Abstract
Persistent pondering over negative self‐related thoughts is a central feature of depressive psychopathology. In this study, we sought to investigate the neural correlates of abnormal negative self‐referential processing (SRP) in patients with Major Depressive Disorder and its impact on subsequent cognitive control‐related neuronal activation. We hypothesized aberrant activation dynamics during the period of negative and neutral SRP in the rostral anterior cingulate cortex (rACC) and in the amygdala in patients with major depressive disorder. Additionally, we assumed abnormal activation in the fronto‐cingulate network during Stroop task execution. 19 depressed patients and 20 healthy controls participated in the study. Using an event‐related functional magnetic resonance imaging (fMRI) design, negative, positive and neutral self‐referential statements were displayed for 6.5 s and followed by incongruent or congruent Stroop conditions. The data were analyzed with SPM8. In contrast to controls, patients exhibited no significant valence‐dependent rACC activation differences during SRP. A novel finding was the significant activation of the amygdala and the reward‐processing network during presentation of neutral self‐referential stimuli relative to baseline and to affective stimuli in patients. The fMRI analysis of the Stroop task revealed a reduced BOLD activation in the right fronto‐parietal network of patients in the incongruent condition after negative SRP only. Thus, the inflexible activation in the rACC may correspond to the inability of depressed patients to shift their attention away from negative self‐related stimuli. The accompanying negative affect and task‐irrelevant emotional processing may compete for neuronal resources with cognitive control processes and lead thereby to deficient cognitive performance associated with decreased fronto‐parietal activation. Hum Brain Mapp 36:2781–2794, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: major depression, self‐referential processing, cognitive control, functional imaging, rostral anterior cingulate cortex, amygdala, nucleus accumbens, dorsolateral prefrontal cortex
INTRODUCTION
The habit of pondering over one's own negative thoughts and feelings, termed rumination, is not only a central feature of major depressive disorder (MDD) but also closely correlates to the onset, number and length of depressive episodes [Alloy and Robinson, 2003; Moberly and Watkins, 2008]. Rumination involves the repetitive focusing on one's distress symptoms or negative emotions, and strong self‐referential attention [Nolen‐Hoeksema et al., 2008; Treynor et al., 2003]. Thus, depressed patients find it difficult to disengage from self‐focusing even though it might be irrelevant in the present moment. This characteristic behavior contributes to disturbances in attention to cognitively demanding tasks [Joormann and Gotlib, 2008; Watkins and Brown 2002].
Neuroimaging studies of the neural underpinnings of self‐related processes in healthy subjects have consistently found evidence for the strong involvement of cortical midline structures, that is, posterior cingulated cortex (PCC), ventromedial prefrontal cortex, (VMPFC, overlapping with rostral anterior cingulate cortex [rACC]), and dorsomedial prefrontal cortex during processing of self‐related information [Northoff et al., 2006]. Moran et al. [2006] demonstrated that the activity in the rACC signified the personal relevance of information. In a recent review of self‐referential processing (SRP), van der Meer et al. [2010] concluded that the rACC/VMPFC is a key structure in processing self‐related stimuli in contrast to other‐related stimuli, and that it might be specifically involved in processing of affective self‐relevant information. Moreover, recent studies have provided evidence that cortical midline structures are strongly involved in rumination in both healthy and depressed subjects [Nejad et al., 2013]. Beside the medial prefrontal cortex and rACC, Freton et al. [2014] recently observed a specific involvement of the PCC/precuneus during two specific self‐focusing conditions in healthy controls and could differentiate subjects with lower and higher brooding scores based on its activation.
Convergent evidence from brain imaging research indicates that in patients with MDD this brain network is anomalous in structure and function [Drevets et al. 1997; Greicius et al., 2007; Sheline et al., 2009; Wagner et al., 2006, 2008]. The importance of the medial prefrontal cortex in the exaggerated self‐focus of depressed patients was highlighted by Lemogne et al. [2012] in a recent review. In addition, fMRI studies using negative affective stimuli have consistently reported that increased negative self‐focus and ruminative thinking are accompanied by abnormal activation in the VMPFC in depressed subjects [Cooney et al., 2010; Grimm et al., 2009]. Similarly, we have shown in our previous study of healthy subjects [Wagner et al., 2013] that the rACC was specifically engaged during negative SRP (relative to positive and neutral self‐referential statements) and was significantly and positively related to the degree of depressive symptoms in healthy subjects.
Another brain structure which is anatomically strongly interconnected with the rACC [Paus, 2001] and plays an important role in processing of motivationally salient, such as self‐relevant information is the amygdala. Northoff et al. [2009] demonstrated in healthy subjects that beside the medial PFC and the rACC activation, higher degrees of self‐relatedness were accompanied by stronger activation of subcortical brain regions, such as the amygdala and the nucleus accumbens, and emphasized the interaction between self‐relatedness and emotional valence. Sharot et al. [2007] reported enhanced rACC and amygdala BOLD activation during processing of personal future expectations. Etkin et al. [2006] reported a modulatory influence of rACC activity on the amygdala during emotional conflict resolution. In MDD, hyperactivity of the amygdala was frequently observed during processing of negative stimuli such as sad faces or negative words [Siegle et al., 2007; Suslow et al., 2010]. An interaction between increased amygdala activation during self‐relevant processing and decreased dorsolateral prefrontal Cortex (DLPFC) BOLD activation has also been previously demonstrated [Ray et al. 2005; Siegle et al., 2007].
However, decreased amygdalar activation in response to emotional face expressions [Lawrence et al., 2004] or affective picture ratings [Ritchey et al., 2011] was also detected. Davey et al. [2011] even detected an increased amygdala response to positive social feedback in MDD patients. This finding indicates that amygdala hyperactivity in depressed patients is not restricted to negatively valenced stimuli only.
The rACC/VMPFC is a central node of the so‐called “default mode network” (DMN). This brain network, which mainly consists of midline brain structures, often appears to “deactivate” during cognitive tasks. Therefore, it has been suggested that self‐reflective thinking may constitute a common “default mode” when individuals are not otherwise engaged [Gusnard et al., 2001]. It seems that successful DMN suppression is of vital importance for proper cognitive functioning [Li et al., 2007]. Using resting‐state functional magnetic resonance imaging (rs‐fMRI) Hamilton et al. [2011] provided evidence for the abnormal interaction between the DMN and the “task‐positive” network in MDD. This network encompasses the lateral prefrontal, for example, DLPFC, parietal and dorsal anterior cingulate regions and shows typically a strong negative correlation (“anticorrelation”) to the DMN. It is activated by tasks that demand attention and cognitive control [Fox et al., 2005; Uddin et al., 2009]. Using resting state functional connectivity analysis Sheline et al. [2010] detected an area, comprising the ACC (BA 32) and medial frontal cortex (BA 9) which showed in patients with MDD increased functional brain connectivity to the cognitive control network, to the DMN and to the affective network. The authors termed this region the dorsal nexus. This study provides a potential model of how different symptoms in depression, that is, increased self‐focus and ruminations, cognitive deficits, and affective/autonomic dysregulations interact in the brains of patients with MDD. In healthy controls, Kelly et al. [2008] demonstrated a competitive relationship between the DMN and the “task‐positive” network, which mediated behavioral performance during and Eriksen flanker task.
