Abstract
The neural systems for phonological processing of written language have been well identified now, while models based on these neural systems are different for different language systems or age groups. Although each of such models is mostly concordant across different experiments, the results are sensitive to the experiment design and intersubject variability. Activation likelihood estimation (ALE) meta‐analysis can quantitatively synthesize the data from multiple studies and minimize the interstudy or intersubject differences. In this study, we performed two ALE meta‐analysis experiments: one was to examine the neural activation patterns of the phonological processing of two different types of written languages and the other was to examine the development characteristics of such neural activation patterns based on both alphabetic language and logographic language data. The results of our first meta‐analysis experiment were consistent with the meta‐analysis which was based on the studies published before 2005. And there were new findings in our second meta‐analysis experiment, where both adults and children groups showed great activation in the left frontal lobe, the left superior/middle temporal gyrus, and the bilateral middle/superior occipital gyrus. However, the activation of the left middle/inferior frontal gyrus was found increase with the development, and the activation was found decrease in the following areas: the right claustrum and inferior frontal gyrus, the left inferior/medial frontal gyrus, the left middle/superior temporal gyrus, the right cerebellum, and the bilateral fusiform gyrus. It seems that adults involve more phonological areas, whereas children involve more orthographic areas and semantic areas. Hum Brain Mapp 35:2607–2618, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: fMRI, activation likelihood estimation, meta‐analysis, language, phonology, Chinese, Alphabetic, development, children, adults
INTRODUCTION
The neural systems of phonological processing have been investigated using neuroimaging methods in various studies for several decades (Petersen et al., 1988), and the results are in general agreement pertaining to the major regions of the brain. These regions lead to different models for different language systems or age groups. Although the activated cortical areas are mostly consistent among different experiments, the results are sensitive to the experiment design and intersubject variability. Activation likelihood estimation (ALE) meta‐analysis can quantitatively synthesize data from functional neuroimaging studies and minimize the interstudy or intersubject differences (Turkeltaub et al., 2002). In a previous meta‐analysis study, Tan et al. (2005) analyzed 13 literatures of either alphabetic language or Chinese language work, which were published before 2005. In this study, we performed meta‐analysis on neural activation patterns of phonological processing using each of two different language systems from papers published after 2005 and made a comparison to the previous analysis (Tan et al., 2005). In addition, we also performed an analysis by applying the same stimuli on different age groups, i.e., children and adults, to investigate the development characteristics of phonological processing in the human brain where the development characteristics were independent.
Alphabetic language (e.g., English and French) and logographic language (e.g., Chinese, Japanese, and Korean) are two different types of written languages, especially in morphologies and mappings among orthography, phonology, and semantics. Most alphabetic languages have a serial left to right structure of letter strings and use grapheme (letters) mapping onto the phonemes (Perfetti et al., 2005). On the other hand, in the writing system of logographic languages, characters are the basic writing units and encode no clear phonological information at the subsyllabic level though most characters have a phonetic radical that can give hints about the pronunciation. Compared to alphabetic languages, logographic languages have less systematic information on phonology (Booth et al., 2006). Two earlier meta‐analyses of fMRI studies on phonological processing showed both similarities and differences between the two types of languages (Bolger et al., 2005; Tan et al., 2005). The analysis of Tan (Tan et al., 2005) showed that both languages activated the left fusiform gyrus and the left inferior frontal gyrus. Furthermore, the study of Bolger (Bolger et al., 2005) suggested that both English and Chinese activated the left middle frontal gyrus, the left inferior frontal gyrus, the mid/anterior portion of the left posterior superior temporal gyrus, and the left occipito‐temporal region. The two meta‐analyses also reported some cross‐language differences that Chinese activated more significantly in the left middle frontal gyrus, and English activated more significantly in the left temporo‐parietal and supramarginal area.
No matter which language system is used, children normally receive training of reading during their early education. Therefore, we would explore the processing model of children and how such model changes with the development into adults to investigate the nature of the brain's organization for processing languages. Some earlier literatures on development studies of brain phonological processing have reported that both children and adults have activation during the rhyming task in the left middle/inferior frontal gyri, the medial frontal gyrus, the left fusiform gyrus, and the bilateral middle occipital gyri (Bitan et al., 2009; Cao et al., 2009; Chee et al., 1999; Chen et al., 2002; Hoeft et al., 2006; Kuo et al., 2004; Shaywitz et al., 2007; Tan et al., 2001; Tan et al., 2003; Temple et al., 2001). It has been shown later that adults activated more greatly in the right middle occipital gyrus on the rhyming task (Cao et al., 2009; Cao et al., 2010), which implies the increased involvement over age of visuo‐orthographic analysis. A more recent study has reported that there are developmental decreases in the activation of the left middle occipital gyrus in the rhyming task, suggesting that the development of reading is marked by the reduced involvement of orthographic representations (Cao et al., 2011). In addition, there appears to be a developmental difference in the phonological processing of the written languages, but due to the differences of the experiments or the intersubject variability, the left inferior occipital gyrus and the left superior temporal gyrus were found to decrease in different studies (Cao et al., 2010; Cao et al., 2011). To statistically integrate the results of these literatures from both alphabetic and logographic language systems, we performed ALE meta‐analysis across two age groups. The goal of this experiment was to determine the changes of neural activation patterns of phonological processing of written languages during the development.
In this study, we conducted two experiments: one to verify the different phonological processing models of different types of written languages which were compared with a previous work (Tan et al., 2005), and the other to analyze the development patterns of the phonological processing during the reading of both language systems.
