Abstract
The ability to imagine the future is a complex mental faculty that depends on an ensemble of cognitive processes supported by an extended set of brain regions. Our aim here was to shed light on one key component of future thinking—personal goal processing—and to determine its neural correlates during both directed and spontaneous forms of thoughts. To address this question, we performed separate ALE meta‐analyses of neuroimaging studies of episodic future thinking (EFT), mind‐wandering, and personal goal processing, and then investigated the commonalities and differences in brain activity between these three domains. The results showed that the three domains activated a common set of brain regions within the default network and, most notably, the medial prefrontal cortex. This finding suggests that the medial prefrontal cortex mediates the processing of personal goals during both EFT and mind‐wandering. Differences in activation were also observed, and notably regions supporting cognitive control processes (the dorsolateral prefrontal cortex) were recruited to a lesser extent during mind‐wandering than experimentally directed future thinking, suggesting that different kinds of self‐generated thoughts may recruit varying levels of attentional control abilities. Hum Brain Mapp 36:2928–2947, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: neuroimaging, default network, prefrontal cortex, future thinking, personal goals, mind‐wandering, meta‐analysis
INTRODUCTION
A remarkable feature of the human mind is the ability to decouple the focus of attention from the immediate environment to project oneself into other places and times [Buckner and Carroll, 2007; D'Argembeau, 2012; Schacter and Addis, 2007; Suddendorf et al., 2009; Tulving, 2005]. Perhaps most importantly, we can picture in our minds a variety of possible forthcoming events and “pre‐experience” what it would be like to encounter these imagined situations (e.g., how one would feel, how one could act, the potential implications of our actions, and so on). Although not without flaws, this capacity—referred to as episodic future thinking (EFT)—contributes to many important aspects of human cognition and behavior, such as planning, decision making, and self‐control [see e.g., Bechara and Damasio, 2005; Boyer, 2008; D'Argembeau et al., 2011; Schacter, 2012], which likely explains why it has become the focus of growing interest in psychology and neuroscience in the last decade [for recent reviews, see Klein, 2013; Mullally and Maguire, 2013; Schacter et al., 2012; Seligman et al., 2013; Szpunar, 2010].
Recent neuroimaging studies have shown that EFT is underpinned by an extended set of brain regions that comprises the medial prefrontal (mPFC), posterior cingulate (PCC) and restrosplenial (Rsp) cortices, medial, and lateral temporal regions, posterior inferior parietal lobules (pIPL), and parts of the lateral prefrontal cortex (PFC) [e.g., Addis et al., 2007; Botzung et al., 2008; D'Argembeau et al., 2008; Schacter et al., 2012; Spreng et al., 2009; Szpunar et al., 2007]. These findings suggest that the ability to project oneself into future scenarios is complex and multifaceted, and depends on an ensemble of various cognitive processes [D'Argembeau et al., 2010a; Klein, 2013; Schacter et al., 2012]. Furthermore, many of the brain regions that have been associated with EFT are also commonly engaged in other cognitive domains, such as episodic/autobiographical memory, social cognition, and mental navigation [e.g., Buckner et al., 2008; Schilbach et al., 2008; Spreng et al., 2009; Van Overwalle, 2009]. These brain areas are indeed part of a functional network—commonly referred to as the default network (DN)—which may play a general role in internally directed or self‐generated thought [for recent reviews, see Andrews‐Hanna, 2012; Andrews‐Hanna et al., 2014b; Smallwood et al., 2012]. However, the precise nature of the cognitive processes involved in EFT and other forms of self‐generated thoughts, as well as their specific neural correlates, are not fully understood and still remain debated [Buckner et al., 2008; Hassabis and Maguire, 2009; Schacter et al., 2008].
Among the various cognitive processes involved in EFT, the processing of personal goals might be particularly important in shaping the mental representations of future events. According to this view, EFT does not solely consist in using episodic details and semantic knowledge to construct novel and coherent event representations [Hassabis and Maguire, 2009; Hassabis et al., 2007; Schacter et al., 2012], but is also strongly influenced by the individual's personal goals and motives [Christian et al., 2013; D'Argembeau and Demblon, 2012; D'Argembeau and Mathy, 2011; D'Argembeau et al., 2010b; Grysman et al., 2013; Johnson and Sherman, 1990]. Personal goals are hierarchically organized mental representations of desired end‐states that impact and drive cognition and behavior [Austin and Vancouver, 1996; Conway, 2005, 2009; Fishbach and Ferguson, 2007], and an important function of EFT may precisely be to construct detailed representations or simulations of goal‐relevant information [D'Argembeau, 2015; Schacter, 2012]. In line with this view, there is evidence that personal goals facilitate access to episodic details when constructing episodic future thoughts [D'Argembeau and Mathy, 2011], help structure and organize these thoughts in meaningful themes and causal sequences [D'Argembeau and Demblon, 2012; Demblon and D'Argembeau, 2014], and shape the content of future simulations to make goal‐relevant features more salient [Christian et al., 2013].
Although behavioral studies suggest that EFT and personal goals are intimately related, relatively little is known about the brain regions supporting goal processing during EFT. Among the few neuroimaging studies that investigated this issue, D'Argembeau et al. [2010b] asked their participants to imagine future events that were either related to their personal goals or plausible but unrelated to their goals. By contrasting these two conditions, the authors found that midline brain regions corresponding to key nodes of the DN (i.e., the mPFC and PCC) were more activated when participants were thinking about goal‐related than goal‐unrelated future events, a result consistent with other studies of personal goal processing [e.g., Johnson et al., 2006; Preminger et al., 2011]. These findings suggest that cortical midline structures may support the processing of personal goals during EFT. Another set of studies [Gerlach et al., 2014; Spreng and Schacter, 2012; Spreng et al., 2010] has further shown that goal‐directed autobiographical planning (i.e., imagining taking various steps and actions in the future to achieve personal goals) depends on the functional coupling between the DN and the frontoparietal control network (e.g., the dorsolateral PFC and anterior IPL). This suggests that, in addition to midline DN structures, the frontoparietal control network also plays an important role in personal goal processing, and may contribute to monitor and integrate future‐oriented thoughts in coherent sequences to achieve imagined end‐states [Gerlach et al., 2014; Spreng et al., 2010].
With the exception of the few studies described above, most neuroimaging studies did not explicitly investigate the contribution of goal‐related processes in EFT. Yet, it is likely that goal‐related representations were often spontaneously activated in these studies (e.g., for constructing future thoughts that are deemed plausible with respect to the individual's life) [D'Argembeau and Van der Linden, 2012] and that some of the activated brain regions reflected such goal‐related processes. Here we investigated this possibility by performing meta‐analyses of neuroimaging studies of EFT on the one hand, and of personal goal processing on the other hand to determine the commonalities and differences in the brain regions associated with these two areas of research. If personal goals are, as suggested, a key component of EFT [D'Argembeau and Mathy, 2011; D'Argembeau et al., 2010b], then substantial overlap should be found between these two domains in terms of associated brain activations. However, as mentioned above, EFT involves multiple cognitive processes [D'Argembeau et al., 2010a] and some of the brain regions supporting the imagination of future events should be unrelated to personal goal processing per se. In particular, EFT involves the construction of detailed representations of specific events (referred to as episodic simulation or scene construction), which in part depends on the medial temporal lobe and Rsp [e.g., Addis and Schacter, 2011; Hassabis and Maguire, 2009; Hassabis et al., 2007]. In contrast personal goal processing can be more or less abstract (e.g., by focusing on general aspects of one's behavior rather than specific situations) [Fishbach and Ferguson, 2007] and thus does not necessarily involve such scene construction processes.
In addition to clarifying the neural correlates of personal goal processing in typical EFT tasks, we also aimed at investigating this question in the context of spontaneous or unconstrained self‐generated thoughts. A long tradition of research suggests that mind‐wandering—the spontaneous occurrence of thoughts whose content is unrelated to our immediate circumstances [Singer, 1993; Smallwood and Schooler, 2006; Stawarczyk et al., 2011]—is closely related to the individual's personal goals and concerns, and contributes to planning and preparing for the future [Antrobus et al., 1966; Klinger, 1978, 2013; Smallwood and Schooler, 2006]. It has indeed been found that a substantial part of mind‐wandering episodes involves goal‐directed and personally relevant future events [e.g., Baird et al., 2011; Song and Wang, 2012; Stawarczyk et al., 2011, 2013], and that priming personal goals influences the content and frequency of subsequent mind‐wandering episodes [e.g., Klinger, 1978; Masicampo and Baumeister, 2011; McVay and Kane, 2013; Stawarczyk et al., 2011]. Furthermore, neuroimaging studies suggest that mind‐wandering is associated with activations in similar DN areas as EFT and personal goal processing [e.g., Christoff et al., 2009; Mason et al., 2007; Stawarczyk et al., 2011].
Although these results suggest that future thinking and personal goal processing are important components of mind‐wandering episodes, the brain regions that specifically support these processes have not been directly investigated. To address this question, we included mind‐wandering as a third domain of investigation in our meta‐analyses. Given the influence of personal goals on the content and frequency of mind‐wandering episodes, as well as their preponderant future temporal orientation [e.g., Stawarczyk et al., 2011], we expected to detect substantial overlap between mind‐wandering, EFT, and personal goal processing in DN regions. However, a key difference between these three domains is that, by definition, mind‐wandering occurs spontaneously (and often involuntarily) during task performance [e.g., Stawarczyk et al., 2011, 2013], whereas studies of EFT and personal goal processing generally involve controlled and voluntary forms of thoughts that are induced by experimental instructions. We therefore expected that regions supporting cognitive control processes, such as the dorsolateral PFC [Spreng et al., 2009, 2010], would show higher activity during directed forms of EFT and personal goal processing compared to mind‐wandering episodes.
In summary, our aim was to investigate the commonalities and differences in the neural correlates of EFT, personal goal processing, and mind‐wandering to shed further light on the contribution of personal goal‐related processes to both directed and spontaneous forms of self‐generated thoughts. To do so, we first performed three distinct meta‐analyses (one for each domain of interest) using the activation likelihood estimation (ALE) method—a quantitative, automated, and highly validated procedure for voxel‐wise meta‐analyses of neuroimaging studies [Eickhoff et al., 2009, 2012; Laird et al., 2005; Turkeltaub et al., 2002, 2012]. Then, we performed (1) conjunction analyses between each domain to assess the potential overlaps between the clusters identified in each meta‐analysis and (2) subtraction analyses to determine the brain regions that are more specifically associated with each domain. To the extent that personal goals play an important role in EFT and mind‐wandering, we expected significant overlaps and convergences in neural activations between the three domains in brain regions that have been previously associated with personal goal processing, that is, midline regions of the DN and in particular the mPFC [e.g., D'Argembeau et al., 2010b; Johnson et al., 2006; Packer and Cunningham, 2009]. Furthermore, although our main goal was to examine the commonalities between the neural correlates associated with these three areas of research, some differences in brain activations were also expected. First, we expected regions involved in cognitive control (e.g., the lateral PFC) [Niendam et al., 2012] to be less active during mind‐wandering than experimentally directed EFT and personal goal processing. Second, we also expected that regions supporting scene construction processes (e.g., the medial temporal areas) [Hassabis and Maguire, 2009; Hassabis et al., 2007) would be more activated in association with EFT than personal goal processing and possibly mind‐wandering.
