Abstract
Behavioral studies indicate that older adults exhibit normal motor sequence learning (MSL), but paradoxically, show impaired consolidation of the new memory trace. However, the neural and physiological mechanisms underlying this impairment are entirely unknown. Here, we sought to identify, through functional magnetic resonance imaging during MSL and electroencephalographic (EEG) recordings during daytime sleep, the functional correlates and physiological characteristics of this age‐related motor memory deficit. As predicted, older subjects did not exhibit sleep‐dependent gains in performance (i.e., behavioral changes that reflect consolidation) and had reduced sleep spindles compared with young subjects. Brain imaging analyses also revealed that changes in activity across the retention interval in the putamen and related brain regions were associated with sleep spindles. This change in striatal activity was increased in young subjects, but reduced by comparison in older subjects. These findings suggest that the deficit in sleep‐dependent motor memory consolidation in elderly individuals is related to a reduction in sleep spindle oscillations and to an associated decrease of activity in the cortico‐striatal network. Hum Brain Mapp 35:3625–3645, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: aging, sleep, memory consolidation, spindles, fMRI, EEG, motor sequence learning, putamen, cerebellum, hippocampus
INTRODUCTION
The use of learned motor skills is ubiquitous in everyday activities: from seemingly simple tasks such as reaching and grasping an object, to highly coordinated and complex sequences of movements like dancing, or the mastery of a musical instrument. Motor skill learning requires repeated practice and occurs in distinct phases (e.g., fast and slow) during which automatization occurs [e.g., Doyon and Benali, 2005; Doyon et al., 2009a, 2009b]. During fast (early) learning, newly formed memories are in a fragile state, susceptible to interference and amnestic agents [Brashers‐Krug et al., 1996]. Yet transformation to an enduring, resilient long‐term memory trace is known to occur offline through a process called memory consolidation [Doyon et al., 2009a,b; Dudai, 2004; Karni and Sagi, 1993].
In young individuals, accumulating evidence indicates that sleep facilitates enhanced consolidation of a newly acquired sequence of movements, as reflected by sleep‐dependent spontaneous gains in performance that are normally observed after an equivalent period of wakefulness [see Diekelmann and Born, 2010; Maquet, 2001, for reviews]. There is also a growing consensus that non‐rapid eye movement (non‐REM) sleep, and sleep spindles in particular, are involved in the consolidation of acquired motor skills [Albouy et al., 2008; Barakat et al., 2012, 2011; Doyon et al., 2009a,b; Fischer and Born, 2009; Fogel and Smith, 2006; Fogel et al., 2007; Milner et al., 2006; Morin et al., 2008; Nishida and Walker, 2007; Peters et al., 2007; and for review: Fogel and Smith, 2011]. Indeed, procedural learning results in increased non‐REM sleep duration and as a result, in a greater number of sleep spindles [Fogel and Smith, 2006; Fogel et al., 2007]. Over‐and‐above this increase, the density (number per minute), duration and amplitude of spindles are also increased [Barakat et al., 2012; Fogel and Smith, 2006; Fogel et al., 2007; Morin et al., 2008; Nishida and Walker, 2007], resulting in a substantial intensification of spindle‐related activity over the course of a night's sleep. Sleep spindles are relatively brief (<3 s), fusiform, phasic events that are cortically generated and thalamically regulated [Bonjean et al., 2011]. They recur in a spaced and repeated pattern, as well as in the same frequency range (e.g., ∼12–16 Hz in humans, and ∼7–14 Hz oscillations in rats) necessary to induce cortical long‐term potentiation [Bergmann et al., 2008; Chapman et al., 1998; Ivanco and Racine, 2000; Rosanova and Ulrich, 2005; Trepel and Racine, 1998; Werk et al., 2005]. Furthermore, sleep spindles are time‐locked to hippocampal ripples, suggesting that spindles are also involved in the hippocampal‐neocortical dialogue [Clemens et al., 2007, 2011; Girardeau and Zugaro, 2011; Mölle et al., 2009; Siapas and Wilson, 1998; Sirota and Buzsáki, 2005; Sirota et al., 2003], a process also believed to be necessary for memory consolidation.
Importantly, recent functional neuroimaging studies combining electroencephalography (EEG) recordings and functional magnetic resonance imaging (fMRI) have shown that sleep spindles activate the putamen [Caporro et al., 2011; Tyvaert et al., 2008]. These findings taken together with the fact that: (1) Motor sequence learning (MSL) is mediated by the striatum, cerebellum, and motor cortical regions [Doyon and Benali, 2005; Doyon et al., 2003, 2009a,b], (2) sleep‐dependent changes of activity in the putamen [Barakat et al., 2011; Debas et al., 2010] and the hippocampus [Albouy et al., 2008; Walker et al., 2005] are associated with sleep‐dependent consolidation of a MSL task, (3) learning‐dependent increases in sleep spindles are observed following procedural and MSL [Barakat et al., 2012; Fogel and Smith, 2006; Fogel et al., 2007; Morin et al., 2008; Nishida and Walker, 2007], (4) spindle density is correlated with offline gains in MSL performance [Barakat et al., 2011], and (5) functional activity in the putamen is increased following MSL and is correlated with sleep spindles [Barakat et al., 2012], strongly suggest that sleep spindles are one of the possible mechanisms that support motor sequence memory consolidation in young subjects.
Yet less is known with regards to the effects of aging on motor memory consolidation and the physiological substrate that mediates this memory process in elderly populations. Recently, investigators have reported that older subjects do not exhibit normal gains in motor skill performance following a retention interval that includes sleep [Nemeth and Janacsek, 2011; Nemeth et al., 2010; Shea et al., 2006; Spencer et al., 2007; Wilson et al., 2012], despite demonstrating a normal rate of learning for new motor skills during initial practice. Furthermore, a meta‐analysis has identified that older subjects exhibit an age‐related increased proportion of light sleep, decreased deep sleep, and decreased REM sleep [Ohayon et al., 2004]. Other studies have also revealed an age‐related decrease in spindle activity [Carrier et al., 2001; Landolt et al., 1996; Nicolas et al., 2001], marked by shorter spindle duration and reduced spindle amplitude [Crowley et al., 2002; Principe and Smith, 1982; Rauchs et al., 2008; Wei et al., 1999] for both slow and fast spindles [Rauchs et al., 2008]. These age‐related differences are greatest at anterior regions, particularly toward the end of the night [Martin et al., 2012]. Lastly, in relation to procedural memory consolidation, one study [Peters et al., 2008] found that the density of Stage 2 sleep spindles increased following task acquisition in young, but not in older adults. The authors also reported that the increase in spindle density in young subjects was correlated with the level of performance during acquisition. By contrast, sleep spindle density was not related to performance during acquisition in older adults, suggesting that the relationship between procedural skills and sleep spindles may deteriorate with age. Taken together, these results raise an intriguing possibility that the breakdown in sleep‐dependent motor memory consolidation seen in older individuals may be associated with an age‐related reduction in sleep spindles [Pace‐Schott and Spencer, 2011; Peters et al., 2008; for review see Fogel et al., 2012], and perhaps to a change of activity in brain regions underlying sleep‐dependent memory consolidation of newly acquired motor skills.
