Abstract
A number of genetic polymorphisms are related to individual differences in cognitive performance. Striatal dopamine (DA) functions, associated with cognitive performance, are linked to the TaqIA polymorphism of the DRD2/ANKK1 gene. In humans, presence of an A1 allele of the DRD2/ANKK1‐TaqIA polymorphism is related to reduced density of striatal DA D2 receptors. The resource‐modulation hypothesis assumes that aging‐related losses of neurochemical and structural brain resources modulate the extent to which genetic variations affect cognitive functioning. Here, we tested this hypothesis using functional MRI during long‐term memory (LTM) updating in younger and older carriers and noncarriers of the A1‐allele of the TaqIa polymorphism. We demonstrate that older A1‐carriers have worse memory performance, specifically during LTM updating, compared to noncarriers. Moreover, A1‐carriers exhibited less blood oxygen level‐dependent (BOLD) activation in left caudate nucleus, a region critical to updating. This effect was only seen in older adults, suggesting magnification of genetic effects on functional brain activity in aging. Further, a positive relationship between caudate BOLD activation and updating performance among non‐A1 carriers indicated that caudate activation was behaviorally relevant. These results demonstrate a link between the DRD2/ANKK1‐TaqIA polymorphism and neurocognitive deficits related to LTM updating, and provide novel evidence that this effect is magnified in aging. Hum Brain Mapp 36:1325–1334, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: functional magnetic resonance imaging (fMRI), memory, aging, dopamine, DRD2, updating, striatum
INTRODUCTION
There is much evidence for a relationship between dopamine (DA) functions and cognition (Cervenka et al., 2008; Slagter et al., 2012). Functional and molecular brain imaging have linked cognitive operations to interactions between the striatum and prefrontal cortex, and suggests a critical role for DA in regulating these interactions. Maintaining stable representations and flexible updating of working‐ and long‐term memory (LTM) representations both depend on these fronto‐striatal interactions (Cools and D'Esposito, 2009).
Differences in cognitive performance can partially be explained by individual differences in DA levels. This is supported by findings that D2 agonists may enhance performance in participants with low working‐memory span, but impair performance in those with high span (Gibbs and D'Esposito, 2005; Kimberg et al., 1997). Such individual performance differences may stem from genetic variability in DA functions.
In humans, the presence of an A1 allele of the DRD2/ANKK1‐TaqIA polymorphism is related to reduced density of striatal DA D2 receptors (Jönsson et al., 1999). In studies of young adults and children, this particular polymorphism has been associated with reduced cognitive abilities in carriers of the A‐allele compared to noncarriers (Berman and Noble, 1995; Klein et al., 2007). Moreover, in line with the hypothesis that striatal DA is critical for cognitive flexibility, DRD2/ANKK1‐TaqIA affects task switching and working memory‐related processes (Berryhill et al., 2013; Jocham et al., 2009; Stelzel et al., 2010). It was also recently shown that the DRD2/ANKK1‐TaqIA polymorphism influences updating of mental representations in epistatic interaction with the COMT Val108/158Met genotype (Garcia‐Garcia et al., 2011).
Aging comes with decline in dopaminergic neurotransmission, as demonstrated in studies assessing brain D1 and D2 receptor density, transporter availability, and DA concentration (Bäckman et al., 2000; Erixon‐Lindroth et al., 2005; Volkow, 1996; Wang et al., 1998). The resource‐modulation hypothesis assumes that genetic influences on cognition are more pronounced for individuals in whom neuroanatomical or neurochemical resources are limited (Lindenberger et al., 2008). This hypothesis rests on the assumption that the function relating brain resources to cognitive performance is nonlinear (Cools and D'Esposito, 2011; Li and Sikström, 2002), so that genetic variability is more likely to result in neurocognitive differences when resources move away from close‐to‐optimal levels, as in aging.
Although DA‐relevant genes have been linked to cognitive performance in young adults, age‐comparative studies have provided support for the resource‐modulation hypothesis, such that variations in these genes (e.g., COMT, DRD2, DAT1) have a stronger influence on cognitive performance in older, compared to younger, adults. This has been demonstrated for working memory (Nagel et al., 2008; Störmer et al., 2012), episodic memory (Li et al., 2013; Papenberg et al., 2013), and inhibition (Colzato et al., 2013). However, empirical evidence directly relating age, the DRD2/ANKK1‐TaqIA polymorphism, and changes in striatal activity during operations that require cognitive flexibility has not been established. The current study was designed to fill this gap.
We investigated the effect of the DRD2/ANKK1 polymorphism on updating of LTM representations, and whether this effect was magnified in aging, as postulated by the resource‐modulation hypothesis. We hypothesized that A1 carriers would show reduced LTM updating performance and reduced functional activation of task‐relevant brain networks. Moreover, we expected that this effect would be larger in older compared to younger adults. To test these predictions, participants were scanned with fMRI while performing a category‐instance task designed to specifically target the process by which LTM representations are manipulated under conditions of proactive interference (from here on simply referred to as updating).
