Abstract
Background: Cerebellar pathology occurs in late multiple sclerosis (MS) but little is known about cerebellar changes during early disease stages. In this study, we propose a new multicontrast “connectometry” approach to assess the structural and functional integrity of cerebellar networks and connectivity in early MS. Methods: We used diffusion spectrum and resting‐state functional MRI (rs‐fMRI) to establish the structural and functional cerebellar connectomes in 28 early relapsing‐remitting MS patients and 16 healthy controls (HC). We performed multicontrast “connectometry” by quantifying multiple MRI parameters along the structural tracts (generalized fractional anisotropy‐GFA, T1/T2 relaxation times and magnetization transfer ratio) and functional connectivity measures. Subsequently, we assessed multivariate differences in local connections and network properties between MS and HC subjects; finally, we correlated detected alterations with lesion load, disease duration, and clinical scores. Results: In MS patients, a subset of structural connections showed quantitative MRI changes suggesting loss of axonal microstructure and integrity (increased T1 and decreased GFA, P < 0.05). These alterations highly correlated with motor, memory and attention in patients, but were independent of cerebellar lesion load and disease duration. Neither network organization nor rs‐fMRI abnormalities were observed at this early stage. Conclusion: Multicontrast cerebellar connectometry revealed subtle cerebellar alterations in MS patients, which were independent of conventional disease markers and highly correlated with patient function. Future work should assess the prognostic value of the observed damage. Hum Brain Mapp 36:1609–1619, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: connectometry, multicontrast, diffusion MRI, resting‐state MRI, multiple sclerosis
INTRODUCTION
Multiple Sclerosis (MS) is an autoimmune inflammatory disease affecting the central nervous system, characterized by local and diffuse inflammation and degeneration [Chang et al., 2008]. The cerebellum is dually implicated in MS pathophysiology. First, it is a region where extensive cortical and subcortical demyelination occurs during late disease phases [Kutzelnigg et al., 2007], and where alterations of fiber bundles [Hulst et al., 2013] and atrophy [Calabrese et al., 2010] reportedly contribute to cognitive and motor dysfunction [Calabrese et al., 2010; Hulst et al., 2013]. Second, it is a structure implicated in functional motor recovery [Saini et al., 2004]. To date, however, little is known about the presence and impact of cerebellar damage and adaptive plasticity in the early phases of MS.
In this work, we aimed to study the structural integrity and functional properties of cerebellar networks and connectivity in early and minimally impaired MS patients. To achieve our goal, we used diffusion spectrum imaging (DSI) and resting‐state fMRI (rs‐fMRI) to construct structural and functional cerebellar connectomes and investigated the presence of axonal degeneration, microinflammation, and demyelination using quantitative MRI relaxometry and magnetization transfer imaging (MTI) (multicontrast connectometry). We then correlated the properties of affected cerebellar connections with clinical tests assessing disability, motor, and cognitive function.
DSI is a high‐b value and high‐angular resolution diffusion MRI technique [Wedeen et al., 2005] that can be used to resolve complex fiber bundle trajectories across the brain as well as to depict intricate cerebellar circuits [Granziera et al., 2009]. Conversely, rs‐fMRI allows computation of functional connections between different regions in a task‐independent manner and has already been applied to construct the cerebellar functional connectome in healthy subjects [Bernard et al., 2012; Buckner et al., 2011]. The combined analysis of DSI and rs‐fMRI, together with multiple contrasts providing microstructural information (multicontrast connectometry), represent a novel approach to assess the complex structure of cerebellar connections in early MS phases and in the presence of minor deficits.
METHODS
Subjects and MRI Acquisition
We enrolled 28 relapsing remitting MS patients (RRMS) and 16 healthy controls (HC), whose demographic characteristics are summarized in Table 1. All patients were diagnosed according to the revised MacDonald criteria [Polman et al., 2011] and at the enrollment had less than 6 years from initial symptoms (32 ± 21, range 2–66 months, early disease stage) and less than 5 years from disease diagnosis (25 ± 19 months, range 0–59 months). All patients were under immunomodulatory treatment (high‐dosage IFN beta or fingolimod) for at least 3 months. Fingolimod is approved as a first‐line treatment in Switzerland.
Table 1.
Demographic characteristics of HC and MS patients
| Demographic data | HC | MS patients | P‐value |
|---|---|---|---|
| Number of subjects | 16 | 28 | – |
| Age (mean years ± std) | 33.06 ± 10.37 | 34.32 ± 8.71 | 0.67 |
| Gender (males:females) | 7:9 | 10:18 | 0.61 |
| Education (mean years ± std) | 16.06 ± 3.51 | 15.93 ± 2.78 | 0.89 |
| Months since initial symptoms(mean ± std) | – | 31.86 ± 20.95 | – |
| Number of cerebellar lesions(mean ± std) | – | 22.21 ± 20.16 | – |
| Volume of cerebellar lesions(mean ratio to TIV ± std) | – | 0.00322 ± 0.00411 | – |
TIV: total intracranial volume.
No patient had received corticosteroid therapy within the three months preceding the enrollment. The protocol was approved by the ethics committee of the Lausanne University Hospital (CHUV) and all the participants gave written informed consent.
