Abstract
Cognitive dysfunction is common in multiple sclerosis (MS). However, the relationship between white matter (WM) damage and cognition remains insufficiently clear. This study investigates the extent and severity of WM diffusion abnormalities in MS patients and relations with cognition. Diffusion tensor imaging scans were obtained in 131 MS patients (88 women, 6 years postdiagnosis) and 49 age‐matched controls (29 women). Patient groups were equal in terms of disease duration, disability, and WM lesion volume. Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were compared between groups. Post hoc analyses calculated the spatial extent and severity of diffusion abnormalities to relate these to cognitive performance. In controls, 31% of WM voxels showed higher FA in men; therefore, all patient analyses were within‐sex. The extent of diffusion changes was higher in male patients than in female patients for all parameters (FA: 24% in women, 53% in men), as was the severity of changes (FA: Z = −0.18 in women, Z = −0.41 in men). Especially the extent of FA abnormalities was strongly related to cognitive performance in all patients (r = −0.42, P < 0.0001). Regionally, thalamic decreases in FA were especially correlated with cognitive performance. Cognitively impaired patients showed greater extent and severity on all diffusion parameters compared to cognitively preserved patients. The WM of male patients was both more extensively and also more severely affected than that of female patients. The extent of WM FA changes, especially in the thalamus, was associated with cognitive performance in this cohort of early MS patients. Hum Brain Mapp 35:2348–2358, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: multiple sclerosis, MRI, cognition, thalamus, gender, DTI, sex, TBSS
INTRODUCTION
Cognitive dysfunction is common in multiple sclerosis (MS) [Chiaravalloti and DeLuca, 2008]. Traditional white matter (WM) measures, such as lesion load, have shown moderate relations with cognition [Filippi et al., 2010], indicating the need for more advanced WM methods, such as diffusion tensor imaging (DTI).
Studies using DTI revealed diffuse changes throughout the brain [Pagani et al., 2007; Roosendaal et al., 2009; Rovaris et al., 2005], also beyond focal MS lesions [Dineen et al., 2009; Moll et al., 2011; Roosendaal et al., 2009; Vrenken et al., 2006], enabling a more advanced model for cognition in MS. Subsequently, common DTI parameters have been related to cognitive dysfunction [Benedict et al., 2007; Dineen et al., 2009; Rocca et al., 2009; Roosendaal et al., 2009; Rovaris et al., 2002; Van Hecke et al., 2010; Yu et al., 2011], although studies using sufficiently broad cognitive evaluations and more sophisticated diffusion analyses are still lacking.
The aim of this study, therefore, was to use DTI to investigate the cognitive implications of WM damage in a large and homogeneous early inception cohort of MS patients, at 6 years postdiagnosis. We applied two individualized quantification methods. First, the number of voxels with abnormal DTI parameters in the WM skeleton (i.e., the “extent” of damage) was investigated. Second, the effect sizes of these abnormalities (i.e., the “severity” of damage) were also assessed throughout the whole skeleton. These two measures were then related to cognitive performance using a multivariate model that also included traditional MR measures.
METHODS
Participants
A total of 180 subjects were included, of which 49 age‐matched healthy controls (29 women) and 131 MS patients (88 women), see Table 1. Physical disability was measured using the Expanded Disability Status Scale (EDSS) [Kurtzke, 1983]. All patients were diagnosed with clinically definite MS (CDMS) [Polman et al., 2011], and were part of an early inception cohort, all of whom presently at 6 years postdiagnosis. MS subtypes [Lublin and Reingold, 1996] included relapsing‐remitting MS (RRMS, 114 patients, 79 women), primary progressive MS (PPMS, 8 patients, 2 women), and secondary progressive MS (SPMS, 9 patients, 6 women). Patients were relapse‐free and without steroid treatment for at least 2 months and have followed standard treatment options since diagnosis. There were no treatment differences between the sexes, as described elsewhere in more detail [Schoonheim et al., 2012]. Healthy controls had no history of neurological or psychiatric disease, and were normal on MRI.
Table 1.
Descriptive and cognitive variables for controls and patients, comparing male and female patient groups
| Female controls | Male controls | Female patients | Male patients | Z/F | P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | |||
| Descriptives | ||||||||||||||
| Age (years) | 41.0 | ± | 11.2 | 39.6 | ± | 11.6 | 40.5 | ± | 8.2 | 42.1 | ± | 9.6 | −0.69 | 0.49 |
| Education (1–7) | 5.5 | ± | 1.5 | 5.7 | ± | 1.6 | 4.9 | ± | 1.5 | 5.0 | ± | 1.7 | −0.40 | 0.69 |
| NGMV (ml)a | 839.4 | ± | 45.2 | 842.4 | ± | 57.9 | 825.2 | ± | 40.6 | 798.1 | ± | 47.4 | 7.4 | 0.0001c |
| NWMV (ml)a | 679.6 | ± | 29.6 | 701.9 | ± | 36.0 | 658.2 | ± | 35.9 | 659.8 | ± | 37.6 | 10.7 | <0.0001c |
| NBV (ml)a | 1519.9 | ± | 63.7 | 1544.3 | ± | 84.8 | 1483.4 | ± | 62.9 | 1457.9 | ± | 71.2 | 10.1 | <0.0001c |
| MS characteristics | ||||||||||||||
| EDSS (1–10)b | 1.5 | (0–8) | 2.0 | (0–8) | −0.07 | 0.94 | ||||||||
| DD (years) | 7.5 | ± | 2.3 | 7.8 | ± | 2.0 | −1.39 | 0.16 | ||||||