METHODS
Participants
Participants were 30 younger [16 females; age range: 20–31 (M = 25.1 years; standard deviation (SD) = 3.4)] and 32 older [18 females; age range: 65–74 (M = 68.2 years; SD = 2.5] adults. Due to technical problems with the response pad, behavioral data were lost for one older woman and one older man. Also, four participants (two younger, two older) were excluded from the fMRI analyses, because of poor image quality arising from movement artifacts. Thus, analyses were based on data from 56 participants (28 younger, 28 older). Medical histories were self reported. All participants were in good health, with no known history of stroke, heart disease, or primary degenerative neurological disorder. All participants were also free from past or present neuropsychiatric diseases, diabetes and/or neurological disorders and were not taking blood‐thinning medication or had contraindications to MRI. For older adults, a radiologist screened a T1‐weighted and a T2‐weighted image and ruled out abnormal levels of atrophy or lesions. All participants gave informed consent, were right‐handed native Swedish speakers, and had normal or corrected‐to‐normal vision. The study was approved by the Ethics Committee in Stockholm.
Table 1 shows demographic information and data from two cognitive tasks (n‐back working memory and vocabulary) across age groups. The cognitive data indicate that the two age groups were representative of their respective cohorts, with an advantage for the young in n‐back and an advantage for the old in vocabulary.
Table 1.
Demographics and off‐line cognitive scores
| Young adults | Older adults | All | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | A+ | A− | A + versus A− | All | A+ | A− | A + versus A− | Y versus O adults | A + versus A− | |
| Demographics | ||||||||||
| N | 28 | 12 | 16 | 28 | 12 | 16 | ||||
| Age, years (range) | 25.0 (20–31) | 23.6 | 25.0 | 0.89 | 68.1 (65–74) | 68.3 | 68.1 | 0.69 | 0.53 | |
| Gender (f/m) | 15/13 | 6/6 | 10/6 | 0.59 | 15/13 | 6/6 | 10/6 | 0.79 | 0.89 | 0.69 |
| Education, yrs (range) | 14.7 (12–19) | 13.7 | 15.2 | 0.19 | 14.6 (9–27) | 14.4 | 14.3 | 0.96 | 0.69 | 0.35 |
| Cognitive scores | ||||||||||
| MMSE (range) | 29.3 (27–30) | 29.2 | 29.3 | 0.78 | 28.9 (27–30) | 29.1 | 28.9 | 0.54 | 0.23 | 0.51 |
| WM 1‐back | 10.5 | 10.4 | 10.5 | 0.79 | 8.8 | 8.6 | 8.9 | 0.76 | <0.002 | 0.86 |
| WM 2‐back | 8.4 | 8.3 | 8.4 | 0.84 | 6.5 | 5.5 | 6.9 | 0.11 | <0.001 | 0.19 |
| WM 3‐back | 7.0 | 6.8 | 7.1 | 0.68 | 5 | 4.6 | 5.1 | 0.39 | <0.001 | 0.74 |
| SRB | 22.6 | 21.7 | 23.1 | 0.38 | 26.0 | 25.6 | 26.3 | 0.51 | <0.001 | 0.69 |
MMSE = Mine‐mental state examination; WM = Working memory N‐back test; SRB = Word comprehension.
Genotyping
Genotyping was performed on DNA extracted from peripheral blood samples. Samples were subsequently labeled anonymously and transferred to the Mutation Analysis Facility at the Karolinska Institute, Huddinge, Sweden, where DNA extraction and genotyping took place. Genotyping was conducted with a single‐nucleotid extention reaction, with allele detection by mass spectrometry (Sequenom MassArray system; Sequenom, San Diego, CA). Polymerase chain reaction (PCR) and extension primers were designed using the MassArray assay design software. The genotype success rate for SNP rs1800497 was 100%. The SNP rs1800497 is usually referred to as the TaqIA polymorphism. Participants with the A1/A1 and A1/A2 genotypes were considered to have A1+ allelic status, whereas those with the A2/A2 homozygous genotype were considered to have A1‐ allelic status, consistent with previous studies (Noble, 2003) and a dominant model of inheritance. There was no departure from Hardy–Weinberg equilibrium in the combined sample (P = 0.24), the younger adults sample (P = 0.62), or the older adults sample (P = 0.17; Table 1).
