Abstract
Background and purpose: Intravascular angioplasty and stenting of intracranial arterial stenosis provided controversial results. Besides the expertise of the practitioners, the selection of the patients remains challenging. BOLD MRI of the cerebral vasoreactivity (BOLD MRI CVR) to hypercapnia provides reproducible maps of the entire brain of the vascular reserve, and could be helpful to assess the best therapeutic strategy. Case history: We report the case of a 63‐year‐old woman referred for a severe stenosis of the proximal portion of the left middle cerebral artery, revealed by a lenticulostriate and precentral infarction. Despite an aggressive medical treatment during 5 months, the occurrence of iterative transient ischemic attacks motivated intravascular stenting. Functional MRI of the vasoreactivity to hypercapnia using both Blood Oxygen Level Dependent (BOLD) and arterial spin labeling sequences showed normal basal perfusion and impaired vasoreactivity in the left middle cerebral artery territory. Three months after stenting, the BOLD MRI CVR showed vasoreactivity normalization. Since, the patient remains free of ischemic disorders one year after stenting. Conclusion: BOLD MRI of the CVR to hypercapnia may be helpful to optimize the treatment of patients with intracranial arterial stenosis, and could be performed in future therapeutic trials. Hum Brain Mapp 35:1320–1324, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: transient ischaemic attack, intracranial stenosis, intravascular stenting, BOLD MRI, cerebral vasoreactivity, hypercapnia
INTRODUCTION
The treatment of symptomatic stenosis of intracranial artery remains debated. After stroke and despite the use of aspirin and usual management of risk factors, patients with intracranial stenosis are at risk of recurrent stroke or transient ischemic attack (TIA) from 20 to 40% at 2 years, and up to 60% in the territory of a hemodynamically significant stenotic artery [Chimowitz et al., 2005; Mazighi et al., 2006]. Aggressive medical treatment including a combination of antiplatelet therapy has been suggested to reduce the incidence of cerebrovascular ischemic events [Diener et al., 2004].
To further reduce the recurrence of stroke and TIA, percutaneous transluminal angioplasty and stenting (PTAS) of severe intracranial stenosis plus aggressive medical management has been advocated with controversial results [Chimowitz et al., 2011; Fiorella et al., 2007]. To better identify hemodynamic alteration related to steno‐occlusive disease, BOLD MRI of the cerebral vasoreactivity (BOLD MRI CVR) to hypercapnia has been proposed to provide maps of the vascular reserve. This technique was already performed to monitor patients before and after surgical recanalization [Haller et al., 2008; Heyn et al., 2010; Mandell et al., 2008].
We report the case of a patient with a symptomatic severe intracranial stenosis despite an aggressive medical treatment. Basal perfusion was normal. BOLD MRI CVR showed a regional impairment of vasoreactivity in the territory of the left middle cerebral artery. Both recurrences of TIA and BOLD MRI CVR results motivated PTAS. After PTAS, fMRI showed a normalization of the vasoreactivity and the patient is still free of ischemic events one year after treatment. This case illustrates the normalization of the vascular reserve after endovascular treatment and suggests potential benefit in the patient selection to optimize therapeutic strategy.
CASE HISTORY AND SYMPTOMATOLOGY
A 63‐year‐old woman was initially admitted for the management of a recent ischemic stroke in the left lenticulo‐striate region and in cortical precentral area revealed by a sudden deficit of the right hemibody, due to a severe stenosis (80%) of the M1 portion of left middle cerebral artery (MCA) demonstrated by MRI and angioCT. Her past medical history was only significant for untreated hypertension and dyslipemia. An aggressive medical treatment using two antiplatelet agents, statins, and blood pressure‐lowering drugs was administrated.
Despite a rapid recovery of the initial deficit, the patient complained of iterative TIAs with progressively worsening of sensory disturbance of the right hemibody within several weeks after the treatment onset.
