Abstract
Eating behavior is crucial in the development of obesity and Type 2 diabetes. To further investigate its regulation, we studied the effects of glucose versus water ingestion on the neural processing of visual high and low caloric food cues in 12 lean and 12 overweight subjects by functional magnetic resonance imaging. We found body weight to substantially impact the brain's response to visual food cues after glucose versus water ingestion. Specifically, there was a significant interaction between body weight, condition (water versus glucose), and caloric content of food cues. Although overweight subjects showed a generalized reduced response to food objects in the fusiform gyrus and precuneus, the lean group showed a differential pattern to high versus low caloric foods depending on glucose versus water ingestion. Furthermore, we observed plasma insulin and glucose associated effects. The hypothalamic response to high caloric food cues negatively correlated with changes in blood glucose 30 min after glucose ingestion, while especially brain regions in the prefrontal cortex showed a significant negative relationship with increases in plasma insulin 120 min after glucose ingestion. We conclude that the postprandial neural processing of food cues is highly influenced by body weight especially in visual areas, potentially altering visual attention to food. Furthermore, our results underline that insulin markedly influences prefrontal activity to high caloric food cues after a meal, indicating that postprandial hormones may be potential players in modulating executive control. Hum Brain Mapp 35:918–928, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: obesity, fMRI, insulin, food
INTRODUCTION
Obesity and type 2 diabetes mellitus together with related diseases are a leading cause of illness and death and the prevalence is dramatically rising worldwide. Eating behavior plays an important role in the development of both disorders [Fung et al., 2004]. The control of eating behavior can be separated in homeostatic and nonhomeostatic components; both are associated with specific brain processes. Although the knowledge on the homeostatic control is rather advanced, the understanding of the cognitive processing and the interaction of both systems is rather limited. This is especially true in respect to the termination of food intake, which is of great importance as overconsumption of food is the major cause of obesity.
In the postprandial state, levels of several hormones are altered. Many of them are candidates for the induction of satiety and thereby for the prevention of excessive food intake [Camilleri, 2009; de Graaf et al., 2004; Schloegl, et al., 2007]. One of the best characterized postprandial hormones is insulin. It is secreted by the pancreatic β‐cells, and its plasma level rise rapidly after eating. The hormone promotes uptake of glucose from the blood into several organs, such as muscle, liver, and adipose tissue. Obesity is associated with elevated fasting insulin as well as postprandial insulin levels [Bagdade et al., 1967]. Besides its effects in peripheral tissues, insulin has been shown to act in the brain, where it is involved, e.g., in the control of food intake and cognitive functions [Hallschmid and Schultes, 2009; Morton et al., 2006].
Visual stimulation with food pictures is well established to analyze the neural processing of eating behavior. Food pictures elicit neuronal activity in brain regions involved in sensory processing, object recognition, hedonic processing, appetite regulation, and homeostatic areas [Frank et al., 2010; Fuhrer et al., 2008; Porubska et al., 2006; Schur et al., 2009; Siep et al., 2009]. Furthermore several studies have shown that body weight is an important factor that is associated with the brain's response to food pictures in regions important for salience processing and executive control. In the hungry state, obese subjects showed an increased activation in the anterior cingulate cortex (ACC) and medial prefrontal cortex (MPFC) whilst viewing food pictures compared with normal‐weight subjects [Martin et al., 2009]. Furthermore, brain regions related to hedonic aspects of food cues, especially the striatum, showed significantly reduced dopamine D2 receptor availability [Wang et al., 2001] and exhibited greater activation to high caloric food stimuli in obese individuals [Rothemund et al., 2007; Stoeckel et al., 2008].
The effect of the important postprandial hormone insulin on the neural processing of food cues has been studied by the exogenous administration of the hormone either intravenously or by an intranasal approach [Guthoff et al., 2010, 2011; Rotte et al., 2005]. It was shown that insulin alters brain activity specifically in areas related to object processing, memory, and executive control, such as fusiform gyrus, hippocampus, and frontal areas [Grichisch et al., 2011; Guthoff et al., 2010; Kullmann et al., 2012a; Rotte et al., 2005]. Of interest, differences between lean and overweight subjects have been observed [Guthoff et al., 2010, 2011], and its importance in human disease is further underlined by the finding of central insulin resistance in overweight persons [Ketterer et al., 2011; Stingl et al., 2010; Tschritter et al., 2006].
To our knowledge, alterations in the neural processing of food cues after glucose‐induced changes in endogenous hormonal levels have not been sufficiently investigated. Following glucose ingestion, levels of glucose, endogenous insulin, and many hormones beyond are rapidly changing physiologically. Investigating the brain response after oral glucose intake therefore allows us to detect the effects of these factors under physiological conditions. A recent study using oral glucose ingestion showed that insulin is critical in regulating neural food‐cue responses in healthy lean adults [Kroemer et al., 2012].
The aim of this study was to investigate the influence of glucose versus water ingestion on the neural processing of food cues in healthy lean and overweight subjects 30 and 120 min post load.
EXPERIMENTAL PROCEDURES
Subjects
Twenty‐four right‐handed subjects participated in the study: 12 lean (six women, body mass index (BMI) range 19.4–22.5 kg/m2, and age range 22–29 years), 12 overweight and obese subjects (six women, BMI range 28.4–34.4 kg/m2, and age range 21–28 years). Lean subjects were required to have a BMI of 18.5–24.0 kg/m2. Overweight and obese subjects were required to have a BMI greater than 25 kg/m2 and are collectively revered to as overweight. To minimize circadian influence, subjects were scanned between 7 and 8 a.m. Before the study, subjects confirmed their fasting state and rated their subjective feeling of hunger on a visual analogue scale from 0 to 10 (0: not hungry at all; 10: very hungry). In addition, blood glucose and plasma insulin levels before the experiment were within the fasting range for all subjects. Before the experiment, subjects filled out a short German version of the Profile of Mood States (POMS; [McNair et al., 1981]), which is a 35 item questionnaire evaluate depression, fatigue, anger, and vigor.