MATERIALS AND METHODS
Literature Selection
Candidate literatures were identified using an online citation indexing service (web of science, SCI) offered by Thomson Reuters with the following Boolean operation: (“brain mapping” OR “fMRI” OR “functional magnetic resonance imaging”) AND (language OR “word recognition” OR “Chinese reading” OR Chinese OR English OR “Alphabetic words” OR Alphabet) AND (“phonological processing” OR phonological OR phonetic OR phonology) AND (“2005”: “2011/12/31”), which was conducted on the topics of the literatures. This step yielded 661 papers. After the languages were changed from Chinese and alphabetic to Japanese and Korean, 68 results were found. The following inclusion and exclusion criteria were used to finalize the articles.
The inclusion criteria:
fMRI as the imaging modality;
Normal and healthy subjects;
Phonological judgment task involvement in the experiments;
Visual presentation method;
The image data acquisition over the whole brain.
The exclusion criteria:
Case study;
Analysis with prespecified anatomically limited ROIs;
Inexplicit coordinate space that the images were normalized to.
Only the papers associated with positive performances of the participants were kept, because we believed those positive responses could be more pertinent to the neural correlates under study. The experiments were excluded if the established ROIs were anatomically prespecified, but functionally selected ROIs were considered as acceptable.
According to these criteria, the titles and the abstracts from these studies were first evaluated, and the unidentifiable ones were left for the full‐text examination. After this step, only 150 papers were kept. The full‐text articles especially the experiment designs were evaluated based on the criteria listed above. Finally, we obtained 15 papers with 19 experiments for alphabetic languages and nine papers with 13 experiments for logographic languages (Table 1).
Table 1.
Summary of literature selected for meta‐analysis
| Literature | N | Task | Baseline | Language Cateragy |
|---|---|---|---|---|
| Cross language analysis | ||||
| Alphabetic words | ||||
| Aparicio et al., 2007 | 12 | Rhyming judgment | Identical strings judgment | English |
| Bitan et al., 2005 | 14 | Rhyming judgment | Line pattern matching | English |
| Booth et al., 2007 | 14 | Rhyming judgment | Line judgment | English |
| Burton et al., 2005 | 12 | Rhyming judgment | Symbol font discrimination | English |
| Cousin et al., 2007 | 11 | Rhyme detection | Visual detection | French |
| Gitelman et al., 2005 | 14 | Homophone judgment | String matching task | English |
| Tham et al., 2005 | 6 | Homophone matching | Fixation | English |
| Logographic characters | ||||
| Booth et al., 2006 | 13 | Rhyming judgment | Line pattern judgment | Chinese |
| Cao et al., 2009 | 13 | Rhyming judgment | Line pattern judgment | Chinese |
| Cao et al., 2010 | 20 | Rhyme judgment | Spelling | Chinese |
| Dong et al., 2005 | 12 | phonological matching | Fixation | Chinese |
| Liu et al., 2006 | 12 | Phonological decision | Orthographic decision | Chinese |
| Liu et al., 2009 | 16 | Rhyme judgment | Line matching | Chinese |
| Matsuo et al., 2010 | 33 | Homophone judgment | Null | Japanese(Kanji) |
| Tham et al., 2005 | 6 | Homophone matching | Fixation | Chinese |
| Development analysis | ||||
| Adult | ||||
| Aparicio et al., 2007 | 12 | Rhyming judgment | Identical strings judgement | English |
| Bitan et al., 2005 | 14 | Rhyming judgment | Line pattern matching | English |
| Booth et al., 2002a | 13 | Rhymejudgment | Line pattern matching | English |
| Booth et al., 2002b | 13 | Rhymejudgment | Spelling | English |
| Booth et al., 2004 | 16 | Rhymejudgment | Letter case decision | English |
| Booth et al., 2007 | 14 | Rhyming judgment | Line judgment | English |
| Burton et al., 2005 | 12 | Rhyming judgment | Symbolfontdiscrimination | English |
| Cousin et al., 2007 | 11 | Rhyme detection | Visual detection | French |
| Gitelman et al., 2005 | 14 | Homophonejudgment | String matching task | English |
| Poldrack et al., 2001 | 8 | Rhymejudgment | Letter case decision | English |
| Price et al., 1997 | 6 | Syllable decision | Semantic judgment | English |
| Sergent et al., 1992 | 8 | Lettersound decision | Letter spatial decision | English |
| Tan et al., 2003 | 12 | Rhymejudgment | Font size decision | English |
| Tham et al., 2005 | 6 | Homophone matching | Fixation | English |
| Xu et al., 2001 | 12 | Homophone matching | Fixation | English |
| Xu et al., 2002 | 18 | Rhymejudgment | Letter feature search | English |
| Booth et al., 2006 | 13 | Rhyming judgment | Line pattern judgment | Chinese |
| Cao et al., 2009 | 13 | Rhyming judgment | Line pattern judgment | Chinese |
| Liu et al., 2006 | 12 | Phonological decision | Orthographic decision | Chinese |
| Tham et al., 2005 | 6 | Homophone matching | Fixation | Chinese |
| Child | ||||
| Bitan et al., 2006 | 15 | Rhyming judgment | Line pattern | English |
| Bitanet al., 2007a | 38 | Rhyming judgment | Symbols /spelling judgment | English |
| Bitan et al., 2007b | 36 | Rhyming judgment | Fixation | English |
| Bitan et al., 2009 | 36 | Rhyming judgment | Fixation | English |
| Booth et al., 2004 | 16 | Rhyme judgment | Letter case decision | English |
| Cao et al., 2006 | 14 | Rhyming judgment | Fixation | English |
| Cao et al., 2008 | 12 | Rhyming judgment | Fixation | English |
| Georgiewa et al., 1999 | 17 | Phonological transformation | Letter identification | German |
| Hoeft et al., 2006 | 10 | Rhyming judgment | Fixation | English |
| McNorgan et al., 2011 | 26 | Rhyming judgment | Fixation | English |
| Temple et al., 2001 | 15 | Rhyming judgment | line matching | English |
| Cao et al., 2009 | 8 | Rhyming judgment | Line pattern judgment | Chinese |
| Siok et al., 2008 | 12 | Rhyme judgment | Font‐size decision | Chinese |
| Xue et al., 2005 | 12 | Rhyming judgment | Fixation | Chinese |
N: number of subject.
