echo time (TE) = 102 ms, flip angle=150°, matrix size = 256 × 256; field of view (FOV) = 248 × 248 mm2; and slice thickness = 3 mm. High‐resolution T1‐weighted images were acquired using a three‐dimensional (3D) magnetization‐prepared 3D gradient echo sequence with the following parameters: TR = 1,560 ms; TE = 3.68 ms; flip angle = 15°; matrix size = 256 × 192 × 208; FOV = 256 × 192 × 208 mm3; resulting in isotropic spatial resolution of 1 mm3. To accurately reconstruct the fiber pathways of the tracts, DSI was used due to its superior ability to resolve crossing fibers within each voxel [Wedeen, et al., 2005]. A spin‐echo diffusion echo planar imaging sequence with twice‐refocused balanced echo [Reese, et al., 2003] was used to acquire 56 contiguous axial DSI images that covered the whole brain; this sequence used the following parameters: TR = 9,600 ms; TE = 130 ms; matrix size = 80 × 80; FOV = 200 × 200 mm2; and slice thickness = 2.5 mm without gap. Based on a modified optimal q‐space sampling scheme [Kuo, et al., 2008], a total of 102 volumes of diffusion‐weighted images were acquired with diffusion‐encoding gradient vectors pointing at the grid points within a half sphere of the q‐space; the maximum diffusion sensitivity (bmax) was 4,000 s/mm2. The scan time for the DSI acquisition was approximately 16 min.
DSI Data Reconstruction
DSI data were reconstructed based on the Fourier relationship between the diffusion MR signals and the diffusion probability density function (PDF). After projecting the diffusion signals to the other half of the sphere in the q‐space and filling the eight corners around the sphere with zeros, Fourier transform was performed on the diffusion MR signals to obtain a PDF at each voxel [Callaghan, et al., 1991]. The orientation distribution function (ODF) was computed by calculating the second moment of the PDF in 362 radial directions (a sixfold tessellated icosahedron). A decomposition method was used to decompose the ODF into several constituent Gaussian ODFs [Yeh et al., 2013]. The decomposed constituent ODFs were used to indicate the local tract orientation in each voxel. Generalized fractional anisotropy (GFA), which is a DSI index that is equivalent to FA in DTI [Fritzsche et al., 2010; Gorczewski et al., 2009], was computed for each voxel using the following formula: (standard deviation of the ODF) / (root mean square of the ODF) [Tuch, 2004].
Tractography‐Based Automatic Analysis
The technical details of TBAA have been described elsewhere [Chen et al., 2014]. In brief, the TBAA method requires two pieces of information: a high quality DSI template and a whole‐brain white matter tract atlas. First, the DSI template, termed NTU‐DSI‐122, was constructed by coregistering 122 healthy subjects' DSI datasets to the Montreal Neurobiology Institute space using a large deformation diffeomorphic metric mapping (LDDMM‐DSI) method [Hsu et al., 2012]. Healthy subjects were recruited via internet advertisement posted throughout the community. The subjects comprised 62 males (age 27.9 ± 5.0 years) and 60 females (age 27.4 ± 5.6 years). All of the subjects were free of the DSM‐IV diagnosis of schizophrenia and other DSM‐IV Axis I diagnoses. None of them had history of clinically significant head trauma, neurological diseases, currently taking psychotropic medication or any first or second‐degree relative with a psychotic disorder. Second, whole‐brain white matter tracts were reconstructed on the DSI template by an expert (W.Y.I. Tseng) using multiple regions of interest (ROIs) and whole brain seeding [Chen et al., 2014; Lo et al., 2011]. The whole‐brain white matter tracts were classified into three categories: (a) association fibers (cortical‐cortical connections), (b) projection fibers (cortical‐spinal, cortical‐caudate, cortical‐putaminal, and cortical‐thalamic connections), and (c) commissural fibers (left‐right hemispheric connections) [Fernandez‐Miranda et al., 2008; Wakana et al., 2004; Zhang et al., 2010]. A total of 74 tracts were reconstructed from the 56 ROIs defined in the automatic anatomical labeling system (See the Supporting Information Table I).
Figure 1 shows a schematic of the TBAA procedures (See the Supporting Information Methods for detail), which included the following steps: (1) a DSI template was built by coregistering the DSI datasets of 122 healthy subjects via LDDMM; (2) seventy‐four white matter tracts were reconstructed on the DSI template using deterministic tractography; (3) a study‐specific template (SST) was created by coregistering the DSI datasets of the 93 studied subjects via LDDMM; (4) the SST was coregistered to the DSI template via LDDMM, and a deformation map of the combined transformations of steps 1 and 3 was determined; (5) the sampling coordinates of the 74 tracts were transformed from the DSI template to the individual DSI datasets with the deformation map; and (6) the GFA values were sampled in the native space along the sampling coordinates. In this study, the mean GFA was calculated for each tract bundle, which resulted in 74 mean GFA values for each participant. The mean GFA of the whole‐brain white matter tracts was obtained by averaging the mean GFA values of 74 tracts.
Figure 1.
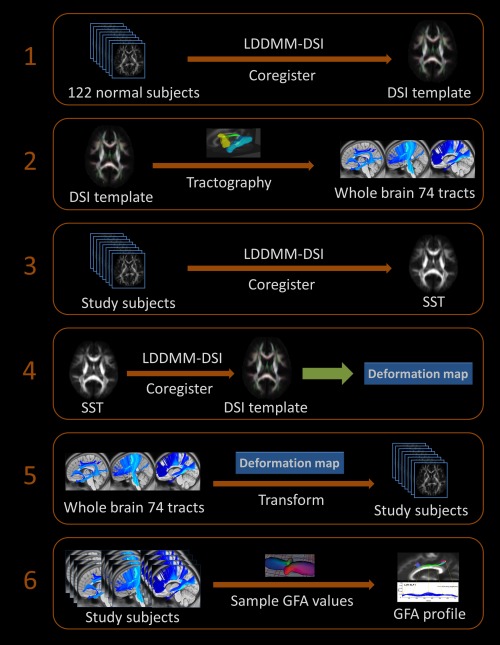
Illustration of the six steps of the TBAA. LDDMM, large deformation diffeomorphic metric mapping; DSI, diffusion spectrum imaging; SST, study‐specific template; GFA, generalized fractional anisotropy.