fMRI Methods
fMRI task
We used the “category‐instance” memory task that allows for independent assessment of different LTM components (Dolan and Fletcher, 1997; Nyberg et al., 2009), including maintenance “old‐old,” updating “old‐new,” and novelty “new‐new.” Prior to scanning, each participant was presented with 34 pairs of words. Each word pair consisted of a category (e.g., “tree”) and an instance of that category (e.g., “pine”). Both words of each pair were presented simultaneously. Each pair was presented twice at a rate of 2 sec/item on a computer screen, and participants were instructed to memorize each word pair. Following presentation of word pairs, participants practiced on one additional task to be performed in the scanner (not reported here). During scanning, an event‐related design was used. A new list of word pairs was visually presented at a rate of 2 sec/item. Participants were informed that some of the word pairs had been presented prior to scanning, that others were new, and that still others had been changed (an old category paired with a new instance). Participants were instructed to memorize these new pairs, and told that they later would be asked to retrieve these new word pairs from memory. The list included three different types of word pairs: (a) the same category‐instance combination as presented prior to scanning “old‐old,” (b) an old category paired with a new instance “old‐new,” and (c) a new category paired with a new instance “new‐new.” Each type of combination consisted of 17 word pairs, with a total of 51 pairs presented during scanning. During the interstimuli interval (ISI) a fixation cross was presented, and the ISI was evenly distributed between 1 s and 6 s. The different types of word pairs were evenly distributed throughout the scanning session. After the scanning session, participants performed a cued recall test in which they were presented with the category names and asked to complete each category with the instance presented during MRI scanning. Additional tasks and protocols that were carried out between the encoding phase in the scanner and off‐line testing included a multisource interference task, a face emotion discrimination task, a functional resting state scan and three structural scans (diffusion tensor imaging (DTI), perfusion and T2). The time between scanning and subsequent cued recall was approximately 50 min.
Image acquisition
Images were acquired using a 3T Siemens Magnetom Tim Trio scanner, equipped with a 32 channel head coil at Huddinge Hospital, Stockholm, Sweden. Functional data was acquired with a T2*‐weighted echo‐planar sequence (repetition time (TR) = 2500 ms, echo time (TE) = 40 ms, flip angle = 90°, field of view (FOV) = 230 mm, voxel size = 3 × 3 × 3 mm). To allow for progressive saturation of the fMRI‐signal, four dummy scans were collected and discarded prior to experimental image acquisition. Thirty‐nine oblique axial slices were positioned parallel to the AC–PC line, and acquired interleaved. High‐resolution T1‐weighted MP‐RAGE structural images were also collected, and used for coregistration with functional images and structural analyses (see below; 176 sagittal slices, TR = 1900 ms, TE = 2.52 ms, flip angle = 9°, FOV = 256 mm, voxel size = 1 × 1 × 1 mm). The scanner tasks were presented on a computer screen, seen through a mirror mounted on the head coil, and responses were collected with a scanner‐compatible response box. Participants were given headphones and earplugs to dampen scanner noise, and cushions inside the head coil minimized head movements.
Image preprocessing
All fMRI data were analyzed using the statistical parametric mapping software (SPM8, Welcome Department of Imaging Neuroscience, University College London, UK) implemented in Matlab 2010b (Mathworks, MA). Preprocessing included slice‐timing correction, motion correction, coregistration of functional images to participants' anatomical scans, spatial normalization using the echo‐planar imaging template from the Montreal Neurological Institute (EPI‐MNI) template provided in SPM8, and smoothing (8‐mm full‐width half maximum Gaussian kernel).
fMRI data analysis
Event‐related effects were modeled separately using the general linear model (GLM) framework, as implemented in SPM8, and these transient responses were modeled as delta functions based on trial onset. All regressors of interest were convolved with the hemodynamic response function. Three translational (x, y, z) and three rotational (pitch, roll, yaw) regressors obtained from the realignment step were included as covariates of no interest in the individual fixed‐effect analysis to account for in‐scanner movement. Single‐subject statistical contrasts were set up using the GLM, and group data were analyzed in a random‐effects model. Statistical parametric maps were generated using t statistics to identify regions activated according to the model. Temporal autocorrelations within a session were corrected using an AR(1) model. All results are reported in MNI space.
Given our a‐priori interest in the striatal complex as potentially contributing to memory updating (Cools and D'Esposito, 2011; Nyberg et al., 2009; O'Reilly and Frank, 2006) and the TaqIA polymorphism of the DRD2/ANKK1 gene, we focused our follow‐up analyses to this particular region (we also report additional regions for each comparison of interest in the text). We report clusters with a voxel‐level threshold of P < 0.001 (uncorrected). In the striatum, for which we had a prior hypotheses, results were small volume corrected for familywise error (FWE; Worsley et al., 1996) within a sphere of 6 mm diameter around peak coordinates. All striatal activations survive a voxel‐level threshold of pFWE < 0.05.
The Marsbar toolbox (http://marsbar.sourceforge.net/) was used to create regions of interest (ROIs) and to extract each ROI's mean BOLD parameter estimate value for each condition and participant. The parameter estimates were then used for plotting the results in SPSS, as well as for performing brain‐behavior correlations. ROIs were functionally defined on the basis of voxels that showed peak activations in the comparison of the new‐old (LTM updating) versus the old‐old (familiarity) and new‐new (novelty) conditions. Each region within the left caudate was created by including activated voxels (P < 0.001, uncorrected) during updating of LTM representations in the contrast of A1‐carriers were compared to noncarriers across all participants (Fig. 2), the age by condition interaction (Fig. 3A), or the contrast of A1‐carriers compared to noncarriers in the older group only (Fig. 3C). Each functional ROI contained a minimum of 10 contiguous voxels. For each ROI, effect sizes (% signal change) for each of the conditions were then extracted and averaged.
Figure 2.
