Abstract
To study the properties of human primary somatosensory (S1) cortex as well as its role in cognitive and social processes, it is necessary to noninvasively localize the cortical representations of the body. Being arguably the most relevant body parts for tactile exploration, cortical representations of fingers are of particular interest. The aim of the present study was to investigate the cortical representation of individual fingers (D1–D5), using human touch as a stimulus. Utilizing the high BOLD sensitivity and spatial resolution at 7T, we found that each finger is represented within three subregions of S1 in the postcentral gyrus. Within each of these three areas, the fingers are sequentially organized (from D1 to D5) in a somatotopic manner. Therefore, these finger representations likely reflect distinct activations of BAs 3b, 1, and 2, similar to those described in electrophysiological work in non‐human primates. Quantitative analysis of the local BOLD responses revealed that within BA3b, each finger representation is specific to its own stimulation without any cross‐finger responsiveness. This finger response selectivity was less prominent in BA 1 and in BA 2. A test‐retest procedure highlighted the reproducibility of the results and the robustness of the method for BA 3b. Finally, the representation of the thumb was enlarged compared to the other fingers within BAs 1 and 2. These findings extend previous human electrophysiological and neuroimaging data but also reveal differences in the functional organization of S1 in human and nonhuman primates. Hum Brain Mapp 35:213–226, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: somatotopy, digit representation, fMRI, 7 tesla
INTRODUCTION
The primary somatosensory cortex (S1) consists of four distinct cytoarchitectonic areas, namely Brodmann areas (BA) 3a, 3b, 1, and 2 [Jones et al., 1978; Powell and Mountcastle, 1959] each of which includes a full somatotopic representation of the contralateral body. Animal studies revealed that body parts are represented at distinct positions in these four areas and that certain body parts are characterized by cortical magnification [Kaas et al., 1979; Merzenich et al., 1978; Nelson et al., 1980]. BA 3a receives proprioceptive information from muscles and joints, whereas signals from the skin are processed in BA 3b, 1, and 2. Within BA 2, skin signals are combined with proprioceptive information [Kandel et al., 2000]. The receptive field size of S1 neurons increases from BA 3b, 1, to 2 and a higher degree of selectivity is progressively exhibited [i.e., orientation and direction; Costanzo and Gardner, 1980; Gardner, 1988; Hyvarinen and Poranen, 1978] suggesting that somatosensory information is processed hierarchically along the rostro‐caudal direction in S1 [Iwamura et al., 1983, 1993].
Human S1 was first mapped by Penfield and Boldrey 1937 by applying intraoperative electrical cortical stimulation in pre‐ and postcentral gyri. They reported that each location of the contralateral body surface is represented at a specific position in the postcentral gyrus and also described cortical magnification. With respect to single finger representations, these electrical stimulation data separated the representations for each digit and reported an enlarged thumb representation [Penfield and Boldrey, 1937; Penfield and Jasper, 1954, Figures 3.15 at page 70 and 3.18 at page 73]. However, in these studies human finger somatotopy was not analyzed for individual patients, nor for the different S1 subregions, and it was not statistically verified. Additionally, their results may not represent S1 in healthy subjects.
Several fMRI studies have investigated finger somatotopy in S1 [Kurth et al., 2000, 1998; Nelson and Chen, 2008; Overduin and Servos, 2004; Sanchez‐Panchuelo et al., 2010; Schweizer et al., 2008; Stringer et al., 2011; van Westen et al., 2004], although this has been challenging due to large functional and anatomical intersubject variability. Several group studies were completed at 1.5T and 3T [e.g. Maldjian et al., 1999; van Westen et al., 2004] using a limited spatial resolution close to the cortical extent of single digits (between 8 and 40 mm3). Thus, in some studies, the cortical representation of only two or three digits was investigated [Gelnar et al., 1998; Kurth et al., 1998; Overduin and Servos, 2004; Weibull et al., 2008] or the finger somatotopy observed was incomplete [Moore et al., 2000; Schweizer et al., 2008]. Finger somatotopy for the different S1 areas was not determined, although previous studies at 1.5T [Kurth et al., 2000] and 3T [Nelson and Chen, 2008] observed that the cortical digit representation is organized somatotopically on average in BA 3b and 1. Recent studies using 7T fMRI described finger somatotopy for all five fingers but only in BA 3b [Sanchez‐Panchuelo et al., 2010], or focused on a limited subset of fingers [Stringer et al., 2011].
All of the above studies employed some form of mechanical stimulation. However, human touch provides a strong, complex, and natural tactile stimulus, which potentially activates not only BA 3b, but also 1 and 2. Moreover, studies on the role of S1 in cognitive or social tasks, such as body ownership [e.g., Dieguez et al., 2009; Petkova et al., 2011] or social interactions [e.g., Ebisch et al., 2011], often use human touch as a stimulus and therefore require an appropriate localizer. The aim of the present study was twofold: first, to obtain reliable somatotopic maps for functional localizers using a human touch stimulus and the increased BOLD signal sensitivity at 7T, and second, to investigate human finger somatotopy in the different Brodmann areas of S1.
MATERIALS AND METHODS
Participants and Experimental Procedures
Ten men aged between 20 and 35 years (mean ± std dev: 25.6 ± 5.6 years) participated in the study. All subjects were right‐handed, as assessed through the Edinburgh Oldfield Handedness Inventory [Oldfield, 1971], and gave written informed consent. Eight of these ten participants were scanned a second time (between 3 and 28 weeks later) with the same protocol to assess the reproducibility of the fMRI data. All procedures were approved by the Ethics Committee of the Faculty of Biology and Medicine of the University of Lausanne and the study was conducted in accordance with the Declaration of Helsinki.
During the experiment, participants lied supine in the scanner with their right arm comfortably stretched along the magnet bore. An experimenter was positioned at the entrance of the bore where he could easily reach and stroke the two distal phalanges of each digit with his own index finger. Each digit was independently stroked for 20 s, followed by 10 s of rest (no stroking). The order in which the digits were stroked was: D1 (thumb)–D3 (middle)–D5 (little)–D2 (index)–D4 (ring). This sequence was repeated four times per run, resulting in a scan time of 10 min per run. Two functional runs were acquired per participant. During the 20 s of stimulation, the two distal phalanges of each finger were repeatedly stroked along the axis of the finger, from the proximal to the distal portion of the finger. To minimize the variability of the stroking procedure, all the stimulations were performed by the same experimenter (RM) keeping similar pace and pressure on the fingers across subjects. Each stroke had a duration of 400–700 ms and digits were stroked at a frequency of ∼1 Hz, leading to approximately 20–25 strokes per stimulation block. The rationale for using the human touch as a stimulus is that it provided not only localized touch but also several features such as motion, texture, and temporal variability. We hypothesized that such a complex and natural tactile input potentially activates not only BA 3b, but also 1 and 2. Moreover, natural stimuli such as human touch have been used in studies on body ownership [e.g., Dieguez et al., 2009; Tsakiris et al., 2007] or agency [Farrer et al., 2008], making the resulting finger maps well suited for use as functional localizers in these types of studies. The stimulation pattern remained fixed during the experiment, similarly to several previous mapping studies involving the visual [e.g., Sereno et al., 1995; Warnking et al., 2002], auditory [e.g., Da Costa et al., 2011; Formisano et al., 2003], and somatosensory cortex [e.g., Sanchez‐Panchuelo et al., 2010; Stringer et al., 2011].
