Abstract
Humans differ widely in their navigational abilities. Studies have shown that self‐reports on navigational abilities are good predictors of performance on navigation tasks in real and virtual environments. The caudate nucleus and medial temporal lobe regions have been suggested to subserve different navigational strategies. The ability to use different strategies might underlie navigational ability differences. This study examines the anatomical correlates of self‐reported navigational ability in both gray and white matter. Local gray matter volume was compared between a group (N = 134) of good and bad navigators using voxel‐based morphometry (VBM), as well as regional volumes. To compare between good and bad navigators, we also measured white matter anatomy using diffusion tensor imaging (DTI) and looked at fractional anisotropy (FA) values. We observed a trend toward higher local GM volume in right anterior parahippocampal/rhinal cortex for good versus bad navigators. Good male navigators showed significantly higher local GM volume in right hippocampus than bad male navigators. Conversely, bad navigators showed increased FA values in the internal capsule, the white matter bundle closest to the caudate nucleus and a trend toward higher local GM volume in the caudate nucleus. Furthermore, caudate nucleus regional volume correlated negatively with navigational ability. These convergent findings across imaging modalities are in line with findings showing that the caudate nucleus and the medial temporal lobes are involved in different wayfinding strategies. Our study is the first to show a link between self‐reported large‐scale navigational abilities and different measures of brain anatomy. Hum Brain Mapp 35:2561–2572, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: navigational abilities, diffusion tensor imaging, voxel‐based morphometry, caudate nucleus, parahippocampal gyrus, hippocampus, sense of direction, volumetry, magnetic resonance imaging
INTRODUCTION
The ability to efficiently find our way in our surroundings is a vital skill for all species. Nevertheless, humans differ widely in their navigational abilities [for a review, see Wolbers and Hegarty, 2010]. This study examines the anatomical correlates in both gray and white matter of self‐reported navigational ability. Navigational abilities pertain to large‐scale spaces, often assessed by map drawing, pointing to invisible landmarks or retracing routes in learned environments (in contrast to small‐scale spatial abilities, such as the ability to mentally rotate objects). A feasible way to capture these large‐scale spatial abilities in a large group is to use questionnaires, such as the Santa Barbara Sense of Direction questionnaire (SBSOD), which asks people to rate their competence on navigation, giving and following directions, reading maps, and orienting oneself in the environment [Hegarty et al., 2002]. The score on this questionnaire predicts navigational performance on different scales in real as well as in virtual environments [Hegarty et al., 2002; 2006], which indicates that people have a good subjective awareness of their spatial abilities. Furthermore, the concept captured by this score is coherent across languages, as the factor loadings of the questions correlated highly between languages [Montello and Xiao, 2011]. Therefore, the SBSOD questionnaire can be treated as a measure of navigational ability. Consequently, from this point onward, we will refer to people with a high self‐reported navigational ability on the SBSOD as good navigators and people with a low score as bad navigators.
Self‐reported navigational abilities are associated with the use of strategies for wayfinding. An individual's navigational ability correlates positively with the use of a survey strategy, which is based on spatial relations between environmental landmarks in a map‐like manner [Prestopnik and Roskos‐Ewoldsen, 2000]. This correlation was not found for the users of a route navigational strategy, who use a sequence of actions to navigate to a goal. The same study showed that a group with poor self‐reported navigational ability makes less use of a survey strategy compared with a group that had a good self‐reported navigational ability. Additional support for a relation between survey strategy use and navigational ability comes from a study that used a modified task from the rodent literature. In this so‐called eight‐arm task, participants had to remember the locations of objects in a virtual radial maze [Iaria et al., 2003]. In a probe trial, the distal spatial cues (e.g., mountains) were removed during the retrieval phase. Participants that relied on these spatial cues made more errors in this probe trial. On the basis of the descriptions of their strategies, people were divided into groups with a survey strategy, a response‐start position strategy (people that counted arms) or a response‐external landmarks strategy (people that used a response strategy that relied on an external landmark). In a different, more realistic large‐scale virtual environment, the survey group outperformed the response‐start position group [Etchamendy and Bohbot, 2007]. The response‐external landmark group, using a strategy that was efficient in the eight‐arm task, also performed best in the realistic environment, suggesting the best navigators are the ones who can use the optimal strategy for the task at hand. Furthermore, a self‐report study characterized people's spatial styles as landmark, route and survey users in a cumulative manner [i.e., people in the route group used both landmark and route, but no survey information; Piccardi et al., 2011]. This study showed that this cumulative characterization correlated positively with self‐reported sense of direction.
Which brain regions contribute to navigational success? The parahippocampal gyrus is involved in the processing of spatial scenes and in the navigational relevance of landmarks [Epstein and Kanwisher, 1998; Janzen and van Turennout, 2004], as well as spatial and nonspatial context [Bar and Aminoff, 2003]. Stronger location‐specific and viewpoint‐invariant representations were found for better navigators, a mechanism that might support successful wayfinding [Epstein et al., 2005]. The hippocampus, a crucial region for navigation and memory for spatial relations [Doeller et al., 2008; Hartley et al., 2003; O'Keefe and Nadel, 1978], showed anatomical changes with navigational training [Maguire et al., 2000, 2003; Woollett and Maguire, 2011]. In the hippocampus, an increase in activation to landmarks learned the previous day compared with landmarks learned on the day of scanning was related to increased navigational ability. This suggests better spatial abilities might arise partly because of a consolidation advantage [Janzen et al., 2008]. In contrast to the survey representations in the hippocampus, the caudate nucleus is associated with stimulus‐response learning in which a stimulus is consistently associated with a correct response [Hartley et al., 2003; Iaria et al., 2003; Packard et al., 1989; Packard and McGaugh, 1996]. An example of stimulus‐response learning enabled by the caudate is egocentric (body‐centered) navigation, for example, following a learned route and using landmarks as turn indicators [Hartley et al., 2003]. A study linking navigational ability to the connectivity between the aforementioned regions found that functional connectivity between the parahippocampal gyrus and the hippocampus increased for good navigators, whereas the connectivity between the parahippocampal gyrus and the caudate nucleus increased for bad navigators after route learning [Wegman and Janzen, 2011].
