Abstract
Parkinson's disease (PD) is characterized by degenerative changes of nigral dopamine neurons, resulting in the dopaminergic denervation of the striatum. Resting state networks studies have demonstrated that dopamine modulates distinct network connectivity patterns in both a linear and a nonlinear fashion, but quantitative analyses of dopamine‐dependent functional connectivity secondary to PD pathology were less informative. In the present study, we performed a correlation analysis between striatal dopamine levels assessed quantitatively by FP‐CIT positron emission tomography imaging and resting‐state functional connectivity in 23 drug naïve de novo patients with PD to elucidate dopamine‐dependent functional networks. The major finding is that the patterns of dopamine‐dependent positive functional connectivity varied depending on the location of striatal seeds. Dopamine‐dependent functional connectivity with the caudate predominantly overlay pericentral cortical areas, whereas dopamine‐dependent structures functionally connected with the posterior putamen predominantly involved cerebellar areas. The dorsolateral frontal area overlapped as a dopamine‐dependent cortical region that was positively connected with the anterior and posterior putamen. On the other hand, cortical areas where functional connectivity from the posterior cingulate was negatively correlated with dopaminergic status in the posterior putamen were localized in the left anterior prefrontal area and the parietal area. Additionally, functional connectivity between the anterior putamen and mesiofrontal areas was negatively coupled with striatal dopamine levels. The present study demonstrated that dopamine‐dependent functional network connectivity secondary to PD pathology mainly exhibits a consistent pattern, albeit with some variation. These patterns may reflect the diverse effects of dopaminergic medication on parkinsonian‐related motor and cognitive performance. Hum Brain Mapp 35:5431–5441, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: dopamine, resting‐state functional connectivity, de novo Parkinson's disease
INTRODUCTION
Parkinson's disease (PD) is characterized by degenerative changes of nigral dopamine neurons in the midbrain, resulting in the dopaminergic denervation of the striatum. Because the striatum has extensive functional and anatomical connections with the cerebral cortex, these neurochemical alterations typically lead to abnormal neuronal processing in several cortical areas. In particular, dopamine neurotransmission is known to play a central role in cognition, attention, motivated behavior, and reward response, and thus, various neuropsychiatric symptoms are closely associated with dopaminergic status in the brain [Nieoullon, 2002; Wise, 2004]. The therapeutic modulation of dopamine to correct hypo‐ or hyperdopaminergic states has produced contradictory results, including both beneficial and detrimental effects on neuropsychiatric symptoms. Thus, ample evidence has suggested an optimal range of dopamine function that leads to improved neuropsychiatric symptoms, implying inverted U‐shaped associations between dopamine and dopamine‐dependent behavior or cognition [Kehagia et al., 2010; Williams‐Gray et al., 2009].
Cognitive impairments are among the most disabling non‐motor symptoms associated with PD. A population‐based cohort study demonstrated that nearly 80% of patients with PD develop cognitive dysfunction [Aarsland et al., 2003]. Furthermore, according to recent epidemiological studies, approximately one‐fifth of PD patients are classified as having mild cognitive impairment (MCI), a transition state between normal aging and dementia, and about 19% of patients with untreated early PD have MCI [Aarsland et al., 2010; Caviness et al., 2007]. In the early stages of cognitive dysfunction or MCI, a general feature of the cognitive profile in patients with PD is dysfunction in frontal lobe‐based cognitive performance, especially executive dysfunction in tasks that require generation of mental sets, planning, and cognitive sequencing [Jacobs et al., 1995; Muslimovic et al., 2005]. Regarding the neurochemical basis of these observations, dopamine appears to play an important role in frontal lobe‐based cognitive performance in patients with PD because dopaminergic therapies lead to improvements in subsets of cognitive performance that require planning and working memory [Gotham et al., 1988; Kehagia et al., 2010; Owen et al., 1995]. Meanwhile, dopamine replacement in patients with advanced or demented PD may have detrimental effects in other subsets of cognitive function, such as visual hallucinations [Diederich et al., 2005].
Resting‐state networks (RSN) are associated with self‐oriented mental activity and offer a means of evaluating the status of functional systems within the brain without externally goal‐directed cognitive performance [Mantini and Vanduffel, 2013; Raichle et al., 2001]. Therefore, RSN analysis may be advantageous for the identification of brain areas that are functionally integrated with dopamine‐dependent processes. Some studies have investigated dopamine‐dependent cortical‐subcortical functional networks using RSN analyses and demonstrated that dopamine modulates distinct large‐scale network connectivity patterns in both a linear and a nonlinear fashion [Cole et al., 2013; Delaveau et al., 2010; Kelly et al., 2009; Nagano‐Saito et al., 2008]. However, these studies generally used healthy subjects or PD patients receiving dopaminergic pharmacological intervention, and thus, quantitative analyses of dopamine‐dependent functional network connectivity secondary to PD pathology‐associated dopamine states have been less informative. Thus, in the present study, we performed a correlation analysis between dopamine levels in the striatum assessed quantitatively by FP‐CIT positron emission tomography (PET) imaging and resting‐state functional connectivity in 23 drug naïve de novo patients with PD to further elucidate dopamine‐dependent functional networks.
