Abstract
Recent studies have demonstrated the working memory impairment in patients with amnestic mild cognitive impairment (aMCI). However, the neurophysiological basis of the working memory deficit in aMCI is poorly understood. The aim of this study was to explore the abnormal activity during encoding and recognition procedures, as well as the reorganization of the background network maintaining the working memory state in aMCI. Using event‐related fMRI during a visuospatial working memory task with three recognition difficulty levels, the task‐related activations and network efficiency of the background network in 17 aMCI patients and 19 matched controls were investigated. Compared with cognitively healthy controls, patients with aMCI showed significantly decreased activity in the frontal and visual cortices during the encoding phase, while during the recognition phase, decreased activity was detected in the frontal, parietal, and visual regions. In addition, increased local efficiency was also observed in the background network of patients with aMCI. The results suggest patients with aMCI showed impaired encoding and recognition functions during the visuospatial working memory task, and may pay more effort to maintain the cognitive state. This study extends our understanding of the impaired working memory function in aMCI and provides a new perspective to investigate the compensatory mechanism in aMCI. Hum Brain Mapp 36:3387–3403, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: Functional MRI, amnestic mild cognitive impairment, visuospatial working memory, brain network, background functional connectivity
INTRODUCTION
“When a person has put something somewhere and cannot find it again” [Freud and Strachey, 1989] always occurs in our daily life, especially for elderly. Amnestic mild cognitive impairment (aMCI), characterized by the impairment of memory function, with or without impairment in other cognitive domains (such as attention, executive function, and visuospatial ability), is considered to have a higher risk of progressing to clinical Alzheimer's disease (AD) [Petersen and Morris, 2005; Petersen et al., 2006]. To extend our understanding of the neural processes of memory function involved in healthy and pathological aging, brain activity patterns in the context of impairment and compensation in healthy aging and patients with MCI/AD have become topics of increasing importance [Bai et al., 2009; Celone et al., 2006; Grady et al., 2005; Jin et al., 2012; Mattson et al., 2014; Remy et al., 2005; Salami et al., 2012].
Episodic and semantic memory impairments are considered as the earliest cognitive deficits in aMCI in previous studies [Hodges et al., 2006; Perry et al., 2000]. However, recent studies have found that deficits in working memory may also be a sensitive reflection of being aMCI [Economou et al., 2007; Saunders and Summers, 2011; Zheng et al., 2012]. Economou et al. [2006] were the first to reveal that the difference between working‐delayed memory is sensitive to detect early memory impairment, which is independent of age and education, using neuropsychological tests. Broster et al. [2013] assessed the repeated contextual working memory of patients with aMCI, mild AD, and normal elderly subjects using a visual working memory paradigm. They found that contextual working memory impairment could distinguish patients from normal elderly despite the observation that they had similar overall cognitive performance. In addition, Alescio‐Lautier et al. [2007] compared the performances of visual and visuospatial short‐term memory in patients with aMCI and AD, and reported that visuospatial short‐term memory deficits appeared earlier than visual short‐term memory. Their results suggested that the deficits of visuospatial short‐term memory performance may originate in the memory itself.
Functional magnetic resonance imaging (fMRI) evidences have demonstrated the altered brain activations in aMCI during episodic or semantic memory tasks [Bai et al., 2009; Jin et al., 2012; Woodard et al., 2009]. Dannhauser et al. [2008] observed decreased left ventrolateral prefrontal activations during verbal episodic memory encoding task and suggested the dysfunction of prefrontal cortex may affect the encoding stage of semantic processing in aMCI patients. Bai et al. [2009] investigated the hippocampus functional connectivity of patients with aMCI during an episodic memory retrieval task and reported altered connectivity in the prefrontal, temporal, parietal and cerebellum lobes for aMCI patients. Jin et al. [2012] investigated the functional abnormalities of aMCI patients during three different domains of episodic memory tasks and found decreased activations in the medial temporal lobes and increased prefrontal/precentral activations. Recently, Wang et al. [2013b] demonstrated the altered topological organization of the default mode network for aMCI patients who performed an episodic memory task using graph theory approaches. Zamboni et al. [2013] investigated the relationship between the performance in the Placing Test and the structure and function of the brain in healthy elderly, and found the performance in this visuospatial associative memory test was related with atrophy as well as functional activation in the medial temporal regions. This implies visuospatial associative memory impairment may be one of the earliest changes predicting cognitive impairments. However, the abnormal activity patterns in aMCI during a visuospatial working memory task remains largely unclear.
In recent years, more and more functional neuroimaging studies have started to investigate the brain activity/connectivity of AD/MCI during working memory tasks. Bokde et al. [2010] first reported on the altered brain activations in aMCI patients during a verbal working memory task using fMRI. A Positron emission tomography (PET) study also found the altered effective connectivity of AD during a delayed match‐to‐sample face recognition task [Rosenbaum et al., 2010]. Recently, Alichniewicz et al. [2012] investigated the structural and functional neural correlates of visuospatial working memory in aMCI patients, healthy elderly, and young adults, and found reduced frontal and parietal activations in the aMCI group during visuospatial information processing. Due to the n‐back task they used, they could not reveal the abnormal activity of aMCI in the encoding phase and recognition phase separately. Kochan et al. [2010] investigated the visuospatial working memory of MCI using a delayed recognition paradigm with graded working memory loads, and reported the variable activations/deactivations around the right anterior cingulare, precuneus, and posterior cingulate‐medial precuneus areas during the encoding process according to different working memory loads. They also found significantly decreased activations in the right lateral parietal lobe and medial parieto‐occipital cortices during the retrieval process. But, whether the variable activation exists in the recognition phase with different task difficulty levels is still unknown.
In addition, previous task‐based fMRI studies in MCI mainly focused on searching for the altered task‐related activations/deactivations, which reflect the response of the brain to external stimuli, but little is known about the internal mechanism which maintains their cognitive states. The fMRI activity during a certain task could be considered as the composition of task‐related activity, which is evoked by the external stimuli, and background activity, which is related to establishing and maintaining the current cognitive state [Turk‐Browne, 2013]. After regressing out the task‐evoked activity and nuisance variables, the background activity retained in the residuals would reflect the trial‐to‐trial fluctuations of different cognitive states, and would, most likely, contribute to the task state functional connectivity estimates [Cole et al., 2014; Rissman et al., 2004]. In recent years, the background connectivity, which derived from the functional connectivity of the background activity, has been used to investigate the functional connectivity of the brain during episodic memory encoding, visual processing, as well as working memory in healthy subjects [Al‐Aidroos et al., 2012; Geerligs et al., 2014c; Norman‐Haignere et al., 2012; Summerfield et al., 2006]. The background connectivity may provide a new insight to explore the cognitive state of brain. A previous study has suggested that the compensatory mechanism of aMCI is capable of maintaining a near‐normal performance as healthy elderly during high‐level cognitive tasks [Bai et al., 2009]. Thus, we hypothesized that background connectivity may play a specific role in the compensatory mechanism of aMCI.
In this study, we investigated the task‐related activations of encoding and recognition phases, as well as the background networks which were task‐activated, during a visuospatial working memory task with varying recognition difficulty levels. Our main purpose was to detect the abnormal activation patterns underlying impaired working memory in aMCI patients. In addition, we were also interested in revealing the influence of recognition difficulty levels during the recognition phase. Finally, we explored whether the background activity plays a compensatory role in aMCI during task execution.
METHODS
Participants
Twenty‐five patients with aMCI and twenty‐one cognitively healthy control elderly (HC) were recruited in this study. All the participants were recruited from local elderly community centers. The study was approved by the Clinical Research Ethics Committee of The Chinese University of Hong Kong (NTEC‐CUHK ethics committee). Written informed consent was obtained from all the participants.