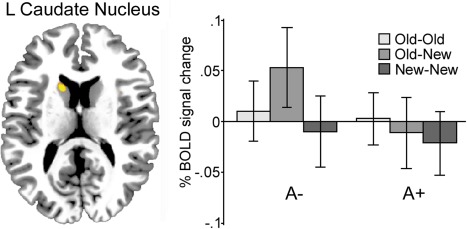
Across age groups, activation in the left caudate nucleus was observed during LTM updating (x, y, z = −10, 10, 16) for noncarriers of the DRD2/ANKK1‐Taq1a polymorphism, but not for carriers. Activation was considered significant at P < 0.001 uncorrected. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
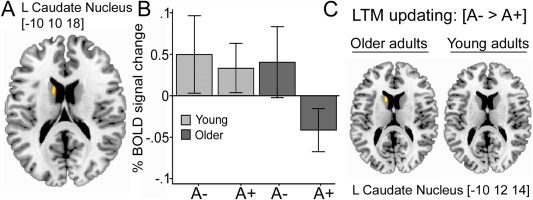
(A) Activation in the left caudate nucleus (x, y, z = −10, 10, 18) during LTM updating was observed for younger carriers and noncarriers of the DRD2/ANKK1‐Taq1a polymorphism, and older noncarriers. (B) Older carriers had significantly reduced activation in this region, as indicated by the gene by age interaction. Activation was considered significant at P < 0.001 uncorrected. (C) Main effect of the DRD2/ANKK1‐Taq1a polymorphism on LTM updating activation in the caudate nucleus for younger and older adults, respectively. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Volumetric Measurements
The FreeSurfer software automated cortical and subcortical parcellation tools (version 5.1; http://surfer.nmr.mgh.harvard.edu) were used for volumetric segmentation, cortical surface reconstruction, and parcellation to quantify the brain volumes of interest. The technical details of these procedures are described elsewhere (e.g., Dale et al., 1999; Fischl et al., 2002; Han et al., 2006). The surface‐based stream has been validated via histological (Rosas et al., 2002) and manual (Kuperberg et al., 2003) measurements, and the subcortical assessment has been shown to be indistinguishable from that derived by manual ratings (Fischl et al., 2002). The subcortical segmentation and surface reconstruction and parcellation procedures are run automatically, but require supervision of the accuracy of spatial registration and tissue segmentation. Using this method, we obtained volumes from caudate, globus pallidus, and putamen from the T1‐weighted images. This procedure generated a set of brain masks for each participant. Intracranial volume (ICV) was also estimated using the “new segment” toolbox in SPM12, and used to adjust for differences in brain size via analysis of covariance according to the formula: adjusted volume = raw volume – b × (ICV – mean ICV), where b is the slope of the regression for a region of interest volume on ICV. Correcting for differences in brain size helps to reduce the variability in measurement of brain structures leading to more accurate volume measurements.
RESULTS
Behavioral Data
Group effects on memory performance were analyzed using a 2 (gene: A1+/A1−) × 2 (age: younger/older adults) × 3 (condition: old‐old/old‐new/new‐new) repeated‐measures ANOVA (Fig. 1). First, the main effect of condition was significant [F(1,52) = 135.9, P < 0.001, η p 2 = 0.708], reflecting that the condition in which intact category‐instance pairs were presented again (old‐old) were remembered better compared to the two other conditions (old‐new, new‐new). Second, the gene by condition interaction was significant, showing that A1 carriers performed selectively worse in the old‐new condition compared to the conditions not requiring LTM updating [F(1,52) = 4.32, P < 0.05, η p 2 = 0.07]. Third, although the age by gene by condition interaction did not approach conventional significance (P > 0.05), a significant gene by condition interaction was observed in older adults [F(1,27) = 5.16, P < 0.01, η p 2 = 0.15], whereas the corresponding interaction for younger adults fell far short of significance (P = 0.714).
Figure 1.
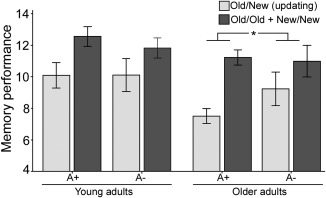
Behavioral results. Effect of genotype (A+/A−), age (younger/older), and condition (old‐new vs. old‐old/new‐new) on memory performance. The results show a gene by condition interaction for older adults, indicating that older carriers of the A allele perform worse on the old‐new (updating) condition compared to noncarriers.
fMRI Data
In the first set of analyses, we contrasted BOLD activity associated with updating (old‐new) with the conditions in which no updating was required (old‐old/new‐new). Using this contrast, we explored differences between TaqIA A1 carriers and noncarriers. In line with our predictions, we found reduced striatal BOLD signal in A1 carriers compared to noncarriers in left caudate nucleus (x, y, z = −10, 10, 16, t = 3.53, k = 21; Fig. 2). Critically, this effect was mainly driven by older noncarriers of the A1 allele in the ANKK1 gene (A1+ < A1−), as indicated by a significant gene by age by condition interaction (x, y, z = −10, 10, 18, t = 3.37; Fig. 3). These results were also significant when left caudate volume was used as a covariate in the analyses. Two additional clusters were more activated for A1 noncarriers compared to carriers. These were located in anterior caudate nucleus (x, y, z = −16, 24, 12; t = 3.18; k = 18) and right precuneus (x, y, z = 18, −48, 38; t = 3.09; k = 12). Two clusters were activated in the reverse contrast (A1+ > A1−) located in the left precentral gyrus (x, y, z = −26, −2, 44; t = 4.15; k = 74) and the subcallosal gyrus (x, y, z = −20, 2, −12; t = 3.96; k = 18). All activated clusters are shown in Supporting Fig. 1.