Strategy use in spatial tasks has also been associated with brain function and structure. Using the same eight‐arm task described earlier, it was shown that participants that relied on spatial cues showed more hippocampal activation during encoding, in contrast to users of a response strategy (counting arms) who showed higher caudate activity [Iaria et al., 2003]. Another study using the eight‐arm task found that the number of errors (more errors indicate the use of a spatial strategy) correlated positively with gray matter in the hippocampus and negatively with gray matter in the caudate nucleus [Bohbot et al., 2007]. Similarly, in a survey navigation task in a virtual environment, hippocampal fractional anisotropy (FA) values predicted shorter learning times [Iaria et al., 2008]. Moreover, in a group of older adults, hippocampal volume predicted navigation performance after learning a survey wayfinding but not a response‐based route task, whereas caudate volume predicted navigation performance after learning the response‐based route but not the survey wayfinding task [Head and Isom, 2010].
In the current study, we investigated whether self‐reported navigational skill scores correlated with local gray matter (GM) volume, regional volumetry, and white matter (WM) FA [for reviews on the link between interindividual variation and gray and white matter measures, see Johansen‐Berg, 2010; Kanai and Rees, 2011]. On the basis of the preceding findings linking navigational abilities to the use of specific navigational strategies and brain regions associated with these strategies, we hypothesized that the hippocampus and the caudate nucleus would show anatomical differences that were related with self‐reported navigational skills. For the hippocampus, we expected this correlation to be positive, whereas for the caudate nucleus we expected a negative correlation. We tested this hypothesis by analyzing the predictive power the SBSOD score has on the local gray matter volume in anatomical scans, regional volumetry, and on the FA values derived from DTI scans.
MATERIALS AND METHODS
Participants
This study combines participants from a number of previous studies (for an overview, see Table SI in the Supporting Information). The VBM analysis is based on 134 participants (60 females, average age 22.7, range 18–32): 21 participants took part in Janzen and Jansen [2010; 9 females], 16 in Janzen et al. [2008; 8 females], 17 in Wegman et al. [in preparation; 9 females], 24 in Wegman and Janzen [2011; 12 females], 16 in Van Ekert et al. [in preparation; 8 females], and 40 in Wegman et al. [in preparation; 14 females]. A subset of these participants also underwent DTI scanning: participants in the studies of Van Ekert et al. [in preparation; 19 participants] and Wegman et al. [in preparation; 35 participants]. All participants were neurologically healthy and right‐handed according to self‐report. Participants received a monetary reward or course credits for their participation, and all gave informed consent according to institutional guidelines of the local ethics committee (CMO region Arnhem‐Nijmegen, The Netherlands).
Questionnaires
Self‐reported navigational ability was assessed using the original English SBSOD [Santa Barbara Sense of Direction; Hegarty et al., 2002], which was administered to all participants after taking part in the experiments to prevent the participants from creating expectations about the purpose of the experiment. The SBSOD comprises 15 self‐referential statements about aspects of environmental spatial cognition, which needed to be rated on a 1–7 scale to indicate agreement with the statement. Approximately half of the statements were phrased positively (e.g., I am very good at giving directions) and half of the statements were phrased negatively (e.g., It's not important to me to know where I am). To the participants for which DTI scans were acquired, two additional questionnaires were administered. First, participants filled out the wayfinding strategy scale [Lawton, 1994], which generates one score characterizing the degree to which participants use a route strategy and one score for their use of a survey strategy. The survey and route scores we collected from our participants were positively correlated with each other (P < 0.05; r = 0.311), which was also reported by Lawton [1994]. As we were interested in individual differences in preference for either a survey or route strategy, a single survey‐route score was created by subtracting the route score from the survey score. Positive values on this difference score indicated an individual's preference for a survey strategy, whereas negative values indicated a preference for a route strategy. Second, the Spatial Anxiety questionnaire was administered, which was developed to “measure the level of anxiety that participants would experience in eight situations presumed to require spatial/navigational skills”, [Lawton, 1994] for example, finding the way out of a complex arrangement of offices that was visited for the first time. Answers indicating the level of anxiety were given on a Likert scale; the average over the eight items was used as the participant's Spatial Anxiety score.
Image Acquisition
Imaging data were acquired on a 3T Siemens Trio scanner (Siemens, Erlangen, Germany). Structural images were acquired on a 3T Trio MRI system with eight‐ and 32‐channel head array radio‐frequency coils (Siemens, Erlangen, Germany). We acquired a structural scan of each participant with small variations (due to the use of scans obtained in different studies) to a standard T1‐weighted three‐dimensional magnetization‐prepared rapid acquisition gradient echo (MP‐RAGE; 192 sagittal slices; FA = 8°; TI = 1100 msec; slice thickness = 1 mm; FOV = 256 * 256 mm; in‐plane voxel resolution 1 * 1 mm). The variations to the scan protocol included a TR/TE of 1960/4.58 ms and 2300/3.03 ms and the use of GRAPPA parallel imaging with an acceleration factor of 2.
Diffusion‐weighted data were collected using a twice‐refocused pulsed‐gradient spin echo‐planar imaging (EPI) sequence [Reese et al., 2003] at 3T using a Siemens Trio scanner with the following imaging parameters: TE = 98 ms, TR = 8800 ms, bandwidth 1924 Hz/pixel, 64 slices with no gap, resolution 2.2 × 2.2 × 2.2 mm, phase encoding direction anterior to posterior. We acquired diffusion‐weighted images in 64 noncollinear directions at a b‐value of 1000 s/mm2 and 4 images with no diffusion weighting. The total acquisition time for this sequence was 10 min.