All the participants underwent a battery of neuropsychological tests to evaluate their cognitive‐related functions. The Cantonese version of Mini‐Mental State Examination (CMMSE) [Chiu et al., 1994; Folstein et al., 1975] was used to evaluate the general cognitive function. The Clinical Dementia Rating (CDR) [Morris, 1993] scale was used to measure the severity of dementia. The Chinese version of the Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐Cog) [Chu et al., 2000; Rosen et al., 1984] was used to assess the global cognitive deficit in patients with aMCI. And, a 10‐minute ADAS‐Cog extended Delayed Recall was used as a measure of episodic memory. In addition, the forward and backward Digit Span tests from the Wechsler Adult Intelligence Scale [Wechsler, 1999] were used to assess the function of attention and working memory. The Category Verbal Fluency Test (CVFT) [Chiu et al., 1997; Lam et al., 2006] was used to examine executive and semantic memory functions.
The diagnosis of the aMCI was made by expert neurologists based on the Mayo Clinic Criteria [Petersen et al., 2001], which includes (1) subjective memory complaints, (2) objective memory impairment (i.e. Delayed Recall scores of at least 1.5 standard deviations below age‐ and education‐matched persons with a CDR of 0), (3) intact daily life activities, (4) CDR score of 0.5, and (5) no clinical dementia (CMMSE > 22 for elderly with more than 2 years of education, CMMSE > 20 for elderly with less than 2 years of education, and CMMSE > 19 for elderly with no education [Lam et al., 2010]). Participants with profound sensory deficits, psychiatric (i.e., dependence on alcohol or other substances) and/or neurological disorders other than dementia (i.e., head trauma, multiple sclerosis, and Parkinson's disease) were excluded in this study. Of the 25 patients with aMCI, 6 participants were excluded because they could not perform the visuospatial working memory task properly (with an accuracy of less than 50% for at least one condition) during the fMRI scanning, and two were excluded from further analysis due to excessive head movement. Of the 21 cognitive healthy controls, one was excluded due to excessive head movement and one was excluded because of poor image quality. In this case, 17 patients with aMCI and 19 control elderly subjects remained for further analysis. The demographics and clinical characteristics for each group are presented in Table 1.
Table 1.
Demographics and clinical characteristics
| HC (N = 19) | aMCI (N = 17) | P value | |
|---|---|---|---|
| Gender (M/F) | 9/10 | 9/8 | 0.738a |
| Age (years) | 71.11 ± 3.84 | 72.18 ± 2.90 | 0.356b |
| Education (years) | 8.68 ± 4.03 | 5.94 ± 5.14 | 0.082b |
| CMMSE | 28.74 ± 1.05 | 26.41 ± 2.81 | 0.003c |
| CDR | 0 | 0.5 | – |
| ADAS‐Cog | 4.91 ± 2.07 | 11.53 ± 4.25 | <0.001b |
| Delayed Recall | 7.21 ± 1.44 | 2.53 ± 1.74 | <0.001c |
| CVFT | 45.26 ± 10.01 | 34.41 ± 9.79 | 0.002b |
| Forward Digit Span | 8.16 ± 1.02 | 7.18 ± 1.43 | 0.040c |
| Backward Digit Span | 4.16 ± 1.83 | 2.69 ± 1.25 | 0.013c |
Data are presented as mean ± standard deviations. M, Male; F, Female; CMMSE, Cantonese version of mini‐mental state examination; CDR, clinical dementia rating scale; ADAS‐Cog, Alzheimer's disease assessment scale‐cognitive subscale; CVFT, category verbal fluency test.
Pearson chi‐square test was used.
Independent two‐sample t test was used.
Mann–Whitney test was used.
Experiment
A visuospatial working memory task (Fig. 1) was implemented using E‐Prime 1.1 (Psychology Software Tools). Subjects viewed the stimuli via a mirror mounted on the head coil. Five BBQ pork buns randomly scattered across the screen with black background were used as stimuli. A cue stimulus with 5 BBQ pork buns scattered across the screen was first presented for 1s (encoding). After a delay of 5–8 s, a probe stimulus was presented for 1s (recognition) and the participants were instructed to judge whether the probe stimulus was the same as the cue stimulus by pressing the button with the right index or the middle finger. The inter‐trial interval lasted for 13–16 s, randomly.
Figure 1.

Schematic description of the visuospatial working memory task. The stimulus was presented with five BBQ pork buns randomly spaced across a screen with black background. A cue stimulus was first presented for 1 s. After a delay of 5–8 s, a probe stimulus was presented for 1 s and the participants were instructed to judge whether the probe stimulus was the same as the cue stimulus by pressing the button with the right index or the middle finger. The difficulty level of the task was varied by 1–3 buns being moved in the probe stimuli compared to the cue stimuli. The inter‐trial interval lasted 13–16 s randomly. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For 25% of the trials, the locations of the BBQ pork buns in cue stimulus were the same as the probe stimulus (non‐changed trial), while for 75% of the trials, the locations were different between the cue and the probe stimuli (changed trial). There were three difficulty levels (T1, T2, and T3) for the changed stimuli with equal possibilities. T1–T3 indicated there were one to three buns located differently between the cue and the probe stimuli. The more buns in different locations, the easier it was to recognize the change. The three difficulty levels of changed stimuli and non‐changed stimuli were randomly intermixed within each run. There were 16 trials, lasting 384s, per run, and 4 runs were completed by each subject. Prior to the scanning, all participants were given instruction and practice outside MRI.
Behavioral Data Analysis
For each participant, the accuracy and mean reaction time for the correct responses were calculated for the three difficulty levels, respectively. Trials with a reaction time shorter than 200 ms or larger than 2000 ms were regarded as incorrect response. Six aMCI participants were excluded due to having an accuracy below 50% for at least one difficulty level, which indicated they could not understand/perform the task properly. The accuracy and reaction time of the changed trails were entered into repeated‐measures analyses of variance (ANOVA) to test for the main effects of group and difficulty level. Significant main effects and interactions measures were post hoc tested using t‐tests for normally distributed data and Mann‐Whitney for non‐normally distributed data.
Image Acquisition
The MRI images were acquired using a 3 Tesla Philips MRI scanner (Achieva TX, Philips Medical System, Best, the Netherlands) with an eight‐channel SENSE head coil. A 3D high resolution T1‐weighted anatomical image was obtained for each participant with the following parameters: repetition time (TR) = 7.4 ms, echo time (TE) = 3.4 ms, flip angle = 8°, voxel size = 1.04 × 1.04 × 0.6 mm3. Four runs of fMRI images during the working memory task were acquired with a T2‐weighted gradient echo‐planar imaging (EPI) sequence: TR = 3000 ms, TE = 35 ms, flip angle = 90°, field of view = 230 × 230 mm2, matrix = 96 × 96, 40 slices, voxel size = 2.4 × 2.4 × 3 mm3. A total of 128 volumes were obtained for each of the four runs. Before the participants performing the working memory task, a 4‐minute resting state fMRI images with eyes open were also collected (80 volumes).
Data Preprocessing
Functional MR imaging data were preprocessed using Statistical Parametric Mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm/). The first five volumes of each run were discarded to allow for T1 equilibration. The remaining 123 task functional images per run and 75 resting sate functional images were corrected for timing offsets between different slices and then realigned to the first volume of each run for rigid‐body head motion correction. The T1‐weighted anatomical image of each participant was then co‐registered to the mean realigned functional images and subsequently segmented into gray matter, white matter, and cerebrospinal fluid using a unified segmentation method [Ashburner and Friston, 2005]. A study‐specific (for aMCI and HC participants together) template was created using the Diffeomorphic Anatomical Registration Exponentiated Lie algebra (DARTEL) toolbox [Ashburner, 2007]. The corrected functional images were spatially normalized to the standard MNI space using the nonlinear normalization parameters estimated by DARTEL toolbox. Functional images were resampled to 2 × 2 × 2 mm3 and spatially smoothed with a 6 mm full‐width half maximum (FWHM) Gaussian kernel. Participants who had head movement larger than 3 mm translation or 3° of rotation in any direction were considered as excessive head movement during the scanning and were excluded. There was no significant difference for head motion in any direction between the two groups.