We observed previously [Wagner et al., 2006, 2008] that unmedicated depressed patients were unable to suppress activation in the rACC (which is anatomically adjacent to the dorsal nexus) during Stroop task performance, in contrast to healthy controls. This result was interpreted in terms of an inability of patients to inhibit affective interferences, which may arise from the increased SRP during the execution of the Stroop task. In a recent study, we tested this assumption in healthy controls [Wagner et al., 2013]. We observed a clear impact of enhanced activation in the DMN and in particular in the rACC during processing of negative self‐referential stimuli on the subsequent activation pattern in the fronto‐cingulate cognitive control network during the incongruent Stroop condition. The interfering effect was more pronounced in healthy subjects with a higher degree of depressive symptoms. These results fit well with a number of previous studies indicating that enhanced self‐focus or rumination scores in healthy subjects and depressed patients impact the activation of the DLPFC, which may reflect the underlying greater cognitive effort required to disengage from self‐focus [Ray et al., 2005; Siegle et al., 2007; Vanderhasselt et al., 2011].
Thus, there is growing evidence for the supposition that there is a potential break‐down of the dynamic interplay between brain regions of the DMN in depressed patients, involving internally focused processes such as self‐reflective thoughts and brain regions of the “task‐positive” network, which comprises the fronto‐cingulo‐parietal regions and which are involved in externally focused processes such as cognitive control, attention, and working memory. However, the postulated interfering effect between enhanced self‐focused attention associated with increased activation in DMN and the potential impairment of cognitive control processes associated with the abnormal activation in the fronto‐cingulo‐parietal network has not yet been explicitly investigated in patients with MDD.
Therefore, the main goal of this study was to investigate the impact of postulated enhanced negative SRP on subsequent cognitive control processes in patients suffering from depression. To achieve this goal subjects were exposed to explicitly self‐referential statements resembling the typical depressive pattern of thinking, which were followed by single Stroop task events. We used relatively long exposure durations (6.5 s) to better focus the attention of patients to the self‐referential stimuli [Gotlib and Joormann, 2010]. Based on our previous study [Wagner et al., 2013] and the current state of knowledge, we hypothesized that depressed patients would specifically exhibit an increased and sustained activation in the VMPFC/rACC during processing of negative self‐referential statements in contrast to controls. We assumed that it might be characterized by missing activation dynamics in the form of a linear BOLD signal increase in the rACC during neutral, positive and negative SRP, as observed in our previous study of healthy subjects [Wagner et al., 2013]. We additionally expected to find significant BOLD signal differences during negative SRP in the amygdala. Furthermore, we hypothesized that we would find an interfering effect of the postulated increased rACC and amygdala BOLD signal on the fronto‐cingulate activation during the incongruent Stroop task condition in patients.
METHODS
Subjects
Twenty patients (11 females) who met the DSM IV criteria for MDD according to the Structured Clinical Interview (SCID) for DSM‐IV Axis I disorders were recruited from the inpatient service of the Department of Psychiatry and Psychotherapy at the University Hospital in Jena. On average, patients were 39.9 ± 13.3 years old and had a mean level of education of 10.6 ± 1.3 years. Patients’ score on the Beck Depression Inventory (BDI) was 20.9 ± 8.2 and 23.1 ± 4.6 on the Hamilton Rating Scale for Depression (HRSD; 21 items). One patient had two documented suicide attempts.
A multiple choice vocabulary test [Lehrl et al., 1995] confirmed that none of the patients was mentally retarded (patients: M=113.2, SD=15.0). Thirteen patients were being treated with selective serotonin and norepinephrine reuptake inhibitors, three with selective serotonin reuptake inhibitors (SSRI), one with norepinephrine‐dopamine reuptake inhibitor, one with noradrenergic and specific serotonergic antidepressants, and one with agomelatine. Patients with current comorbid Axis I disorder (according to SCID), with a history of manic episodes or with any neurological disorders were excluded from this study. One patient had to be excluded from the study due to brain abnormalities in the MRI scan.
Twenty control subjects (12 females) matched for age and gender were recruited through a local newspaper advertisement. The mean age was 34.1 ± 11.3 and the mean level of education 11.5 ± 0.9 years. The subjects’ score on the BDI was 3.8 ± 3.5. There was no overlap between the present sample and the sample of healthy controls investigated in our previous study [Wagner et al., 2013]. Subjects with past or current neurological or psychiatric diseases according to M.I.N.I [Sheehan et al., 1998] and/or first‐degree relatives with Axis I psychiatric disorders were excluded from the study. None of the study participants were taking any psychopharmacological medications.
All participants were German native speakers, right‐handed according to the modified version of Annetts handedness inventory [Briggs and Nebes, 1975] and provided written informed consent prior to participating in the study. The study protocol was approved by the Ethics Committee of the University of Jena. All subjects were paid 10 Euro per hour for their participation.
Paradigm Design
Self‐referential processing task
The paradigm used in this study was described in full detail in our previous article [Wagner et al., 2013]. In brief, during the SRP task, twenty negative, twenty positive, and twenty neutral self‐referential statements were presented. The self‐referential stimuli, which were used in this study, were selected from a pilot study with 20 inpatients with MDD and 20 matched healthy controls. Based on this pilot study, negative and positive self‐referential statements were chosen which were capable of discriminating between depressive patients and healthy controls. Neutral self‐referential statements, which did not provoke differing responses between patients and healthy controls regarding their self‐relatedness, were selected from this pilot study and used in this study. The negative self‐referential statements dealt with negative views about the patient's own self, for example: “I consider myself to be a loser,” while the positive self‐referential statements related to the positive view about the own self, such as “I have a lot of positive qualities.” Parts of these statements were used in previous studies conducted by our research group to induce negative affect in healthy subjects as well as in patients with MDD [Terhaar et al., 2009; Wagner et al., 2009]. The neutral statements described neutral traits or attitudes such as “I prefer to spend money instead of saving it.” All stimuli were matched according to the word number and length (no significant differences in the mean number) as well as syntax.
Stroop task
As in our previous study with healthy controls [Wagner et al., 2013], the cognitive Stroop task (manual version) (2006) was used to examine the effect of negative SRP on subsequent cognitive control processing. The Stroop task consisted of two conditions: a congruent and an incongruent condition. In the congruent condition, color words were presented in the color denoted by the corresponding word; in the incongruent condition, color words were displayed in one of three colors not denoted by the word. Two possible answers (color words in black type) were presented below the target stimulus (in the lower visual field) in order to minimize contextual memory demand. The subjects had to indicate as quickly as possible the correct color by pressing one of two buttons (with index or middle finger), which corresponded spatially to the two possible answers. Correct answers were counterbalanced on the right and left sides of the display. We defined the “baseline” condition, with which single regressors were contrasted, as the intertrial interval starting after the Stroop task presentation (see Supporting Information Fig. S1).