VBM Analysis
The T1 anatomical images were manually checked for scanner artifacts and gross anatomical abnormalities. Next, the image origin was set to the anterior commissure. The anatomical images were subsequently segmented into gray matter, white matter, and cerebrospinal fluid (CSF) using the ‘New Segment’ tool in SPM8. Subsequently, we performed diffeomorphic anatomical registration through exponentiated lie algebra [DARTEL; Ashburner, 2007] for intersubject registration of the GM images. In the final step, the registered images were smoothed (FWHM = 8 mm), Jacobian modulated, thresholded at 0.2 and transformed into MNI152 standard space for a two‐sample t‐test second‐level statistical test. The GM images were entered into a factorial model, with the factors gender and navigational ability (bad or good, according to their self‐reported navigational ability, as measured by the SBSOD questionnaire, using a median split per gender). Age, total brain volume (TBV; total white matter plus gray matter volumes) and scan protocol were added to the model as covariates of no interest. All statistical tests were performed at the voxel level, statistically family‐wise error corrected for the entire brain (P FWE) or across all voxels in a region of interest using small volume correction (P SVC). Given our a priori hypotheses, our regions of interest (ROIs) were the hippocampus, parahippocampal gyrus, and the caudate nucleus. We created masks for each hemisphere in these regions based on the automated anatomical labeling [AAL library; Tzourio‐Mazoyer et al., 2002]. To look into the particular parahippocampal region that was previously found to label objects according to navigational relevance, we also created a bilateral ROI based on 10 mm spheres around the peak coordinates in Janzen and van Turennout [2004], which were converted to MNI152 space using the tal2mni MATLAB algorithm (available at http://imaging.mrc-cbu.cam.ac.uk/downloads/MNI2tal/tal2mni.m).
Volumetric Analysis
For the automatic segmentation of the hippocampus and the caudate nucleus in our T1 images we used FIRST v1.2 (available at: http://www.fmrib.ox.ac.uk/fsl/first/index.html) in FSL 4.1.4 (available at: http://www.fmrib.ox.ac.uk/fsl) [Smith et al., 2004]. This method is based on Bayesian statistical models of shape and appearance for fifteen subcortical structures from 336 manually labeled T1‐weighted MR images. To fit the models, the probability of the shape given the observed intensities is used [Patenaude et al., 2011]. The segmented caudate and hippocampal regions were visually inspected and overlaid on the anatomical image using FSL's “slicesdir” function to check for obvious segmentation errors (such as large parts of a structure located in the ventricles). Datasets in which the segmentation method failed were removed from further analysis (1 of 134).
The volumes of the segmentations for both the left and right caudate nucleus and hippocampus were entered into a multiple regression analysis, which was performed in SPSS 19.0 (SPSS, Chicago, IL). The regional volumes of our ROIs were the dependent variables, and SBSOD, gender, age, total brain volume, and scan protocol were included as predictors.
Diffusion Imaging Analysis
Each participant's diffusion weighted data was preprocessed using the Diffusion toolbox developed at the Donders Institute for Brain, Cognition and Behavior [Zwiers, 2010]. The images were realigned and then corrected for motion artifacts as well as cardiac and table‐vibration artifacts using the PATCH algorithm [Zwiers, 2010]. Then, we performed tract‐based spatial statistics analysis [TBSS; Smith et al., 2006] using the TBSS toolbox routines implemented in FSL. TBSS consists of the following steps: first, FA maps were nonlinearly registered to the standard FMRIB58–FA–1mm template included as part of FSL and a mean FA map was generated in MNI space. Then, the mean FA map was thresholded at an FA value of 0.2 and from it a white matter tract skeleton was generated representing the center of the tracts common across participants (Supporting Information Fig. S1). This procedure is aimed at reducing intersubject variability, thereby eliminating the need to smooth the images. Finally, each participant's FA values were projected onto this skeleton. A two‐sample t‐test was performed to compare FA values between a good navigator group and a bad navigator group, created by a median split based on the SBSOD scores. We included gender and age as covariates of no interest. Note that, in contrast to our VBM analysis and in line with common practice in the literature, we did not include TBV as a covariate in our analysis. However, including TBV in our FA analysis did not change the effects we found, rendering these partial‐volume confounds unlikely to be consequential.
The two‐sample t‐test was performed on the skeletonized FA values, using a voxel‐wise extent threshold of P < 0.05 corrected for multiple comparisons using the threshold‐free cluster enhancement algorithm [TFCE; Smith and Nichols, 2009], as implemented in the “randomize” permutation statistics tool in FSL. Similar to the VBM analysis, we performed ROI analyses on a priori defined regions of interest: the white matter structures closest to the hippocampus and caudate, which are the bilateral anterior limb of the internal capsule and the bilateral cingulum, respectively. We created the ROI masks by overlapping the white matter skeleton with the ICBM‐DTI‐81 white matter label regions [Mori et al., 2005] closest to our gray matter regions of interest: the bilateral anterior limb of the internal capsule (adjacent to the caudate nucleus) and the bilateral cingulum (hippocampus). To verify that the masked white matter of the caudate nucleus feeds into the anterior limb of the internal capsule, we ran probabilistic tractography separately for each hemisphere by seeding from the AAL caudate nucleus projected in subject space. To this end, we first fitted a local diffusion model with (maximally) 2 anisotropic compartments and one isotropic compartment [the ball‐and‐sticks model; Behrens et al., 2007] using a Markov Chain Monte Carlo algorithm and Automatic Relevance Detection for estimating the number of compartments per voxel. We then used samples from the posterior distribution on the direction of the anisotropic compartments for probabilistic tractography. Tractography was performed in the participants' native space.
The average FA values of these ROIs were extracted and entered into a multivariate linear regression analysis, in which all covariates were entered simultaneously, to estimate associations between ROI FA value and our measures of interest. This allowed us to look into the influence of all behavioral measures on the regional FA values simultaneously. To this end, for each ROI, we created two models within a hierarchical multiple regression analysis with the average FA value as dependent variable. The first model contained gender, age, and SBSOD value as independent variables. The second model was exploratory and contained the same variables as the first model plus the remaining behavioral measures: the survey‐route difference strategy score and spatial anxiety. We report standardized regression coefficients and the model fit value (R 2). P values reported with adjusted R 2 indicated whether the addition of the variables in that model led to a significant improvement in model fit over the previous model (or compared with the inclusion of no variables in the case of Model 1), adjusting for the number of variables in the model.