General Linear Model Analysis
A General Linear Model (GLM) was used to estimate the activity of the brain during the visuospatial working memory task. The onsets of probe stimuli for each difficulty level, non‐changed stimuli, as well as their cue stimuli were entered as different regressors. The onsets of cue/probe stimuli for incorrect trials were modeled as separate regressors. The regressors were convolved with the canonical hemodynamic response function (HRF), as well as its temporal derivative and dispersion. Additionally, six realignment parameters, ventricle, white matter, and whole brain signals, along with their first derivatives were included as nuisance covariates. We also performed the analysis without regressing out the whole brain signal and found the main results were similar with and without whole brain signal regression. For resting state fMRI, only the six realignment parameters, ventricle, white matter, and whole brain signals along with their first derivatives were modeled in the GLM. A high‐pass filter (128s cutoff period) was used to remove low‐frequency confounds, and a first‐order autoregressive model was used to correct for serial correlations.
For the task fMRI, the beta weights of the three HRF terms were combined by calculating the average area under the curve (AUC) for each condition [Geerligs et al., 2014a, 2014b; Kokal et al., 2009]. The resulting AUC images of each condition were entered into one sample t‐test and the task‐related activations during encoding and recognition procedures were identified for the HC group and aMCI group separately. To assess the between‐group differences during the encoding phase, the AUC values of the encoding phase were tested using an independent two sample t‐test. Then, with regard to the AUC values in the recognition phase, a random‐effects‐level repeated‐measures ANOVA with difficulty level (T1, T2, and T3) as within‐factor and group (aMCI and HC) as between‐factor was used to examine the effects of group and difficulty level during the recognition phase. The Monte Carlo simulation was used for multiple comparisons correction (P < 0.01 uncorrected, 10,000 iterations) to achieve a cluster‐level false‐positive rate of 0.05. All the between‐group comparisons were performed with gender, age, and education years as covariates. The regions showed significant main effect of group, main effect of difficulty level, or interaction of group by difficulty level were identified and the averaged beta weight AUC values in these regions were extracted for post‐hoc analysis. In addition, the relationships between the performance of task and the encoding activations as well as recognition activations (across task difficulty) were also investigated (P < 0.005, uncorrected, cluster number ≧30).
Background Brain Network Analysis
In this study, we mainly focused on the activated brain regions during the task, i.e., the working memory network. Firstly, the working memory network (i.e., task‐activated regions of the brain derived from the GLM analysis) was identified. The residuals of the GLM analysis were used to analyze the background activity of the working memory network. Twenty‐nine 10‐mm diameter‐spherical regions of interest (ROIs) in the working memory network (Fig. 2) were selected based on Power's 264 putative functional regions [Power et al., 2011], which represent the centers of putative regions defined using a combination of task‐fMRI meta‐analysis and resting‐state functional connectivity analysis [Cohen et al., 2008] and has been demonstrated with higher reliabilities for global and local functional network properties [Cao et al., 2014]. Average time course of the residual images in the 29 ROIs were extracted and mean‐centered for each run for both the task fMRI and resting fMRI. To reflect the background activity of subjects maintaining the task state, only the sample points in the task epochs, that is, from the first point after the onset of cue stimulus to the third point after the onset of the probe stimulus, were selected. As previous studies [Fair et al., 2007; Van Dijk et al., 2010] have demonstrated the functional connectivity derived from concatenation of discontinuous time series provided similar results as the continuous time series, the task epochs concatenated across runs were used to compute the correlation matrix as done previously [Cole et al., 2014; Geerligs et al., 2014c]. Only the correct changed trials were entered in the analysis. The Pearson correlation was used to evaluate the correlation between different ROI time series. The functional connectivity in the working memory network during the resting state was also estimated. Only those correlations whose corresponding P‐values pass through a statistical threshold (P < 0.05, FDR‐correction) were retained and a weighted‐undirected network was constructed for each subject.
Figure 2.
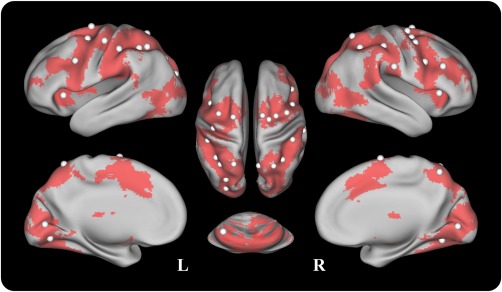
The node distribution of the working memory network for the background functional network analysis. The 29 ROIs were defined from Power's 264 putative functional regions [Power et al., 2011] and the MNI coordinates of the ROIs are shown in Supporting Information Table S1. The nodes are posted on an inflated surface rendering of the human brain using the CARET program [Van Essen et al., 2001]. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
The topological organization of the background working memory network was characterized by the network efficiency based on graph theory approaches, which was demonstrated to be susceptible to MCI/AD for the functional brain network during both resting and task states [Wang et al., 2013b; Zhao et al., 2012], as well as the structural brain network [Bai et al., 2012]. The global efficiency, Eglob, is used to measure the capacity of parallel information propagation in the network and defined as the inverse of harmonic mean of the shortest path length between each pair of nodes in a network as follows [Latora and Marchiori, 2001; Rubinov and Sporns, 2010],
where, is the shortest weighted path length between node i and node j in the network G, and NG indicates the number of nodes in the network.
The local efficiency of the whole network, E loc, is measured as
where Gi denotes the subgraph composed of the nearest neighbors of node i. The local efficiency reveals the extent of local cliquishness of information transfer in a network. The network efficiency in this study was estimated using the Brain Connectivity Toolbox (BCT, https://sites.google.com/site/bctnet/) [Rubinov and Sporns, 2010].
The background network efficiency comparisons between aMCI and HC groups were implemented using a non‐parametric permutation method (10,000 permutations) [Nichols and Holmes, 2002] adopted in other brain graph theoretical analysis studies [Meng et al., 2014; van den Heuvel et al., 2010; Wang et al., 2013a]. Before implementing the permutation tests, the influences of age, gender, and education were regressed out using multiple linear regressions.
The Relationships between Background Network Efficiency and Task‐Activations and Performance
The associations between background network efficiency and task‐activations in the brain regions with significant between‐group differences were also investigated. The AUC values in each region with significant between‐group differences were averaged. For the recognition phase, the averaged AUC values were calculated across the three difficulty levels. The Pearson correlation was used to assess the relationships for the aMCI and HC groups, separately. In addition, the relationships between the background network efficiency and the performance of the subjects during the task were also investigated for the aMCI group and HC group using Spearman test. Age, sex, and education years were also controlled and the associations were deemed significant if P < 0.05 (uncorrected).
RESULTS
Behavioral Data
Figure 3 illustrates the accuracy and average reaction time of the changed trials with different difficulty levels for HC and aMCI groups. Separate repeated ANOVA showed lower accuracy for aMCI patients compared to HC (F 1,34 = 5.86, P = 0.021), and no group difference was found in mean reaction time (F 1,34 = 2.45, P = 0.127). The accuracy was decreased (F 2,68 = 39.74, P < 0.001) and mean reaction time was increased (F 2,68 = 15.36, P < 0.001) with an increase of difficulty level (from T3 to T1) in both groups. There was no difficulty level by group interaction in either accuracy (F 2,68 = 0.40, P = 0.67) or mean reaction time (F 2,68 = 0.42, P = 0.66).
Figure 3.
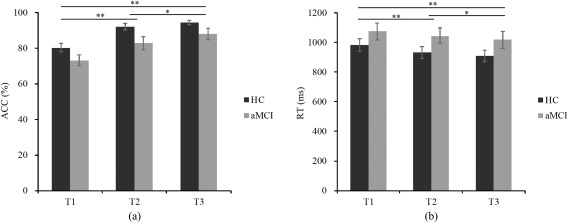
Behavioral data. (a) Accuracy and (b) mean reaction time of the recognition performance (T1‐T3 indicates 1‐3 buns moved in the probe stimuli compared to the cue stimuli. ACC, accuracy; RT, mean reaction time; * P < 0.05; ** P < 0.01. Error bars reflect the standard error of the mean (SEM)).