METHODS
Subject
The present study included 23 drug‐naïve de novo PD patients from a university hospital who were recruited between September 2011 and May 2013. PD was diagnosed according to the clinical criteria of the United Kingdom (UK) PD Society Brain Bank [Hughes et al., 1992]. All subjects completed the magnetic resonance imaging (MRI) protocol and FP‐CIT PET imaging and exhibited decreased dopamine transporter (DAT) uptake in the posterior putamen. All study subjects completed the MRI and FP‐CIT PET imaging within a 1‐week interval. Exclusion criteria included evidence of focal brain lesions based on MRI data, evidence of Parkinsonian Plus syndromes, a history of using drugs that can cause parkinsonism (antipsychotics, gastrointestinal kinetics, antiepileptic drugs, or L‐type calcium channel blockers), scores <26 on the Korean version of the Mini‐Mental State Examination (K‐MMSE), and/or scores >21 on the Beck Depression Inventory (BDI).
18F‐FP‐CIT PET
The 18F‐FP‐CIT PET scan was performed using a GE PET‐CT DSTe scanner (GE Discovery STE, GE Healthcare Technologies; Milwaukee, WI), which obtains images with three‐dimensional resolution of 2.3‐mm full width at half maximum (FWHM). All subjects had fasted for at least 6 h before PET scan, and then 5mCi (185 MBq) of 18F‐FP‐CIT was injected intravenously. After 90 min following the injection, images were acquired for 20 min with three‐dimensional mode at 120 KVp and 380 mAs.
Quantitative Analysis of 18F‐FP‐CIT PET Data
Quantitative analyses were performed following a modified version of a previously described procedure [Oh et al., 2012]. Image processing was performed using SPM2 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, UCL, London, UK) under Matlab 6.5.1 for Windows (Math Works, Natick, MA) and MRIcro version 1.37 (Chris Rorden, Columbia, SC). Quantitative analyses were based on volumes of interest (VOIs), which were defined based on a template in standard space. All reconstructed PET images were spatially normalized to Talairach space using a standard 18F‐FP‐CIT PET template which was made using 18F‐FP‐CIT PET and T1 MR images of 13 normal controls to remove inter‐subject anatomical variability. The 8 VOIs of bilateral striatal subregions and one occipital VOI were drawn on a co‐registered spatially normalized single T1 MR and 18F‐FP‐CIT PET template image. The striatum was divided into the caudate, ventral striatum, anterior putamen, and posterior putamen. The VOI for the ventral striatum was defined according to previously defined criteria [Mawlawi et al., 2001], and the boundary between the anterior and posterior putamen was the anterior commissure coronal plane. The outer boundaries of the striatal subregions were visually determined based on the characteristic dense gray signal of the striatum, which readily distinguishes these subregions from adjacent structures. DAT activity was calculated by the non‐displaceable binding potential, which was defined as follows: (mean standardized uptake value of the striatal subregions VOI—mean standardized uptake value of the occipital VOI)/mean standardized uptake value of the occipital VOI [Innis et al., 2007]. Of the VOIs, DAT activity in the posterior putamen was selected because this area is the most severely affected in early stage of PD [Brooks, 2004] and thus, likely reflects dopamine levels in the striata of individual patients.
MR Imaging Analysis
Image acquisition
All participants underwent functional MRI (fMRI) scanning with a 3.0 Tesla MRI scanner (Achieva, Philips Medical System, Best, Netherlands) to obtain T2* weighted single shot echo planar imaging sequences. Each participant was axially scanned using the following parameters: voxel size, 2.8 × 2.8 × 3.0 mm3; slice number, 31 (interleaved); matrix, 80 × 80; slice thickness, 3.0 mm; gap, 1.0 mm; repetition time (TR), 2,000 ms; echo time (TE), 30 ms; flip angle = 90°; and field of view, 220 mm. Each 330‐s scan produced 165 fMRI images, which is known to be sufficient to evaluate resting state functional connectivity and to obtain low frequency oscillation for the resting state functional connectivity [Van Dijk et al., 2010]. During functional MR imaging, the subjects were instructed to stay awake with their eyes closed, without focusing on a specific thought and without moving.
Anatomical segmentation
The anatomical native T1 images were normalized to a stereotaxic space using a linear transformation [Collins et al., 1994] and were corrected for intensity non‐uniformities resulting from inhomogeneities in the magnetic field [Sled et al., 1998]. Then, the registered and corrected images were classified into white matter, gray matter, cerebrospinal fluid, and background using an advanced neural‐net classifier [Zijdenbos et al., 1996]. Additionally, four large ventricles were automatically identified using automated nonlinear image matching and anatomical labeling, a well‐established nonlinear warping algorithm that uses a multi‐scale approach to deform one image to match a previously labeled template [Collins et al., 1995]. All results in the standard stereotaxic space were transformed into the native space. From this, mask volumes of the whole brain, gray matter, white matter, and the four large ventricles in the native space were generated for each individual subject.