RESULTS
Behavioral Data
For the 134 participants included in the gray matter analyses, the SBSOD scores averaged 67.82, SD = 14.45, range 27–98. The SBSOD score was significantly correlated with gender (r = 0.265, P < 0.01; males coded as 1). Furthermore, TBV (gray + white matter) was correlated with SBSOD (r = 0.24, P < 0.01) and gender (r = 0.63, P < 0.001). All other pairwise correlations between age, gender, SBSOD and TBV did not reach significance.
For the 54 participants included in the FA value regression analyses, the average SBSOD, SD, and range of each of the scores are given in Table 1. The SBSOD score was significantly positively correlated with the survey‐route score (r = 0.488; P < 0.01). Spatial anxiety was negatively correlated with the survey‐route score (r = ‐0.425; P < 0.01) and SBSOD score (r = −0.300; P < 0.05). Finally, gender was positively correlated with survey‐route score (r = 0.433; P < 0.01; males coded as 1). All other pairwise correlations between age, gender, SBSOD, survey‐route score, and spatial anxiety score did not reach significance.
Table 1.
Questionnaire and task scores for the participants included in the FA regression (N = 54)
| Average | SD | Range | Possible range | |
|---|---|---|---|---|
| SBSOD | 65.24 | 14.94 | 27–93 | 15–105 |
| Survey‐route score | −0.62 | 0.80 | −2.76 to 0.98 | −4 to 4 |
| Spatial anxiety score | 2.38 | 0.65 | 1.13–3.75 | 1–5 |
SD = standard deviation.
To ensure that the self‐reported navigational ability in the SBSOD was not influenced by the task they had performed before filling out the questionnaire, we asked participants in the study of Wegman et al. (in preparation) to fill out the SBSOD again months after they had participated in the experiment. In that study they had performed a virtual navigation experiment. Thirty‐nine participants filled out the SBSOD again over the internet, an average of 352 days after they were tested in the lab (range 115–462 days). The correlation between scores on the two administrations of the scale (test‐retest reliability) was 0.73. As a reference, Hegarty et al. [2002] reported a test‐retest reliability of 0.91 with 40 days between administrations. Next, we investigated performance on the virtual navigation task performed before filling out the SBSOD, to test whether performance on the task influenced self‐reported navigational skills. Importantly, we did not observe a correlation between the SBSOD difference (test–retest) with performance on any of the navigation task conditions (all Ps > .5). Finally, we did not observe a relationship between the test–retest difference score and the time between these administrations (r = −0.17, P > .3; correlation with absolute difference score r = −0.19, P > 0.24). We therefore conclude that performance on this task did not have an influence on self‐reported navigational ability. Of all the tasks performed in the studies from which participants are included in this study [e.g., recognition of objects placed along a previously learned route; Janzen et al., 2008; Janzen and Jansen, 2010; Wegman and Janzen, 2011], this virtual navigation task resembled everyday navigation the most and we therefore assume this conclusion also holds for the other tasks performed before filling out the SBSOD.
Relationships Between Navigational Ability and GM
Local GM differences were investigated using a factorial model containing the factors navigational ability and gender (see Methods), with these groups: bad male navigators (SBSOD range 27–71), bad female navigators (SBSOD range 35–63), good male navigators (SBSOD range 72–98), and good female navigators (SBSOD range 66–95). Good navigators showed a trend toward more regional GM in the right anterior parahippocampal gyrus, in the rhinal cortex (P SVC = 0.098; peak coordinate x = 26, y = 3, z = −35) compared with bad navigators (Fig. 1A, Supporting Information Table SII). When we compared good with bad male navigators, a significant difference in the right hippocampus was observed (Fig. 1B, Supporting Information Table SII; P SVC = 0.046; peak coordinate x = 32, y = −9, z = −26). Comparing regional GM in good female navigators to bad female navigators yielded no significant voxel‐wise results. When we compared bad versus good navigators, we observed a trend in the right caudate nucleus (Fig. 1C, Supporting Information Table SII; P SVC = 0.07; peak coordinate x = 15, y = 15, z = 0). Furthermore, we observed a trend toward an interaction in right hippocampus (Fig. 1D, Supporting Information Table SII; P SVC = 0.088; peak coordinate x = 34, y = −7, z = −24) and right parahippocampal gyrus (P SVC = 0.077; peak coordinate x = 36, y = −18, z = −26).
Figure 1.
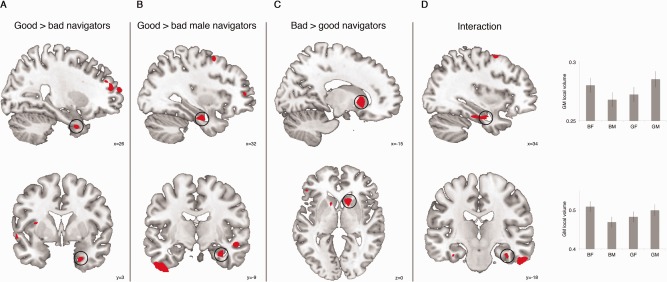
Results of GM volume voxel‐based morphometry analysis. A: increased gray matter (GM) in right anterior parahippocampal gyrus (PHG)/rhinal cortex for good navigators compared with bad navigators. B: increased GM in right hippocampus for good compared with bad male navigators. C: increased GM in right caudate nucleus for Bad compared with good navigators. D: Left panels: GM gender * navigational ability interaction in right hippocampus (top) and right PHG (bottom). Right panels: local GM volume in right hippocampus at the peak (top; x = 34, y = −7, z = −24) and right PHG at the peak (bottom; x = 36, y = −18, z = −26). BF = bad female navigators, BM = bad male navigators, GF = good female navigators, GM = good male navigators. Images thresholded at P < 0.005 uncorrected.
