Abstract
A large body of evidence supports the view that psychopathy is associated with anomalous emotional processing, reduced guilt and empathy, which are important risk factors for criminal behaviors. However, the precise nature and specificity of this atypical emotional processing is not well understood, including its relation to moral judgment. To further our understanding of the pattern of neural response to perceiving and evaluating morally‐laden behavior, this study included 155 criminal male offenders with various level of psychopathy, as assessed with the Psychopathy Check List‐Revised. Participants were scanned while viewing short clips depicting interactions between two individuals resulting in either interpersonal harm or interpersonal assistance. After viewing each clip, they were asked to identify the emotions of the protagonists. Inmates with high levels of psychopathy were more accurate than controls in successfully identifying the emotion of the recipient of both helpful and harmful actions. Significant hemodynamic differences were detected in the posterior superior temporal sulcus, amygdala, insula, ventral striatum, and prefrontal cortex when individuals with high psychopathy viewed negative versus positive scenarios moral scenarios and when they evaluated the emotional responses of the protagonists. These findings suggest that socioemotional processing abnormalities in psychopathy may be somewhat more complicated than merely a general or specific emotional deficit. Rather, situation‐specific evaluations of the mental states of others, in conjunction with sensitivity to the nature of the other (victim vs. perpetrator), modulate attention to emotion‐related cues. Such atypical processing likely impacts moral decision‐making and behavior in psychopaths. Hum Brain Mapp 36:2015–2026, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: emotion, empathy, moral evaluation, psychopathy, decision‐making, temporoparietal junction, insula, ventromedial prefrontal cortex, ventral striatum
INTRODUCTION
Most social interactions hinge on the degree to which individuals understand one another's emotional and cognitive states and process general socioemotional information. The study of psychopathy, a construct characterized by symptoms of emotional detachment, a propensity of disinhibited impulsive conduct, combined with a general callousness and lack of insight for the impact such actions have on others [Cleckley, 1941], can generate valuable information for our understanding of how affective and cognitive computations are integrated when perceiving morally‐laden behaviors. Over the past 50 years, the field of psychopathy research has been dominated by clinical descriptions and theories that emphasize emotional deficits as core features of this disorder [Kiehl, 2014]. A growing body of evidence supports the view that psychopathy is associated with anomalous emotional processing, and deficits in empathy, which are important risk factors for criminal and violent behaviors [Anderson and Kiehl, 2011; Blair, 2007; Decety and Michalska, 2012; Rogstad and Rogers, 2008; Seara‐Cardoso and Viding, 2014]. The level of specificity of this atypical emotional and empathetic processing is not well understood, and both behavioral and neuroscience studies have reported mixed results [Book et al., 2007; Glass and Newman, 2006; Hastings et al., 2008; Lishner et al., 2012; Pham and Philippot, 2010, Seara‐Cardoso et al., 2012]. However, most neuroimaging studies do report abnormal neural processing in the perception and recognition of elicited emotions of others in psychopathy [Dawel et al., 2012; Decety, Skelly, Yoyer and Kiehl, 2014; Marsh, 2013].
There is also solid evidence for the role of emotion in moral judgment [Haidt and Graham, 2007]. Importantly, moral insensitivity in psychopathy is often attributed to callousness and lack of empathy [e.g., Blair, 1997; Decety and Cowell, 2014a; Cheng et al., 2012; Harenski and Kiehl, 2011; Maibom, 2009]. Empathy, the natural ability to perceive and be sensitive to the emotional states of others coupled with a motivation to care for their well‐being is a multifaceted construct that is comprised of affective, motivational, and cognitive components [Batson, 2012; Decety and Jackson, 2004; Decety, 2015]. While there is general agreement that both the emotional and motivational components of empathy are affected in psychopathy [Decety and Skelly, 2014; Decety et al., 2013a; Seara‐Cardoso and Viding, 2014], there is extensive debate regarding whether or not cognitive empathy is impaired and how this impairment impacts moral cognition and behavior.
Despite the general consensus that psychopaths have an intact ability to make inferences about another person's mental states, there some evidence suggesting that psychopathy is inversely associated with empathic accuracy, which is the inference of another person's emotional state [Brook and Kosson, 2013; Brook et al., 2013]. One study found that inmates with elevated psychopathic traits were deficient in inferring affective states (emotions) but not cognitive states (beliefs) in a theory of mind task [Shamay‐Tsoory et al., 2010]. These cognitive aspects of emotional understanding are closely related to processes involved in theory of mind, the capacity to infer the explicit content of others' mental states such as intentions and beliefs. Neuroimaging and lesion studies converge to indicate that the posterior superior temporal sulcus (pSTS) cortex at the junction with the temporal parietal cortex (TPJ) and medial prefrontal cortex are essential to the perception of intentionality and mental states of others, as well as cognitive empathy [Decety and Lamm, 2007; Gallagher and Frith, 2003; Lamm et al., 2007; Moll et al., 2007; Saxe et al., 2004; Silani et al., 2013; Yoder and Decety, 2014a]. Atypical information processing in this network in people with psychopathy when viewing others' morally‐laden actions may have downstream consequences that can impact their moral judgment and interpersonal behaviors.
Overall, while research clearly indicates atypical emotion processing in psychopathy, the nature of these deficits and how they relate to moral cognition are still a matter of discussion. In addition, neuroimaging studies of moral judgment in psychopathy have shown that psychopaths often make the same moral evaluations as nonpsychopaths, but use different brain circuits to do so [Aharoni et al., 2012]. But atypical neural response is found in several regions involved in moral decision‐making, particularly the amygdala, pSTS/TPJ and ventromedial prefrontal cortex (vmPFC) [Harenski et al., 2010]. Thus, while psychopaths show abnormal brain activation during moral judgment, it is not understood why, or in which contexts. In particular, there is little neurobiological work regarding where such abnormalities originate, especially when the emotions of others are perceived in social contexts. Finally, while many theories consider that psychopaths' cognitive deficits are secondary to their abnormal emotion processing systems, a number of studies have documented situation‐specific abnormalities in attention, which could account for psychopaths' socioemotional deficiencies [Newman and Lorenz, 2003; Newman et al., 2011]. Thus, explicitly requiring psychopaths to focus their attention on the emotional consequences of others' actions may modulate the neural processing of affective cues that are critical in for moral decision‐making. The study of basic socioemotional processes underlying implicit moral evaluations of others' behaviors and the identification of the affective consequences of their actions is quite critical, as it is generally believed that the psychopath may be quite skilled in understanding another person's perspective and may be uniquely adept in their ability to manipulate others [Book et al., 2007], with significant implications for legal proceedings, justice, and the law [Maibom, 2008].