Before the measurement day, all subjects underwent a medical examination to assure that they did not suffer from neither psychiatric, neurological, nor metabolic diseases. Any volunteer treated for chronic disease or taking any kind of medication other than oral contraceptives was excluded. To address psychiatric diseases, the Patient Health Questionnaire [Löwe et al., 2002] was used; to assess eating behavior subjects took the German Three Factor Eating Questionnaire (TFEQ) [Pudel and Westenhöfer, 1989]. All subjects were normal sighted or had corrected‐to‐normal vision. Informed written consent was obtained from all subjects and the protocol was approved by the local ethics committee of the medical faculty of the University of Tübingen. The demographic characteristics of the subjects are shown in Table 1.
Table 1.
Subjects' characteristics in lean vs. overweight group
| Lean group | Overweight group | p | |
|---|---|---|---|
| Gender (female/male) | 6/6 | 6/6 | — |
| Age (y) | 22.91 ± 2.10 | 24.66 ± 2.42 | 0.08 |
| BMI (kg/m²) | 21.16 ± 1.13 | 30.46 ± 1.77 | 0.001 |
| Fasting glucose (mmol/l) | 4.94 ± 0.34 | 4.96 ± 0.41 | 0.9 |
| Fasting insulin (pmol/l) | 37 ± 15 | 77 ± 33 | 0.0014 |
| OGTT‐derived insulin sensitivity index (AU) | 19.0 ±7.1 | 11.5 ±7.0 | 0.0067 |
| TFEQ | |||
| Restraint eating | 4.75 ± 4.55 | 9.33 ± 4.7 | 0.024 |
| Disinhibition | 3.91 ± 1.24 | 6.08 ± 3.57 | 0.06 |
| Hunger | 5.66 ± 3.14 | 4.50 ± 2.31 | 0.321 |
| POMS‐scale: Depression (%) | 0.82 ± 1.45 | 2.84 ± 5.31 | 0.236 |
| POMS‐scale: Fatigue (%) | 21.53 ± 10.6 | 18.15 ± 14.1 | 0.526 |
| POMS‐scale: Anger (%) | 1.40 ± 1.8 | 3.76 ± 7.3 | 0.307 |
| POMS‐scale: Vigor (%) | 32.79 ± 17.29 | 42.33 ± 15.9 | 0.183 |
| Accuracy (%) One‐back visual recognition task | 87 ± 0.07 | 88 ± 0.08 | 0.442 |
| Reaction time (sec) One‐back visual recognition task | 0.84 ± 0.16 | 0.73 ± 0.09 | 0.08 |
Data are given as means ± SD.
TFEQ = Three Factor Eating Questionnaire.
P = P‐values, comparison of unadjusted loge‐transformed data by ANOVA.
Oral Glucose Tolerance Test/Water Experiment
All subjects participated in two experiments, Oral Glucose Tolerance Test (OGTT) or water, on 2 different days in randomized order with a time‐lag of 7–28 days. Experiments were conducted after an overnight fast of 10 h and started at 7.00 a.m. with an fMRI measurement under basal conditions before glucose or water administration (fMRI basal). After this basal measurement, subjects ingested a solution containing 75 g glucose or an equal volume of water (300 ml). After 30 min, the second fMRI measurement was performed (fMRI 30) and after 120 min, the third measurement was performed (fMRI 120). Venous blood samples were obtained at 0, 30, 60, 90, 120, and 150 min, and plasma glucose and insulin concentrations were determined (Fig. 1; Supporting Information Table 1).
Figure 1.
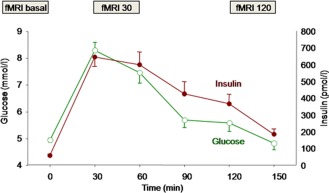
Schematic time points for fMRI measurements, glucose (green open symbols) and insulin (red filled symbols) levels (see also Supporting Information Table 1 for values). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Analytical Procedures
Blood glucose was determined using a bedside glucose analyzer (glucose oxidase method; Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was measured by commericial chemiluminescence assays for ADVIA Centaur (Siemens Medical Solutions, Fernwald, Germany). Insulin sensitivity during OGTT was estimated according to Matsuda and DeFronzo 2002.
Stimulus Material
A stimulus set of 48 color food and 48 non‐food related pictures matched for physical complexity, arousal, and valence were used in this study. The food images consisted of different kinds of dishes (salad, meat, soup, desserts, fruits, vegetables, and complex meals) and the non‐food images comprised objects that had no association with eating (i.e., chair, umbrella, toy, money, car, and jewelry).
Experimental Protocol
A block design was used with food and non‐food pictures, presented either during a one‐back visual recognition task or a null‐back control task separated by baseline blocks (fixation cross). For the one‐back task subjects had to press a button at each picture depending whether the presented picture was the same or was different as the one before (yes = left button, no = right button). For the control task (food and non food pictures combined), subjects had to press only the left button as soon as they saw the picture.