| T 1 (ml) | 2.0 | ± | 2.9 | 2.1 | ± | 2.6 | −0.48 | 0.63 | ||||||
| T 2 (ml) | 3.7 | ± | 4.2 | 5.3 | ± | 6.1 | −1.71 | 0.09 | ||||||
| Cognitive Z scoresa | ||||||||||||||
| Executive functioning | 0.01 | ± | 0.68 | −0.02 | ± | 0.62 | −0.39 | ± | 0.96 | −1.23 | ± | 1.85 | 8.39 | <0.0001c |
| Verbal memory | −0.04 | ± | 0.93 | 0.08 | ± | 0.89 | −0.21 | ± | 0.92 | −1.01 | ± | 0.95 | 9.42 | <0.0001c |
| Information processing | 0.01 | ± | 1.09 | −0.02 | ± | 0.87 | −0.51 | ± | 1.17 | −1.13 | ± | 1.28 | 6.42 | 0.0004c |
| Visuospatial memory | −0.17 | ± | 1.15 | 0.25 | ± | 0.66 | −0.51 | ± | 1.22 | −0.30 | ± | 1.08 | 2.03 | 0.1126 |
| Working memory | −0.08 | ± | 0.85 | 0.11 | ± | 0.92 | −0.49 | ± | 0.87 | −1.21 | ± | 1.31 | 9.68 | <0.0001c |
| Attention | −0.02 | ± | 0.65 | 0.03 | ± | 0.55 | −0.31 | ± | 0.74 | −0.87 | ± | 1.18 | 7.19 | 0.0001c |
| Psychomotor speed | −0.01 | ± | 0.88 | 0.01 | ± | 0.66 | −0.46 | ± | 1.01 | −1.28 | ± | 1.62 | 8.64 | <0.0001c |
| Average cognition | −0.04 | ± | 0.64 | 0.06 | ± | 0.46 | −0.42 | ± | 0.70 | −1.03 | ± | 1.04 | 12.43 | <0.0001c |
NGMV: normalized gray matter volume, NWMV: normalized white matter volume, NBV: normalized brain volume, EDSS: Expanded Disability Status Scale, DD: disease duration, T 1: T 1‐hypointense lesion volume, T 2: T 2‐hyperintense lesion volume. Z/F: Mann‐Whitney Z values or GLM F values.
indicates GLM F values.
indicates median and range.
indicate significant differences between male or female patients (respectively) compared to their sex‐matched controls.
The study was approved by the institutional ethics review board, and all subjects gave written informed consent before participation.
Neuropsychological Evaluation
Subjects underwent a comprehensive set of neuropsychological tests comprising of the Brief Repeatable Battery for Neurological disease (BRB‐N) [Rao, 1990], as well as the concept shifting test, the Stroop color‐word test, and the memory comparison test, performed by an experienced neuropsychologist. This resulted in an evaluation of eight cognitive domains, which have been used in earlier publications [Schoonheim et al., 2012, 2013], see Table 1. Patients who scored at least 1.5 standard deviations (i.e., Z ≤ 1.5) below the average of controls in two or more domains, excluding the average cognition domain, were defined as “cognitively impaired.”
Magnetic Resonance Imaging
Subjects received structural 3T‐MRI (GE Signa HDxt), using a three‐dimensional T 1‐weighted fast spoiled gradient‐echo sequence (TR 7.8 ms, TE 3.0 ms, TI 450 ms, FA 12, 0.9 × 0.9 mm in‐plane resolution, 1 mm slice thickness), two‐dimensional (2D) dual‐echo PD/T 2 (TR 9,680 ms, TE 22/112 ms, flip angle 90, 48 contiguous axial slices of 3 mm, covering the entire brain, in‐plane resolution 0.6 × 0.6 mm), and 2D spin‐echo T 1‐weighted imaging (TR 475 ms, TE 9 ms, FA 90, 48 contiguous axial slices of 3 mm, in‐plane resolution 0.7 × 1 mm). DTI based on EPI also covered the entire brain, using five volumes without directional weighting (i.e., b 0) and 30 volumes with noncollinear diffusion gradients (i.e., 30 directions, b = 1,000 s/mm2, TR 13,000 ms, TE 91 ms, FA 90, 53 contiguous axial slices of 2.4 mm, in‐plane resolution 2 × 2 mm).
Brain and Lesion Volumes
T 2‐hyperintense and T 1‐hypointense lesion volumes were marked by an experienced rater (MMS) and measured using a local thresholding technique. Brain volumes were analyzed using SIENAX version 2.5 (FSL4.1, http://www.fmrib.ox.ac.uk/fsl), providing total gray matter (NGMV), total WM (NWMV), and whole‐brain volumes (NBV), corrected for head size.
Diffusion Tensor Imaging Preprocessing
All preprocessing steps were performed using FSL, including motion‐ and eddy‐current correction on images and gradient‐vectors, followed by diffusion tensor fitting. Fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD, L1), and radial diffusivity (RD, average of L2 and L3) were derived for each voxel. Each subject's FA image was used to calculate nonlinear registration parameters to the FMRIB58_FA brain, which were then applied to all four parameter images. The registered FA images were averaged into a mean FA image, which was skeletonized for tract‐based spatial statistics (TBSS) [Smith et al., 2006]. The skeleton was thresholded at 0.2 to include only WM and used for TBSS statistics in all diffusion parameters. For each subject the maximum FA value perpendicular to each voxel of the skeleton was projected onto the skeleton. The projection parameters for each voxel were then also applied to the MD, AD, and RD data to create skeletonized data in standard space for each subject.
Statistical Analysis: DTI
Main effects: Group differences
Differences in diffusion parameters between groups within the WM skeleton were analyzed using randomise [Smith et al., 2006] (part of FSL), using 500 permutations, threshold‐free cluster enhancement [Smith and Nichols, 2009], and familywise error, using a final threshold of P < 0.05 and correcting for age and level of education. Male and female patients were separately compared to sex‐matched controls, as significant sex effects were found in controls (see below), comparable to earlier studies [Menzler et al., 2011].
Individual quantification of main effects
After the abovementioned analysis, detailing the number and location of voxels that are significant between groups, an individual quantification of severity and extent was performed.