DIAGNOSIS
Five months after the stroke, we performed a BOLD MRI CVR in order to estimate the vascular reserve of the whole brain on a 3 T clinical MRI. Data acquisition was performed using T2*‐weighted image gradient echo‐echo planar imaging (WI GE‐EPI) sensitive to BOLD contrast [time of repetition (TR): 3,000 ms, time of echo (TE): 35 ms, α: 90°, voxel size: 4 mm × 4 mm × 4 mm, 32 axial planes]. CVR was elicited using a block‐designed inhalation paradigm of alternating medical air and hypercapnic gas mixture (CO2 7%, O2 21%, balanced N2). The capnic modulation was administered using a non‐rebreathing high concentration mask according to the following paradigm: [air (1 min) − hypercapnia (2 min) − air (1 min)] × 3, for a total duration of 12 min. End‐tidal CO2 (EtCO2) pressure was simultaneously recorded using a MR compatible Maglife C® device (Schiller Medical, Switzerland) via a nasal canula. No simultaneous recordings of arterial CO2 pressure were performed. This EtCO2 time course was used as a physiological regressor to estimate the BOLD signal change per mm Hg EtCO2 change [Cantin et al., 2011]. No adverse reaction to the hypercapnic stimulus was reported.
Basal perfusion was quantified using pseudocontinuous arterial spin labeling (ASL). Whereas basal perfusion was normal, especially in MCA territories, BOLD MRI CVR showed a regional impairment in the left MCA territory (Fig. 1), suggesting a decreased vascular reserve.
Figure 1.
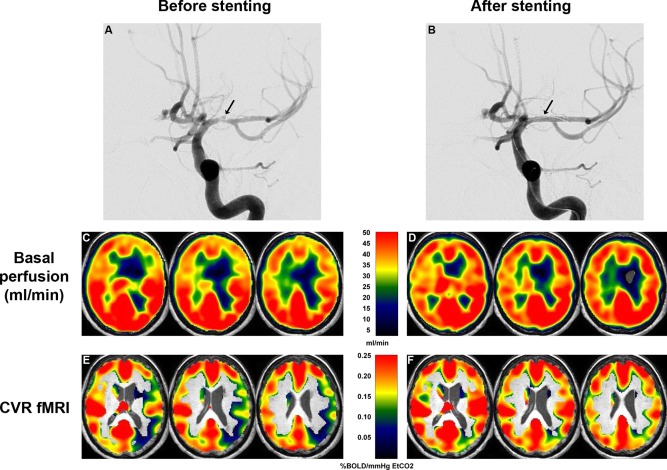
Arteriography showed a severe stenosis of the M1 portion of the left MCA (arrow) before (A) and after successful endovascular stenting (B). In MCA territories, basal perfusion estimated using pseudocontinuous ASL was normal and symmetrical before (C) and after stenting (D), except in the left lenticulostriate infarct. Before endovascular treatment, CVR BOLD MRI of CVR to hypercapnia showed a CVR decrease in the left MCA territory (m ± sd = 0.13 ± 0.07 %BOLD/mm Hg EtCO2) compared to the right MCA territory (0.19 ± 0.07), accounting for a decrease of vascular reserve of 31.6% (E). After treatment, CVR was normalized and symmetrical in the left MCA territory (0.20 ± 0.09) compared to the right MCA territory (0.21 ± 0.08) (F). A persistent CVR decrease was detected in the left posterior junctional area, only.
TREATMENT
Because of both clinical and MRI data, a PTAS was proposed to the patient after having given appropriate explanations on benefits and risks.
Angiography was performed using a transfemoral approach, the left carotid artery was supraselectively accessed and confirmed the severe left MCA stenosis. Then, the stenosis was successfully treated by the stent delivery, without periprocedural complication (Fig. 1).
Three months after the intravascular treatment, no further TIA was reported. Postoperative BOLD MRI CVR showed a normalization of the vascular reserve in the left MCA territory (Fig. 1). Basal perfusion remained normal. Since, the patient remains free of TIA or stroke one year after treatment.
DISCUSSION
Symptomatic intracranial stenosis is associated with a high risk of recurrent ischemic events [Chimowitz et al., 2011; Diener et al., 2004; Mazighi et al., 2006]. In this case, and despite an aggressive medical therapy, the patient presented iterative TIA and we considered PTAS.
PTAS emerged as an efficient therapeutic option for symptomatic intracranial stenosis [Fiorella et al., 2007]. Indeed, no adverse event occurred during and after the procedure and the patient remains free of any stroke or TIA after nine months.
However, SAMMPRIS trial that was performed to compare PTAS using Wingspan stent system plus aggressive medical management to aggressive medical management alone for the prevention of recurrent stroke in patients with major intracranial stenosis (without perfusion imaging selection of patients) was stopped at the primary end point because the 30‐day rate of stroke in the territory of the stenotic artery was higher in the PTAS group than expected [Mazighi et al., 2006]. In this study, a later benefit of preventing stroke remains to be estimated. Identifying subgroups at high risk for stroke despite aggressive medical management would be also important [Chimowitz et al., 2011]. Thus, CVR measurement may help to better identify patients with hemodynamically significant stenosis among patient with atherothrombotic disease.