The experiment consisted of six sessions. Two sessions were performed at each experimental phase (fMRI basal, fMRI 30, and fMRI 120). Within each session, subjects were presented with 12 different stimuli blocks at random: four blocks consisted of the control task and eight blocks of the one‐back task, being four blocks with food pictures, and four blocks with non‐food pictures. The food picture blocks were further divided into blocks with high caloric and low caloric food pictures. Before each block, subjects were informed whether the upcoming task is a control or a one‐back task. Stimuli were presented for 1.5 s with an interstimulus interval varied randomly between 2 and 3 s. Before and after each block, a blank screen with a red fixation cross in the middle was shown for 14 s. The Presentation® software (Version 10.2, http://www.neurobs.com) was used for stimulus presentation and response data collection via button press. The stimulus presentation was synchronized with the trigger input from the scanner. Subjects were instructed to look at the middle of the picture or the fixation cross that would appear between stimulus presentations. During both days, the same stimulus set was used but to avoid confounding effects, the sequence of the six sessions was inverted on the second day. The sequence order was counterbalanced.
Imaging Procedures
Whole‐brain fMRI data was obtained by using a 3.0 T scanner (Siemens Tim Trio, Erlangen, Germany). Each of the six sessions consisted of 226 scans (Repetition time (TR) = 2 s, Echo time (TE) = 30 ms, matrix 64 × 64, flip angle 90°, voxel size 3 × 3 × 3.75 mm3, slice thickness 3 mm, 0.75 mm gap, 30 slices) lasting ∼ 8 min; the images were acquired in ascending order. In addition, high‐resolution T1 weighted anatomical images (MPRage: 176 slices, matrix: 256 × 224, 1 × 1 × 1 mm3) of the brain were obtained. During the scan procedure, subjects were lying in a supine position. Their heads were stabilized by foam padding around the head within the head coil to minimize head movements. Pictures were projected on a tilted mirror mounted on the head coil.
Preprocessing and statistical analysis of the fMRI data was done using SPM8 (Wellcome Trust Centre for Neuroimaging, London, UK). Images were realigned and resliced to the first image after unwarping in phase encoding direction (anterior–posterior) to account for susceptibility by movement artifacts. A mean image was created and coregistered to the T1 structural image. The anatomical image was normalized in MNI space, and the resulting parameter file was used to normalize the functional images (voxel size: 3 × 3 × 3 mm3). Finally, the normalized images were smoothed with a three‐dimensional isotropic Gaussian kernel (FWHM: 6 mm). FMRI data were highpass (cut‐off period 128 s) filtered and global AR(1) auto correlation correction was performed.
Image Processing
For the first level analysis, a design matrix was created individually for glucose and water day. For each subject, the durations of high and low caloric food pictures (in relation to the implicit baseline) were modulated separately for the three different time points (fMRI basal, fMRI 30, and fMRI 120) using a fixed effect analyses. Food pictures were selected for the analysis based on our recent finding of a specific insulin modulation on brain activation elicited by food pictures [Guthoff et al., 2010]. For each condition, a separate regressor was modeled and convolved with a canonical hemodynamic response function and its time derivative. The movement regressors, separately for each session were included as covariates in the model to account for possible movement‐induced variance. Individual contrast images were computed to estimate activation changes to high caloric and low caloric food cues after oral glucose and water ingestion compared with the basal measurement, i.e., between fMRI 30 and fMRI basal (fMRI 30 minus fMRI basal) and between fMRI 120 and fMRI basal (fMRI 120 minus fMRI basal).
These differential contrasts were used for random effects second level analyses comprising a four‐way repeated measurement analysis of variance (ANOVA) using the full factorial model in SPM8. The model included a between‐subject factor “body weight” (lean vs. overweight subjects) and 3 within‐subject factors: “condition” (glucose vs. water administration), “caloric content” (high vs. low caloric food) and “time” (30 vs. 120 min post load), and three covariates were included to model out between‐subject differences (change in glucose levels, insulin levels and subjective feeling of hunger 30 and 120 min after glucose and water administration). Activations exceeding an uncorrected threshold of P < 0.001 with a minimal cluster size of k = 10 (which corresponds to the expected voxels per cluster in SPM) were considered significant.
Furthermore, we analyzed the effect of high and low caloric food cues in the fMRI basal session only, to investigate the effect of food images before glucose or water administration in lean and overweight subjects (see Supporting Information text and Supporting Information Table 2).
A multiple regression approach was used to evaluate the association between the change in insulin levels, glucose levels, and subjective feeling of hunger 30 and 120 min after glucose/water administration with the brain's post load response to high and low caloric food pictures (P < 0.05, corrected for multiple comparisons).
Statistical Analyses
Unless otherwise stated, data are given as mean ± SD. Data that were not normally distributed were logarithmically transformed before statistical analysis. For the analyses of questionnaires and hunger ratings, the software package SPSS (version 19; SPSS, Chicago, IL) was used.
The statistical analysis of metabolic parameters was performed with JMP 7.0 (SAS Institute, Cary, NC). All data are given as unadjusted mean ± SD. The parameters were log transformed to approximate normal distribution before statistical analysis. T‐tests were used to test for significant differences between lean and overweight subjects. Results with values of P < 0.05 were considered statistically significant.
RESULTS
Imaging Results
We investigated the effect of glucose versus water ingestion on the brain's response to high and low caloric food cues 30 and 120 min post load (fMRI 30 minus fMRI basal) (fMRI 120 minus fMRI basal) in lean and overweight subjects.
After glucose administration, we found a significant increase in activity to food pictures 30 and 120 min post load in the right fusiform gyrus, left precuneus, and right hippocampus (Table 2). After water administration, we found a significant increase in activity to food pictures 30 and 120 min post load in the left superior occipital gyrus (Table 2). However, we observed no main effect of condition (glucose vs. water). Nevertheless, we found a main effect of caloric content (low caloric > high caloric food) in the bilateral middle temporal, right middle orbital frontal, right superior medial frontal, and right middle frontal gyrus and a main effect of time (30 min > 120 min post load) distributed from the prefrontal cortex to the cerebellum (Table 2). Interestingly, we found a significant main effect of body weight (overweight > lean) in the left putamen and right middle occipital gyrus (Table 2, Fig. 2); significant interactions were found between body weight (lean vs. overweight) and condition (glucose vs. water) in the lingual gyrus, (Table 2) and between body weight, condition, and caloric content of food cues in the fusiform gyrus and precuneus (Table 2, Fig. 3).