First, in the area affected in both male and female patient groups across all diffusion parameters (i.e., the “common area”) average diffusion values were calculated to investigate the severity of damage.
Second, whole‐skeleton effect sizes were investigated by converting all diffusion metrics to Z scores on a voxel basis. For each skeleton voxel of a patient the average value of the (sex‐corresponding) control group was subtracted, and divided by the control group's standard deviation for that voxel. Then, a whole‐skeleton mean (unthresholded) Z score was calculated for each diffusion parameter, indicating the severity across the entire WM skeleton per subject.
Third, to investigate differences in the extent of relatively severe damage, the individual voxelwise Z scores (see above) were thresholded at P < 0.001, that is, Z > 3.1 or Z < −3.1 compared to the control group. Afterward, the voxels exceeding this threshold were counted, at Z ≤ −3.1 for FA and Z ≥ 3.1 for MD, AD, and RD. These counts were log‐transformed to a normal distribution for further analysis.
Statistical Analysis: General Linear Model
All variables were checked for normality using Kolmogorov–Smirnov tests and histogram inspection. T 1‐ and T 2‐lesion volumes were log‐transformed. All analyses were corrected for age and level of education where applicable. Multivariate general linear model (GLM) analyses were used to assess diffusion extent/severity, cognitive, and volumetric measures, using one factor with four levels: male control, female control, male patient, and female patient, comparable to the TBSS analysis model. Post hoc group comparisons were made using Bonferroni corrections (P < 0.05).
Relations with Cognition
Cognitive performance was related to all measures using stepwise linear regression, which used age, sex, education level, disease duration, EDSS, MS phenotype, NGMV, NWMV, T 1 and T 2 lesion loads, and diffusion extent and severity as predictors. Variables like EDSS and education level were dichotomized using a median split, whereas MS phenotype was dichotomized into RRMS or progressive MS.
To investigate regional correlations with the most predictive DTI parameter resulting from the linear regression, voxelwise correlations with those cognitive domains found to be impaired in the GLM analyses were calculated with randomise, which is part of the TBSS pipeline [Smith et al., 2006].
As a final exploration of cognition, we applied a second GLM analysis to compare cognitively impaired and cognitively preserved patients (using the threshold for cognitive impairment as defined above) on the extent and severity of DTI abnormalities and brain and lesion volumes.
RESULTS
Cognition and Brain Volume
Multivariate GLM analyses showed a main effect of group (average F = 8.0, P = 0.01) for all cognitive domains except visuospatial memory (see Table 1). Post hoc Bonferroni pairwise comparisons revealed all remaining cognitive Z scores to be significantly lower in male patients compared to male controls (average cognition: mean Z of male patients −1.03, P < 0.0001) and compared to female patients (average cognition mean Z = −0.42, P = 0.0003). Female patients did not score significantly lower than female controls in any cognitive domain. A significant interaction between sex and diagnosis was found for verbal memory (F = 7.40, P < 0.01), working memory (F = 6.59, P = 0.01), and average cognition (F = 6.83, P = 0.01).
Brain volumes showed a significant effect of group for NGMV, NWMV, and NBV (see Table 1, average F = 9.42, P < 0.0001). Bonferroni analyses revealed significant reductions of NGMV in male patients only, compared to male controls (effect size Cohen's d = 0.84, P = 0.002) as well as female patients (Cohen's d = 0.62, P = 0.01). NWMV was reduced in both female (d = 0.65, P = 0.02) and male (d = 1.14, P < 0.0001) patient groups compared to their sex‐respective controls. NBV was reduced in both female (d = 0.56, P = 0.03) and male (d = 1.11, P = 0.001) patient groups compared to their controls. The interaction between sex and diagnosis showed a trend for NBV (F = 3.81, P = 0.053).
Regional Diffusion Changes: Tract‐Based Spatial Statistics
Controls
Comparing individual voxels of the WM skeleton between groups showed that male controls displayed higher FA values than female controls in 31% of investigated WM voxels (i.e., in the skeleton of major WM bundles), in areas like the thalamus, corpus callosum (CC), internal capsule, and external capsule (see Fig. 1). Diffusivity measures did not differ between sexes.
Figure 1.
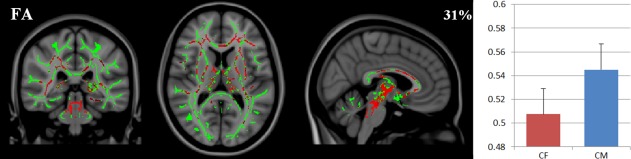
Diffusion in healthy controls. Left: areas of the white matter skeleton (green) displaying higher FA (red, X = 86, Y = 93, Z = 83) in healthy control males (CM) compared to healthy control females (CF), overlayed on the MNI‐standard brain. Right: the average FA (and standard deviations) in those areas.
Patients
Female MS patients showed decreased FA and increased diffusivity values compared to female controls in the CC, temporal WM and posterior periventricular areas (FA: 24%, MD: 17%, AD: 6%, and RD: 21% of the WM skeleton, see Fig. 2). Male MS patients showed affected voxels in similar regions as female patients, when compared to male controls. However, they also showed additional abnormalities in the posterior CC, thalamus, cerebellum, pons, and juxtacortical fronto‐parietal WM (FA: 53%, MD: 54%, AD: 28%, RD: 60% of investigated WM voxels, see Fig. 2).
Figure 2.