Paradigm timing
As illustrated in Supporting Information Figure S1, in a single experimental trial participants viewed a cue (1 s) indicating an SRP condition, followed by a positive, negative, or neutral self‐referential statement (6.5 s). Subjects had to judge these statements on a four‐point scale, as to whether they properly described the participants themselves. This was followed by a variable fixation baseline. Subsequently, a cue (1 s) indicating the Stroop task was presented, followed by a congruent or incongruent Stroop stimulus (2.5 s). Subjects had to indicate by pressing a button the color in which the color‐word was written. No feedback was given to the subjects thereafter. The whole paradigm consisted of 60 trials which were presented during the fMRI experiment in a pseudorandomized order and consisted of a combination of 60 self‐referential statements and 60 Stroop stimuli. To guarantee that subjects concentrated on the reading of the statements, the self‐referential statement was presented during the first 3.5 s without a digit bar with four possible responses, and during the last 3 s with the bar. The subjects’ responses were recorded using an MRI‐compatible fiber optic response device (Lightwave Medical Industries, Canada) operated by the right hand of the subject using a four‐button keypad.
MRI Parameters
Functional images were recorded on a 3 Tesla whole body scanner (MAGNETOM Trio, Siemens, Germany). 600 EPI volumes were acquired in one run with 48 parallel slices, each with a thickness of 2.7 mm and an isotropic voxel size of 2.7 × 2.7 × 2.7 mm3. TR was 2,700 ms, TE 30 ms, the flip angle was α = 90° and the field of view (FOV) was 192 mm × 192 mm. A high‐resolution structural scan was made for coregistration using a 3D MP‐RAGE sequence with 192 contiguous axial slices of 1 mm thickness (TR 2,300 ms; TE 3 ms; flip angle 9°; matrix size 256×256; isotropic voxel dimensions of 1 × 1 × 1 mm3).
Functional Data Analyses
Data preprocessing
The first four EPI images were discarded from further analysis to avoid T1 saturation effects. The functional images were preprocessed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The preprocessing included slice timing correction, 3D motion correction, within‐subject registration between functional and anatomical images, segmentation of the coregistered anatomical images, and spatial normalisation using parameters estimated during the segmentation process. The data was smoothed using a Gaussian filter of 8 mm FWHM, and a temporal high‐pass filter of 128 s was applied. In the first level analysis, 12 regressors of interest were defined and represented the three levels of SRP valence corresponding to negative, positive and neutral SRP conditions, six Stroop task × SRP valence combinations and three levels of fixation baseline after the SRP conditions and before the Stroop task. Individual movement parameters were entered as covariates into the design matrix. All regressors were convolved with a model of the HRF.
In an additional analysis, a design matrix incorporating a single regressor for all SRP items together and a parametric regressor, which indicated the subject's response to each sentence, was created. The function of the parametric modulation was considered as linear. The other regressors were identical to the design matrix used in the main statistical analysis. By means of this analysis, the height of the expected BOLD signal was parametrically modulated as a function of each subject's own self‐relevance ratings for each self‐relevant sentence.
Univariate analysis
The single‐subject contrasts were submitted to the second level group analyses with the subject as the random‐effect variable. A two‐way analysis of variance (ANOVA) was performed with three levels of the within‐subjects factor SRP VALENCE, that is, negative, positive, neutral SRP condition, and one between‐subjects factor GROUP (patients vs. controls). The statistical analyses were focused on the hypothesis‐driven post hoc t‐tests from the significant F‐tests of the main effect of GROUP and GROUP × SRP VALENCE interaction.
To investigate the brain network involved in the interaction between SRP and the Stroop task, a two‐way ANOVA with the first factor GROUP (patients vs. controls) and the second factor VALENCE with three levels (negative, positive and neutral) was set up. This statistical analysis focused on the incongruent Stroop condition (interference condition) as a dependent variable according to our initial hypothesis. Due to the relatively small sample size in the fMRI analysis and in order to balance between Type I and II error rates (Lieberman and Cunningham, 2009), all functional comparisons were thresholded at an uncorrected voxel‐level significance of P<0.005 and an FWE corrected cluster‐level significance of P<0.05. All MNI coordinates were converted to Talairach coordinates using the mni2tal algorithm [Brett et al., 2001].
Correlative analyses
The significance of a relationship between the BOLD signal in the rACC and amygdala, clinical characteristics and ratings of the self‐referential statements in patients and healthy controls were investigated. The parameter estimates were extracted from the rACC as well as from clusters that showed different activation in patients compared to controls.
RESULTS
Behavioral Results
SRP task
As expected, there was a significant difference between patients and controls in judging whether negative (MDD: Mean=2.45, SD=0.5; HC: Mean=1.51, SD=0.3, t(36)=6.7, P<0.001, Cohen's d= 2.1) and positive stimuli (MDD: Mean =2.38, SD=0.5; HC: Mean=3.15, SD=0.4, t(36)=5.3, P<0.001, Cohen's d= 1.7) referred to the participants themselves, but not with regard to the neutral stimuli (MDD: Mean=2.87, SD=0.3; HC: Mean=2.82, SD=0.3, t(36)=0.49, P=n.s., Cohen's d= 0.16).
Stroop task
The two‐way ANOVA with the between‐subjects factors GROUP (patients vs. controls) and the within‐subject factor VALENCE (incongruent Stroop condition after negative vs. positive vs. neutral SRP conditions) revealed a significant main effect of GROUP [F(1,36)=4.7, P<0.05] for the reaction time (RT). Post hoc t‐tests showed slower patients’ performance in the incongruent condition after presentation of negative (t(36)=2.25, P=0.03) and positive (t(36)=2.1, P=0.04), but not after presentation of neutral self‐referential stimuli. There was further a significant main effect of VALENCE [F(2,72)=5.9, P<0.01], but no significant GROUP × VALENCE interaction.
There were no significant differences between patients and controls with regard to the number of correct responses in the incongruent Stroop conditions.
FMRI Results
SRP task: Within group comparisons
In agreement with our previous study [Wagner et al., 2013], healthy subjects showed a significant linear BOLD signal increase and thus activated more strongly during negative SRP in contrast to neutral SRP, predominantly in the midline brain structures. These activation patterns included the rACC (x=4, y=41, z=7, BA 24/32, t=4.7, cluster=2,153) and the right rostrolateral prefrontal cortex (RLPFC, x=20, y=60, z=−3, BA10, t=4.8) as the first cluster, and the posterior cingulate cortex (PCC, x=6, y=−38, z=24, BA 23, t=4.3, cluster=3,537) and precuneus/superior parietal lobe (SPL, x=12, y=−68, z=33, BA 7, t=5.6) as the second cluster. In patients with MDD, no such significant linear valence‐dependent BOLD signal increase could be detected.
SRP task: Main effect of GROUP
Only the post hoc t‐tests revealed a significantly stronger BOLD signal in healthy controls compared to patients in the cuneus (x=10, y=−88, z=28, BA 19, t=3.8, cluster=647) for the negative SRP condition, but not for the neutral or positive SRP condition as well as in the opposite contrast (patients vs. healthy controls).
SRP task: GROUP × VALENCE interaction
As illustrated in Figure 1, the post hoc t‐test revealed significant group differences for the contrast negative vs. neutral SRP (Fig. 1 and Table 1) in three significant clusters (GROUP × negative vs. neutral SRP) including the right putamen, the globus pallidus, the left amygdala, insula, the ventral tegmental area (VTA), the rACC, the left nucleus accumbens, and the left cerebellum and occipital lobe.
Figure 1.