Further, we observed a positive correlation between activation in left striatum (x, y, z = −10, 10, 16) and old‐new updating performance (r = .38, P < 0.05; Fig. 4) among noncarriers of the A allele. Such an association was not found among A carriers (P > 0.70; Fig. 4). The relationship in noncarriers remained marginally significant when age was entered as a covariate in a partial correlation (r = 0.34; P = 0.054) suggesting that age only marginally affected this association. Note however, that although the correlations with performance in the old‐old and new‐new conditions and BOLD signal were not significant (old‐old: r = 0.21, P = 0.22; new‐new: r = −0.04; P = 0.81), the difference between correlations (A+ vs. A−) was not significant either (old‐old vs. old‐new: P = 0.45; new‐new vs. old‐new: P = 0.08). Regardless, this interactive pattern suggests that ANKK1 A1 carriers may suffer from less efficient dopaminergic modulation during LTM updating.
Figure 4.
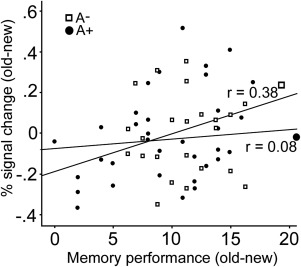
Activity in the left caudate nucleus was correlated with LTM updating performance in A1 noncarriers, but not in A1 carriers of the DRD2/ANKK1‐Taq1a polymorphism.
Structural MR Data
To examine whether the DRD2/ANKK1 genotype also had an effect on striatal volume, thereby potentially contributing to allelic differences in the striatal BOLD signal during LTM updating, we obtained volumes for bilateral caudate nucleus, putamen, and globus pallidus. Although these analyses revealed a main effect of age [F(1, 52) = 31.14, P < 0.001, η p 2 = 0.36], there was no main effect of DRD2/ANKK1 genotype (P = 0.16) and no gene by age interaction (P = 0.68). Follow‐up analyses revealed that age differences (younger > older) were significant in all regions (all Ps < 0.01), and the effect of genotype was not significant in any of the structures (all Ps > 0.10). Also, correlations between volumes of these nuclei and the magnitude of the striatal BOLD signal were nonsignificant (all Ps > 0.10) indicating that activation in basal ganglia was unrelated to their volumes.
Follow‐Up Analysis Including Only Subsequently Remembered Items
In the analyses presented above, we included all trials, irrespective of subsequent recall success. A potential concern is that all trials were included, and not only trials that were subsequently recalled. We therefore analyzed all data by only including regressors that coded for trials that were later remembered. As each of the conditions only included 17 trials, for some participants the number of trials that were included in each condition was low. Thus, in order for the analyses to be meaningful, we excluded participants which had less than six correct trials in any of the conditions. Based on this criterion, 16 participants were excluded from analysis. The remaining 40 participants were included in the random‐effects model that mirrored the analyses previously reported. Even though this analysis had much less power, we were able to replicate the main effect of the Taq1A polymorphism reported in Figure 2, showing stronger activation during updating (old‐new compared to old‐old and new‐new) in left caudate nucleus (A noncarriers > A carriers; uncorrected at 0.001; peak coordinates: −10 20 14 with a subpeak at −10 10 18). This cluster overlapped with the previously reported cluster showing a left caudate BOLD signal difference between Taq1A carriers and noncarriers in the whole sample of participants with all trials included.
Moreover, although the whole‐brain gene by age interaction did not reach significance (Fig. 3A,B) with only correct trials included, likely reflecting reduced power, the ROI analysis on the BOLD signal estimates from this region showed a significant gene by age effect as previously reported. Finally, we were able to replicate the finding of stronger left caudate BOLD signal activation in Taq1A noncarriers compared to carriers in old adults (0.001 uncorrected, peak coordinates: −10 14 18). Similar to our results reported above, no difference in left caudate activation between Taq1A noncarriers and carriers were found in young adults (nonsignificant at an uncorrected threshold of 0.001).
DISCUSSION
We found that carriers of the A allele of the DRD2/ANKK1‐TaqIA polymorphism (A+) have worse LTM updating performance compared to noncarriers. Moreover, carriers of this allele exhibited less activation of left caudate in the old‐new condition, a region known to be involved in LTM updating (Bäckman et al., 2011; Dahlin et al., 2008). This effect was seen in the older age group only. Further, we demonstrate a positive relationship between left caudate activation and updating performance among non‐A carriers, suggesting that caudate activation is behaviorally relevant. These results are among the first to demonstrate a link between the DRD2/ANKK1‐TaqIA polymorphism and neurocognitive deficits related to LTM updating and provide novel evidence that this effect is magnified in aging. Note that the allelic groups were indistinguishable on tests of global cognitive functioning, working memory, and vocabulary. This fact indicates discriminant validity regarding the role of the DRD2/ANKK1‐TaqIA polymorphism, and extends previous findings to the domain of LTM updating.