Task‐Related Activations
The task‐related activations of the encoding (Supporting Information Fig. S1) and recognition (Supporting Information Fig. S2) procedure were identified using the one sample t‐test (P < 0.01, cluster size >872 mm3), separately. The task‐related activations were mainly distributed in the frontal, parietal and visual areas, and comprised the frontoparietal network (FPN), the dorsal attention network (DAN), and the visual network (VN).
Between‐Group Differences
Regarding the encoding phase, a two‐sample t test was used to test the group differences of the task‐related activity. The aMCI group showed significantly decreased activity around the bilateral inferior frontal gyrus, bilateral middle frontal gyrus, calcarine sulcus/right cuneus, left lingual gyrus, as well as the bilateral cerebellum (lobule VI) compared to HC group (P < 0.01, cluster size >520 mm3, see Fig. 4, Table 2). There was no significantly increased activity in aMCI group compared to HC group.
Figure 4.
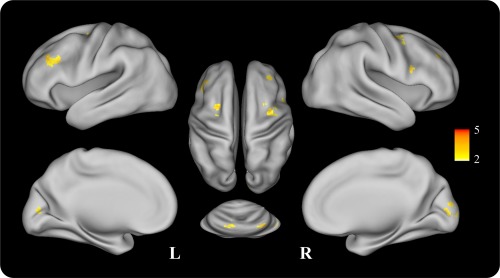
t‐map for the significantly decreased activity regions of aMCI compared to HC group during the encoding phase. The t‐map was projected on an inflated surface rendering of the human brain using the CARET program [Van Essen et al., 2001]. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Areas showed significantly decreased activity in aMCI group compared to HC group during the encoding phase
| Peak coordinates | ||||
|---|---|---|---|---|
| Cluster size | Regions | x | y | z |
| 147 | L Inferior/Middle Frontal Gyrus | −38 | 36 | 32 |
| 65 | R Inferior Frontal Gyrus | 50 | 10 | 18 |
| 156 | L Middle/Superior Frontal Gyrus | −22 | 4 | 56 |
| 126 | R Middle Frontal Gyrus/Precentral | 36 | 0 | 52 |
| 85 | R Middle Frontal Gyrus | 34 | 38 | 30 |
| 198 | Calcarine/R Cuneus | 16 | −92 | 6 |
| 116 | L Cerebelum 6/Lingual Gyrus | −14 | −74 | −14 |
| 104 | R Cerebelum 6/Crus1 | 36 | −72 | −24 |
L = Left, R = Right.
During the recognition phase, the repeated ANOVA yielded a main effect of group (P < 0.01, cluster size >568 mm3) in the left intraparietal sulcus, left postcentral gyrus, right cuneus, right angular gyrus, right inferior frontal gyrus, and right medial frontal gyrus (Fig. 5, Table 3). Post hoc Mann–Whitney tests indicated significantly reduced activations for aMCI patients in all the regions except the right inferior frontal gyrus/precentral gyrus for all three difficulty level conditions, while significantly increased activation was found in the right inferior frontal gyrus/precentral gyrus for all three difficulty levels in the aMCI group compared to the HC group (Fig. 6a).
Figure 5.
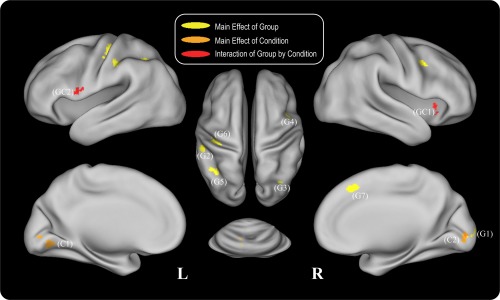
Repeated ANOVA results for the recognition phase. Areas which showed a main effect of group are depicted in yellow, areas which showed a main effect of difficulty level condition are depicted in orange, and areas which showed an interaction effect of group by condition are depicted in red. The indexed numbers next to the areas correspond to the ROI indices in the post‐hoc analysis in Figure 6. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 3.
Areas showed main effect of group, main effect of condition, and interaction of group by condition during the recognition phase.
| Peak coordinates | |||||
|---|---|---|---|---|---|
| Cluster size | Regions | x | y | z | |
| Main effect of group | 120 | R Cuneus/Calcarine | 22 | −90 | 8 |
| 178 | L Intraparietal Sulcus/Inferior Parietal Lobule | −52 | −30 | 44 | |
| 121 | R Angular | 32 | −60 | 44 | |
| 76 | R Inferior Frontal Gyrus/Precentral Gyrus | 44 | 6 | 38 | |
| 125 | L Intraparietal Sulcus/Inferior Parietal Lobule | −50 | −50 | 38 | |
| 205 | L Postcentral/Precentral Gyrus | −34 | −26 | 56 | |
| 83 | R Medial Frontal Gyrus | 6 | 28 | 46 | |
| Main effect of condition | 168 | L Lingual Gyrus | −18 | −72 | −6 |
| 81 | R Lingual Gyrus/Calcarine | 14 | −88 | 0 | |
| Interaction of group by condition | 124 | R Putamen/Insula | 32 | 16 | −8 |
| 110 | L Precentral Gyrus | −52 | 6 | 10 | |
L = Left, R = Right.
Figure 6.
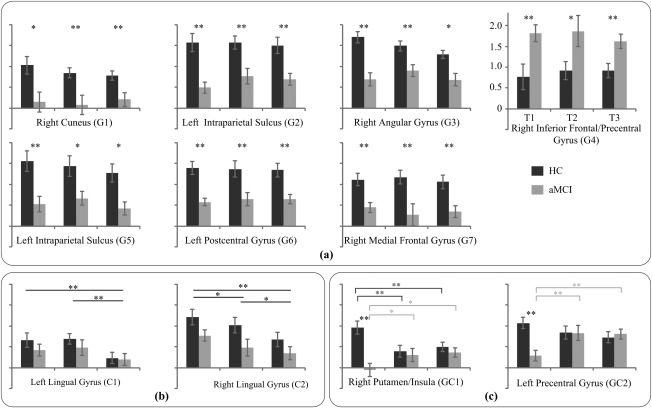
The average AUC values in the regions with (a) a main effect of group, (b) a main effect of difficulty level, and (c) an interaction of group by condition derived from the ANOVA analysis during the recognition phase (*P < 0.05, **P < 0.01. Error bars reflect the SEM).
Effect of Difficulty Level in Recognition Phase
A main effect of difficulty level was found in the bilateral lingual gyrus (P < 0.01, cluster size >568 mm3, Fig. 5, Table 3). Post hoc analysis showed increased activations in conditions T1 and T2 compared with T3 in the bilateral lingual gyrus, and significantly increased activation in T1 condition compared with T2 condition was also observed in the right lingual gyrus (Fig. 6b).
Interactions between Group and Difficulty Level in Recognition Phase
A group by difficulty level interaction was found in right putamen/insula and left precentral gyrus (P < 0.01, cluster size > 568 mm3, Fig. 5, Table 3). Post hoc analysis showed significantly reduced activations in patients with aMCI in both regions for the T1 condition compared to healthy controls. In addition, for the HC group, the activation of the T1 condition was increased compared to the T2 and T3 conditions in the right putamen/insula region, while the activation was decreased in the T1 condition compared with the T2 and T3 conditions for the aMCI group. The decreased activation of the T1 condition was also found in the left precentral gyrus for the aMCI group (Fig. 6c).
Correlations between Brain Activations and Task Performance
In the aMCI group, the accuracy showed a positive correlation with encoding activations in the left inferior frontal gyrus/precentral (x = −52, y = 8, z = 12, k = 32), and recognition activations in the right medial frontal gyrus (x = 6, y = 22, z = 48, k = 39), right superior parietal lobule (x = 26, y = −62, z = 54, k = 30), left inferior parietal lobule (x = −50, y = −38, z = 40, k = 54), and left fusiform gyrus (x = −40, y = −60, z = −18, k = 45). In the HC group, only a negative correlation between the reaction time and encoding activations in the left precentral gyrus (x = −48, y = −4, z = 50, k = 34) was found.