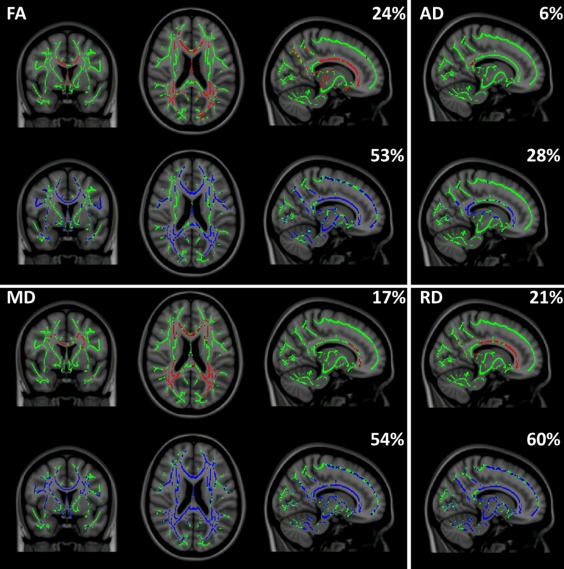
Spatial extent of the white matter voxels in the TBSS skeleton (green) that show lowered FA or increased diffusivity (red or blue, X = 77, Y = 128, Z = 92) in patients and the percentage of the skeleton involved. Red: female patients compared to female controls, blue: male patients compared to male controls. FA: fractional anisotropy, MD: mean diffusivity, AD: axial diffusivity, RD: radial diffusivity.
Subject‐Level Quantification: Common Areas
Regions that were involved in both sexes in the TBSS analyses were mainly posterior periventricular areas. Average diffusion values in these areas were more severely affected in male patients (see Fig. 3). RD showed the largest effect sizes in these areas, with male patients showing 25% higher RD than male controls versus female patients showing 15% higher RD than female controls. Within the control group, these areas showed no significant sex differences in any diffusion parameter (average Z = −1.05, P = 0.3), while all diffusivity values except FA were significantly higher in male patients compared to female patients (average Z = −2.61, P = 0.02, see Table 2).
Figure 3.

Common areas. Left: severity of diffusion abnormalities in areas of the white matter skeleton (green) affected in both male and female patients (red) and all DTI parameters (X = 64, Y = 78, Z = 95), shown as percentage change in diffusion parameters in female (F) and male (M) patients in those areas (right).
Table 2.
DTI variables for controls and patients
| Controls | Patients | GLM | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Main effect | ||||||||||
| Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | Mean | ± | SD | F | P | |
| DTI: common areaa | ||||||||||||||
| Average FA | 0.58 | ± | 0.03 | 0.59 | ± | 0.03 | 0.55 | ± | 0.04 | 0.53 | ± | 0.05 | –1.92 | 0.0555 |
| Average MD (10−3 mm2/s) | 0.79 | ± | 0.03 | 0.77 | ± | 0.03 | 0.86 | ± | 0.08 | 0.89 | ± | 0.09 | –2.82 | 0.0049b |
| Average AD (10−3mm2/s) | 1.37 | ± | 0.04 | 1.37 | ± | 0.03 | 1.44 | ± | 0.08 | 1.49 | ± | 0.08 | –3.23 | 0.0012b |
| Average RD (10−3 mm2/s) | 0.49 | ± | 0.04 | 0.48 | ± | 0.03 | 0.57 | ± | 0.08 | 0.60 | ± | 0.09 | –2.49 | 0.0123b |
| DTI: Severity (Z score) | ||||||||||||||
| FA | 0.00 | ± | 0.29 | 0.00 | ± | 0.27 | –0.18 | ± | 0.34 | –0.41 | ± | 0.37 | 11.11 | <0.0001b |
| MD | 0.00 | ± | 0.32 | 0.00 | ± | 0.28 | 0.17 | ± | 0.44 | 0.49 | ± | 0.44 | 11.09 | <0.0001b |
| AD | 0.00 | ± | 0.21 | 0.00 | ± | 0.15 | 0.04 | ± | 0.23 | 0.16 | ± | 0.20 | 5.877 | 0.0008b |
| RD | 0.00 | ± | 0.33 | 0.00 | ± | 0.31 | 0.21 | ± | 0.46 | 0.54 | ± | 0.48 | 11.78 | <0.0001b |
| DTI: extent (# of voxels, log‐transformed) | ||||||||||||||
| FA | 1.38 | ± | 0.45 | 1.07 | ± | 0.58 | 2.67 | ± | 0.49 | 3.07 | ± | 0.50 | 121.57 | <0.0001b |
| MD | 2.52 | ± | 0.30 | 2.40 | ± | 0.42 | 3.22 | ± | 0.44 | 3.61 | ± | 0.40 | 66.20 | <0.0001b |
| AD | 2.33 | ± | 0.26 | 2.17 | ± | 0.40 | 3.01 | ± | 0.36 | 3.31 | ± | 0.34 | 81.60 | <0.0001b |
| RD | 2.47 | ± | 0.29 | 2.38 | ± | 0.36 | 3.19 | ± | 0.44 | 3.58 | ± | 0.43 | 67.86 | <0.0001b |
FA: fractional anisotropy, MD: mean diffusivity, AD: axial diffusivity, RD: radial diffusivity.
indicates Mann–Whitney Z and P values depicting the difference between male and female patients only.
indicate significant differences between male or female patients (respectively) compared to their sex‐matched controls.
Subject‐Level Quantification: Severity
The severity of diffusion changes was further quantified per subject by calculating Z scores for every voxel and averaging these over the entire skeleton. In the female patient group, the average Z score was mildly abnormal, mostly for RD (FA: −0.18, MD: 0.17, AD: 0.04, RD: 0.21, see Table 2). In the male patient group Z scores showed stronger abnormalities (FA −0.41, MD 0.49, AD 0.16, RD 0.54, see Table 2), indicating a stronger deviation from normal values in all parameters in male patients compared to male controls, than female patients compared to female controls. This was confirmed using the GLM analyses (see Table 2 for main effect F and P values), which showed all Z scores to be significantly abnormal in male patients compared to controls (average post hoc P = 0.003), but not in female patients (average P = 0.4), where only FA showed a trend (P = 0.06). All Z scores were significantly different between male and female patients (average P = 0.003).