Table 2.
Regions of significant change in response to food cues 30 and 120 min after glucose versus water administration in lean and overweight subject
| MNI‐coordinatesa | |||||||
|---|---|---|---|---|---|---|---|
| Region | Hemisphere | Brodmann's area | X | Y | Z | z‐value | k |
| Increase in activity to food cues after glucose administration | |||||||
| Fusiform gyrus | R | 37 | 42 | −45 | −12 | 4.37 | 34 |
| Hippocampus | R | 24 | −27 | −9 | 3.94 | 17 | |
| Precuneus | L | 7 | −21 | −63 | 30 | 3.88 | 40 |
| Increase in activity to food cues after water administration | |||||||
| Superior occipital gyurs | L | −21 | −87 | 42 | 4.33 | 50 | |
| Main effect of body weight: overweight > lean | |||||||
| Middle occipital gyurs | R | 19 | 36 | −87 | 24 | 3.63 | 31 |
| Putamen | L | −18 | 12 | 0 | 3.51 | 11 | |
| Main effect of caloric content: low > high caloric food cues | |||||||
| Middle temporal gyrus | L | 21 | −63 | −51 | 0 | 4.32 | 54 |
| Middle orbital frontal gyrus | R | 47 | 39 | 54 | −6 | 4.17 | 93 |
| Middle temporal gyrus | R | 21 | 54 | −42 | −3 | 4.15 | 125 |
| Superior medial frontal gyrus | R | 8 | 12 | 45 | 45 | 3.94 | 126 |
| Middle frontal gyrus | R | 8 | 39 | 12 | 48 | 3.71 | 21 |
| Main effect of time: 30 > 120 min after glucose and water administration | |||||||
| Middle temporal gyrus | R | 37 | 42 | −63 | −3 | 4.54 | 85 |
| Fusiform gyrus | L | 19 | −30 | −66 | −12 | 4.27 | 209 |
| Inferior frontal gyrus | R | 47 | 39 | 18 | −18 | 3.92 | 32 |
| Middle temporal gyrus | L | 37 | −48 | −66 | 0 | 3.90 | 31 |
| Cerebellum | R | 21 | −63 | −18 | 3.86 | 18 | |
| Postcentral gyrus | L | 4 | −48 | −15 | 24 | 3.78 | 36 |
| Inferior parietal gyrus | L | 2 | −36 | −33 | 39 | 3.68 | 18 |
| Superior temporal gyrus | L | 42 | −66 | −24 | 9 | 3.59 | 18 |
| Amygdala | R | 24 | 0 | −18 | 3.55 | 16 | |
| Superior occipital gyrus | R | 19 | 33 | −90 | 21 | 3.54 | 13 |
| Superior temporal gyrus | R | 22 | 54 | −24 | 3 | 3.51 | 14 |
| Interaction body weight * condition | |||||||
| Lingual gyrus | L | 30 | −18 | −54 | −3 | 3.74 | 21 |
| Interaction body weight * condition * caloric content of food cues | |||||||
| Precuneus | R | 7 | 21 | −57 | 27 | 4.71 | 36 |
| Fusiform gyrus | R | 30 | 24 | −66 | −9 | 3.82 | 32 |
Results of four‐way repeated measurement ANOVA with one between‐subject factor body weight (lean vs. overweight) and three within‐subject factors (condition: glucose vs. water administration, caloric content of food cues: high vs. low caloric and time: 30 vs. 120 min post load) including three covariates (change in hunger, plasma glucose, and insulin). No main effect of condition was observed. P 0.001, uncorrected, k > 10.
Montreal Neurological Institute.
Figure 2.
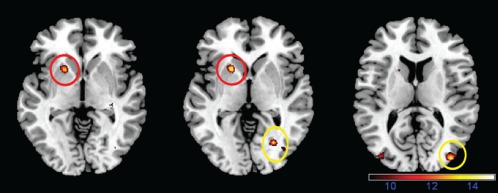
Main effect of body weight on the post load brain response to food pictures. Overweight subjects showed an increased response in the putamen (red) and occipital cortex (yellow) 30 and 120 min after water and glucose ingestion (color‐coded F‐value map; P < 0.001, uncorrected).
Figure 3.
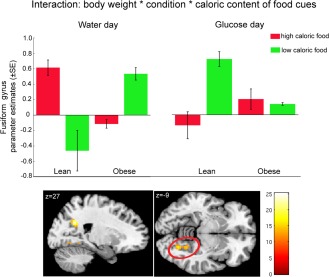
Interaction between body weight (lean vs. overweight), condition (glucose vs. water administration) and caloric content was revealed in the right precuneus and fusiform gyrus (color‐coded F‐value map; P < 0.001, uncorrected). Plots show post load response to high and low caloric food cues in the fusiform gyrus (red circle) after glucose and water ingestion in lean and overweight subjects expressed as parameter estimates (±SE). The response 30 and 120 min after ingestion were combined.