To investigate whether the total regional volume was related to SBSOD, we applied automatic volumetry on our anatomical MRI data. All regional volumes were significantly related to total brain volume (all P < 0.001; Supporting Information Table SIII). Furthermore, left caudate (P = 0.031) and right caudate (P = 0.029) volume was negatively related to SBSOD, that is, bad navigators have bilaterally enlarged caudate nuclei compared with good navigators.
Relationships Between Sense of Direction and FA
A comparison of the good and bad navigator groups did not give any whole‐brain significant regions. We then compared the skeletonized FA values in our masked dataset for white matter label regions in the hippocampus and white matter surrounding the caudate nucleus. Bad navigators showed significantly higher FA values than good navigators inside the left and right anterior limb of the internal capsule close to the caudate nucleus (Fig. 2). The contrast of good versus bad navigators revealed no significant results.
Figure 2.
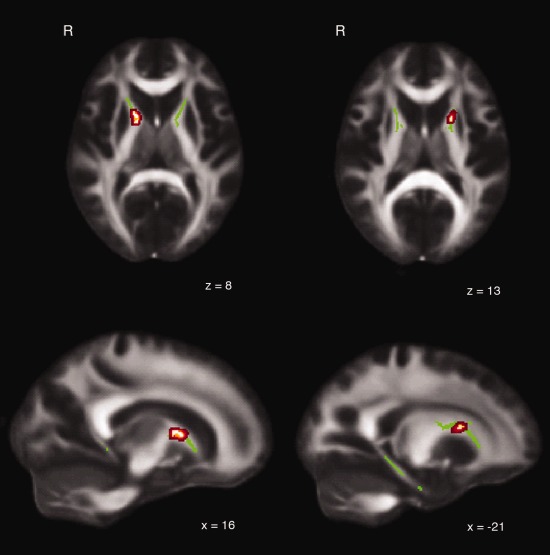
ROI skeletal white matter (WM) voxels (green) that show higher FA values for bad navigators compared with good navigators. Cluster‐based thresholding, corrected for multiple comparisons (P < 0.05).
To gain insight into the influence of our behavioral measures on regional FA volume, we performed multiple regressions analysis on the average FA values from our four ROIs (WM in the left and right hippocampus and surrounding the caudate nucleus; bilateral cingulum and bilateral anterior limb of the internal capsule). To determine the relationship between our behavioral measures and the average FA values in our ROIs, we conducted a multiple regression analysis with the FA value in a region as dependent variable. For each ROI, we created two models. Model 1 contained gender, age, and SBSOD value as independent variables. This basic model was aimed at testing our hypothesized link between SBSOD and anatomical differences in our ROIs. Model 2 was exploratory and contained the same variables as the first model plus the remaining behavioral measures: the survey‐route difference strategy score and spatial anxiety. In the left anterior capsule (WM surrounding the caudate nucleus), the SBSOD reached significance in both Models, although only Model 1 trended toward significance (Table 2). In the right anterior capsule, the SBSOD was a significant negative predictor of average FA value in both Models, although only Model 1 reached significance (Table 2). In the left cingulum (WM in the hippocampus), Model 1 failed to reach significance, but Model 2 showed a trend toward significance (Table 3). Within that model, the SBSOD (P = 0.053) and the spatial anxiety score were positive predictors of average FA values (Table 3). For the right cingulum, none of the explanatory variables reached significance, nor did the models themselves (Table 3). To verify that the masked white matter of the caudate nucleus feeds into the anterior limb of the internal capsule, we ran probabilistic tractography separately for each hemisphere by seeding from the AAL caudate nucleus projected in subject space. Figure S2 (Supporting Information) shows that our assumptions are confirmed.
Table 2.
FA regression analysis: associations between explanatory variables and regional FA values in left and right anterior capsule (caudate)
| Left | Right | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| β | P | β | P | β | P | β | P | |
| Age | 0.08 | 0.55 | 0.08 | 0.56 | 0.08 | 0.53 | 0.08 | 0.56 |
| Gender | 0.11 | 0.42 | 0.14 | 0.37 | 0.14 | 0.31 | 0.15 | 0.31 |
| SBSOD score | −0.37 | 0.01b | −0.35 | 0.03b | −0.45 | 0.001b | −0.44 | 0.006b |
| Strategy: survey‐route | −0.12 | 0.49 | −0.03 | 0.88 | ||||
| Spatial anxiety | −0.11 | 0.47 | 0.02 | 0.87 | ||||
| Adjusted R 2 | 0.07 | 0.078a | 0.05 | 0.68 | 0.14 | 0.02b | 0.1 | 0.97 |
β = standardized regression coefficient.
P <0.1,
P < 0.05.
Table 3.
FA regression analysis: associations between explanatory variables and regional FA values in left and right cingulum (hippocampus)
| Left | Right | |||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| β | P | β | P | β | P | β | P | |
| Age | 0.14 | 0.31 | 0.12 | 0.39 | 0.1 | 0.51 | 0.08 | 0.6 |
| Gender | 0.05 | 0.73 | 0.12 | 0.42 | −0.14 | 0.34 | −0.04 | 0.81 |
| SBSOD score | 0.19 | 0.19 | 0.31 | 0.053a | −0.01 | 0.97 | 0.12 | 0.46 |
| Strategy: survey‐route | −0.06 | 0.75 | −0.26 | 0.15 | ||||
| Spatial anxiety | 0.33 | 0.03b | 0.07 | 0.68 | ||||
| Adjusted R 2 | 0.022 | 0.253 | 0.099 | 0.053a | −0.03 | 0.74 | −0.01 | 0.24 |
β = standardized regression coefficient.
P < 0.1,
P < 0.05.
DISCUSSION
Our results show that self‐reported navigational abilities have different neural underpinnings in local gray matter volume, regional volumes, and white matter FA. We observed trends toward higher local GM volume in right anterior parahippocampal gyrus/rhinal cortex for good versus bad navigators and in right caudate nucleus for bad versus good navigators. Good male navigators showed higher local GM volume in right hippocampus than bad male navigators. This result could be explained by trends toward an interaction between gender and navigational ability in the right medial temporal lobe. White matter integrity in the left hippocampus was positively correlated with navigational ability and spatial anxiety. Bad navigators showed increased FA values in the internal capsule, which feeds into the caudate nucleus. Bilaterally, caudate nucleus regional volume showed the same inverse correlation with navigational ability.