Preprocessing of fMRI data
Preprocessing of fMRI data was carried out using Analysis of Functional NeuroImages, (http://afni.nimh.nih.gov/afni) software [Cox, 1996]. The first five volumes from each functional image were discarded to allow for stabilization of the magnetic field. Images were de‐spiked, and then corrected for slice time acquisition differences and head motion [Cox and Jesmanowicz, 1999]. At the motion‐correction stage, displacement due to head motion was estimated using the motion‐correction parameters of the x, y, and z translations and three rotation axes. In all subjects, estimated displacement due to head motion was <1 mm between successive time‐series volumes and <2 mm in any of the three translation directions or <2.0° maximum rotation around any of the axes during the resting‐state scans. The anatomical T1 image was coregistered to the functional images using the affine registration with Local Pearson Correlation cost function [Saad et al., 2009], and all masks were transformed to echo planar imaging space. To reduce partial volume effects, the white matter mask and the large ventricle mask were eroded by one voxel. Then, a seed‐based analysis with improved preprocessing was carried out as previously reported [Jo et al., 2010]. To remove noise from the data, we performed regression analysis using several noise sources: (1) six parameters obtained by rigid body correction of head motion, (2) signal from the eroded large ventricle mask, and (3) signal from a region of the eroded local white matter mask. Head coil and hardware artifacts were modeled with eroded local white matter and eroded large ventricle masks. To reduce partial voluming effects from gray matter on white matter and large ventricle masks, we eroded these masks by one voxel. Subsequently, data were temporally band‐pass filtered (0.009 < f < 0.08). Data were then masked out using the gray matter mask to reduce the inclusion of unwanted blood oxygen level‐dependent or other physiological signals that occur due to large draining vessels that tend to course on the outer surface of gray matter. Images were normalized to a standard MNI152 template and resampled at an isotropic voxel size of 2 mm before spatial smoothing was carried out with a 6‐mm FWHM Gaussian kernel.
Functional connectivity analyses
Four regions of interest (ROIs) were defined to study dopamine‐dependent resting‐state functional networks in patients with PD. First, the posterior cingulate cortex (PCC) was chosen to investigate alterations within the default mode network (DMN), which is highly associated with cognitive function in resting‐state fMRI analyses. This ROI was defined using voxels with a 6‐mm radius spherical mask at the peak voxel (x/y/z = 0/−52/30 mm) [Hahn et al., 2012]. Second, the caudate and putamen (anterior and posterior), which play key roles in PD pathophysiology, were selected to study functional connectivity between the basal ganglia and cerebral cortices. These areas were defined according to the automated anatomical labeling template [Tzourio‐Mazoyer et al., 2002]. The putamen was separated into anterior (y < 0) and posterior (y > 0) aspects according to the same method used in the PET analysis [Oh et al., 2012]. Except for the PCC, ROIs in striatal seeds where a within‐subject DAT activity in the posterior putamen of one hemisphere was lower relative to the other hemisphere were selected and used for the functional connectivity analyses. If the left side demonstrated a lower within‐subject DAT activity, there was no additional processing. However, if the right posterior putamen exhibited a lower within‐subject DAT activity, it was flipped in the x direction to align the images of the more affected hemisphere on the same body side. The time series for data in each ROI were averaged, and Pearson's correlation coefficient maps were created for each individual subject. The correlation coefficient maps were converted to a z‐value using Fisher's z transformation. Thus, the z‐scores refer to the functional connectivity levels, and the z‐score maps were used in the following correlation analysis.
Correlation Analysis
To investigate the relationship between corticostriatal or corticocortical functional connectivity from the striatal or PCC seeds and DAT activity in the posterior putamen, a multiple regression analysis was performed with age, sex, education, and K‐MMSE scores entered as covariates. The dependent variable was z‐scores of functional connectivity maps and the independent variables were DAT activity value in the posterior putamen and covariates. The t value on the coefficient of DAT activity describes the size of the effect that the DAT activity is having on functional connectivity. Therefore, the dopamine‐dependent functional connectivity refers to the t value on the coefficient of DAT activity. The statistical maps were corrected for multiple comparisons to a significance level of Pα < 0.05. Using the AFNI's AlphaSim program, Monte Carlo simulations were performed to control Type I error (parameters: individual voxel P value = 0.02, 10,000 simulations, FWHM = 6 mm, with group‐specific grey matter mask). The AlphaSim program provides an estimate of the overall significance levels for various combinations of individual voxel probability thresholds and cluster size thresholds [Poline et al., 1997]. The group‐specific grey matter mask created using individual normalized grey matter masks. We combined them to make one group‐specific grey matter mask. We used a corrected significance level of Pα < 0.05 (uncorrected individual voxel height threshold of P < 0.02 with a minimum cluster size of 1648 mm3).
RESULTS
Demographic Features
The demographic characteristics of the participants are presented in Table 1. Mean age at onset of PD was 61 years, and 9 (39%) patients were female. Subjects were diagnosed a median of 1.1 year after the onset of motor symptoms, and the mean Unified PD Rating Scale motor score at diagnosis 20.7. Mean years of education was 10.6 years, and the mean K‐MMSE score was 27.8.
Table 1.
Demographic characteristics in patients with Parkinson's disease
| Study subjects | |
|---|---|
| Disease onset age (yr) | 61.0 ± 8.2 |
| Female (%) | 9 (39) |
| Disease duration (yr) | 1.1 ± 1.1 |
| K‐MMSE | 27.3 ± 1.7 |
| Education level (yr) | 10.6 ± 4.5 |
| UPDRS‐III | 20.7 ± 11.6 |
| BDI | 6.2 ± 2.7 |
| DAT uptake in the posterior putamen | 1.9 ± 0.6 |
Data are expressed as mean ± SD, K‐MMSE: Korean version of the Mini‐Mental State Examination, UPDRS: Unified Parkinson's disease Rating Scale, BDI: Beck Depression Inventory, DAT: dopamine transporter.