Each subject was scanned at 3T MRI (TIM Trio, Siemens, Erlangen, Germany) using a 32 channel head‐coil and the following protocol (Supporting Information Table 1): MPRAGE for anatomic reference; 3DFLAIR, double inversion recovery (DIR) and MP2RAGE [Marques et al., 2010] for lesion count [Kober et al., 2012] and T1 relaxometry (rt) maps respectively. MTI with (MT) and without a (M0) transfer saturation pulse were used to compute magnetization transfer ratio (MTR) maps [MTR = (M0 − MT)/M0 × 100]. T2 relaxometry (rt) maps were obtained from a model‐based reconstruction with blocked sampling [Sumpf et al., 2011]. Diffusion spectrum imaging (DSI) was acquired for structural connectome construction and computation of generalized fractional anisotropy (GFA) maps [Tuch, 2004]. A fast multiband T2*‐sensitive sequence yielding blood oxygen level dependent (BOLD) contrast was used for resting‐state fMRI [Xu et al., 2013]. During rs‐fMRI, the subjects were asked to remain alert, close their eyes, and not to think anything specific. Both DSI and rsfMRI data were visually inspected for detection of motion artefacts. No image was discarded from the analysis.
MRI Images Postprocessing
MPRAGE, DIR, and 3DFLAIR images as well as MTR and T2 rt maps were registered to the MP2RAGE/T1 rt map space with 6 degrees of freedom using FNIRT (FMRIB's Non Linear Image Registration Tool, http://fsl.fmrib.ox.ac.uk/fsl/). All subsequent computations were performed using these same registered volumes.
Lesions were manually counted by consensus between an expert neurologist (Cristina Granziera, CG) and radiologist (David Rotzinger, DR) in the 3DFLAIR, DIR, and MP2RAGE uniform images as reported [Kober et al., 2012]. Lesion volume was then manually segmented by a technician using itk‐SNAP (http://www.itksnap.org/) and subsequently verified by DR. A single set union mask was computed as reported [Kober et al., 2012]. Total brain lesion volume was computed as the sum of all lesion volumes divided by the total intracranial volume as supported by an in‐house based software.
Disability, Motor, Cognitive, and Behavioral Functions Assessment
Expanded disability status scale (EDSS) [Kurtzke, 1983] and multiple sclerosis functional composite (MSFC) [Rudick et al., 2002] scores were assessed by a certified neurologist. A brief repeatable battery of neuropsychological tests (BRB‐N) [Rao et al., 1991] was also assessed to measure cognitive performances. Cognitive z‐scores were computed for each test. Mood and fatigue were quantified using the Hospital anxiety and depression scale [Zigmond and Snaith, 1983] and the fatigue scale for motor and cognitive functions (FSMC) [Penner et al., 2009]. Clinical scores are reported in Table 2.
Table 2.
Clinical tests
| Disability and motor tests | Assessed function | HC | MS patients | P‐value |
|---|---|---|---|---|
| EDSS | Disability | – | 1.55 ± 0.21 | – |
| MSFC | Disability | – | −0.08 ± 0.24 | – |
| Arm function (MSFC) | Motor | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.19 |
| Leg function (MSFC) | Motor | 0.28 ± 0.05 | 0.26 ± 0.05 | 0.22 |
| BRB‐N | ||||
| SRT‐LTS | Verbal memory | 66.06 ± 6.72 | 64.14 ± 7.12 | 0.39 |
| SRT‐CLTR | Verbal memory | 63.19 ± 8.76 | 59.64 ± 11.94 | 0.31 |
| SRT‐D | Verbal memory | 11.75 ± 1.00 | 11.54 ± 0.79 | 0.44 |
| SPART | Visual memory | 23.75 ± 4.91 | 23.68 ± 4.23 | 0.96 |
| SPART‐D | Visual memory | 8.63 ± 1.93 | 8.82 ± 1.98 | 0.75 |
| SDMT | Attention | 56.88 ± 12.24 | 58.54 ± 9.89 | 0.63 |
| PASAT | Attention | 48.06 ± 12.41 | 48.39 ± 8.97 | 0.92 |
| WLG | Execution | 27.56 ± 7.23 | 28.54 ± 5.65 | 0.62 |
| Mood and fatigue tests | ||||
| HADA | Anxiety | 5.38 ± 2.45 | 6.50 ± 4.32 | 0.35 |
| HADD | Depression | 1.38 ± 1.26 | 3.07 ± 2.62 | 0.02 |
| FSMC_COG | Cognitive fatigue | 16.13 ± 5.30 | 23.00 ± 9.17 | 0.01 |
| FSMC_MOT | Motor fatigue | 14.19 ± 4.25 | 22.39 ± 11.01 | 0.01 |
EDSS: expanded disability status scale; MSFC: multiple sclerosis functional composite, BRB‐N: brief repeatable battery of neuropsychological tests, SRT‐LTS: Simple reaction time‐long term storage, SRT‐CLTR: SRT‐consistent long‐term retrieval, SRT‐D: SRT‐delayed recall, SPART: 10/36 spatial recall test, SPART‐D: 10/36 spatial recall test‐delayed, SDMT: symbol digit modalities test, PASAT: paced auditory serial addition test, WLG: word list generation, HADA and HADD: hospital anxiety and depression scale‐anxiety and hospital anxiety and depression scale‐depression, FSMC_MOT and FSMC_COG: fatigue scale for motor and cognitive functions. P < 0.05 in bold.