METHODS
Selection of Studies
We performed a systematic literature search to select functional neuroimaging studies on EFT, personal goal processing, and mind‐wandering. The search was conducted in the Medline database and only peer‐reviewed articles written in English from January 1990 up to August 2014 were considered for inclusion in the meta‐analyses. We searched for studies using functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) with the specific terms “future thinking,” “future events,” or “prospection” for the EFT domain, “personal goals,” “future goals,” “goal states,” “autobiographical planning,” and “hopes and aspirations,” for the personal goals domain, and “mind‐wandering,” “task‐unrelated thoughts,” or “stimulus‐independent thoughts” for the mind‐wandering domain.1 This search process yielded 132 articles for EFT, 41 for personal goal processing, and 64 for mind‐wandering. Additional studies were also added in each domain after examining the reference list of the articles found in the initial search process. Finally, studies that were included in previous meta‐analyses on EFT [Spreng et al., 2009; Viard et al., 2012] and mind‐wandering [Fox et al., [Link]] were also considered.
Inclusion Criteria
The general inclusion criteria for the articles were as follows: only peer‐reviewed articles that reported original experiments were included in the analyses; theoretical articles, reviews of the literature, and studies that reexamined already published data were excluded. Only studies that used PET or fMRI and reported their results in a standard reference frame (MNI or Talairach space) were included; studies that only reported region‐of‐interest (ROI) analyses were excluded. Studies that solely focused on functional connectivity analyses such as independent component analyses [Kiviniemi et al., 2003] or psychophysical interaction analyses [Friston et al., 1997] were excluded. Studies on clinical samples were excluded except when they reported separate results for the control group or common results for both the control group and the clinical group [e.g., Johnson et al., 2009]. Studies involving healthy older adults were included [e.g., Spreng and Schacter, 2012]. For articles reporting several experiments with independent samples, each of the experiment was considered individually and all appropriate data were included [e.g., Johnson et al., 2006; McGuire et al., 1996]. Only one contrast per experiment was selected for inclusion in the analyses.
The specific inclusion criteria for EFT were as follows: only studies that involved the mental simulation of specific events were included; studies that asked participants to merely talk about their future [e.g., Okuda et al., 2003] or to make judgment about personal future events without the explicit instruction to mentally simulate specific situations [e.g., Abraham et al., 2008; Andrews‐Hanna et al., 2010] were excluded. Studies that contrasted different categories of future events (e.g., familiar versus unfamiliar events) [Szpunar et al., 2009] but did not include a control task that did not require EFT were also excluded. In total, 16 studies were included in the analyses (for details, see Table 1).
Table 1.
Studies included in the meta‐analysis for the EFT domain
| No | Study | Task | Comparison task | N | Foci | Comments |
|---|---|---|---|---|---|---|
| 1. | Addis et al., 2007 | Future and past event elaborations | Semantic memory and visual imagery | 14 | 23 | |
| 2. | Sharot et al., 2007 | Future event imagination | Fixation | 15 | 24 | Coordinates not provided in the article |
| 3. | Szpunar et al., 2007 | Future and past event imagination | Imagining Bill Clinton in similar events | 21 | 15 | |
| 4. | Botzung et al., 2008 | Future event imagination | Semantic decision task | 10 | 12 | |
| 5. | D'Argembeau et al., 2008 | Near and far future event imagination | Imagining routine activities | 12 | 6 | |
| 6. | Addis et al., 2009 | Future and past event imagination (TR 4) | Semantic/visual imagery task | 18 | 15 | Multivariate analyses |
| 7. | Spreng and Grady, 2010 | Future and past event imagination | Theory of mind | 16 | 17 | |
| 8. | Weiler et al., 2010 | Future and past event elaborations | Imagining Angela Merkel in similar events | 17 | 49 | |
| 9. | Addis et al., 2011 | Future and past event imagination | Semantic memory and visual imagery | 15 | 9 | |
| 10. | Addis et al., 2011 | Future and past event imagination | Semantic/visual imagery task | 28 | 15 | Multivariate analyses/activity common to young and older adults |
| 11. | Benoit et al., 2011 | Future event imagination | Semantic estimation | 12 | 25 | Conditions included in a delay discounting task |
| 12. | Martin et al., 2011 | Future event imagination | Semantic/visual imagery task | 25 | 20 | |
| 13. | Viard et al., 2011 | future and past event imagination | Perceptual decision task | 12 | 29 | Participants were older adults |
| 14. | Gaesser et al., 2013 | Future event imagination | Semantic/visual imagery task | 24 | 13 | |
| 15. | Van Hoeck et al., 2013 | Future, past and counterfactual event imagination | Semantic memory | 13 | 22 | |
| 16. | van Mulukom et al., 2013 | Future event imagination | Semantic/visual imagery task | 20 | 10 | |
| Total | 272 | 304 |
For the personal goal domain, we only included studies in which participants were explicitly instructed to reflect on personal goals; studies examining the neural activity related to the incidental exposition to personal goals without explicit instructions to reflect upon these goals were excluded from the analyses [e.g., Eddington et al., 2007; Strauman et al., 2012]. Studies involving goal‐directed thoughts in scenarios that were not selected on the basis of the participants' personal goals were also excluded [e.g., Gerlach et al., 2011]. In total, nine studies were included in the analyses (see Table 2). It should be noted that four of these studies required participants to separately reflect on hopes and aspirations (promotion focus) versus duties and obligations (prevention focus) [e.g., Johnson et al., 2006; Packer and Cunningham, 2009]. Here, we only included the contrasts associated with hopes and aspirations because the other studies that did not explicitly distinguish between different kinds of personal goals generally referred to aspirations rather than obligations. Indeed, the study by D'Argembeau et al. [2010b] clearly investigated hopes and aspirations, and the instructions in Preminger et al. [2011] were to “generate novel/unrehearsed thoughts and think what you want and can do in order to advance your research project [italics added]” and thus also had a clear promotion focus. For the remaining studies [Gerlach et al., 2014; Spreng and Schacter, 2012; Spreng et al., 2010], we asked 24 independent individuals (16 females; mean age = 28.71, SD = 7.04) who were blind to the purpose of the present meta‐analysis to decide whether each personal goal used in these studies reflected mainly hopes and aspirations or duties and obligations. The data showed that personal goals in each of these studies were mostly hopes and aspirations, with a mean of 77% (SD = 12) of hopes and aspirations in Gerlach et al. [2014], 73% (SD = 17) in Spreng et al. [2010], and 68% (SD = 17) in Spreng and Schacter [2012].
Table 2.
Studies included in the meta‐analysis for the personal goal domain
| No | Study | Task | Comparison task | N | Foci | Comments |
|---|---|---|---|---|---|---|
| 1. | Johnson et al., 2006 | Thinking about goals with promotion focuses | Thinking about goals with prevention focuses and semantic memory | 19 | 2 | First experiment of the article |
| 2. | Johnson et al., 2006 | Thinking about goals with promotion focuses | Thinking about goals with prevention focuses and semantic memory | 11 | 1 | Second experiment of the article |
| 3. | Johnson et al., 2009 | Thinking about goals with promotion focuses | Thinking about goals with prevention focuses and semantic memory | 44 | 1 | Activity common to normal and depressed young adults |
| 4. | Mitchell et al., 2009 | Thinking about goals with promotion focuses | Thinking about goals with prevention focuses | 21 | 1 | No significant results for the aging group |
| 5. | Packer and Cunningham, 2009 | Thinking about goals with promotion focuses | Thinking about goals with prevention focuses | 20 | 3 | |
| 6. | D'Argembeau et al., 2010b | Personal future event imagination | Nonpersonal future event imagination | 20 | 5 | |
| 7. | Spreng et al., 2010 | Autobiographical planning | Visuospatial planning | 20 | 24 | Multivariate analyses |
| 8. | Preminger et al., 2011 | Volitional and prospective thinking towards a personal goal | Alphabet backwards task | 15 | 15 | |
| 9. | Spreng and Schacter, 2012 | Autobiographical planning | Visuospatial planning | 36 | 17 | Multivariate analyses/activity common to young and older adults |
| 10. | Gerlach et al., 2014 | Goal simulation | Odd‐even judgment | 28 | 14 | Multivariate analyses |
| Total | 234 | 83 |
Regarding the mind‐wandering domain, we only included studies that had some measure of mind‐wandering: studies in which mind‐wandering was assessed online in the scanner [e.g., Christoff et al., 2009; Stawarczyk et al., 2011], studies in which mind‐wandering was retrospectively assessed after the scanning session [e.g., Dumontheil et al., 2010; McGuire et al., 1996], and studies in which mind‐wandering was measured in conditions similar to those employed during scanning [e.g., Binder et al., 1999; Mason et al., 2007]. Thus, studies on spontaneous thoughts in which mind‐wandering episodes were not explicitly measured were excluded [e.g., Christoff et al., 2004]. Studies on spontaneous thoughts that did not distinguish between mind‐wandering and task‐related thoughts were also excluded [e.g., Spiers and Maguire, 2006a, 2006b]. In addition, we decided to exclude the study by D'Argembeau et al. [2005] because the contrast reported in that study was of a different nature than the contrasts used in other studies of mind‐wandering. Specifically, the tasks to which rest was compared in D'Argembeau et al. [2005] involved (directed) internal thinking and a recent review of the literature [Dixon et al., 2014] has shown that spontaneous versus directed forms of internal cognition recruits highly similar brain regions. Thus, many of the processes associated with mind‐wandering during rest were similarly involved in the control tasks and, consequently, the brain regions supporting these processes were probably subtracted out in the reported contrast. We also excluded the study by Wang et al. [2009], for two reasons. First, this study did not perform voxel‐wise correlations between brain activity and reports of mind‐wandering frequency during scanning, but instead reported correlations with a questionnaire investigating the general tendency to mind‐wander in daily life. Second, and most importantly, the authors did not report all coordinates in their correlation analyses but only regions that also showed greater activity during a control memory task (in which there was the lowest rate of mind‐wandering) compared to rest. Whether the reported coordinates reflect the entire brain activations associated with mind‐wandering is thus highly questionable. Finally, studies involving experienced meditators were excluded [e.g., Hasenkamp et al., 2012], as meditation experience might be associated with differences in mind‐wandering experience and its associated neural correlates [Brewer et al., 2011]. It should be noted, however, that including these latter studies did not alter the general patterns of findings. In total, nine studies were included in the analyses (for details, see Table 3).