A deficit in LTM updating was observed for carriers of the A1+ DRD2/ANKK1‐TaqIA polymorphism, carriers previously characterized by a 30–40% reduction in striatal D2 receptor density (Jönsson et al., 1999; Pohjalainen et al., 1998). However, this disadvantage was seen in older, but not in younger, A1 carriers. The observation that the genetic effect was more pronounced in older adults, known to suffer from compromised DA functions (Bäckman et al., 2006; for reviews, see Bäckman et al., 2010) is in line with the resource‐modulation hypothesis (Lindenberger et al., 2008). This finding corroborates previous observations showing interactive effects of DRD2 genotypes, with a specific cognitive disadvantage in older adults (Colzato et al., 2013; Li et al., 2013; Papenberg et al., 2013). For example, in a recent study, carriers of the C/C genotype of the C957T polymorphism (rs6277), which is related to greater DA D2 receptor availability, showed reduced LTM forgetting compared to noncarriers (Papenberg et al., 2013). However, our study is the first to show a selective deficit (i.e., discriminant validity) in updating of LTM representations, which is a well‐known function of the dopaminergic system, among older individuals carrying the A1 allele of the DRD2/ANKK1‐TaqIA polymorphism. As such, our behavioral results extend previous observations linking DRD2 polymorphisms to memory functioning in aging, and provide further support for the resource‐modulation hypothesis.
We also found that A1 carriers had reduced striatal BOLD activation compared to noncarriers during LTM updating. Similar to the behavioral findings, this effect was present in older adults only. Although the neuroimaging literature on different DRD2 genotypes in relation to cognition is scarce, there are demonstrations of altered striatal BOLD activation associated with the DRD2/ANKK1‐TaqIA polymorphism. Jocham et al. (2009) found that carriers of the A1 allele were less likely to maintain a new correct response in a reversal‐learning task compared to noncarriers, and this effect was associated with less reversal‐related striatal BOLD activity. Our findings extend these observations by showing compromised caudate BOLD activation in A1 carriers related to updating of LTM representations. Toward this end, reversal learning and LTM updating may rely on common mechanisms associated with behavioral flexibility.
Moreover, in agreement with the resource‐modulation hypothesis, reduced striatal BOLD activation was evident in older A1 carriers only. These findings provide initial evidence that individual variability in striatal D2 receptor density associated with a DRD2 polymorphism leads to compromised striatal efficiency in older adults, who have reduced striatal D2 functions compared to younger adults (e.g., Bäckman et al., 2000; Cervenka et al., 2008). Previous demonstrations of age‐related reductions in striatal activity during memory updating (Podell et al., 2012), along with reduced striatal BOLD activation among A1 carriers during reversal learning (Jocham et al., 2009) suggest a possibility for age ‐ genotype associations. Given the known neuromodulatory role of DA in the fronto‐striatal circuitry, and its suggested role for memory updating and behavioral flexibility that are impaired in aging (Braver and Barch, 2002), it is conceivable that the effects of age and the DRD2 polymorphism on the DA system underlie the observed BOLD signal differences in striatum.
Although the focus of the current study is on updating processes and striatal activation during episodic memory, evidence indicates that hippocampal‐striatal and hippocampal‐midbrain modulation are also important for different LTM functions. For instance, animal work has shown that midbrain DA modulation of hippocampal functions is critical to encoding and maintaining memory associations in LTM (Lisman and Grace, 2005). Relatedly, pharmacological imaging in humans has shown that the agonist Levodopa may enhance hippocampal activity and episodic memory functioning in older adults (Chowdhury et al., 2012)
The size of the striatal BOLD signal was positively linked to task performance in the old‐new condition, further underscoring the significance of this region for LTM updating. This association between activation during the updating condition and performance was only significant in A1 noncarriers. This selective pattern suggests a deficit among A1‐carriers in modulating striatal activity in response to demands for LTM updating. Speculatively, carriers of the DRD2/ANKK1‐TaqIA polymorphism, conversely, may suffer from decreased efficiency and less selective stimulus processing in this particular region that decouples neural activity from subsequent memory performance.
Given previous observations of relations between aging and caudate volume (Raz et al., 2003, 2005) as well as striatal volume reductions among DRD2/ANKK1‐TaqIA A1‐carriers (Bartres‐Faz et al., 2002), we tested for a relationship between the polymorphism and volumes of caudate, putamen, and pallidum. Although we found age‐related volume reductions for all these regions, there was no association between volume and genotype. Moreover, volumetric measures did not correlate with striatal BOLD signal. This pattern indicates that the observed reduction in striatal activation for older A1 carriers during LTM updating was not driven by volume losses in this region. This also suggests that functional brain‐imaging measures of brain function may be more sensitive than structural measures to detect differences between individuals, including genetic effects. Toward this end, previous studies have also shown that genetic variability may have a greater impact on functional, compared to structural, estimates of brain integrity in aging (e.g. Damoiseaux et al., [Link]; Matura et al., 2014).