The self‐reported navigational ability score correlated with the self‐reported use of a survey navigational strategy, which is in line with behavioral literature [Piccardi et al., 2011; Prestopnik and Roskos‐Ewoldsen, 2000]. Besides being more likely to use a survey (spatial) strategy, people with good self‐reported navigational ability can more flexibly make use of an effective navigation strategy [Kato and Takeuchi, 2003], which could also explain why route and survey scores in the strategy questionnaire were correlated. On the basis of the self‐reported strategy use in the aforementioned eight‐arm task [a virtual radial maze; Etchamendy and Bohbot, 2007], spatial (survey) users and those who used a response strategy that relied on an external landmark performed best in a more realistic city environment, again suggesting that the best navigators are the ones who can use the optimal strategy for the task at hand.
The negative relationship between navigational ability and caudate WM microstructure was corroborated by volumetric analysis of the caudate nucleus and a trend toward higher local GM volume in the right caudate nucleus for bad compared with good navigators in the VBM analysis. This suggests that both structural integrity of WM around the caudate, local GM volume and the regional volume of the caudate negatively underlie navigational abilities. In older adults, caudate volume was positively related to (response‐based) route learning performance but not to survey learning performance [Head and Isom, 2010]. Consistent with this finding, good and bad performers on a navigation task showed an opposite activation pattern in the caudate nucleus between people with navigational performance [Hartley et al., 2003]. In that fMRI study, bad navigational performers activated the caudate nucleus stronger in a survey wayfinding task than when following a well‐learned route, whereas good navigational performers activated the caudate nucleus more in route following than wayfinding. The current findings suggest that bad navigational skills are related to volume and microstructure of the caudate, a region known to be involved in response‐based navigation. Although we cannot conclude that these anatomical differences are a result of increased use of the caudate based on this cross‐sectional study, longitudinal studies suggest increased use of a brain structure was related to higher FA values in that structure [Johansen‐Berg, 2010].
Partial volume effects are a known nuisance effect in DTI analyses [Jones and Cercignani, 2010]. As a result, our observed effects in the caudate nucleus and surrounding WM might not be independent effects. Larger caudate volume could lead to smaller adjacent WM. A smaller WM region is more affected by partial volume effects, leading to lower average FA values. However, the relationships we observed between self‐reported navigational ability and both caudate volume and FA in surrounding WM were in the same (negative) direction. Therefore, this possible confound is unlikely.
The reported relationships in brain microstructure, as measured by DTI, could not be explained by gender or age. The absence of an effect probably stems from the narrow age range in our young group of participants, as navigational skills are known to alter as humans grow older [Jones and Cercignani, 2010]. Although gender can have an impact on different spatial tasks [Grön et al., 2000; Moffat et al., 1998], it did not have a significant effect on the WM microstructure in our regions of interest.
We also observed a trend toward more local GM volume in right anterior parahippocampal gyrus/rhinal cortex for good compared with bad navigators. The rhinal cortex comprises the entorhinal and perirhinal cortex. The entorhinal cortex contains grid cells whose firing locations within an environment form a regular, hexagonal grid‐like pattern [Hafting et al., 2005], together functioning as a map of a navigator's position in space. The cluster showing more local GM volume for good compared with bad navigators is located very close to a region exhibiting grid cell population signals in a human fMRI navigation study, showing modulation by running direction with six‐fold rotational symmetry [Doeller et al., 2010]. The coherence of the signal in this area correlated with spatial memory, suggesting that this region can contribute to better spatial abilities. The perirhinal cortex was activated more by good than bad navigational performers during wayfinding [Hartley et al., 2003]. This region is suggested to be vital for recognition memory [for reviews, see Brown and Aggleton, 2001; Davachi, 2006; Diana et al., 2007; Eichenbaum et al., 2007]. Related to spatial memory, the perirhinal cortex was found to be involved in source memory and object‐in‐place encoding [Awipi and Davachi, 2008]. Also, the perirhinal cortex does seem to play a role in solving tasks that require spatial awareness [Kealy and Commins, 2011]. These are both functions that can contribute to successful navigation. Furthermore, Bohbot et al. [2007] found that entorhinal and perirhinal GM was correlated with hippocampal and parahippocampal GM densities, suggesting that the coactivation of this network leads to GM changes and might support good navigational skills. Future research is necessary to investigate anterior parahippocampal/rhinal GM contributions to good spatial abilities.
When we compared good with bad male navigators, we found GM differences in the right hippocampus. Since the discovery of place cells in the hippocampus, which represent the location of an animal in its environment, this region is viewed as providing the navigator with a cognitive map [Burgess et al., 2002; O'Keefe and Nadel, 1978]. Place cell representations could be computed from upstream entorhinal grid cells [see Derdikman and Moser, 2010], for a review]. More local GM volume in the hippocampus is therefore consistent with good navigational abilities. It is, however, unclear how these results relate to decreases in local GM volume in the anterior hippocampus after navigational training, occurring alongside an increase in posterior hippocampus GM volume [Maguire et al., 2003; 2000]. We did not observe GM differences related to self‐reported navigational abilities in females. This might be explained by the more pronounced differences between good and bad navigators in males. For instance, males had on average higher SBSOD scores than females and a higher survey‐route difference score. Previous research on a greater number of participants has shown that males use a survey strategy more often than females do [Montello et al., 1999]. Also, western males were found to have more childhood wayfinding experience than western females [Lawton and Kallai, 2002]. We also observed trends toward an interaction between gender and navigational ability in right hippocampus and right parahippocampal gyrus. This could explain why the overall differences between good and bad navigators in medial temporal lobe are weaker than when only looking at males. Gender differences in spatial cognition are commonly found [Hegarty et al., 2006; Voyer et al., 2007], as are structural and functional differences between males and females in the brain [Cahill, 2006]. For instance, males and females were found to use different brain networks during a navigation task [Grön et al., 2000]. The use of different networks for navigation might explain the trending interactions in the medial temporal lobe, although further investigation is necessary to elucidate how functional and structural differences in males and females contribute to good spatial abilities.