Multicontrast Cerebellar Connectometry
A summary of the pipeline applied to perform cerebellar multicontrast connectometry is reported in Figure 1.
Figure 1.

Multicontrast cerebellar connectometry. On the left side: (1a) diffusion tractography and the cerebellum atlas (SUIT) used to perform structural cerebellar connectomics; (1b) Quantitative maps coregistered to diffusion data; (1c) Quantitative structural matrices extracted for GFA, T1, MTR, and T2. On the right side: (2a) Mean filtered fMRI data and the cerebellum atlas (SUIT) were used to perform functional cerebellar connectomics; (2b) Functional connectivity matrices for the blood oxygenation level dependent (BOLD) contrast.
Structural connectome analysis was computed using the connectome mapping toolkit (CMTK, http://www.cmtk.org) [Daducci et al., 2012], which was developed by coauthors of this study.
First, we isolated the cerebellum and the brainstem from MPRAGE images using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/); cerebellar lobes and dentate nuclei were then identified using the SUIT toolbox [Diedrichsen, 2006] for statistical parametric mapping (SPM, http://www.fil.ion. http://ucl.ac.uk/spm/). The lobule vermis_Crus I was excluded as some subjects were lacking the corresponding label due to its small size. Combining the two parcellations, we obtained an anatomical atlas with 30 regions of interest (ROIs), including: 1 brain stem, 27 cerebellum lobules, and 2 dentate nuclei.
The MP2RAGE/T1 map was nonlinearly registered to the b0 image from DSI using FNIRT (http://fsl.fmrib.ox.ac.uk/fsl/) and the transformation applied to the anatomical atlas, the T1, T2, and MTR maps as well as the lesion mask [Fig. 1 (1a and 1b)]. The orientation density function from DSI data using the diffusion toolkit (http://trackvis.org/dtk/) and performed streamline tractography with 32 random seeds per voxel and 60° angular threshold using trackvis (http://trackvis.org/) [Granziera et al., 2012]. Mean values along the tracts and structural connectivity matrices [T1 rt, T2 rt, MTR, and GFA, Fig. 1 (1c)] were calculated using CMTK (http://www.cmtk.org, [Daducci et al., 2012]).
Using an in‐house software, we also estimated the number of distinct lesions per connection bundle by counting the number of different lesions encountered along the fibers connecting every pair of anatomical ROIs.
As to functional connectome analysis, the 340 functional volumes of each subject were realigned to the first acquired volume using SPM (http://www.fil.ion.ucl.ac.uk/spm/) and then averaged in a mean functional image. The mean functional image was then registered the DSI b0 using SPM (http://www.fil.ion.ucl.ac.uk/spm/) and the transformation was applied to the anatomical atlas [Fig. 1 (2a)].
Figure 2.

(a) Adjacency matrix of the CPN (average path length L = 2.00, number of nodes N = 30, number of edges e = 109, SΔ = 1.68, 99% confidence interval for SΔ of 10,000 equivalent Erdös‐Rényi random graphs = [0.697 1.434]). In red: connections that show structural differences between MS patients and HC (C1–C5). (b) Degree of distribution of the CPN. (c) Schematic 3D view of the CPN. The colored spheres represent the 30 ROIs and the lines between them their connections. (d) Schematic 3D view of the C1–C5 network, which is altered in MS patients compared to HC.
Mean BOLD activity was computed for each region, zero‐meaned, and decomposed into four sub‐bands using the discrete wavelet transform with orthogonal wavelet of degree alpha = 3 [Achard et al., 2006; Richiardi et al., 2012]. Functional connectivity matrices were computed as Pearson correlations between the coefficients of the fourth sub‐band between all different pairs of ROIs [Richiardi et al., 2012], Fig. 1 (2b). Given the fast TR, signal in the fourth sub‐band mainly captures fluctuations in the frequency range 0.06–0.125Hz. Thus, respiration and heart beat contributions are conveniently discarded from the signal.
Connectometry: Statistical Analysis
Statistics was performed using the Connectome Analyzer (http://www.cmtk.org/analyzer) and aimed at assessing the presence of structural and functional differences between patients and controls, both for local connections and network properties. Age and gender were regressed before performing statistical tests.
Local structural connection and network analysis were performed for all parameters (T1 rt, T2 rt, MTR, or GFA) on the cerebellar prevalent network (CPN), which is constituted by connections with at least one DSI fiber trajectory for every subject (Fig. 2a,c). Mean values along the tracts were considered.
Local connection differences between MS patients and HC were assessed using the soft thresholding screening and filtering (STSF) [Meskaldji et al., 2013]. This two‐steps method is adapted to connectivity analysis as it exploits the structure of complex networks and the positive dependence that could exist between nodes and connections. The STSF first step consists in exploring group differences when clustering subsets of connections defined as inter/intra connectivity between/within communities of nodes. This information is then used in a the second step to infer group differences at the single connection level while controlling the family‐wise error rate (FWER), [Meskaldji et al., 2013].