For the cross‐language difference analysis, we chose the experiments from the selected papers where the participants were adults. Eleven experiments for the logographic language group and eight experiments for the alphabetic language group were finalized, respectively (Table 1). We analyzed these results to identify the cross‐language differences in phonological processing in the brain and compared them with the previous studies (Tan et al., 2005).
For the second experiment, we classified the papers into two age groups regardless of the language types: there were 14 experiments for the children group (three on logographic languages and 11 on alphabetic languages), and 19 experiments for the adults group (11 on logographic languages and eight on alphabetic languages). The experiments published before 2005 were included by combining the adults group experiments mentioned above with the previous meta‐analysis in the published studies on adults (seven for Chinese and 12 for alphabetic words) (Tan et al., 2005). Yet, two cases were exclude due to the ROI analysis (Gold and Buckner, 2002) and the missing of standard coordinate space referred to explicitly (Petersen et al., 1988). To cover the earlier publications for the children group, we conducted the Boolean operation mentioned above, adding “child” and changing the publication date to the year before 2005. After filtering with inclusion and exclusion criteria, only three experiments on alphabetic languages were left for the analysis. In summary, 17 experiments were identified for the children group (three logographic and 14 alphabetic) and 38 experiments were identified for the adults group (18 logographic and 20 alphabetic). To balance the experiment amount between the language types, we randomly chose four out of 18 experiments on the logographic languages for the adults group, resulting in 24 final experiments (20 alphabetic and four logographic) in this group(Table 1).
To view the meta‐analysis results, we used the desktop version of Mango (Multi‐Image Analysis GUI, http://ric.uthscsa.edu/mango/).
Activation Likelihood Estimation
For each study, we analyzed all the reported experiments meeting the inclusion criteria. The MNI coordinates were converted into Talairach space using the icbm2tal transform (Lancaster et al., 2007) implemented in the GingerALE software package (Eickhoff et al., 2011; Eickhoff et al., 2009). ALE maps were generated using the activation likelihood estimate (ALE) method (Turkeltaub et al., 2002), which was implemented in the GingerALE software package, with the subject‐based FWHW values (Eickhoff et al., 2009; Eickhoff et al., 2011), 10 mm additional FWHM, 0.05 FDR threshold and 100 mm3 minimum volume size. The ALE method treats each reported coordinate as the center of a Gaussian probability distribution and uses a permutation test of randomly distributed foci to determine the statistical significance. ALE maps were created for logographic characters and alphabetic words, respectively. The same method was used for both the adults and children groups. For the contrary analysis, all parameters were the same as above except that the threshold was set to uncorrected P value of 0.001.
RESULTS
Cross‐Language Analysis
Table 2 and Figure 1 illustrate the results of our ALE meta‐analysis of the studies published in the chosen literatures corresponding to our first experiment design for cross‐language investigation, which are consistent with the results reported in the articles. Specifically, the activation regions for phonological processing of logographic characters were from left precentral gyrus (BA6), left middle frontal gyrus (BA46,9), right middle occipital gyrus, left insula (BA13), left fusiform gyrus (BA37), left inferior frontal gyrus (BA44), and left sublobar extranuclear (BA47) in our analysis. As for the experiments on alphabetic words, the ALE analysis resulted in eight clusters of significant ALE values: left inferior frontal gyrus (BA46), left middle temporal gyrus (BA21), left angular gyrus (BA39), cerebellum, left medial frontal gyrus (BA8), bilateral superior frontal gyrus (BA10, BA8), and left lentiform nucleus.
Table 2.
ALE meta‐analysis results of phonological processing in visual word recognition
| Anatomical region | Brodmann area | x | y | z | ALE (10−2) | Volume (mm3) |
|---|---|---|---|---|---|---|
| Alphabetic words | ||||||