Therefore, this study sought to test this hypothesis and to identify the functional and physiological changes associated with the age‐related motor memory consolidation deficit. Groups of young and older subjects were scanned using fMRI while practicing a finger MSL task adapted from Karni et al. [1995]. MSL training and re‐testing took place on two separate sessions, before and after a retention interval filled with either sleep or wake. We hypothesized that: (1) older participants would show a deficit in sleep‐dependent memory consolidation of the sequence task as compared with young subjects, (2) sleep spindles would be correlated with gains in performance in young, but not older subjects, (3) older participants would have reduced non‐REM sleep and sleep spindles, (4) a sleep‐dependent increase in cerebral activation would be observed for young participants in structures thought to be involved in the consolidation process of MSL (e.g., striatum and hippocampus), whereas older participants would show no change, or perhaps reduced activity in those regions as compared to young subjects, and (5) sleep spindles would be correlated in young (but not older subjects) with task‐related cerebral activation in the striatum, and possibly the hippocampus.
MATERIALS AND METHODS
Participants
Ethical and scientific approval was obtained from the Ethics Committee at the “Institut de Gériatrie de Montréal”, Montreal, Quebec, Canada, and informed written consent was obtained prior to entering the study. Groups of young healthy volunteers (n = 37, 23 females) between the age of 20 and 35 years (M = 24.0, SD = 3.8) and older healthy individuals (n = 49, 32 females) between the age of 55 and 75 years (M = 62.6, SD = 5.0) were recruited for the study. All subjects met the following inclusion and exclusion criteria. Information was obtained through a telephone pre‐screening interview and battery of online questionnaires. From these questionnaires, subjects were included if they were right handed (scored > 40 on the Edinburgh Handedness Inventory; [Oldfield, 1971]), reported they were non‐smokers, reported they were not taking any prescription medications, reportedly had a normal body mass index (≤25) and reported that they had never been diagnosed with any neurological, psychological, psychiatric, or sleep disorders. They were also included if they had no previous formal training as a typist or musician, nor considered themselves to be expert video game players. Young and older subjects had to score ≤ 8 on the short version of the Beck Depression Scale [Beck et al., 1974] and ≤ 8 on the Beck Anxiety Scale [Beck et al., 1988] to be included. In addition, older subjects were screened for signs of cognitive impairment using the Mini Mental State Examination, and were only included if they scored ≥ 24, Mean score = 27, ± 1.7; [Cockrell and Folstein, 1988; Tombaugh and McIntyre, 1992]. By contrast, subjects who worked at night or had taken a transmeridian trip ≤ 3 months prior to the study were not allowed to participate. Subjects that were categorized as either “extreme morning” or “extreme evening” types were also excluded (i.e., subjects had to score between 30 “moderate evening” and 70 “moderate morning” on the Horne Ostberg Morningness‐Eveningness Scale; [Horne and Ostberg, 1976] to be included).
In addition to the prescreening criteria, all participants were required to abstain from alcohol, nicotine, and caffeine consumption during the experiment to be included. They were also required to keep a regular sleep‐wake cycle (bed‐time: 10:00 PM – 1:00 AM, wake‐time: 07:00 AM – 10:00 AM) and to abstain from daytime naps to be included. Participants' sleep‐wake cycles were monitored throughout the experiment with an actigraph worn on the nondominant wrist, and a sleep journal (using a modified version of the National Sleep Foundation “Sleep Diary”: http://sleepfoundation.org) was used to ensure compliance with sleep‐wake instructions. Subjects who did not comply with these instructions were excluded from further participation in the study.
Each participant underwent a 90‐min acclimatization nap beginning at 1:00 PM, which was scheduled less than three days prior to the beginning of the study (see Fig. 1, “Day 7”). At least 5 min of consolidated stage 2 sleep during the acclimatization nap was required to be included in the study. This was to ensure that all subjects were able to sleep in a laboratory setting during the day, and to increase the probability that subjects would sleep during the experimental daytime nap opportunity. In addition, older subjects underwent an overnight polysomnographic (PSG) screening night (see Fig. 1, “Day 1”) to exclude subjects with an apnea‐hypoapnea index (AHI) > 5, and a periodic limb movement (PLM) index > 10. In total, 19 older subjects were excluded before completing the protocol; 10 were excluded for an elevated AHI or PLM index, 2 for sleeping <5 min during the acclimatization nap, 2 for MRI safety concerns and 5 voluntarily withdrew from the study. A total of 7 young subjects were also excluded before completing the protocol; 6 voluntarily withdrew from the study and one unexpectedly had a vascular abnormality revealed upon visual inspection of the T1‐weighted MRI scan.
Figure 1.

Overall experimental design. Older subjects underwent an overnight screening night (Day 1), one week prior to the screening nap. Then all subjects underwent a screening nap (Day 7) from 13:00 to 14:30 and returned on the following day for the remainder of the experimental protocol (Day 8). The SSS and Psychomotor Vigilance Test (PVT) were administered at 10:30 and 15:30, preceding both MRI scanning sessions. The morning MRI scanning session began at 11:00 and lasted ∼45 min. It consisted of a training session on the MSL task. After a light lunch, subjects were assigned to either the Nap or No‐Nap condition. The retention interval began at 13:00 and lasted 90 min, with an additional 1+ h for recovery from any sleep inertia effects. The afternoon MRI scanning session began at 16:00 and lasted ∼45 min. The latter comprised a retest session on the MSL task and a T1‐weighted anatomical scan.