Specifically, in our study, the STSF first step consisted in clustering anatomical cerebellar ROIs into six regions (left motor, left cognitive, vermis motor, vermis cognitive, right motor, and right cognitive), according to their laterality and main function as described in [Bernard et al., 2012; Stoodley and Schmahmann, 2010]. The screening threshold was set at α1 = 0.05. The second step consisted in performing a univariate t‐test to assess whether T1 rt, T2 rt, GFA, or MTR were increased or decreased in CPN connections of the patient group vs the control group. Connections with P‐values <0.05 after correction for FWER with Bonferroni were considered significantly different between groups [Meskaldji et al., 2013]. For multivariate analysis a permutation‐based Hoteling test was applied instead of a t‐test for the second step [Meskaldji and Ville, 2014]. Connections with P‐values < 0.05 after correction for FWER with Bonferroni were considered significantly different between groups [Meskaldji and Ville, 2014].
The local connections that were found to be significantly different between MS patients and HC in the univariate and multivariate analyses are referred to as C1, C2, C3, C4, and C5 (C1–C5 network) (Fig. 2d).
Network analysis was performed globally (on the whole CPN) and at the subnetwork level (within the six anatomical clusters detailed above). Using the Connectome Analyzer (http://www.cmtk.org/analyzer), we computed measures of small worldness (presence of high local connectivity and few long‐range connections), degree betweenness, closeness, diameter, and efficiency on T1 rt, T2 rt, GFA, and MTR connectomes [Humphries and Gurney, 2008; Rubinov and Sporns, 2010]; subsequently, each measure was compared between patients and HC using Student's t‐tests.
Local functional connection analysis was performed using the STSF method, to perform between‐groups comparison of correlation values between BOLD in signals couples of ROIs; P‐values ≤0.05 after correction for multiple comparisons with Bonferroni were considered statistically significant [Meskaldji et al., 2013]. Global functional network and subnetwork analyses were also performed as for structural data.
Correlation to Lesion Load, Disease Duration, and Clinical Scores
Correlation analysis was performed by calculating Pearson correlation coefficients between (a) GFA, MTR, T1 rt, T2 rt, and rsfMRI correlation coefficients along each edge of the C1–C5 network and (b) lesion load (total brain lesion volume and the number of lesions on the connection), disease duration (months since the first clinical relapse) and motor and cognitive scores. Age, gender, years of education, anxiety, depression, and fatigue scores were linearly detrended from the clinical scores before performing the correlation since they have been reported to be linked to functional performance in MS [DeLuca et al., 1995; Phillips and Stuifbergen, 2010]. For each score, we performed multiple‐comparison correction using Bonferroni.
RESULTS
Demographic Data and Clinical Scores
There were no differences in demographic data, disability, motor function, or cognitive performance between patients and HC. However, the MS and HC groups differed in depression and motor/cognitive fatigue (P < 0.05 and P ≤ 0.01), Table 2.
Connectometry: Statistical Analysis
The CPN showed small‐network properties as its small worldness (SΔ = 1.680, average path length L = 2.00) significantly differed from equivalent random networks (confidence interval for SΔ of 10,000 random graphs = [0.697 1.434], [Humphries and Gurney, 2008], Figure 2a. Furthermore, as for random networks, its degree distribution was close to a Poisson distribution (Fig. 2b).
Local connection analysis showed a number of differences between groups (Fig. 3c): univariate structural local connection analysis revealed that MS subjects had an increased T1 rt in Right_V‐Right_VI (C1, P = 0.02) as well as a decreased GFA in Left_I_IV‐Left_IX (C2, P = 0.03) and Left_IX‐Left dentate (C3, P = 0.02). In addition, multivariate analysis showed altered structural connectivity measures for Left_I_IV‐Left_VI (C4, P = 0.04), Left_V‐Left_VI (C5, P = 0.03), and Left_IX‐Left dentate (C3, P = 0.03). Local functional connectivity did not differ between groups. In addition, structural and functional network properties (both at the global and subnetwork level) in MS patients were comparable to those of HC.
Figure 3.