In this study, instead of using complex moral dilemmas that pit consequentialism versus deontology (which are unlikely to happen to most people) or static pictures that evoke moral feelings (which are very simple), we used more ecologically valid dynamic visual scenarios depicting either intentional interpersonal harm or interpersonal assistance. Brain response was measured when participants viewed these scenarios and subsequently evaluated the emotional states of the protagonists. The general emotional deficit theory predicts that psychopathy should be associated with deficits in affective processing during implicit moral evaluation of outcomes of the actions, as well as during the inference of the emotional state of the protagonists. Conversely, the specific emotional deficit theory predicts selective impairment in processing negative affect such as sadness and distress, and in the present study for inferring the emotional states of victims of harm.
MATERIAL AND METHODS
Participants
One hundred fifty‐five adult males between the ages of 19 and 54, incarcerated in a medium‐security North American correctional facility, volunteered for the study and provided informed consent to the procedures described here, which were approved by the Institutional Review Boards of the University of New Mexico and the University of Chicago. Participants underwent the PCL‐R assessment, a reliable and valid instrument, designed to assess psychopathic traits such as antisocial behavior, shallowness, impulsivity, callousness, criminal history, and lack of moral emotions, based on evidence obtained from medical and juridical records and documents, as well as extensive interviews with the forensic patients. The PCL‐R assessment was conducted by trained and qualifies research professionals. Participants scoring 30 and above on the PCL‐R were assigned to the high‐psychopathy group (n = 38; age 32.4 ± 6.6; IQ 97 ± 13.1), which was the primary target of the study. To create the medium‐ and low‐psychopathy groups, two groups of volunteers were matched to high scorers on age, race and ethnicity, IQ (WAIS), comorbidity for DSM‐IV Axis II disorders, and past drug abuse and dependence, from pools of inmates scoring between 21 and 29 (n = 67; age 33.1 ± 7.6; IQ 98.72 ± 13.82), and those scoring below 20 on the PCL‐R (n=50; age 31.6 ± 7; IQ 97.42 ± 13.38), respectively. The latter group was used as the control group. There was no difference between groups in terms of IQ [F (2,152) = 0.69, P = 0.5] and age [F (2,152) = 0.24, P = 0.79]. Participants were paid at a rate consistent with the facility hourly labor wage.
Participants were included if they met the following criteria: age > 18 or < 55 years, fluent in English, reading level higher than 4th grade, IQ score higher than 80, no history of seizures, no prior head injury with loss of consciousness > 30 min, current Diagnostic and Statistical Manual of Mental Disorders (4th ed.; American Psychiatric Association, 1994), Axis I diagnosis, no lifetime history of a psychotic disorder or psychotic disorder in a first degree relative, and no current alcohol or drug use.
Task Design
A set of 48 dynamic visual stimuli depicting ecologically valid dyadic social interactions that resulted in either harm or assistance (e.g., pulling hair or helping up off of the floor) was used. Importantly, the faces of protagonists were not visible; thus, there was no emotional reaction of either protagonist visible to participants. Three still frames were extracted from each clip and presented in succession to create apparent motion (1,000, 200, and 1,000 ms, respectively). The stimuli had been previously assessed and validated with 91 subjects (43 males and 48 females; age 25 ± 10.5 years) who rated with seven‐point Likert scales the outcome of the actions, their valence, and motives of the acting agent, and have also been used in functional MRI (fMRI) and high‐density EEG/ERPs studies with healthy participants [Yoder and Decety, 2014a].
In the scanner, participants were shown the morally‐laden scenarios. Following each scenario, and separated by a jittered ISI, a final screen was presented depicting either the agent or the recipient of the previously shown action and an emotional face of either the perpetrator/agent or victim/beneficiary of the action. Participants were instructed to identify how likely the emotional state of the specified target (perpetrator/agent or victim/beneficiary of the action) matched the provided face using a visual analog scale (Fig. 1 e.g., on the experimental procedure). A total of six evenly distributed facial expressions (sad, happy, angry, fearful, pain, and disgust) were used in the emotional state evaluations of the scenarios. The order of harm/assistance scenarios and the match between scenarios and facial expressions was randomized with the same number of same‐sex/different sex pairs.
Figure 1.
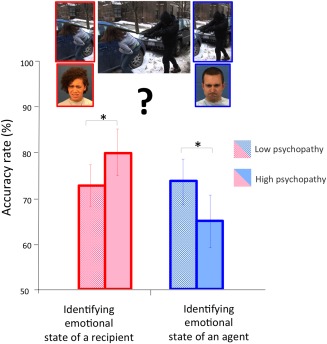
Accuracy rates from the 20% of trials in which the facial expressions of the protagonists were congruent with the outcome of the behavior shown in the clip, such as an angry face for a perpetrator of a harmful action, or a pained face for a victim of a harmful action. Both the perpetrator and victim are provided in the figure to illustrate all potential task mappings. However, only one face appeared in each trial during the scanning session. Participants with high psychopathy scores, compared to participants with low psychopathy scores (79 ± 5% vs. 72 ± 4%) identified the emotional states of a victim/recipient more accurately, but were less accurate (65 ± 6% vs. 73 ± 5%) in identifying the emotional states of a perpetrator (P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The confidence rating was determined by the absolute value of each participant's response on the scale (SR) minus 3 (| SR‐3 |). The SR was derived from the original rating subjects provided (i.e., higher rating = larger SR, ranging from 0 to 6). Both “very likely” and “very unlikely” were considered as confident responses. Hence, the single‐trial confidence was assessed with participants' ratings of emotional states as “very likely” or “very unlikely” of how the person in the clip would be feeling.
Analysis of the participants ratings during the identification of emotions showed a wide distribution from very certain (very likely or very unlikely) to very ambiguous (perhaps or doubtful). Hence, a trial‐to‐trial analysis was applied, using the single‐trial confidence for each participant as a parametric modulator of the condition‐dependent BOLD responses to response making at the single‐subject level. Contrast images from these first‐level models were then introduced into a one‐sample t test at the second level to test for group effects. Each viewing trial lasted 2.2 s and consisted of one scenario, and interstimuli intervals were jittered between 4.6 and 14.1 s. Each emotional identification trial was time‐locked to a cropped frame following each clip to target either the agent/perpetrator or recipient/victim. These durations were jittered between 3.8 and 9.1 s. The interstimulus interval between the viewing phase of the trial and the emotional expression judgment phase of the trial jittered between 0.79 and 6.01 s. Finally, participants' subjective responses were modeled using each trial's reaction time as well as the single‐trial confidence. Timing parameters were generated using a genetic optimization algorithm [Wager and Nichols, 2003].
Eye‐tracking was monitored in the scanner to ensure that participants were paying attention to the stimuli. Prescan practice and postscan debriefings were also conducted to ensure that participants followed the instructions and understood the task.
MRI Acquisition: Scanning was conducted on a 1.5 Tesla Siemens Magnetom Avanto mobile unit equipped with advanced SQ gradients and a 12 element head coil. Functional images were collected using an EPI gradient‐echo pulse sequence with TR/TE = 2,000/39 ms, flip angle = 90°, field of view = 240 × 240 mm, matrix = 64 × 64 cm, in‐plane resolution = 3.4 × 3.4 mm, slice thickness = 5 mm, and 30 slices, full‐brain coverage. Task presentation was implemented using the commercial software package E‐Prime 1.0 (Psychology Software Tools, Pittsburgh PA).