In patients with intracranial arterial stenosis, autoregulatory vasodilatation maintains regional cerebral blood flow and perfusion pressure roughly constant despite changes of arterial pressure. When this vascular reserve is decreased, the risk of ischemic event increases, showing the importance of hemodynamic factors in the pathogenesis and treatment focal cerebral ischemia [Powers, 1991].
Some patients, like the present case, that have normal basal perfusion with reduced vascular reserve may represent a particular risk profile [Markus and Cullinane, 2001]. We hypothesize that these patients would have a greater benefit of PTAS that may relieve symptomatic hemodynamic disorders. Indeed, others patients with a severe arterial stenosis do not have a reduced CVR, given the potential collateral flow via the circle of Willis and corticopial anastomosis [Han et al., 2011]. For such patients, aggressive medical treatment alone would be sufficient, limiting the additional risk of PTAS adverse event.
To estimate the vascular reserve, BOLD MRI CVR to hypercapnia has been used to monitor surgical treatment of patients with occlusive carotid disease or intracranial stenosis [Haller et al., 2008; Han et al., 2011; Mandell et al., 2011]. In our case, the normal CVR values obtained in the healthy hemisphere before PTAS and in both hemispheres after PTAS were in line with previous work using a similar method [Han et al., 2011; Mandell et al., 2011]. In the arterial territory of the PTAS, CVR value was decreased by 31.6%. This decrease was weaker than that observed in some patients previously reported [Mandell et al., 2011]. Several differences in data acquisition, processing, and analyses may account for this amplitude difference. Among these, we reported a mean value of the arterial territory with an important variability. In some areas such as the posterior junctional region, CVR decreased by over 80% (Fig. 1).
Although BOLD signal change is a complex measure of changes in oxygenation, cerebral blood flow, and volume, BOLD contrast to hypercapnia is correlated with cerebral blood flow in patients with steno‐occlusive disease [Mandell et al., 2008]. However, the quality of the EtCO2 measurement is questionable. Indeed, we previously attempted to compare EtCO2 to arterial pressure in CO2 using a transcutaneous capnometer (TINA‐TCM4®, Radiometer, Denmark) [Jiang et al., 2010]. Using the same gas mixture (CO2 7%, O2 21%, balanced N2) inhaled during 2 min, PaCO2 increased by 4.3 mm Hg whereas EtCO2 increased by 9.0 mm Hg (unpublished data). Combined measurements of CO2 pressures revealed both amplitude and temporal discrepancies. This is a limitation for absolute quantification of CVR using EtCO2 measurements. However in patients with severe arterial stenosis, the aim of the CVR imaging is to identify regional impairment in the parenchyma downstream the stenotic artery by comparison to the contralateral territory. In addition to the variations of the BOLD signal due to field strength and acquisition parameters, BOLD MRI provides a semiquantitative map of CVR. For clinical purpose, regional CVR should be tested against healthy parenchymal reference, here the contralateral MCA territory and global CVR should be tested against values obtained in a healthy subjects' population. Here and before PTAS, the decrease of vascular reserve in the stenotic MCA territory was 31.6% when compared to the contralateral territory.
To avoid potential confound, perfusion imaging has been proposed. However, dynamic susceptibility contrast imaging requires gadolinium injection and quantification remains difficult. Moreover, functional imaging supposes a conditional procedure with two contrast administrations and a significant delay. ASL perfusion imaging has been also proposed [Bokkers et al., 2010]. This attractive technique does not require contrast administration and allow measuring dynamic change of cerebral blood flow (CBF) across conditions [Noth et al., 2008]. However, large changes in arterial transit time downstream of a hemodynamically significant stenosis may lead to underestimate CBF via ASL [Bokkers et al., 2010]. Thus, BOLD MRI CVR to hypercapnia should remain the imaging method of choice to investigate CVR in patients with arterial stenosis using MRI, until advances in ASL may resolve the sensitivity of ASL to severely prolonged arterial transit times. The choice of the vasomotor stimulus is also debated. Acetazolamide requires data acquisitions prior and after administration. Several contraindications and side effects have to be considered.