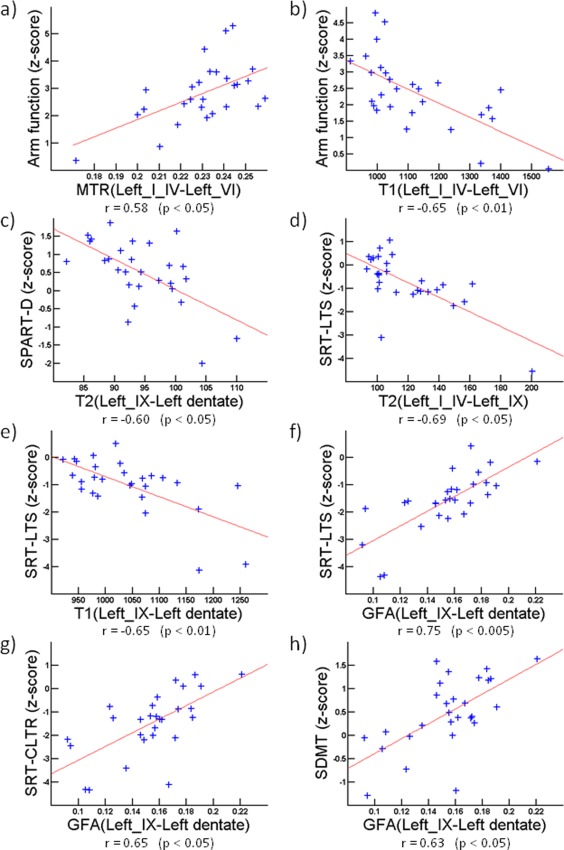
Correlations between clinical scores and metrics of structural connectivity integrity (a) and (b) Arm function correlates with MTR and T1 values along C4 (r = 0.58, P < 0.05 and r = −0.65, P < 0.01 respectively) (c) SPART‐D correlates with T2 along C3 (r = –0.60, P < 0.05) (d–f) SRT‐LTS correlates with T2 along C2 (r = –0.69, P < 0.05), and with T1 and GFA along C3 (r = −0.65, P < 0.01 and r = 0.75, P <0.005 respectively) (g) SRT‐CLTR correlates with GFA along C3 (r = 0.65, P < 0.05). (h) SDMT correlates with GFA along C3 (r = 0.63, P < 0. 05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Lesion Load
Brain lesion volume and lesion number are reported in Supporting Information Table 2; the number of cerebellar lesions per bundle are reported in Supporting Information Figure 1.
Correlations of Connectivity Measures to Lesion Load, Disease Duration, and Clinical Scores
Altered cerebellar connectivity did not correlate with total lesion volume and number nor disease duration. However, structurally altered connections strongly correlated with clinical performance in patients (Fig. 3): along C4, MTR correlated positively (r = 0.58, P < 0.05) whereas T1 rt correlated negatively (r = −0.65, P < 0.01) with MSFC arm function score. T2 rt values along C2 correlated negatively with the SRT‐LTS score (r = −0.69, P < 0.05); along C3, T1 rt correlated negatively (r = −0.65, P < 0.01) whereas GFA correlated positively (r = 0.75, P < 0.005) with the SRT‐LTS score. The GFA along C3 correlated positively with (SRT)‐CLTR (r = 0.65, P < 0.05) and SDMT (r = 0.63, P < 0.05), while T2 rt values correlated negatively with SPART‐D (r = −0.60, P < 0.05).
DISCUSSION
Multicontrast connectometry of the cerebellum evidenced altered local structural connectivity in early RRMS patients with minimal deficits. The observed alterations were independent from conventional measures of disease impact and highly correlated with clinical function. Cerebellar network and functional properties in patients appeared to be similar to HCs.
Previous neuro‐pathological works reported widespread cerebellar demyelination in advanced MS [Kutzelnigg et al., 2007; Wegner and Stadelmann, 2009] and MRI studies provided in vivo evidence of cerebellar atrophy in both RRMS and in secondary progressive MS (SPMS) [Anderson et al., 2011; Calabrese et al., 2010; Riccitelli et al., 2012]. Cerebellum volume loss in MS patients moderately correlated with motor disability [Anderson et al., 2011; Calabrese et al., 2010] and cognitive impairment [Weier et al., 2014], whereas volume loss in selected cerebellar lobes (IV, V, VI, and VIII) was associated with balance impairment [Prosperini et al., 2013]. Damage in cerebellar white matter tracts, as measured by diffusion MRI, was described in cohorts of MS patients, including both RRMS and SPMS, with different degrees of disability and cognitive impairment [Hulst et al., 2013; Preziosa et al., 2014]. Conversely, compensatory plasticity in cerebellar‐frontal lobe connections was evident in RRMS, but not at later disease stages (SPMS) [Rocca et al., 2012]. Nonetheless, it remains unclear whether cerebellar alterations and plasticity are present since the first years after MS diagnosis.
In this study, we applied multicontrast connectomics to investigate the presence and extent of structural and functional cerebellar abnormalities in MS patients with less than 5 years disease duration. Brain connectomics provides comprehensive information about cerebral connectivity properties [Griffa et al., 2013] and might represent a sensitive way to detect subtle alterations in early disease stages. To date, no connectomic studies of the cerebellum have been reported.
Likewise, available brain connectomics studies are limited by the fact that applied measures of connectivity integrity lack specificity to different components of white matter microstructure. To overcome these limits, it has been recently proposed to characterize single white matter tracts by combining multicomponent relaxometry measures with diffusion MRI (Bells Cerciignani M, et al., 2011; De Santis et al., 2014]. Our work is an attempt to expand this approach to perform both local and network analysis as well as structural–functional evaluation (multicontrast connectometry).
We first established structural and functional cerebellar connectomes from rs‐fMRI and DSI tractography data. Rs‐fMRI measurements permitted the calculation of measures of functional cerebellar connectivity at rest. DSI tractography provided two different structural fiber trajectories: (i) cortical fiber paths between lobules, probably representing anisotropy coherence of parallel fibers (PF); we considered the PF structure as a modulatory system influencing adjacent lobules, since the granule cells generating them are receiving input from mossy fibers branching through numerous cerebellar folia and (ii) fiber trajectories between the brain stem and cerebellar lobules, between lobules and dentate nuclei as well as between dentate nuclei and brain stem; these fiber paths are most probably corresponding to cerebellar afferent and efferent fibers. To our knowledge, this is the first study combining structural and functional connectivity analysis in the cerebellum and characterizing at the same time both intracortical and cortico‐subcortical cerebellar connectivity.