Background Network Efficiency Analysis
During the visuospatial working memory task, the background network efficiency analysis showed that the local efficiency of the working memory network in aMCI patients was significantly higher compared to the cognitively healthy controls (P = 0.005), and there was no significant difference between the two groups in global efficiency (P = 0.054). In contrast, no significant difference was found in either the local efficiency (P = 0.32) or global efficiency (P = 0.46) between the aMCI and the HC groups in the resting state (Fig. 7).
Figure 7.
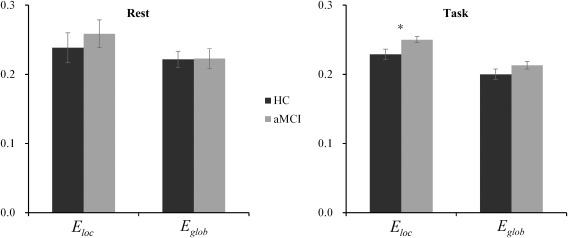
The background network efficiency of HC and aMCI groups during resting state and visuospatial working memory task. (Rest = resting state; Task = visuospatial working memory task; *P < 0.05. Error bars reflect the SEM).
A significant correlation between the background network efficiency and task‐related activations was only found in the right inferior frontal gyrus/precentral gyrus in the aMCI group during the recognition phase. The activations of right inferior frontal gyrus/precentral gyrus were negatively correlated with both the global efficiency (r = −0.60, P = 0.023) and the local efficiency (r = −0.65, P = 0.013).
No significant correlation between the network efficiency and the performance of visuospatial working memory task was found in the aMCI group. Within the HC group, the correlation analysis showed that higher global efficiency (r = −0.52, P = 0.037) and local efficiency (r = −0.58, P = 0.019) were associated with poor task performance as measured by accuracy.
DISCUSSION
To reveal the abnormal activity of the working memory network in patients with aMCI, in this study, we investigated the task‐related activations as well as the network topology of the background activity among the activated regions during a visuospatial working memory task. In particular, the impaired encoding and recognition in aMCI were studied separately. And different abnormal activity patterns during these two phases were observed. In addition, the background activity of the working memory network, which was derived from the residual of the GLM analysis, was used to assess the maintained cognitive state during the task.
Task‐Related Activations
During both the encoding and recognition phases, the task‐related activations were mainly distributed around the fronto‐parietal network, dorsal attention network, as well as the visual network. The activated patterns were well in line with previous working memory studies [Bittner et al., 2014; Meulenbroek et al., 2010; van den Bosch et al., 2014], which confirmed the involvement of visual input encoding, maintenance, task planning, attention, as well as recognition during the visuospatial working memory task.
Correlation analysis between task‐related activations and task performance revealed that the task performance of aMCI patients were positively correlated to the left inferior frontal encoding activations, and to the right medial frontal, right superior parietal, left inferior parietal, and left fusiform recognition activations. Only the left precentral encoding activations showed a negative correlation with the performance of reaction time in the HC group. The results implied that both encoding and recognition procedures are critical for performance in the aMCI group, while only the encoding procedure seems to play a critical role in the performance of the HC group. Grady et al. [Grady et al., 2003; Bokde et al., 2010] have reported the task performance during semantic and episodic memory tasks was correlated to a compensatory network in AD. For the HC group, the negative correlation between reaction time and left precentral activations may indicate that greater precentral activations during the encoding phase would lead to more efficient recognition in the working memory task. Because the task performance of the aMCI group positively correlated with brain activations in regions not found in HC group, the aMCI group would use alternative regions to improve the performance in the task.
Group Differences in Encoding Activations
Compared to cognitively healthy elderly, aMCI patients showed impaired encoding activity in the frontal (bilateral inferior/middle frontal gyrus) and visual areas (bilateral calcarine sulcus, left lingual gyrus, and cerebellum lobule VI). The decreased frontal activity had been widely reported in previous studies [Alichniewicz et al., 2012; Dannhauser et al., 2008; Machulda et al., 2009; Petrella et al., 2006]. Petrella et al. [2006] investigated the abnormal brain activations of patients with MCI during a face‐name encoding‐retrieval task, and found MCI patients showed decreased activations in the bilateral frontal cortex and left cerebellum during encoding compared to healthy controls. Dannhauser et al. [2008] reported decreased activation in left ventrolateral prefrontal cortex in aMCI during verbal episodic memory encoding. Machulda et al. [2009] investigated the activations of encoding and recognition of MCI patients using a block design paradigm, and reported decreased activations in the bilateral temporoparietal and frontal regions in MCI during the encoding phase. Alichniewicz et al. [2012] investigated the abnormal activations of aMCI patients performing a visuospatial working memory task and found that they showed a reduced activation in the bilateral inferior/middle frontal gyrus, left superior parietal lobule, and right inferior parietal lobule. Decreased frontal activity has been consistently reported in these studies. The inconsistent abnormal activations in these studies may be due to differences in the nature of the tasks the subjects performed. Kochan et al. [2010] have demonstrated the variability of activation/deactivation in patients with MCI during encoding with different task difficulties.
Reduced activations around the visual areas, including left lingual gyrus, bilateral calcarine sulcus, as well as cerebellum lobule VI, were also found in this study. Harrison and Tong [2009] have demonstrated the orientations held in working memory could be decoded from the activity patterns in early visual areas. Recent meta‐analysis also reported that activations in bilateral lobule VI and left Crus 2 of the cerebellum to be consistently associated with working memory [Keren‐Happuch et al., 2014]. Cohen et al. [2014] reported the correspondence between the encoding and maintenance phases in regional activity in the lateral prefrontal cortex as well as connectivity between lateral prefrontal cortex and extrastriate cortex was associated with successful working memory performance. The results in the current study imply that the reduced working memory performance in patients with aMCI occurs from the encoding phase with a dysfunction in frontal and visual areas.
Group Differences in Recognition Activations
During the recognition phase, patients with aMCI showed decreased activations in the inferior parietal cortex, medial frontal gyrus, and visual area, and increased activation in right inferior frontal/precentral gyrus compared to healthy elderly for all three difficulty levels. Decreased frontal and parietal activations in aMCI patients were consistently reported in previous studies [Alichniewicz et al., 2012; Kochan et al., 2010; Machulda et al., 2009; Petrella et al., 2006]. During the recognition phase, participants would match the locations of BBQ pork buns in the incoming probe stimulus to that of the internal cue stimulus held in the visuospatial working memory. The aMCI‐sensitive regions found in this study are essential for working memory recognition [Bledowski et al., 2006; Rahm et al., 2014; Wagner et al., 2005]. The intraparietal sulcus was reported to be sensitive to the spatial information retained in visual working memory [Harrison et al., 2010] and the right angular gyrus was considered to be involved in visuospatial fact recollection/retrieval [Arsalidou and Taylor, 2011; Seghier, 2013]. The medial frontal and motor cortex activations were reported to reflect the action selection cognitive process by Bledowski et al. [2006] using a combined fMRI and event‐related potentials approach. The decreased parietal and frontal recognition activations indicate that impaired recognition may be a syndrome of information maintenance, information recollection, as well as action selection impairment in aMCI.
Similarly to the encoding phase, decreased activity in the visual region (right cuneus) was also observed during the recognition phase. de Rover et al. [2008] found enhanced lingual gyrus/fusiform and cuneus activity when subjects used a spatial‐associative structure for retrieval, which demonstrated that higher‐order visual regions are also involved in the retrieval process. Alichniewicz et al. [2012] also observed the increased bilateral cuneus activity in healthy elderly compared to younger adults during the visuospatial information processing and speculated it as a task‐dependent compensatory mechanism in healthy elderly adults. However, no significant difference in cuneus regions between healthy elderly and aMCI patients was found, which may be due to the n‐back task they used being unable to dissociate the encoding and retrieval phases effectively. The decreased cuneus activity during the retrieval phase may imply that patients with aMCI could not effectively recruit the compensatory mechanism of the cuneus as could healthy elderly. Our findings of encoding and recognition dysfunction in the visual areas may provide new insights in the role of visual regions in visuospatial working memory impairment in aMCI.