A limitation of this study is that it did not include a direct measure of DA system integrity. This prevents firm conclusions about the effect of age and the DRD2/ANKK1‐TaqIA polymorphism on updating performance and striatal BOLD signal activation. Toward this end, it has proven problematic to predict the direction of brain activity (more or less BOLD activity) in response to higher or lower DA levels (Braskie et al., 2008; Bäckman et al., 2011; Landau et al., 2009). There is evidence, however, for a positive link between striatal D2 binding and prefrontal BOLD activation during LTM updating (Nyberg et al., 2009). Also, recent animal work using simultaneous positron‐emission tomography (PET) and fMRI assessment demonstrates a relationship between striatal receptor occupancy and BOLD activation (Sander et al., 2013).
These results provide evidence for a relationship between striatal DA availability and functional brain activity, and suggest that the current observations of age and DRD2 genotype effects on striatal BOLD activation may reflect dopaminergic alterations critical to memory updating. In sum, the present study provides novel evidence for a link between neurocognitive deficits related to LTM updating and the DRD2/ANKK1‐TaqIA polymorphism, and show that this effect is magnified in aging. These results thus support the resource‐modulation hypothesis, which assumes that age‐related losses of brain resources modulate the effects of genetic variation on brain and cognition (Lindenberger et al., 2008). Our genetic, brain imaging, and behavioral data suggest that one such resource may be striatal DA functions.
Supporting information
Supplementary Information
Supplementary Information
ACKNOWLEDGMENTS
This research was conducted at the Karolinska Institute MR‐Center, Huddinge Hospital, Stockholm, Sweden. The authors wish to thank Joakim Svärd for assistance in data collection and entry. The authors declare no competing financial interests.
REFERENCES
- Bartres‐Faz D, Junque C, Serra‐Grabulosa JM, Lopez‐Alomar A, Moya A, Bargallo N, Clemente IC (2002): Dopamine DRD2 Taq I polymorphism associates with caudate nucleus volume and cognitive performance in memory impaired subjects. Neuroreport 13:1121–1125. [DOI] [PubMed] [Google Scholar]
- Berman SM, Noble EP (1995): Reduced visuospatial performance in children with the D2 dopamine receptor A1 allele. Behav Genet 25:45–58. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Wiener M, Stephens JA, Lohoff FW, Coslett HB (2013): COMT and ANKK1‐Taq‐Ia genetic polymorphisms influence visual working memory. PLoS ONE 8:e55862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braskie MN, Wilcox CE, Landau SM, O'Neil JP, Baker SL, Madison CM, Jagust WJ (2008): Relationship of striatal dopamine synthesis capacity to age and cognition. J Neurosci 28:14320–14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Barch DM (2002): A theory of cognitive control, aging cognition, and neuromodulation. Neurosci Biobehav Rev 26:809–817. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Ginovart N, Dixon RA, Wahlin TB, Wahlin A, Halldin C, Farde L (2000): Age‐related cognitive deficits mediated by changes in the striatal dopamine system. Am J Psychiatry 157:635–637. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L (2006): The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev 30:791–807. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li SC, Nyberg L (2010): Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neurosci Biobehav Rev 34:670–677. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Soveri A, Johansson J, Andersson M, Dahlin E, Rinne JO (2011): Effects of working‐memory training on striatal dopamine release. Science 333:718. [DOI] [PubMed] [Google Scholar]
- Cervenka S, Bäckman L, Cselényi Z, Halldin C, Farde L (2008): Associations between dopamine D2‐receptor binding and cognitive performance indicate functional compartmentalization of the human striatum. Neuroimage 40:1287–1295. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Guitart‐Masip M, Bunzeck N, Dolan RJ, Düzel E (2012): Dopamine modulates episodic memory persistence in old age. J Neurosci 32:14193–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colzato LS, van den Wildenberg WPM, Hommel B (2013): The genetic impact (C957T‐DRD2) on inhibitory control is magnified by aging. Neuropsychologia 51:1377–1381. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M (2009): Dopaminergic modulation of flexible control in humans In: Bjorklund A, Dunnett SB, Iversen LL, Iversen SD, editors. Dopamine Handbook. Oxford: Oxford University Press; pp 249–261. [Google Scholar]
- Cools R, D'Esposito M (2011): Inverted‐U‐shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L (2008): Transfer of learning after updating training mediated by the striatum. Science 320:1510–1512. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, Greicius MD. (Alzheimer's Disease Neuroimaging Initiative 2012). Gender modulates the APOE ε4 effect in healthy older adults: Convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci 32:8254–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan NJ, Fletcher P (1997): Dissociating prefrontal and hippocampal function in episodic memory encoding. Nature 388:582–585. [DOI] [PubMed] [Google Scholar]
- Erixon‐Lindroth N, Farde L, Wahlin TB, Sovago J, Halldin C, Bäckman L (2005): The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res 138:1–12. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM (2002): Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Garcia‐Garcia M, Barceló F, Clemente IC, Escera C (2011): COMT and ANKK1 gene‐gene interaction modulates contextual updating of mental representations. Neuroimage 56:1641–1647. [DOI] [PubMed] [Google Scholar]