Table 3.
Studies included in the meta‐analysis for the mind‐wandering domain
| No | Study | Task | Comparison task | N | Foci | Comments |
|---|---|---|---|---|---|---|
| 1. | McGuire et al., 1996 | Postscan rating of mind‐wandering during various cognitive tasks | 5 | 5 | Correlational analyses | |
| 2. | Binder et al., 1999 | Resting state | Phonetic monitoring task | 30 | 8 | Fourteen other participants reported more mind‐wandering during rest than during the task in a mock MRI scanner |
| 3. | Mason et al., 2007 | Resting state | Working memory task | 19 | 20 | The participants reported more mind‐wandering during rest than the task outside the scanner |
| 4. | Christoff et al., 2009 | Periods of mind‐wandering | Periods of on‐task focus | 15 | 17 | |
| 5. | Dumontheil et al., 2010 | Low demanding tasks | Higher demanding tasks | 16 | 18 | The Participants retrospectively reported more mind‐wandering in the low demanding tasks after scanning |
| 6. | Vanhaudenhuyse et al., 2011 | Rating of internal awareness during the resting state | 22 | 4 | Correlational analyses | |
| 7. | Stawarczyk et al., 2011 | Periods of mind‐wandering | Periods of on‐task focus | 22 | 21 | |
| 8. | Kucyi et al., 2013 | Periods of attention to something other than pain | Periods of attention to pain | 51 | 17 | |
| 9. | Maillet and Rajah, 2014 | Periods of mind‐wandering | Periods of on‐task focus | 14 | 8 | |
| Total | 194 | 118 |
Data Analysis
All analyses were performed using the Brain Map Application Ginger ALE, Version 2.3 (http://brainmap.org/ale/). Out of the 35 studies included, 23 used the MNI stereotactic space as the standard reference frame. Coordinates for the 12 remaining studies reported in the Talairach space were converted to the MNI stereotactic space using the icbm2tal transformation algorithm [Lancaster et al., 2007].
A detailed description of the procedure underlying ALE meta‐analyses can be found elsewhere [Eickhoff et al., 2009, 2012; Turkeltaub et al., 2002, 2012] and will be only briefly summarized here. In short, in order for ALE analyses to find convergences of 3D peak coordinates across contrasts, the reported foci for each study are modelled as the center of a 3D Gaussian probability distribution whose width is empirically determined and automatically included [Eickhoff et al., 2009]. Probability values of all foci in a particular experiment are calculated and combined for each voxel, resulting in a modelled activation (MA) map that represents a summary of the results of that specific experiment taking into account the spatial uncertainty associated with each reported coordinate. ALE scores are then calculated on a voxel‐by‐voxel basis by taking the union of these individual MA maps and thus reflect the convergence of results at each location. In the present study, a non‐additive ALE method was chosen to restrict the number of inflated ALE values resulting from contrasts with many closely located activation foci. An advantage of this method is to reduce the risk for within‐experiment effects rather than the between‐experiment concordance to be the cause of significant ALE values [Turkeltaub et al., 2012]. Next, the significance of ALE values is assessed using a random‐effects significance test against the null hypothesis that localization of activity is independent between studies. To correct for multiple comparisons, we used cluster‐level inference in the present study. A cluster forming threshold of P < 0.001 (uncorrected at the voxel‐level) was used and the size of the resulting supra‐threshold clusters was compared (with a threshold of P < 0.05) to a null distribution of cluster sizes determined by 5000 random permutations of the data. This latter threshold corresponds to a cluster size identical or >5% of the clusters obtained when applying the above mentioned cluster forming threshold to 5000 ALE maps with random relocations of activation foci within each experiment [Eickhoff et al., 2012].
Next, conjunction analyses were performed to identify the voxels commonly activated across the three domains. These analyses were computed using the voxel‐wise minimum value of the input ALE images such that only significant clusters in the individual analyses were included [Eickhoff et al., 2011]. Finally, the ALE maps were compared between contrasts of interest using the ALE subtraction analyses [Laird et al., 2005]. A particularity of the ALE subtraction analyses is that they are performed on the already thresholded ALE maps and it is therefore advised to use a more lenient threshold than in the individual meta‐analyses to avoid inflating false negative results. As cluster‐level inference is not available in Ginger ALE for meta‐analytic subtractions, 5000 permutations were used in the present study to obtain the null distribution and a threshold of P < 0.05 (uncorrected) was chosen for each analysis with a minimum cluster size of 200 mm³. This cluster size of 200 mm³ corresponds to the minimal size determined by the cluster‐level inference for the individual meta‐analyses (see Table 4). Using smaller minimal cluster size for the subtraction analyses did not change the general pattern of findings.
Table 4.
Peaks of activation for the EFT, personal goal, and mind‐wandering domains
| MNI coordinates | |||||
|---|---|---|---|---|---|
| Vol. (mm³) | X | Y | Z | Studies contributing to cluster | |
| EFT | |||||
| mPFC | 1912 | −2 | 58 | −4 | 1, 2, 4–6, 11, 12, 14, 16 |
| −8 | 46 | −12 | |||
| PCC/Rsp/PHC | 4616 | −4 | −46 | 34 | 1–3, 5–8, 10–16 |
| −8 | −58 | 24 | |||
| 8 | −52 | 12 | |||
| 16 | −46 | 0 | |||
| R pIPL | 2048 | 50 | −62 | 30 | 4, 6, 8–13, 15, 16, |
| L pIPL | 2480 | −48 | −68 | 30 | 2, 4–6, 8–10, 12, 13, 16 |
| R PHC/Hippocampus | 2440 | 26 | −38 | −10 | 2, 3, 6, 7, 10–12, 16 |
| 26 | −18 | −22 | |||
| L posterior PHC | 2224 | −24 | −40 | −12 | 3, 6, 7, 10–13, 16 |
| L anterior PHC/Hippocampus | 880 | −22 | −16 | −20 | 1, 6, 7, 10, 12 |
| R mid. temporal gyrus | 1960 | 58 | −6 | −18 | 2, 4, 8, 10, 12–14, 16 |
| L mid. temporal gyrus | 1704 | −58 | −6 | −18 | 1, 2, 6, 10, 12–14, 16 |
| R mid./sup. frontal gyrus | 632 | 22 | 30 | 44 | 1, 14–16 |
| L mid./sup. frontal gyrus | 1296 | −22 | 32 | 46 | 4, 8, 10–12, 14 |
| Personal Goals | |||||
| mPFC | 2408 | −4 | 50 | −10 | 1, 3–7, 9 |
| Dorsal mPFC | 784 | −6 | 58 | 14 | 5, 7, 9 |
| PCC | 336 | −8 | −46 | 36 | 8, 9 |
| R pIPL | 408 | 48 | −60 | 24 | 8, 10 |
| L pIPL | 896 | −50 | −72 | 40 | 1, 6, 10 |
| R anterior PHC/amygdala | 312 | 24 | −10 | −22 | 7, 9 |
| L mid. temporal gyrus | 528 | −54 | −8 | −22 | 7, 9 |
| L sup. frontal gyrus | 600 | −12 | 34 | 52 | 6, 7, 9 |
| R inf. frontal gyrus | 392 | 52 | 32 | −4 | 7, 9 |
| L inf. frontal gyrus | 312 | −52 | 26 | 14 | 10, 8 |
| L orbital inf. frontal gyrus | 392 | −40 | 28 | −16 | 9, 10 |
| L caudate nucleus | 408 | −12 | 12 | 14 | 7, 10 |
| Mind‐wandering | |||||
| mPFC | 384 | −2 | 58 | −4 | 7, 8 |
| PCC/Precuneus | 792 | −6 | −50 | 40 | 2–4, 7 |
| −8 | −54 | 28 | |||
| L pIPL | 328 | −44 | −70 | 32 | 2, 7 |
| L posterior PHC | 1032 | −28 | −40 | −14 | 2, 3, 7, 8 |
| L Rsp/posterior PHC | 376 | −12 | −44 | 6 | 6, 7, 8 |
| L mid. Temporal gyrus | 272 | −56 | −16 | −22 | 7, 8 |
Note: L: left; R: right; mPFC: medial prefrontal cortex; PCC: posterior cingulate cortex; pIPL: posterior inferior parietal lobule; Rsp: restrosplenial cortex; PHC: parahippocampal; FG: frontal gyrus. The minimal cluster sizes determined by cluster‐level inferences were, respectively, 400 mm³ for EFT, 224 mm³ for personal goals, and 272 mm³ for mind‐wandering. The numbers reported for the studies contributing to each cluster correspond to the numbers associated with each study in Tables 1, 2, 3.
All the activations presented below are reported in MNI coordinates and overlaid on a MNI‐normalized template (Colin27_T1_seg_MNI.nii) using Mango (http://www.nitrc.org/projects/mango) and MRIcroGL (http://www.mccauslandcenter.sc.edu/mricrogl/).
RESULTS
Individual Meta‐Analyses
To examine the brain regions associated with EFT, personal goal processing, and mind‐wandering, we first performed individual ALE meta‐analyses for each domain of investigation considered individually.
Episodic future thinking
The meta‐analysis of activation foci associated with EFT revealed significant convergence of activation in 11 clusters. These clusters were located in the mPFC and PCC extending into the right Rsp and posterior parahippocampal cortices (PHC), as well as bilaterally in the anterior PHC extending into the hippocampi, pIPL, middle temporal gyri, and middle/superior frontal gyri (see Table 4, Fig. 1).
Figure 1.
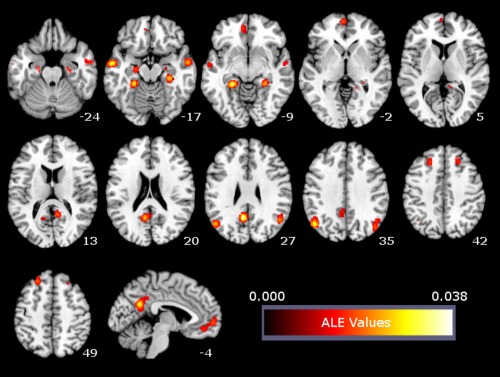
ALE meta‐analysis map for the EFT domain. P < 0.05 (cluster‐level corrected inference using P < 0.001 uncorrected at voxel‐level as the cluster‐forming threshold). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Personal goal processing
For personal goal processing, the meta‐analysis identified 12 clusters of significant activation likelihood. As expected, several of these clusters were located in areas similar to those found for EFT, including the mPFC, PCC, right PHC, left middle temporal gyrus, left superior frontal gyrus, and bilateral pIPL. In addition, this analysis also revealed clusters of activation likelihood in the left caudate nucleus and bilaterally in the inferior frontal gyri (IFG; see Table 4, Fig. 2).