Statistical Analyses
Data normality was verified using the Kolmogorov–Smirnov test, and descriptive analysis results were expressed as the means and the standard deviations. To assess whether the groups differed on demographic variables, chi‐square tests were conducted for categorical variables. One‐way analyses of variance (ANOVAs) were used for continuous variables when the data exhibited normal distributions; otherwise Kruskal–Wallis ANOVAs were used. To compare the mean GFA values of the 74 major white matter tracts among the three groups, we used analyses of covariance (ANCOVAs) with Benjamini–Hochberg corrections for multiple comparisons, and a false discovery rate (FDR) was set to 0.05 [Benjamini, 2010; van Beveren et al., 2012]. In addition, ANCOVA was performed to compare the mean GFA values of the whole‐brain white matter tracts. Age and gender were used as covariates to minimize their effects on the study variables. To determine the differences between the groups, posthoc Fisher's least significant difference (LSD) tests were applied [Belforte et al., 2010]. Additionally, a trend analysis using the Jonckheere–Terpstra (J–T) test and Kendall's tau rank correlation was performed to examine whether the data from each group were significantly ordered [Filbey et al., 2008].
To study the associations between the altered tracts as determined by the ANCOVAs, partial correlation analyses were performed between the patients' mean GFA values and the clinical parameters (i.e., positive and negative symptom scores of the PANSS, duration of illness, and dose of medication). Gender [Walder et al., 2006] and age [Zhang et al., 2014] were controlled for in the individual tracts based on the dependencies reported in the literature. The significance level for the correlation analyses was defined as 0.05.
RESULTS
Demographic Features
There were no significant differences in age, gender, education, or handedness between the patients, unaffected siblings, and normal controls (Table 1). The patients were clinically stable, and their mean total PANSS score was below 60. The mean duration of illness was 9.16 years (SD = 5.30). All of the patients received antipsychotic medication; the mean chlorpromazine equivalent dose of the 31 patients was 319 mg/day (range 79–731 mg/day) [Andreasen et al., 2010].
Table 1.
Demographic and clinical characteristics of study groups
| Schizophrenia patients (N = 31) | Unaffected siblings (N = 31) | Healthy controls (N = 31) | X 2‐value | P‐value | |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 33.90 ± 8.09 | 33.29 ± 9.80 | 31.26 ± 9.14 | 1.905 | 0.386a |
| Gender, male (%) | 17 (54.8%) | 18 (58.1%) | 15 (48.3%) | 0.611 | 0.739a |
| Education (years), mean ± SD | 14.06 ± 2.06 | 15.25 ± 1.91 | 15.41 ± 2.45 | 5.922 | 0.052a |
| Right‐handed (%) | 31 (100%) | 30 (96.8%) | 31 (100%) | 2.022 | 0.313a |
| Duration of illness | 9.16 ± 5.30 | ||||
| Total antipsychotic dose, mean CPZ equivalent (mg) ± SD | 319.71 ± 156.76 |
Statistical comparison of the demographics across groups by a Kruskal‐Wallis ANOVA.
Chi‐square test.
Abbreviations: CPZ, chlorpromazine.
Integrity of the White Matter Tracts
Repeated measures ANCOVA with the mean GFA values of the 74 white matter tracts as within subject variables, group (patients, siblings and controls) as between subject variables, and age and gender as covariates revealed a significant effect of group for the mean GFA values (P < 0.001; partial Eta‐squared = 0.388). ANCOVAs of the mean GFA values of the 74 white matter tracts over the entire brain revealed 10 tracts that were significantly different among the three study groups after Benjamini–Hochberg corrections for multiple comparisons (FDR = 0.05) (Fig. 2, Supporting Information Tables II and III).
Figure 2.
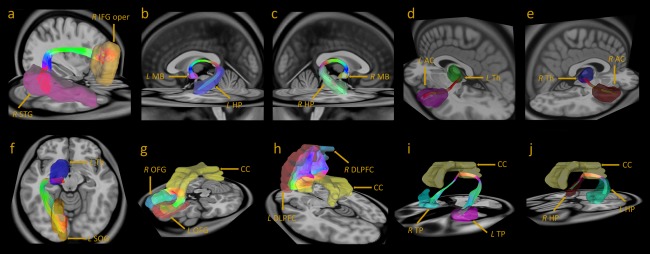
Three‐dimensional fiber tracking of the 10 tracts. (a) Right arcuate fasciculus: ROIs, R STG and R IFG oper. (b) Left fornix: ROIs, L MB and L HP. (c) Right fornix: ROIs, R MB and R HP. (d) Left auditory tract: ROIs, L Th, and L AC. (e) Right auditory tract: ROIs, R Th, and R AC. (f) Left optic radiation: ROIs, L Th, and L SOG. (g) The genu of the corpus callosum: ROIs, R OFG, and L OFG. (h) The corpus callosum to the bilateral DLPFC: ROIs, R DLPFC, and L DLPFC. (i) The corpus callosum to the bilateral temporal poles: ROIs, R TP, and L TP. (j) The corpus callosum to the bilateral hippocampi: ROIs, R HP, and L HP. ROIs, regions of interest; R, right; L, left; STG, superior temporal gyrus; IFG oper, the opercular portion of the inferior frontal gyrus; MB, mammillary body; Th, thalamus; AC: auditory cortex; HP, hippocampus; SOG, superior occipital gyrus; CC, corpus callosum; OFG, orbital frontal gyrus; DLPFC, dorsal lateral prefrontal gyrus; TP, temporal pole.