Subject‐Level Quantification: Extent
The extent of diffusion abnormalities was calculated using aforementioned Z score maps, by counting the number of voxels showing Z ≤ −3.1 for FA and Z ≥ 3.1 for MD, AD, and RD. Both patient groups showed a significant amount of affected voxels in all parameters (See Table 2 for main effect F and P values, both groups: average post hoc P < 0.0001, see Table 2), similar to the TBSS results. Male patients showed significantly more abnormal voxels than female patients (post hoc P = 0.0002, see Table 2).
Stepwise Linear Regression
The linear regression model predicting average cognition in the entire patient group resulted in an adjusted R 2 of 0.39 (F = 14.36, P < 0.0001). There were six significant predictors in the model: the extent of abnormal (Z < −3.1) voxels based on FA (standardized β = −0.43, t = −4.62, P < 0.0001), level of education (standardized β = 0.28, t = 3.91, P = 0.0002), NGMV (standardized β = 0.19, t = 2.52, P = 0.01), MS phenotype (standardized β = −0.17, t = −2.28, P = 0.02), T 1 lesion volume (standardized β = 0.20, t = 2.27, P = 0.02), and EDSS (standardized β = −0.16, t = −2.21, P = 0.03). Repeating the regression analysis using only RRMS patients did not change these results, with the exception of NGMV, which was no longer a significant predictor of cognition. Extent of FA abnormalities correlated significantly with cognition in the entire patient group (r = −0.42, P < 0.0001), as well as in male (r = −0.42, P = 0.005) and female patients (r = −0.30, P = 0.006) separately, while, for example, EDSS scores did not (rho=0.07, P = 0.47), in the entire patient group.
Voxelwise Correlations
Correlation results are summarized in Figures 4 and 5. Average cognition is shown in Figure 4 with P < 0.05 for all significant voxels, shown in blue‐light blue (P = 0.05 and below, respectively) in men, and red‐yellow in women. Especially strong correlations are seen in the thalamus in both groups. In the female group, no areas were significant at the P < 0.01 level, in the male group only within the thalamus (r = 0.65, P < 0.0001). Specific cognitive domains were correlated to FA in male patients only (see Fig. 5), as female patients showed no significant cognitive dysfunction as a group. Attention showed the strongest correlations in the thalamus and corpus callosum, extending primarily toward frontal areas, especially on the left, including WM near the anterior cingulate, orbitofrontal and middle frontal gyri, as well as the frontal pole. Other WM areas included the left capsula externa, and right capsula interna (anterior limb), the right superior longitudinal fasciculus and (mainly right) occipitotemporal areas. Executive function showed significant correlations within the thalamus only, although none at P < 0.01. Information processing speed was the most extensively relatable domain, involving almost the entire WM skeleton, apart from the cerebellum, brain stem, capsula interna, and the WM surrounding the right superior frontal gyrus. Interestingly, FA values in the body of the corpus callosum significantly correlated to this domain, while those in the genu and splenium did not. Psychomotor speed only included the thalamus at P < 0.01. Verbal memory showed the strongest relations within the thalamus and the corpus callosum, extending upward into anterior and posterior cingulate areas, especially on the right side. Although working memory was significantly impaired in the male patient group, the correlation analyses did not survive multiple comparison corrections.
Figure 4.
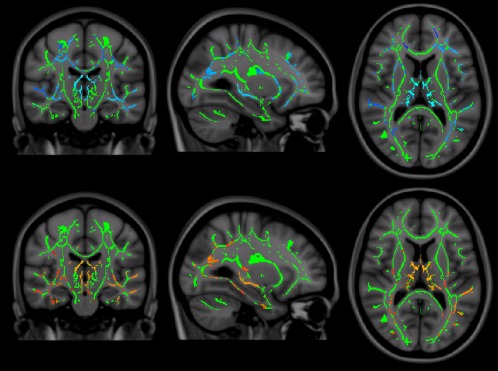
Correlations between average cognition and FA abnormalities in male (top row) and female patients (bottom row, (X = 90, Y = 107, Z = 83). Note that the choice of color scales [gradual from blue to light blue (least to most significant) for men, and red to yellow for women] is purely for illustration, these indicate the P value of the given voxel at P < 0.05. None of the significant voxels reached P < 0.01 in female patients, while in male patients, voxels did reach P < 0.01 in throughout the thalamus.
Figure 5.
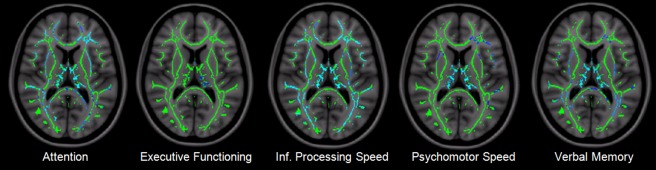
Cognitive domains and their correlated FA values in WM regions in male patients (Z = 83). From left to right: voxelwise correlations using the attention, executive functioning, information processing speed, psychomotor speed, and verbal memory domains (P < 0.05).
Cognitively Impaired Versus Cognitively Preserved Patients
A final GLM analysis was applied, where cognitively impaired (n = 40, 20 men) and cognitively preserved patients (n = 91, 26 men) were contrasted. Here, cognitively impaired patients showed higher extent and severity on all DTI parameters: Extent was higher on FA (F = 18.825, P < 0.001), MD (F = 15.002, P < 0.001), AD (F = 13.647, P < 0.001), and RD (F = 18.040, P < 0.001); Severity was higher on FA (F = 22.648 <0.001), MD (F = 20.449, P < 0.001), AD (F = 8.699, P = 0.004), and RD (F = 23.469, P < 0.001). Brain volumes were lower for NGMV (F = 13.121, P < 0.001), NWMV (F = 7.540, P = 0.007), and NBV (F = 15.361, P < 0.001); Lesion volumes were higher on T 2 only (F = 8.884, P = 0.003).