Figure 2.
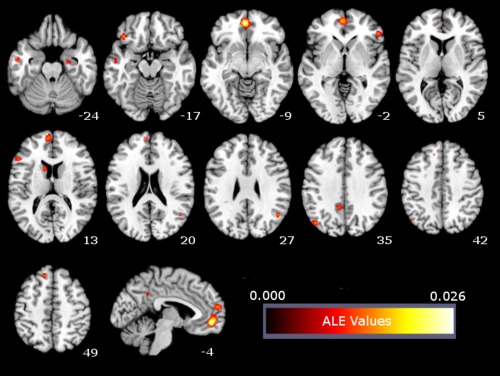
ALE meta‐analysis map for the personal goal domain. P < 0.05 (cluster‐level corrected inference using P < 0.001 uncorrected at voxel‐level as the cluster‐forming threshold). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Mind‐wandering
The meta‐analysis of brain activity related to mind‐wandering yielded six clusters of significant activation likelihood in areas highly similar to those found for EFT and personal goal processing. These areas included the mPFC, PCC/precuneus, left pIPL, left Rsp/PHC, and left middle temporal gyrus (see Table 4, Fig. 3).
Figure 3.
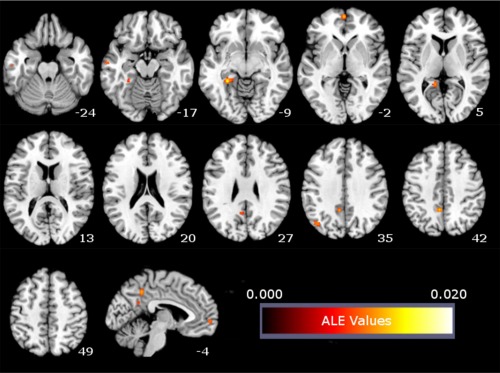
ALE meta‐analysis map for the mind‐wandering domain. P < 0.05 (cluster‐level corrected inference using P < 0.001 uncorrected at voxel‐level as the cluster‐forming threshold). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Conjunction Analyses
We next performed conjunction analyses to examine the extent to which the clusters of significant activation likelihood found for each domain of investigation overlapped. In its current version, Ginger ALE does not permit to perform conjunction analyses between more than two ALE images. We therefore computed conjunction analyses for each combination of domains considered two‐by‐two and then overlaid the three sets of results on the same template to visually examine the potential overlaps between all of them. As expected, substantial overlap was found between the different domains. More specifically, the conjunction analyses revealed common activations in (1) the mPFC, PCC, left pIPL, left PHC, and left middle temporal gyrus for EFT and mind‐wandering, (2) the mPFC, PCC, bilateral pIPL, right PHC, and left middle temporal gyrus for EFT and personal goals,2 and (3) the mPFC, PCC, and left pIPL for personal goals and mind‐wandering (see Table 5, Fig. 4). Overlaying these maps on a normalized brain revealed overlaps between the three domains in the mPFC, PCC, and left pIPL (Fig. 4).
Table 5.
Correspondence across the domains considered two‐by‐two
| Vol. (mm³) | MNI coordinates | |||
|---|---|---|---|---|
| X | Y | Z | ||
| EFT and MW | ||||
| mPFC | 312 | −2 | 58 | −4 |
| PCC | 344 | −6 | −50 | 36 |
| −8 | −54 | 28 | ||
| L pIPL | 200 | −44 | −70 | 32 |
| L post. PHC | 912 | −28 | −40 | −14 |
| L mid. temporal gyrus | 64 | −62 | −12 | −18 |
| EFT and PG | ||||
| mPFC | 832 | −6 | 48 | −12 |
| −2 | 56 | −6 | ||
| PCC | 168 | −8 | −50 | 36 |
| R pIPL | 232 | 48 | −60 | 24 |
| L pIPL | 312 | −44 | −72 | 32 |
| R PHC | 48 | 24 | −12 | −22 |
| L mid. temporal gyrus | 192 | −54 | −6 | −22 |
| MW and PG | ||||
| mPFC | 184 | −2 | 56 | −4 |
| PCC | 192 | −8 | −50 | 36 |
| L pIPL | 176 | −44 | −70 | 32 |
Note: L: left; R: right; EFT: episodic future thinking; MW: mind‐wandering; PG: personal goals; mPFC: medial prefrontal cortex; PCC: posterior cingulate cortex; pIPL: posterior inferior parietal lobule; PHC: parahippocampal cortex.
Figure 4.
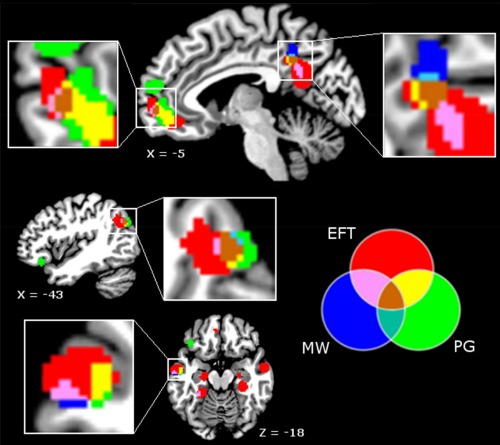
Correspondence across meta‐analysis maps in the mPFC, PCC, left pIPL, and lateral temporal cortex. P < 0.05 (cluster‐level corrected inference using P < 0.001 uncorrected at voxel‐level as the cluster‐forming threshold); EFT: episodic future thinking; PG: personal goals; MW: mind‐wandering. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Subtraction Analyses
Finally, we aimed at examining the brain activations that were specifically associated with EFT, personal goal processing, and mind‐wandering. The current version of Ginger ALE does not support contrasts between more than two ALE datasets. We therefore performed the subtractions analyses for each combination of domains considered two‐by‐two. These meta‐analytic subtractions revealed clusters of larger activations for EFT relative to the two other domains, specifically (1) in the PCC extending into the right Rsp and PHC, left PHC and hippocampus, bilateral pIPL, bilateral temporal gyrus, and bilateral middle/superior frontal gyrus for EFT compared to personal goals and (2) in the PCC, right Rsp extending into the PHC, bilateral pIPL, bilateral temporal gyrus, and left middle/superior frontal gyrus for EFT compared with mind‐wandering (see Table 6, Fig. 5). Interestingly, we also found that the mPFC was more activated for personal goals than both EFT and mind‐wandering (Table 6). Finally, two clusters in the left Rsp and PHC were more activated during mind‐wandering than personal goal processing (Table 6). No cluster was more activated during mind‐wandering than EFT.
Table 6.
Peaks of activation of meta‐analytic subtractions across the domains considered two‐by‐two
| Vol. (mm³) | MNI coordinates | |||
|---|---|---|---|---|
| X | Y | Z | ||
| EFT > PG | ||||
| PCC/R Rsp/R post. PHC | 2936 | −8 | −62 | 28 |
| 4 | −54 | 12 | ||
| 14 | −42 | 0 | ||
| R pIPL | 664 | 42 | −66 | 32 |
| L pIPL | 1000 | −54 | −70 | 24 |
| R ant. PHC | 1472 | 30 | −32 | −12 |
| L PHC | 2184 | −20 | −38 | −14 |
| L ant. PHC/Hippocampus | 560 | −18 | −12 | −20 |
| R mid. temporal gyrus | 768 | 64 | −4 | −14 |
| L mid. temporal gyrus | 880 | −62 | −4 | −16 |
| R mid./sup. frontal gyrus | 408 | 22 | 26 | 42 |
| L mid./sup. frontal gyrus | 920 | −24 | 34 | 42 |
| PG > EFT | ||||
| mPFC | 648 | 0 | 50 | −8 |
| EFT > MW | ||||
| PCC | 464 | −10 | −58 | 18 |
| R Rsp/PHC | 592 | 8 | −46 | 12 |
| 18 | −46 | 1 | ||
| R PHC | 640 | 26 | −32 | −20 |
| R pIPL | 1808 | 44 | −62 | 32 |
| L pIPL | 920 | −48 | −62 | 26 |
| R mid. temporal gyrus | 1848 | 58 | −6 | −18 |
| L mid. Temporal gyrus | 672 | −58 | −2 | −20 |
| L mid./sup. frontal gyrus | 624 | −24 | 28 | 48 |
| MW > EFT | ||||
| No cluster found | ||||
| PG > MW | ||||
| mPFC | 632 | 0 | 50 | −8 |
| Dorsal mPFC | 248 | −8 | 62 | 16 |
| MW > PG | ||||
| L Rsp/PHC | 248 | −10 | −44 | 4 |
| L PHC | 984 | −26 | −44 | −10 |
Note: L: left; R: right; EFT: episodic future thinking; MW: mind‐wandering; PG: personal goal; PCC: posterior cingulate cortex; pIPL: posterior inferior parietal lobe; Rsp: retrosplenial cortex; PHC: parahippocampal cortex.
Figure 5.
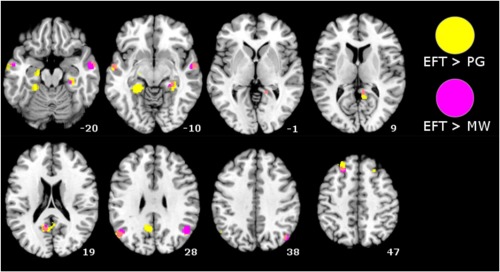
Results of meta‐analytic subtractions between EFT and personal goals/mind‐wandering. P < 0.05 (uncorrected for multiple comparison) with a minimal cluster size of 200 mm³; EFT: episodic future thinking; PG: personal goals; MW: mind‐wandering. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
EFT is a complex mental faculty that depends on an ensemble of cognitive processes supported by an extended set of brain regions. Our main aim here was to test the hypothesis that personal goal processing is one important component of EFT and to identify its specific neural correlates. Furthermore, we also sought to determine the contribution of goal processing and future thinking in spontaneous forms of self‐generated thought. To address these questions, we performed a systematic literature search of functional neuroimaging studies on EFT, personal goal processing, and mind‐wandering before computing three distinct ALE meta‐analyses of the peak activation foci reported for each of these areas of research. We then performed conjunction analyses to determine the overlaps between the clusters identified in each meta‐analysis, as well as subtraction analyses to identify the brain regions that are more specifically associated with each domain.