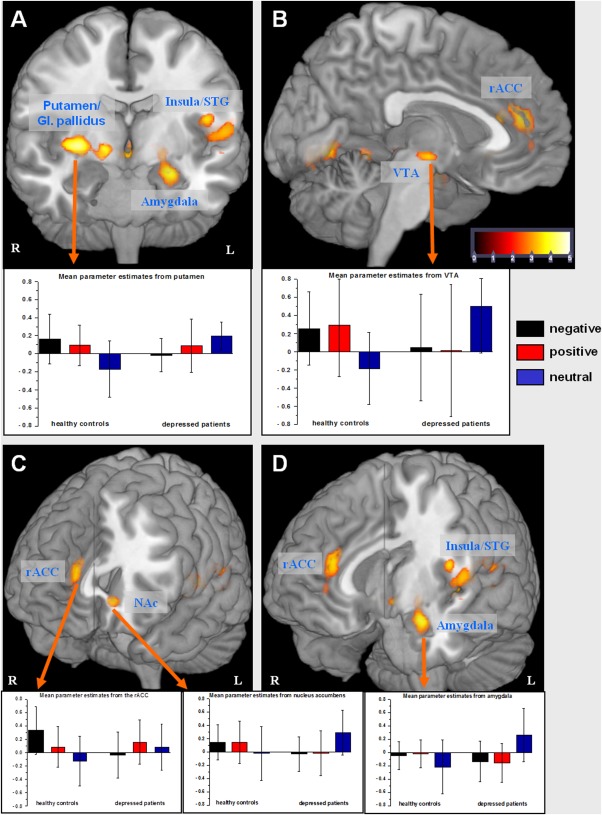
Brain regions showing significant BOLD signal in the post hoc t‐test of the GROUP by VALENCE (neutral vs. negative self‐referential stimuli) interaction (P<0.005 uncorrected, P<0.05 FWE cluster‐level corrected). The error bars represent standard deviation. rACC, rostral anterior cingulate cortex; NAc, nucleus accumbens; VTA, ventral tegmental area; G. pallidus, globus pallidus; STG, superior temporal gyrus.
Table 1.
Maxima of regions showing (P<0.005 uncorrected, P<0.05 FWE cluster‐level corrected) a significant GROUP by VALENCE (neutral vs. negative as well as neutral vs. positive self‐referential stimuli) interaction in post hoc t‐test
| Group X Valence interaction: post hoc t‐test MDD vs. HC, negative vs. neutral SRP | |||||||
|---|---|---|---|---|---|---|---|
| Region of activation | Right/Left | Brodmann's Area | Cluster sizea | Talairach coordinate | T value | ||
| x | y | Z | |||||
| Putamen | R | 1928 | 28 | −6 | −1 | 5.1 | |
| Globus pallidus | R | 14 | 0 | −5 | 4.6 | ||
| Amygdala | L | −24 | −7 | −15 | 4.2 | ||
| Insula | L | 13 | −34 | −23 | 10 | 3.7 | |
| Vetral tegmental area | R/L | 0 | −12 | −6 | 3.7 | ||
| Rostral ACC | R | 24/32 | 844 | 12 | 30 | 12 | 4.3 |
| Nucleus accumbens | L | −10 | 25 | −5 | 4.1 | ||
| Cerebellum | 782 | −6 | −45 | −1 | 3.7 | ||
| Occipital cortex | L | 18 | −4 | −72 | 0 | 3.7 | |
| Group X Valence interaction: post hoc t‐test MDD vs. HC, positive vs. neutral SRP | |||||||
|---|---|---|---|---|---|---|---|
| Region of activation | Right/Left | Brodmann's Area | Cluster sizeb | Talairach coordinate | T value | ||
| x | y | Z | |||||
| Lentiform nucleus | R | 1806 | −26 | −19 | −1 | 4.7 | |
| Amygdala | L | −24 | −7 | −15 | 4.6 | ||
| Vetral tegmental area | R/L | 0 | −16 | −6 | 3.7 | ||
| Lentiform nucleus | L | 788 | −26 | −19 | −1 | 3.8 | |
| Insula | R | 13 | 684 | 59 | −36 | 17 | 4.7 |
| Occipital cortex | R | 18 | 675 | 18 | −70 | 2 | 3.5 |
FWE corrected
FWE corrected
A similar activation pattern was also observed for the group differences regarding the contrast positive vs. neutral SRP, but without a significant GROUP × VALENCE interaction in the rACC and nucleus accumbens (Fig. 2). As depicted in the box plots of parameter estimates in Figure 1, this result mainly indicates a BOLD signal increase in patients in the neutral—relative to negative or positive—SRP condition. In controls, the expected BOLD signal decrease was observed in these contrasts. A very strong positive correlation was observed in patients’ parameter estimates from the amygdala and the NAc in the neutral SRP condition (r=0.8, P<0.001), but not among healthy controls (r=0.39, P=0.1). The correlation coefficients were significantly different between groups (Z=2.01, P=0.04).
Figure 2.
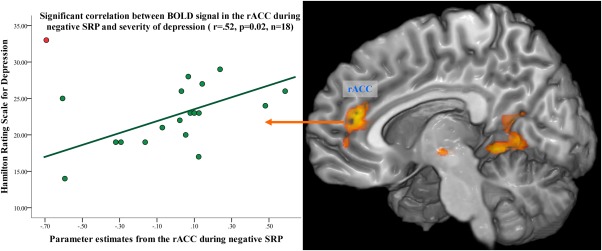
Scatterplot of the correlation between parameter estimates from the rACC (based on the GROUP by VALENCE interaction) and the HRSD. The exclusion of one patient (red dot) with suicidal behavior from the analysis resulted in a significant correlation in 18 patients (green dots, r=0.52, P=0.02).
Interestingly, patients revealed a lack of valence‐dependent BOLD activation in the rACC, which was observed in healthy controls (Fig. 1). Further significant interaction was detected in the contrast of positive vs. negative SRP in the precentral gyrus (x=36, y=7, z=29, BA 6, t=4.7, cluster=703), indicating a BOLD signal increase in patients in the positive relative to the negative SRP condition and a BOLD signal decrease in controls in this contrast.
Parametric modulation
Using parametric modulation of neural activation with respect to self‐referential stimuli, we investigated in which brain regions the BOLD signal varied depending on the subjects’ response to self‐related stimuli. The overall contrast (both groups together) is depicted in Supporting Information Figure S2 and Table S1. The degree of subjective agreement with the self‐referential stimuli was accompanied by a significant increase in BOLD signal activation in the contralateral motor system, comprising the motor cortex, SMA and midcinguate cortex, in the right inferior parietal lobe and in the left superior temporal gyrus. We further observed a parametric modulation of the BOLD signal in the amygdala, putamen, insula and nucleus accumbens. Testing for group differences in the parametric modulation we did not observe significant differences between healthy controls and patients with MDD on the uncorrected P voxel‐level<0.005 and an FWE corrected P cluster‐level=0.005 threshold.
Correlational analyses
A strong positive correlation between the BOLD signal in the left amygdala and the judgment of positive self‐referential statements was detected in healthy controls (r=0.7, P<0.001), but not in patients. We did not observe any significant correlations between the degree of depressive symptoms (assessed by the BDI or HRSD) or the degree of agreement with self‐referential statements and the BOLD signal in the rACC or in the amygdala in the total group of patients. However, when excluding the single patient with suicidal behavior from the correlational analysis between BOLD signal in rACC and depression severity as assessed by HRSD, the significance of this relationship markedly changed from r=0.15–0.52 (P=0.02) as illustrated in Figure 2. As observed in previous studies [Jollant et al., 2011; Wagner et al., 2011, 2012] depressed patients with suicidal behavior may have distinct neurobiological alteration in the fronto‐cingulate system and in particular in the rACC/VMPFC in contrast to depressed patients without suicidal behavior.