From those who met the inclusion/exclusion criteria and completed the protocol, groups of young (n = 30, 17 females; n = 15 Young Nap [YN] condition, n = 15 Young No‐Nap [YNN] condition) and older adults (n = 30, 20 females; n = 15 Older Nap [ON] condition, n = 15 Older No‐Nap [ONN] condition) were included in the study. Of these 60 subjects, three subjects were excluded from the data analyses (two from the YN condition and one from the ON condition). One participant reportedly suffered from severe sleepiness in the last five blocks of training and succumbed to extreme tiredness during practice, thus producing lapses in performance during the training session. A second subject performed poorly on the MSL task (as this participant was outside the 99% confidence interval for all 14 blocks) and did not comply well with the instructions given throughout the experiment. Finally, a third subject failed to correctly memorize the sequence, hence showing poor performance in terms of overall accuracy on the task (<75% correct sequences).
Overall Experimental Design
Following the screening process (see Fig. 1, “Day 8”), all participants were asked to arrive in the laboratory around 10:30 AM. Upon arrival, they were administered the stanford sleepiness scale (SSS) [MacLean et al., 1992] and the psychomotor vigilance task (PVT) [Dinges and Powell, 1985] to collect both subjective and objective measures of sleepiness immediately prior to the MRI scanning sessions (for more information, see Section “Subjective and Objective Sleepiness”). The MSL task (starting at 11:00 AM) was performed while obtaining functional blood oxygen level dependent (BOLD) images using a block design (see Section “Brain Imaging Data Acquisition and Analysis” for details). A light lunch was given following the first MRI scanning session. Participants were then randomly assigned to either the Nap or No‐Nap condition for the 90‐min retention interval that began at 1:00 PM. All subjects in the Nap condition had >5 min of uninterrupted stage 2 sleep (see Table 1 for details). By contrast, participants in the No‐Nap condition were required to remain awake during the same 90‐min interval, and were allowed to read quietly while supine in bed under dim ambient light. PSG measures were recorded in the Nap condition for further sleep stage analyses as well as in the No‐Nap condition to verify that all participants remained awake. If signs of drowsiness (e.g., elevated alpha activity, lower muscle tension, and slow rolling eye movements) were observed in the No‐Nap condition, the experimenter entered the room to alert the participant. The retest session took place at 4:00 PM, > 1.5 h after the end of the nap or wake period to avoid sleep inertia effects.
Table 1.
Mean (and standard error; SEM) sleep parameters recorded during the 90‐minute daytime nap retention interval in both young and older participants
| Young | Older | t | P | |||
|---|---|---|---|---|---|---|
| M | SEM | M | SEM | |||
| Wake (min) | 19.85 | 3.44 | 47.09 | 7.47 | −3.31 | 0.004a |
| Stage 1 (min) | 7.87 | 1.36 | 6.18 | 0.76 | 1.28 | 0.270 |
| Stage 2 (min) | 43.31 | 2.28 | 31.02 | 5.22 | 2.16 | 0.044a |
| SWS (min) | 11.45 | 2.41 | 4.60 | 1.75 | 2.35 | 0.027a |
| Non‐REM (min) | 54.77 | 3.13 | 35.62 | 6.20 | 2.76 | 0.012a |
| REM (min) | 9.44 | 2.10 | 4.20 | 2.18 | 1.72 | 0.098 |
| Total sleep time (min) | 72.08 | 3.56 | 46.00 | 7.26 | 3.23 | 0.004a |
| Stage 1% TST | 11.51 | 2.36 | 20.14 | 4.15 | −1.08 | 0.084 |
| Stage 2% TST | 60.98 | 2.86 | 67.40 | 3.33 | −1.44 | 0.162 |
| SWS% TST | 15.41 | 3.10 | 7.01 | 2.61 | 2.09 | 0.047a |
| Non‐REM% TST | 76.39 | 2.74 | 74.41 | 3.96 | 0.41 | 0.684 |
| REM% TST | 12.10 | 2.61 | 5.45 | 2.73 | 1.75 | 0.093 |
| Sleep onset latency (min) | 11.92 | 1.91 | 18.40 | 5.26 | −1.16 | 0.263 |
| Wake after sleep onset (min) | 10.87 | 2.69 | 31.13 | 6.79 | −2.77 | 0.012a |
| Total recording time (min) | 94.87 | 0.74 | 93.76 | 0.73 | 1.06 | 0.297 |
| Sleep efficiency (%) | 76.05 | 3.85 | 49.31 | 7.83 | 3.06 | 0.006a |
Indicates P < 0.05, df = 26, corrected for heterogeneity of variance where appropriate.
Abbreviations: min: Minutes; TST: total sleep time; REM: rapid eye movement sleep.
Finger Sequence Task
MSL was tested using an adapted version of the sequential finger‐tapping task [Karni et al., 1995]. An MR‐compatible response box (model HH‐1x4‐L; Current Designs, Inc., Philadelphia PA) comprising four push buttons located in a horizontal row at equal distance from each other was used. First, the experimenter demonstrated the on‐screen visual appearance of the task and the task instructions in order to familiarize subjects with the MSL task. The subjects were then positioned supine in the MR scanner. Prior to the training sessions, participants were instructed to perform the 5‐item sequence “4‐1‐3‐2‐4” (where 1 stands for the index finger and 4 for the little finger of the non‐dominant hand) slowly and accurately until they were able to reproduce it three times in a row without making an error. This procedure was intended to verify that subjects understood the instructions, had memorized the sequence of finger movements, and could perform the 5‐item sequence reliably prior to performing the task while being scanned during the subsequent training and retest sessions. During those sessions, participants were required to practice the same explicitly known sequence of finger movements (4‐1‐3‐2‐4) with their non‐dominant hand, but were instructed “to perform the sequence by tapping the fingers as quickly as possible, while making as few errors as possible”. Each session consisted of 14 blocks of practice (indicated by a green cross in the center of the screen, displayed via a projector). Each block terminated after subjects had produced 60 key presses (equivalent to 12 repetitions of the sequence). Each block was separated by 15 s periods of rest (indicated by a red cross displayed in the center of the screen). Participants were also instructed that in the event they were aware they had made an error, they were to continue practicing by starting at the beginning of the 5‐item sequence. This ensured that errors were isolated events in the train of 60 key presses within each block and helped subjects to continue practicing despite an error in performance. The task was un‐cued (i.e., self‐initiated) to avoid age‐related differences in reaction time, although it should be noted that this does not ensure that age‐related differences in execution time were controlled for. Finally, the timing of all key presses was recorded and speed was measured by calculating inter‐key‐press intervals for correct responses. All subjects in the analysis performed the sequence with an accuracy above 83.3% overall, corresponding to 10/12 correct sequences per block.