The caudate nucleus and the hippocampus have been suggested to support spatial cognition by working in parallel [Voermans et al., 2004]. However, a competition between these structures is suggested by Bohbot et al. [2007], who found an inverse correlation between hippocampal and caudate nucleus gray‐matter density. These latter findings are in line with our WM findings in which we observed a negative correlation between navigational ability and caudate FA values. In the left hippocampus, we found a trend toward a positive correlation between navigational ability and FA. Similarly, our VBM results show higher local GM volume in the right medial temporal lobe for good navigators. In the caudate nucleus, bad navigators showed higher local GM volumes in right caudate nucleus and we observed negative correlations between navigational ability and regional volumetry. Taken together, our results support different neural underpinnings of good and bad navigational ability in the caudate nucleus and the hippocampus through the different representations that they subserve.
We found left hippocampal white matter integrity to be positively related (P = 0.053 for both the regression model as the navigational ability predictor) to navigational abilities. Although spatial memory is often associated with the right hippocampus [Burgess et al., 2002; Iaria et al., 2008], the evidence for lateralization of spatial memory in the hippocampus is mixed and depends on the type of spatial task [Burgess et al., 2001; Glikmann‐Johnston et al., 2008; Iglói et al., 2010; Kessels et al., 2002; Spiers et al., 2001]. Therefore, this left hippocampal finding does not seem to contradict previous findings, promoting the idea that both hippocampi support navigation.
The regression model also showed a positive correlation between left hippocampal FA values and spatial anxiety, which was not hypothesized. However, in light of previous literature this finding is less surprising, as a positive relationship between hippocampal volume and trait anxiety has been reported by Rusch et al. [2001].
All the effects revealed by our multimodal approach were in hypothesized directions in the regions where they were expected. Therefore, although the effect sizes in gray and white matter are relatively small, the strong overlap between effects with our expectations and between imaging modalities strengthens our confidence in the relationship between navigational ability and brain anatomy.
This study shows that large‐scale navigational abilities, assessed through self‐report, are related to differences in white matter microstructure, gray matter local and regional volumes. To our knowledge, it is the first study to combine different structural imaging methods with a large sample of participants to show anatomical correlates underlying these abilities. Our combined findings point to a larger reliance on the caudate nucleus for bad navigators. The medial temporal lobes support better navigational abilities through hippocampal white matter integrity and hippocampal and parahippocampal gray matter differences that support the formation and use of cognitive maps.
ACKNOWLEDGMENTS
The authors thank Mark Rijpkema for helpful discussions, Jaap Rohof for assistance during data acquisition and two anonymous reviewers for valuable comments.
Supporting information
Supporting Information
REFERENCES
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. NeuroImage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Awipi T, Davachi L (2008): Content‐specific source encoding in the human medial temporal lobe. J Exp Psychol Learn Mem Cogn 34:769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E (2003): Cortical analysis of visual context. Neuron 38:347–358. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW (2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage 34:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot VD, Lerch J, Thorndycraft B, Iaria G, Zijdenbos AP (2007): Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J Neurosci 27:10078–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP (2001): Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Publishing Group 2:51–61. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O′Keefe J (2002): The human hippocampus and spatial and episodic memory. Neuron 35:625–641. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O′Keefe J (2001): A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. NeuroImage 14:439–453. [DOI] [PubMed] [Google Scholar]
- Cahill L (2006): Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484. [DOI] [PubMed] [Google Scholar]
- Davachi L (2006): Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16:693–700. [DOI] [PubMed] [Google Scholar]
- Derdikman D, Moser EI (2010): A manifold of spatial maps in the brain. Trends Cogn Sci (Regul Ed) 14:561–569. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C (2007): Imaging recollection and familiarity in the medial temporal lobe: A three‐component model. Trends Cogn Sci (Regul Ed) 11:379–386. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Barry C, Burgess N (2010): Evidence for grid cells in a human memory network. Nature 463:657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, King JA, Burgess N (2008): Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proc Natl Acad Sci USA 105:5915–5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C (2007): The medial temporal lobe and recognition memory. Annu Rev Neurosci 30:123–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N (1998): A cortical representation of the local visual environment. Nature 392:598–601. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Thompson‐Schill SL (2005): Learning places from views: variation in scene processing as a function of experience and navigational ability. J Cogn Neurosci 17:73–83. [DOI] [PubMed] [Google Scholar]
- Etchamendy N, Bohbot VD (2007): Spontaneous navigational strategies and performance in the virtual town. Hippocampus 17:595–599. [DOI] [PubMed] [Google Scholar]
- Glikmann‐Johnston Y, Saling MM, Chen J, Cooper KA, Beare RJ, Reutens DC (2008): Structural and functional correlates of unilateral mesial temporal lobe spatial memory impairment. Brain 131:3006–3018. [DOI] [PubMed] [Google Scholar]
- Grön G, Wunderlich AP, Spitzer M, Tomczak R, Riepe MW (2000): Brain activation during human navigation: gender‐different neural networks as substrate of performance. Nat Neurosci 3:404–408. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser M‐B, Moser EI (2005): Microstructure of a spatial map in the entorhinal cortex. Nature 436:801–806. [DOI] [PubMed] [Google Scholar]