High‐resolution T1‐weighted structural MRI scans were acquired using a multiecho MPRAGE pulse sequence (repetition time = 2,530 ms, echo times = 1.64, 3.50, 5.36, and 7.22 ms, inversion time = 1,100 ms, flip angle = 7°, slice thickness = 1.3 mm, matrix size = 256 × 256) yielding 128 sagittal slices with an in‐plane resolution of 1.0 mm × 1.0 mm.
Image Processing and Analysis
Functional images were processed with SPM8 (Wellcome Department of Imaging Neuroscience, London, UK) in Matlab (Mathworks, Sherborn, MA). For each participant, functional data were realigned to the first image acquisition of the series and resampled to a voxel size of 2 × 2×2 mm3. Structural T1 images were coregistered to the mean functional image and segmented using the ‘New Segment’ routine. A group‐level structural template and individual flow fields were created using DARTEL, and the flow fields were in turn used to spatially normalize functional images to standard MNI space. Data were smoothed with an 8 mm full‐width at half maximum isotropic Gaussian kernel.
Statistics were calculated at the first level using the general linear model. The design matrix included seven regressors (viewing harm; viewing help; identifying the emotional reaction to harm for perpetrator; identifying the emotional reaction to harm for victim; identifying the emotional reaction to help for recipient; identifying the emotional reaction to help for agent; confidence during response making) representing the event onsets and their time and dispersion derivatives. Single‐trial confidence was parametrically modeled (ranging from 0.0007 to 1.6432; mean = 0.4419; SD =0.3127) for the “confidence during response making” regressor.
Movement parameters from the realignment output were included as regressors of no interest. All participants were entered into a second‐level pooled analysis, and full brain activations were thresholded voxelwise at P < 0.005 and with an extent threshold k > 20 based on Gaussian random fields set to control the whole‐brain false discovery rate (FDR) rate at P < 0.05. For analysis of group differences, participants with PCL‐R total score at or above 30 were included in the psychopathy group, while participants scoring at 20 or below comprised the incarcerated control group.
Finally, mean activity within a 6 mm‐radius sphere (FWE‐corrected, P < 0.05) was extracted from predefined regions of interest (ROIs) using the MarsBar toolbox. Coordinates for the ROIs (pSTS/TPJ: x = 66, y = −34, z = 8; dmPFC: x = −5, y = 54, z = 34; vmPFC: x = 4, y = 58, z = −8; dlPFC: x = 54, y = 20, z = 16; right anterior insula: x = 36, y = 22, z = −8; right amygdala: x = 22, y = −2, z = −16) were taken from a recent meta‐analysis of fMRI studies of morality [Bzdok et al., 2012] as well as a previous fMRI study of affective perspective taking in 121 male incarcerated participants (dlPFC: x = 48, y = 30, z = 0) [Decety et al., 2013a]. An additional ROI for the ventral striatum (x = −10, y = 10, z = −2) was taken from a recent meta‐analysis of fMRI studies [Diekhof et al., 2012] to examine the atypical processing in antisocial personality disorders.
RESULTS
Behavioral Evaluations in the Scanner
Results from an analysis of variance (ANOVA) with moral valence of the scenarios (harmful vs. helpful) and agency (agent vs. recipient) as within‐subject variables and group (high psychopathy group vs. low psychopathy group) as the between‐subject variable, revealed a main effect of moral valence [F (1, 86) = 136.5, P < 0.001] qualified by an interaction between moral valence and agency [F (1, 86) = 19.65, P < 0.001] on behavioral ratings in the scanner. The emotional states of the protagonists were easier for participants to identify in scenarios depicting harmful actions (Mean ± S.E.: 2.76 ± 0.026), compared to scenarios depicting helpful actions (2.4 ± 0.031). Post hoc comparisons showed that participants were more confident in evaluating the emotional states of a victim after viewing interpersonal harm than the emotional state of a beneficiary in interpersonal helping. No significant effects of PCL‐R scores were found in confidence ratings. Accuracy rate was assessed for 20% of the trials where the facial expressions of the agent and of the recipient matched in terms of expected outcomes of the behavior (i.e., an angry perpetrator, victim expressing pain). Results from a 2 (moral valence) × 2 (agency) × 2 (group) Repeated Measures ANOVA revealed a significant interaction between group and agency [F (1, 86) = 2.93, one‐tailed P < 0.05] on accuracy scores. Post hoc comparisons showed that high psychopathy participants compared to low psychopathy participants (79 ± 5% vs. 72 ± 4%) identified the emotional states of a recipient more accurately but were less accurate (65 ± 6% vs. 73 ± 5%) in identifying the emotional states of an agent (Fig. 1).
Neurohemodynamic Response to the Scenarios
The entire sample of 155 participants (regardless of their psychopathy level) showed significant neurohemodynamic increase in the network of regions involved in moral valence evaluation (k > 10, P < 0.05, FDR corrected). Regions with greater activity when viewing scenarios depicting interpersonal assistance vs. interpersonal harm included the middle and inferior temporal gyri, vlPFC, dlPFC, vmPFC, dmPFC, insula, and hippocampus (Supporting Information Table I). The reverse contrast showed increased signal during scenarios depicting interpersonal harm in the midcingulate cortex, precuneus, right pSTS/TPJ. When participants made a decision about inferring the emotional states of the protagonists, a similar pattern of response was observed, with the addition of the supplementary motor area (SMA), thalamus, inferior frontal gyrus and fusiform gyrus, in the interpersonal harm vs. interpersonal assistance condition (Supporting Information Table II). Parametric modulation of single‐trial difficulty showed that when a decision was more difficult to make, greater activation was found in the right fusiform gyrus.
Regions of Interest Analyses
Results from the ROI analyses are presented in Tables 1 and 2. When participants with low scores on the PCL‐R were compared with individuals scoring high on the PCL‐R during the viewing phase of the harmful vs. helpful scenarios, greater response was detected in right pSTS/TPJ, dmPFC, and bilaterally in dlPFC, temporal pole and anterior cingulate cortex (ACC). The opposite pattern was found (high psychopathy > low psychopathy) when participants inferred the emotional states of the protagonists elicited by harmful actions compared to helpful actions (Fig. 2). When participants identified the emotional states of others (either agent and recipient), high psychopathy was associated with bilateral increased activity in the pSTS/TPJ, dmPFC, dlPFC, vlPFC, hippocampus, amygdala, and ACC (Fig. 2). Importantly, during the identification of the emotional state of an agent/perpetrator, individuals with low scores on the PCL‐R compared with individuals with high scores on the PCL‐R, showed greater signal change in the anterior insula, vmPFC, dlPFC, and right pSTS/TPJ, consistent with previous studies on perceiving intentional harm (Decety and Porges, 2011; Decety, et al., 2012; Yoder and Decety, 2014a). Conversely, participants with high scores on the PCL‐R (>30) show decreased signal in all regions (see Fig. 3, bottom).