In addition, partially consistent with the findings of [Petrella et al., 2006], increased activity in the inferior frontal gyrus, notably in the right precentral gyrus, was found in this study. The result was consistent with the frontal compensatory mechanism in aMCI for both task and resting states [Petrella et al., 2006; Qi et al., 2010]. The frontal compensatory mechanism was also reported in healthy elderly for both encoding and recognition tasks and was interpreted to compensate for the medial temporal lobe impairment [Grady et al., 2005; Gutchess et al., 2005]. Our result confirms the frontal compensatory mechanism presented in aMCI.
Effect of Task Difficulty Level during the Recognition Procedure
There may be two strategies (“where” or “what”) for the subjects to perform the working memory task [Alescio‐Lautier et al., 2007; Grady et al., 2008; Harrison et al., 2010]. For the “where” strategy, the locations of the buns in the cue stimuli were encoded/maintained and matched with the probe stimuli. The difficulty of the task should dependent on number of the locations changed. For the “what” strategy, the content of stimuli would be simplify encoded as a whole, and compared with the probe “image”; the difficulty of the task would rely on the content of the stimuli. As the main effect of task difficulty was found in both the behavioral and neuroimaging results, it is possible that subjects focused more on the locations' information and performed the task using the “where” strategy.
Task difficulty has been consistently reported to influence the activity of the visual cortex using various neuroimaging techniques (i.e., fMRI, PET, EEG) [Banko et al., 2011; Garrett et al., 2014; Grady et al., 1996]. In this study, with the increase of recognition difficulty levels, the activations of the bilateral lingual gyrus were also increased. Schmidt et al. [2007] observed the activity of lingual gyrus was enhanced with increasing changes of the point of view in 3D space between the encoding and retrieval processes. This area was considered to reflect the matching process between the incoming sensory information and the stored information. In this study, with the increasing level of recognition difficulty, a more intensive matching process would be required. Our findings indicate the lingual gyrus involves not only the matching process in 3D space, but also in 2D space. Similar to the role of fusiform area in face matching task [Bokde et al., 2005], task difficulty increased the activations of the lingual gyrus to enhance the spatial matching processing and implies a specific role of the lingual gyrus in spatial matching.
The significantly decreased activations in left precentral gyrus and right putamen/insula in aMCI patients were only found for the highest difficulty level recognition process (T1 condition). For patients with aMCI, they could not activate these areas effectively and significantly decreased activity was found compared to easier recognition conditions (T2 and T3 conditions). For cognitively healthy controls, increased activity was observed at the highest level of difficulty condition compared to easier conditions. Complementing the study of Kochan et al. [2010], the current results demonstrate the variable activation in aMCI patients during recognition phase with different task difficulty.
Background Network Efficiency
Moreover, we also investigated the background activity of the working memory network using network efficiency based on graph theory approaches for both the task‐state and resting‐state fMRI. The background activity constitutes part of the “residual” time‐series and is considered to reflect the activity related to establishing and maintaining the current cognitive state [Al‐Aidroos et al., 2012; Geerligs et al., 2014c; Norman‐Haignere et al., 2012; Summerfield et al., 2006; Turk‐Browne, 2013]. In this study, the background connectivity but not the psycho‐physiological interaction (PPI) approach was used to estimate the functional connectivity as we were interested in the consistently maintained activity during the task while the PPI method would remove it out [Cole et al., 2014]. The aMCI group showed a significantly increased local efficiency compared to the HC group, which indicated the reorganization of the background working memory network in aMCI. Wang et al. also found the increased local efficiency of the default mode network in aMCI patients during an episodic memory task, but no significant network efficiency was found between the two groups during the resting state [Wang et al., 2013b]. Similar to their study, no significant network efficiency difference was found between the two groups during the resting state. Our findings suggested the topologic reorganization of the network not only exists in the deactivated default mode network but also in the activated working memory network. Compared to resting state, the increased local network efficiency was more apparent in the aMCI group during the working memory task, which may imply that aMCI patients have to pay more effort during the task due to their memory deficits. Within the HC group, both the global and local efficiency were negatively correlated with the accuracy of the task performance, which indicated the worse of the subjects' performance, the higher global and local efficiencies. The increased network efficiency may suggest the compensatory property of the background network, and subjects have to pay more effort to complete the task for patients with mild cognitive impairment or healthy elderly without cognitive impairment but worse performance.
In addition, we found a negative correlation between the network efficiency and the recognition activations of the right inferior frontal/precentral gyrus in the aMCI group. The right inferior frontal/precentral gyrus recognition activations were increased and considered as compensatory mechanism presented in aMCI. Our current results indicated that the more effort the subject paid to complete the task, the less activations of the prefrontal cortex. Recently, Lara et al. [Lara and Wallis, 2014] reported the role of prefrontal cortex is in controlling the allocation of resources to support working memory using local field potential analysis. However, the causality between the prefrontal activations and background connectivity is not clear based on current result. The compensation presented in aMCI during the working memory task might by a mechanism that coordinates the prefrontal activations and background activity so that the aMCI patients could maintain a near‐normal performance akin to healthy elderly. It is possible that when the balance of the prefrontal activations and background activity breakdown, the compensatory mechanism would disappear and aMCI might progress to dementia. Further study should be investigated to assess whether the balance breakdown exists for AD patients.
Limitations
Several aspects in this study are still limited and could be explored in future research. First, this study only focused on the task‐positive networks, the task‐negative network (default mode network, DMN) activations and the background network efficiency of the DMN should also be important in aMCI pathology and working memory task. In addition, the relationships of the task‐positive networks and task‐negative networks from the perspective of the background network are intriguing and should also be investigated in future. Second, the sample size is relatively small, a larger sample size should be recruited to increase the statistical power. Finally, we postulated both the prefrontal activations and the background network efficiency play a compensatory role in patients with aMCI, and their balance may be broken down when the subjects progress to dementia. Whether the balance is breakdown should be investigated in AD patients in the future. Moreover, a long‐term longitudinal study should be performed to further unravel the mechanism of compensation.
CONCLUSIONS
In conclusion, we investigated the task‐related activations of the brain during the encoding and recognition phases as well as the cognitive maintained background network during a visuospatial working memory task separately in patients with aMCI and cognitively healthy controls. Impaired frontal and visual areas encoding activity, abnormal frontal, parietal and visual regions recognition activity, as well as increased background local efficiency were found in patients with aMCI compared to healthy elderly controls. The results indicated the impaired encoding and recognition functions in aMCI and implied the specific role of visual area in aMCI during the visuospatial working memory task. In addition, the background network efficiency analysis suggests that the background activity may play a compensatory role in aMCI. This study enhances the understanding of the mechanism of impaired working memory function in aMCI and provides a new perspective to study the compensatory mechanism in aMCI.
Supporting information
Supporting Information
ACKNOWLEDGMENT
We would like to thank the three anonymous reviewers for their constructive comments.