- Hartley T, Maguire EA, Spiers HJ, Burgess N (2003): The well‐worn route and the path less traveled: Distinct neural bases of route following and wayfinding in humans. Neuron 37:877–888. [DOI] [PubMed] [Google Scholar]
- Head D, Isom M (2010): Age effects on wayfinding and route learning skills. Behav Brain Res 209:49–58. [DOI] [PubMed] [Google Scholar]
- Hegarty M, Montello D, Richardson A, Ishikawa T, Lovelace K (2006): Spatial abilities at different scales: Individual differences in aptitude‐test performance and spatial‐layout learning. Intelligence 34:151–176. [Google Scholar]
- Hegarty M, Richardson AE, Montello DR, Lovelace K, Subbiah I (2002): Development of a self‐report measure of environmental spatial ability. Intelligence 30:425–447. [Google Scholar]
- Iaria G, Lanyon LJ, Fox CJ, Giaschi D, Barton JJS (2008): Navigational skills correlate with hippocampal fractional anisotropy in humans. Hippocampus 18:335–339. [DOI] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD (2003): Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: variability and change with practice. J Neurosci 23:5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglói K, Doeller CF, Berthoz A, Rondi‐Reig L, Burgess N (2010): Lateralized human hippocampal activity predicts navigation based on sequence or place memory. Proc Natl Acad Sci USA 107:14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen G, Jansen C (2010): A neural wayfinding mechanism adjusts for ambiguous landmark information. NeuroImage 52:364–370. [DOI] [PubMed] [Google Scholar]
- Janzen G, Jansen C, van Turennout M (2008): Memory consolidation of landmarks in good navigators. Hippocampus 18:40–47. [DOI] [PubMed] [Google Scholar]
- Janzen G, van Turennout M (2004): Selective neural representation of objects relevant for navigation. Nat Neurosci 7:673–677. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H (2010): Behavioural relevance of variation in white matter microstructure. Curr Opin Neurol 23:351–358. [DOI] [PubMed] [Google Scholar]
- Jones DK, Cercignani M (2010): Twenty‐five pitfalls in the analysis of diffusion MRI data. NMR Biomed 23:803–820. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G (2011): The structural basis of inter‐individual differences in human behaviour and cognition. Nat Rev Neurosci 12:231–242. [DOI] [PubMed] [Google Scholar]
- Kato Y, Takeuchi Y (2003): Individual differences in wayfinding strategies. J Environ Psychol 23:171–188. [Google Scholar]
- Kealy J, Commins S (2011): The rat perirhinal cortex: A review of anatomy, physiology, plasticity, and function. Prog Neurobiol 93:522–548. [DOI] [PubMed] [Google Scholar]
- Kessels RPC, Jaap Kappelle L, de Haan EHF, Postma A (2002): Lateralization of spatial‐memory processes: Evidence on spatial span, maze learning, and memory for object locations. Neuropsychologia 40:1465–1473. [DOI] [PubMed] [Google Scholar]
- Lawton C (1994): Gender differences in way‐finding strategies: Relationship to spatial ability and spatial anxiety. Sex Roles 30:765–779. [Google Scholar]
- Lawton C, Kallai J (2002): Gender differences in wayfinding strategies and anxiety about wayfinding: A cross‐cultural comparison. Sex Roles 47:389–401. [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD (2000): Navigation‐related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA 97:4398–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Spiers HJ, Good CD, Hartley T, Frackowiak RS, Burgess N (2003): Navigation expertise and the human hippocampus: A structural brain imaging analysis. Hippocampus 13:250–259. [DOI] [PubMed] [Google Scholar]
- Moffat S, Hampson E, Hatzipantelis M (1998): Navigation in a “Virtual” maze: Sex differences and correlation with psychometric measures of evolution and human behavior19: 73–87.
- Montello D, Lovelace K, Golledge R, Self C (1999): Sex‐related differences and similarities in geographic and environmental spatial abilities. Ann Assoc Am Geograph 89:515–534. [Google Scholar]
- Montello D, Xiao D (2011): Linguistic and cultural universality of the concept of sense‐of‐direction. In: Egenhofer MJ, Giudice NA, Moratz R, Worboys MF, editors. Berlin: Springer; pp 264–282. [Google Scholar]
- Mori S, Wakana S, Nagae‐Poetscher L, van Zijl P (2005): MRI Atlas of Human White Matter. Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L (1978): The Hippocampus as a Cognitive Map. Oxford: Clarendon Press. [Google Scholar]
- Packard MG, Hirsh R, White NM (1989): Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: Evidence for multiple memory systems. J Neurosci 9:1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL (1996): Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Memory 65:65–72. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccardi L, Risetti M, Nori R (2011): Familiarity and envorinmental representations of a city: A self‐report study. Psychol Rep 109:309–326. [DOI] [PubMed] [Google Scholar]
- Prestopnik J, Roskos‐Ewoldsen B (2000): The relations among wayfinding strategy use, sense of direction, sex, familiarity, and wayfinding ability. J Environ Psychol 20:177–191. [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ (2003): Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med 49:177–182. [DOI] [PubMed] [Google Scholar]
- Rusch BD, Abercrombie HC, Oakes TR, Schaefer SM, Davidson RJ (2001): Hippocampal morphometry in depressed patients and control subjects: relations to anxiety symptoms. Biol Psychiatry 50:960–964. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23 (Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, O'Keefe J (2001): Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain 124:2476–2489. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Petersson KM, Daudey L, Weber B, van Spaendonck KP, Kremer HPH, Fernández G (2004): Interaction between the human hippocampus and the caudate nucleus during route recognition. Neuron 43:427–435. [DOI] [PubMed] [Google Scholar]
- Voyer D, Postma A, Brake B, Imperato‐McGinley J (2007): Gender differences in object location memory: A meta‐analysis. Psychon Bull Rev 14:23–38. [DOI] [PubMed] [Google Scholar]
- Wegman J, Janzen G (2011): Neural encoding of objects relevant for navigation and resting state correlations with navigational ability. J Cogn Neurosci 23:3841–3854. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Hegarty M (2010): What determines our navigational abilities? Trends Cogn Sci 14:138–146. [DOI] [PubMed] [Google Scholar]
- Woollett K, Maguire EA (2011): Acquiring the knowledge of london's layout drives structural brain changes. Curr Biol 21:2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiers MP (2010): Patching cardiac and head motion artefacts in diffusion‐weighted images. NeuroImage 53:565–575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