Table 1.
Groupwise results and PCL‐R correlations from harmful > helpful contrasts when participants viewed morally‐laden scenarios and identified the emotional states of the protagonists
| MNI coordinates | PCL‐R Factor 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region of interest | x | y | z | Peak T | x | y | z | Peak T | |
| DIRECT COMPARISON BETWEEN VIEWING AND IDENTIFYING MORALLY‐LADEN BEHAVIORS | |||||||||
| Viewing > identifying | |||||||||
| L | Lingual gyrus | −12 | −74 | −2 | 4.83 | ||||
| L | Posterior cingulate cortex | −6 | −46 | 32 | 3.67 | ||||
| L | Middle temporal gyurs | −58 | −62 | 10 | 3.52 | ||||
| L | Precentral gyrus | −60 | 10 | 32 | 3.43 | ||||
| — | ventromedial prefrontal cortex | −2 | 58 | −10 | 2.09* | ||||
| — | ventromedial prefrontal cortex | 14 | 42 | −12 | 2.84 | ||||
| R | Supramarginal gyrus | 52 | −24 | 30 | 3.28 | ||||
| Identifying > viewing | |||||||||
| L | dorsolateral prefrontal cortex | −52 | 36 | −6 | 3.59 | ||||
| L | anterior insula | −36 | 18 | −4 | 3.44 | ||||
| L | dorsomedial prefrontal cortex | −12 | 32 | 48 | 3.98 | ||||
| L | Superior temporal gyrus | −54 | −18 | −4 | 4.73 | ||||
| L | pSTS | −50 | −36 | 16 | 3.6 | ||||
| L | Inferior parietal lobule | −42 | −30 | 32 | 3.18 | ||||
| — | anterior cingulate cortex | 0 | 2 | 28 | 3.37 | ||||
| R | pSTS | 52 | −42 | 6 | 2.57* | ||||
| R | dorsolateral prefrontal cortex | 46 | 12 | 26 | 3.97 | ||||
| R | Superior temporal gyrus | 44 | −24 | 6 | 4.47 | ||||
| R | dorsomedial prefrontal cortex | 2 | 24 | 44 | 3.66 | ||||
| R | Precentral gyrus | 26 | −32 | 70 | 3.18 | ||||
| R | Lingual gyrus | 14 | −84 | −6 | 2.96 | ||||
| VIEWING MORALLY‐LADEN BEHAVIORS | |||||||||
| Controls > Psychopaths | |||||||||
| L | Dorsolateral prefrontal cortex | −48 | 38 | 0 | 3.2 | −52 | 38 | 4 | −2.75* |
| L | Temporal pole | −52 | 2 | −6 | 3.44 | −52 | 4 | −4 | −4.35 |
| L | Dorsomedial prefrontal cortex | −8 | 54 | 34 | 2.19* | −8 | 38 | 40 | −3.76 |
| L | Anterior cingulate cortex | −12 | 28 | 18 | 3.99 | −12 | 28 | 18 | −3.76 |
| R | dorsolateral prefrontal cortex | 48 | 30 | 0 | 3.21* | 48 | 24 | 2 | −3.74 |
| R | pSTS/TPJ | 60 | −30 | 8 | 2.61* | 56 | −30 | 10 | −4.29 |
| R | precentral gyrus | n.s. | 58 | −8 | 16 | −4.11 | |||
| R | temporal pole | n.s. | 58 | 6 | 0 | −3.5 | |||
| Psychopaths > Controls | |||||||||
| L | postcentral gyrus | −24 | −42 | 48 | 4.13 | n.s. | |||
| IDENTIFYING EMOTIONAL STATES | |||||||||
| Controls > psychopaths | |||||||||
| L | postcentral gyrus | −24 | −40 | 50 | 4.35 | −24 | −40 | 48 | −3.46 |
| Psychopaths > controls | |||||||||
| L | dorsolateral prefrontal cortex | −56 | 32 | 10 | 2.63* | −56 | 34 | 10 | 3.01* |
| — | dorsomedial prefrontal cortex | −4 | 50 | 32 | 3.07* | −2 | 50 | 32 | 3.45 |
| R | dorsolateral prefrontal cortex | 46 | 26 | 0 | 2.74* | 50 | 24 | 16 | 3.16 |
| R | pSTS/TPJ | 60 | −34 | 8 | 2.25* | 56 | −30 | 10 | 3.86 |
| R | precentral gyrus | n.s. | 56 | −6 | 18 | 3.81 | |||
Negative and positive peak T‐values represent negative and positive correlations, respectively. All clusters are significant at FDR‐corrected P < 0.05 [thresholded at P < 0.005, cut‐off, t = 2.608 (uncorrected) with a spatial extent threshold of k > 20], except those marked with a star, which are taken from a priori predefined ROIs and significant at uncorrected P < 0.05. The FDR correction is used for exploratory purposes only as some have argued that it underestimates Type I error.
Table 2.