As expected, for each domain, the ALE meta‐analyses revealed clusters of significant activation likelihood in several regions of the DN [Buckner et al., 2008; Raichle et al., 2001], including the mPFC, PCC, pIPL, as well as medial and lateral temporal regions. Many studies have shown that the DN is generally more active at rest than during cognitive tasks that require focused attention on external stimuli [e.g., Mazoyer et al., 2001; Shulman et al., 1997], and these findings have led to the view that the DN is a “task negative” network, implying that it would not contribute to active, goal‐directed cognition [for further discussion, see Spreng, 2012]. However, this view has recently been challenged by a number of studies showing that the DN is indeed engaged in many active cognitive tasks, provided that they are internally focused, such as autobiographical memory, future thinking, self‐reference, and social cognition [e.g., Buckner et al., 2008; Kim, 2012; Qin and Northoff, 2011; Schacter et al., 2012; Schilbach et al., 2008; Spreng et al., 2009; Van Overwalle, 2009]. This suggests that the DN is not antithetical to goal‐directed cognition per se but instead is characterized by the type of information processing it supports: this network would play a general role in self‐generated thought, that is, the capacity to generate mental contents that are not derived directly from immediate perceptual input [see Andrews‐Hanna, 2012; Andrews‐Hanna et al., 2014b; Klinger, 2009; Schooler et al., 2011; Smallwood et al., 2012; Spreng, 2012]. The present results support this view and further demonstrate that the DN contributes to personal goal‐related processes during both experimentally induced self‐generated thoughts (such as in EFT studies) and thoughts that are spontaneously generated when our mind wanders.
Crucially, our conjunction analyses revealed that EFT, personal goal processing, and mind‐wandering were associated with overlapping activation in the mPFC, and the direct contrast between the three domains showed that activity in this region was highest for personal goal processing. This activation profile supports the hypothesis that the mPFC plays a key role in the processing of personal goals and that such processing is an important component of both EFT and mind‐wandering. The exact processing operations that are mediated by the mPFC in relation to personal goals remain to be fully specified, however. One possibility is that this region contributes to the subjective appraisal of the personal relevance of mental contents, resulting in an abstract representation of their affective meaning or value that may be of critical importance to guide later thoughts, decisions, and behaviors [D'Argembeau, 2013; Northoff and Hayes, 2011; Northoff et al., 2006; Roy et al., 2012; Schmitz and Johnson, 2007]. This account is, for example, supported by the finding that activity in the mPFC increases in a linear fashion with the personal importance of the mental representations under consideration [Andrews‐Hanna et al., 2010; D'Argembeau et al., 2012]. In the particular case of EFT, the mPFC may contribute to appraise and code the relevance of represented events with respect to personal goals, which might then modulate one's motivation and effort to attain the imagined state of affairs [D'Argembeau et al., 2010b]. This idea that the motivational impact of future thinking might be mediated by the mPFC is supported by recent research on the role of EFT and mind‐wandering in economic decision making. It has indeed been shown that engaging in EFT [Lin and Epstein, 2014] or mind‐wandering [Smallwood et al., 2013] is associated with advantageous decision making for monetary rewards with higher long‐term pay‐offs during economic choice tasks. Furthermore, neuroimaging studies have revealed that this effect is mediated by neural activity and cortical thickness within the mPFC, indicating that this region might indeed play a direct role in adaptive decision making processes [Benoit et al., 2011; Bernhardt et al., 2014; Peters and Buchel, 2010].
Another, not necessarily mutually exclusive, possibility is that the mPFC mediates the integration of imagined experiences within higher‐order autobiographical knowledge structures. It has indeed been suggested that the mPFC contributes to the creation of abstract knowledge or schemas derived from extracted regularities in episodic experiences, and mediates the integration of novel experiences into these pre‐existing networks of knowledge [Brod et al., 2013; Kroes and Fernandez, 2012; Preston and Eichenbaum, 2013; van Kesteren et al., 2012]. In the particular case of EFT, such integrative processes might play a role in contextualizing imagined events within an individual's life story by linking them to autobiographical knowledge and self‐models [Conway, 2005; D'Argembeau and Mathy, 2011]. Recent behavioral studies indeed suggest that many future events are not represented in isolation, but instead are linked to other related events and form part of higher‐order autobiographical knowledge structures that organize imagined events in broader themes and causal sequences [D'Argembeau and Demblon, 2012; Demblon and D'Argembeau, 2014]. The outcome of this integrative process might be the constitution of an overarching personal meaning of the event currently under consideration (e.g., its place in a particular's individual life story), and it could be that the mPFC contributes to such process. More generally, the integration of anticipated events within autobiographical knowledge structures might contribute to the creation of a continual sense of self‐identity across time that lays the foundation for long‐term motivation, decision making, and planning processes [Metzinger, 2013].
It is also worth noting that the mPFC has been frequently associated with self‐referential processing in the neuroimaging literature [e.g., Northoff et al., 2006; van der Meer et al., 2010; Wagner et al., 2012]. It could therefore be argued that the common activation of the mPFC in the three domains under investigation here reflects self‐processing. While we would certainly not dispute this interpretation, it should be noted that “the self” is a multifaceted construct that depends on multiple processes [Klein, 2012; Klein and Gangi, 2010], and therefore it is important to specify which aspect of self‐processing is under investigation to avoid misinterpretations of neuroimaging findings [Zahavi and Roepstorff, 2011]. In this context, the processing of personal goals can be conceived as one particular kind of self‐processing [McAdams, 2013]. Previous studies have shown that other forms of self‐processing such as the evaluation of one's personality traits and retrieval of autobiographical memories also engage the mPFC [for a recent meta‐analysis, see Martinelli et al., 2013], suggesting that this region contributes to the processing of various self‐related characteristics and experiences. Although this awaits further confirmation, the two processing operations proposed above—appraisal of personal relevance and integration of information within higher‐order knowledge structures—might prove useful in accounting for the involvement of the mPFC across these different self‐related domains.
Besides the mPFC, the second region of the DN that showed overlapping activations between EFT, personal goals, and mind‐wandering was the left pIPL. This brain region is a cross‐modal association area [Seghier, 2013] that is functionally connected to multiple areas of the DN [Andrews‐Hanna et al., 2010, 2014a], and it has been associated with a wide range of cognitive functions, including semantic processing, reading, theory of mind, spatial cognition, working memory, and episodic memory [for reviews, see Cabeza et al., 2012; Olson and Berryhill, 2009; Seghier, 2013]. Several recent theoretical accounts have been proposed to explain the contributions of the pIPL to these different domains, including the maintenance of information in working memory [Vilberg and Rugg, 2008; Wagner et al., 2005], the integration of spatiotemporal knowledge about event concepts [Binder and Desai, 2011], or the feeling of vividness and re‐living that accompanies episodic memory retrieval [Ally et al., 2008; Yazar et al., 2012, 2014]. Another parsimonious theory regarding the functional role of the pIPL is that this region supports bottom‐up attentional processes by which salient information automatically captures attention [Cabeza et al., 2012; Ciaramelli et al., 2008]. Following this view, the common activation of the left pIPL during EFT, personal goal processing, and mind‐wandering that was evidenced in the present meta‐analysis could reflect the bottom‐up capture of attention by the different elements that compose self‐generated thoughts. Further studies should be conducted to test this hypothesis more directly.
The third region in which the three domains showed convergent activations in the present meta‐analysis was the PCC. More specifically, the three domains showed overlapping activity in the dorsal PCC (Z = 36) and we also found overlapping activation in the ventral part of the PCC (Z = 22), but this time only for EFT and mind‐wandering. These differences in overlap between the three domains suggest that distinct regions of the PCC might support different functions, a proposal supported by connectivity studies showing a functional and anatomic fractionation of this area into distinct subregions [Cauda et al., 2010; Leech and Sharp, 2014; Zhang et al., 2014]. In particular, the most ventral part of the PCC, which was associated with EFT and mind‐wandering in the present study, is more strongly associated with the other DN regions and may contribute to episodic memory retrieval [Cauda et al., 2010; Huijbers et al., 2012; Spreng et al., 2013; Zhang et al., 2014]. In contrast recent research suggests that the dorsal part of the PCC is more strongly associated with fronto‐parietal areas involved in attentional control abilities [Leech et al., 2012; Spreng et al., 2013; Vincent et al., 2008]. More specifically, the dorsal PCC might be involved in the control of the attentional balance between internal thoughts and external stimuli [Leech and Sharp, 2014] through the detection of environmental changes [Henseler et al., 2011; Pearson et al., 2011]. Although studies on EFT and reflection on personal goals mostly involve internally self‐generated thoughts, a minimum continuous monitoring of the external environment is nonetheless required during these experiments to follow the task instructions. Furthermore, recent studies on mind‐wandering have shown that the detection of unpredictable external events is not always impaired when the mind wanders [Kam et al., 2013], and might even be faster for frequent mind‐wanderers [Thomson et al., 2015]. A tentative proposal would then be that these external monitoring processes during internal thoughts are supported by the dorsal PCC, although further studies should be conducted to directly assess this proposal.
In addition to the mPFC, PCC, and left pIPL, the three domains also showed convergent (although not perfectly overlapping) activation in the left middle temporal gyrus. This area is commonly activated in autobiographical and semantic memory tasks, and may support the processing of semantic and conceptual information [Binder et al., 2009; Burianova et al., 2010; Svoboda et al., 2006]. There is indeed substantial evidence for the contribution of semantic memory in remembering the past [e.g., Greenberg and Verfaellie, 2010] and imagining the future [Irish et al., 2012)], and it has been suggested that semantic memory may in fact provide the foundation for many, if not all, complex cognitive functions [Binder and Desai, 2011]. The lateral temporal cortex may in particular be involved in representing social [Andrews‐Hanna et al., 2010, 2014a; Denny et al., 2012] and personal [Renoult et al., 2012] semantic information. For example, a recent study has shown that the left temporal cortex is more activated when imagining social than non‐social scenarios during EFT [Szpunar et al., 2014], and another research has found that specific personality traits processed in the left lateral temporal cortex and PCC are assembled in the mPFC, probably to form general personality models that are then used to predict how others are likely to act in different situations [Hassabis et al., 2014]. The left lateral temporal region that was observed here may thus mediate the retrieval of semantic knowledge (including social and personal information) that provides the foundation for representing various forms of self‐generated thoughts.