The multiple regression analysis revealed a significant negative association between the increase in insulin and the brain's post load response to high caloric food in the prefrontal and parietal cortex 120 min after glucose ingestion (for details, see Table 3). We also found a significant negative association between the increase in blood glucose and the hypothalamic response to high caloric food cues 30 min post load. All these associations were limited to high caloric pictures, and no significant correlation was found for the brain's post load response to low caloric food cues (for details, see Supporting Information Table 3). Also, no association was found between the change in subjective feeling of hunger after glucose and water ingestion and the brain's post load response to food cues. As insulin sensitivity and BMI are highly correlated, further statistical analyses were applied to adjust the correlation between insulin/glucose with the post load brain response to food cues for BMI. For this purpose, we extracted the mean signal from the activated cluster with the highest t max (see peak coordinates in Table 3). The post load response of the hypothalamus to high caloric food cues 30 min after glucose ingestion correlated negatively with the respective increase in plasma glucose adjusted for BMI (r adj = −0.613, P = 0.002). The post load response of the brain to high caloric food cues 120 min after glucose ingestion correlated negatively with the respective increase in plasma insulin adjusted for BMI in the inferior frontal gyrus/orbitofrontal cortex (OFC) (BA47) (r adj = −0.727, P < 0.001), middle frontal gyrus/dorsolateral prefrontal cortex (DLPFC) (BA8) (r adj = −0.595, P = 0.001), inferior parietal gyrus (BA40) (r adj = −0.623, P = 0.001), and the cingulate gyrus (BA32) (r adj = −0.633, P = 0.001; Fig. 4).
Table 3.
Regions showing a significant negative correlation with the increase in plasma glucose and insulin 30 and 120 min after glucose ingestion
| MNI‐coordinatesb | |||||||
|---|---|---|---|---|---|---|---|
| Region | Hemisphere | Brodmann's area | X | Y | Z | z‐value | k |
| Plasma glucose a brain response to high caloric food cues 30 min post load | |||||||
| Hypothalamus | R/L | ±9 | 0 | −6 | 2.96a | 7 | |
| Plasma insulin a brain response to high caloric food cues 120 min post load | |||||||
| Inferior frontal gyrus | R | 47 | 48 | 42 | −12 | 4.66 | 144 |
| Middle frontal gyrus | R | 8 | 33 | 15 | 42 | 4.44 | 183 |
| Cingulate gyrus | R | 32 | 12 | 24 | 42 | 4.38 | 128 |
| Inferior parietal gyrus | L | 40 | 48 | −60 | 42 | 4.10 | 164 |
Results of multiple regression analyses. The brain's post load response (glucose day) (fMRI 30 – fMRI basal) (fMRI 120 – fMRI basal) to food cues was correlated with the change in plasma glucose and insulin and subjective feeling of hunger (P 0.05, corrected for multiple comparisons)
(small volume corrected).
No significant correlation was observed for subjective feeling of hunger.
Montreal Neurological Institute.
Figure 4.
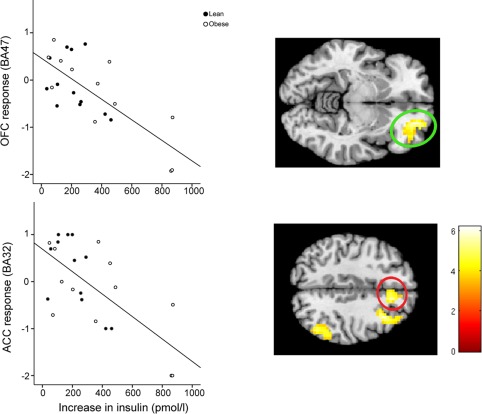
Negative relationship between increase in plasma insulin 120 min after glucose ingestion and the post load response to high caloric food in the prefrontal and parietal cortex (color‐coded T‐value map; P < 0.001, uncorrected for display). Plot shows significant correlation between the increase in insulin and the response of the OFC (r adj = −0.727, P < 0.001) (green circle, BA47) and ACC (r adj = −0.633, P = 0.001) (red circle, BA32) adjusted for BMI.
BEHAVIORAL DATA
Questionnaires
There was no statistical significant difference in mood states (i.e., depression, fatigue, anger, and vigor) assessed by POMS between lean and overweight subjects on the day of the measurement (P > 0.05). The TFEQ revealed a significant difference between lean and overweight subjects for the restraint eating scale (P = 0.024) and a trend for disinhibition (P = 0.06; Table 1).
Hunger Rating
The ratings for the subjective feeling of hunger showed significant within‐subject and between‐subject differences. The ANOVA revealed a significant main effect of time (F(2,33) = 33.861, P < 0.001), a significant interaction condition*time (F(2,32) = 3.641, P = 0.045) and a significant main effect for body weight (F(1,19) = 5.760, P = 0.027). The post hoc analysis (paired t‐tests) showed in lean subjects a significant increase in hunger 30 min (t(11) = 2.954, P = 0.013) and 120 minutes (t(11) = 6.671, P < 0.001) after water intake but no increase in hunger after glucose intake. In overweight subjects, a significant increase in subjective hunger was observed 30 and 120 min after drinking water (t(10) = 3.767), P = 0.004, (t(10) = 6.876, P < 0.001) and 120 min after glucose intake (t(10) = 3.333, P = 0.008) (Supporting Information Table 4).
Metabolic Parameters
Fasting plasma levels of glucose (Table 1) and glucose area under the curve (Supporting Information Table 1) did not differ between lean and overweight subjects (P > 0.05). As expected, fasting plasma insulin levels (P = 0.001; Table 1), OGTT‐derived insulin sensitivity index (P = 0.006; Table 1) and insulin area under the curve (P = 0.03) significantly differed between lean and overweight subjects with higher insulin levels and relative insulin resistance in overweight (for detail, see Supporting Information Table 1 and Supporting Information text).
Accuracy
For the one‐back visual recognition task, there was no significant difference in accuracy between lean and obese subjects and conditions. The average accuracy was 88% (SD = 0.08).