Groupwise results and PCL‐R correlations on harmful > helpful contrast when participants identified the emotional states of the recipient/victim and the agent/perpetrator involved in the morally laden scenarios
| MNI coordinates | PCL‐R total scores | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region of interest | x | y | z | Peak T | x | y | z | Peak T | |
| DIRECT COMPARISON BETWEEN AGENT AND RECIPIENT SENARIOS | |||||||||
| Recipient > agent | |||||||||
| L | anterior insula | −36 | 14 | −14 | 2.15* | ||||
| — | ventromedial prefrontal cortex | 14 | 38 | −2 | 2.46* | ||||
| R | anterior cingulate cortex | 12 | 36 | −2 | 2.89 | ||||
| R | anterior insula | 46 | −2 | −2 | 3.11 | ||||
| Agent > recipient | |||||||||
| L | dorsolateral prefrontal cortex | −52 | 40 | 0 | 2.24* | ||||
| L | dorsolateral prefrontal cortex | −44 | 32 | 30 | 3.36 | ||||
| L | Fusiform gyrus | −42 | −54 | −20 | 4.95 | ||||
| L | Angular gyrus | −42 | −66 | 38 | 5.14 | ||||
| L | TPJ | −56 | −50 | 36 | 4.06 | ||||
| — | Mid cingulate cortex | 8 | −34 | 36 | 4.92 | ||||
| R | dorsolateral prefrontal cortex | 50 | 18 | 36 | 3.19* | ||||
| R | pSTS | 48 | −46 | 14 | 2.06* | ||||
| R | dorsolateral prefrontal cortex | 42 | 20 | 34 | 3.82 | ||||
| R | Occipital cortex | 46 | −68 | 28 | 5.74 | ||||
| R | TPJ | 58 | −32 | 34 | 3.08 | ||||
| R | Fusiform gyrus | 48 | −56 | −18 | 4.59 | ||||
| R | Angular gyrus | 46 | −62 | 26 | 5.52 | ||||
| IDENTIFYING EMOTIONAL STATE OF AN AGENT/PERPETRATOR | |||||||||
| Controls > psychopaths | |||||||||
| L | anterior insula | −34 | 12 | −8 | 2.4* | −36 | 14 | −10 | −2.46* |
| — | ventromedial prefrontal cortex | −6 | 58 | −4 | 1.91* | 14 | 38 | −2 | −2.84* |
| R | dorsolateral prefrontal cortex | 50 | 22 | 34 | 2.62* | 52 | 24 | 32 | −2.67* |
| R | pSTS/TPJ | 46 | −46 | 12 | 3.28 | 46 | −46 | 12 | −3.16 |
| Psychopaths > controls | |||||||||
| L | amygdala | −26 | 0 | −26 | 3.1 | −26 | 0 | −26 | 3.12 |
| L | dorsolateral prefrontal cortex | −52 | 38 | 0 | 2.76* | −46 | 38 | 0 | 2.34* |
| L | dorsomedial prefrontal cortex | −4 | 48 | 32 | 3.2 | −2 | 50 | 32 | 3.46 |
| R | hippocampus | 30 | −38 | −2 | 4.46 | 30 | −38 | −2 | 3.48 |
| R | temporal pole | 30 | 6 | −18 | 3.42 | 30 | 6 | −20 | 3.92 |
| R | anterior cingulate cortex | 12 | 34 | 14 | 3.23 | 12 | 34 | 12 | 2.78 |
| R | SMA | 16 | −8 | 54 | 3.71 | 16 | −8 | 54 | 3.94 |
| IDENTIFYING EMOTIONAL STATE OF A RECIPIENT/VICTIM | |||||||||
| Controls > psychopaths | |||||||||
| L | postcentral gyrus | −24 | −40 | 50 | 3.39 | −24 | −40 | 50 | −3.19 |
| Psychopaths > controls | |||||||||
| L | pSTS/TPJ | −42 | −46 | 18 | 2.99 | −60 | −30 | 18 | 2.93 |
| L | dorsomedial prefrontal cortex | −2 | 50 | 32 | 2.81* | −2 | 48 | 34 | 3.7 |
| L | anterior cingulate cortex | −10 | 28 | 20 | 3.09 | −8 | 26 | 20 | 3.26 |
| R | hippocampus | 30 | −38 | −2 | 4.67 | 32 | −40 | −4 | 3.76 |
| R | amygdala | 30 | 2 | −20 | 2.33* | 30 | 2 | −20 | 3.01 |
| R | ventrolateral prefrontal cortex | 40 | 38 | −6 | 2.61 | 42 | 40 | −8 | 3.05 |
| R | pSTS/TPJ | 58 | −30 | 8 | 2.30* | 58 | −30 | 8 | 3.3 |
| R | dorsolateral prefrontal cortex | 54 | 20 | 16 | 2.46* | 52 | 22 | 20 | 2.24* |
Negative and positive peak T‐values represent negative and positive correlations, respectively. All clusters are significant at FDR‐corrected P < 0.05 [thresholded at P < 0.005, cut‐off, t = 2.608 (uncorrected) with a spatial extent threshold], except those marked with a star, which are taken from predefined ROIs and significant at uncorrected P < 0.05.
Figure 2.
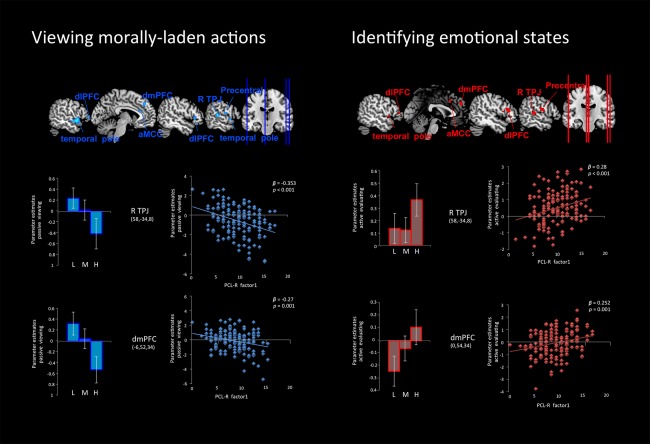
Neural response in individuals with different levels of psychopathy (low, L; medium, M; and high, H) while they viewed morally‐laden actions (left) and when they were requested to identify the emotional states of the victims/recipients or perpetrator of the behavior (right). Neural responses to harmful actions compared to helpful actions, modeled to the onset of the clips, suprathreshold voxels (P < 0.05 corrected for multiple comparisons) are displayed on 4 anatomical sections. There was significantly reduced activity in the rTPJ, dmPFC, dlPFC, temporal pole, precentral gyrus, and ACC in individuals with high levels of psychopathy while they viewed morally laden scenarios. Conversely, when identifying the emotional states of the recipient/victim of an action, participants with high scores on PCL‐R Factor 1, showed greater hemodynamic response in the rTPJ and dmPFC. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
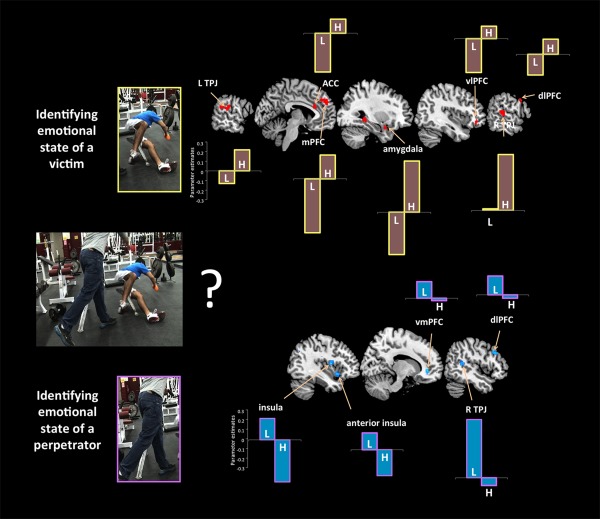
Contextualized socioemotional understanding in psychopathy. Groupwise effects in bar graphs (L, low; H, high on total PCL‐R scores). During the identification of the emotional states of the protagonists, participants with low scores on the PCL‐R compared with individuals with high scores on the PCL‐R, showed greater signal change in the anterior insula (−34 12 −8), vmPFC (14 38 −2), dlPFC (50 22 34), and rTPJ (46 −46 12). When participants were asked to identify the emotions of the protagonists, participants with high psychopathy showed increased activity bilaterally in the TPJ (left: −42 −46 18; right: 58 −30 8), dmPFC (‐2 46 32), dlPFC (52 20 20), vlPFC (40 38 −6), hippocampus (30 −38 −2), amygdala (30 2 −20), and ACC (−10 28 20). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
When inferring the emotional states of a victim of harm, those with high scores on psychopathy showed increased signal in bilateral TPJ, amygdala, dlPFC, dmPFC, ACC and vlPFC. Participants with low levels of psychopathy exhibited an attenuation of response in these regions (Fig. 3, top).