MR Data Acquisition
Images were acquired on a short‐bore 7T scanner (Siemens Medical, Germany) with an 8‐channel Tx/Rx rf‐coil (Rapid Biomedical, Germany). Functional images were acquired using a sinusoidal readout EPI sequence [Speck et al., 2008] and comprised 28 axial slices (in‐plane resolution 1.3 × 1.3 mm2; slice thickness 1.3 mm; gap 0.13 mm) placed over the postcentral gyrus (approximately orthogonal to the central sulcus) in order to cover the primary somatosensory cortex (matrix size 160×160, FOV = 210 mm, TE = 27 ms, TR = 2.5 s, GRAPPA = 2). Two functional runs were acquired, comprising 241 volumes each. To aid coregistration, a single whole‐brain EPI volume with 64 slices (1.3 × 1.3 × 1.3 mm3 resolution) was also acquired. To aid in the delineation of the Brodmann areas, an anatomical volume was acquired using the MP2RAGE sequence [Marques et al., 2010], with TE = 2.63 ms, TR = 7.2 ms, TI1 = 0.9 s, TI2 = 3.2 s, TRmprage = 5 s.
Data Analysis
Statistical analyses were conducted using SPM8 software (Wellcome Department of Cognitive Neurology, London, UK). Functional volumes were temporally realigned to the first slice acquired, spatially realigned to the first volume acquired, and smoothed with an isotropic Gaussian kernel (FWHM = 2 mm).
Statistical analysis was performed according to the General Linear Model using the canonical hemodynamic response function (HRF) and its time derivative as basis functions. The model included two regressors (the HRF and its temporal derivative) per finger and the motion parameters as nuisance regressors. Using this model, the response for stimulation of each single finger was estimated independently from the others.
The MP2RAGE volume was coregistered with the whole brain EPI image by means of rigid body transformations and both were subsequently coregistered to the mean EPI functional volume. Susceptibility induced distortions are small in the area of the brain covered by our imaging slab, notably the superior part of the central sulcus [van der Zwaag et al., 2009] and were further limited by the use of a limited matrix size in combination with parallel imaging to keep the read‐out duration short. As a result, co‐registration between the functional images and the MP2RAGE data using standard SPM8 routines was successful for all subjects. Cortical reconstruction and volumetric segmentation was performed with the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/).
Only for the extraction of the centers of mass coordinates, the anatomical images and the somatotopic maps were normalized to the standard brain template of the Montreal Neurological Institute (MNI) using the normalization algorithms included in SPM8. All other analyses were conducted in the individual subject space (i.e., in the space of individual functional acquisitions).
For the somatotopic mapping, an F‐contrast (P < 0.001 uncorrected) was computed including the HRF regressors for all five fingers, including all voxels responding to the stimulation of at least one finger. The result was used as an S1 mask. Maps of single finger responses were computed by means of a t‐contrast over the HRF regressors of each individual finger. Within the S1 mask, each voxel was independently labeled as representing the digit demonstrating the highest t‐value for that particular voxel.
The borders of BAs 3b, 1, and 2 were identified using the guidelines proposed by cytoarchitectonic studies [Geyer et al., 2000; Grefkes et al., 2001]. Within the postcentral gyrus, BA 3b is located on the anterior wall, BA1 occupies the crown, and BA2 lies on the posterior wall. Once we had segmented each BA of S1 on these anatomical borders, we identified for each BA the cluster showing the finger representations organized in an ordered manner. To compare the localization of the anatomically defined subregions with the location of cytoarchitectonically defined BAs, for each subject we computed the overlap between each identified functional subregion with the probabilistic cytoarchitectonic maps of BAs 3b, 1 [Geyer et al., 1999; Geyer et al., 2000], and 2 [Grefkes et al., 2001], as included in the Anatomy toolbox for SPM [Eickhoff et al., 2006; Eickhoff et al., 2007; Eickhoff et al., 2005].
Within each subregion and finger representation, the average BOLD responses to each of the five finger stimulations were tested to assess whether they were different from zero using a two‐tailed t‐test (P < 0.05). In addition, the Euclidean distance in the 3D space between the centers of mass of the representations of adjacent fingers and the volume of each finger representation were computed. Differences in interdigit distances and volumes across finger and functional subregions were assessed by 2‐way repeated measures ANalysis of VAriance (ANOVA), followed by a Tukey Honestly Significant Difference (HSD) test as post‐hoc test. The significance level was set to P < 0.05 for both the ANOVA and the Tukey HSD tests.
To assess the robustness and reliability of our fMRI results, eight subjects repeated the experiment, between 3 and 28 weeks after the first session. We first compared the positions of the center of mass of the finger representations across sessions in the MNI space. We also calculated the mean beta values of the BOLD responses in the second session within the finger representations as identified in the first session, and we qualitatively compared the BOLD response patterns with those obtained in the first session.
RESULTS
For all subjects, finger maps were observed within the postcentral gyrus contralateral to the stimulated fingers. Results for a single participant (number X) are presented in Figures 1 and 2, and one representative slice per participant is shown in Figure 3 (see also Supporting Information Fig. 1 for a display of several slices). All slices are displayed relative to the scanner coordinates and have not been reoriented relative to the bicommissural (i.e. AC‐PC) plane. The data revealed three regions with single finger representations that were all organized in a somatotopic manner. In all subjects, we observed that single finger representations along the anterior wall of the postcentral gyrus have an orderly spatial organization. In this representation, the little finger (shown in red in Figs. 1 and 2) was localized to a more superior and medial position and the thumb (blue) to a more inferior and lateral region (Figs. 1 and 2). The remaining fingers, ring (orange), middle (light green), and index (dark green), were located at intermediate positions between these extremes (Figs. 1 and 2).
Figure 1.
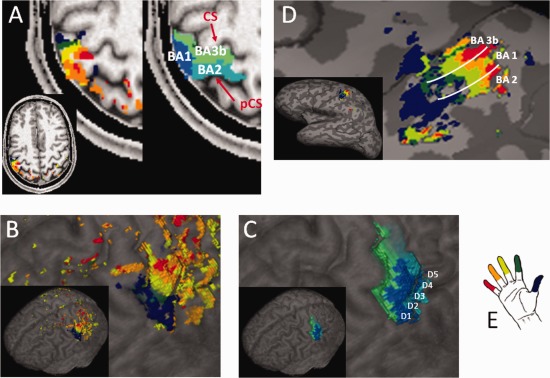
Results of the finger mapping procedure. The finger maps and BAs of one representative subject are displayed (A) on a selected axial plane, (B) and (C) on the cortical surface, and (D) on the inflated brain (including a schematic representation of the borders between the three BAs). The color code for the fingers is represented in (E), while different tones of blue indicate the three functional subregions. As anatomical references, the central sulcus (CS) and postcentral sulcus (pCS) are indicated on the axial slice.
Figure 2.