PSG Recording and Analysis
A Brain Products GmbH (Gilching, Germany) 16‐channel, V‐Amp16 system (high pass filter = 0.3 Hz, low pass filter = 70 Hz) was used to record PSG data including EEG from 10 scalp derivations (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4, and Oz). Signals were digitalized at 500 samples per second. Standard PSG measures included EEG, electrooculographic (EOG), and bipolar submental electromyogram (EMG). During the preliminary screening night, recordings from a nasal/oral thermistor and respiratory effort belts were used to screen for signs of sleep apnea. Recordings from EMG leg electrodes were also used to screen for signs of periodic leg movements. For all PSG recordings, sleep stages were visually scored in 20‐s epochs displaying EEG (high pass filter = 0.5 Hz, low pass filter = 35 Hz) from central and occipital derivations (C3, C4, and Oz) re‐referenced to average mastoids (A1 and A2). EOG (high pass filter = 0.5 Hz, low pass filter = 35 Hz) from the lateral outer canthus of each eye and bipolar submental EMG (high pass filter of 10 Hz) was also recorded. Sleep stage scoring was done by an expert PSG technologist according to standard criteria defined by the American Academy of Sleep Medicine [Iber, 2007] and using the “fMRI Artifact rejection and Sleep Scoring Toolbox (FASST)” (ver. 0.302; http://www.montefiore.ulg.ac.be/~phillips/FASST.html) for Matlab (Mathworks, Natick, MA; [Leclercq et al., 2011]). Periods of cortical arousal or movement during sleep were identified by an automated detector when movement continuously exceeded 100 μV for more than 100 ms. Sections automatically marked as bad data were subsequently visually verified and excluded from further quantitative analysis of the EEG data, including spindle detection.
Sleep spindles were automatically detected from Fz, Cz, and Pz in non‐REM sleep (Fig. 2) by Brain Products Analyzer software (Version 2.1) using a method inspired by an algorithm used in an analogue system developed by Campbell et al. [1980]. Similar to the root mean square (RMS) transformation commonly used to transform the raw EEG signal prior to spindle detection [Mölle et al., 2002], this method used a complex demodulation transformation [Walter, 1968] to extract the power for each data point between 11 and 17 Hz. This approach has an advantage over the RMS transform by extracting the signal of interest with a very good signal to noise ratio, and of yielding only positive data point values (Fig. 2B), hence making event detection straightforward. Also, similar to other methods that employ an individualized amplitude threshold [Barakat et al., 2011] calculated from a percentile score of the whole recording (e.g., 95%), the present data from each channel and for each participant were normalized using a Z‐score transformation (Fig. 2C) derived from a 60‐s sliding window. The use of a sliding window allows the amplitude threshold to adapt to the U‐shaped changes in sigma activity that occur within each NREM cycle [Aeschbach and Borbely, 1993; Himanen et al., 2002] as well as to the increased sigma that occurs over the entire sleep episode [Aeschbach and Borbely, 1993; Werth et al., 1997], hence taking into account the dynamic changes in the ratio of spindle‐related sigma activity to ongoing background sigma over the course of the sleep cycle throughout the whole recording. Events were detected on the transformed signal using a cut‐off z‐score = 3.10, equivalent to the 99.9th percentile. Importantly, the present method also has the advantage of normalizing the amplitude of each spindle across channels, and thus to account for the significant intraindividual differences in sleep spindles and to address the challenges inherent in reliable spindle detection across individuals [De Gennaro et al., 2005; Ray et al., 2010; Silverstein and Levy, 1976]. Also, this allows a single threshold to be used for all channels throughout the recording, across individuals, and importantly without bias across age groups. Contrary to other approaches, however, this method does not use any explicit minimum duration criteria for spindle detection, because many existing definitions are based on arbitrary minimum duration criteria derived from spindles large enough to be observed in the raw, mixed‐frequency (∼ 0.5–35 Hz) EEG by the naked eye alone (e.g., 0.5 s). In fact, excluding spindles below 0.5 s in duration can possibly exaggerate age differences, especially in a daytime nap, when spindles are thought to be shorter in duration than in the later part of overnight sleep, for example. Despite having no minimum duration criteria, the current method did not detect spindles <0.2 s. Using this detection method, the number of spindles in non‐REM sleep, amplitude (μV2), duration (s), and peak frequency (Hz) were derived along the midline recordings (Fz, Cz, and Pz), as age‐differences have been found to vary systematically along the anterior‐posterior axis [Martin et al., 2012]. Spindle events were then visually verified for correct identification, and were found to have a high inter‐rater reliability with an established method used in young [Barakat et al., 2012] and older subjects [Martin et al., 2012] for spindle number [all subjects: r(26) = 0.92, P < 0.001; YN: r(11) = 0.73, P = 0.004; ON: r(13) = 0.98, P < 0.001], amplitude [all subjects: r(26) = 0.95, P < 0.001; YN: r(11) = 0.94, P < 0.001; ON: r(13) = 0.95, P < 0.001], duration [all subjects: r(26) = 0.55, P = 0.002; YN: r(11) = 0.29, P = 0.331; ON: r(13) = 0.45, P = 0.090] and peak frequency [all subjects: r(26) = 0.83, P < 0.001; YN: r(11) = 0.82, P < 0.001; ON: r(13) = 0.70, P = 0.004]. Taken together, the findings presented above suggest that the detection method used here had a high agreement with other published automated detection methods. Consequently, the latter was chosen here because it appears to perform particularly well in a daytime nap, and because it is well suited for use in older subjects.
Figure 2.
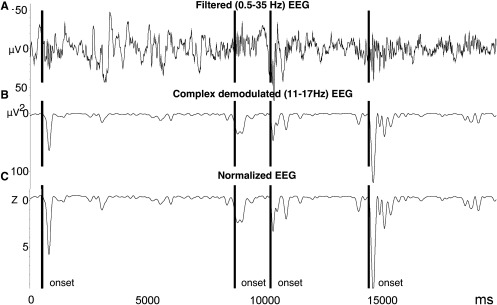
A: Spindle detection method. The raw EEG (0.5 − 35 Hz) was transformed, and power (μV2) was extracted for each data point using a complex demodulation algorithm in the range of 11–17 Hz. Spindle onsets were detected on the normalized, demodulated signal using a Z‐score transformation that employed a 60 s sliding window (used to calculate the mean and standard deviation about each data point). A threshold of z = 3.10 was used, equivalent to the 99.9th percentile (see text for more details).