Correlation Analysis Between PCL‐R Scores and Signal Change in ROIs
To examine psychopathy from a dimensional perspective rather that a categorical one, PCL‐R scores were computed as a continuous variable and related to signal change. PCL‐R Factor 1 inversely predicted response in right pSTS/TPJ (β = −0.35, P < 0.001) and dmPFC (β = −0.27, P = 0.001) during the viewing phase, but was directly related to signal change in right pSTS/TPJ (β = 0.28, P < 0.001) and dmPFC (β = 0.25, P = 0.001) during the identification phase of the protagonists (Fig. 2).
PCL‐R total score negatively predicted the hemodynamic response in dlPFC during moral evaluation of the perpetrator doing the harmful versus helpful actions (β = −0.21, P = 0.009). Activity in the right pSTS/TPJ was positively associated with PCL‐R total score (β = 0.258, P = 0.001) when participants identified the emotions of the victim in harmful vs. helpful scenarios. Finally, a positive association was found between the response in the ventral striatum (−10, 10, −2) and scores on PCL‐R Factor 1 (β = 0.254, P = 0.002) when participants evaluated the emotional states of perpetrators engaging in harmful actions (Fig. 4).
Figure 4.
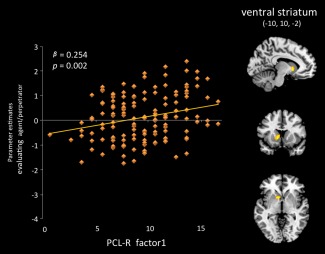
Differential activity in the ventral striatum in participants with psychopathy while they were evaluating a perpetrator engaging in harmful actions (compared to an agent engaging in helpful behavior) as positively associated with PCL‐R scores on Factor 1. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
Psychopaths are characterized by a general lack of empathy and shallow affect, traits associated with callous disregard for the wellbeing of others, guiltlessness, and little appreciation of moral wrongdoing. However, the precise nature of their emotional, moral and empathetic deficits remains unclear [Carré et al., 2013; Sato et al., 2011; Seara‐Cardoso and Viding, 2014]. While most behavioral and neuroimaging studies report that psychopaths are impaired in their capacity to experience empathic concern [Decety et al., 2013a], resonate with the affective states of others [Decety et al., 2014; Marsh, 2013], or process stimuli depicting moral violations in a typical way [Harenski et al., 2010], some work also suggests that they may have difficulties in correctly identifying the emotional states of others [Brook and Kosson, 2013]. Such impairments can have downstream effects in decision‐making and moral behavior.
In this study, we examined basic socioemotional processing of morally‐laden behavior depicting interpersonal harm or interpersonal assistance in criminal offenders with high levels of psychopathy (PCL‐R ≥ 30) and low levels of psychopathy (PCL‐R ≤ 20), by measuring the neurohemodynamic response elicited by viewing and subsequently interpreting the emotional consequences of such actions on others.
Importantly, online behavioral ratings indicate that all participants were engaged in both segments of the task while being scanned and paid attention to the outcomes of the scenarios. In addition, and like healthy subjects viewing a similar stimuli [Decety, et al., 2012; Yoder and Decety, 2014a], harmful actions were more discernable than helpful actions. This has been interpreted along the line of the negativity bias in social emotional processing, which plays a critical role in moral judgment [Decety and Cacioppo, 2012; Peeters and Czapinski, 1990; Schupp et al., 2004; Yoder and Decety, 2014b]. Interestingly, at the cognitive level, psychopaths had no massive deficit in either intention or emotion understanding. In fact, individuals scoring high on the PCL‐R had a higher accuracy rate in identifying the emotions of the victim of a harmful action or the recipient of a helpful interaction. These results support a previous study with inmates which reported that psychopathy was not associated with any deficit in rating individuals on assertiveness after viewing short interpersonal interactions, and that psychopathic traits were significantly positively correlated with accuracy in ratings of facial expressions of emotions [Book et al., 2007].
Despite this seemingly absent cognitive deficit in reading the emotional states of others in social context, a number of striking differences were detected in neural response patterns between high and low psychopaths. At the whole group level, viewing harmful vs. helpful actions and evaluating the emotional states of the protagonists of those actions was associated with increased activity in the insula, ACC, SMA, dorsomedial and dorsolateral PFC, temporal pole, inferior frontal gyrus, and pSTS/TPJ, and was stronger in the right hemisphere. This pattern of response is consistent with previous neuroimaging studies using similar stimuli with nonforensic populations [Decety et al., 2012; Yoder and Decety, 2014a].
In individuals with high psychopathy, when viewing harmful vs. helpful scenarios, the first segment of the task, the neural response was significantly reduced in the right pSTS/TPJ, dmPFC, dlPFC, temporal pole, and ACC (Fig. 2). These regions are involved in extracting the intentions and mental states of others, which is required for moral judgment [Decety and Lamm, 2007; Pelphrey et al., 2004; Saxe et al., 2004; Yoder and Decety, 2014a]. Additionally, an inverse association between the neural responses to harmful versus helpful actions in this network during viewing the scenarios was found with PCL‐R Factor 1 which clusters the interpersonal/affective aspects of psychopathy. This finding supports the theory that psychopaths suffer from a general deficit in both emotional and cognitive processing, but not necessarily in the case of positively valenced actions, as previously reported by Brook and Kosson [2013]. However, during the emotional evaluation segment of the task (identifying the emotional states of the individuals interacting), there was a significant increase of activity in right pSTS/TPJ, dmPFC, dlPFC, temporal pole, and anterior midcingulate cortex for individuals scoring high on psychopathy compared with those scoring low. In addition, when high psychopaths evaluated the emotional consequences of harmful actions for a victim, there was an augmentation of the hemodynamic activity bilaterally in the pSTS/TPJ, hippocampus, amygdala, and ACC. This shift in the pattern of response fits well with the attentional theory of psychopathy [Newman and Lorenz, 2003]. Such a pattern, which could be viewed as compensatory neural activity in brain regions extracting intentionality and emotional saliency, may explain why psychopaths were not impaired in behavioral ratings of the scenarios' agents and could distinguish the moral valence of the actions.
Moreover, and most interestingly, differential neural response detected in individuals scoring high in psychopathy depended on whether the target of emotional identification was a victim or a perpetrator of a harmful action (Fig. 3). When focusing on a perpetrator vs. a victim engaging in harmful actions, significantly decreased activity was detected in areas related to empathic concern and perspective taking, including the anterior insula, vmPFC, dlPFC, and right pSTS/TPJ. Conversely, inferring the emotional state of a victim of harmful actions was associated with increased signal change in a large portion of the same network. This distinction in neurohemodynamic response between victim and perpetrator is quite intriguing, as one would have expected just the opposite from previous work documenting a lack of empathic concern and perspective taking for victims in high psychopathy [Decety et al., 2013a].