Effects of self‐referential processing on brain activation during the Stroop task
Here, we tested for potential group differences with respect to prior presentation of negative SRP items on brain activation during the incongruent Stroop condition and masked this contrast with the Stroop interference contrast (incongruent > congruent condition over all valence conditions) in healthy controls. We aimed to test for significant group differences in brain regions predominantly involved in cognitive control.
A significantly reduced BOLD signal was observed after negative SRP in patients with MDD relative to controls in the right DLPFC (BA 9, x =48, y=23, z=34, t=3.6, k=533, FWE corr.), and in the inferior parietal lobule (BA 40, x=46, y=−46, z=42, t=3.97, k=527, FWE corr.) extending to the superior temporal gyrus (BA 22, x=61, y=−42, z=13, t=3.34) as illustrated in Figure 3. There was no significantly increased BOLD activation in patients in the incongruent condition after negative SRP relative to controls.
Figure 3.
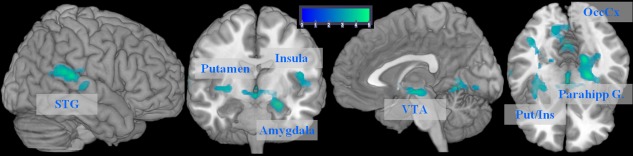
Brain regions showing significant BOLD signal in the post hoc t‐test of the GROUP by VALENCE (neutral vs. positive self‐referential stimuli) interaction (P<0.005 uncorrected, P<0.05 FWE cluster‐level corrected). VTA, ventral tegmental area; Put, putamen; Ins., insula; STG, superior temporal gyrus; OccCx, occipital cortex; Parahipp. G., parahippocampal gyrus.
Figure 4.
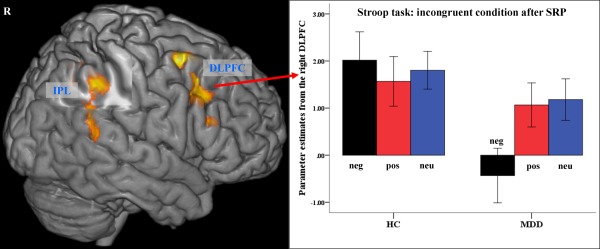
Brain regions showing significant BOLD signal difference (controls vs. patients) in the incongruent Stroop after negative SRP condition (P<0.005 uncorrected, P<0.05 FWE cluster‐level corrected). The error bars represent standard deviation. DLPFC, dorsolateral prefrontal cortex; IPL, inferior parietal lobule.
No significant group differences were observed when comparing the BOLD signal of the incongruent Stroop condition after positive as well as after neutral SRP. We further did not observe any significant GROUP × VALENCE interactions in the incongruent condition.
Correlational analyses
No significant relationship between BOLD signal in the right DLPFC during the incongruent Stroop condition after negative SRP and BOLD signal in the rACC as well as in the amygdala during negative SRP was observed.
DISCUSSION
This study investigated the neural underpinnings of abnormal negative SRP in patients with MDD and its impact on subsequent cognitive control processing. As expected, during the self‐relevance judgment task, depressed patients reported greater self‐relatedness of negative and lower self‐relatedness of positive statements in comparison to controls. However, no significant differences in the judgment of neutral stimuli were found. In the fMRI analysis of the SRP conditions two main important differences between patients and healthy controls were observed, shedding new light on the depressive mode of thinking. First, and in agreement with our initial hypothesis, we observed abnormal activation dynamics in the rACC in patients, indicating a lack of dynamic activation in the rACC as a function of the valence manipulation during the processing of self‐referential stimuli. A second novel and surprising finding was the activation of the amygdala and the reward‐processing network during evaluation of non‐affective “neutral” self‐referential stimuli in patients.
Furthermore with respect to the Stroop task performance, we observed significantly prolonged response times in the incongruent condition after negative and positive SRP in patients and a reduced BOLD activation in the right fronto‐parietal executive network after negative SRP relative to controls.
Rostral ACC and Self‐Referential Processing
MDD has been associated with negative automatic thoughts and an attentional bias toward negative stimuli [Mathews and MacLeod, 2005]. However, in contrast to anxiety disorders, for which an anxiety‐congruent bias has been observed in the early (already preattentive) stage of information processing [Bradley et al., 1995], the neuropsychological data for depressive patients tend to detect differences in attentional bias toward negative stimuli only when the stimuli were presented for a long duration [Mathews and MacLeod, 2005]. Therefore, it was suggested that depressed patients have difficulties in shifting their attention away from negative stimuli. This was mainly observed for self‐relevant negative information in particular when elaborative processing was facilitated [Gotlib and Joormann, 2010; Mogg and Bradley, 2005]. By using a study design based on these considerations, this study revealed a lack of a dynamic and valence‐dependent activation in the rACC in patients in contrast to what is found in healthy subjects. This was statistically confirmed by the significant GROUP × VALENCE interaction (Fig. 1). The detected dynamic rACC activation in the new sample of healthy controls confirmed our previous findings [Wagner et al., 2013]. In the neutral SRP condition the rACC as well as the amygdala showed negative BOLD response in controls relative to fixation baseline. This finding is in agreement with the recent meta‐analysis conducted by van der Meer et al. [2010], who suggested that the rACC/VMPFC might be specifically involved in processing of affective self‐relevant information. As a central component of the DMN [Uddin et al., 2009], the rACC showed a negative BOLD signal during cognitive tasks and an increased BOLD signal during specific emotion‐related tasks [Bush et al., 2000; Vogt, 2005].
Sheline et al. [2009] detected during active reappraisal of negative pictures a significantly higher activation in central DMN regions, that is, VMPFC, rACC as well as in lateral parietal and temporal cortices in depressed patients than controls. They further observer greater BOLD activation in patients in amygdala and hippocampal complex during looking at negative pictures. The authors interpreted these findings in terms of a failure of depressives to down‐regulate activity mainly within the DMN, which fits very well with the observation of the present study.
The absence of the valence‐dependent neural activation in the rACC in depressed patients may indicate a strong attentional bias toward negative aspects of the self. Thus, once negative stimuli capture the attention, patients may exhibit difficulties disengaging from the negative self‐focus [Gotlib and Joormann, 2010]. The positive correlation between depression severity and BOLD activation in the rACC at least in a significant portion of depressed patients (Fig. 2), suggests a direct relationship between depression severity, rACC BOLD signal and potentially stronger negative self‐focus.
Furthermore, because fMRI activation parameters are computed relative to an arbitrary baseline, the lack of modulation in depressed patients might be caused by a high sustained baseline activity in the rACC due to the abnormal focus of patients on internal mental contents [Northoff and Sibille, 2014]. This might limit further increase in rACC activation (ceiling effect) during processing of negative SRP stimuli. Previous PET studies in MDD provided some evidence for this speculation by reporting an increased resting state metabolism in the rACC [Drevets, 2004; Konarski et al., 2007]. Furthermore, MR spectroscopic studies detected an abnormal relationship between GABAergic‐mediated neural inhibition as well as glutamatergic‐mediated neural excitation and BOLD signal in that region in MDD [Northoff and Sibille, 2014]. These observations point in the direction of an imbalance between excitation and inhibition in the rACC in MDD. The abnormally strong role of glutamatergic‐mediated neural excitation may support the notion of chronically increased rACC BOLD signal in MDD [Walter et al., 2009].