- Gibbs SE, D'Esposito M (2005): Individual capacity differences predict working memory performance and prefrontal activity following dopamine receptor stimulation. Cogn Affect Behav Neurosci 5:212–221. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Fischl B (2006): Reliability of MRI‐derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. Neuroimage 32:180–194. [DOI] [PubMed] [Google Scholar]
- Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M (2009): Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. J Neurosci 29:3695–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson EG, Nöthen MM, Grünhage F, Farde L, Nakashima Y, Propping P, Sedvall GC (1999): Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry 4:290–296. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, D'Esposito M, Farah MJ (1997): Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport 8:3581–3585. [DOI] [PubMed] [Google Scholar]
- Klein TA, Neumann J, Reuter M, Hennig J, Von Cramon DY, Ullsperger M (2007): Genetically determined differences in learning from errors. Science 318:1642–1645. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Fischl B (2003): Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 60:878–888. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ (2009): Striatal dopamine and working memory. Cereb Cortex 19:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SC, Sikström S (2002): Integrative neurocomputational perspectives on cogntive aging, neuromodulation, and representation. Neurosci Biobehav Rev 26:795–808. [DOI] [PubMed] [Google Scholar]
- Li SC, Papenberg G, Nagel IE, Preuschhof C, Schröder J, Nietfeld W, Bäckman L (2013): Aging magnifies the effects of dopamine transporter and D2 receptor genes on backward serial memory. Neurobiol Aging 34: 358.e1–10. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Bäckman L (2008): Age‐related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci 2:234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA (2005): The hippocampal‐VTA loop: controlling the entry of information into long‐term memory. Neuron 46:703–713. [DOI] [PubMed] [Google Scholar]
- Matura S, Prvulovic D, Jurcoane A, Hartmann D, Miller J, Scheibe M, Pantel J (2014): Differential effects of the ApoE4 genotype on brain structure and function. NeuroImage 89:81–91. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, von Oertzen T, Sander T, Villringer A, Lindenberger U (2008): Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci 2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble EP (2003): D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B Neuropsychiatr Genet 116B:103–125. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Andersson M, Forsgren L, Jakobsson‐Mo S, Larsson A, Marklund P, Bäckman L (2009): Striatal dopamine D2 binding is related to frontal BOLD response during updating of long‐term memory representations. Neuroimage 46:1194–1199. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Frank MJ (2006): Making working memory work: A computational model of learning in the prefrontal cortex and basal ganglia. Neural Comput 18:283–328. [DOI] [PubMed] [Google Scholar]
- Papenberg G, Bäckman L, Nagel IE, Nietfeld W, Schröder J, Bertram L, Li SC (2013): Dopaminergic gene polymorphisms affect long‐term forgetting in old age: Further support for the magnification hypothesis. J Cogn Neurosci 25:571–579. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Någren K, Lehikoinen P, Anttila K, Syvälahti EK, Hietala J (1998): The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry 3:256–260. [DOI] [PubMed] [Google Scholar]
- N Raz, F Gunning‐Dixon, D Head, KM Rodrigue, A Williamson, JD Acker (2004): Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging 25:377–396. [DOI] [PubMed] [Google Scholar]
- N Raz, U Lindenberger, KM Rodrigue, KM Kennedy, D Head, A Williamson, C Dahle, D Gerstorf, JD Acker (2005): Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, Fischl B (2002): Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58:695–701. [DOI] [PubMed] [Google Scholar]
- Sander CY, Hooker JM, Catana C, Normandin MD, Alpert NM, Knudsen GM, Mandeville JB (2013): Neurovascular coupling to D2/D3 dopamine receptor occupancy using simultaneous PET/functional MRI. Proc Natl Acad Sci USA 110:11169–11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Tomer R, Christian BT, Fox AS, Colzato LS, King CR, Davidson RJ (2012): PET evidence for a role for striatal dopamine in the attentional blink: Functional implications. J Cogn Neurosci 24:1932–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ (2010): Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci 30:14205–14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Störmer VS, Passow S, Biesenack J, Li SC (2012): Dopaminergic and cholinergic modulations of visual‐spatial attention and working memory: Insights from molecular genetic research and implications for adult cognitive development. Dev Psychol 48:875–889. [DOI] [PubMed] [Google Scholar]
- Volkow ND (1996): Measuring age‐related changes in dopamine D2 receptors with 11C‐raclopride and 18F‐N‐methylspiroperidol. Psychiatry Res 67:11–16. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Stoessl AJ (1998): Age‐dependent decline of dopamine D1 receptors in human brain: A PET study. Synapse 30:56–61. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 1:58–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information