The response in the ventral striatum to the emotional evaluation of a perpetrator engaging in harmful actions was predicted by scores on PCL‐R factor 1 (Fig. 4), and is consistent with previous work that demonstrates that psychopaths and adolescents with conduct disorder show enhanced activation in this region when viewing an individual hurting another [Decety et al., 2009], imagining another individual in pain [Decety et al., 2013a], or viewing facial expressions of pain [Decety et al., 2013b]. This has been interpreted as reflecting feelings of pleasure toward the emotional distress of others. Neurons in the ventral striatum have access to central representations of reward and thereby participate in the processing of information underlying the motivational control of goal‐directed behavior [Delgado, 2007; Diekhof et al., 2012]. However, some studies suggest that the human striatum is not only involved in appetitive and reward processing, but also in aversive processing [Fox, et al., 2013; Jensen et al., 2003; Porges and Decety, 2013]. Yet, the relation between Factor 1 and activation in ventral striatum is unlikely to be driven by aversive reactivity. For instance, psychopathy measured in a community sample is directly associated with reports of positive valence in response to viewing facial expressions of sadness [Ali et al., 2009]. Importantly, individuals with psychopathy often show higher activity in regions associated with reward processing in tasks involving moral decision‐making [Seara‐Cardoso and Viding, 2014].
Overall, individuals high in psychopathy appear to be able to distinguish between right and wrong actions, as well as interpret the emotional consequences of these actions on other people. This is in line with a study reporting that high‐psychopathy offenders were not deficient in discerning moral from conventional transgressions when compared to low‐psychopathy offenders [Aharoni et al., 2012]. However, the neural underpinnings of these computations are dramatically different from normative populations [Decety et al., 2012; Yoder and Decety, 2014a], as well as incarcerated controls.
CONCLUSION
Deficits in emotional functioning have been critical to many etiological theories of psychopathy. Importantly, our results support theories indicating that individuals with psychopathy do seem to make the “cognitive” distinction between morally good and bad actions, as shown by behavioral ratings. Moreover, when they focus on the emotional consequences of others' negative relative to positive actions, increased neural response is detected in regions that are critical for affective reactivity, including the amygdala and theory of mind such as the TPJ and dmPFC. However, the pattern of brain activation during the evaluation of positive and negative behavior is clearly dissociable from incarcerated nonpsychopaths. The findings of this study further suggest that socioemotional processing abnormalities in psychopathy may be somewhat more complicated than a mere general emotional deficit. Rather, situation‐specific evaluations of the mental states of others, in conjunction with sensitivity to the nature of the other (victim vs. perpetrator) modulate attention to emotion‐related cues. Such atypical neural processing impacts moral judgment and decision‐making.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
Dr. Decety takes full responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all the data in the study.
Conflict of interest: Drs. Decety, Chen, Harenski and Kiehl have no conflicts of interest to disclose.
REFERENCES
- Aharoni E, Sinnott‐Armstrong W, Kiehl KA (2012): Can psychopathic offenders discern moral wrongs? A new look at the moral/conventional distinction. J Abnorm Psychol 121:484–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali F, Amorim IS, Chamorro‐Premuzic T (2009): Empathy deficits and trait emotional intelligence in psychopathy and Machiavellianism. Pers Individ Dif 47:758–762. [Google Scholar]
- Anderson NA, Kiehl KA (2011): The psychopath magnetized: Insights from brain imaging. Trends Cogn Sci 16:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batson CD (2012): The empathy‐ altruism hypothesis: Issues and implications In: Decety J, editor. Empathy—From Bench to Bedside. Cambridge: MIT Press; pp. 41–54. [Google Scholar]
- Blair RJR (2007): The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends Cogn Sci 11:387–392. [DOI] [PubMed] [Google Scholar]
- Book AS, Quinsey VL, Langford D (2007): Psychopathy and the perception of affect and vulnerability. Crim Justice Behav 34:531–544. [Google Scholar]
- Brook M, Kosson DS (2013): Impaired cognitive empathy in criminal psychopathy: Evidence from a laboratory measure of empathic accuracy. J Abnorm Psychol 122:156–166. [DOI] [PubMed] [Google Scholar]
- Brook M, Brieman CL, Kosson DS (2013): Emotion processing in Psychopathy Checklist‐assessed psychopathy: A review of the literature. Clin Psychol Rev 33:979–995. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Schilbach L, Vogeley K, Schneider K, Laird AR, Langner R, Eickhoff SB (2012): Parsing the neural correlates of moral cognition: ALE meta‐analysis on morality, theory of mind, and empathy. Brain Struct Funct 217:783–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carré JM, Hyde LW, Neumann CS, Viding E, Hariri AR (2013): The neural signatures of distinct psychopathic traits. Soc Neurosci 8:122–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Hung A, Decety J (2012): Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Dev Psychopathol 24:623–636. [DOI] [PubMed] [Google Scholar]
- Cleckley H (1941): The Mask of Sanity: An Attempt to Reinterpret the So‐Called Psychopathic Personality. St. Louis: Mosby Company. [Google Scholar]
- Dawel A, Kearney RO, Mckone E, Palermo R (2012): Not just fear and sadness: Meta‐analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neurosci Biobehav Rev 36:2288–2304. [DOI] [PubMed] [Google Scholar]
- Decety J (2015): The neural pathways, development and functions of empathy. Curr Opin Behav Sci 3:1–6. [Google Scholar]
- Decety J, Cacioppo S (2012): The speed of morality: A high‐density electrical neuroimaging study. J Neurophysiol 108:3068–3072. [DOI] [PubMed] [Google Scholar]
- Decety J, Cowell JM (2014a): The complex relation between morality and empathy. Trends Cogn Sci 18:337–339. [DOI] [PubMed] [Google Scholar]
- Decety J, Cowell JM (2014b): Friends or foes: Is empathy necessary for moral behavior? Perspect Psychol Sci 9:525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Jackson PL (2004): The functional architecture of human empathy. Behav Cogn Neurosci Rev 3:71–100. [DOI] [PubMed] [Google Scholar]
- Decety J, Lamm C (2007): The role of the right temporoparietal junction in social interaction: How low‐level computational processes contribute to meta‐cognition. Neuroscientist 13:580–593. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska K (2012): How children develop empathy—The contribution of affective neuroscience In Decety J. J, editor. Empathy: From Bench to Bedside. Cambridge: MIT Press; pp. 167–190 [Google Scholar]
- Decety J, Porges EC (2011): Imagining being the agent of actions that carry different moral consequences: An fMRI study. Neuropsychologia 49:2994–3001. [DOI] [PubMed] [Google Scholar]