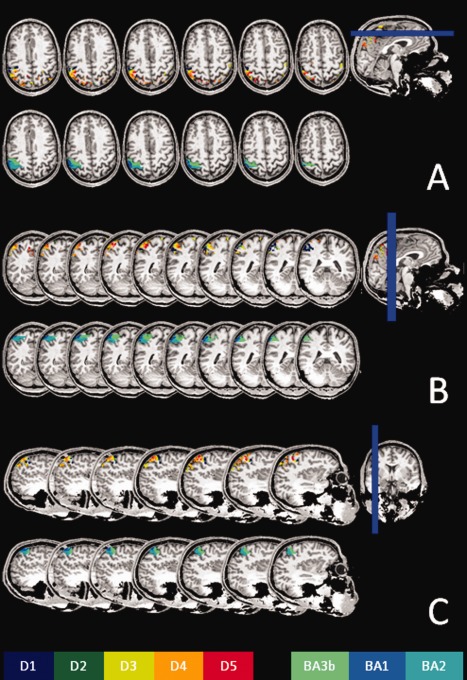
A set of axial (A), coronal (B), and sagittal (C) slices extracted from the same subject as in Fig. 1, showing the representations of the five fingers and BAs. All slices are displayed relative to the scanner coordinates, and have not been reoriented relative to the bicommissural plane.
Figure 3.
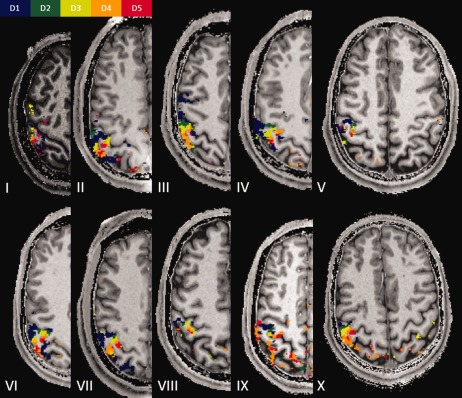
Robustness of the mapping procedure. One representative slice for each of the participants in the experiment is displayed, showing the robustness of the mapping procedure as well as the intersubject variability of finger representations.
The data obtained for each subject revealed yet another region with a precise spatial organization of single fingers (Figs. 1A and 3). This region was located on the crown of the postcentral gyrus. Finally, six subjects exhibited a third functional subregion located on the posterior wall of the postcentral gyrus, adjacent to the previous area, again displaying an ordered sequence of single fingers (Figs. 1A and 3, subjects number II, III, V, VI, VII, and X). In all three mentioned subregions, the thumb was found to be most inferior and the little finger most superior. Because of the relative inclination between the postcentral gyrus and the axial plane, the three subregions appeared for most subjects on the same axial slice. We note, however, that—depending on the position of the slice—the axial plane cuts the postcentral gyrus at different positions on the inferior–anterior axis and accordingly, at the level of different finger representations for the three S1 finger representations. The three subregions may therefore appear to include a variable number of digits and may even appear to be incomplete.
On a surface display (Fig. 1B–D), the labeled areas of the three BAs form continuous bands that are oriented parallel to the central sulcus, characterized by a sequence that has the thumb in the most inferior and the little finger in the most superior location.
The consistency of the finger maps across subjects is shown in Figure 3 and in the Supporting Information Figure 1. This anatomico‐functional architecture partly replicates patterns of somatotopic organization in nonhuman primates [Kaas et al., 1979], with the three functional regions oriented in three stripes parallel to the central sulcus. Moreover, in each stripe, a strikingly similar representation of the five digits was found, with the little finger located at the top of the stripe and the thumb at the bottom.
Because the three observed regions were located on the anterior wall, on the crown, and on the posterior wall of the postcentral gyrus, and because within each region the finger representations were organized in a somatotopic manner, we propose that the three functional subregions that we observed likely represent three regions of primary somatosensory cortex, namely BAs 3b, 1, and 2.
To assess the spatial correspondence between the anatomically defined BAs 3b, 1, and 2 and cytoarchitectonic maps, we computed the overlap between each of the anatomically defined BAs (including the representation of all the five fingers) and the probabilistic cytoarchitectomic maps of BAs 3b, 1, and 2 representing the likelihood that a certain voxel belongs to a particular BA [Geyer et al., 1999; Geyer et al., 2000; Grefkes et al., 2001]. This analysis shows that on average 83% of the volume we labeled as BA 3b overlaps with the probabilistic map of BA 3b (60 and 22% with the probabilistic maps of BAs 1 and 2, respectively). Similarly, 83% of the volume we labeled as BA 1 overlaps with the probabilistic map of BA 1 (46 and 52% with the probabilistic maps of BAs 3b and 2, respectively) and 75% of the volume we labeled as BA 2 overlaps with the probabilistic map of BA 2 (49 and 86% with the probabilistic maps of BAs 3b and 1, respectively).
Interdigit Distances
In the following sections we will describe the results separately for the three regions, BAs 3b, 1 and 2. First, we computed the position of the center‐of‐mass of each finger representation in the MNI space (see Table 1) and the inter‐digit distances (see Fig. 4A). To better account for the three‐dimensional orientation of the postcentral sulcus, the Euclidean distance between the centers of mass of all adjacent fingers was computed in 3D space.
Table 1.
Mean (± StdDev) coordinates of the centers of mass (mm) in the MNI space in BA 3b (N = 10), in BA 1 (N = 10), and in BA 2 (N = 6)
| BA 3b | BA 1 | BA 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| X | y | z | x | y | z | x | y | z | |
| D1 | −48.4 ± 2.2 | −18.4 ± 2.5 | 52.0 ± 3.4 | −54.6 ± 2.3 | −22.2 ± 3.6 | 54.0 ± 4.0 | −46.4 ± 5.5 | −35.5 ± 3.6 | 58.4 ± 6.4 |
| D2 | −44.4 ± 2.3 | −19.7 ± 2.4 | 53.7 ± 2.4 | −51.6 ± 2.9 | −23.1 ± 3.0 | 58.4 ± 4.0 | −47.6 ± 5.0 | −33.4 ± 4.3 | 58.6 ± 5.0 |
| D3 | −42.7 ± 1.9 | −22.4 ± 3.2 | 57.5 ± 3.7 | −49.1 ± 2.7 | −25.4 ± 3.9 | 60.9 ± 2.7 | −46.7 ± 4.1 | −35.2 ± 3.4 | 60.6 ± 4.8 |
| D4 | −40.4 ± 2.4 | −25.5 ± 2.9 | 59.1 ± 4.2 | −46.9 ± 2.1 | −28.7 ± 3.1 | 63.8 ± 3.1 | −45.8 ± 2.2 | −35.6 ± 3.5 | 62.1 ± 3.4 |
| D5 | −39.9 ± 3.1 | −27.0 ± 3.2 | 61.2 ± 3.1 | −46.7 ± 2.8 | −29.6 ± 3.7 | 64.2 ± 4.0 | −45.4 ± 1.8 | −33.9 ± 5.1 | 61.6 ± 5.4 |
Figure 4.
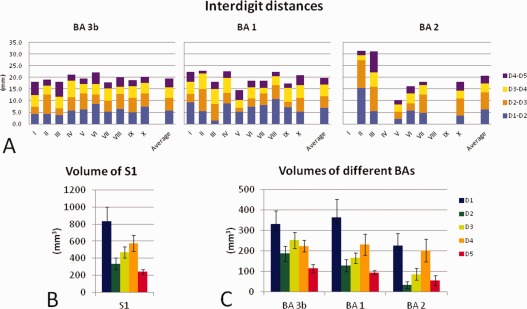
Quantitative analyses conducted on the finger representations. A: Interdigit distances between adjacent fingers for all subjects and the average across participants for each BA. B: Graphics representing the average volume (with the error bars indicating the standard error of the mean) of each single finger representation within the entire S1. C: The volume of each finger representation within each BA (error bars indicate the standard error). Note the overrepresentation of the thumb region relative to the other fingers.