DISCUSSION
In this study, we investigated diffusion changes in a well‐defined early inception cohort of MS patients, measured 6 years postdiagnosis. Mild cognitive dysfunction and brain atrophy were found. DTI analyses revealed the highest extent and severity of damage in RD, most likely reflecting myelin integrity [Klawiter et al., 2011; Song et al., 2005]. Regionally, posterior periventricular WM areas showed abnormalities in all diffusion parameters, a region that commonly contains lesions. Severity of diffusion changes was especially severe in radial diffusion as opposed to axial diffusion, indicative of mostly demyelination, as opposed to axonal integrity loss. Sex effects were very strong in cognitive and DTI measures, with changes of RD in 21% of the measured WM voxels in female patients, and 60% in male patients. This is remarkable, as lesion volumes were not different between the sexes, which indicates that there are more severe changes in normal appearing tissue.
A regression model that included demographic, clinical and MRI variables showed that the extent of FA changes in the MS WM was found to best predict cognition, in addition to level of education, gray matter atrophy, MS disease type, T 1‐hypointense lesion load and disability. Our measure of “extent” in FA abnormalities was the main predictor of cognition. The fact that FA was the primary predictor, and not RD, is interesting, given the larger effect in RD (e.g., in the male MS group: 60% of the skeleton was abnormal on RD, and 53% on FA). FA, however, combines both AD and RD; and therefore, AD abnormalities are taken into account as well. The extent of diffusion changes was also more important for cognitive decline than the severity of change, perhaps indicating that it is especially important whether or not a specific area is affected, more so than if the abnormality is very severe. The fact that level of education predicts cognition is not surprising, as indicated by prior studies [Benedict and Zivadinov, 2011; Sumowski et al., 2010a, 2010b]. Total T 2 lesion volume was not a significant predictor in the model, while the extent of FA changes was, which indicates that damage in nonlesional areas is important for cognition. This was also supported by earlier studies, showing the importance of NABT changes for cognitive dysfunction in MS [Deloire et al., 2005, 2011]. Although sex was not a separate significant predictor in the model, both the extent of FA changes and cognitive changes were worse in male patients, which could have eliminated sex as a separate predictor from the model. Interestingly, although the extent based on FA was the most important predictor of cognitive performance, a final exploration using GLM revealed that patients suffering from cognitive impairment showed significantly greater extent (especially on FA) and severity (especially on RD) values on all diffusion parameters than those in patients without cognitive impairment, as well as more atrophy and higher T 2‐lesion volumes. This indicates that while the extent based on FA is the most important predictor of cognition, all measures of extent and severity are related to cognition in MS.
To identify specific WM tracts that are particularly relevant for cognition, post hoc voxelwise correlations were performed. These especially implicated thalamic regions of the skeleton as important for cognitive impairment in MS. Although such voxelwise correlations have been investigated before [Roosendaal et al., 2009; Yu et al., 2011], our study now allowed for a much broader cognitive evaluation as well as for sex‐specific explorations. When selecting a more conservative threshold of P < 0.01, the average cognition domain showed no significant voxels in the female patient group, probably due to their limited cognitive dysfunction. In male patients, the more conservative threshold revealed only thalamic voxels, suggesting an especially strong role for the thalamus in MS cognitive decline in this phase of the disease. This is also supported by earlier studies on thalamus atrophy in MS [Batista et al., 2012; Benedict et al., 2004; Houtchens et al., 2007; Schoonheim et al., 2012].
Looking at areas outside the thalamus, attention deficits were also related to diffusion changes in mainly frontal and parietal (superior longitudinal fasciculus) WM areas, confirming an earlier study showing primarily frontal and parietal lesions to explain variance in the Stroop task [Pujol et al., 2001], as well as functional MRI studies implicating these areas as important in Stroop performance in MS [Filippi et al., 2012; Rocca et al., 2009]. Interestingly, information processing speed seems to involve almost all of the cerebral WM, making it very sensitive to the seemingly random damage seen in MS. This could provide an explanation as to why this cognitive domain is affected so early in the disease [Amato et al., 2006; Chiaravalloti and DeLuca, 2008]. Executive functioning was only related to thalamic changes in FA, whereas it would be expected to lie mostly in frontal areas. Perhaps, this is due to power issues, given the mild cognitive dysfunction after 6 years, or due to the sensitivity of the tests used to assess the domain (concept shifting task and word list generation). Attention, information processing speed and verbal memory also showed involvement of cingulate areas, which is a region of the cortex frequently implicated in cognition in MS [Cader et al., 2006; Filippi et al., 2012; Hawellek et al., 2011; Rocca et al., 2010].
The sex effect on FA values in healthy controls confirms many previous studies showing differences in brain structure and network topology between healthy male and female brains [Cosgrove et al., 2007; Gong et al., 2009, 2011; Menzler et al., 2011], and relates to sex‐specific differences that are apparent during development [Kumar et al., 2012]. If these normal differences between the sexes are not taken into account they can bias disease‐specific findings, perhaps missing sex effects such as those in this MS cohort. Previous studies have also shown other clear sex effects in MS cognition [Benedict and Zivadinov, 2011; Savettieri et al., 2004; Schoonheim et al., 2012], GM atrophy [Antulov et al., 2009; Schoonheim et al., 2012], and clinical progression [Koch et al., 2010], all specifying a poorer prognosis for male patients, while clinically eloquent sex effects in MS‐related WM damage have not been shown as clearly before. A previous postmortem study investigating the histopathology of end‐stage MS lesions did not find sex differences in lesion characteristics [Kuhlmann et al., 2009]. This could indicate that our findings of strong sex effects in diffusion metrics are only present in earlier phases of the disease. While T 1‐hypointense and T 2‐lesion volumes did not differ between the sexes, male patients showed more extensive diffusion abnormalities, seemingly within the normal‐appearing WM, underlining the importance of diffusion changes in areas beyond focal lesions in cognitive dysfunction in MS.