Among the association fibers, the tracts with significant differences included the right arcuate fasciculus (P = 0.043), left fornix (P = 0.003), and right fornix (P = 0.003). Among the projection fibers, the left auditory tract (P = 0.021), right auditory tract (P = 0.021), left optic radiation (P = 0.043) was found to be significantly different. Among the commissural fibers, the significant tracts included the genu of the corpus callosum (P = 0.021), the corpus callosum to the bilateral dorsolateral prefrontal cortices (DLPFC) (P = 0.018), the corpus callosum to the bilateral temporal poles (P = 0.018), and the corpus callosum to the bilateral hippocampi (P = 0.027). In addition, the mean GFA values of the whole‐brain white matter tracts were significantly different (P = 0.044) among the three study groups. To determine whether whole‐brain white matter tracts affected the results of the tract‐specific differences, the mean GFA values of the whole‐brain white matter tracts were used as an additional covariate. The 10 tracts remained significant after covarying the mean GFA of the whole‐brain white matter tracts (Table 2).
Table 2.
Comparisons of the mean GFA values of the white matter tracts of the three groups (patients, siblings, and controls)
| Fiber tract | Schizophrenic patients | Unaffected siblings | Healthy controls | ANCOVA | Posthoc LSD | ||
|---|---|---|---|---|---|---|---|
| Patients versus controls | Siblings versus controls | Patients versus siblings | |||||
| GFA value Mean ± SD | GFA value Mean ± SD | GFA value Mean ± SD | P‐value | P‐value | P‐value | P‐value | |
| Whole‐brain white matter tracts | 0.285 ± 0.008 | 0.287 ± 0.010 | 0.290 ± 0.005 | 0.044a | 0.018a | 0.232 | 0.233 |
| Rt. AF | 0.262 ± 0.017 | 0.269 ± 0.017 | 0.278 ± 0.012 | b0.043a | 0.001a | c0.035a | 0.108 |
| Lt. FX | 0.140 ± 0.026 | 0.170 ± 0.020 | 0.177 ± 0.015 | b0.003a | <0.001a | 0.205 | <0.001a |
| Rt. FX | 0.185 ± 0.025 | 0.211 ± 0.019 | 0.214 ± 0.015 | b0.003a | <0.001a | 0.593 | <0.001a |
| Lt. AT | 0.196 ± 0.011 | 0.200 ± 0.010 | 0.204 ± 0.010 | b0.021a | 0.005a | 0.129 | 0.171 |
| Rt. AT | 0.199 ± 0.013 | 0.204 ± 0.014 | 0.209 ± 0.007 | b0.021a | 0.002a | 0.100 | 0.136 |
| Lt. OR | 0.308 ± 0.015 | 0.316 ± 0.015 | 0.324 ± 0.015 | b0.043a | <0.001a | 0.057 | 0.047a |
| Genu of CC | 0.294 ± 0.021 | 0.305 ± 0.022 | 0.314 ± 0.021 | b0.021a | 0.001a | 0.136 | 0.055 |
| CC to DLPFC | 0.321 ± 0.018 | 0.332 ± 0.018 | 0.336 ± 0.015 | b0.018a | 0.001a | 0.318 | 0.014a |
| CC to TP | 0.186 ± 0.022 | 0.199 ± 0.015 | 0.205 ± 0.013 | b0.018a | <0.001a | 0.198 | 0.003a |
| CC to HP | 0.176 ± 0.032 | 0.195 ± 0.018 | 0.201 ± 0.022 | b0.027a | <0.001a | 0.371 | 0.005a |
Statistically significant.
A significant difference among three study groups was detected by ANCOVA test while controlling for age and sex and survived Benjamini–Hochberg correction for multiple comparisons (FDR = 0.05).
A significant difference between siblings and controls was detected by posthoc LSD test.
Abbreviations: Rt., right; Lt., left; AF, arcuate fasciculus; FX, fornix; AT, auditory tract; OR, optic radiation; CC, corpus callosum; DLPFC, dorsolateral prefrontal cortex; TP, temporal pole; HP, hippocampus; LSD, least significant difference.
The tracts showing significant differences were analyzed to determine whether they were trait markers or state markers. The J–T tests revealed significant (P < 0.05) linear, stepwise orders from healthy controls to unaffected siblings to patients in all ten of the tracts. To identify the directions of these tendencies, Kendall's tau rank correlations were applied to analyze the white matter tracts that were significantly ordered. The results indicated that the white matter tracts exhibited significant positive correlations such that patients < siblings < controls.
Results of Posthoc Analyses (See Table 2)
To further discriminate the differences among the three study groups, we performed posthoc between‐group analyses of each pair of groups. Compared to the healthy controls, we found significant decreases in the mean GFA values of the 10 tracts in the patients (P ≤ 0.05). In the right arcuate fasciculus, the controls had significantly higher mean GFA values than did the siblings (P = 0.035) and patients (P = 0.001), whereas the difference between the patients and siblings was not significant (P = 0.108). In the other nine tracts, there were no significant differences between the siblings and controls. Comparisons between the patients and siblings revealed that the patient group exhibited significant decreases in the mean GFA values of the bilateral fornices (P < 0.001), left optic radiation (P = 0.047), the corpus callosum to the bilateral DLPFC (P = 0.014), bilateral temporal poles (P = 0.003), and bilateral hippocampi (P = 0.005) and a borderline significant difference in the genu of the corpus callosum (P = 0.055).