We performed an in‐depth characterization of the cerebellar structural connectome by combining deterministic DSI tractography with multimodal MRI measures of microstructural integrity. In fact, increases in T1 rt and T2 rt along a fiber trajectory point at a loss of microstructure [Granziera et al., 2013; Helms, in press) and enhanced water content (i.e., microoedema [Bonnier et al., 2014; Helms, in press; Whittall et al., 1997]), which are frequently observed in early MS [Chang et al., 2008]. Conversely, a reduction of MTR and GFA suggests alterations in myelination and neuroaxonal integrity [Granziera et al., 2012, 2013], which might be consequent to multiple inflammatory attacks.
We identified a prevalent structural cerebellar network that exhibited small‐world properties, a feature that has been previously described in human brain network topology [Achard et al., 2006]. Furthermore, we established that the degree of distribution of the CPN fits a Poisson distribution similarly to that of large, random networks (Fig. 2b), a characteristic that renders it particularly resilient to targeted and random attacks [Achard et al., 2006] such as inflammatory events.
The prevalent cerebellar network exhibited some local structural alterations in edges that linked cerebellar lobules with prevalent motor (C1, C4, and C5), cognitive (C3), or motor‐cognitive (C2) functions [Bernard et al., 2012; Stoodley and Schmahmann, 2010]. Univariate local connection analysis revealed microstructural loss (high T1 rt) and decreased axonal integrity (low GFA) in C1, C2, and C3 of MS patients compared to controls. In addition, multivariate analysis showed that two other connections (C4‐C5) had different structural properties in MS patients compared to the healthy population. Interestingly, no network alterations nor functional differences were observed in our MS cohort, confirming the ability of the cerebellar connectivity to resisting autoimmune attacks. This aspect is in accord with the lack of major motor and cognitive deficits in our MS cohort, yet it might also suggest that the cerebellar network organization exhibits different properties than the brain, since altered brain network topology has been reported, using different methodology and connectivity measures, in early MS [Li et al., 2013]. Future studies are required to further investigate the interplay between cerebellar and brain networks in early MS.
Lastly, microstructural alterations in C1–C5 were independent from conventional measures of lesion load (number and volume) as well as from disease duration. Nevertheless, C1–C5 properties highly correlated with both motor and cognitive performance in MS patients. Arm function correlated with C4 motor connection integrity between the Left_I_IV and Left_VI lobule, known to be involved in adaptation of movement trajectories to external stimuli [Donchin et al., 2012]. In addition, verbal memory function correlated with C2 and C3 integrity, which mainly represents cognitive connections between Left_I_IV‐Left_IX (C2) and Left_IX‐Left dentate (C3); Left_IX is indeed a lobule involved in verbal working memory tasks [Cooper et al., 2012]. Visual and verbal memory as well as attention function were influenced by the integrity of C3 connectivity. Lobule IX is, in fact, not only involved in verbal working memory [Cooper et al., 2012] but also in visual memory [Robinson et al., 1984] and attention [Loitfelder et al., 2012].
In summary, multicontrast cerebellar connectometry revealed subtle local connectivity disruptions in a group of early MS patients but no changes in structural network organization properties or functional connectivity. Multicontrast measures of local connectivity alterations suggested loss of axonal integrity and tissue microstructure and highly correlated with motor and cognitive function in patients.
Limitations of our current approach include the differing spatial resolution of quantitative MRI maps, the choice of the streamline tractography method (for review see [Descoteaux et al., 2009], and the lack of reproducibility in assessment of both functional and structural data in healthy subjects. Reproducibility and longitudinal studies in larger and heterogeneous MS cohorts are currently being performed to determine whether these new biomarkers might be helpful to monitor disease evolution and patient response to therapy.
Supporting information
Supplementary Information
Supplementary Information
Supplementary Information
Disclosures: A. Roche and G. Krueger are Siemens AG employees. The other authors have nothing to disclose.