REFERENCES
- Al‐Aidroos N, Said CP, Turk‐Browne NB (2012): Top‐down attention switches coupling between low‐level and high‐level areas of human visual cortex. Proc Natl Acad Sci USA 109:14675–14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alescio‐Lautier B, Michel BF, Herrera C, Elahmadi A, Chambon C, Touzet C, Paban V (2007): Visual and visuospatial short‐term memory in mild cognitive impairment and Alzheimer disease: Role of attention. Neuropsychologia 45:1948–1960. [DOI] [PubMed] [Google Scholar]
- Alichniewicz KK, Brunner F, Klunemann HH, Greenlee MW (2012): Structural and functional neural correlates of visuospatial information processing in normal aging and amnestic mild cognitive impairment. Neurobiol Aging 33:2782–2797. [DOI] [PubMed] [Google Scholar]
- Arsalidou M, Taylor MJ (2011): Is 2 + 2=4? Meta‐analyses of brain areas needed for numbers and calculations. Neuroimage 54:2382–2393. [DOI] [PubMed] [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Bai F, Shu N, Yuan YG, Shi YM, Yu H, Wu D, Wang JH, Xia MR, He Y, Zhang ZJ (2012): Topologically convergent and divergent structural connectivity patterns between patients with remitted geriatric depression and amnestic mild cognitive impairment. J Neurosci 32:4307–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Zhang ZJ, Watson DR, Yu H, Shi YM, Yuan YG, Zang YF, Zhu CZ, Qian Y (2009): Abnormal functional connectivity of hippocampus during episodic memory retrieval processing network in amnestic mild cognitive impairment. Biol Psychiatry 65:951–958. [DOI] [PubMed] [Google Scholar]
- Banko EM, Gal V, Kortvelyes J, Kovacs G, Vidnyanszky Z (2011): Dissociating the effect of noise on sensory processing and overall decision difficulty. J Neurosci 31:2663–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner RA, Linden DEJ, Roebroeck A, Härtling F, Rotarska‐Jagiela A, Maurer K, Goebel R, Singer W, Haenschel C (2014): The when and where of working memory dysfunction in Early‐onset schizophrenia—a functional magnetic resonance imaging study. Cereb Cortex (in press). doi: 10.1093/cercor/bhu050 [DOI] [PubMed] [Google Scholar]
- Bledowski C, Kadosh KC, Wibral M, Rahm B, Bittner RA, Hoechstetter K, Scherg M, Maurer K, Goebel R, Linden DEJ (2006): Mental chronometry of working memory retrieval: a combined functional magnetic resonance imaging and event‐related potentials approach. J Neurosci 26:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde ALW, Dong W, Born C, Leinsinger G, Meindl T, Teipel SJ, Reiser M, Hampel H (2005): Task difficulty in a simultaneous face matching task modulates activity in face fusiform area. Brain Res Cogn Brain Res 25:701–710. [DOI] [PubMed] [Google Scholar]
- Bokde ALW, Karmann M, Born C, Teipel SJ, Omerovic M, Ewers M, Frodl T, Meisenzahl E, Reiser M, Moller HJ, Hampel H (2010): Altered brain activation during a verbal working memory task in subjects with amnestic mild cognitive impairment. J Alzheimers Dis 21:103–118. [DOI] [PubMed] [Google Scholar]
- Broster LS, Li J, Smith CD, Jicha GA, Schmitt FA, Jiang Y (2013): Repeated retrieval during working memory is sensitive to amnestic mild cognitive impairment. J Clin Exp Neuropsychol 35:946–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Plichta MM, Schafer A, Haddad L, Grimm O, Schneider M, Esslinger C, Kirsch P, Meyer‐Lindenberg A, Tost H (2014): Test‐retest reliability of fMRI‐based graph theoretical properties during working memory, emotion processing, and resting state. Neuroimage 84:888–900. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA (2006): Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci 26:10222–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu FK, Lee HC, Chung WS, Kwong PK (1994): Reliability and validity of the cantonese version of the Mini‐mental state examination: A preliminary study. J Hong Kong Col Psychiatrists 4:25–28. [Google Scholar]
- Chiu HFK, Chan CKY, Lam LCW, Ng KO, Li SW, Wong M, Chan WF (1997): The modified fuld verbal fluency test: A validation study in Hong Kong. J Gerontol Ser B‐Psychol Sci Soc Sci 52:P247–P250. [DOI] [PubMed] [Google Scholar]
- Chu LW, Chiu KC, Hui SL, Yu GKK, Tsui WJC, Lee PWH (2000): The reliability and validity of the Alzheimer's disease assessment scale cognitive subscale (ADAS‐cog) among the elderly Chinese in Hong Kong. Ann Acad Med Singapore 29:474–485. [PubMed] [Google Scholar]
- Cohen AL, Fair DA, Dosenbach NUF, Miezin FM, Dierker D, Van Essen DC, Schlaggar BL, Petersen SE (2008): Defining functional areas in individual human brains using resting functional connectivity MRI. Neuroimage 41:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Sreenivasan KK, D'Esposito M (2014): Correspondence between stimulus Encoding‐ and Maintenance‐related neural processes underlies successful working memory. Cereb Cortex 24:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE (2014): Intrinsic and Task‐evoked network architectures of the human brain. Neuron 83:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannhauser TM, Shergill SS, Stevens T, Lee L, Seal M, Walker RWH, Walker Z (2008): An fMRI study of verbal episodic memory encoding in amnestic mild cognitive impairment. Cortex 44:869–880. [DOI] [PubMed] [Google Scholar]
- de Rover M, Petersson KM, van der Werf SR, Cools AR, Berger HJ, Fernandez G (2008): Neural correlates of strategic memory retrieval: differentiating between spatial‐associative and temporal‐associative strategies. Hum Brain Mapp 29:1068–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economou A, Papageorgiou S, Karageorgiou C (2006): Working‐delayed memory difference detects mild cognitive impairment without being affected by age and education. J Clin Exp Neuropsychol 28:528–535. [DOI] [PubMed] [Google Scholar]
- Economou A, Papageorgiou SG, Karageorgiou C, Vassilopoulos D (2007): Nonepisodic memory deficits in amnestic MCI. Cogn Behav Neurol 20:99–106. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE (2007): A method for using blocked and event‐related fMRI data to study "resting state" functional connectivity. Neuroimage 35:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR (1975): “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res 12:189–198. [DOI] [PubMed] [Google Scholar]
- Freud S, Strachey J 1989: Introductory Lectures on Psychoanalysis. Norton, New York.