The position of individual centers‐of‐mass (see Table 1) confirms the sequential organization of the previously identified five finger representations within each S1 subregion.
Within BA 3b, the interdigit distances (± standard deviation) were 5.7 ± 1.4 mm for D1‐D2, 5.4 ± 1.8 for D2‐D3, 4.7 ± 1.0 for D3‐D4, and 3.7 ± 1.5 for D4‐D5. Within BA 1, the distances were 6.9 ± 2.7 mm for D1‐D2, 4.9 ± 2.3 for D2‐D3, 5.0 ± 1.5 for D3‐D4 and 3.0 ± 1.1 for D4‐D5. For BA 2 they were 6.2 ± 4.7 mm for D1‐D2, 7.3 ± 3.7 for D2‐D3, 3.8 ± 1.3 for D3‐D4, and 3.5 ± 2.8 for D4‐D5. The analysis of the interdigit distances revealed a main effect of fingers within BAs 3b and 1 (BA 3b: F (3,27) = 3.17, P = 0.04; BA 1: F (3,27) = 5.51, P = 0.004; BA 2: F (3,12) = 2.26, P = 0.12). Post‐hoc analyses (Tukey HSD test) revealed that for both BA 3b and 1, the distance between D4 and D5 was smaller than the distance between D1 and D2 (BA 3b: P = 0.04; BA 1: P = 0.002).
In addition, we computed the distance between D1 and D5, which was 15.5 ± 2.4 mm in BA 3b, 15.1 ± 4.3 mm in BA 1, and 8.6 ± 4.2 mm in BA 2. The distance between D1 and D5 in BA 2 was significantly shorter than the same distance in BA 3b (P = 0.016) and BA 1 (P = 0.035).
Volume of S1 Finger Representations
The total volumes (± standard deviation) of the three regions were 1110 ± 390 mm3 for BA 3b, 980 ± 440 mm3 for BA 1, and 600 ± 319 mm3 for BA 2. No significant difference was observed between the total volumes of BAs 3b and 1 (P = 0.21) while BA 2 was significantly smaller than both BA 3b (P = 0.003) and BA 1(P = 0.033). To evaluate whether each single finger was evenly represented within the three functional subregions of S1, the volume of each single finger representation was computed. Results are shown schematically in Figure 4B,C. Statistical analysis revealed a main effect of finger in all three regions (F (4,36) = 5.1, P = 0.002 in BA 3b; F (4,36) = 6.2, P = 0.001 in BA 1; F (4,20) = 8.7, P = 0.0003 in BA 2).
Further analysis (Tukey HSD test) revealed that, in BA 3b, the D1 representation was larger than the representation of D2 (P = 0.049) and D5 (P = 0.001) but not of D3 (P = 0.508) and D4 (P = 0.218). Within BA 1 and BA2, the D1 representation was significantly larger than those of D2, D3, and D5 (all P < 0.018 within BA 1 and all P < 0.023 within BA 2). Additionally, we observed that within BA 2, the D4 representation was significantly larger than those of D2 (P < 0.006) and D5 (P < 0.018). Quantification of the thumb magnification with respect to the average volume of the other digits yielded factors of 2 for all S1, 1.7 for BA 3b, 2.3 for BA 1, and 2.4 for BA 2. These results show that the thumb has a significantly larger representation than the other fingers in BA 1 and BA 2, while a trend towards magnification was observed in BA 3b. A pictorial representation of the volumes of the finger representations estimated in the present and previous studies is shown in Figure 5.
Figure 5.
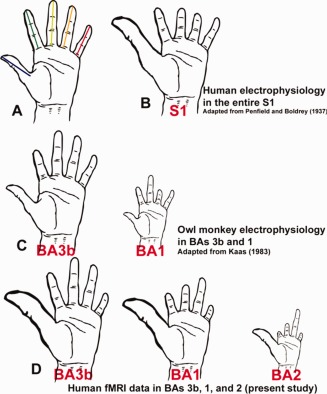
Schematic representation by different authors of the size of cortical finger representations in different species. The size of the cortical representations of each finger is indicated by the length of that finger. All finger sizes are plotted onto a human hand. A: Human hand. The colored lines represent for each finger the length of the finger that was scaled according to the results from the different studies. B: Single finger representation in human S1 as measured with intraoperative stimulation in humans (Penfield and Boldrey, 1937). C: Single finger representation in owl monkeys for BAs 3b and 1 as measured with electrophysiology (Kaas, 1983). The hand sizes were scaled with the size of BAs 3b and 1 reported in the paper. D: Single finger representation in human BAs 3b, 1, and 2 as measured with fMRI in the present study. The hand sizes were scaled with the size of BAs 3b, 1 and 2 measured in this study.
Finger Selectivity
To investigate the selectivity of each of the single finger regions to the tactile stimulation of the respective finger as compared to the tactile stimulation of the other four fingers, we computed the mean BOLD response for each finger (represented by the beta values for the relevant GLM regressors) within each of the five single finger representations in BAs 3b, 1, and 2. Results (see Fig. 6) showed that within BA 3b, finger representations are specialized and respond uniquely to stimulation of the target finger: the target finger was the only digit showing a significant positive BOLD response to stimulation except for areas of D4 and D5, which also showed a significant response to the tactile stimulation of D3 and D4 respectively. Qualitatively, the response gradually decreased as the digital distance increased from the stimulated finger, resulting in a negative BOLD response for the most distant fingers. A similar digit distance effect was observed in BAs 1 and 2, but with a less pronounced reduction of the BOLD response with the distance from the stimulated finger compared to BA 3b. In addition, in BAs 1 and 2 each finger representation also showed limited cross‐finger responses as they responded also to the stimulation of the adjacent fingers.
Figure 6.
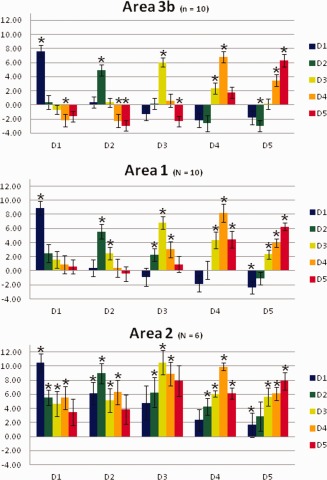
Average BOLD response (beta values) to finger stimulation within each finger representation within the three BAs. Asterisks indicate values significantly different from zero (P < 0.05). Error bars show the standard error.
Robustness of the Finger Representations
To assess the reliability of our somatotopic finger maps, we compared the topography of finger representations obtained in two different sessions. Data obtained in the second fMRI session (from the same subject as depicted in Figs. 1 and 2) are shown in Figure 7A–C. Generally, the responses to the tactile stimulation during the second session were reduced both in size of the activated area and in amplitude of the BOLD signal, compared to the first session. In all eight re‐tested participants, we obtained a somatotopic map within BA 3b, as in the first session. The coordinates of the centers of mass of the finger representations within BA 3b of the second session (Table 2) were located close to those identified in the first session. Across sessions, the mean displacement (± standard deviation) of the centers of mass was 1.7 ± 1.2 mm for D1, 3.2 ± 3.1 mm for D2, 2.3 ± 1.3 mm for D3, 2.2 ± 1.5 mm, for D4, and 2.2 ± 2.5 mm for D5. In the second session, the average interdigit distance was 5.44 ± 2.0 mm and the distance between thumb and little finger was 16.6 ± 1.7 mm. These values are comparable with those from the first session. Activations in BAs 1 and 2 were less reproducible. In the second session, complete finger topography was observed in 5 subjects in BA 1, while no subject had a representation of all the five fingers in BA 2. Because BAs 1 and 2 were observed in only few subjects, we limited the comparison of the results across sessions to BA 3b.