Brain Imaging Data Acquisition and Analysis
MR sequence parameters
Brain images were acquired using a 3.0T TIM TRIO magnetic resonance imaging system (Siemens, Erlangen, Germany) and a 12‐channel head coil. For all subjects, a structural T1‐weighted MRI image was acquired using a 3D MPRAGE sequence (TR = 2300 ms, TE = 2.98 ms, TI = 900 ms, FA = 9°, 176 slices, FoV = 256 × 256 mm², matrix size = 256 × 256 × 176, voxel size = 1 × 1 × 1 mm³). Multislice T2*‐weighted fMRI images were acquired during the training and retest sessions of the MSL task with a gradient echo‐planar sequence using axial slice orientation (TR = 2650 ms, TE = 30 ms, FA = 90°, 43 transverse slices, 3 mm slice thickness, 10% inter‐slice gap, FoV = 220 × 220 mm², matrix size = 64 × 64 × 43, voxel size = 3.44 × 3.44 × 3 mm³).
Preprocessing
Functional volumes were preprocessed and analyzed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/; Welcome Department of Imaging Neuroscience, London, UK) implemented in Matlab (ver. 7.10 R2010a) for OS X (Apple, Cupertino, CA). First, each participant's functional volumes were realigned to the first functional volume of the session, and coregistered to the high‐resolution T1‐weighted anatomical image. The coregistered structural images were then segmented into grey matter, white matter and cerebrospinal fluid. An average subject‐based template was created using DARTEL in SPM8. All functional and anatomical images were normalized using the resulting template, which was generated from the structural scans. Finally, spatial smoothing was applied on all functional images [Gaussian kernel, 8 mm full‐width at half‐maximum (FWHM)].
Fixed‐effects analysis
Brain responses were estimated using the fixed‐effect (within subject level) general linear model (GLM) with a box‐car function for each block of practice convolved with the canonical hemodynamic response function. Movement parameters were not included in the model, as this is not recommended in a block design [Johnstone et al., 2006]. Slow drifts were removed from the time series using a high pass filter with a cut‐off period of 128 s. Serial correlations in the fMRI signal were estimated using an autoregressive (order 1) plus white noise model and a restricted maximum likelihood algorithm. Linear contrasts tested (1) the main effect of practice of the learned sequence during training, (2) the main effect of practice for the learned sequence during retest, and (3) the effect of session (retest – training) on practice of the learned sequence. These linear contrasts generated statistical parametric maps [SPM(T)]. The resulting contrast images were then further spatially smoothed (Gaussian kernel 6 mm FWHM) and entered in a second‐level analysis, corresponding to a random‐effect model, accounting for inter‐subject variance.
Random‐effects analyses
In the second level analysis of the training session, a one‐sample t‐test characterized the main effect of practice over all subjects (including the YN, YNN, ON, and ONN groups together). Two‐sample independent t‐tests were used to compare the main effect of practice during the training session between young and older subjects. All analyses carried out on the main effect of practice in the training session were set at a threshold of P < 0.05, controlling for multiple comparisons using Bonferroni family wise error correction.
To investigate the changes in cerebral activation due to the nap, another second level analysis identified brain activated areas for the interaction between practice sessions (Retest – Training), age (Young – Older), and sleep/wake condition (Nap – No‐Nap). To do so, an ANOVA compared the main effect of session (Retest − Training) between the four different groups (ON, ONN, YN, and YNN). This was followed up by an exploration of the simple effects, creating contrasts to specifically investigate the effect of age in those that slept (YN‐ON, ON‐YN) and the effect of sleep within each age group (YN‐YNN and ON‐ONN).
Furthermore, to assess the relationship between sleep spindles and changes in brain activation from the training to the retest session in those that napped (YN vs. ON), we regressed the individual within‐subjects contrast images (Retest − Training) with spindle density, duration, amplitude, and peak frequency. Spindle parameters from Fz were used as regressors of interest because, similar to previous reports [Martin et al., 2012], the differences between age groups were maximal at that location. Because the main goal of this study was to investigate the role of sleep spindles per se for memory consolidation as opposed to sleep duration, the number of minutes of non‐REM sleep was used as covariate of no‐interest. This contrast was used as a mask (P < 0.05) to exclude voxels whose activity was significantly correlated with non‐REM sleep duration within the two‐sample t‐test comparing YN and ON groups. The resulting set of voxel values for each group (YN‐ON and ON‐YN) and between session (Retest − Training) interaction contrast constituted a map of t statistic [SPM(T)], thresholded at P < 0.001 (uncorrected for multiple comparisons).
The average signal intensity (i.e., parameter estimates) for regions of interest (ROI) from statistically significant clusters were extracted using the MarsBaR ROI toolbox (http://marsbar.sourceforge.net/; ver. 0.42). The signal intensity was extracted individually for each subject and scaled to a mean of 100, so that the unit of measurement, while still arbitrary [a.u.], can be interpreted as reflecting a proportion of the whole brain signal.
RESULTS
MSL Task
Speed
A mixed design, block (blocks 1‐14) by age group (young vs. older) by sleep/wake condition (Nap vs. No Nap) ANOVA was used to verify that participants were able to improve on the MSL task as a function of practice during the training session. This analysis revealed a significant effect of block (F(13,689) = 32.13, P < 0.0001) and a significant age group by block interaction (F(13,689) = 4.08, P = 0.001). Separate repeated‐measures ANOVAs in young and older subjects were used to further decompose this interaction. The results confirmed that both young (F(13, 338) = 14.41, P < 0.0001) and older subjects (F(13, 351) = 19.93, P < 0.0001) significantly improved on the MSL task over the course of the training session. Indeed, performance during this session increased at a rate that followed an inverse function curve in both young (best‐fit: R 2 = 0.95, F(1,12) = 229.57, P < 0.001) and older subjects (best fit: R 2 = 0.96, F(1,12) = 259.76, P < 0.001), indicating that the rate of the improvement over time was similar in both age groups. Yet, as expected, older participants' performance was slower overall during the training session (F(1,53) = 14.69, P < 0.0001). Results from the overall mixed‐design ANOVA showed that there was no significant sleep/wake condition by block interaction during training, or any significant age group by sleep/wake condition interaction. Altogether, these results suggest that both Nap and No‐Nap groups had similar performance during the initial learning phase of the motor skill, and that while age did have an impact on performance in the training session, the older subjects exhibited similar learning as compared with the younger subjects.