To further investigate patients with symptomatic severe intracranial arterial stenosis, CVR MRI may help to better identify those at high risk of stroke. We hypothesize that impaired CVR in the territory of the stenotic artery would be associated with a higher recurrence of ischemic events. To test this hypothesis, a group study has to be conducted. If this hypothesis is confirmed, the risks and benefits of PTAS should take into account individual CVR results.
AUTHORS CONTRIBUTION
All authors contributed to the manuscript as follows: A.A: data collection, analysis and interpretation, drafting, and final approval of the article; M.V.: data collection, analysis and interpretation, critical review, and final approval of the article; F.T.: data collection, analysis and interpretation, critical review and final approval of the article; J.W.: data analysis and interpretation, critical review and final approval of the article; O.D.: data collection, analysis and interpretation, critical review, final approval of the article; A.K.: data collection, analysis and interpretation, drafting, critical review, final approval of the article.
ACKNOWLEDGMENTS
Authors acknowledge the precious help of Irène Troprès for data collection and Patrice Jousse for editing artwork.
REFERENCES
- Bokkers RP, van Osch MJ, et al. (2010). Symptomatic carotid artery stenosis: Impairment of cerebral autoregulation measured at the brain tissue level with arterial spin‐labeling MR imaging. Radiology 256:201–208. [DOI] [PubMed] [Google Scholar]
- Cantin S, Villien M, et al. (2011). Impaired cerebral vasoreactivity to CO2 in Alzheimer's disease using BOLD fMRI. Neuroimage 58:579–587. [DOI] [PubMed] [Google Scholar]
- Chimowitz MI, Lynn MJ, et al. (2005). Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 352:1305–1316. [DOI] [PubMed] [Google Scholar]
- Chimowitz MI, Lynn MJ, et al. (2011). Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 365:993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener HC, Bogousslavsky J, et al. (2004). Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): Randomised, double‐blind, placebo‐controlled trial. Lancet 364:331–337. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Levy EI, et al. (2007). US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: Periprocedural results. Stroke 38:881–887. [DOI] [PubMed] [Google Scholar]
- Haller S, Bonati LH, et al. (2008). Reduced cerebrovascular reserve at CO2 BOLD MR imaging is associated with increased risk of periinterventional ischemic lesions during carotid endarterectomy or stent placement: Preliminary results. Radiology 249:251–258. [DOI] [PubMed] [Google Scholar]
- Han JS, Abou‐Hamden A, et al. (2011). Impact of extracranial‐intracranial bypass on cerebrovascular reactivity and clinical outcome in patients with symptomatic moyamoya vasculopathy. Stroke 42:3047–3054. [DOI] [PubMed] [Google Scholar]
- Heyn C, Poublanc J, et al. (2010). Quantification of cerebrovascular reactivity by blood oxygen level‐dependent MR imaging and correlation with conventional angiography in patients with Moyamoya disease. AJNR Am J Neuroradiol 31:862–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Krainik A, et al. (2010). Impaired fMRI activation in patients with primary brain tumors. Neuroimage 52:538–548. [DOI] [PubMed] [Google Scholar]
- Mandell DM, Han JS, et al. (2008). Mapping cerebrovascular reactivity using blood oxygen level‐dependent MRI in Patients with arterial steno‐occlusive disease: Comparison with arterial spin labeling MRI. Stroke 39:2021–2028. [DOI] [PubMed] [Google Scholar]
- Mandell DM, Han JS, et al. (2011). Quantitative measurement of cerebrovascular reactivity by blood oxygen level‐dependent MR imaging in patients with intracranial stenosis: Preoperative cerebrovascular reactivity predicts the effect of extracranial‐intracranial bypass surgery. AJNR Am J Neuroradiol 32:721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H, Cullinane M (2001). Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 124:457–467. [DOI] [PubMed] [Google Scholar]
- Mazighi M, Tanasescu R, et al. (2006). Prospective study of symptomatic atherothrombotic intracranial stenoses: The GESICA study. Neurology 66:1187–1191. [DOI] [PubMed] [Google Scholar]
- Noth U, Kotajima F, et al. (2008). Mapping of the cerebral vascular response to hypoxia and hypercapnia using quantitative perfusion MRI at 3 T. NMR Biomed 21:464–472. [DOI] [PubMed] [Google Scholar]
- Powers WJ (1991). Cerebral hemodynamics in ischemic cerebrovascular disease. Ann Neurol 29:231–240. [DOI] [PubMed] [Google Scholar]