- Decety J, Skelly L (2014): The neural underpinnings of the experience of empathy: Lessons for psychopathy In Ochsner KN, Kosslyn SM, editors. The Oxford Handbook of Cognitive Neuroscience, Vol.2. New York: Oxford University Press; pp. 228–243. [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB (2009): Atypical empathic responses in adolescents with conduct disorder: A functional MRI investigation. Biol Psychol 80:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Kinzler KD (2012): The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cereb Cortex 22:209–220. [DOI] [PubMed] [Google Scholar]
- Decety J, Chen C, Harenski CL, Kiehl KA (2013a): An fMRI study of affective perspective taking in individuals with psychopathy: Imagining another in pain does not evoke empathy. Front Hum Neurosci 7:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Skelly L, Kiehl KA (2013b): Brain response to empathy‐eliciting scenarios involving pain in incarcerated psychopaths. JAMA Psychiatry 70:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decety J, Skelly L, Yoder KJ, Kiehl KA (2014): Neural processing of dynamic emotional facial expressions in psychopaths. Soc Neurosci 9:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR (2007): Reward‐related responses in the human striatum. Ann N Y Acad Sci 1104:70–88. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O (2012): The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude—An activation likelihood estimation meta‐analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50:1252–1266. [DOI] [PubMed] [Google Scholar]
- Fox GR, Sobhani M, Aziz‐Zadeh L (2013): Witnessing hateful people in pain modulates brain activity in regions associated with physical pain and reward. Front Psychol 4:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD (2003): Functional imaging of “theory of mind.” Trends Cogn Sci 7:77–83. [DOI] [PubMed] [Google Scholar]
- Glass SJ, Newman JP (2006): Recognition of facial affect in psychopathic offenders. J Abnorm Psychol 115:815–820. [DOI] [PubMed] [Google Scholar]
- Haidt J, Graham J. (2007): When morality opposes justice: Concervatives have moral intuitions that liberals may not recognize. Soc Justice Res 20:98–116. [Google Scholar]
- Harenski CL, Kiehl KA (2011): Emotion and morality in psychopathy and paraphilias. Emotion Rev 3:299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harenski CL, Harenski KA, Shane MS, Kiehl KA (2010): Aberrant neural processing of moral violations in criminal psychopaths. J Abnorm Psychol 119:863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings M, Tangney J, Stuewig J (2008): Psychopathy and identification of facial expressions of emotion. Pers Individ Dif 44:1474–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S (2003): Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron 40:1251–1257. [DOI] [PubMed] [Google Scholar]
- Kiehl KA (2014): The Psychopath Whisperer: Inside the Minds of Those Without a Conscience. New York: Crown Publishers. [Google Scholar]
- Lamm C, Batson CD, Decety J (2007): The neural substrate of human empathy: Effects of perspective‐taking and cognitive appraisal. J Cogn Neurosci 19:42–58. [DOI] [PubMed] [Google Scholar]
- Lishner DA, Vitacco MJ, Hong PY, Mosley J, Miska K, Stocks EL (2012): Evaluating the relation between psychopathy and affective empathy: Two preliminary studies. Int J Offender Ther Comp Criminol 56:1161–1181. [DOI] [PubMed] [Google Scholar]
- Maibom HL (2008): The mad, the bad, and the psychopath. Neuroethics 1:167–184. [Google Scholar]
- Maibom HL (2009): Feeling for others: Empathy, sympathy and morality. Inquiry 52:483–499. [Google Scholar]
- Marsh A (2013): What can we learn about emotion by studying psychopathy? Front Hum Neurosci 7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Adalio CJ, Jurkowitz ITN, Schechter JC, Pine DS, Decety J, Blair RJR (2013): Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J Child Psychol Psychiatry 54:900−110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll J, de Oliveira‐Souza R, Garrido GJ, Bramati IE, Caparelli‐Daquer EM, Paiva MLM F, … Grafman J (2007): The self as a moral agent: Linking the neural bases of social agency and moral sensitivity. Soc Neurosci 2:336–352. [DOI] [PubMed] [Google Scholar]
- Newman JP, Lorenz AR (2003): Response modulation and emotion processing: Implications for psychopathy and other dysregulatory psychopathology In: Davidson RJ, editor. Handbook of Affective Sciences. New York: Oxford University Press; pp 904–929. [Google Scholar]
- Newman JP, Curtin JJ, Bertsch JD, Baskin‐Sommers AR (2011): Attention moderates the fearlessness of psychopathic offenders. Biol Psychiatry 67:66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G (2004): Grasping the intentions of others: The perceived intentionality of and action influences activity in the superior temporal sulculs during social perception. J Cogn Neurosci 16:1706–1716. [DOI] [PubMed] [Google Scholar]
- Peeters G, Czapinski J (1990): Positive‐negative asymmetry in evaluations: The distinction between affective and informational negativity effects. Eur Rev Soc Psychol 1:33–60. [Google Scholar]
- Pham TH, Philippot P (2010): Decoding of facial expression of emotion in criminal psychopaths. J Pers Disord 24:445–459. [DOI] [PubMed] [Google Scholar]
- Porges EC, Decety J (2013): Violence as a source of pleasure or displeasure is associated with specific functional connectivity with the nucleus accumbens. Front Hum Neurosci 7:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogstad JE, Rogers R (2008): Gender differences in contributions of emotion to psychopathy and antisocial personality disorder. Clin Psychol Rev 28:1472–1484. [DOI] [PubMed] [Google Scholar]
- Saxe R, Xiao DK, Kovacs G, Perrett DI, Kanwisher N (2004): A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia 42:1435–1446 [DOI] [PubMed] [Google Scholar]
- Sato JR, de Oliveira‐Souza R, Thomaz CE, Basílio R, Bramati IE, Amaro E, Tovar‐Moll F, Hare RD, Moll J (2011): Identification of psychopathic individuals using pattern classification of MRI images. Soc Neurosci 6:627–639. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Oehmann A, Weike AI, Stockburger J, Hamm AO (2004): The facilitated processing of threatening faces: An ERP analysis. Emotion 4:189–200. [DOI] [PubMed] [Google Scholar]
- Seara‐Cardoso A, Viding E (2014): Functional neuroscience of psychopathic personality in adults. J Pers, epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Seara‐Cardoso A, Neumann CS, Roiser J, McCrory E, Viding E (2012): Investigating associations between empathy, morality and psychopathic personality traits in the general population. Pers Individ Dif 52:67–71. [Google Scholar]
- Shamay‐Tsoory SG, Harari H, Aharon‐Peretz J, Levkovitz Y (2010): The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex 46:668–677. [DOI] [PubMed] [Google Scholar]
- Silani G, Lamm C, Ruff CC, Singer T (2013): Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. J Neurosci 33:15466–15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Nichols TE (2003): Optimization of experimental design in fMRI: A general framework using a genetic algorithm. Neuroimage 18:293–309. [DOI] [PubMed] [Google Scholar]
- Yoder KJ, Decety J (2014a): The good, the bad, and the just: Justice sensitivity predicts neural response during moral evaluation of actions performed by others. J Neurosci 34:4161–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KJ, Decety J (2014b): Spatiotemporal neural dynamics of moral judgments: A high‐density EEG/ERP study. Neuropsychologia 60:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