However, previous fMRI studies detected both decreased activation in the rACC during SRP of positive or negative picture stimuli [Grimm et al., 2009] and increased activation during SRP of positive and negative personality trait words [Lemogne et al., 2009]. Cooney et al. [2010] observed group differences only, when the SRP condition was contrasted with nonaffective statements (interaction contrast). Johnson et al. [2009] reported no significant group differences in the rACC activation during unrestricted thinking about hopes and responsibilities.
Thus, it seems that different stimulus material (pictures, words or sentences), different presentation durations, and the implementation of a control and/or distraction condition as well as patient variables may influence activation patterns, not only in the rACC, but also in other depression‐relevant regions such as the limbic, striatal or frontal regions.
Amygdala and Reward‐Processing Network
Contrary to our initial hypothesis, we did not observe a hyperactive amygdala in patients during negative SRP. Instead, we observed strong left amygdalar activation in depressed patients during processing of neutral statements relative to baseline and to affective statements as well as relative to amygdalar deactivation in healthy controls. This finding needs to be discussed in more detail.
Amygdalar hyperactivity was observed in MDD during processing of negative stimuli [Siegle et al., 2007; Suslow et al., 2010]. However, decreased amygdalar activation induced by emotional face expressions [Lawrence et al., 2004] and affective picture ratings [Ritchey et al., 2011] or no differences in processing of emotional faces [Almeida et al., 2010] has also been observed in patients with MDD. Furthermore, increased amygdalar activation brought about by stimuli of positive valence [van Tol et al., 2012] as well as by positive social feedback [Davey et al., 2013] has been reported. Thus, despite some evidence for amygdalar hyperactivity induced by negative stimuli in MDD, the empirical picture remains complex, which may be due to distinct methodological differences between these studies.
Furthermore, one would also expect that positive self‐referential stimuli might be experienced as mood‐incongruent. However, on the behavioral level we observed a highly significant group difference regarding the judgment of positive statements, indicating lower self‐relatedness of positive stimuli in patients. Furthermore, fMRI results showed (Figs 1 and 3) that relative to neutral stimuli, the processing of both negative and positive self‐referential stimuli produced similar activation differences in patients. This was confirmed by the contrast in which the neural differences in the processing of positive vs. negative stimuli were directly tested. Thus, the behavioral and fMRI results point to the notion that negative and positive self‐relevant stimuli used in the present study appear to be processed in a similar way in acutely depressed inpatients.
In healthy controls, but not in patients with MDD, we observed a significant correlation between the degree of subjective agreement with positive self‐referential statements and amygdala activation (r=0.7). Using parametric modulation of neural activation with respect to all self‐referential stimuli we observed in the total group a modulation of the BOLD activation depending on the degree of self‐relatedness in the amygdala, nucleus accumbens (NAc), insula, PCC as well as in the motor regions and in the inferior parietal cortex (Supporting Information Fig. S2). This observation is in accordance with previous study of Moran et al. [2006] as well as with Northoff et al. [2009] who demonstrated stronger activation of the amygdala to go along with higher degree of self‐relatedness. Northoff et al. [2009] also observed a differential activation in the NAc depending on the degree of self‐relatedness and have reasoned that the association of the NAc with self‐relatedness might be related to the process of evaluating the rewarding value of a stimulus and might be considered as an important part of self‐relatedness. Here, as depicted in Figure 1 the bars of parameter estimates indicate in healthy controls a differential activation pattern in subcortical regions, that is, amygdala, NAc, VTA, and dorsal striatum during processing of affective relative to neutral self‐referential stimuli. Conversely, we detected a reversed activation pattern in patients with MDD. Schmitz et al. [2007] provided in their review some evidence that the amygdala, and NAc, together with ventral ACC and MPFC are involved in self‐relevant processes by identification the affective significance and by mediating the emotional response.
The amygdala has widespread projections to the striatum, the NAc, the hippocampus, and the sensory cortices as well as to the prefrontal and subgenual anterior cingulate cortices [Vuilleumier and Brosch, 2009]. Due to this strong anatomical connectivity, the contribution of the amygdala‐accumbens system was shown to be important when appetitive and aversive motivational values are processed [Manella et al., 2013]. In this study, we observed a strong correlation between the hyperactive amygdala and the NAc during processing of neutral self‐referential statements in patients, illustrating strong functional coupling between the amygdala and the NAc during neutral SRP in patients. This indicates that the neutral stimuli might have higher significance for patients than for controls as well as relative to negative and positive stimuli.
Moreover, the ventral tegmental area (VTA), ventral and dorsal striatum and the medial frontal cortex, have been found to be involved in processing diverse kinds of rewarding stimuli, including money [Delgado et al., 2000; Elliott et al., 2003; Gehring and Willoughby, 2002; Knutson et al., 2001] and rewarding drugs [Breiter et al., 1997]. In the present study, a strong relative activation of the whole reward‐processing network was observed in patients with MDD during evaluating of neutral self‐referential statements. One might therefore speculate that neutral statements have higher self‐relevance for patients, because they are experienced as a relief from “painful” processing of affective self‐referential stimuli. The relief from pain has been considered as rewarding and is therefore regarded as a strong motivational parameter for maintaining homeostatic balance [Seymour et al., 2005].
In a recent meta‐analysis, Rotge et al. [2015], on the basis of 46 fMRI studies mainly using the cyberball paradigm, provided evidence that the perigenual ACC is also involved in social rejection and social pain. Thus, an additional interpretation of the present rACC finding might be that the abnormal rACC activation during processing of affective SRP stimuli might be related to the affective experience of social rejection and more generally of social pain. This might in turn explain why neutral, potentially nonthreatening sentences might be associated with some relief or even pleasure among MDD patients.
However, we should be cautious with this interpretation based on the activation pattern, since we did not use a classical reward task. Whereas this form of deductive reasoning, known as reverse inference, may be misleading when applied to other brain regions, Ariely and Berns [2010] demonstrated that it may be less problematic for the interpretation of NAc activation in actively engaging in reward‐related processes.
The Impact of Increased SRP on Cognitive Control Related Brain Network
The present study tested the hypothesis that an enhanced self‐focus on negative aspects of the self in depression may compete for neuronal resources for cognitive processing and may thus interfere with corresponding brain networks [Elliss and Ashbrook, 1988]. The analysis of response times indicated a significantly slower performance in patients with MDD in the incongruent condition after presentation of negative and positive stimuli, but not after neutral SRP. The fMRI analysis revealed a reduced BOLD activation in the incongruent condition in the right fronto‐parietal network only after negative SRP. Thus, the behavioral results are in agreement with previous studies [Gillanders and Fleming, 2006] and lend support to the hypothesis that enhanced negative self‐focus in depression may interfere with executive functions, which were consistently shown to be impaired in MDD [Ottowitz et al., 2002].