- Garrett DD, McIntosh AR, Grady CL (2014): Brain signal variability is parametrically modifiable. Cereb Cortex 24:2931–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Maurits NM, Renken RJ, Lorist MM (2014a): Reduced specificity of functional connectivity in the aging brain during task performance. Hum Brain Mapp 35:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Saliasi E, Maurits NM, Renken RJ, Lorist MM (2014b): Brain mechanisms underlying the effects of aging on different aspects of selective attention. Neuroimage 91:52–62. [DOI] [PubMed] [Google Scholar]
- Geerligs L, Saliasi E, Renken RJ, Maurits NM, Lorist MM (2014c): Flexible connectivity in the aging brain revealed by task modulations. Hum Brain Mapp 35:3788–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Horwitz B, Pietrini P, Mentis MJ, Ungerleider LG, Rapoport SI, Haxby JV (1996): Effect of task difficulty on cerebral blood flow during perceptual matching of faces. Hum Brain Mapp 4:227–239. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Beig S, Keightley ML, Burian H, Black SE (2003): Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. J Neurosci 23:986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FIM (2005): Task‐related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia 43:1466–1481. [DOI] [PubMed] [Google Scholar]
- Grady CL, Yu H, Alain C (2008): Age‐related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cereb Cortex 18:189–199. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, Park DC (2005): Aging and the neural correlates of successful picture encoding: frontal activations compensate for decreased medial‐temporal activity. J Cogn Neurosci 17:84–96. [DOI] [PubMed] [Google Scholar]
- Harrison A, Jolicoeur P, Marois R (2010): "what" and "where" in the intraparietal sulcus: An fMRI study of object identity and location in visual Short‐term memory. Cereb Cortex 20:2478–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Tong F (2009): Decoding reveals the contents of visual working memory in early visual areas. Nature 458:632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges JR, Erzinclioglu S, Patterson K (2006): Evolution of cognitive deficits and conversion to dementia in patients with mild cognitive impairment: a very‐long‐term follow‐up study. Dement Geriatr Cogn Disord 21:380–391. [DOI] [PubMed] [Google Scholar]
- Jin MW, Pelak VS, Curran T, Nandy RR, Cordes D (2012): A preliminary study of functional abnormalities in aMCI subjects during different episodic memory tasks. Magn Reson Imaging 30:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren‐Happuch E, Chen SHA, Ho MHR, Desmond JE (2014): A Meta‐analysis of cerebellar contributions to higher cognition from PET and fMRI studies. Hum Brain Mapp 35:593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan NA, Breakspear M, Slavin MJ, Valenzuela M, McCraw S, Brodaty H, Sachdev PS (2010): Functional alterations in brain activation and deactivation in mild cognitive impairment in response to a graded working memory challenge. Dement Geriatr Cogn Disord 30:553–568. [DOI] [PubMed] [Google Scholar]
- Kokal I, Gazzola V, Keysers C (2009): Acting together in and beyond the mirror neuron system. Neuroimage 47:2046–2056. [DOI] [PubMed] [Google Scholar]
- Lam LCW, Ho P, Lui VWC, Tam CWC (2006): Reduced semantic fluency as an additional screening tool for subjects with questionable dementia. Dement Geriatr Cogn Disord 22:159–164. [DOI] [PubMed] [Google Scholar]
- Lam LCW, Tam CWC, Leung GTY, Lui VWC, Fung AWT, Chiu HFK, Chan SSM, Chan WC, Ng S, Chan WM (2010): Combined clinical and cognitive criteria to identify mild cognitive impairment in a southern chinese community. Alzheimer Dis Assoc Dis 24:343–347. [DOI] [PubMed] [Google Scholar]
- Lara AH, Wallis JD (2014): Executive control processes underlying multi‐item working memory. Nat Neurosci 17:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latora V, Marchiori M (2001): Efficient behavior of small‐world networks. Phys Rev Lett 87:198701. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Senjem ML, Weigand SD, Smith GE, Ivnik RJ, Boeve BF, Knopman DS, Petersen RC, Jack CR (2009): Functional magnetic resonance imaging changes in amnestic and nonamnestic mild cognitive impairment during encoding and recognition tasks. J Int Neuropsychol Soc 15:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson JT, Wang TH, de Chastelaine M, Rugg MD (2014): Effects of age on negative subsequent memory effects associated with the encoding of item and item–context information. Cereb Cortex 24:3322–3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng C, Brandl F, Tahmasian M, Shao JM, Manoliu A, Scherr M, Schwerthoffer D, Bauml J, Forstl H Zimmer C, et. al. (2014): Aberrant topology of striatum's connectivity is associated with the number of episodes in depression. Brain 137:598–609. [DOI] [PubMed] [Google Scholar]
- Meulenbroek O, Kessels RPC, de Rover M, Petersson KM, Rikkert M, Rijpkema M, Fernandez G (2010): Age‐effects on associative object‐location memory. Brain Res 1315:100–110. [DOI] [PubMed] [Google Scholar]
- Morris JC (1993): The clinical dementia rating (CDR): current version and scoring rules. Neurology 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman‐Haignere SV, McCarthy G, Chun MM, Turk‐Browne NB (2012): Category‐selective background connectivity in ventral visual cortex. Cereb Cortex 22:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Watson P, Hodges JR (2000): The nature and staging of attention dysfunction in early (minimal and mild) Alzheimer's disease: Relationship to episodic and semantic memory impairment. Neuropsychologia 38:252–271. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Morris JC (2005): Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 62:1160–1163. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E (2006): Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 63:665–672. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST (2001): Practice parameter: early detection of dementia: mild cognitive impairment (an evidence‐based review) ‐ report of the quality standards subcommittee of the american academy of neurology. Neurology 56:1133–1142. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Krishnan S, Slavin MJ, Tran TTT, Murty L, Doraiswamy PM (2006): Mild cognitive impairment: evaluation with 4‐T functional MR imaging. Radiology 240:177–186. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE (2011): Functional network organization of the human brain. Neuron 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi ZG, Wu X, Wang ZQ, Zhang N, Dong HQ, Yao L, Li KC (2010): Impairment and compensation coexist in amnestic MCI default mode network. Neuroimage 50:48–55. [DOI] [PubMed] [Google Scholar]
- Rahm B, Kaiser J, Unterrainer JM, Simon J, Bledowski C (2014): fMRI characterization of visual working memory recognition. Neuroimage 90:413–422. [DOI] [PubMed] [Google Scholar]
- Remy F, Mirrashed F, Campbell B, Richter W (2005): Verbal episodic memory impairment in Alzheimer's disease: a combined structural and functional MRI study. Neuroimage 25:253–266. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M (2004): Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage 23:752–763. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Mohs RC, Davis KL (1984): A new rating scale for Alzheimer's disease. Am J Psychiatry 141:1356–64. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Furey ML, Horwitz B, Grady CL (2010): Altered connectivity among emotion‐related brain regions during short‐term memory in Alzheimer's disease. Neurobiol Aging 31:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O (2010): Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Salami A, Eriksson J, Nyberg L (2012): Opposing effects of aging on Large‐scale brain systems for memory encoding and cognitive control. J Neurosci 32:10749–10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NLJ, Summers MJ (2011): Longitudinal deficits to attention, executive, and working memory in subtypes of mild cognitive impairment. Neuropsychology 25:237–248. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Krause BJ, Weiss PH, Fink GR, Shah NJ, Amorim MA, Muller HW, Berthoz A (2007): Visuospatial working memory and changes of the point of view in 3D space. Neuroimage 36:955–968. [DOI] [PubMed] [Google Scholar]
- Seghier ML (2013): The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield C, Greene M, Wager T, Egner T, Hirsch J, Mangels J (2006): Neocortical connectivity during episodic memory formation. PloS Biol 4:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk‐Browne NB (2013): Functional interactions as big data in the human brain. Science 342:580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch GE, El Marroun H, Schmidt MN, Tibboel D, Manoach DS, Calhoun VD, White TJH (2014): Brain connectivity during verbal working memory in children and adolescents. Hum Brain Mapp 35:698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Stam CJ, Kahn RS, Pol HEH (2010): Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci 30:15915–15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH (2001): An integrated software suite for surface‐based analyses of cerebral cortex. J Am Med Inf Assoc 8:443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL (2010): Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL (2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9:445–453. [DOI] [PubMed] [Google Scholar]
- Wang JH, Zuo XN, Dai ZJ, Xia MR, Zhao ZL, Zhao XL, Jia JP, Han Y, He Y (2013a): Disrupted functional brain connectome in individuals at risk for Alzheimer's disease. Biol Psychiatry 73:472–481. [DOI] [PubMed] [Google Scholar]
- Wang L, Li H, Liang Y, Zhang JY, Li X, Shu N, Wang YYY, Zhang ZJ (2013b): Amnestic mild cognitive impairment: topological reorganization of the Default‐mode network. Radiology 268:501–514. [DOI] [PubMed] [Google Scholar]
- Wechsler D ( 1999): Wechsler Adult Intelligence Scale. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Woodard JL, Seidenberg M, Nielson KA, Antuono P, Guidotti L, Durgerian S, Zhang Q, Lancaster M, Hantke N, Butts A, Rao SM (2009): Semantic memory activation in amnestic mild cognitive impairment. Brain 132:2068–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni G, de Jager CA, Drazich E, Douaud G, Jenkinson M, Smith AD, Tracey I, Wilcock GK (2013): Structural and functional bases of visuospatial associative memory in older adults. Neurobiol Aging 34:961–972. [DOI] [PubMed] [Google Scholar]
- Zhao XH, Liu Y, Wang XB, Liu B, Xi Q, Guo QH, Jiang H, Jiang TZ, Wang PJ (2012): Disrupted Small‐world brain networks in moderate Alzheimer's disease: a Resting‐state fMRI study. PloS One 7: e33540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DM, Dong XY, Sun HZ, Xu YC, Ma Y, Wang XM (2012): The overall impairment of core executive function components in patients with amnestic mild cognitive impairment: a cross‐sectional study. BMC Neurol 12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