| L Middle Frontal Gyrus | 46 | −44 | 24 | 20 | 1.46 | 1752 |
| L Middle Temporal Gyrus | 21 | −62 | −34 | −2 | 1.45 | 712 |
| L Angular Gyrus | 39 | −44 | −70 | 30 | 0.97 | 704 |
| L Middle Temporal Gyrus | 39 | −46 | −70 | 26 | 0.91 | |
| L Cerebellum | −42 | −56 | −20 | 1.46 | 648 | |
| L Medial Frontal Gyrus | 8 | −14 | 30 | 44 | 1.21 | 520 |
| L Superior Frontal Gyrus | 10 | −10 | 58 | 22 | 1.07 | 472 |
| R Superior Frontal Gyrus | 8 | 8 | 30 | 50 | 0.89 | 384 |
| L Lentiform Nucleus | − | −28 | −16 | −4 | 0.80 | 248 |
| L Hippocampus | −24 | −16 | −10 | 0.73 | ||
| Logographic characters | ||||||
| L Precentral Gyrus | 6 | −46 | 4 | 34 | 3.35 | 4768 |
| L Middle Frontal Gyrus | 46 | −42 | 26 | 20 | 2.08 | |
| L Middle Frontal Gyrus | 9 | −40 | 18 | 26 | 1.99 | |
| L Middle Frontal Gyrus | 9 | −44 | 16 | 32 | 1.80 | |
| R Middle Occipital Gyrus | ‐ | 26 | −86 | 0 | 1.62 | 1344 |
| L Insula | 13 | −30 | 18 | 10 | 1.96 | 968 |
| L Fusiform Gyrus | 37 | −40 | −54 | −18 | 1.30 | 752 |
| L Inferior Frontal Gyrus | 44 | −50 | 6 | 18 | 1.58 | 728 |
| Adult | ||||||
| L Middle Frontal Gyrus | 46 | −44 | 24 | 16 | 2.59 | 10728 |
| L Middle Frontal Gyrus | 46 | −50 | 24 | 26 | 2.57 | |
| L Inferior Frontal Gyrus | 44 | −50 | 10 | 22 | 2.55 | |
| L Precentral Gyrus | 6 | −42 | 0 | 30 | 2.41 | |
| L Precentral Gyrus | 6 | −48 | 0 | 42 | 1.49 | |
| L Precentral Gyrus | 6 | −42 | −10 | 48 | 1.14 | |
| L Superior Temporal Gyrus | 22 | −52 | 12 | 0 | 1.01 | |
| L Cerebellum Declive | −42 | −56 | −20 | 3.65 | 2784 | |
| L Superior Frontal Gyrus | 6 | −2 | 14 | 50 | 1.50 | 1568 |
| L Superior Frontal Gyrus | 6 | −2 | 4 | 56 | 1.21 | |
| R Cerebellum Uvula | ‐ | 8 | −68 | −30 | 2.60 | 1496 |
| L Medial Frontal Gyrus | 8 | −12 | 28 | 44 | 1.40 | 1400 |
| R Superior Frontal Gyrus | 8 | 4 | 28 | 48 | 0.98 | |
| R Middle Occipital Gyrus | 18 | 20 | −86 | −8 | 1.46 | 1160 |
| L Inferior Parietal Lobule | 40 | −54 | −44 | 22 | 2.20 | 1112 |
| L Middle Temporal Gyrus | 21 | −62 | −34 | −2 | 1.74 | 784 |
| L Angular Gyrus | 39 | −26 | −54 | 32 | 1.40 | 680 |
| L Middle Temporal Gyrus | 22 | −46 | −40 | 2 | 1.30 | 648 |
| R Inferior Frontal Gyrus | 47 | 36 | 24 | −2 | 1.18 | 504 |
| L Extra‐Nuclear | 47 | −36 | 24 | −4 | 1.29 | 328 |
| L Middle Occipital Gyrus | 18 | −22 | −84 | −10 | 1.05 | 312 |
| L Angular Gyrus | 39 | −44 | −70 | 30 | 0.98 | 232 |
| L Middle Temporal Gyrus | 39 | −46 | −70 | 26 | 0.92 | |
| L Superior Frontal Gyrus | 10 | −10 | 58 | 22 | 1.08 | 224 |
| Child | ||||||
| L Inferior Frontal Gyrus | 46 | −46 | 26 | 14 | 4.34 | 2864 |
| L Fusiform Gyrus | 37 | −40 | −50 | −16 | 2.56 | 2456 |
| L Cerebellum Declive | ‐ | −42 | −64 | −14 | 1.83 | |
| L Medial Frontal Gyrus | 6 | −6 | 4 | 56 | 2.63 | 2384 |
| L Precentral Gyrus | 6 | −44 | 2 | 34 | 1.87 | 2296 |
| L Inferior Frontal Gyrus | 9 | −46 | 10 | 28 | 1.51 | |
| R Insula | 13 | 34 | 22 | 4 | 2.39 | 2104 |
| L Superior Temporal Gyrus | 22 | −56 | −42 | 10 | 2.2 | 1824 |
| R Fusiform Gyrus | 19 | 26 | −84 | −12 | 1.31 | 952 |
| L Inferior Occipital Gyrus | 19 | −34 | −76 | −6 | 1.38 | 456 |
| R Cerebellum Culmen | 32 | −50 | −14 | 1.57 | 432 | |
| Adult‐Child | ||||||
| L Middle Frontal Gyrus | 9 | −48 | 28 | 30 | 2.76 | 1200 |
| L Inferior Frontal Gyrus | 9 | −54 | 18 | 22 | 2.21 | |
| L Inferior Frontal Gyrus | 45 | −56 | 14 | 20 | 2.16 | |
| R Cerebellum Pyramis | ‐ | 12 | −70 | −24 | 2.15 | 744 |
| R Cerebellum Pyramis | ‐ | 13 | −73 | −30 | 2 | |
| R Cerebellum Uvula | ‐ | 10.42 | −65.5 | −31.33 | 1.91 | |
| L Superior Frontal Gyrus | 8 | −12 | 30 | 48 | 1.98 | 576 |
| L Medial Frontal Gyrus | 8 | −14 | 30 | 40 | 1.69 | |
| L Cerebellum Tuber | ‐ | −45 | −60 | −24 | 1.97 | 408 |
| L Cerebellum Tuber | ‐ | −38 | −60 | −24 | 1.9 | |
| Child‐Adult | ||||||
| R Claustrum | 29 | 20 | 10 | 3.01 | 1288 | |
| R Inferior Frontal Gyrus | 45 | 32 | 28 | 8 | 2.75 | |
| L Inferior Frontal Gyrus | 13 | −44 | 26 | 4 | 2.51 | 1192 |
| L Inferior Frontal Gyrus | 46 | −51 | 26 | 10 | 2.3 | |
| L Medial Frontal Gyrus | 6 | −12 | 6 | 52 | 3.04 | 1144 |
| L Middle Temporal Gyrus | 22 | −52 | −36 | 8 | 2.77 | 976 |
| L Superior Temporal Gyrus | 22 | −56 | −42 | 8 | 2.65 | |
| R Cerebellum Culmen | ‐ | 30.8 | −48 | −11.6 | 2.18 | 432 |
| R Fusiform Gyrus | 37 | 36 | −49 | −12 | 2.1 | |
| L Fusiform Gyrus | 37 | −38 | −46 | −12 | 2.03 | 280 |
| L Inferior Frontal Gyrus | 9 | −44 | 12 | 30 | 2.3 | 240 |
L: left, R: right
Figure 1.