However, the hypothesis that the hyperactive rACC or amygdala during negative SRP will lead to an abnormal BOLD activation pattern during cognitive control, was not supported by the present study. The BOLD signal in the rACC as well as amygdala during negative SRP was not significantly related to the reduced activation of the cognitive control associated fronto‐parietal network in patients after negative SRP. Furthermore, an increased activation in the amygdala during neutral SRP relative to baseline and control subjects did neither lead to significant group differences in the activation pattern during the incongruent Stroop condition nor to significant behavioral differences.
This result stands in some contrast to our previous findings. We observed previously an increased BOLD activation in the rACC and a hyperactive fronto‐cingulate network in the incongruent Stroop condition in acutely depressed patients [Wagner et al., 2006]. However, the behavioral performance in patients and controls was similar. The main difference to the current study is however that the negative SRP was not experimentally manipulated and patients showed normal task performance. In a recent study including only healthy subjects, an increased valence‐dependent interaction (negative vs. neutral SRP) of the rACC BOLD signal was found in fronto‐cingulate regions during Stroop task performance [Wagner et al., 2013]. Thus, the findings of the present study suggest that such a model as observed in healthy subjects cannot directly be transferred to clinically depressed patients.
Nevertheless, our results imply that the lack of a flexible SRP valence‐dependent activation in the rACC may potentially indicate an increased focus on negative self‐referential contents and difficulties to disengage from it. The accompanying negative affect and task‐irrelevant emotional processing may compete for neuronal resources with cognitive control processes, in particular in the DLPFC, which was shown to be crucially involved in emotion regulation [Ochsner and Gross, 2005]. This may lead thereby to deficits in cognitive performance in terms of observed increased response times and decreased neuronal activation in the task‐associated fronto‐parietal brain network.
Limitations
The majority of patients were treated with modern antidepressants inhibiting the reuptake of serotonin and norepinephrine. Recently, Di Simplicio et al. [2012] demonstrated a selective effect of citalopram, an SSRI on BOLD activation in the medial PFC during processing of negative, but not during processing of positive and neutral self‐referential words in healthy controls with a high risk for depression. Thus, based on this study a selective influence of antidepressant medication on the processing of self‐referential stimuli cannot be entirely ruled out, while seeming to more strongly affect processing of negative, compared to positive and neutral SRP stimuli. A further potential limitation of the study is the absence of measures of dispositional rumination tendencies, which may potentially explain some variance in the activation of the rACC and amygdala, especially in the control group.
Supporting information
Supplementary Information
Supplementary Information
Supplementary Information
REFERENCES
- Alloy LB, Robinson MS (2003): Negative cognitive styles and stress‐reactive rumination interact to predict depression: A prospective study1. Cogn Therapy Res 27:275–292. [Google Scholar]
- Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML (2010): Elevated amygdala activity to sad facial expressions: A state marker of bipolar but not unipolar depression. Biol Psychiatry 67:414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariely D, Berns GS (2010): Neuromarketing: The hope and hype of neuroimaging in business. Nat Rev Neurosci 11:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, Millar N, White J (1995): Selective processing of negative information: effects of clinical anxiety, concurrent depression, and awareness. J Abnorm Psychol 104:532–536. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE (1997): Acute effects of cocaine on human brain activity and emotion. Neuron 19:591–611. [DOI] [PubMed] [Google Scholar]
- Brett M, Christoff K, Lancaster J (2001): Using Talairach atlas with the MNI template. NeuroImage 13:S85. [Google Scholar]
- Briggs GG, Nebes RD (1975): Patterns of hand preference in a student population. Cortex 11:230–238. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI (2000): Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci 4:215–222. [DOI] [PubMed] [Google Scholar]
- Cooney RE, Joormann J, Eugene F, Dennis EL, Gotlib IH (2010): Neural correlates of rumination in depression. Cogn Affect Behav Neurosci 10:470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CE, Grayden DB, Egan GF, Johnston LA (2013): Filtering induces correlation in fMRI resting state data. Neuroimage 64:728–740. [DOI] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Yucel M (2011): Increased amygdala response to positive social feedback in young people with major depressive disorder. Biol Psychiatry 69:734–741. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA (2000): Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 84:3072–3077. [DOI] [PubMed] [Google Scholar]
- Di Simplicio M, Norbury R, Harmer CJ (2012): Short‐term antidepressant administration reduces negative self‐referential processing in the medial prefrontal cortex in subjects at risk for depression. Mol Psychiatry 17:503–510. [DOI] [PubMed] [Google Scholar]
- Drevets WC (2004): Neuroplasticity in mood disorders. Dialogues Clin Neurosci 6:199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr ., Todd RD, Reich T, Vannier M, Raichle ME (1997): Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386:824–827. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF (2003): Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: A parametric functional magnetic resonance imaging study. J Neurosci 23:303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliss HC, Ashbrook PW (1988): Resource allocation model of the effects of depressed mood states on memory In: Fiedler K, Forgas JP, editors. Affect, Cognition, and Social Behavior. Göttingen: Hogrefe; pp. 25–43. [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J (2006): Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron 51:871–882. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freton M, Lemogne C, Delaveau P, Guionnet S, Wright E, Wiernik E, Bertasi E, Fossati P (2014): The dark side of self‐focus: brain activity during self‐focus in low and high brooders. Soc Cogn Affect Neurosci 9:1808–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR (2002): The medial frontal cortex and the rapid processing of monetary gains and losses. Science 295:2279–2282. [DOI] [PubMed] [Google Scholar]
- Gillanders D, Fleming PFJ (2006): A Test of the Interacting Cognitive Subsystems Model using a laboratory analogue of Depressive Interlock. Clin Psychol Psychother 13:297–305. [Google Scholar]
- Gotlib IH, Joormann J (2010): Cognition and depression: current status and future directions. Annu Rev Clin Psychol 6:285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G (2009): Altered negative BOLD responses in the default‐mode network during emotion processing in depressed subjects. Neuropsychopharmacology 34:932–943. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME (2001): Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH (2011): Default‐mode and task‐positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry 70:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Nolen‐Hoeksema S, Mitchell KJ, Levin Y (2009): Medial cortex activity, self‐reflection and depression. Soc Cogn Affect Neurosci 4:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F, Lawrence NL, Olie E, Guillaume S, Courtet P (2011): The suicidal mind and brain: A review of neuropsychological and neuroimaging studies. World J Biol Psychiatry 12:319–339. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH (2008): Updating the contents of working memory in depression: Interference from irrelevant negative material. J Abnorm Psychol 117:182–192. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP (2008): Competition between functional brain networks mediates behavioral variability. Neuroimage 39:527–537. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D (2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21:RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski JZ, Kennedy SH, McIntyre RS, Rafi‐Tari S, Soczynska JK, Mayberg HS (2007): Relationship between regional brain metabolism, illness severity and age in depressed subjects. Psychiatry Res 155:203–210. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML (2004): Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 55:578–587. [DOI] [PubMed] [Google Scholar]