REFERENCES
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E (2006): A resilient, low‐frequency, small‐world human brain functional network with highly connected association cortical hubs. J Neurosci 26:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson VM, Wheeler‐Kingshott CA, Abdel‐Aziz K, Miller DH, Toosy A, Thompson AJ, Ciccarelli O (2011): A comprehensive assessment of cerebellar damage in multiple sclerosis using diffusion tractography and volumetric analysis. Mult Scler 17:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bells SCM, Deoni S, Assaf Y, Pasternak O, Evans CJ, Leemans A, Jones DK (2011): Tractometry: Comprehensive multimodal imaging quantitative assessment of white matter along specific tracts. Proceedings of International Society of Magnetic Resonance in Medecine 2011, Montréal, Québec, Canada. [Google Scholar]
- Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, Jaeggi SM, Buschkuehl M, Monk CS, Jonides J, Peltier SJ (2012): Resting state cortico‐cerebellar functional connectivity networks: A comparison of anatomical and self‐organizing map approaches. Front Neuroanat 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnier G, Roche A, Romascano D, Simioni S, Meskaldji D, Rotzinger D, Lin YC, Menegaz G, Schluep M, Du Pasquier R, Sumpf TJ, Frahm J, Thiran JP, Krueger GCG (2014): Advanced MRI unravels the nature of tissue alterations in early multiple sclerosis. Ann Clin Transl Neurol 1:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011): The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese M, Mattisi I, Rinaldi F, Favaretto A, Atzori M, Bernardi V, Barachino L, Romualdi C, Rinaldi L, Perini P, Gallo P (2010): Magnetic resonance evidence of cerebellar cortical pathology in multiple sclerosis. J Neurol Neurosurg Psychiatry 81:401–404. [DOI] [PubMed] [Google Scholar]
- Chang A, Smith MC, Yin X, Fox RJ, Staugaitis SM, Trapp BD (2008): Neurogenesis in the chronic lesions of multiple sclerosis. Brain: J Neurol 131:2366–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper FE, Grube M, Von Kriegstein K, Kumar S, English P, Kelly TP, Chinnery PF, Griffiths TD (2012): Distinct critical cerebellar subregions for components of verbal working memory. Neuropsychologia 50:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daducci A, Gerhard S, Griffa A, Lemkaddem A, Cammoun L, Gigandet X, Meuli R, Hagmann P, Thiran JP (2012): The connectome mapper: An open‐source processing pipeline to map connectomes with MRI. PloS one 7:e48121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca J, Johnson SK, Beldowicz D, Natelson BH (1995): Neuropsychological impairments in chronic fatigue syndrome, multiple sclerosis, and depression. J Neurol Neurosurg Psychiatry 58:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis S, Drakesmith M, Bells S, Assaf Y, Jones DK (2014): Why diffusion tensor MRI does well only some of the time: Variance and covariance of white matter tissue microstructure attributes in the living human brain. NeuroImage 89:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux M, Deriche R, Knosche TR, Anwander A (2009): Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Trans Med Imaging 28:269–286. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J (2006): A spatially unbiased atlas template of the human cerebellum. NeuroImage 33:127–138. [DOI] [PubMed] [Google Scholar]
- Donchin O, Rabe K, Diedrichsen J, Lally N, Schoch B, Gizewski ER, Timmann D (2012): Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophysiol 107:134–147. [DOI] [PubMed] [Google Scholar]
- Granziera C, Schmahmann JD, Hadjikhani N, Meyer H, Meuli R, Wedeen V, Krueger G (2009): Diffusion spectrum imaging shows the structural basis of functional cerebellar circuits in the human cerebellum in vivo. PloS One 4:e5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granziera C, Daducci A, Meskaldji DE, Roche A, Maeder P, Michel P, Hadjikhani N, Sorensen AG, Frackowiak RS, Thiran JP, Meuli R, Krueger G (2012): A new early and automated MRI‐based predictor of motor improvement after stroke. Neurology 79:39–46. [DOI] [PubMed] [Google Scholar]
- Granziera C, Daducci A, Simioni S, Cavassini M, Roche A, Meskaldji D, Kober T, Metral M, Calmy A, Helms G, Hirschel B, Lazeyras F, Meuli R, Krueger G, Du Pasquier RA (2013): Micro‐structural brain alterations in aviremic HIV+ patients with minor neurocognitive disorders: A multi‐contrast study at high field. PloS One 8:e72547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffa A, Baumann PS, Thiran JP, Hagmann, P (2013): Structural connectomics in brain diseases. NeuroImage 80:515–526. [DOI] [PubMed] [Google Scholar]
- Helms G: Tissue properties from quantitiative MRI. In: AT, editor. Brain Mapping: An Encyclopedic Reference: Elsevier (in press).