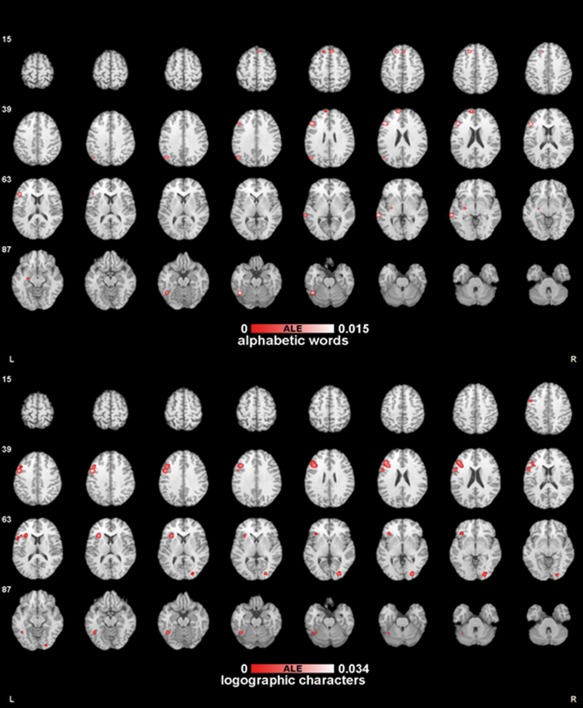
The ALE maps of the different language systems.
Development Analysis
First, we performed the ALE analysis of the selected experiments on phonological processing of the printed words for the adults and children groups, respectively, and then compared the two groups. Table 2 and Figure 2 illustrate the regions of the two groups and their subtractions. The activation for the adults group was of extremely high concordance in the left frontal lobe (BA46, BA44, BA6, and BA47), left superior temporal gyrus (BA22), bilateral cerebellum, left medial frontal gyrus and superior frontal gyrus (BA8), right middle occipital gyrus (BA18), left inferior parietal lobule (BA40), left middle temporal gyrus (BA21, BA22), and left angular gyrus. The ALE analysis of the adults group demonstrated the highest convergence in the left dorsal frontal gyrus (BA46) with a cluster size of 10728 mm3 and ALE value 0.026.
Figure 2.
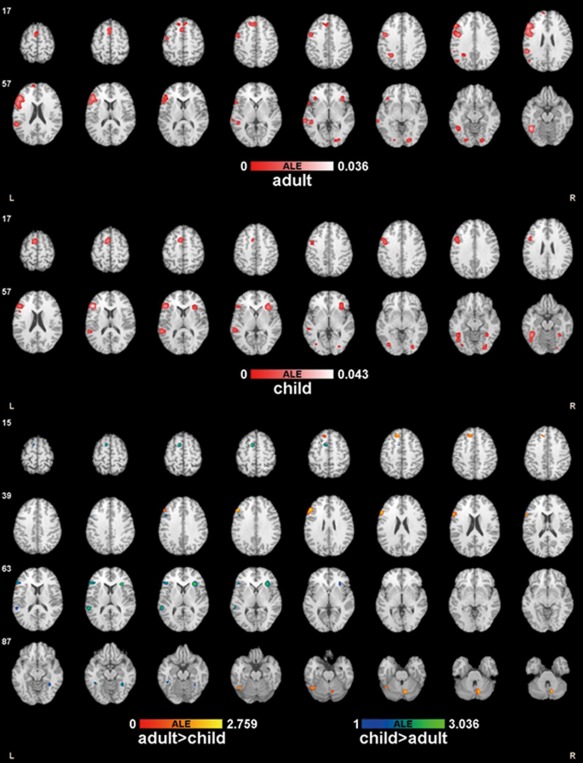
ALE maps across ages of phonological processing of written word.
The ALE apparent activation of the children group indicated high convergence in the left inferior frontal gyrus (BA46), fusiform gyrus (BA37) and cerebellum, left medial frontal gyrus (BA6), left precentral gyrus (BA6) and left inferior frontal gyrus (BA9), right insula (BA13), left superior temporal gyrus (BA22), right fusiform gyrus (BA19), left inferior occipital gyrus (BA19), and right cerebellum. The highest convergence was obtained in the inferior frontal gyrus, with a cluster size of 2864 mm3 and ALE value 0.043.
Both the adults and children groups showed great activation in the left inferior frontal gyrus (BA46, BA44, BA47), left precentral gyrus (BA6), left medial frontal gyrus (BA6, BA8), left superior/middle temporal gyrus (BA22), and bilateral middle/superior occipital gyrus (BA18, BA19).
The contrast between the adults and children groups showed significant differences between their relative ALE maps (Fig. 2). The left middle/inferior frontal gyrus (BA9, BA45) was more consistently activated in the phonological processing of the adults group. Other areas included the bilateral cerebellum and left superior frontal gyrus (BA8). Brain regions that were more concordantly implicated by the children group included right claustrum and inferior frontal gyrus (BA45). In addition, another four clusters were involved in the left inferior frontal (BA13, BA46), left medial frontal gyrus (BA6), left middle/superior temporal gyrus (BA22), right cerebellum, bilateral fusiform gyrus (BA37), and left inferior frontal gyrus (BA9).
DISCUSSION
Cross‐Language Analysis
For both language systems, the activation was concordantly shown in left frontal gyrus and left temporal gyrus. For alphabetic languages, the clusters were consistently in middle frontal gyrus and temporal gyrus, and for logographic language system, the clusters were most consistently in left precentral/middle frontal gyrus (BA6/46/9). These patterns were consistent with the result of the previous work (Tan et al., 2005), which demonstrated that the results of the studies after 2005 (see Table 1) were in general the same as previous ones (Booth et al., 2002a; Booth et al., 2004; Booth et al., 2002b; Georgiewa et al., 1999; Gold and Buckner, 2002; Petersen et al., 1988; Poldrack et al., 2001; Price et al., 1997; Sergent et al., 1992; Siok et al., 2003; Tan et al., 2001; Tan et al., 2003; Temple et al., 2001; Xu et al., 2001; Xu et al., 2002).