Figure 7.
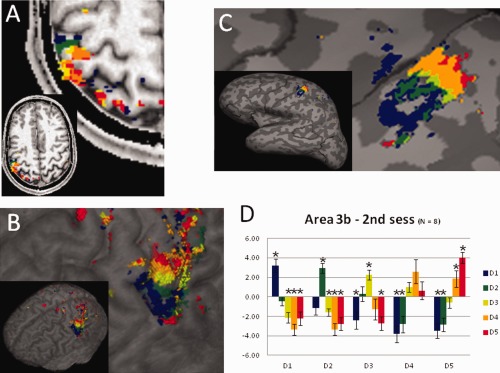
Reproducibility of the mapping procedure. Finger mapping obtained during the second session in the same subject shown in Fig. 1A shown on (A) a selected axial plane, on (B) the cortical surface, and on (C) the inflated brain, using the same color code as in Fig. 1. D: Average beta values (error bars indicate the standard error) obtained during the second session within BA 3b defined using the results of the first session. Asterisks indicate values significantly different from zero (P < 0.05).
Table 2.
Mean (± StdDev) coordinates of the centers of mass (mm) in the MNI space for the second session in area 3b (N = 8)
| x | y | z | |
|---|---|---|---|
| D1 | −48.4 ± 2.6 | −19.0 ± 3.3 | 52.7 ± 3.7 |
| D2 | −44.2 ± 1.7 | −21.3 ± 3.3 | 56.0 ± 3.3 |
| D3 | −43.1 ± 1.8 | −22.5 ± 3.3 | 58.9 ± 3.3 |
| D4 | −41.0 ± 2.8 | −24.7 ± 2.6 | 60.3 ± 4.8 |
| D5 | −38.4 ± 2.2 | −27.3 ± 3.2 | 62.4 ± 4.3 |
To further characterize the consistency of the finger topographies across sessions, the mean BOLD responses of the second session were computed within the single finger regions, as defined in the first session. Analysis for session 2 (see Fig. 7D) showed that each finger representation (as defined in Session 1) also responded most strongly to the same finger stimulation and exhibited a BOLD response that gradually decreased with the digit distance from the stimulated finger, replicating the pattern observed in the first session. The generally reduced BOLD amplitude observed in the second session can also be seen in Figure 7D.
DISCUSSION
We took advantage of the increased BOLD sensitivity and spatial resolution available at 7T (Shmuel et al., 2007; van der Zwaag et al., 2009] to precisely and non‐invasively investigate the cortical representation of single fingers in human S1, using human touch as the stimulus. In the postcentral gyrus (extending from the anterior wall bordering the central sulcus to the posterior wall bordering the postcentral sulcus) we found each finger represented within three areas in S1, identified as BAs 3b, 1, and 2. Within each of these areas, the finger representations were organized in a somatotopic order. Quantitative analysis revealed that within each of the three regions, each finger representation responded specifically to that particular finger stimulation with no or low cross‐finger responsiveness and that, especially in BA 3b, the BOLD signal decreased during the stimulation of other fingers. A test‐retest procedure highlighted the reproducibility of our results within BA 3b and the robustness of the method despite a reduction in BOLD signal in the second recording.
Finger Somatotopy
In each participant, we observed a sequential representation of the five fingers on the anterior wall of the postcentral sulcus (BA 3b); the little finger was in a superior and medial position and the thumb in a more inferior and lateral position (Fig. 1). Additionally, we were able to determine finger somatotopy for two other regions adjacent to BA 3b. These regions were located on the crown of the postcentral gyrus (BA 1) and on the posterior wall of the postcentral gyrus (BA 2). When observed on an inflated brain surface, these three regions are positioned in parallel to each other and the central sulcus (Fig. 1C). In each of these three regions the thumb is located more inferior and the little finger more superior (see Fig. 1B,D). This organization is comparable to that of BAs 3b, 1, and 2 observed in non‐human primates [Kaas et al., 1979] and as proposed in humans based on histological post‐mortem analysis [Geyer et al., 1999; Geyer et al., 2000; Grefkes et al., 2001]. The location of our three anatomically defined BAs as defined here was in good agreement with reported probabilistic cytoarchtectonic maps for the same BAs [Geyer et al., 1999; Geyer et al., 2000; Grefkes et al., 2001]. Yet, our data also show the high anatomical and functional variability of these primary somatosensory regions across subjects. This highlights the need for a method that separates regions at the single subject level and determines boundaries independently of and orthogonal to digit or other body representations. In the absence of clear anatomical landmarks to guide the segmentation of the three BAs, in this paper we adopted an anatomically driven strategy, labeling the activations on the anterior wall of the postcentral gyrus as belonging to BA 3b, those within the crown as belonging to BA 1 and those on the posterior wall as belonging to BA 2. Though, some misalignment on the individual pixel levels may have occurred, the general trends we observed were averaged over large numbers of voxels and should be valid irrespective of minor segmentation errors. Further research is required to improve the segmentation method in order to achieve more objective and fully automated ways for segmenting subregions in S1, similar to those commonly used for segmenting visual [e.g., Sereno et al., 1995] and auditory [Da Costa et al., 2011] cortices.
Extending recent fMRI studies [Nelson and Chen, 2008; Sanchez‐Panchuelo et al., 2010; Stringer et al., 2011] we show that similar to monkey S1, the three anatomically distinct finger representations in humans each have an orderly somatotopic organization. Because of cortical folding, the three finger representations (one in each BA) are closer in the three‐dimensional space than what would be expected by the inflated maps. This feature is similar to that observed in the tonotopic regions of human auditory cortex [Da Costa et al., 2011; Formisano et al., 2003] and in the retinotopic regions of the human visual cortex [Rajimehr and Tootell, 2009; Sereno et al., 1995], suggesting that this is an organizational feature common to human sensory cortex.
These results extend previous fMRI findings obtained at 3 and 7T [Nelson and Chen, 2008; Sanchez‐Panchuelo et al., 2010; Stringer et al., 2011]. We have however employed a natural stimulus (i.e. human touch). The method proposed here therefore also allows for the creation of a functional localizer of digit representations, which can subsequently be used to study the role of the different portions of S1 in cognitive neuroscience research. In particular, the use of a natural stimulus such as human touch makes the method well suited to investigate body ownership [e.g. Dieguez et al., 2009; Ionta et al., 2011; Tsakiris et al., 2007], agency [e.g., Blakemore et al., 1998; Farrer et al., 2008], and self‐other discrimination [e.g., Cardini et al., 2011], given that the stimulus of the functional localizer is similar in nature to those used in these types of investigations. The present method was designed to be used as a functional localizer in subsequent functional MRI studies at 7T. We believe that the translation of our results to 3T fMRI should be possible with the latest technological improvements of MR scanners and head coils, allowing functional images of sufficient resolution and SNR to be acquired on a 3T MR scanner (i.e. resolution of 1.5 mm isotropic).
Robustness of Finger Representations
The reliability of our results was assessed by comparing the somatotopic maps obtained in the same subject in two separate recording sessions. Somatotopic maps were highly similar between the two sessions. Across sessions, the mean distance between the centers of mass was approximately 2.3 mm, which corresponds to less than 2 voxels (i.e., less than the size of the finger representation). This observation was confirmed by the analysis of the BOLD responses of the second session, using the ROIs as defined in the first session, replicating the specificity of each finger representation and the BOLD response decrease with the digit distance with the stimulated finger (Fig. 7). These observations also confirm the reliability of the human touch as a stimulus.