We then used the percent change across the sleep/wake retention interval to assess sleep‐dependent consolidation changes in performance. However, to ensure that there were no differences at the end of training between groups that could have confounded the gains used in subsequent analyses, a between groups ANOVA was first carried out to test for the effect of age (Young vs. Older) and sleep/wake condition (Nap vs. No Nap) using mean interkey‐press intervals of the last four blocks of the training session. This analysis did not reveal any main effect of age (F(1,53) = 138.25, P = 0.054), or sleep/wake condition (F(1,53) = 3.28, P = 0.321). Importantly, there was no age by sleep/wake condition interaction (F(1,53) = 0.10, P = 0.749), nor any simple effect of sleep/wake condition in the older (ON vs. ONN) (t(27) = 0.49, P = 0.698), or young (YN vs. YNN) subjects (t(26) = 0.39, P = 0.630). Thus, to investigate the effects of sleep and age on offline gains in performance from training to retest (i.e., reflecting consolidation), gains scores were calculated by taking the percent change from the mean of the last four blocks of the training session compared to the mean of the first four blocks of the retest session. This measure was used to minimize individual differences in the level of performance achieved at the end of the training session and in the overall speed of performance. An ANOVA (sleep/wake condition [Nap, No‐Nap] × age [Young, Older]) was conducted using the gains scores and revealed a significant interaction (F(1,53) = 4.87, P = 0.032). Holm‐Bonferroni corrected independent t‐tests demonstrated that the YN group had greater performance gains than the ON group (Fig. 3; t(26) = 3.87, P = 0.003). Yet there was no significant difference between the YNN and ONN (t(27) = 0.19, P = 0.851), nor between the ON and ONN groups (t(27) = 2.11, P = 0.134). In addition, Holm‐Bonferroni corrected one‐sample t‐tests were used to identify groups that had a significant change from training to retest (i.e., gains significantly different from zero). We found that the YN group had significant gains in performance (Fig. 3; t(12) = 4.32, P = 0.004), but not the YNN (t(14) = 2.28, P = 0.116), ON (t(14) = ‐1.47, P = 0.163) or ONN (t(13) = 1.51, P = 0.310) groups.
Figure 3.
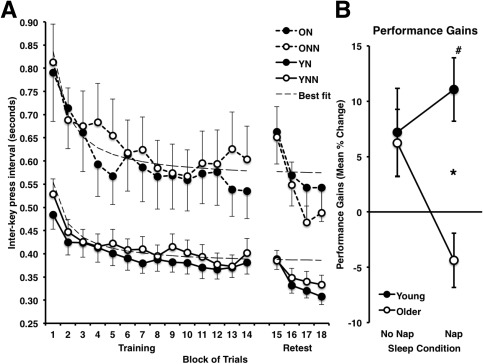
A: Performance speed [interkey press interval (s)] during training (14 blocks) for both young and older participants in the Nap and No‐Nap conditions. Dashed line indicates the mean fitted performance (from individually fitted inverse function: y = C + B/x) at training. B: Performance gains (percent change mean interkey‐press interval) from the end of training (mean last 4 blocks) to the beginning of retest (mean first 4 blocks) for both Young and Older participants in the Nap and No‐Nap conditions. * Indicates significantly greater mean gains in performance (P < 0.05, Holm‐Bonferroni corrected) at retest in Young participants as compared with older participants in the Nap condition. # Indicates significant gain in performance in the Young Nap condition. YN: Young Nap; YNN: Young No‐Nap; ON: Older Nap; ONN: Older No‐Nap.
Accuracy
The number of correctly executed sequences per block was analyzed using the same strategy described above. Briefly, accuracy improved with practice (main effect of block: F(13, 689) = 2.11, P = 0.012), but there was no effect of age group (F(1,53) = 0.03, P = 0.873) or sleep/wake condition (F(1,53) = 0.19, P = 0.662) on this performance measure. As subjects were pre‐trained to criterion and due to the simple (and explicit) nature of the sequence, as expected, there was no difference in accuracy between age groups, or the sleep/wake condition. Accuracy also improved from the training to the retest session (F(1,53) = 6.21, P = 0.016), irrespective of the age group or sleep/wake condition.
Gains scores were calculated for accuracy in the same way as for speed. There was no significant sleep/wake condition (Nap, No‐Nap) × age (Young, Older) interaction observed for accuracy gains scores (F(1,53) = 1.42, P = 0.238).
Sleep Data
Subjective and objective sleepiness
At retest, there was no main effect of age group (F(1,54) = 0.342, P = 0.561), sleep/wake condition (F(1,54) = 0.096, P = 0.758) or sleep/wake condition x age group interaction (F(1,54) = 0.342, P = 0.561) on the subjective sleepiness scores as assessed by the SSS. Not surprisingly, however, there was a significant main effect of age group for the objective sleepiness scores from the PVT (F(1,54) = 4.912, P = 0.031), likely due to well‐established age‐differences in reaction time. Importantly, however, there was no main effect of sleep/wake condition (F(1,54) = 0.710, P = 0.681) or sleep/wake condition × age group interaction (F(1,54) = 0.302, P = 0.585), suggesting that sleepiness was consistent across the sleep/wake condition, and thus that it could not have had a confounding effect on MSL performance.
Sleep architecture
As expected, young and older subjects differed with respect to the sleep characteristics measured during the 90‐min daytime nap (Table 1). ON subjects spent significantly more time awake (t(26) = 3.31, P = 0.004). This was reflected by an increased wake time after sleep onset (t(26) = 2.77, P = 0.012), decreased total sleep time (t(26) = 3.23, P = 0.004) and reduced sleep efficiency (t(26) = 3.06, P = 0.006). Due to the highly variable duration of stage 2 and slow wave sleep (SWS) across subjects in a daytime nap, stage 2 and SWS were collapsed into a total measure of non‐REM sleep. ON subjects had significantly less non‐REM sleep (t(26) = 2.76, P = 0.012) than YN subjects. By contrast, stage 1 and REM sleep did not significantly differ between the YN and ON subjects, hence indicating that sleep deficits in older individuals were predominantly a result of changes in non‐REM sleep. The difference between YN and ON subjects for REM sleep did approach statistical significance (P < 0.10). However, it should be noted that this was largely due to a floor effect, whereby 73% of ON subjects had no REM sleep at all, as compared to only 23% of YN subjects. Thus, this non‐significant age difference is somewhat difficult to interpret, but likely represents age‐related differences in the initiation of REM sleep in a daytime nap, and not necessarily REM sleep duration per se.