Development Analysis
Both children and adults showed activation in the left inferior frontal gyrus, left precentral gyrus, left medial frontal gyrus, left superior/middle temporal gyrus, and bilateral middle/superior occipital gyrus. This activated pattern was consistent with the previous results (Chee et al., 1999; Hoeft et al., 2006; Shaywitz et al., 2007; Tan et al., 2005).
In a previous work (Cao et al., 2010), the left inferior frontal gyrus showed increased activation with development. However, the result of our ALE meta‐analysis demonstrated that there was not only a simple increase of activation in the activated areas during the development but also a shift of activation areas to the dorsolateral prefrontal cortex, from BA13/46 to BA 9/8/45. In the earlier studies, the left inferior frontal gyrus (pars operculars) was suggested as part of the phonological routes to subserve the phonological decoding of words (Cao et al., 2008), and the left inferior frontal gyrus (pars triangularis) was reported as part of the lexico‐semantic routes for the semantic retrieval of words (Bokde et al., 2001). Hence, the inferior frontal gyrus was associated with the search and retrieval of information about meanings, and syntactic and phonological patterning. It makes sense that the activation of this area is increased with age and reading proficiency.
Another region showing increased activation in our analysis was bilateral cerebellum. The cerebellum is an important region for language process. In 2001, Marien et al. (2001) proposed the concept of a “lateralized linguistic cerebellum”. And in the same year, Middleton and Strick (2001) demonstrated that the cerebellum is anatomically projected to the prefrontal cortex of the primate and influences several areas of prefrontal cortex via the thalamus. Further studies also demonstrated that the cerebellum is associated with semantic discrimination (Xiang et al., 2003) and phonemic process (Leggio et al., 2000). In our meta‐analysis, developmentally increased activation was showed in the cerebellum, which may be involved in the development of processing ability of the frontal gyrus.
Moreover, developmental decrease of neural activation were found in the following regions : right claustrum/inferior frontal gyrus (BA45), left middle/superior temporal gyrus (BA22), bilateral fusiform gyrus (BA37). A recent published study reported that the right superior frontal gyrus was involved in processing orthography and visuospatial attributes of Chinese characters for literate and illiterate subjects, respectively (Wu et al., 2012). We hypothesized that although the experiment design on phonological processing was intended to avoid orthography processing, during the experiments children still tended to process the visuospatial attributes automatically, whereas adults did not process the orthography. Therefore, the developmental decrease of activation in the right inferior frontal gyrus was of significance. As for the left middle/superior temporal gyrus, the development characteristics are ambiguous. According to many studies, the activation of these regions was increased during an early age (before school age) and was stable across infancy (Bitan et al., 2007a; Hoeft et al., 2006; Petitto et al., 2012; Shaywitz et al., 2007; Turkeltaub et al., 2003). This enhanced the previous evidence that posterior language areas mature earlier than the anterior parts (Balsamo et al., 2002; Simos et al., 2001). However, our study found the activation of left middle/superior temporal gyrus decreased developmentally, which is consistent with previous studies (Bitan et al., 2007a; Cao et al., 2010). Temporo‐occipital regions (including fusiform gyrus) segment the linguistic stream into elementary phonetic‐syllabic units and their underlying phonemic categories (Petitto et al., 2012). The activation of these regions was decreased with the development in the phonological processing, suggesting the reduced reliance on phonological information with age and reading skills (Cao et al., 2011). The neural activation of the left middle/superior temporal gyrus (BA22) may increase during early ages and then decrease until mature. In a recent study, Cao et al. (2010) has presented this development attribute. The increase during early age may result from the lack of top‐down control and the decrease indicates developmental increase in top‐down control.
In addition, we noted that the phonological processing during reading showed different patterns between the adults and children groups. Adults engaged more to phonological areas (dorsal inferior frontal gyrus), whereas children engaged more to orthographic areas (fusiform gyrus) and semantic areas (ventral inferior frontal gyrus and middle temporal gyrus). In a previous study, Cao et al. (2009) compared the activation areas between children and adults during rhyming task and reported a greater specialization of phonological processing in adults. In another study, Bitan et al. (2007a) found that children involve more of phonological and semantic processing in the rhyming task. The developmental decreases in the activation of the left fusiform gyrus in the rhyming task were observed, which suggests that the development of reading is marked by reduced involvement of orthography (Cao et al., 2011). These experimental results demonstrated that during rhyming task children engage more orthographic and semantic areas to mapping orthography to phonology and to help identify the phonology; however, adults engage more phonological areas, due to their skilled reading abilities.
Limitations of this Study
It should be noted that this work only focuses on the phonological processing patterns of different language systems for adults and development characteristics based on alphabetic and logographic languages. Limited by the insufficient literatures, the analysis of cross‐cultural impact on adults and development characteristics was not conducted. As mentioned in the previous work, the left dorsal lateral frontal system is responsible for the logographic language, and the posterior sites of temporoparietal regions are essential for the alphabetic language (Tan et al., 2005). Given the cross‐language differentiations for the adults, there could be cross‐language differences on the development characteristics too. It has been found that the left inferior frontal gyrus increases and left superior temporal gyrus decreases with age in both English and Chinese phonological processing patterns (Bitan et al., 2007b; Cao et al., 2010). However, we notice that the left posterior parietal cortex increases with age in English language system (Bitan et al., 2007b), which could be caused by the developmental difference of the cultures.