In addition to common patterns of activation, we also found that EFT, personal goal processing, and mind‐wandering engaged specific brain areas. Notably, EFT significantly activated the ventral PCC, Rsp, PHC, hippocampus, pIPL, and lateral temporal and prefrontal cortices to a larger extent than reflections on personal goals. Most of these regions have been associated with episodic memory retrieval and recent studies suggest that they form a functional subsystem of the DN that supports the construction of detailed mental representations of specific events—episodic simulation or scene construction processes [Andrews‐Hanna et al., 2010, 2014a; Hassabis and Maguire, 2009; Hassabis et al., 2007; Schacter et al., 2007, 2012; Szpunar et al., 2014]. EFT is indeed associated with the subjective experience of detailed scenes involving vivid sensorial and contextual details, as well as the subjective sense of pre‐experiencing the imagined event [D'Argembeau and Van der Linden, 2012; Klein, 2013; Mullally and Maguire, 2013; Schacter et al., 2012]. Such scene construction processes are integral to the generation of specific event representations during EFT. By contrast, personal goal processing can be more abstract in nature [Fishbach and Ferguson, 2007], thus relying on scene construction to a lesser extent, which may explain the difference in brain activation found in the present study for scene construction areas between these two forms of self‐generated thoughts.3
A second prediction that we made regarding the meta‐analytic contrasts was that, given its spontaneous and often involuntary nature [Stawarczyk et al., 2011, 2013], mind‐wandering would recruit brain areas involved in cognitive control to a lesser extent than experimentally directed thoughts. As expected, we found larger activity in the middle/superior frontal gyrus, one of the main regions of the fronto‐parietal control network [Niendam et al., 2012; Spreng et al., 2013; Vincent et al., 2008], during directed EFT compared to mind‐wandering. This result suggests that a difference between directed EFT and mind‐wandering might be the extent to which the construction of self‐generated thoughts requires some effortful cognitive processes. These results are in line with recent neuroimaging findings on involuntary versus voluntary episodic memory retrieval showing a greater involvement of lateral prefrontal regions during voluntary retrieval, with otherwise extensive overlaps in DN activity between voluntary and involuntary memories [Hall et al., 2008, 2014; Kompus et al., 2011]. In the same vein, a recent review of the literature proposed that a key difference in the neural basis of voluntary and involuntary cognition relates to the involvement of the lateral prefrontal cortex [Dixon et al., 2014].
It is important to note, however, that the finding that EFT recruits the lateral prefrontal cortex to a larger extent than mind‐wandering does not necessarily imply that the latter reflects the absence or breakdown of attentional control [McVay and Kane, 2010]. A recent study has indeed shown that transcranial direct current stimulation of the lateral PFC during a monotonous task resulted in an increase in mind‐wandering frequency without reducing task performance [Axelrod et al., [Link]]. Furthermore, neuroimaging findings have revealed that during mind‐wandering episodes, the anterior midline regions show a positive functional connectivity with both the other DN regions and lateral prefrontal areas commonly involved in attentional control abilities [Christoff, 2012]. Another recent meta‐analysis [Fox et al., [Link]] found a cluster of activation in the ventral lateral PFC in association with mind‐wandering and spontaneous thoughts more generally.4 Thus, although we can conclude that EFT recruits some prefrontal control areas to a larger extent than mind‐wandering, it cannot be excluded that some attentional control processes might nonetheless be engaged when the focus of attention switches from the task at hand to internal thoughts during mind‐wandering episodes [for further discussion of this issue, see Smallwood, 2013; Stawarczyk et al., 2014; Unsworth and McMillan, 2014].
In summary, the present meta‐analysis shows that EFT, reflections on personal goals, and mind‐wandering depend on a similar set of brain regions that strongly overlap with the DN of the brain. We have proposed that these common activations reflect the use of similar mental processes in each domain and, most notably, that the mPFC plays a role in integrating or evaluating self‐generated thoughts with respect to personal goals. Furthermore, our results suggest that similar personal goal‐related processes occur in the DN when participants are engaged in the effortful imagination of their personal future and when their mind spontaneously wanders from the current task at hand. These data are in line with the view that the DN of the brain is fundamentally goal‐oriented [Andrews‐Hanna, 2012] and may support the continuous generation and updating of internal predictions that adaptively guide our behaviors in a complex and ever changing environment [Bar et al., 2007; Metzinger, 2013; Raichle, 2011].
ACKNOWLEDGMENTS
The authors would like to thank Dr. Steven Laureys for clarifying the use of whole‐brain analyses in Vanhaudenhuyse et al. [2011], as well as Dr. Nathan Spreng for providing the lists of personal goals used in Spreng et al. [2010] and Spreng and Schacter [2012], and the peak coordinates from Sharot et al. [2007].
Conflict of interest: The authors declare that there are no conflicts of interest.
Footnotes
For instance, the search terms used in Pubmed for the future thinking domain were: “(fmri OR PET OR neuroimaging) and (“future thinking” or “future events” or “prospection”) and (“1990/01/01”[Date ‐ Publication]:“2014/08/31”[Date ‐ Publication]).”
It should be noted that the contrasts selected from three studies included in the personal goals meta‐analysis not only involved goal processing, but also EFT [i.e., Gerlach et al., 2014; Spreng and Schacter, 2012; Spreng et al., 2010]. To examine whether the peaks from these three studies explained the similarities between EFT and personal goal processing in our meta‐analyses, we checked the studies that contributed to each reported cluster (see Table IV). This showed that both groups of studies (i.e., those involving goal processing only and those involving goal processing plus EFT) contributed to the main clusters (i.e., mPFC, PCC, and pIPL) that overlapped between the two domains of investigation. Therefore, the similarities between the EFT and personal goal processing domains cannot simply be explained by the three studies that not only involved personal goal processing but also EFT.
It should be not that the contrasts selected from the three studies included in our meta‐analysis on personal goals that also included EFT [i.e., Gerlach et al., 2014; Spreng and Schacter, 2012; Spreng et al., 2010] probably involved some scene construction processes (in addition to personal goal processing). Our investigation of the differences between EFT and personal goal processing was thus quite conservative, and the increased activation of scene construction areas during EFT can be considered a robust finding.
The difference in prefrontal activation between this and our own meta‐analysis is probably due to differences in the criteria used for study inclusion and statistical threshold. Fox et al. ([Link]) indeed used false discovery rate (FDR) corrections rather than cluster‐level inference for their analyses (for a discussion of the advantages of cluster‐level inferences over FDR, see Eickhoff et al., 2012). However, besides this difference and a few activations in other regions outside the DN (e.g., the insula), the results of the two meta‐analyses are largely consistent.
REFERENCES
- Abraham A, Schubotz RI, von Cramon DY (2008): Thinking about the future versus the past in personal and non‐personal contexts. Brain Res 1233:106–119. [DOI] [PubMed] [Google Scholar]
- Addis DR, Schacter DL (2011): The hippocampus and imagining the future: Where do we stand? Front Human Neurosci 5:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Cheng T, Roberts RP, Schacter DL (2011a): Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus 21:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL (2009): Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia 47:2222–2238. [DOI] [PubMed] [Google Scholar]
- Addis DR, Roberts RP, Schacter DL (2011b): Age‐related neural changes in autobiographical remembering and imagining. Neuropsychologia 49:3656–3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL (2007): Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45:1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Simons JS, McKeever JD, Peers PV, Budson AE (2008): Parietal contributions to recollection: Electrophysiological evidence from aging and patients with parietal lesions. Neuropsychologia 46:1800–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR (2012): The brain's default network and its adaptive role in internal mentation. Neuroscientist 18:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Saxe R, Yarkoni T (2014a): Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting‐state connectivity, and fMRI meta‐analyses. Neuroimage 91:324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Smallwood J, Spreng RN (2014b): The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus JS, Singer JL, Greenberg S (1966): Studies in the stream of consciousness: Experimental enhancement and suppression of spontaneous cognitive processes. Percept Mot Skills 23:399–417. [Google Scholar]
- Austin JT, Vancouver JB (1996): Goal constructs in psychology: Structure, process, and content. Psychol Bull 120:338–375. [Google Scholar]
- Axelrod V, Rees G, Lavidor M, Bar M (2015): Increasing propensity to mind‐wander with transcranial direct current stimulation. Proc Natl Acad Sci USA 112:3314–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Schooler JW (2011): Back to the future: Autobiographical planning and the functionality of mind‐wandering. Conscious Cogn 20:1604–1611. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Mason MF, Fenske M (2007): The units of thought. Hippocampus 17:420–428. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR (2005): The somatic marker hypothesis: A neural theory of economic decision. Games Econ Behav 52:336–372. [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW (2011): A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci 31:6771–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Smallwood J, Tusche A, Ruby FJ, Engen HG, Steinbeis N, Singer T (2014): Medial prefrontal and anterior cingulate cortical thickness predicts shared individual differences in self‐generated thought and temporal discounting. Neuroimage 90:290–297. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH (2011): The neurobiology of semantic memory. Trend Cogn Sci 15:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW (1999): Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci 11:80–95. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL (2009): Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzung A, Denkova E, Manning L (2008): Experiencing past and future personal events: Functional neuroimaging evidence on the neural bases of mental time travel. Brain Cogn 66:202–212. [DOI] [PubMed] [Google Scholar]
- Boyer P (2008): Evolutionary economics of mental time travel? Trends Cogn Sci 12:219–224. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Gray JR, Tang Y‐Y, Weber J, Kober H (2011): Meditation experience is associated with differences in default mode network activity and connectivity. Proc Natl Acad Sci USA 108:20254–20259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod G, Werkle‐Bergner M, Shing YL (2013): The influence of prior knowledge on memory: A developmental cognitive neuroscience perspective. Front Behav Neurosci 7:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC (2007): Self‐projection and the brain. Trends Cogn Sci 11:49–57. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease In: Kingstone A, Miller MB, editors. The Year in Cognitive Neuroscience. Malden: Blackwell Publishing; p 1–38. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, Grady CL (2010): A common functional brain network for autobiographical, episodic, and semantic memory retrieval. Neuroimage 49:865–874. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M (2012): Cognitive contributions of the ventral parietal cortex: An integrative theoretical account. Trends Cogn Sci 16:338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, Geminiani G, D'Agata F, Sacco K, Duca S, Bagshaw AP, Cavanna AE (2010): Functional connectivity of the posteromedial cortex. PLoS One 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian BM, Miles LK, Fung FH, Best S, Macrae CN (2013): The shape of things to come: Exploring goal‐directed prospection. Conscious Cogn 22:471–478. [DOI] [PubMed] [Google Scholar]
- Christoff K (2012): Undirected thought: Neural determinants and correlates. Brain Res 1428:51–59. [DOI] [PubMed] [Google Scholar]