- Hulst HE, Steenwijk MD, Versteeg A, Pouwels PJ, Vrenken H, Uitdehaag BM, Polman CH, Geurts JJ, Barkhof F (2013): Cognitive impairment in MS: Impact of white matter integrity, gray matter volume, and lesions. Neurology 80:1025–1032. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Gurney, K (2008): Network 'small‐world‐ness': A quantitative method for determining canonical network equivalence. PloS One 3:e0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober T, Granziera C, Ribes D, Browaeys P, Schluep M, Meuli R, Frackowiak R, Gruetter R, Krueger G (2012): MP2RAGE multiple sclerosis magnetic resonance imaging at 3 T. Invest Radiol 47:346–352. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF (1983): Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 33:1444–1452. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Faber‐Rod JC, Bauer J, Lucchinetti CF, Sorensen PS, Laursen H, Stadelmann C, Bruck W, Rauschka H, Schmidbauer M, Lassmann H (2007): Widespread demyelination in the cerebellar cortex in multiple sclerosis. Brain Pathol 17:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Jewells V, Kim M, Chen Y, Moon A, Armao D, Troiani L, Markovic‐Plese S, Lin W, Shen D (2013): Diffusion tensor imaging based network analysis detects alterations of neuroconnectivity in patients with clinically early relapsing‐remitting multiple sclerosis. Hum Brain Mapp 34:3376–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loitfelder M, Filippi M, Rocca M, Valsasina P, Ropele S, Jehna M, Fuchs S, Schmidt R, Neuper C, Fazekas F, Enzinger C (2012): Abnormalities of resting state functional connectivity are related to sustained attention deficits in MS. PloS One 7:e42862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R (2010): MP2RAGE, a self bias‐field corrected sequence for improved segmentation and T1‐ mapping at high field. NeuroImage 49:1271–1281. [DOI] [PubMed] [Google Scholar]
- Meskaldji D‐E, Ville DVD (2014): Multimodal graph theoretical analysis of functional connectivity using adaptive two‐step strategy. IEEE International Symposium on Biomedical Imaging, Beijing, China, 2014. [Google Scholar]
- Meskaldji DE, Fischi‐Gomez E, Griffa A, Hagmann P, Morgenthaler S, Thiran JP (2013): Comparing connectomes across subjects and populations at different scales. NeuroImage 80:416–425. [DOI] [PubMed] [Google Scholar]
- Penner IK, Raselli C, Stocklin M, Opwis K, Kappos L, Calabrese P (2009): The fatigue scale for motor and cognitive functions (FSMC): Validation of a new instrument to assess multiple sclerosis‐related fatigue. Mult Scler 15:1509–1517. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, Stuifbergen AK (2010): The relevance of depressive symptoms and social support to disability in women with multiple sclerosis or fibromyalgia. Int Journal Rehabil Res 33:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg‐Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011): Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preziosa P, Rocca MA, Mesaros S, Pagani E, Drulovic J, Stosic‐Opincal T, Dackovic J, Copetti M, Caputo D, Filippi M (2014): Relationship between damage to the cerebellar peduncles and clinical disability in multiple sclerosis. Radiology 271:822–830. [DOI] [PubMed] [Google Scholar]
- Prosperini L, Sbardella E, Raz E, Cercignani M, Tona F, Bozzali M, Petsas N, Pozzilli C, Pantano P (2013): Multiple sclerosis: white and gray matter damage associated with balance deficit detected at static posturography. Radiology 268:181–189. [DOI] [PubMed] [Google Scholar]
- Rao SM, Leo GJ, Bernardin L, Unverzagt, F (1991): Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 41:685–691. [DOI] [PubMed] [Google Scholar]
- Riccitelli G, Rocca MA, Pagani E, Martinelli V, Radaelli M, Falini A, Comi G, Filippi M (2012): Mapping regional grey and white matter atrophy in relapsing‐remitting multiple sclerosis. Mult Scler 18:1027–1037. [DOI] [PubMed] [Google Scholar]
- Richiardi J, Gschwind M, Simioni S, Annoni JM, Greco B, Hagmann P, Schluep M, Vuilleumier P, Van De Ville, D (2012): Classifying minimally disabled multiple sclerosis patients from resting state functional connectivity. NeuroImage 62:2021–2033. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Cohen JL, May J, Sestokas AK, Glickstein M (1984): Cerebellar targets of visual pontine cells in the cat. J Comput Neurol 223:471–482. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Bonnet MC, Meani A, Valsasina P, Colombo B, Comi G, Filippi M (2012): Differential cerebellar functional interactions during an interference task across multiple sclerosis phenotypes. Radiology 265:864–873. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Cutter G, Reingold S (2002): The multiple sclerosis functional composite: A new clinical outcome measure for multiple sderosis trials. Mult Scler 8:359–365. [DOI] [PubMed] [Google Scholar]
- Saini S, DeStefano N, Smith S, Guidi L, Amato MP, Federico A, Matthews PM (2004): Altered cerebellar functional connectivity mediates potential adaptive plasticity in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 75:840–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann, JD (2010): Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpf TJ, Uecker M, Boretius S, Frahm J (2011): Model‐based nonlinear inverse reconstruction for T2 mapping using highly undersampled spin‐echo MRI. J Magn Reson Imaging: JMRI 34:420–428. [DOI] [PubMed] [Google Scholar]
- Tuch DS (2004): Q‐ball imaging. Magn Reson Med 52:1358–1372. [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM (2005): Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 54:1377–1386. [DOI] [PubMed] [Google Scholar]
- Wegner C, Stadelmann C (2009): Gray matter pathology and multiple sclerosis. Curr Neurol Neurosci Rep 9:399–404. [DOI] [PubMed] [Google Scholar]
- Weier K, Penner IK, Magon S, Amann M, Naegelin Y, Andelova M, Derfuss T, Stippich C, Radue EW, Kappos L, Sprenger T (2014): Cerebellar abnormalities contribute to disability including cognitive impairment in multiple sclerosis. PloS One 9:e86916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittall KP, MacKay AL, Graeb DA, Nugent RA, Li DK, Paty DW (1997): In vivo measurement of T2 distributions and water contents in normal human brain. Magn Reson Med 37:34–43. [DOI] [PubMed] [Google Scholar]
- Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, Yacoub E, Ugurbil K (2013): Evaluation of slice accelerations using multiband echo planar imaging at 3 T. NeuroImage 83:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP (1983): The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information
Supplementary Information