Additionally, we observed that activation maps were much smaller in the second session. Because the majority of the subjects were naïve to the MR environment prior to the first session and because the stimulation protocol was completely passive, subjects may have been less attentive to the task in the second session. As attention is known to modulate the amplitude of BOLD signals, even in primary sensory cortices [e.g., Nelson et al., 2004], it is likely that the reduction in the BOLD signal was due to reduced attention levels in the second session.
Interdigit Distances
The interdigit distances between the thumb and the little finger that we observed (i.e., 15.5 mm in area 3b, 15.1 mm in area 1, and 8.6 mm in area 2) are comparable to what has been reported previously [i.e., 17.2–17.9 mm in BA 3b (Kurth et al., 2000; Nelson and Chen, 2008; van Westen et al., 2004)), 14.3–14.9 mm in BA 1 (Kurth et al., 2000; Nelson and Chen, 2008), and 6.8 mm in BA 2 (Nelson and Chen, 2008). Similarly to the interdigit distances between the thumb and the little finger, we also observed that the total volumes of finger representations within BA 3b (1110 mm3) and BA 1 (983 mm3) were both significantly larger than those within BA 2 (604 mm3). This result is in line with previous studies showing that BA 2 is smaller than BAs 3b and 1 [e.g., Nelson and Chen, 2008]. Although a significant main effect of interdigit distances was observed within BA 3b and 1, further testing only confirmed a single significantly different distance (D1‐D2 versus D4‐D5), This result is consistent with results in previous mapping studies [e.g., Duncan and Boynton, 2007; Nelson and Chen, 2008] showing that D4‐D5 is the shortest interdigit distance.
Finally, we note the large inter‐subject variability of finger representation. Thus, the standard deviation of the MNI coordinates of the centers of mass was comparable to the interdigit distances, highlighting the importance of performing single subject analysis in somatotopic fMRI.
Thumb Magnification
We observed that the thumb had a larger cortical representation than the other fingers within BAs 1 and 2. An S1 over‐representation of the thumb in the postcentral gyrus was observed by Penfield and Boldrey 1937, based on data of 126 patients and after pooling data across a large number of individuals with often incomplete finger mappings. These patients suffered from different neurological diseases that may have affected patterns of cortical organization. Moreover, the employed stimulation techniques in humans typically used relatively large electrodes and used high currents leading to the spread of the electrical current within and beyond S1 [i.e. Blanke et al., 1999; Blanke et al., 2000; Lesser et al., 1987]. Extrapolating the data from Fig. 26 in Penfield and Boldrey 1937, which represents the number of stimulations that led to a finger‐specific somatosensory response, we estimated that the thumb representation is 2 times larger than the average representation of the other four fingers (thumb magnification for S1 globally; Penfield and Boldrey, 1937; Penfield and Jasper, 1954, Figures 3.15 at page 70 and 3.18 at page 73). Our data extend these earlier findings by showing that that thumb enlargement was significantly present in both BA 1 and 2. A trend to enlargement was also observed in BA 3b. Thumb magnification is compatible with what was reported by Penfield and colleagues for S1 globally, but suggests that thumb enlargement may predominate in BAs 1 and 2. Recent studies also reported data that are compatible with thumb magnification [Nelson and Chen, 2008; Sanchez‐Panchuelo et al., 2010].
Our analysis shows that cortical magnification is not due to a larger skin region of the thumb being stimulated, but more likely reflects a higher number of neurons and/or a larger volume of cortex in BA 1 and 2, linked to the great importance of the human thumb for tactile perception (and haptic explorations).
The size of a cortical digit representation is thought to be correlated with the tactile acuity of that digit [Duncan and Boynton, 2007; Tegenthoff et al., 2005]. Thus, Duncan and Boynton 2007 reported that the interdigit distance between D4‐D5 is significantly smaller than those between D2‐D3 and D3‐D4 (with D3‐D4 not being significantly different from D2‐D3). They also found that there is a non‐linear correlation between tactile acuity and cortical magnification. In addition, Sathian & Zangadze 1996, showed that D5 has a poorer tactile acuity than the other fingers while no such differences were observed between D1, D2, D3, and D4. Moreover, the results of Vega‐Bermudez & Johnson 2001 showed that the tactile acuity of D2 is higher than D3 which in turn is higher than D4. Based on these previous findings, it can be speculated that D5 has the smallest cortical representation, followed by D4, D3 and finally by D2 and D1. This is compatible with the present findings on interdigit distances and the volume of cortical representations in which we observed that the D5 representation is the smallest (significantly different from D1) and D2, D3, and D4 representations have comparable volumes. Although Duncan and Boynton 2007 did not directly investigate the thumb representation and the correlation between magnification factor and tactile acuity for the thumb, by extrapolating the data from the studies of Duncan and Boynton 2007 and Sathian & Zangadze 1996 it can be speculated that D1 and D2 should be similar in size as these two fingers have been reported to have comparable tactile acuity [Sathian and Zangaladze, 1996]. This proposal, though, does not fit easily with the thumb magnification that was observed in our present data as well as in the results of previous studies [Nakamura et al., 1998; Nelson and Chen, 2008; Penfield and Boldrey, 1937; Sanchez‐Panchuelo et al., 2010]. The relationship between cortical magnification and tactile acuity is an interesting issue but is beyond the scope of the present study and should be investigated by future imaging studies.
Interestingly, thumb magnification has not been observed in nonhuman primates. This potential species difference between squirrel and macaque monkeys on the one hand, and humans on the other, may reflect central nervous system differences that are related to increased tactile function in humans due to thumb opposition and precision grip [i.e. Johansson and Westling, 1984; Maier and Hepp‐Reymond, 1995; Napier, 1961]. Thus, thumb‐related motor functions (and likely haptic and tactile functions) are characteristically expanded in humans due to the increased importance of the thumb for fine motor tasks such as grasping and tool handling.
Finger Selectivity
By analyzing the BOLD responses in single finger representations, we observed that these digit representations responded only to the tactile stimulation of the corresponding finger in BA 3b (i.e., no cross‐finger responsiveness), but less so in BA 1 and 2. BA 1 showed increased cross‐finger responsiveness for neighboring digits compared to BA 3b and no negative BOLD responses. BA 2 showed strong cross‐finger responsiveness for most digits, with BOLD signal amplitudes of up to half that of the mainly represented digit during stimulation of adjacent fingers. Such finger selective activations are biased by the fact that the same data were used for the definition of the cortical finger representations and for the extraction of the beta values. However, this bias cannot explain why the stimulation of the other four fingers consistently led to much smaller or even negative BOLD responses. Importantly, we confirmed finger selective activations in a second session (see Fig. 7D). In this particular analysis, finger regions and activation data were drawn from two independent datasets, thereby overcoming the circularity problem of the analysis of the data from the first session, without significant changes in the results. Additional analysis exploited the fact that the data recording included two consecutive functional runs. In this analysis, described in detail in the Supporting Information material, we used the data of the first run to create the finger maps and we extracted the beta values from the data acquired in the second run. The results of this analysis (see Supporting Information Fig. 2) replicated the findings observed using both runs together. That is, within BA 3b, finger representations are specialized and respond uniquely to stimulation of the target finger. A similar effect was also observed in BA 1, but with less pronounced finger selectivity. Single finger representations in BA 2 tended to respond to stimulation of any finger (yet this was only based on 3 subjects and therefore was not found to be significant).