Correlations with Clinical Parameters in the Patients
Table 3 summarizes the results of the partial correlation analyses of the GFA values and the positive and negative symptom PANSS scores. The results were uncorrected for multiple comparisons. The right arcuate fasciculus exhibited a significant negative correlation with the severity of the positive symptoms (r = −0.410; P = 0.030). The left and right fornices, left auditory tract, corpus callosum to the bilateral temporal poles and the bilateral hippocampi exhibited significant negative correlations with the severity of the negative symptoms (left fornix, r = −0.405; P = 0.033; right fornix, r = −0.450; P = 0.016; left auditory tract, r = −0.449; P = 0.017; corpus callosum to bilateral temporal poles, r = −0.465, P = 0.013; corpus callosum to bilateral to bilateral hippocampi, r = −0.542, P = 0.004). The left optic radiation, the genu of the corpus callosum and the corpus callosum to the bilateral DLPFC exhibited no significant correlations with the severities of either positive or negative symptoms. In addition, the correlations with duration of illness were significant in the genu of the corpus callosum (r = −0.404, P = 0.027), the corpus callosum to the bilateral DLPFC (r = −0.425, P = 0.019) and the bilateral hippocampi (r = −0.388, P = 0.034) but failed to reach statistical significance in the other seven tracts. For the correlations with medication dosage, significances were only observed in the left optic radiation (r = −0.386, P = 0.035) and genu of the corpus callosum (r = −0.413, P = 0.023). All of the correlations did not survive after the correction for multiple comparisons.
Table 3.
Relationships between the mean GFA values of the white matter tracts and the PANSS positive and negative symptom scores of the schizophrenia patients
| Fiber tract | PANSS symptoms | r | P‐value |
|---|---|---|---|
| Rt. AF | Positive symptoms | −0.410 | a0.030a |
| Lt. FX | Negative symptoms | −0.405 | b0.033a |
| Rt. FX | Negative symptoms | −0.450 | b0.016a |
| Lt. AT | Negative symptoms | −0.449 | b0.017a |
| Rt. AT | — | — | NS |
| Lt. OR | — | — | NS |
| Genu of CC | — | — | NS |
| CC to DLPFC | — | — | NS |
| CC to TP | Negative symptoms | −0.465 | b0.013a |
| CC to HP | Negative symptoms | −0.542 | b0.004a |
Statistically significant (uncorrected for multiple comparisons). The partial correlation analyses between the patients' mean GFA values and the PANSS symptom scores were performed while controlling for a sex. b age.
Statistically significant (uncorrected for multiple comparisons).
Abbreviations: PANSS, Positive and Negative Symptom Scale; Rt., right; Lt., left; AF, arcuate fasciculus; FX, fornix; AT, auditory tract; OR, optic radiation; CC, corpus callosum; DLPFC, dorsolateral prefrontal cortex; TP, temporal pole; HP, hippocampus; NS, nonsignificant.
DISCUSSION
To the best of our knowledge, this is the first study to discover trait/state markers of schizophrenia via TBA of the major white matter tracts over the whole brain. We compared the tract integrities across three study groups using ANCOVAs and found 10 tracts with significant differences. These 10 tracts exhibited a significantly linear, stepwise order from healthy controls to unaffected siblings to patients. Further analyses demonstrated that the GFA of the right arcuate fasciculus was significantly reduced in patients and siblings compared to the controls, and the GFA exhibited a trend toward a correlation with the severity of the positive symptom scores. Our results suggest that the right arcuate fasciculus may be a candidate trait marker of schizophrenia.
Based on the hypothesis that the siblings should possess tract alterations that are similar to those of patients, we identified 10 tracts that exhibited gradations of alterations that distinguished the patients from the controls with the siblings located in between. The 10 tracts found in this study are consistent with those identified in previous studies in patients with schizophrenia that have reported decreased diffusion nisotropy in the frontal and temporal white matter [Lee et al., 2013; Leitman et al., 2007; Phillips et al., 2009; Shergill et al., 2007] and alterations in the optic radiation [Butler et al., 2006]. After identifying these 10 tracts, we performed posthoc analyses of the between‐group differences. We found that the right arcuate fasciculus exhibited significantly decreased microstructural integrity in both the patients and the unaffected siblings compared to the controls (Table 2). In contrast, the other nine tracts exhibited significantly decreased integrities only in patients and not in the siblings. These results indicate that the right arcuate fasciculus is statistically qualified to be a trait marker.
Impairments of the right arcuate fasciculus have been repeatedly reported in schizophrenia [Catani et al., 2011; de Weijer et al., 2013] and are associated with the severity of positive symptoms [de Weijer et al., 2011]. Similarly, we found a trend toward a correlation between positive symptom scores and the right arcuate fasciculus in the patients (Table 3). Given the qualifications of the right arcuate fasciculus as a candidate trait marker, impairments to this structure might exist early in the course of the disease, and such impairments might be predictive of positive symptoms.
In addition to the right arcuate fasciculus, the other nine tracts also exhibited significant trends across the three groups. However, these nine tracts failed to pass the statistical tests in the posthoc between‐group analyses. Compared to the controls, white matter integrity was significantly decreased only in the patients and not in the siblings. Furthermore, comparisons between the patients and siblings revealed that most of the tracts exhibited significant differences with the exception of the bilateral auditory tracts and the genu of the corpus callosum. These results imply that the integrities of these nine tracts might be less affected by the inheritance than by disease course.