CONCLUSIONS
In this study, we performed two ALE meta‐analysis experiments: the first one examined the neural activation patterns of two language systems for phonological processing of written languages, and the second one examined the development characteristics based on both alphabetic language and logographic language. By conducting the first experiment, we found that logographic languages significantly activated the left middle‐superior frontal lobe, the right middle occipital gyrus, and the left fusiform gyrus, whereas the alphabetic languages led to significant activations in the left inferior/medial frontal gyrus, left middle temporal gyrus, left angular gyrus, cerebellum, bilateral superior frontal gyrus, and left lentiform nucleus. The second experiment on the development analysis has suggested that both adults and children showed noticeable activations in the left frontal lobe, left superior/middle temporal gyrus, and bilateral middle/superior occipital gyrus. The neural activation of the left middle/inferior frontal gyrus was found to increase with the development. Moreover, we found that the activation decreased in the following regions: right claustrum and inferior frontal gyrus, left inferior/medial frontal gyrus, left middle/superior temporal gyrus, right cerebellum, bilateral fusiform gyrus, and left inferior frontal gyrus. It seems that adults engage more to phonological areas (dorsal inferior frontal gyrus), whereas children engage more to orthographic areas (fusiform gyrus) and semantic areas (ventral inferior frontal gyrus and middle temporal gyrus).
REFERENCES
- Aparicio M, Gounot D, Demont E, Metz‐Lutz MN (2007): Phonological processing in relation to reading: An fMRI study in deaf readers. Neuroimage 35:1303–1316. [DOI] [PubMed] [Google Scholar]
- Balsamo LM, Xu B, Grandin CB, Petrella JR, Braniecki SH, Elliott TK, Gaillard WD (2002): A functional magnetic resonance Imaging study of left hemisphere language dominance in children. Arch Neurol 59:1168–1174. [DOI] [PubMed] [Google Scholar]
- Bitan T, Booth JR, Choy J, Burman DD, Gitelman DR, Mesulam MM (2005): Shifts of effective connectivity within a language network during rhyming and spelling. J Neurosci 25:5397–5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Lu D, Cone NE, Gitelman DR, Mesulam MM, Booth JR (2006): Weaker top‐down modulation from the left inferior frontal gyrus in children. Neuroimage 33:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Chou T‐L, Lu D, Cone NE, Cao F, Bigio JD, Booth JR (2007a): The interaction between orthographic and phonological information in children: An fMRI study. Hum Brain Mapp 28:880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Gitelman DR, Mesulam MM, Booth JR (2007b): Developmental changes in activation and effective connectivity in phonological processing. Neuroimage 38:564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitan T, Cheon J, Lu D, Burman DD, Booth JR (2009): Developmental increase in top‐down and bottom‐up processing in a phonological task: An effective connectivity, fMRI study. J Cogn Neurosci 21:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde ALW, Tagamets MA, Friedman RB, Horwitz B (2001): Functional interactions of the inferior frontal cortex during the processing of words and word‐like stimuli. Neuron 30:609–617. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W (2005): Cross‐cultural effect on the brain revisited: Universal structures plus writing system variation. Hum Brain Mapp 25:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM (2002a): Modality independence of word comprehension. Hum Brain Mapp 16:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TD, Mesulam MM (2002b): Functional anatomy of intra‐ and cross‐modal lexical tasks. Neuroimage 16:7–22. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM (2004): Development of brain mechanisms for processing orthographic and phonologic representations. J Cogn Neurosci 16:1234–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, Zhang L, Ding GS, Deng Y, Liu L (2006): Specialization of phonological and semantic processing in Chinese word reading. Brain Res 1071:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Wood L, Lu D, Houk JC, Bitan T (2007): The role of the basal ganglia and cerebellum in language processing. Brain Res 1133:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MW, LoCasto PC, Krebs‐Noble D, Gullapalli RP (2005): A systematic investigation of the functional neuroanatomy of auditory and visual phonological processing. Neuroimage 26:647–661. [DOI] [PubMed] [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR (2006): Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J Child Psychol Psychiatry 47:1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Bitan T, Booth JR (2008): Effective brain connectivity in children with reading difficulties during phonological processing Brain Lang 107:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Peng D, Liu L, Jin Z, Fan N, Deng Y, Booth JR (2009): Developmental differences of neurocognitive networks for phonological and semantic processing in Chinese word reading. Hum Brain Mapp 30:797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Lee R, Shu H, Yang Y, Xu G, Li K, Booth JR (2010): Cultural constraints on brain development: Evidence from a developmental study of visual word processing in mandarin chinese. Cereb Cortex 20:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Khalid K, Lee R, Brennan C, Yang Y, Li K, Bolger DJ, Booth JR (2011): Development of brain networks involved in spoken word processing of Mandarin Chinese. Neuroimage 57:750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Tan EWL, Thiel T (1999): Mandarin and English single word processing studied with functional magnetic resonance imaging. J Neurosci 19:3050–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Fu SM, Iversen SD, Smith SM, Matthews PM (2002): Testing for dual brain processing routes in reading: A direct contrast of chinese character and pinyin reading using fMRI. J Cogn Neurosci 14:1088–1098. [DOI] [PubMed] [Google Scholar]