Reaction time
The repeated measurement ANOVA of the reaction time showed a main effect of condition (F(3,66) = 164.653, P < 0.001). During the control task, both lean and obese subjects were significantly faster (mean = 0.54 ± 0.13 s) than during the one‐back visual recognition task (mean = 0.79 ± 0.14 s). No main effect of body weight (F(1,22) = 3.362, P = 0.08) or interaction (F(3,66) = 1.464, P = 0.232) was observed.
DISCUSSION
With this fMRI study, we investigated the complex postprandial neural processing of food cues in lean versus overweight adults. For this purpose, we used a standard OGTT with water intake as a control condition. Therefore, we were also able to simultaneously evaluate changes in blood glucose and insulin in response to the OGTT on the neural processing of food cues. To our knowledge, this is the first study assessing food cue related brain activity at multiple time points after glucose and water ingestion (i.e., before, 30 and 120 min post load) in lean as well as overweight subjects. We were able to show that body weight has a substantial impact on the brain's response to visual food cues after glucose versus water ingestion. Specifically, we found that overweight subject showed increased activity to food cues in response to glucose and water ingestion in the putamen and occipital cortex. Furthermore, our design revealed a significant interaction between body weight, condition (glucose versus water), and caloric content of visual food cues. Hereby, overweight subjects showed a generalized reduced post load response to food objects, while the lean group showed a differential response to high vs. low caloric foods depending on the condition (glucose vs. water), reacting more strongly to low caloric foods after glucose ingestion in the fusiform gyrus and precuneus. Interestingly, we found no correlation with subjective feeling of hunger. However, we did observe an insulin and glucose associated effect. In response to high caloric food cues, the hypothalamus activity significantly correlated with changes in blood glucose 30 min post load; while especially brain regions in the prefrontal cortex showed a significant relationship with increases in plasma insulin 120 min post load.
These results coincide with previous studies showing that a complex network including the hypothalamus, paralimbic regions involved in reward processing and the prefrontal cortex are modulated following a meal [Del Parigi et al., 2002; Tataranni et al., 1999] or after glucose ingestion [Kroemer et al., 2012; Liu et al., 2000; Page et al., 2009; Smeets et al., 2005b], with a attenuated response in obese individuals in the hypothalamus [Matsuda et al., 1999] and prefrontal cortex [Del Parigi et al., 2002; Le et al., 2006; Page et al., 2011].
Interestingly, this study suggests that insulin mediates the response of the prefrontal cortex to food cues after glucose ingestion, specifically to high caloric food pictures. Concomitantly, a recent study proposed [Page et al., 2011] that circulating glucose levels are associated with prefrontal activity for food cues, and this response was absent in obese subjects. This study used a stepped hyperinsulinemic hypoglycemic clamp that lowered glucose to subphysiological levels in the presence of high insulin. By contrast, we used a standardized oral glucose tolerance increasing both glucose and insulin as it usually occurs after eating a meal to evaluate the brain response to glucose. In our study, subjects with a stronger increase in insulin showed reduced activity in the cingulate cortex, OFC, DLPFC, and parietal cortex 120 min after glucose ingestion independent of BMI. As insulin sensitivity inversely correlated with the increase in insulin after glucose ingestion, our results further substantiate the possible presence of cerebral insulin resistance [Guthoff et al., 2011; Heni et al., 2012; Kullmann et al., 2012b; Tschritter et al., 2006]. As the prefrontal cortex plays a central role in executive function including inhibitory control of feeding [Appelhans, 2009], cerebral insulin resistance may promote reduced inhibitory control toward food cues after food intake and could thereby contribute to overeating behavior.
Besides the prefrontal cortex, we also found a significant increase in occipital and parietal cortex activity after glucose administration, which is consistent with a recent report on neural response to food cues after glucose ingestion [Kroemer et al., 2012]. However, this study analyzed the post load response immediately after ingestion of 75 g of glucose in lean subjects only. In this study, we measured the brain response 30 and 120 min after ingestion in lean and overweight subjects. At these time points, glucose is absorbed into the blood and distinct endocrine responses are present. Nonetheless, there seems to be a strong modulation of the visual cortex by food cues even immediately after glucose ingestion [Kroemer et al., 2012] and up to 120 min after ingestion as shown in our study. Interestingly, we also found the visual cortex to be modulated by food cues after water ingestion. This could be due to gastric extension caused by the water bolus or by anticipation of food intake.
Recent research has shown the importance of the visual system in discriminating high from low caloric foods. Especially fMRI studies have identified the fusiform gyrus to elicit an enhanced response to high caloric foods [Frank et al., 2010; Killgore and Yurgelun‐Todd, 2007; Siep et al., 2009]. On a behavioral level, an attentional bias toward food pictures was observed to be enhanced by body weight and hunger [Nijs et al., 2010]. In response to insulin, we found in a recent magnetoencephalography study that intranasal insulin modulates food‐related occipital brain activity in lean but not obese adults [Guthoff et al., 2011]. Concurrently, we found a significant interaction between body weight, condition, and caloric content of food cues in the fusiform gyrus and precuneus. After water ingestion, lean subjects responded with increased activity to high caloric foods, while glucose ingestion resulted in an enhanced activity to low caloric stimuli in lean subjects. Overweight subjects, on the other hand, showed no such differential pattern. As lean subjects experienced a reduction of subjective feeling of hunger after glucose ingestion, low caloric foods probably had a higher incentive value leading to heightened visual processing. Alternatively, changes in eating behavior could also potentially explain this interaction between body weight and caloric content of food cues. Overweight subjects in our study revealed higher scores for restraint eating, which is frequently initiated as a reaction to weight gain as a cognitive mediated effort to eat less than desired [Johnson et al., 2011]. Therefore, it can be speculated that overweight and obese subjects might reduce their visual attention to food cues to restrain from eating.