Among these nine tracts, the integrities of the left and right fornices, the left auditory tract, the corpus callosum to the bilateral temporal poles and the bilateral hippocampi exhibited a trend toward negative correlations with negative symptom scores. Decreased integrities of the genu of the corpus callosum, corpus callosum to the bilateral DLPFC and the bilateral hippocampi were associated with a trend toward longer duration of illness. The integrities of the left optic radiation and the genu of the corpus callosum were associated with a trend toward dose of mediation. These findings are consistent with previous studies reporting that alteration of these structures were associated with the severity of the negative symptom in patients with schizophrenia [Anderson et al., 2002; Innocenti et al., 2003; Kunimatsu et al., 2012], duration of illness [Downhill et al., 2000] and dose of medication [Goghari et al., 2013; Reis Marques et al., 2014]. In contrast to the right arcuate fasciculus, these tracts are associated with a trend toward negative symptoms or influenced by antipsychotic treatment during their illness. This indicates that these tracts may form a set of biologically distinct markers that behave differently from the right arcuate fasciculus.
The TBAA method was developed in‐house, and its accuracy has been validated [Chen et al., 2014]. Comparison with manual tractography in five selected fiber tracts (i.e., the arcuate fasciculus, cingulum bundle, corticospinal tract, the genu and splenium of the corpus callosum) revealed that TBAA outperforms manual tractography in geometric similarity of the regions traversed by the tracts and in functional variability of the GFA values sampled along the tracts. The advantages TBAA in automation and objectivity allowed us to investigate the majority of the major white matter tracts in a brain‐wise manner over a large group of subjects. In this study, the mean GFA values of the individual tract bundles were measured and compared across three groups. However, some abnormalities may have been local and thus the mean GFA measures may have been unable to localize the lesions or to detect differences. To search for local abnormalities, one could resort to GFA profiles in TBAA to scrutinize the fine‐grained information from each tract. This approach would require a more stringent statistic approach to correct for multiple comparisons.
There are limitations to our study. First, this is a cross‐sectional study of patients being in the chronic stage (duration of illness: 9.16 ± 5.30 years). The trait maker found in our study requires further testing to fulfill criteria for a trait marker. For example, a longitudinal study of first‐episode patients is warranted to verify that the right arcuate fasciculus is impaired in the early stage and that this impairment is stable over the course of disease. Second, we included some unaffected siblings who were unrelated to patients of current study. The altered white matter integrities of both the patient and sibling groups may not be solely attributed to genetic risk. Although some studies [Ceaser et al., 2008; Groom et al., 2008; Simmonite et al., 2012] that have searched for trait markers have included unrelated siblings among the subjects, for a study designed to search for endophenotypes recruitment of the unaffected sibling related to the patients under investigation would provide better control over the influence of family factors. Third, the statistical issues associated with multiple comparisons also need to be addressed. The results of partial correlations with clinical parameters did not survive correction for multiple analyses. Although we used them to elucidate the clinical relevance of the 10 tracts, the results should be interpreted with the support of previous reports. Finally, the sample size of this study was moderate; thus, the differences between the siblings and controls remained statistically insignificant for some tracts; for example, the genu of the corpus callosum and bilateral auditory tracts. A larger cohort may increase the statistical power and reveal additional trait markers.
CONCLUSION
The right arcuate fasciculus was significantly impaired in both the unaffected siblings and the patients with schizophrenia in a predicted, graded pattern. Therefore, the right arcuate fasciculus is a candidate trait marker and may be potentially valuable for establishing the links between genetic liabilities and specific clinical phenotypes.
Supporting information
Supplementary Information
Supplementary Information
Supplementary Information Table 1.
Supplementary Information Table 2.
Supplementary Information Table 3.
REFERENCES
- Allen AJ, Griss ME, Folley BS, Hawkins KA, Pearlson GD (2009): Endophenotypes in schizophrenia: A selective review. Schizophr Res 109:24–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JE, Wible CG, McCarley RW, Jakab M, Kasai K, Shenton ME (2002): An MRI study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophr Res 58:123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC (2010): Antipsychotic dose equivalents and dose‐years: A standardized method for comparing exposure to different drugs. Biol Psychiatry 67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A (2000): In vivo fiber tractography using DT‐MRI data. Magn Reson Med 44:625–632. [DOI] [PubMed] [Google Scholar]
- Beedie SA, Benson PJ, St Clair DM (2011): Atypical scanpaths in schizophrenia: evidence of a trait‐ or state‐dependent phenomenon? J Psychiatry Neurosci 36:150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, Quinlan EM, Nakazawa K (2010): Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia‐like phenotypes. Nat Neurosci 13:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y (2010): Discovering the false discovery rate. J R Stat Soc Ser B 72:405–416. [Google Scholar]