Dopaminergic Modulation of Striatocortical Functional Connectivity
Using the caudate as a seed for the functional connectivity analysis of the RSN, dopamine‐dependent cortical areas that were functionally connected with the caudate were identified in right postcentral areas that extended into the precentral area (Fig. 1). However, there were no regions where cortical functional connectivity from the caudate was negatively correlated with dopaminergic status in the posterior putamen. When the anterior putamen was used as a seed for RSN analysis, the right dorsolateral frontal area was found to exhibit functional connectivity with the anterior putamen that was positively correlated with dopaminergic status in the posterior putamen (Fig. 2). Additionally, the mesiofrontal areas exhibited a functional interaction with the anterior putamen that was negatively correlated with dopaminergic status in the posterior putamen (Fig. 2 and Supporting Information Fig. S1). When the posterior putamen was used as a seed for RSN analysis, the patterns of functional connectivity were unique. The bilateral cerebellar cortices were found to exhibit functional connectivity with the posterior putamen that was positively correlated with dopaminergic status in the posterior putamen. Additionally, the right dorsolateral frontal area exhibited a dopamine‐dependent functional connectivity with the posterior putamen (Fig. 3). There were no regions where cortical functional connectivity with the posterior putamen was negatively correlated with dopaminergic status in the posterior putamen. The anatomical locations of the significant peaks based on seed region are listed in Table 2.
Figure 1.
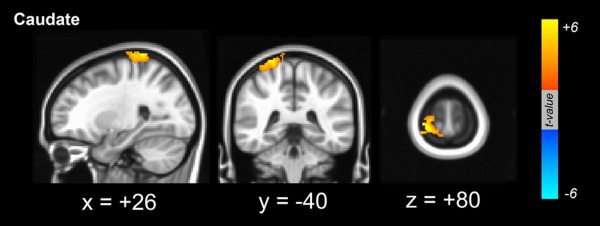
Dopamine‐dependent functional connectivity between the cortex and seed region of interest in the caudate. The pericentral cortical areas exhibited significant functional connectivity with the caudate that was positively coupled with dopamine levels in the posterior putamen. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 2.
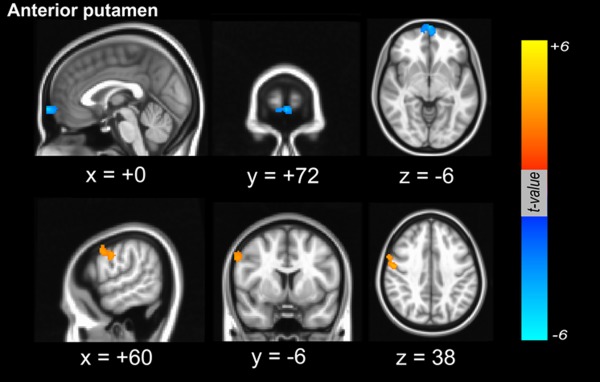
Dopamine‐dependent functional connectivity between the cortex and seed region of interest in the anterior putamen. Dopamine‐dependent positive functional connectivity was localized in the right dorsolateral frontal area (orange color), whereas in the mesiofrontal areas, functional connectivity between the cortex and the anterior putamen was negatively correlated with dopaminergic status in the posterior putamen (blue color). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
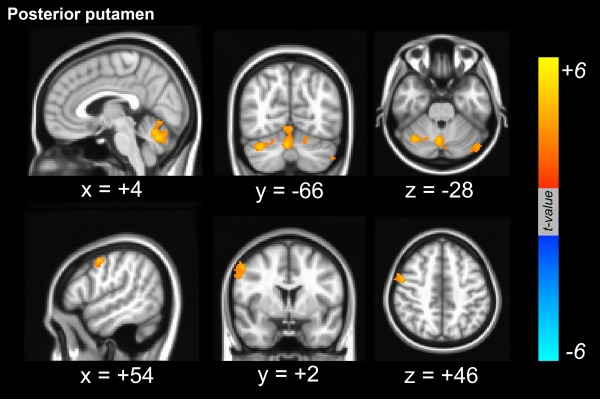
Dopamine‐dependent functional connectivity from the seed region of interest in the posterior putamen. Dopamine‐dependent positive functional connectivity was localized in the right dorsolateral frontal area. Additionally, functional connectivity between the cerebellar areas and the posterior putamen was positively correlated with dopaminergic status in the posterior putamen. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Clusters Displaying Significant Dopamine‐dependent Functional Connectivity from Seed Regions of Interest in the Striatum or Posterior Cingulate Cortex
| Stereotaxic coordinates (mm) | |||||||
|---|---|---|---|---|---|---|---|
| Seed | Brain regions | Side | x | y | z | Maximum t | Number of voxels |
| Caudate | Postcentral gyrus, extending into precentral gyrus | R | 26 | −40 | 80 | 6.59 | 613 |
| AP | |||||||
| Medial frontal gyrus | L | 0 | 72 | −6 | −4.60 | 265 | |
| Middle frontal gyrus | R | 60 | −6 | 38 | 3.43 | 219 | |
| PP | |||||||
| Cerebellum | L | −40 | −84 | −44 | 5.19 | 702 | |
| Cerebellum | R | 4 | −66 | −28 | 4.99 | 501 | |
| Middle frontal gyrus | R | 54 | 2 | 46 | 4.34 | 248 | |
| PC | |||||||
| Angular gyrus | L | −46 | −50 | 34 | −5.99 | 243 | |
| Superior frontal gyrus | L | −18 | 36 | 42 | −4.11 | 227 | |
AP: anterior putamen, PP: posterior putamen, PC: posterior cingulate, R: right, L; left.