- Lehrl S, Triebig G, Fischer B (1995): Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91:335–345. [DOI] [PubMed] [Google Scholar]
- Lemogne C, le Bastard G, Mayberg H, Volle E, Bergouignan L, Lehericy S, Allilaire JF, Fossati P (2009): In search of the depressive self: Extended medial prefrontal network during self‐referential processing in major depression. Soc Cogn Affect Neurosci 4:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C, Delaveau P, Freton M, Guionnet S, Fossati P (2012): Medial prefrontal cortex and the self in major depression. J Affect Disord 136:e1–e11. [DOI] [PubMed] [Google Scholar]
- Li CS, Yan P, Bergquist KL, Sinha R (2007): Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage 38:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA (2009): Type I and Type II error concerns in fMRI research: Re‐balancing the scale. Soc Cogn Affect Neurosci 4:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C (2005): Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol 1:167–195. [DOI] [PubMed] [Google Scholar]
- Mannella F, Gurney K, Baldassarre G (2013): The nucleus accumbens as a nexus between values and goals in goal‐directed behavior: a review and a new hypothesis. Front Behav Neurosci 7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberly NJ, Watkins ER (2008): Ruminative self‐focus, negative life events, and negative affect. Behav Res Ther 46:1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP (2005): Attentional bias in generalized anxiety disorder versus depressive disorder. Cogn Ther Res 29:29–45. [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM (2006): Neuroanatomical evidence for distinct cognitive and affective components of self. J Cogn Neurosci 18:1586–1594. [DOI] [PubMed] [Google Scholar]
- Nejad AB, Fossati P, Lemogne C (2013): Self‐referential processing, rumination, and cortical midline structures in major depression. Front Hum Neurosci 7:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen‐Hoeksema S, Wisco BE, Lyubomirsky S (2008): Rethinking rumination. Perspect Psychol Sci 3:400–424. [DOI] [PubMed] [Google Scholar]
- Northoff G, Sibille E (2014): Why are cortical GABA neurons relevant to internal focus in depression? A cross‐level model linking cellular, biochemical and neural network findings. Mol Psychiatry 19:966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J (2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. Neuroimage 31:440–457. [DOI] [PubMed] [Google Scholar]
- Northoff G, Schneider F, Rotte M, Matthiae C, Tempelmann C, Wiebking C, Bermpohl F, Heinzel A, Danos P, Heinze HJ, Bogerts B, Walter M, Panksepp J (2009): Differential parametric modulation of self‐relatedness and emotions in different brain regions. Hum Brain Mapp 30:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005): The cognitive control of emotion. Trends Cogn Sci 9:242–249. [DOI] [PubMed] [Google Scholar]
- Ottowitz WE, Dougherty DD, Savage CR (2002): The neural network basis for abnormalities of attention and executive function in major depressive disorder: Implications for application of the medical disease model to psychiatric disorders. Harv Rev Psychiatry 10:86–99. [DOI] [PubMed] [Google Scholar]
- Paus T (2001): Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci 2:417–424. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ (2005): Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn Affect Behav Neurosci 5:156–168. [DOI] [PubMed] [Google Scholar]
- Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R (2011): Neural correlates of emotional processing in depression: Changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatry Res 45:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotge JY, Lemogne C, Hinfray S, Huguet P, Grynszpan O, Tartour E, George N, Fossati P (2015): A meta‐analysis of the anterior cingulate contribution to social pain. Soc Cogn Affect Neurosci 10:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, O'Doherty JP, Koltzenburg M, Wiech K, Frackowiak R, Friston K, Dolan R (2005): Opponent appetitive‐aversive neural processes underlie predictive learning of pain relief. Nat Neurosci 8:1234–1240. [DOI] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA (2007): Neural mechanisms mediating optimism bias. Nature 450:102–105. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry 59(Suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME (2009): The default mode network and self‐referential processes in depression. Proc Natl Acad Sci USA 106:1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA (2010): Resting‐state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci USA 107:11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME (2007): Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry 61:198–209. [DOI] [PubMed] [Google Scholar]
- Suslow T, Konrad C, Kugel H, Rumstadt D, Zwitserlood P, Schoning S, Ohrmann P, Bauer J, Pyka M, Kersting A, Arolt V, Heindel W, Dannlowski U (2010): Automatic mood‐congruent amygdala responses to masked facial expressions in major depression. Biol Psychiatry 67:155–160. [DOI] [PubMed] [Google Scholar]
- Terhaar J, Boettger MK, Schwier C, Wagner G, Israel AK, Bar KJ (2009): Increased sensitivity to heat pain after sad mood induction in female patients with major depression. Eur J Pain 14:559–563. [DOI] [PubMed] [Google Scholar]
- Treynor W, Gonzales R, Nolen‐Hoeksema S (2003): Rumination reconsidered: A psychometric analysis. Cogn Ther Res 27:247–259. [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP (2009): Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp 30:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS (2010): Self‐reflection and the brain: A theoretical review and meta‐analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev 34:935–946. [DOI] [PubMed] [Google Scholar]
- van Tol MJ, Demenescu LR, van der Wee NJ, Kortekaas R, Marjan MAN, Boer JA, Renken RJ, van Buchem MA, Zitman FG, Aleman A, Veltman DJ (2012): Functional magnetic resonance imaging correlates of emotional word encoding and recognition in depression and anxiety disorders. Biol Psychiatry 71:593–602. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt MA, Kuhn S, De Raedt R (2011): Healthy brooders employ more attentional resources when disengaging from the negative: An event‐related fMRI study. Cogn Affect Behav Neurosci 11:207–216. [DOI] [PubMed] [Google Scholar]
- Vogt BA (2005): Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci 6:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Brosch T (2009): Interactions of emotion and attention in perception In: Gazzaniga MS, editor. The Cognitive Neurosciences IV. Cambridge, MA: MIT Press; pp. 925–934. [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RG (2006): Cortical inefficiency in patients with unipolar depression: An event‐related FMRI study with the Stroop task. Biol Psychiatry 59:958–965. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Reichenbach JR, Sauer H, Schlösser RG (2008): Enhanced rostral anterior cingulate cortex activation during cognitive control is related to orbitofrontal volume reduction in unipolar depression. J Psychiatry Neurosci 33:199–208. [PMC free article] [PubMed] [Google Scholar]
- Wagner G, Koschke M, Leuf T, Schlosser R, Bar KJ (2009): Reduced heat pain thresholds after sad‐mood induction are associated with changes in thalamic activity. Neuropsychologia 47:980–987. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Schultz CC, Sauer H, Schlosser RG (2011): Structural brain alterations in patients with major depressive disorder and high risk for suicide: Evidence for a distinct neurobiological entity? Neuroimage 54:1607–1614. [DOI] [PubMed] [Google Scholar]
- Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG (2012): Prefrontal cortical thickness in depressed patients with high‐risk for suicidal behavior. J Psychiatr Res 46:1449–1455. [DOI] [PubMed] [Google Scholar]
- Wagner G, Koch K, Schachtzabel C, Peikert G, Schultz CC, Reichenbach JR, Sauer H, Schlosser RG (2013): Self‐referential processing influences functional activation during cognitive control: An fMRI study. Soc Cogn Affect Neurosci 8:828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Henning A, Grimm S, Schulte RF, Beck J, Dydak U, Schnepf B, Boeker H, Boesiger P, Northoff G (2009): The relationship between aberrant neuronal activation in the pregenual anterior cingulate, altered glutamatergic metabolism, and anhedonia in major depression. Arch Gen Psychiatry 66:478–486. [DOI] [PubMed] [Google Scholar]
- Watkins E, Brown RG (2002): Rumination and executive function in depression: An experimental study. J Neurol Neurosurg Psychiatry 72:400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information
Supplementary Information