- Christoff K, Ream JM, Gabrieli JDE (2004): Cognitive and neural basis of spontaneous thought processes. Cortex 40:623–630. [DOI] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Schooler JW, Smith R (2009): Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc Natl Acad Sci USA 106:8719–8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Grady CL, Moscovitch M (2008): Top‐down and bottom‐up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia 46:1828–1851. [DOI] [PubMed] [Google Scholar]
- Conway MA (2005): Memory and the self. J Mem Lang 53:594–628. [Google Scholar]
- Conway MA (2009): Episodic memories. Neuropsychologia 47:2305–2313. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A (2012): Autobiographical memory and future thinking In: Berntsen D, Rubin DC, editors. Understanding autobiographical memory: Theories and approaches. Cambridge University Press; p 311–330. [Google Scholar]
- D'Argembeau A (2013): On the role of the ventromedial prefrontal cortex in self‐processing: The valuation hypothesis. Front Hum Neurosci 7:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A (2015): Knolwedge structures involved in episodic future thinking In: Feeney A, Thompson VA, editors. Reasoning as memory. Hove, UK: Psychology Press; p 128–145. [Google Scholar]
- D'Argembeau A, Mathy A (2011): Tracking the construction of episodic future thoughts. J Exp Psychol Gen 140:258–271. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Demblon J (2012): On the representational systems underlying prospection: Evidence from the event‐cueing paradigm. Cognition 125:160–167. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Van der Linden M (2012): Predicting the phenomenology of episodic future thoughts. Conscious Cogn 21:1198–1206. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, Luxen A, Salmon E (2005): Self‐referential reflective activity and its relationship with rest: A PET study. Neuroimage 25:616–624. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Feyers D, Majerus S, Collette F, Van der Linden M, Maquet P, Salmon E (2008): Self‐reflection across time: Cortical midline structures differentiate between present and past selves. Soc Cogn Affect Neurosci 3:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Ortoleva C, Jumentier S, Van der Linden M (2010a): Component processes underlying future thinking. Mem Cognit 38:809–819. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Stawarczyk D, Majerus S, Collette F, Van der Linden M, Feyers D, Maquet P, Salmon E (2010b): The neural basis of personal goal processing when envisioning future events. J Cogn Neurosci 22:1701–1713. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Renaud O, Van der Linden M (2011): Frequency, characteristics, and functions of future‐oriented thoughts in daily life. Appl Cogn Psychol 25:96–103. [Google Scholar]
- D'Argembeau A, Jedidi H, Balteau E, Bahri M, Phillips C, Salmon E (2012): Valuing one's self: medial prefrontal involvement in epistemic and emotive investments in self‐views. Cereb Cortex 22:659–667. [DOI] [PubMed] [Google Scholar]
- Demblon J, D'Argembeau A (2014): The organization of prospective thinking: Evidence of event clusters in freely generated future thoughts. Conscious Cogn 24:75–83. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN (2012): A meta‐analysis of functional neuroimaging studies of self‐ and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci 24:1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Fox KC, Christoff K (2014): A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia 62:321–330. [DOI] [PubMed] [Google Scholar]
- Dumontheil I, Gilbert SJ, Frith CD, Burgess PW (2010): Recruitment of lateral rostral prefrontal cortex in spontaneous and task‐related thoughts. Q J Exp Psychol 63:1740–1756. [DOI] [PubMed] [Google Scholar]
- Eddington KM, Dolcos F, Cabeza R, Krishnan KR, Strauman TJ (2007): Neural correlates of promotion and prevention goal activation: An fMRI study using an idiographic approach. J Cogn Neurosci 19:1152–1162. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009): Coordinate‐based activation likelihood estimation meta‐analysis of neuroimaging data: A random‐effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT (2011): Co‐activation patterns distinguish cortical modules, their connectivity and functional differentiation. Neuroimage 57:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT (2012): Activation likelihood estimation meta‐analysis revisited. Neuroimage 59:2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbach A, Ferguson MJ (2007): The goal construct in social psychology In: Kruglanski AW, Higgins ET, editors. Social psychology: Handbook of basic principles, 2nd ed. New York: Guilford Press; p 490–515. [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews‐Hanna JR, Christoff K (2015): The wandering brain: Meta‐analysis of functional neuroimaging studies of mind‐wandering and related spontaneous thought processes. Neuroimage 111:611–621. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ (1997): Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6:218–229. [DOI] [PubMed] [Google Scholar]
- Gaesser B, Spreng RN, McLelland VC, Addis DR, Schacter DL (2013): Imagining the future: Evidence for a hippocampal contribution to constructive processing. Hippocampus 23:1150–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Gilmore AW, Schacter DL (2011): Solving future problems: Default network and executive activity associated with goal‐directed mental simulations. Neuroimage 55:1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Madore KP, Schacter DL (2014): Future planning: Default network activity couples with frontoparietal control network and reward‐processing regions during process and outcome simulations. Soc Cogn Affect Neurosci 9:1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Verfaellie M (2010): Interdependence of episodic and semantic memory: Evidence from neuropsychology. J Int Neuropsychol Soc 16:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grysman A, Prabhakar J, Anglin SM, Hudson JA (2013): The time travelling self: Comparing self and other in narratives of past and future events. Conscious Cogn 22:742–755. [DOI] [PubMed] [Google Scholar]
- Hall NM, Gjedde A, Kupers R (2008): Neural mechanisms of voluntary and involuntary recall: A PET study. Behav Brain Res 186:261–272. [DOI] [PubMed] [Google Scholar]
- Hall SA, Rubin DC, Miles A, Davis SW, Wing EA, Cabeza R, Berntsen D (2014): The neural basis of involuntary episodic memories. J Cogn Neurosci 26:2385–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson‐Mendenhall CD, Duncan E, Barsalou LW (2012): Mind wandering and attention during focused meditation: A fine‐grained temporal analysis of fluctuating cognitive states. Neuroimage 59:750–760. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Maguire EA (2009): The construction system of the brain. Philos Trans R Soc London Ser B 364:1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA (2007): Using imagination to understand the neural basis of episodic memory. J Neurosci 27:14365–14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Spreng RN, Rusu AA, Robbins CA, Mar RA, Schacter DL (2014): Imagine all the people: How the brain creates and uses personality models to predict behavior. Cereb Cortex 24:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henseler I, Kruger S, Dechent P, Gruber O (2011): A gateway system in rostral PFC? Evidence from biasing attention to perceptual information and internal representations. Neuroimage 56:1666–1676. [DOI] [PubMed] [Google Scholar]
- Huijbers W, Vannini P, Sperling RA, C MP, Cabeza R, Daselaar SM (2012): Explaining the encoding/retrieval flip: Memory‐related deactivations and activations in the posteromedial cortex. Neuropsychologia 50:3764–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, Piguet O (2012): Considering the role of semantic memory in episodic future thinking: Evidence from semantic dementia. Brain 135(Pt 7):2178–2191. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Sherman SJ (1990): Constructing and reconstructing the past and the future in the present In: Higgins ET, Sorrentino RM, editors. Handbook of motivation and cognition: Foundations of social behavior, Vol 2. New York, NY: Guilford Press; US; p 482–526. [Google Scholar]
- Johnson MK, Raye CL, Mitchell KJ, Touryan SR, Greene EJ, Nolen‐Hoeksema S (2006): Dissociating medial frontal and posterior cingulate activity during self‐reflection. Soc Cogn Affect Neurosci 1:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MK, Nolen‐Hoeksema S, Mitchell KJ, Levin Y (2009): Medial cortex activity, self‐reflection and depression. Soc Cogn Affect Neurosci 4:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam JW, Dao E, Stanciulescu M, Tildesley H, Handy TC (2013): Mind wandering and the adaptive control of attentional resources. J Cogn Neurosci 25:952–960. [DOI] [PubMed] [Google Scholar]
- Kim H (2012): A dual‐subsystem model of the brain's default network: Self‐referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage 61:966–977. [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Kantola JH, Jauhiainen J, Hyvarinen A, Tervonen O (2003): Independent component analysis of nondeterministic fMRI signal sources. Neuroimage 19(2 Pt 1):253–260. [DOI] [PubMed] [Google Scholar]
- Klein SB (2012): The self and science: Is it time for a new approach to the study of human experience? Curr Dir Psychol Sci 21:253–257. [Google Scholar]
- Klein SB (2013): The complex act of projecting oneself into the future. Wiley Interdisciplinary Reviews: Cognitive Science 4:63–79. [DOI] [PubMed] [Google Scholar]
- Klein SB, Gangi CE (2010): The multiplicity of self: Neuropsychological evidence and its implications for the self as a construct in psychological research. Ann N Y Acad Sci 1191:1–15. [DOI] [PubMed] [Google Scholar]
- Klinger E (1978): Modes of normal conscious flow In: Pope KS, Singer JL, editors. The stream of consciousness. New York: Plenum; p 225–258. [Google Scholar]
- Klinger E (2009): Daydreaming and fantasizing: Thought flow and motivation In: Markman KD, Klein WMP, Suhr JA, editors. Handbook of imagination and mental simulation. New York: Psychology Press; p 225–239. [Google Scholar]
- Klinger E (2013): Goal commitments and the content of thoughts and dreams: Basic principles. Front Psychol 4:415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kompus K, Eichele T, Hugdahl K, Nyberg L (2011): Multimodal imaging of incidental retrieval: The low route to memory. J Cogn Neurosci 23:947–960. [DOI] [PubMed] [Google Scholar]
- Kroes MC, Fernandez G (2012): Dynamic neural systems enable adaptive, flexible memories. Neurosci Biobehav Rev 36:1646–1666. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Salomons TV, Davis KD (2013): Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA 110:18692–18697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT (2005): ALE meta‐analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp 25:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. (2014): The role of the posterior cingulate cortex in cognition and disease. Brain 137(Pt 1):12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Braga R, Sharp DJ (2012): Echoes of the brain within the posterior cingulate cortex. J Neurosci 32:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Epstein LH (2014): Living in the moment: Effects of time perspective and emotional valence of episodic thinking on delay discounting. Behav Neurosci 128:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet D, Rajah MN (2014): Dissociable roles of default‐mode regions during episodic encoding. Neuroimage 89:244–255. [DOI] [PubMed] [Google Scholar]
- Martin VC, Schacter DL, Corballis MC, Addis DR (2011): A role for the hippocampus in encoding simulations of future events. Proc Natl Acad Sci USA 108:13858–13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli P, Sperduti M, Piolino P (2013): Neural substrates of the self‐memory system: New insights from a meta‐analysis. Hum Brain Mapp 34:1515–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masicampo EJ, Baumeister RF (2011): Consider it done! Plan making can eliminate the cognitive effects of unfulfilled goals. J Personal Soc Psychol 101:667–683. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN (2007): Wandering minds: The default network and stimulus‐independent thought. Science 315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio‐Mazoyer N (2001): Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull 54:287–298. [DOI] [PubMed] [Google Scholar]
- McAdams DP (2013): The psychological self as actor, agent, and author. Perspect Psychol Sci 8:272–295. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD (1996): Brain activity during stimulus independent thought. Neuroreport 7:2095–2099. [PubMed] [Google Scholar]