Dopaminergic Modulation of Corticocortical Functional Connectivity
To evaluate dopamine‐dependent corticocortical functional connectivity, the PCC was used as a seed for RSN analysis. In contrast to dopamine‐dependent striatocortical functional connectivity, there were no significant clusters where the functional interaction between specific neocortical areas and the PCC were positively correlated with dopaminergic status in the posterior putamen. On the other hand, the left anterior prefrontal area and the parietal area was found to exhibit functional connectivity with the PCC that were negatively correlated with dopaminergic status in the posterior putamen (Fig. 4 and Supporting Information Fig. S1). The anatomical locations of the significant peaks based on PCC seed are listed in Table 2.
Figure 4.
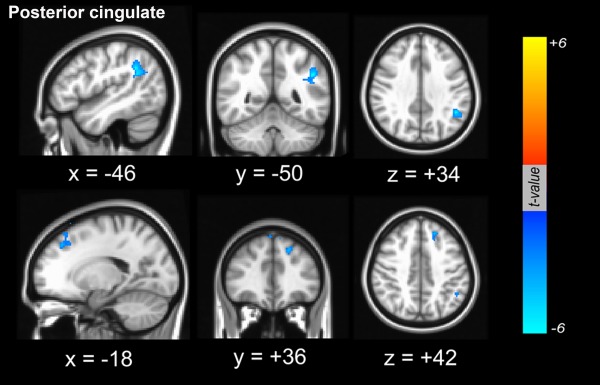
Dopamine‐dependent functional connectivity between the cortex and seed region of interest in the posterior cingulate cortex. Functional connectivity from the posterior cingulate that was negatively associated with striatal dopaminergic status was involved in the left anterior prefrontal area and the parietal area (blue color). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The present study provides novel information concerning dopamine‐dependent corticostriatal functional connectivity in patients with de novo PD. The major findings of the present study are as follows: (1) the dorsolateral frontal areas were the primary dopamine‐dependent cortical regions that were functionally connected with the anterior and posterior putamen, (2) patterns of dopamine‐dependent positive functional connectivity varied depending on the location of the striatal seeds; dopamine‐dependent functional connectivity from the caudate predominantly overlaid pericentral cortical areas, whereas dopamine‐dependent structures that were functionally connected to the posterior putamen predominantly involved cerebellar areas, and (3) there were cortical areas where the corticocortical or striatocortical connectivity were negatively associated with dopaminergic status in the posterior putamen.
A number of studies have demonstrated that corticostriatal functional connectivity follows the anatomy of corticostriatal loops [Kelly et al., 2009; Postuma and Dagher, 2006]. More specifically, the posterior putamen is functionally connected to large parts of the cortical motor region including the primary motor, supplementary motor, and premotor cortices as well as the parietal cortex and cerebellum. The anterior putamen is predominantly coupled with the anterior cingulate cortex and middle frontal areas, and the caudate is functionally connected to large parts of the prefrontal cortex as well as the parietal, temporal, and cerebellar cortices. Patients with PD appear to show a distribution of corticostriatal functional connectivity similar to that in healthy subjects, but the specific patterns of this connectivity differ. Compared with controls, PD patients exhibit decreased cortical connectivity from the posterior putamen, whereas the anterior putamen shows enhanced cortical connectivity [Helmich et al., 2010]. Additionally, patients with PD show decreased brainstem and cerebellar connectivity from the striatum relative to controls [Hacker et al., 2012]. The PCC is largely connected to parietal, temporal, and prefrontal cortices in healthy subjects, but PD patients exhibit decreased connectivity in the frontal areas in conjunction with worsening cognitive performance [Seibert et al., 2012]. Thus, in patients with PD, dopamine deficiency may lead to alterations in resting‐state functional connectivity and reorganization of striatocortical functional network. This may be one of mechanisms underlying impaired sensorimotor integration in PD.