- Cousin E, Peyrin C, Pichat C, Lamalle L, Le Bas JF, Baciu M (2007): Functional MRI approach for assessing hemispheric predominance of regions activated by a phonological and a semantic task. Eur J Radiol 63:274–285. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nakamura K, Okada T, Hanakawa T, Fukuyama H, Mazziotta JC, Shibasaki H (2005): Neural mechanisms underlying the processing of Chinese words: An fMRI study. Neuroscience Research 52(2):139–145. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011): Co‐activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiewa P, Rzanny R, Hopf JM, Knab R, Glauche V, Kaiser WA, Blanz B (1999): fMRI during word processing in dyslexic and normal reading children. Neuroreport 10:3459–3465. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam MM (2005): Language network specializations: An analysis with parallel task designs and functional magnetic resonance imaging. Neuroimage 26:975–985. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL (2002): Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron 35:803–812. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor‐Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, et al. (2006): Neural basis of dyslexia: A comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci 26:10700–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee JR, Chen LF, Lee PL, Chen SS, Ho LT, Hung DL, Tzeng OJL, Hsieh JC (2004): Orthographic and phonological processing of Chinese characters: An fMRI study. Neuroimage 21:1721–1731. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, ZilleS K, Mazziotta JC, Fox PT (2007): Bias between MNI and talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio MG, Silveri MC, Petrosini L, Molinari M (2000): Phonological grouping is specifically affected in cerebellar patients: A verbal fluency study. J Neurol Neurosurg Psychiatry 69:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Hue CW, Chen CC, Chuang KH, Liang KC, Wang YH, Wu CW, Chen JH (2006): Dissociated roles of the middle frontal gyri in the processing of Chinese characters. Neuroreport 17:1397–401. [DOI] [PubMed] [Google Scholar]
- Liu L, Deng X, Peng D, Cao F, Ding G, Jin Z, Zeng Y, Li K, Zhu L, Fan N, et al (2009): Modality‐ and task‐specific brain regions involved in Chinese lexical processing. J Cogn Neurosci 21:1473–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marien P, Engelborghs S, Fabbro F, De Deyn PP (2001): The lateralized linguistic cerebellum: A review and a new hypothesis. Brain Lang 79:580–600. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Chen SH, Hue CW, Wu CY, Bagarinao E, Tseng WY, Nakai T (2010): Neural substrates of phonological selection for Japanese character Kanji based on fMRI investigations. Neuroimage 50:1280–1291. [DOI] [PubMed] [Google Scholar]
- McNorgan C, Alvarez A, Bhullar A, Gayda J, Booth JR (2011): Prediction of reading skill several years later depends on age and brain region: Implications for developmental models of reading. J Neurosci 31:9641–9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2001): Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 21:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA, Liu Y, Tan LH (2005): The lexical constituency model: Some implications of research on Chinese for general theories of reading. Psychol Rev 112:43–59. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331:585–589. [DOI] [PubMed] [Google Scholar]
- Petitto LA, Berens MS, Kovelman I, Dubins MH, Jasinska K, Shalinsky M (2012): The “Perceptual Wedge Hypothesis” as the basis for bilingual babies' phonetic processing advantage: New insights from fNIRS brain imaging. Brain Lang 121:130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrieli JDE (2001): Relations between the neural bases of dynamic auditory processing and phonological processing: Evidence from fMRI. J Cogn Neurosci 13:687–697. [DOI] [PubMed] [Google Scholar]
- Price CJ, Moore CJ, Humphreys GW, Wise RJS (1997): Segregating semantic from phonological processes during reading. J Cogn Neurosci 9:727–733. [DOI] [PubMed] [Google Scholar]
- Sergent J, Zuck E, Levesque M, MacDonald B (1992): Positron emission tomography study of letter and object processing: Empirical findings and methodological considerations. Cereb Cortex 2:68–80. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Skudlarski P, Holahan JM, Marchione KE, Constable RT, Fulbright RK, Zelterman D, Lacadie C, Shaywitz SE (2007): Age‐related changes in reading systems of dyslexic children. Ann Neurol 61:363–370. [DOI] [PubMed] [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Mouzaki A, Papanicolaou AC (2001): Age‐related changes in regional brain activation during phonological decoding and printed word recognition. Develop Neuropsychol 19:191–210. [DOI] [PubMed] [Google Scholar]
- Siok WT, Jin Z, Fletcher P, Tan LH (2003): Distinct brain regions associated with syllable and phoneme. Hum Brain Mapp 18:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH (2008): A structural‐functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci USA 105:5561–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH (2001): The neural system underlying Chinese logograph reading. Neuroimage 13:836–846. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, Fox PT, Gao JH (2003): Neural systems of second language reading are shaped by native language. Hum Brain Mapp 18:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT (2005): Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta‐analysis. Hum Brain Mapp 25:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple E, Poldrack RA, Salidis J, Deutsch GK, Tallal P, Merzenich MM, Gabrieli JDE (2001): Disrupted neural responses to phonological and orthographic processing in dyslexic children: An fMRI study. Neuroreport 12:299–307. [DOI] [PubMed] [Google Scholar]
- Tham WWP, Liow SJR, Rajapakse JC, Leong TC, Ng SES, Lim WEH, Ho LG (2005): Phonological processing in Chinese‐English bilingual biscriptals: An fMRI study. Neuroimage 28:579–587. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: Method and validation. Neuroimage 16:765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. (2003): Development of neural mechanisms for reading. Nat Neurosci 6:767–773. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang B, Yan T, Li X, Bao X, Guo Q (2012): Different roles of the posterior inferior frontal gyrus in Chinese character form judgment differences between literate and illiterate individuals. Brain Res 1431:69–76. [DOI] [PubMed] [Google Scholar]
- Xiang HD, Lin CY, Ma XH, Zhang ZQ, Bower JM, Weng XC, Gao JH (2003): Involvement of the cerebellum in semantic discrimination: An fMRI study. Hum Brain Mapp 18:208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Ishii K, Vega‐Bermudez F, Pietrini P, Reeves‐Tyer P, DiCamillo P, Theodore W (2001): Conjoint and extended neural networks for the computation of speech codes: The neural basis of selective impairment in reading words and pseudowords. Cereb Cortex 11:267–277. [DOI] [PubMed] [Google Scholar]
- Xu B, Grafman J, Gaillard WD, Spanaki M, Ishii K, Balsamo L, Makale M, Theodore WH (2002): Neuroimaging reveals automatic speech coding during perception of written word meaning. Neuroimage 17:859–870. [PubMed] [Google Scholar]
- Xue G, Dong Q, Chen K, Jin Z, Chen C, Zeng Y, Reiman EM (2005): Cerebral asymmetry in children when reading Chinese characters. Brain research. Cogn Brain Res 24:206–214. [DOI] [PubMed] [Google Scholar]