Sleep spindles
In addition to age‐related differences in sleep architecture, ON subjects had significantly shorter sleep spindles measured during non‐REM sleep at Fz (t(26) = 3.70, P = 0.003) and Cz (t(26) = 3.55, P = 0.003), and their spindles were significantly slower at Fz (t(26) = 3.33, P = 0.009; Table 2). Yet sleep spindle number, density and amplitude were not significantly different between YN and ON subjects. The equivalent spindle density between YN and ON subjects observed here is consistent with previous reports, which found that spindle density does not differ between these two age groups at the beginning of the night [Martin et al., 2012]. These results also support earlier studies [Carrier et al., 2001; Landolt et al., 1996; Martin et al., 2012; Ohayon et al., 2004] and confirm that over and above age‐related changes in sleep architecture, spindles were smaller and slower in terms of peak frequency in ON individuals, perhaps representing an age‐related reduction in fast spindles during a daytime nap.
Table 2.
Number, density (#/min NREM), duration (s), amplitude (uV2), and peak frequency (Hz) of non‐REM sleep spindles at Fz (midline frontal), Cz (midline central), and Pz (midline posterior) during the 90‐min daytime nap retention interval in young and older participants
| Young | Older | t | P | |||
|---|---|---|---|---|---|---|
| M | SE | M | SE | |||
| Number | ||||||
| Fz | 241.54 | 20.59 | 176.60 | 31.10 | 1.68 | 0.212 |
| Cz | 251.54 | 21.31 | 186.13 | 31.73 | 1.65 | 0.112 |
| Pz | 259.54 | 22.84 | 181.07 | 32.33 | 1.91 | 0.204 |
| Density | ||||||
| Fz | 4.33 | 0.25 | 4.82 | 0.11 | −1.83 | 0.780 |
| Cz | 4.51 | 0.25 | 5.21 | 0.15 | −2.39 | 0.072 |
| Pz | 4.64 | 0.26 | 4.88 | 0.17 | −0.76 | 0.908 |
| Duration | ||||||
| Fz | 0.47 | 0.02 | 0.36 | 0.02 | 3.70 | 0.003a |
| Cz | 0.48 | 0.03 | 0.34 | 0.02 | 3.55 | 0.002a |
| Pz | 0.49 | 0.04 | 0.38 | 0.03 | 2.29 | 0.030a |
| Amplitude | ||||||
| Fz | 59.45 | 7.68 | 40.00 | 4.61 | 2.16 | 0.123 |
| Cz | 90.18 | 12.32 | 67.24 | 8.12 | 1.50 | 0.298 |
| Pz | 73.28 | 10.99 | 71.80 | 11.55 | 0.09 | 0.930 |
| Frequency | ||||||
| Fz | 12.99 | 0.08 | 12.65 | 0.06 | 3.33 | 0.009a |
| Cz | 13.36 | 0.07 | 13.29 | 0.10 | 0.55 | 0.585 |
| Pz | 13.42 | 0.09 | 13.51 | 0.10 | −0.64 | 1.000 |
Indicates P < 0.05, two‐tailed.
df = 26, corrected for homogeneity of variance, where appropriate and Holm‐Bonferroni corrected for multiple comparisons for number of sites.
Based on previously reported physiological and functional differences between slow and fast spindles [Barakat et al., 2012; Schabus et al., 2007, 2008], sleep spindles were also categorized as either slow (11 – 14 Hz) or fast (14 – 17 Hz) based on the peak frequency for each spindle event. These analyses, however, revealed a similar pattern (e.g., both slow and fast spindles had similar age‐differences) as the results presented for spindles in the 11 – 17 Hz range, and were thus not considered separately in subsequent analyses.
Functional MRI Data
Training session
As previously observed [Doyon and Benali, 2005; Doyon et al., 2003, 2009a,b] and as expected, a large network of regions implicated in MSL and motor performance was activated across subjects, irrespective of age, during the training session. Activations were maximal at, but not limited to, the primary motor, sensorimotor, and supplementary motor areas, as well as the striatum, and cerebellum (Table 3.1). This finding is consistent with a number of studies that have established the crucial role of the cortico‐striatal and cortico‐cerebellar systems in MSL [e.g., Doyon and Benali, 2005; Doyon et al., 2003, 2009a,b]. Older subjects had greater activation compared to young subjects in the left frontal cortex and in the occipital cortex bilaterally (Table 3.3). Due to the possible confounding effects of the pre‐existing inter‐individual differences between task‐related brain activation in young and older subjects, and to investigate the changes associated with sleep per se across the retention interval, subsequent fMRI analyses described below were conducted on the difference between the training and retest sessions (Retest − Training) instead of at retest only.
Table 3.
Functional imaging results from a one‐sample t‐test (1) and independent two sample t‐tests (2‐3) for the main effect of training between young (Y) and older (O) subjects
| Hemisphere | Region | Subregion | cluster | X | Y | Z | z | P |
|---|---|---|---|---|---|---|---|---|
| Training Session: Effect of Training | ||||||||
| 1. Main effect training (all participants) | ||||||||
| Right | Parietal | Somatosensory | 31399 | 40 | −30 | 60 | ∞ | 0.000 |
| Left | Cerebellum | V | 9583 | −14 | −52 | −18 | ∞ | 0.000 |
| Left | Frontal | Middle | 203 | −36 | 42 | 26 | 5.59 | 0.000 |
| Left | Hippocampus | 14 | −24 | −36 | 8 | 5.55 | 0.000 | |
| Right | Temporal | Inferior | 81 | 56 | −38 | −18 | 5.28 | 0.002 |
| Right | Temporal | Middle | 36 | 50 | −50 | 4 | 5.26 | 0.002 |
| Right | Occipital | Calcarine | 37 | 28 | −68 | 6 | 5.08 | 0.005 |
| Right | Hippocampus | 3 | 22 | −34 | 10 | 4.80 | 0.016 | |
| Left | Caudate | 9 | −10 | 18 | 2 | 4.79 | 0.017 | |
| Left | Temporal | Inferior | 3 | −54 | −40 | −18 | 4.71 | 0.023 |
| Right | Hippocampus | 3 | 30 | −40 | 6 | 4.66 | 0.029 | |
| Left | Caudate | 1 | −20 | 16 | 14 | 4.63 | 0.032 | |
| Right | Cerebellum | CrusII | 2 | 26 | −88 | −34 | 4.62 | 0.034 |
| Training Session: Effect of Age | ||||||||
| 2. Main effect training (Y‐O) | No significant voxels | |||||||
| 3. Main effect training (O‐Y) | ||||||||
| Left | Frontal | Superior Medial | 42 | −8 | 36 | 56 | 5.43 | 0.001 |
| Left | Occipital | Middle | 120 | −26 | −92 | 0 | 5.20 | 0.003 |
| Right | Occipital | Middle | 62 | 26 | −88 | 0 | 4.98 | 0.007 |
Note: Main effect of training (all participants) and age thresholded at P < 0.05 FWE.