In the present study, compared with general functional connectivity, areas of corticostriatal functional connectivity that were closely coupled with dopaminergic levels in the posterior putamen were more selectively localized and showed a distinctive spatial distribution. Additionally, the spatial distribution of dopamine‐dependent connectivity exhibited distinctive patterns depending on which subregion of the striatum is evaluated. In the caudate, there was a positive effect of dopamine levels on functional connectivity with primary sensorimotor areas, whereas in the anterior putamen, there was a positive association of dopamine‐dependent functional connectivity with lateral frontal areas. Finally, in the posterior putamen, there was a positive correlation of dopamine‐dependent functional connectivity with a large portion of the cerebellar cortex and lateral premotor areas. A previous study using pharmacological interventions has examined the effects of dopaminergic modulation on corticostriatal functional connectivity in healthy subjects [Cole et al., 2013]. This group found that, relative to a placebo, striatal connectivity with left sensorimotor cortices was greater in subjects treated with levodopa but lower in subjects treated with haloperidol. This suggests the existence of a linear dopamine‐dependent functional connectivity between the striatal RSN and regions of the somatomotor and premotor cortices. Accordingly, the topography of dopamine‐dependent functional connectivity between the striatum and sensorimotor or premotor areas corresponds to the motor manifestations of PD as a primary movement disorder. The fact that this pattern of dopamine‐dependent connectivity is quite similar to that of decreased connectivity during a motor activation paradigm also supports the proposition that PD‐related motor symptoms may be ascribed to this connectivity. In other respects, dopamine‐dependent functional connectivity between the striatum and lateral frontal areas (observed in the anterior putamen and posterior putamen seeds) may be associated with cognitive processing. Cognitive dysfunction is relatively common among PD patients, manifesting in about one‐fifth of patients with untreated early PD, and frontal lobe‐based executive dysfunction is a key feature of cognitive profiles in PD patients [Aarsland et al., 2010; Muslimovic et al., 2005]. In conjunction with evidence showing that the lateral prefrontal areas and the striatum are the anatomical bases of these cognitive tasks, ample evidence demonstrates that dopamine plays a key role in a wide range of cognitive tasks that involve working memory, planning and organization, and flexible shifts in attention [Nagano‐Saito et al., 2008; Robbins, 2000]. In addition, several lines of clinical data demonstrating that dopaminergic treatment in patients with PD improved cognitive performance, especially frontal lobe‐based cognitive subsets also support this relationship [Gotham et al., 1988; Kehagia et al., 2010; Owen et al., 1995].
Interestingly, we found that the posterior putamen had a wide range of dopamine‐dependent functional connectivity with the cerebellum. Although earlier studies did not consider a subcortical connection between the striatum and cerebellum, recent reports have provided ample evidence of a direct interaction between these areas. Ichinohe et al. [2000] reported the existence of a cerebellothalamostriatal pathway extending from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus using anterograde and retrograde tracing techniques. Additionally, serial studies have demonstrated a direct interconnection between the cerebellum and the basal ganglia via disynaptic projections mediated either by the ventrolateral and intralaminar nuclei or by the subthalamic nucleus [Bostan et al., 2010, 2013; Hoshi et al., 2005]. In patients with PD, functional connectivity between the basal ganglia and the cerebellum is significantly decreased compared with control subjects [Hacker et al., 2012]. Moreover, exogenous levodopa administration to patients with PD, as well as healthy subjects, leads to a significant increase in functional connectivity involving the cerebellum compared to off medication or placebo medications [Jech et al., 2013; Kelly et al., 2009]. By analyzing the relationships between the in vivo status of dopaminergic levels and resting‐state functional connectivity in patients with drug‐naïve PD, the present findings are well in accordance with previous results and provide further information regarding the positive effects of the modulation of striatal dopamine on functional connectivity between the striatum and the cerebellum. According to recent studies, this striatocerebellar functional connectivity represents a major subcortical network that influences not only movement but also cognition and affect. For example, in PD patients, the oscillatory activity of parkinsonian tremors has been observed in thalamic areas that receive cerebellar outputs [Lenz et al., 1988]. Furthermore, deep brain stimulation of the subthalamic nucleus, which has disynaptic connections with the cerebellum, normalizes cerebellar activity [Payoux et al., 2004]. Moreover, in conjunction with the basal ganglia, the cerebellum also participates in cognitive processing. Whereas the basal ganglia are thought to be involved in dopamine‐dependent reward‐based learning, the cerebellum is known to participate in the adaptive modification of behavior and error‐based learning [Bostan et al., 2013; O'Doherty et al., 2003]. Additionally, these two systems may be closely coupled during reward‐related cognitive tasks [Swain et al., 2011]. Accordingly, as suggested by Jech et al. [2013], the present data support the notion that levodopa treatment may normalize the functional connectivity between the cerebellum and the posterior putamen, which is significantly attenuated in PD. However, further research is required to clarify which types of specific parkinsonian motor symptoms or cognitive dysfunction would be modulated by the normalization of the striatocerebellar functional interconnections via dopaminergic intervention.