- Bertisch H, Li D, Hoptman MJ, Delisi LE (2010): Heritability estimates for cognitive factors and brain white matter integrity as markers of schizophrenia. Am J Med Genet B Neuropsychiatr Genet 153b:885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos HB, Aleman A, Cahn W, Hulshoff Pol H, Kahn RS (2007): Brain volumes in relatives of patients with schizophrenia: A meta‐analysis. Arch Gen Psychiatry 64:297–304. [DOI] [PubMed] [Google Scholar]
- Boos HB, Mandl RC, van Haren NE, Cahn W, van Baal GC, Kahn RS, Hulshoff Pol HE (2013): Tract‐based DTI in patients with schizophrenia and their non‐psychotic siblings. Eur Neuropsychopharmacol 23:295–304. [DOI] [PubMed] [Google Scholar]
- Bramon E, Dempster E, Frangou S, Shaikh M, Walshe M, Filbey FM, McDonald C, Sham P, Collier DA, Murray R (2008): Neuregulin‐1 and the P300 waveform—A preliminary association study using a psychosis endophenotype. Schizophr Res 103:178–185. [DOI] [PubMed] [Google Scholar]
- Brunelin J, D'Amato T, van Os J, Dalery J, Suaud‐Chagny MF, Saoud M (2007): Serotonergic response to stress: A protective factor against abnormal dopaminergic reactivity in schizophrenia? Eur Psychiatry 22:362–364. [DOI] [PubMed] [Google Scholar]
- Brunelin J, d'Amato T, van Os J, Cochet A, Suaud‐Chagny MF, Saoud M (2008): Effects of acute metabolic stress on the dopaminergic and pituitary‐adrenal axis activity in patients with schizophrenia, their unaffected siblings and controls. Schizophr Res 100:206–211. [DOI] [PubMed] [Google Scholar]
- Butler PD, Hoptman MJ, Nierenberg J, Foxe JJ, Javitt DC, Lim KO (2006): Visual white matter integrity in schizophrenia. Am J Psychiatry 163:2011–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan PT, Coy A, MacGowan D, Packer KJ, Zelaya FO (1991): Diffraction‐like effects in NMR diffusion studies of fluids in porous solids. Nature 351:467–469. [Google Scholar]
- Camchong J, Lim KO, Sponheim SR, Macdonald AW (2009): Frontal white matter integrity as an endophenotype for schizophrenia: Diffusion tensor imaging in monozygotic twins and patients' nonpsychotic relatives. Front Hum Neurosci 3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Craig MC, Forkel SJ, Kanaan R, Picchioni M, Toulopoulou T, Shergill S, Williams S, Murphy DG, McGuire P (2011): Altered integrity of perisylvian language pathways in schizophrenia: Relationship to auditory hallucinations. Biol Psychiatry 70:1143–1150. [DOI] [PubMed] [Google Scholar]
- Ceaser AE, Goldberg TE, Egan MF, McMahon RP, Weinberger DR, Gold JM (2008): Set‐shifting ability and schizophrenia: A marker of clinical illness or an intermediate phenotype? Biol Psychiatry 64:782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG (1998): Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry 155:1214–1220. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bidwell LC, Norton D (2006): Trait vs. state markers for schizophrenia: Identification and characterization through visual processes. Curr Psychiatry Rev 2:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Hsu YC, Lo YC, Tseng WY (2014): Validation of a tract‐based automatic analysis by comparison with manual tractography. In: 22th Scientific Meeting and Exhibition, International Society for Magnetic Resonance in Medicine, Milan, Italy, May 10–16, 2014.
- Craddock N, O'Donovan MC, Owen MJ (2006): Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull 32:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weijer AD, Mandl RC, Diederen KM, Neggers SF, Kahn RS, Hulshoff Pol HE, Sommer IE (2011): Microstructural alterations of the arcuate fasciculus in schizophrenia patients with frequent auditory verbal hallucinations. Schizophr Res 130:68–77. [DOI] [PubMed] [Google Scholar]
- de Weijer AD, Neggers SF, Diederen KM, Mandl RC, Kahn RS, Hulshoff Pol HE, Sommer IE. (2013): Aberrations in the arcuate fasciculus are associated with auditory verbal hallucinations in psychotic and in non‐psychotic individuals. Hum Brain Mapp 34:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson H, Laurens KR, Cullen AE, Hodgins S (2012): Meta‐analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med 42:743–755. [DOI] [PubMed] [Google Scholar]
- Downhill JE, Jr ., Buchsbaum MS, Wei T, Spiegel‐Cohen J, Hazlett EA, Haznedar MM, Silverman J, Siever LJ (2000): Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophr Res 42:193–208. [DOI] [PubMed] [Google Scholar]
- Ellison‐Wright I, Bullmore E (2009): Meta‐analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 108:3–10. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Miranda JC, Rhoton AL, Jr ., Alvarez‐Linera J, Kakizawa Y, Choi C, de Oliveira EP. (2008): Three‐dimensional microsurgical and tractographic anatomy of the white matter of the human brain. Neurosurgery 62:989–1026; discussion 1026–1028. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Toulopoulou T, Morris RG, McDonald C, Bramon E, Walshe M, Murray RM (2008): Selective attention deficits reflect increased genetic vulnerability to schizophrenia. Schizophr Res 101:169–175. [DOI] [PubMed] [Google Scholar]
- Fritzsche KH, Laun FB, Meinzer HP, Stieltjes B (2010): Opportunities and pitfalls in the quantification of fiber integrity: What can we gain from Q‐ball imaging? Neuroimage 51:242–251. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Almasy L, Blangero J, Burk GM, Estrada J, Peralta JM, Meyenberg N, Castro MP, Barrett J, Nicolini H, Raventos H, Escamilla MA (2007): Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet B Neuropsychiatr Genet 144B:242–249. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Smith GN, Honer WG, Kopala LC, Thornton AE, Su W, Macewan GW, Lang DJ (2013): Effects of eight weeks of atypical antipsychotic treatment on middle frontal thickness in drug‐naive first‐episode psychosis patients. Schizophr Res 149:149–155. [DOI] [PubMed] [Google Scholar]