Independent of the condition, overweight and obese subjects showed enhanced activity in the putamen after water and glucose ingestion. The putamen is part of the dorsal striatum, which is highly involved in reward processes, potentially mediating the motivational effects of food cues. Reduced dopamine D2 receptor availability in the dorsal striatum has been observed in overweight subjects [Stice et al., 2008]. Thus, functional differences between lean and obese are plausible, which is also consistent with previous neuroimaging studies showing that obese and overweight individuals exemplify increased activity to food cues in the dorsal striatum [e.g., Rothemund et al., 2007; Stoeckel et al., 2008] and with psychological literature indicating exaggerated reactivity to food cues in overweight persons [Braet and Crombez, 2003; Halford et al., 2004]. Our study adds to the current knowledge showing a hyperreactive reward system to food in obese participants.
Additionally, we observed a significant relationship between the hypothalamic response to high caloric food cues and the increase in blood glucose. This is in agreement with previous studies demonstrating an inhibition of the fMRI signal in the hypothalamus after glucose ingestion, which is attenuated in obese adults [Matsuda et al., 1999; Smeets et al., 2005b]. The observed negative correlation between blood glucose and the hypothalamus is probably related to decreases in neuronal activity in the lateral hypothalamic area, which is known to contain glucose‐sensitive neurons [Oomura et al., 1974]. Our results add to the current notion that the hypothalamic response is trigged by glucose, which is in accordance with Smeets et al. [2009a] reporting that only glucose can trigger a decrease in fMRI signal in the upper hypothalamus but not plasma insulin. The reduction of hypothalamic activity leads to an increase in satiety and to an attenuated response to food, giving the hypothalamus a central role in the regulation of appetite and energy homeostasis.
A limitation of this study is the rather small sample size. We analyzed the effect of post load ingestion on neural processing of high and low caloric food cues in lean and overweight participants. Due to the dichotomization of the sample and the multiple conditions, a larger sample would be beneficial to investigate these complex interactions.
CONCLUSION
In this study, we detected an altered response to glucose versus water intake in overweight subjects in regions important for visual attention, reward, and executive control. Furthermore, we identified a significant relationship between prefrontal activity and increase in insulin levels 120 min post glucose load, pointing toward a contribution of endocrine factors to the postprandial termination of food intake. The detected interactions between metabolic factors and processing of food cues can help to gain new insights in the regulation of eating behavior and in the pathogenesis of obesity.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors thank all study participants for their cooperation. They gratefully acknowledge the excellent technical assistance of Maike Borutta, Anna Bury, Alke Guirguis, and Roman Werner.
REFERENCES
- Appelhans BM (2009): Neurobehavioral inhibition of reward‐driven feeding: implications for dieting and obesity. Obesity 17:640–647. [DOI] [PubMed] [Google Scholar]
- Bagdade JD, Bierman EL, Porte D Jr (1967): The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest 46:1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet C, Crombez G (2003): Cognitive interference due to food cues in childhood obesity. J Clin Child Adolesc Psychol 32:32–39. [DOI] [PubMed] [Google Scholar]
- Camilleri M (2009): Peripheral mechanisms in the control of appetite and related experimental therapies in obesity. Regul Pept 156:24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf C, Blom WA, Smeets PA, Stafleu A, Hendriks HF (2004): Biomarkers of satiation and satiety. Am J Clin Nutr 79:946–961. [DOI] [PubMed] [Google Scholar]
- Del Parigi A, Gautier JF, Chen K, Salbe AD, Ravussin E, Reiman E, Tataranni PA (2002): Neuroimaging and obesity: Mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann NY Acad Sci 967:389–397. [PubMed] [Google Scholar]
- Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, Fritsche A, Preissl H (2010): Processing of food pictures: Influence of hunger, gender and calorie content. Brain Res 1350:159–166. [DOI] [PubMed] [Google Scholar]
- Fuhrer D, Zysset S, Stumvoll M (2008): Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity 16:945–950. [DOI] [PubMed] [Google Scholar]
- Fung TT, Schulze M, Manson JE, Willett WC, Hu FB (2004): Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 164:2235–2240. [DOI] [PubMed] [Google Scholar]
- Grichisch Y, Cavusoglu M, Preissl H, Uludag K, Hallschmid M, Birbaumer N, Haring HU, Fritsche A, Veit R (2011): Differential effects of intranasal insulin and caffeine on cerebral blood flow. Hum Brain Mapp 33:280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthoff M, Grichisch Y, Canova C, Tschritter O, Veit R, Hallschmid M, Haring HU, Preissl H, Hennige AM, Fritsche A (2010): Insulin modulates food‐related activity in the central nervous system. J Clin Endocrinol Metab 95:748–755. [DOI] [PubMed] [Google Scholar]
- Guthoff M, Stingl KT, Tschritter O, Rogic M, Heni M, Stingl K, Hallschmid M, Haring HU, Fritsche A, Preissl H, Hennige AM (2011): The insulin‐mediated modulation of visually evoked magnetic fields is reduced in obese subjects. PLoS One 6:e19482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Gillespie J, Brown V, Pontin EE, Dovey TM.2004Effect of television advertisements for foods on food consumption in children. Appetite 42:221–225. [DOI] [PubMed] [Google Scholar]
- Hallschmid M, Schultes B (2009): Central nervous insulin resistance:A promising target in the treatment of metabolic and cognitive disorders? Diabetologia 52:2264–2269. [DOI] [PubMed] [Google Scholar]
- Heni M, Kullmann S, Ketterer C, Guthoff M, Linder K, Wagner R, Stingl KT, Veit R, Staiger H, Haring HU, Preissl H, Fritsche A (2012): Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward‐related human brain regions. Diabetologia 55:1773–1782. [DOI] [PubMed] [Google Scholar]