Another interesting finding in the present study is that corticocortical functional connectivity from the PCC seed showed a negative relationship with dopamine levels in the posterior putamen. Additionally, functional connectivity between the anterior putamen and mesiofrontal areas was negatively coupled with striatal dopamine levels. The PCC plays a central role in the DMN and has a wide range of functional connectivity with cortical areas including the medial prefrontal, dorsolateral prefrontal, inferior parietal, and temporal cortices [Greicius et al., 2003]. Thus, functional connectivity with the PCC is associated with memory encoding, retrieval tasks, and/or visuospatial orientation. According to the current findings, dopamine levels in the posterior putamen may negatively influence the functional interconnections between the PCC and parietal areas as well as prefrontal areas. This is partially in accordance with a previous functional study, which found that levodopa decreased functional connectivity between the PCC and prefrontal areas in healthy subjects relative to placebo treatment [Kelly et al., 2009]. Therefore, these data indicate that dopaminergic intervention in patients with PD may aggravate cognitive performance that is closely coupled with functional connection between PCC and prefrontal or parietal areas. In fact, dopaminergic interventions in patients with PD have been shown to have deleterious effects on visual perception and promote visual hallucinations [Diederich et al., 2005]. Although the exact mechanisms of visual hallucinations in PD remain unknown, dysfunction across the attentional control networks has been suggested to be an important mechanism [Shine et al., 2014]. According to this hypothesis, visual hallucinations in PD result from a relative inability to recruit activation in the dorsal attention network in the presence of an ambiguous percept, leading to an overreliance on the ventral attention network and DMN. Because the parietal and prefrontal areas are the main anatomical areas responsible for dorsal attention network [Asplund et al., 2010], the current data suggest that dopamine treatment in PD may have deleterious effects in this area and result in increased distortion of visual perception‐related information. Regarding the mesiofrontal areas that show another dopamine‐dependent negative connectivity with the anterior putamen, it is cautiously speculated that may underpin some of the psychotic complications associated with dopamine replacement therapy This assumption is based on evidence indicating that the ventromedial frontal cortices may underlie the development of impulse‐control disorders such as hypersexuality, pathological gambling, excessive shopping, and excessive eating [Best et al., 2002; Potenza et al., 2003].
There are several limitations of this study that need to be addressed. First, even though this study used drug‐naïve de novo PD patients, the number of study subjects was too small to draw firm conclusions. Second, because only de novo PD patients were enrolled, it was impossible to directly evaluate the clinical significance of dopamine‐dependent functional connectivity, especially in terms of its relationship with cognitive performance. Therefore, a study with a longitudinal design entailing repeated fMRI analyses is needed to clarify this issue and to suggest clinical applications. Third, contralateral dopamine‐dependent functional connectivity was observed between the cortex and the striatum. This contralaterality was prominent in the dopamine‐dependent positive relationships between the striatal seeds and the cortex and occurred with both the caudate and putamen. According to basal ganglia functional connectivity based on a meta‐analysis, coactivation was more prominent on the contralateral side than the ipsilateral side [Postuma and Dagher, 2006]. Although contralateral anatomical connections between the corticostriatal systems have been described in humans [Lehericy et al., 2004], it is unknown whether this contralateral connectivity represents direct anatomical connectivity or indicates functional connectivity in the absence of anatomical connectivity. Future study combining structural and functional connectivity imaging studies will clarify this issue.
In summary, the present study demonstrated that dopamine‐dependent functional network connectivity secondary to PD pathology mainly exhibits a consistent pattern, albeit with some variation. These patterns of functional connectivity may reflect the diverse effects of dopaminergic medication on parkinsonian‐related motor and cognitive performance.
Supporting information
Supplementary Information
KyoungWon Baik and Jungho Cha contributed equally to this work.
REFERENCES
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh‐Sorensen P (2003): Prevalence and characteristics of dementia in Parkinson disease: An 8‐year prospective study. Arch Nurol 60:387–392. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Bronnick K, Williams‐Gray C, Weintraub D, Marder K, Kulisevsky J, Burn D, Barone P, Pagonabarraga J, Allcock L, Santangelo G, Foltynie T, Janvin C, Larsen JP, Barker RA, Emre M (2010): Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 75:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asplund CL, Todd JJ, Snyder AP, Marois R (2010): A central role for the lateral prefrontal cortex in goal‐directed and stimulus‐driven attention. Nat Neurosci 13:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best M, Williams JM, Coccaro EF (2002): Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci USA 99:8448–8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL (2010): The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107:8452–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL (2013): Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci 17:241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ (2004): Neuroimaging in Parkinson's disease. NeuroRx 1:243–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness JN, Driver‐Dunckley E, Connor DJ, Sabbagh MN, Hentz JG, Noble B, Evidente VG, Shill HA, Adler CH (2007): Defining mild cognitive impairment in Parkinson's disease. Mov Disord 22:1272–1277. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Oei NY, Both S, van Gerven JM, Rombouts SA (2013): Differential and distributed effects of dopamine neuromodulations on resting‐state network connectivity. Neuroimage 78:59–67. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192–205. [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC (1995): Automatic 3‐D model‐based neuroanatomical segmentation. Hum Brain Mapp 3:190–208. [Google Scholar]
- Cox RW (1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A (1999): Real‐time 3D image registration for functional MRI. Magn Reson Med 42:1014–1018. [DOI] [PubMed] [Google Scholar]
- Delaveau P, Salgado‐Pineda P, Fossati P, Witjas T, Azulay JP, Blin O (2010): Dopaminergic modulation of the default mode network in Parkinson's disease. Eur Neuropsychopharmacol 20:784–792. [DOI] [PubMed] [Google Scholar]