- McVay JC, Kane MJ (2010): Does mind wandering reflect executive function or executive failure? Comment on Smallwood and Schooler (2006) and watkins (2008). Psychol Bull 136:188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ (2013): Dispatching the wandering mind? Toward a laboratory method for cuing "spontaneous" off‐task thought. Front Psychol 4:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzinger T (2013): The myth of cognitive agency: Subpersonal thinking as a cyclically recurring loss of mental autonomy. Front Psychol 4:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Ebner NC, Tubridy SM, Frankel H, Johnson MK (2009): Age‐group differences in medial cortex activity associated with thinking about self‐relevant agendas. Psychol Aging 24:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA (2013): Memory, imagination, and predicting the future: A common brain mechanism? Neuroscientist 20:2020–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS (2012): Meta‐analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Hayes DJ (2011): Is our self nothing but reward? Biol Psychiatry 69:1019–1025. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J (2006): Self‐referential processing in our brain–a meta‐analysis of imaging studies on the self. Neuroimage 31:440–457. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A (2003): Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. Neuroimage 19:1369–1380. [DOI] [PubMed] [Google Scholar]
- Olson IR, Berryhill M (2009): Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiol Learn Mem 91:155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer DJ, Cunningham WA (2009): Neural correlates of reflection on goal states: The role of regulatory focus and temporal distance. Soc Neurosci 4:412–425. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML (2011): Posterior cingulate cortex: Adapting behavior to a changing world. Trends Cogn Sci 15:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Buchel C (2010): Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal‐mediotemporal interactions. Neuron 66:138–148. [DOI] [PubMed] [Google Scholar]
- Preminger S, Harmelech T, Malach R (2011): Stimulus‐free thoughts induce differential activation in the human default network. Neuroimage 54:1692–1702. [DOI] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H (2013): Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23:R764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Northoff G (2011): How is our self related to midline regions and the default‐mode network? Neuroimage 57:1221–1233. [DOI] [PubMed] [Google Scholar]
- Raichle ME (2011): The restless brain. Brain Connect 1:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoult L, Davidson PS, Palombo DJ, Moscovitch M, Levine B (2012): Personal semantics: At the crossroads of semantic and episodic memory. Trends Cogn Sci 16:550–558. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD (2012): Ventromedial prefrontal‐subcortical systems and the generation of affective meaning. Trends Cogn Sci 16:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL (2012): Adaptive constructive processes and the future of memory. Am Psychol 67:603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR (2007): The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos Trans R Soc London Ser B 362:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL (2007): Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci 8:657–661. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL (2008): Episodic simulation of future events: Concepts, data, and applications In: Kingstone A, Miller MB, editors. The year in cognitive neuroscience 2008. Malden: Blackwell Publishing; p 39–60. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK (2012): The future of memory: remembering, imagining, and the brain. Neuron 76:677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska‐Jagiela A, Fink GR, Vogeley K (2008): Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the "default system" of the brain. Conscious Cogn 17:457–467. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC (2007): Relevance to self: A brief review and framework of neural systems underlying appraisal. Neurosci Biobehav Rev 31:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooler JW, Smallwood J, Christoff K, Handy TC, Reichle ED, Sayette MA (2011): Meta‐awareness, perceptual decoupling and the wandering mind. Trends Cogn Sci 15:319–326. [DOI] [PubMed] [Google Scholar]
- Seghier ML (2013): The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist 19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman ME, Railton P, Baumeister RF, Sripada C (2013): Navigating into the future or driven by the past. Perspect Psychol Sci 8:119–141. [DOI] [PubMed] [Google Scholar]
- Sharot T, Riccardi AM, Raio CM, Phelps EA (2007): Neural mechanisms mediating optimism bias. Nature 450:102–105. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE (1997): Common blood flow changes across visual tasks: II: Decreases in cerebral cortex. J Cogn Neurosci 9:648–663. [DOI] [PubMed] [Google Scholar]
- Singer JL (1993): Experimental studies of ongoing conscious experience. Ciba Found Symp 174:100–122. [PubMed] [Google Scholar]
- Smallwood J (2013): Distinguishing how from why the mind wanders: A process‐occurrence framework for self‐generated mental activity. Psychol Bull 139:519–535. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW (2006): The restless mind. Psychol Bull 132:946–958. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW (2012): Cooperation between the default mode network and the frontal‐parietal network in the production of an internal train of thought. Brain Res 1428:60–70. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Ruby FJ, Singer T (2013): Letting go of the present: Mind‐wandering is associated with reduced delay discounting. Conscious Cogn 22:1–7. [DOI] [PubMed] [Google Scholar]
- Song X, Wang X (2012): Mind wandering in Chinese daily lives ‐ an experience sampling study. PLoS ONE 7:e44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA (2006a): Spontaneous mentalizing during an interactive real world task: An fMRI study. Neuropsychologia 44:1674–1682. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA (2006b): Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage 31:1826–1840. [DOI] [PubMed] [Google Scholar]
- Spreng RN (2012): The fallacy of a "task‐negative" network. Front Psychol 3:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady CL (2010): Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci 22:1112–1123. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Schacter DL (2012): Default network modulation and large‐scale network interactivity in healthy young and old adults. Cerebral Cortex 22:2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS (2009): The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta‐analysis. J Cogn Neurosci 21:489–510. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL (2013): Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. J Cogn Neurosci 25:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL (2010): Default network activity, coupled with the frontoparietal control network, supports goal‐directed cognition. Neuroimage 53:303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Cassol H, D'Argembeau A (2013): Phenomenology of future‐oriented mind‐wandering episodes. Front Psychol 4:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Catale C, D'Argembeau A (2014): Relationships between mind‐wandering and attentional control abilities in young adults and adolescents. Acta Psychol 148:25–36. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maj M, Van der Linden M, D'Argembeau A (2011a): Mind‐wandering: phenomenology and function as assessed with a novel experience sampling method. Acta Psychol 136:370–381. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, D'Argembeau A (2011b): Neural correlates of ongoing conscious experience: Both task‐unrelatedness and stimulus‐independence are related to default network activity. PLoS One 6:e16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauman TJ, Detloff AM, Sestokas R, Smith DV, Goetz EL, Rivera C, Kwapil L (2012): What shall I be, what must I be: Neural correlates of personal goal activation. Front Integr Neurosci 6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Addis DR, Corballis MC (2009): Mental time travel and the shaping of the human mind. Philos Trans R Soc London Ser B 364:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B (2006): The functional neuroanatomy of autobiographical memory: A meta‐analysis. Neuropsychologia 44:2189–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK (2010): Episodic future thought: An emerging concept. Perspect Psychol Sci 5:142–162. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, Watson JM, McDermott KB (2007): Neural substrates of envisioning the future. Proc Natl Acad Sci USA 104:642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpunar KK, Chan JC, McDermott KB (2009): Contextual processing in episodic future thought. Cereb Cortex 19:1539–1548. [DOI] [PubMed] [Google Scholar]
- Szpunar KK, St Jacques PL, Robbins CA, Wig GS, Schacter DL (2014): Repetition‐related reductions in neural activity reveal component processes of mental simulation. Soc Cogn Affect Neurosci 9:712–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson DR, Ralph BC, Besner D DS (2015): The more your mind wanders, the smaller your attentional blink: An individual differences study. Q J Exp Psychol 68:181–191. [DOI] [PubMed] [Google Scholar]
- Tulving E (2005): Episodic memory and autonoesis: Uniquely human? In: Terrace HS, Metcalfe J, editors. The missing link in cognition: Origins of self‐reflective consciousness. New York: Oxford University Press; p 3–56. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA (2002): Meta‐analysis of the functional neuroanatomy of single‐word reading: method and validation. Neuroimage 16(3 Pt 1):765–780. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P (2012): Minimizing within‐experiment and within‐group effects in activation likelihood estimation meta‐analyses. Hum Brain Mapp 33:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, McMillan BD (2014): Similarities and differences between mind‐wandering and external distraction: A latent variable analysis of lapses of attention and their relation to cognitive abilities. Acta Psychol 150:14–25. [DOI] [PubMed] [Google Scholar]
- Van Hoeck N, Ma N, Ampe L, Baetens K, Vandekerckhove M, Van Overwalle F (2013): Counterfactual thinking: An fMRI study on changing the past for a better future. Soc Cogn Affect Neurosci 8:556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren MT, Ruiter DJ, Fernandez G, Henson RN (2012): How schema and novelty augment memory formation. Trends Neurosci 35:211–219. [DOI] [PubMed] [Google Scholar]
- van Mulukom V, Schacter DL, Corballis MC, Addis DR (2013): Re‐imagining the future: Repetition decreases hippocampal involvement in future simulation. PLoS ONE [Electronic Resource] 8:e69596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F (2009): Social cognition and the brain: A meta‐analysis. Hum Brain Mapp 30:829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, Phillips C, Soddu A, Luxen A, Moonen G, Laureys S (2011): Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci 23:570–578. [DOI] [PubMed] [Google Scholar]
- Viard A, Chetelat G, Lebreton K, Desgranges B, Landeau B, de La Sayette V, Eustache F, Piolino P (2011): Mental time travel into the past and the future in healthy aged adults: An fMRI study. Brain Cogn 75:1–9. [DOI] [PubMed] [Google Scholar]
- Viard A, Desgranges B, Eustache F, Piolino P (2012): Factors affecting medial temporal lobe engagement for past and future episodic events: An ALE meta‐analysis of neuroimaging studies. Brain Cogn 80:111–125. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD (2008): Memory retrieval and the parietal cortex: A review of evidence from a dual‐process perspective. Neuropsychologia 46:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL (2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9:445–453. [DOI] [PubMed] [Google Scholar]
- Wang K, Yu C, Xu L, Qin W, Li K, Jiang T (2009): Offline memory reprocessing: Involvement of the brain's default network in spontaneous thought processes. PLoS One 4:e4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler JA, Suchan B, Daum I (2010): When the future becomes the past: Differences in brain activation patterns for episodic memory and episodic future thinking. Behav Brain Res 212:196–203. [DOI] [PubMed] [Google Scholar]
- Yazar Y, Bergstrom ZM, Simons JS (2012): What is the parietal lobe contribution to long‐term memory? Cortex 48:1381–1382. [DOI] [PubMed] [Google Scholar]
- Yazar Y, Bergstrom ZM, Simons JS (2014): Continuous theta burst stimulation of angular gyrus reduces subjective recollection. PLoS One [Electronic Resource] 9:e110414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahavi D, Roepstorff A (2011): Faces and ascriptions: Mapping measures of the self. Conscious Cogn 20:141–148. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fan L, Zhang Y, Wang J, Zhu M, Zhang Y, Yu C, Jiang T (2014): Connectivity‐based parcellation of the human posteromedial cortex. Cereb Cortex 24:719–727. [DOI] [PubMed] [Google Scholar]