Some limitations apply to the current work. As the study design is cross‐sectional, it is unclear whether the observed clinically relevant sex effects we found for WM damage are only present at this early stage of the disease with mostly relapsing‐remitting MS. Additionally, although our results showed no difference in lesion volumes, lesion location was not studied in detail. Brain volumes were not corrected by lesion filling. As a result, lesion misclassification could have influenced NWMV. Also, relations between GM and WM damage were not investigated thoroughly. Later stages of MS can be dramatically different in terms of, for example, GM atrophy rates [Fisher et al., 2008], and there appears to be a clear link between WM changes and GM atrophy in early PPMS [Bodini et al., 2009]. It is unclear, however, whether this relationship is the same in different disease phenotypes and stages. Future longitudinal studies investigating WM and GM damage will therefore be important, and need to take our cross‐sectional results on sex effects in MS and the importance of the thalamus for cognition into account. These new insights should then help further unravel the currently insufficiently understood relationship between structural damage and cognitive decline in MS.
CONCLUSION
In summary, the extent of WM FA changes, especially in the thalamus, was strongly associated with cognitive performance in this cohort of early MS patients. The WM of male MS patients was both more extensively and also more severely affected than that of female patients, while groups were equal in terms of disease duration, disability and WM lesion volume, indicating an important and sex‐specific role for NAWM changes in cognitive dysfunction in MS.
REFERENCES
- Amato MP, Zipoli V, Portaccio E (2006): Multiple sclerosis‐related cognitive changes: A review of cross‐sectional and longitudinal studies. J Neurol Sci 245:41–46. [DOI] [PubMed] [Google Scholar]
- Antulov R, Weinstock‐Guttman B, Cox JL, Hussein S, Durfee J, Caiola C, Dwyer MG, Bergsland N, Abdelrahman N, Stosic M, Hojnacki D, Munschauer FE, Miletic D, Zivadinov R (2009): Gender‐related differences in MS: A study of conventional and nonconventional MRI measures. Mult Scler 15:345–354. [DOI] [PubMed] [Google Scholar]
- Batista S, Zivadinov R, Hoogs M, Bergsland N, Heininen‐Brown M, Dwyer MG, Weinstock‐Guttman B, Benedict RH (2012): Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J Neurol 259:139–146. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Bruce J, Dwyer MG, Weinstock‐Guttman B, Tjoa C, Tavazzi E, Munschauer FE, Zivadinov R (2007): Diffusion‐weighted imaging predicts cognitive impairment in multiple sclerosis. Mult Scler 13:722–730. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Weinstock‐Guttman B, Fishman I, Sharma J, Tjoa CW, Bakshi R (2004): Prediction of neuropsychological impairment in multiple sclerosis: Comparison of conventional magnetic resonance imaging measures of atrophy and lesion burden. Arch Neurol 61:226–230. [DOI] [PubMed] [Google Scholar]
- Benedict RH, Zivadinov R (2011): Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol 7:333–342. [DOI] [PubMed] [Google Scholar]
- Bodini B, Khaleeli Z, Cercignani M, Miller DH, Thompson AJ, Ciccarelli O (2009): Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: An in vivo study with TBSS and VBM. Hum Brain Mapp 30:2852–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cader S, Cifelli A, Abu‐Omar Y, Palace J, Matthews PM (2006): Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain 129:527–537. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J (2008): Cognitive impairment in multiple sclerosis. Lancet Neurol 7:1139–1151. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK (2007): Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 62:847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloire MS, Ruet A, Hamel D, Bonnet M, Dousset V, Brochet B (2011): MRI predictors of cognitive outcome in early multiple sclerosis. Neurology 76:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloire MS, Salort E, Bonnet M, Arimone Y, Boudineau M, Amieva H, Barroso B, Ouallet JC, Pachai C, Galliaud E, Petry KG, Dousset V, Fabrigoule C, Brochet B (2005): Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J Neurol Neurosurg Psychiatry 76:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dineen RA, Vilisaar J, Hlinka J, Bradshaw CM, Morgan PS, Constantinescu CS, Auer DP (2009): Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain 132:239–249. [DOI] [PubMed] [Google Scholar]
- Filippi M, Riccitelli G, Mattioli F, Capra R, Stampatori C, Pagani E, Valsasina P, Copetti M, Falini A, Comi G, Rocca MA (2012): Multiple sclerosis: Effects of cognitive rehabilitation on structural and functional MR imaging measures—An explorative study. Radiology 262:932–940. [DOI] [PubMed] [Google Scholar]
- Filippi M, Rocca MA, Benedict RH, DeLuca J, Geurts JJ, Rombouts SA, Ron M, Comi G (2010): The contribution of MRI in assessing cognitive impairment in multiple sclerosis. Neurology 75:2121–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E, Lee JC, Nakamura K, Rudick RA (2008): Gray matter atrophy in multiple sclerosis: A longitudinal study. Ann Neurol 64:255–265. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC (2011): Brain connectivity: Gender makes a difference. Neuroscientist 17:575–591. [DOI] [PubMed] [Google Scholar]
- Gong G, Rosa‐Neto P, Carbonell F, Chen ZJ, He Y, Evans AC (2009): Age‐ and gender‐related differences in the cortical anatomical network. J Neurosci 29:15684–15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, Engel AK (2011): Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci U S A 108:19066–19071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtchens MK, Benedict RH, Killiany R, Sharma J, Jaisani Z, Singh B, Weinstock‐Guttman B, Guttmann CR, Bakshi R (2007): Thalamic atrophy and cognition in multiple sclerosis. Neurology 69:1213–1223. [DOI] [PubMed] [Google Scholar]
- Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL (2011): Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 55:1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Kingwell E, Rieckmann P, Tremlett H (2010): The natural history of secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 81:1039–1043. [DOI] [PubMed] [Google Scholar]
- Kuhlmann T, Goldschmidt T, Antel J, Wegner C, Konig F, Metz I, Bruck W (2009): Gender differences in the histopathology of MS? J Neurol Sci 286:86–91. [DOI] [PubMed] [Google Scholar]
- Kumar R, Nguyen HD, Macey PM, Woo MA, Harper RM (2012): Regional brain axial and radial diffusivity changes during development. J Neurosci Res 90:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke JF (1983): Rating neurologic impairment in multiple‐sclerosis—An Expanded Disability Status Scale (Edss). Neurology 33:1444–1452. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC (1996): Defining the clinical course of multiple sclerosis: Results of an international survey. Neurology 46:907–911. [DOI] [PubMed] [Google Scholar]
- Menzler K, Belke M, Wehrmann E, Krakow K, Lengler U, Jansen A, Hamer HM, Oertel WH, Rosenow F, Knake S (2011): Men and women are different: Diffusion tensor imaging reveals sexual dimorphism in the microstructure of the thalamus, corpus callosum and cingulum. Neuroimage 54:2557–2562. [DOI] [PubMed] [Google Scholar]
- Moll NM, Rietsch AM, Thomas S, Ransohoff AJ, Lee JC, Fox R, Chang A, Ransohoff RM, Fisher E (2011): Multiple sclerosis normal‐appearing white matter: Pathology‐imaging correlations. Ann Neurol 70:764–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagani E, Bammer R, Horsfield MA, Rovaris M, Gass A, Ciccarelli O, Filippi M (2007): Diffusion MR imaging in multiple sclerosis: Technical aspects and challenges. AJNR Am J Neuroradiol 28:411–420. [PMC free article] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg‐Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011): Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Deus J, Junque C, Bello J, Marti‐Vilalta JL, Capdevila A (2001): The effect of medial frontal and posterior parietal demyelinating lesions on stroop interference. Neuroimage 13:68–75. [DOI] [PubMed] [Google Scholar]
- Rao SM (1990): A Manual for the Brief Repeatable Battery of Neuropsychological Tests in Multiple Sclerosis. Milwaukee, WI: Medical College of Wisconsin. [Google Scholar]
- Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, Rossi P, Falini A, Comi G, Filippi M (2010): Default‐mode network dysfunction and cognitive impairment in progressive MS. Neurology 74:1252–1259. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Valsasina P, Ceccarelli A, Absinta M, Ghezzi A, Riccitelli G, Pagani E, Falini A, Comi G, Scotti G, Filippi M (2009): Structural and functional MRI correlates of Stroop control in benign MS. Hum Brain Mapp 30:276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal SD, Geurts JJ, Vrenken H, Hulst HE, Cover KS, Castelijns JA, Pouwels PJ, Barkhof F (2009): Regional DTI differences in multiple sclerosis patients. Neuroimage 44:1397–1403. [DOI] [PubMed] [Google Scholar]
- Rovaris M, Gass A, Bammer R, Hickman SJ, Ciccarelli O, Miller DH, Filippi M (2005): Diffusion MRI in multiple sclerosis. Neurology 65:1526–1532. [DOI] [PubMed] [Google Scholar]
- Rovaris M, Iannucci G, Falautano M, Possa F, Martinelli V, Comi G, Filippi M (2002): Cognitive dysfunction in patients with mildly disabling relapsing‐remitting multiple sclerosis: An exploratory study with diffusion tensor MR imaging. J Neurol Sci 195:103–109. [DOI] [PubMed] [Google Scholar]
- Savettieri G, Messina D, Andreoli V, Bonavita S, Caltagirone C, Cittadella R, Farina D, Fazio MC, Girlanda P, Le PF, Liguori M, Lugaresi A, Nocentini U, Reggio A, Salemi G, Tedeschi G, Trojano M, Valentino P, Quattrone A (2004): Gender‐related effect of clinical and genetic variables on the cognitive impairment in multiple sclerosis. J Neurol 251:1208–1214. [DOI] [PubMed] [Google Scholar]
- Schoonheim MM, Geurts JJ, Landi D, Douw L, van der Meer ML, Vrenken H, Polman CH, Barkhof F, Stam CJ (2013): Functional connectivity changes in multiple sclerosis patients: A graph analytical study of MEG resting state data. Hum Brain Mapp 34:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheim MM, Popescu V, Rueda Lopes FC, Wiebenga OT, Vrenken H, Douw L, Polman CH, Geurts JJ, Barkhof F (2012): Subcortical atrophy and cognition: Sex effects in multiple sclerosis. Neurology 79:1754–1761. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005): Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 26:132–140. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Wylie GR, Chiaravalloti N, Deluca J (2010a): Intellectual enrichment lessens the effect of brain atrophy on learning and memory in multiple sclerosis. Neurology 74:1942–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N (2010b): Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: Functional magnetic resonance imaging evidence for cognitive reserve. Brain 133:362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hecke W., Nagels G, Leemans A, Vandervliet E, Sijbers J, Parizel PM (2010): Correlation of cognitive dysfunction and diffusion tensor MRI measures in patients with mild and moderate multiple sclerosis. J Magn Reson Imaging 31:1492–1498. [DOI] [PubMed] [Google Scholar]
- Vrenken H, Pouwels PJ, Geurts JJ, Knol DL, Polman CH, Barkhof F, Castelijns JA (2006): Altered diffusion tensor in multiple sclerosis normal‐appearing brain tissue: Cortical diffusion changes seem related to clinical deterioration. J Magn Reson Imaging 23:628–636. [DOI] [PubMed] [Google Scholar]
- Yu HJ, Christodoulou C, Bhise V, Greenblatt D, Patel Y, Serafin D, Maletic‐Savatic M, Krupp LB, Wagshul M (2011): Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. Neuroimage 59:3713–3722. [DOI] [PubMed] [Google Scholar]