In BA 3b, cross‐finger responsiveness was observed only in the representations of D4 and D5. This response pattern may have originated from the particular organization of tactile receptive fields of these two digits in humans, which often function together in haptic exploration. We cannot exclude completely that this cross‐finger responsiveness was due to partial‐volume effects because the D4 and D5 regions were the two smallest regions in BA 3b. We note, however, that partial volume effects cannot account for the differences we observed between BAs 3b and 1 as their overall size was similar.
These 7T fMRI results are compatible with the notion of hierarchical processing of tactile information from BA 3b to 1 and 2. The finding of decreased finger selectivity and increased cross‐finger responsiveness from BA 3b to 1 and 2 may reflect activation of neuronal populations with receptive fields mostly responsive to a single finger in BA 3b to several fingers in BA 2. These findings are compatible with electrophysiological studies investigating the hierarchical processing of tactile information [e.g. Costanzo and Gardner, 1980; Costanzo and Gardner, 1981; Gardner and Costanzo, 1980; Iwamura et al., 1983; Iwamura et al., 1993]. We note, however, that cross‐finger responsiveness has recently been described to occur also in BA 3b [Lipton et al., 2010]. Yet, the latter data stem from single or multiple unit recordings and are thus sampled from a highly restricted region whereas our data are based on the average signal over the entire finger representation. Moreover, Lipton et al. analyzed multi‐unit activity (MUA), whereas the BOLD signal is assumed to mostly reflect local field potential [Logothetis et al., 2001].
CONCLUSIONS
In this study we presented a method for mapping single finger representations in human S1 using 7T fMRI and a natural stimulus. Using this method we showed that, consistent with animal models, finger specificity of the cortical regions was reduced moving from BA 3b to 1 and 2. We further observed thumb magnification in BAs 1 and 2, which seems to be specific to humans. Finally, the use of human touch as a stimulus makes this method appropriate as a functional S1 localizer in experiments studying the cognitive neuroscience of touch.
Supporting information
Supporting Information
REFERENCES
- Blakemore SJ, Wolpert DM, Frith CD (1998): Central cancellation of self‐produced tickle sensation. Nat Neurosci 1:635–640. [DOI] [PubMed] [Google Scholar]
- Blanke O, Morand S, Thut G, Michel CM, Spinelli L, Landis T, Seeck M (1999): Visual activity in the human frontal eye field. Neuroreport 10:925–930. [DOI] [PubMed] [Google Scholar]
- Blanke O, Perrig S, Thut G, Landis T, Seeck M (2000): Simple and complex vestibular responses induced by electrical cortical stimulation of the parietal cortex in humans. J Neurol Neurosurg Psychiatry 69:553–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardini F, Costantini M, Galati G, Romani GL, Ladavas E, Serino A (2011): Viewing one's own face being touched modulates tactile perception: an fMRI study. J Cogn Neurosci 23:503–513. [DOI] [PubMed] [Google Scholar]
- Costanzo RM, Gardner EP (1980): A quantitative analysis of responses of direction‐sensitive neurons in somatosensory cortex of awake monkeys. J Neurophysiol 43:1319–1341. [DOI] [PubMed] [Google Scholar]
- Costanzo RM, Gardner EP (1981): Multiple‐joint neurons in somatosensory cortex of awake monkeys. Brain Res 214:321–333. [DOI] [PubMed] [Google Scholar]
- Da Costa S, van der Zwaag W, Marques JP, Frackowiak RS, Clarke S, Saenz M (2011): Human primary auditory cortex follows the shape of Heschl's gyrus. J Neurosci 31:14067–14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieguez S, Mercier MR, Newby N, Blanke O (2009): Feeling numbness for someone else's finger. Curr Biol 19:R1108–R1109. [DOI] [PubMed] [Google Scholar]
- Duncan RO, Boynton GM (2007): Tactile hyperacuity thresholds correlate with finger maps in primary somatosensory cortex (S1). Cereb Cortex 17:2878–2891. [DOI] [PubMed] [Google Scholar]
- Ebisch SJ, Ferri F, Salone A, Perrucci MG, D'Amico L, Ferro FM, Romani GL, Gallese V (2011): Differential involvement of somatosensory and interoceptive cortices during the observation of affective touch. J Cogn Neurosci 23:1808–1822. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K (2006): Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. Neuroimage 32:570–582. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K (2007): Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage 36:511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frey SH, Van Horn JD, Tunik E, Turk D, Inati S, Grafton ST (2008): The angular gyrus computes action awareness representations. Cerebral Cortex 18:254–261. [DOI] [PubMed] [Google Scholar]
- Formisano E, Kim D‐S, Di Salle F, van de Moortele P‐F, Ugurbil K, Goebel R (2003): Mirror‐symmetric tonotopic maps in human primary auditory cortex. Neuron 40:859–869. [DOI] [PubMed] [Google Scholar]
- Gardner EP (1988): Somatosensory cortical mechanisms of feature detection in tactile and kinesthetic discrimination. Can J Physiol Pharmacol 66:439–454. [DOI] [PubMed] [Google Scholar]
- Gardner EP, Costanzo RM (1980): Spatial integration of multiple‐point stimuli in primary somatosensory cortical receptive fields of alert monkeys. J Neurophysiol 43:420–443. [DOI] [PubMed] [Google Scholar]
- Gelnar PA, Krauss BR, Szeverenyi NM, Apkarian AV (1998): Fingertip representation in the human somatosensory cortex: An fMRI study. Neuroimage 7:261–283. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schleicher A, Zilles K (1999): Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage 10:63–83. [DOI] [PubMed] [Google Scholar]
- Geyer S, Schormann T, Mohlberg H, Zilles K (2000): Areas 3a, 3b, and 1 of human primary somatosensory cortex. II. Spatial normalization to standard anatomical space. Neuroimage 11(6 Part 1):684–696. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Geyer S, Schormann T, Roland P, Zilles K (2001): Human somatosensory area 2: Observer‐independent cytoarchitectonic mapping, interindividual variability, and population map. Neuroimage 14:617–631. [DOI] [PubMed] [Google Scholar]
- Hyvarinen J, Poranen A (1978): Movement‐sensitive and direction and orientation‐selective cutaneous receptive fields in the hand area of the post‐central gyrus in monkeys. J Physiol 283:523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionta S, Heydrich L, Lenggenhager B, Mouthon M, Fornari E, Chapuis D, Gassert R, Blanke O (2011): Multisensory mechanisms in temporo‐parietal cortex support self‐location and first‐person perspective. Neuron 70:363–374. [DOI] [PubMed] [Google Scholar]
- Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O (1983): Converging patterns of finger representation and complex response properties of neurons in area‐1 of the 1st somatosensory cortex of the conscious monkey. Exp Brain Res 51:327–337. [Google Scholar]
- Iwamura Y, Tanaka M, Sakamoto M, Hikosaka O (1993): Rostrocaudal gradients in the neuronal receptive field complexity in the finger region of the alert monkey's postcentral gyrus. Exp Brain Res 92:360–368. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G (1984): Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56:550–564. [DOI] [PubMed] [Google Scholar]