- Gorczewski K, Mang S, Klose U (2009): Reproducibility and consistency of evaluation techniques for HARDI data. MAGMA 22:63–70. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD (2003): The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry 160:636–645. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J (1973): Genetic theorizing and schizophrenia. Br J Psychiatry 122:15–30. [DOI] [PubMed] [Google Scholar]
- Gouzoulis‐Mayfrank E, Voss T, Morth D, Thelen B, Spitzer M, Meincke U (2003): Semantic hyperpriming in thought‐disordered patients with schizophrenia: State or trait?—A longitudinal investigation. Schizophr Res 65:65–73. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ (2007): Initial heritability analyses of endophenotypic measures for schizophrenia: The consortium on the genetics of schizophrenia. Arch Gen Psychiatry 64:1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom MJ, Jackson GM, Calton TG, Andrews HK, Bates AT, Liddle PF, Hollis C (2008): Cognitive deficits in early‐onset schizophrenia spectrum patients and their non‐psychotic siblings: A comparison with ADHD. Schizophr Res 99:85–95. [DOI] [PubMed] [Google Scholar]
- Hao Y, Yan Q, Liu H, Xu L, Xue Z, Song X, Kaneko Y, Jiang T, Liu Z, Shan B (2009): Schizophrenia patients and their healthy siblings share disruption of white matter integrity in the left prefrontal cortex and the hippocampus but not the anterior cingulate cortex. Schizophr Res 114:128–135. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Nierenberg J, Bertisch HC, Catalano D, Ardekani BA, Branch CA, Delisi LE (2008): A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res 106:115–124. [DOI] [PubMed] [Google Scholar]
- Hsu YC, Hsu CH, Tseng WY (2012): A large deformation diffeomorphic metric mapping solution for diffusion spectrum imaging datasets. Neuroimage 63:818–834. [DOI] [PubMed] [Google Scholar]
- Innocenti GM, Ansermet F, Parnas J (2003): Schizophrenia, neurodevelopment and corpus callosum. Mol Psychiatry 8:261–274. [DOI] [PubMed] [Google Scholar]
- Jones DK, Cercignani M (2010): Twenty‐five pitfalls in the analysis of diffusion MRI data. NMR Biomed 23:803–820. [DOI] [PubMed] [Google Scholar]
- Kanaan RA, Kim JS, Kaufmann WE, Pearlson GD, Barker GJ, McGuire PK (2005): Diffusion tensor imaging in schizophrenia. Biol Psychiatry 58:921–929. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M, Kaprio J, Cohen MS, Lonnqvist J, Cannon TD (2007): The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co‐twins, and control subjects. Schizophr Res 89:191–197. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA (1987): The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Knochel C, O'Dwyer L, Alves G, Reinke B, Magerkurth J, Rotarska‐Jagiela A, Prvulovic D, Hampel H, Linden DE, Oertel‐Knochel V (2012): Association between white matter fiber integrity and subclinical psychotic symptoms in schizophrenia patients and unaffected relatives. Schizophr Res 140:129–135. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Jolesz FA, Shenton ME (2007): A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res 41:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Maciejewski PK, Pelavin PE, Hawley KJ, Ballinger T, Swisher T, Jabbar GA, Thermenos HW, Keshavan MS, Seidman LJ, Delisi LE (2013): Decreased axial diffusivity within language connections: A possible biomarker of schizophrenia risk. Schizophr Res 148:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimatsu N, Aoki S, Kunimatsu A, Abe O, Yamada H, Masutani Y, Kasai K, Yamasue H, Ohtomo K (2012): Tract‐specific analysis of white matter integrity disruption in schizophrenia. Psychiatry Res 201:136–143. [DOI] [PubMed] [Google Scholar]
- Kuo LW, Chen JH, Wedeen VJ, Tseng WY (2008): Optimization of diffusion spectrum imaging and q‐ball imaging on clinical MRI system. Neuroimage 41:7–18. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Bellivier F, Nosten‐Bertrand M, Jouvent R, Pauls D, Mallet J (1998): Psychiatric genetics: Search for phenotypes. Trends Neurosci 21:102–105. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kubicki M, Asami T, Seidman LJ, Goldstein JM, Mesholam‐Gately RI, McCarley RW, Shenton ME (2013): Extensive white matter abnormalities in patients with first‐episode schizophrenia: A diffusion tensor iimaging (DTI) study. Schizophr Res 143:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Hoptman MJ, Foxe JJ, Saccente E, Wylie GR, Nierenberg J, Jalbrzikowski M, Lim KO, Javitt DC. (2007): The neural substrates of impaired prosodic detection in schizophrenia and its sensorial antecedents. Am J Psychiatry 164:474–482. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF (2013): Endophenotype, intermediate phenotype, biomarker: Definitions, concept comparisons, clarifications. Depress Anxiety 30:185–189. [DOI] [PubMed] [Google Scholar]
- Lo YC, Soong WT, Gau SS, Wu YY, Lai MC, Yeh FC, Chiang WY, Kuo LW, Jaw FS, Tseng WY. (2011): The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: A study using diffusion spectrum imaging tractography. Psychiatry Res 192:60–66. [DOI] [PubMed] [Google Scholar]
- Loberg EM, Jorgensen HA, Hugdahl K (2004): Dichotic listening in schizophrenic patients: Effects of previous vs. ongoing auditory hallucinations. Psychiatry Res 128:167–174. [DOI] [PubMed] [Google Scholar]
- Maddah M, Kubicki M, Wells WM, Westin CF, Shenton ME, Grimson WE (2008): Findings in schizophrenia by tract‐oriented DT‐MRI analysis. Med Image Comput Comput Assist Interv 11:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelis M, Cavalier E, Gielen J, Delespaul P, Van Os J (2004): Abnormal response to metabolic stress in schizophrenia: Marker of vulnerability or acquired sensitization? Psychol Med 34:1103–1111. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A (2000): Trait and state aspects of P300 amplitude reduction in schizophrenia: A retrospective longitudinal study. Biol Psychiatry 47:434–449. [DOI] [PubMed] [Google Scholar]