- Johnson F, Pratt M, Wardle J (2011): Dietary restraint and self‐regulation in eating behavior. Int J Obes 36:665–674. [DOI] [PubMed] [Google Scholar]
- Ketterer C, Tschritter O, Preissl H, Heni M, Haring HU, Fritsche A (2011): Insulin sensitivity of the human brain. Diabetes Res Clin Pract 93( Suppl 1):S47–S51. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun‐Todd DA (2007): Positive affect modulates activity in the visual cortex to images of high calorie foods. Int J Neurosci 117:643–653. [DOI] [PubMed] [Google Scholar]
- Kroemer NB, Krebs L, Kobiella A, Grimm O, Vollstadt‐Klein S, Wolfensteller U, Kling R, Bidlingmaier M, Zimmermann US, Smolka MN (2012): (Still) longing for food: Insulin reactivity modulates response to food pictures. Hum Brain Mapp. DOI: 10.1002/hbm.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Frank S, Heni M, Ketterer C, Veit R, Haring HU, Fritsche A, Preissl H (2012. a): Intranasal insulin modulates intrinsic reward and prefrontal circuitry of the human brain in lean women. Neuroendocrinology. DOI: 10.1159/000341406. [DOI] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Haring HU, Fritsche A, Preissl H (2012. b): The obese brain: Association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 33:1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DS, Pannacciulli N, Chen K, Del Parigi A, Salbe AD, Reiman EM, Krakoff J (2006): Less activation of the left dorsolateral prefrontal cortex in response to a meal: A feature of obesity. Am J Clin Nutr 84:725–731. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gao JH, Liu HL, Fox PT (2000): The temporal response of the brain after eating revealed by functional MRI. Nature 405:1058–1062. [DOI] [PubMed] [Google Scholar]
- Löwe B, Spitzer RL, Zipfel S, Herzog W (2002): PHQ‐D. Gesundheitsfragebogen für Patienten. Karlsruhe,Germany:Pfitzer. [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, Butler MG, Savage CR (2009): Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. (Silver Spring) 18:254–260. [DOI] [PubMed] [Google Scholar]
- Matsuda M, DeFronzo RA (1999): Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH (1999): Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 48:1801–1806. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelmann LF (1981): Manual for the Profile of Mood States. San Diego:Educational and Industrial Testing Service. [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006): Central nervous system control of food intake and body weight. Nature 443:289–295. [DOI] [PubMed] [Google Scholar]
- Nijs IM, Muris P, Euser AS, Franken IH (2010): Differences in attention to food and food intake between overweight/obese and normal‐weight females under conditions of hunger and satiety. Appetite 54:243–254. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y (1974): Glucose inhibition of the glucose‐sensitive neurone in the rat lateral hypothalamus. Nature 247:284–286. [DOI] [PubMed] [Google Scholar]
- Page KA, Arora J, Qiu M, Relwani R, Constable RT, Sherwin RS (2009): Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counterregulatory hormones. Diabetes 58:448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KA, Seo D, Belfort‐DeAguiar R, Lacadie C, Dzuira J, Naik S, Amarnath S, Constable RT, Sherwin RS, Sinha R (2011): Circulating glucose levels modulate neural control of desire for high‐calorie foods in humans. J Clin Invest 121:4161–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N (2006): Subjective feeling of appetite modulates brain activity: An fMRI study. Neuroimage 32:1273–1280. [DOI] [PubMed] [Google Scholar]
- Pudel D, Westenhöfer J (1989): Fragebogen zum Eßverhalten (FEV). Handanweisung. Göttingen:Hogrefe. [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF (2007): Differential activation of the dorsal striatum by high‐calorie visual food stimuli in obese individuals. Neuroimage 37:410–421. [DOI] [PubMed] [Google Scholar]
- Rotte M, Baerecke C, Pottag G, Klose S, Kanneberg E, Heinze HJ, Lehnert H (2005): Insulin affects the neuronal response in the medial temporal lobe in humans. Neuroendocrinology 81:49–55. [DOI] [PubMed] [Google Scholar]
- Schloegl H, Percik R, Horstmann A, Villringer A, Stumvoll M (2011): Peptide hormones regulating appetite‐focus on neuroimaging studies in humans. Diabetes Metab Res Rev 27:104–112. [DOI] [PubMed] [Google Scholar]
- Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K (2009): Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes 33:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A (2009): Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res 198:149–158. [DOI] [PubMed] [Google Scholar]
- Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J (2005. a): Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr 82:1011–1016. [DOI] [PubMed] [Google Scholar]
- Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J (2005. b): Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage 24:363–368. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Small DM (2008): Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 322:449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl KT, Kullmann S, Guthoff M, Heni M, Fritsche A, Preissl H (2010): Insulin modulation of magnetoencephalographic resting state dynamics in lean and obese subjects. Front Syst Neurosci 4:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EWIII, Twieg DB, Knowlton RC, Cox JE (2008): Widespread reward‐system activation in obese women in response to pictures of high‐calorie foods. Neuroimage 41:636–647. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E (1999): Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96:4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschritter O, Preissl H, Hennige AM, Stumvoll M, Porubska K, Frost R, Marx H, Klosel B, Lutzenberger W, Birbaumer N, Haring HU, Fritsche A (2006): The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: A magnetoencephalographic study. Proc Natl Acad Sci USA 103:12103–12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS (2001): Brain dopamine and obesity. Lancet 357:354–357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