- Jones EG, Coulter JD, Hendry SH (1978): Intracortical connectivity of architectonic fields in the somatic sensory, motor and parietal cortex of monkeys. J Comp Neurol 181:291–347. [DOI] [PubMed] [Google Scholar]
- Kaas JH (1983): What, if anything, is SI? Organization of first somatosensory area of cortex. Physiol Rev 63:206–231. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Nelson RJ, Sur M, Lin CS, Merzenich MM (1979): Multiple representations of the body within the primary somatosensory cortex of primates. Science 204:521–523. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM.2000. Principles of Neural Science.New York:McGraw‐Hill, Health Professions Division; 1414 p. [Google Scholar]
- Kurth R, Villringer K, Curio G, Wolf KJ, Krause T, Repenthin J, Schwiemann J, Deuchert M, Villringer A (2000): fMRI shows multiple somatotopic digit representations in human primary somatosensory cortex. Neuroreport 11:1487–1491. [PubMed] [Google Scholar]
- Kurth R, Villringer K, Mackert BM, Schwiemann J, Braun J, Curio G, Villringer A, Wolf KJ (1998): fMRI assessment of somatotopy in human Brodmann area 3b by electrical finger stimulation. Neuroreport 9:207–212. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Luders H, Klem G, Dinner DS, Morris HH, Hahn JF, Wyllie E (1987): Extraoperative cortical functional localization in patients with epilepsy. J Clin Neurophysiol 4:27–53. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Liszewski MC, O'Connell MN, Mills A, Smiley JF, Branch CA, Isler JR, Schroeder CE (2010): Interactions within the hand representation in primary somatosensory cortex of primates. J Neurosci 30:15895–15903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A (2001): Neurophysiological investigation of the basis of the fMRI signal. Nature 412:150–157. [DOI] [PubMed] [Google Scholar]
- Maier MA, Hepp‐Reymond MC (1995): EMG activation patterns during force production in precision grip. I. Contribution of 15 finger muscles to isometric force. Exp Brain Res 103:108–122. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Gottschalk A, Patel RS, Detre JA, Alsop DC (1999): The sensory somatotopic map of the human hand demonstrated at 4 tesla. Neuroimage 10:55–62. [DOI] [PubMed] [Google Scholar]
- Marques JP, Kober T, Krueger G, van der Zwaag W, Van de Moortele PF, Gruetter R (2010): MP2RAGE, a self bias‐field corrected sequence for improved segmentation and T1‐mapping at high field. Neuroimage 49:1271–1281. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Sur M, Lin CS (1978): Double representation of the body surface within cytoarchitectonic areas 3b and 1 in "SI" in the owl monkey (Aotus trivirgatus). J Comp Neurol 181:41–73. [DOI] [PubMed] [Google Scholar]
- Moore CI, Stern CE, Corkin S, Fischl B, Gray AC, Rosen BR, Dale AM (2000): Segregation of somatosensory activation in the human rolandic cortex using fMRI. J Neurophysiol 84:558–569. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Yamada T, Goto A, Kato T, Ito K, Abe Y, Kachi T, Kakigi R (1998): Somatosensory homunculus as drawn by MEG. Neuroimage 7(Part 1):377–386. [DOI] [PubMed] [Google Scholar]
- Napier JR (1961): Prehensility and opposability in the hands of primates. Symp Zool Soc 5:115–132. [Google Scholar]
- Nelson AJ, Chen R (2008): Digit somatotopy within cortical areas of the postcentral gyrus in humans. Cereb Cortex 18:2341–2351. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Staines WR, Graham SJ, McIlroy WE (2004): Activation in SI and SII: The influence of vibrotactile amplitude during passive and task‐relevant stimulation. Brain Res Cogn Brain Res 19:174–184. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Sur M, Felleman DJ, Kaas JH (1980): Representations of the body surface in postcentral parietal cortex of Macaca fascicularis. J Comp Neurol 192:611–643. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Overduin SA, Servos P (2004): Distributed digit somatotopy in primary somatosensory cortex. Neuroimage 23:462–472. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E (1937): Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443. [Google Scholar]
- Penfield W, Jasper HH (1954): Epilepsy and the Functional Anatomy of the Human Brain.Boston:Little; 896 p. [Google Scholar]
- Petkova VI, Bjornsdotter M, Gentile G, Jonsson T, Li TQ, Ehrsson HH (2011): From part‐ to whole‐body ownership in the multisensory brain. Curr Biol 21:1118–1122. [DOI] [PubMed] [Google Scholar]
- Powell TP, Mountcastle VB. (1959): The cytoarchitecture of the postcentral gyrus of the monkey Macaca mulatta. Bull Johns Hopkins Hosp 105:108–131. [PubMed] [Google Scholar]
- Rajimehr R, Tootell RB (2009): Does retinotopy influence cortical folding in primate visual cortex? J Neurosci 29:11149–11152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Panchuelo RM, Francis S, Bowtell R, Schluppeck D (2010): Mapping human somatosensory cortex in individual subjects with 7T functional MRI. J Neurophysiol 103:2544–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A. (1996): Tactile spatial acuity at the human fingertip and lip: Bilateral symmetry and interdigit variability. Neurology 46:1464–1466. [DOI] [PubMed] [Google Scholar]
- Schweizer R, Voit D, Frahm J (2008): Finger representations in human primary somatosensory cortex as revealed by high‐resolution functional MRI of tactile stimulation. Neuroimage 42:28–35. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB (1995): Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268:889–893. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Chaimow D, Logothetis NK, Ugurbil K (2007): Spatio‐temporal point‐spread function of fMRI signal in human gray matter at 7 Tesla. Neuroimage 35:539–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O, Stadler J, Zaitsev M (2008): High resolution single‐shot EPI at 7T. MAGMA 21(1–2):73–86. [DOI] [PubMed] [Google Scholar]
- Stringer EA, Chen LM, Friedman RM, Gatenby C, Gore JC (2011): Differentiation of somatosensory cortices by high‐resolution fMRI at 7T. Neuroimage 54:1012–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Forster AF, Nicolas V, Dinse HR (2005): Improvement of tactile discrimination performance and enlargement of cortical somatosensory maps after 5 Hz rTMS. PLoS Biol 3:e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR (2007): Neural signatures of body ownership: A sensory network for bodily self‐consciousness. Cereb Cortex 17:2235–2244. [DOI] [PubMed] [Google Scholar]
- van der Zwaag W, Francis S, Head K, Peters A, Gowland P, Morris P, Bowtell R (2009): fMRI at 1.5, 3 and 7 T: Characterising BOLD signal changes. Neuroimage 47:1425–1434. [DOI] [PubMed] [Google Scholar]
- van Westen D, Fransson P, Olsrud J, Rosen B, Lundborg G, Larsson EM (2004): Fingersomatotopy in area 3b: An fMRI‐study. BMC Neurosci 5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega‐Bermudez F, Johnson KO (2001): Differences in spatial acuity between digits. Neurology 56:1389–1391. [DOI] [PubMed] [Google Scholar]
- Warnking J, Dojat M, Guerin‐Dugue A, Delon‐Martin C, Olympieff S, Richard N, Chehikian A, Segebarth C (2002): fMRI retinotopic mapping—Step by step. Neuroimage 17:1665–1683. [DOI] [PubMed] [Google Scholar]
- Weibull A, Bjorkman A, Hall H, Rosen B, Lundborg G, Svensson J (2008): Optimizing the mapping of finger areas in primary somatosensory cortex using functional MRI. Magn Reson Imaging 26:1342–1351. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