- Diederich NJ, Goetz CG, Stebbins GT (2005): Repeated visual hallucinations in Parkinson's disease as disturbed external/internal perceptions: Focused review and a new integrative model. Mov Disord 20:130–140. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD (1988): “Frontal” cognitive function in patients with Parkinson's disease “on” and “off” levodopa. Brain 111:299–321. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V (2003): Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ (2012): Resting state functional connectivity of the striatum in Parkinson's disease. Brain 135:3699–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Wadsak W, Windischberger C, Baldinger P, Hoflich AS, Losak J, Nics L, Philippe C, Kranz GS, Kraus C, Mitterhauser M, Karanikas G, Kasper S, Lanzenberger R(2012): Differential modulation of the default mode network via serotonin‐1A receptors. Proc Natl Acad Sci USA 109:2619–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I (2010): Spatial remapping of cortico‐striatal connectivity in Parkinson's disease. Cereb Cortex 20:1175–1186. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL (2005): The cerebellum communicates with the basal ganglia. Nat Neurosci 8:1491–1493. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992): Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe N, Mori F, Shoumura K (2000): A di‐synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res 880:191–197. [DOI] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE(2007): Consensus nomenclature for in vivo imaging of reversibly binding radioligands J Cereb Blood Flow Metab 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Marder K, Cote LJ, Sano M, Stern Y, Mayeux R (1995): Neuropsychological characteristics of preclinical dementia in Parkinson's disease. Neurology 45:1691–1696. [DOI] [PubMed] [Google Scholar]
- Jech R, Mueller K, Schroeter ML, Ruzicka E (2013): Levodopa increases functional connectivity in the cerebellum and brainstem in Parkinson's disease. Brain 136:e234. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW (2010): Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage 52:571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Barker RA, Robbins TW (2010): Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 9:1200–1213. [DOI] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K. (2009): L‐dopa modulates functional connectivity in striatal cognitive and motor networks: A double‐blind placebo‐controlled study. J Neurosci 29:7364–7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS (2004): Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol 55:522–529. [DOI] [PubMed] [Google Scholar]
- Lenz FA, Tasker RR, Kwan HC, Schnider S, Kwong R, Murayama Y, Dostrovsky JO, Murphy JT (1988): Single unit analysis of the human ventral thalamic nuclear group: Correlation of thalamic “tremor cells” with the 3–6 Hz component of parkinsonian tremor. J Neurosci 8:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Vanduffel W (2013): Emerging roles of the brain's default network. Neuroscientist 19:76–87. [DOI] [PubMed] [Google Scholar]
- Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M (2001): Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21:1034–1057. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B (2005): Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65:1239–1245. [DOI] [PubMed] [Google Scholar]
- Nagano‐Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A (2008): Dopamine depletion impairs frontostriatal functional connectivity during a set‐shifting task. J Neurosci 28:3697–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A (2002): Dopamine and the regulation of cognition and attention. Prog Neurobiol 67:53–83. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ (2003): Temporal difference models and reward‐related learning in the human brain. Neuron 38:329–337. [DOI] [PubMed] [Google Scholar]
- Oh M, Kim JS, Kim JY, Shin KH, Park SH, Kim HO, Moon DH, Oh SJ, Chung SJ, Lee CS (2012): Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple‐system atrophy. J Nucl Med 53:399–406. [DOI] [PubMed] [Google Scholar]
- Owen AM, Sahakian BJ, Hodges JR, Summers BA, Polkey CE, Robbins TW (1995): Dopamine‐dependent frontostriatal planning deficits in early Parkinson's disease. Neuropsychology 9:126–140. [Google Scholar]
- Payoux P, Remy P, Damier P, Miloudi M, Loubinoux I, Pidoux B, Gaura V, Rascol O, Samson Y, Agid Y (2004): Subthalamic nucleus stimulation reduces abnormal motor cortical overactivity in Parkinson disease. Arch Neurol 61:1307–1313. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A (2006): Basal ganglia functional connectivity based on a meta‐analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 16:1508–1521. [DOI] [PubMed] [Google Scholar]
- Potenza MN, Leung HC, Blumberg HP, Peterson BS, Fulbright RK, Lacadie CM, Skudlarski P, Gore JC (2003): An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. Am J Psychiatry 160:1990–1994. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW (2000): Chemical neuromodulation of frontal‐executive functions in humans and other animals. Exp Brain Res 133:130–138. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW (2009): A new method for improving functional‐to‐structural MRI alignment using local Pearson correlation. Neuroimage 44:839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert TM, Murphy EA, Kaestner EJ, Brewer JB (2012): Interregional correlations in Parkinson disease and Parkinson‐related dementia with resting functional MR imaging. Radiology 263:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JM, Halliday GM, Gilat M, Matar E, Bolitho SJ, Carlos M, Naismith SL, Lewis SJ(2014): The role of dysfunctional attentional control networks in visual misperceptions in Parkinson's disease. Hum Brain Mapp 35:2206–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97. [DOI] [PubMed] [Google Scholar]
- Swain RA, Kerr AL, Thompson RF (2011): The cerebellum: A neural system for the study of reinforcement learning. Front Behav Neurosci 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010): Intrinsic functional connectivity as a tool for human connectomics: Theory, properties, and optimization. J Neurophysiol 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams‐Gray CH, Evans JR, Goris A, Foltynie T, Ban M, Robbins TW, Brayne C, Kolachana BS, Weinberger DR, Sawcer SJ, Barker RA (2009): The distinct cognitive syndromes of Parkinson's disease: 5 year follow‐up of the CamPaIGN cohort. Brain 132:2958–2969. [DOI] [PubMed] [Google Scholar]
- Wise RA (2004): Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494. [DOI] [PubMed] [Google Scholar]
- Zijdenbos A, Evans A, Riahi F, Sled J, Chui J, Kollokian V(1996): Automatic quantification of multiple sclerosis lesion volume using stereotaxic space. Lect Notes Comput Sci 1131:439–448. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