- Melonakos ED, Shenton ME, Rathi Y, Terry DP, Bouix S, Kubicki M (2011): Voxel‐based morphometry (VBM) studies in schizophrenia‐can white matter changes be reliably detected with VBM? Psychiatry Res 193:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, van Zijl PC (2002): Fiber tracking: principles and strategies—A technical review. NMR Biomed 15:468–480. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Lee SK, Melhem ER (2007): Diffusion‐tensor MR imaging and tractography: Exploring brain microstructure and connectivity. Radiology 245:367–384. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr ., Blehar MC, Kaufmann CA, York‐Cooler C, Simpson SG, Harkavy‐Friedman J, Severe JB, Malaspina D, Reich T (1994): Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH genetics initiative. Arch Gen Psychiatry 51:849–859; discussion 863–844. [DOI] [PubMed] [Google Scholar]
- Oertel V, Knochel C, Rotarska‐Jagiela A, Schonmeyer R, Lindner M, van de Ven V, Haenschel C, Uhlhaas P, Maurer K, Linden DE (2010): Reduced laterality as a trait marker of schizophrenia—Evidence from structural and functional neuroimaging. J Neurosci 30:2289–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Peters BD, de Haan L, Dekker N, Blaas J, Becker HE, Dingemans PM, Akkerman EM, Majoie CB, van Amelsvoort T, den Heeten GJ, Linszen DH (2008): White matter fibertracking in first‐episode schizophrenia, schizoaffective patients and subjects at ultra‐high risk of psychosis. Neuropsychobiology 58:19–28. [DOI] [PubMed] [Google Scholar]
- Pflueger MO, Gschwandtner U, Stieglitz RD, Riecher‐Rossler A (2007): Neuropsychological deficits in individuals with an at risk mental state for psychosis—Working memory as a potential trait marker. Schizophr Res 97:14–24. [DOI] [PubMed] [Google Scholar]
- Phillips OR, Nuechterlein KH, Clark KA, Hamilton LS, Asarnow RF, Hageman NS, Toga AW, Narr KL (2009): Fiber tractography reveals disruption of temporal lobe white matter tracts in schizophrenia. Schizophr Res 107:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ (2003): Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med 49:177–182. [DOI] [PubMed] [Google Scholar]
- Reis Marques T, Taylor H, Chaddock C, Dell'acqua F, Handley R, Reinders AA, Mondelli V, Bonaccorso S, Diforti M, Simmons A, David AS, Murray RM, Pariante CM, Kapur S, Dazzan P (2014): White matter integrity as a predictor of response to treatment in first episode psychosis. Brain 137:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadock BJ, Sadock VA (2007): Kaplan and Sadock's Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry, 10th ed Philadelphia: Lippincott Williams & Wilkins; p 467. [Google Scholar]
- Samartzis L, Dima D, Fusar‐Poli P, Kyriakopoulos M (2014): White matter alterations in early stages of schizophrenia: A systematic review of diffusion tensor imaging studies. J Neuroimaging 24:101–110. [DOI] [PubMed] [Google Scholar]
- Shaikh M, Hall MH, Schulze K, Dutt A, Li K, Williams I, Walshe M, Constante M, Broome M, Picchioni M, Toulopoulou T, Collier D, Stahl D, Rijsdijk F, Powell J, Murray RM, Arranz M, Bramon E (2013): Effect of DISC1 on the P300 Waveform in Psychosis. Schizophr Bull 39:161–167. [DOI] [PMC free article] [PubMed]
- Shergill SS, Kanaan RA, Chitnis XA, O'Daly O, Jones DK, Frangou S, Williams SC, Howard RJ, Barker GJ, Murray RM, McGuire P (2007): A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry 164:467–473. [DOI] [PubMed] [Google Scholar]
- Simmonite M, Bates AT, Groom MJ, Jackson GM, Hollis C, Liddle PF (2012): Error processing‐associated event‐related potentials in schizophrenia and unaffected siblings. Int J Psychophysiol 84:74–79. [DOI] [PubMed] [Google Scholar]
- Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, Tamminga CA, Clementz BA, O'Neil K, Pearlson GD (2013): Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry 170:886–898. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Holcomb HH (2005): Phenotype of schizophrenia: A review and formulation. Mol Psychiatry 10:27–39. [DOI] [PubMed] [Google Scholar]
- Tensaouti F, Lahlou I, Clarisse P, Lotterie JA, Berry I (2011): Quantitative and reproducibility study of four tractography algorithms used in clinical routine. J Magn Reson Imaging 34:165–172. [DOI] [PubMed] [Google Scholar]
- Tuch DS (2004): Q‐ball imaging. Magn Reson Med 52:1358–1372. [DOI] [PubMed] [Google Scholar]
- Turner JA, Smyth P, Macciardi F, Fallon JH, Kennedy JL, Potkin SG (2006): Imaging phenotypes and genotypes in schizophrenia. Neuroinformatics 4:21–50. [DOI] [PubMed] [Google Scholar]
- van Beveren NJ, Buitendijk GH, Swagemakers S, Krab LC, Roder C, de Haan L, van der Spek P, Elgersma Y (2012): Marked reduction of AKT1 expression and deregulation of AKT1‐associated pathways in peripheral blood mononuclear cells of schizophrenia patients. PLoS One 7:e32618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S (2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230:77–87. [DOI] [PubMed] [Google Scholar]
- Walder DJ, Seidman LJ, Cullen N, Su J, Tsuang MT, Goldstein JM (2006): Sex differences in language dysfunction in schizophrenia. Am J Psychiatry 163:470–477. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM (2005): Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 54:1377–1386. [DOI] [PubMed] [Google Scholar]
- Yeh FC, Verstynen TD, Wang Y, Fernandez‐Miranda JC, Tseng WY (2013): Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 8:e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Li B, Shan B (2014): Age‐related white matter degradation rule of normal human brain: The evidence from diffusion tensor magnetic resonance imaging. Chin Med J (Engl) 127:532–537. [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X, Akhter K, Rosa‐Neto P, Pike GB, Evans A, Toga AW, Woods R, Mazziotta JC, Miller MI, van Zijl PC, Mori S (2010): Atlas‐guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage 52:1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information
Supplementary Information Table 1.
Supplementary Information Table 2.
Supplementary Information Table 3.