Retest‐training session
We first tested for the possible change in activation over the retention interval (Retest − Training) between young and older subjects in those that either napped or remained awake (Fig. 4). In general, sleep had a differential effect on brain activity between the young and older adults. This analysis revealed significant differences of activation bilaterally in the frontal cortex (middle and medial prefrontal), left temporal cortex (superior and middle), right anterior cingulate, left posterior cingulate, bilateral parietal cortex (cuneus), bilateral posterior hippocampus, and the left cerebellum (lobules V; Table 4; Fig. 4). Examination of the mean parameter estimates revealed a very consistent pattern of activation across these brain regions in the four experimental conditions. YN and ONN subjects had increased activations, whereas YNN and ON subjects had decreased levels of BOLD activity between training and retest sessions (Fig. 4B). There was no significant interaction in the opposite direction that would indicate decreased activity in the YN, or increases in ON group (Table 4.2).
Figure 4.
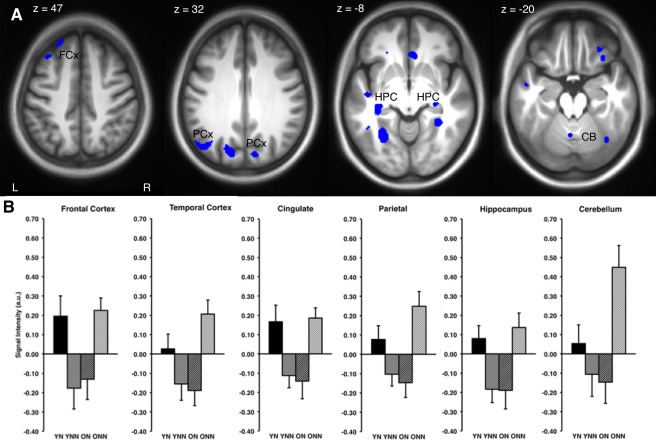
A: Brain regions with significant differences in activation indicated in blue (P < 0.001, unc.) during practice for the session (retest‐training) by age group (Young vs. Older) by sleep/wake condition (Nap vs. No‐Nap) interaction. B: A similar pattern of increased activation (mean voxel intensity, arbitrary units; a.u.) between groups, across regions (FCx: frontal cortex; PCx: parietal cortex; HPC: hippocampus; CB: cerebellum) in the session by group interaction. Error bars represent standard error.
Table 4.
Functional imaging results from independent two sample t‐tests for the sleep by age interaction (1‐2) and the effect of age (3‐4) and sleep (5‐6) for the session by group interaction effects in the young Nap (YN), young No‐Nap (YNN), older Nap (ON) and older No‐Nap (ONN) groups
| Hemisphere | Region | Subregion | cluster | X | Y | Z | z | P |
|---|---|---|---|---|---|---|---|---|
| Retest‐Training Session: Sleep × Age Interaction | ||||||||
| 1. Session × group interaction (YN‐YNN)‐(ON‐ONN) | ||||||||
| Left | Occipital | Middle | 415 | −46 | −72 | 34 | 3.87 | 0.000 |
| Right | Cingulate | Anterior | 51 | 8 | 28 | −6 | 3.86 | 0.000 |
| Left | Temporal | Superior | 64 | −46 | −16 | −4 | 3.86 | 0.000 |
| Left | Fusiform | 178 | −30 | −58 | −4 | 3.81 | 0.000 | |
| Left | Hippocampus | 42 | −32 | −26 | −8 | 3.71 | 0.000 | |
| Right | Fusiform | 63 | 32 | −44 | −6 | 3.62 | 0.000 | |
| Right | Fusiform | 36 | 38 | −64 | −18 | 3.54 | 0.000 | |
| Left | Cingulate | Posterior | 7 | −6 | −38 | 14 | 3.48 | 0.000 |
| Left | Parahippocampal | 4 | −24 | 2 | −24 | 3.48 | 0.000 | |
| Left | Frontal | Middle | 50 | −36 | 28 | 48 | 3.42 | 0.000 |
| Right | Occipital | Superior | 48 | 20 | −94 | 24 | 3.41 | 0.000 |
| Right | Hippocampus | 5 | 30 | −24 | −8 | 3.38 | 0.000 | |
| Left | Temporal | Middle | 8 | −46 | −50 | −4 | 3.33 | 0.000 |
| Right | Parietal | Cuneus | 33 | 12 | −80 | 32 | 3.30 | 0.000 |
| Right | Frontal | Medial Prefrontal | 8 | 6 | 46 | −14 | 3.24 | 0.001 |
| Left | Temporal | Middle | 9 | −50 | −4 | −18 | 3.22 | 0.001 |
| Left | Frontal | Inferior | 4 | −24 | 30 | −10 | 3.20 | 0.001 |
| Left | Occipital | Middle | 3 | −36 | −66 | 24 | 3.16 | 0.001 |
| Left | Temporal | Superior | 3 | −54 | −4 | 0 | 3.15 | 0.001 |
| Left | Cerebellum | V | 4 | −2 | −60 | −20 | 3.13 | 0.001 |
| Left | Occipital | Middle | 1 | −34 | −86 | 20 | 3.12 | 0.001 |
| Left | Frontal | Superior Medial | 1 | −10 | 40 | 30 | 3.10 | 0.001 |
| Right | Occipital | Superior | 1 | 22 | −84 | 22 | 3.10 | 0.001 |
| 2. Session × group interaction (ON‐ONN)‐(YN‐YNN) | No significant voxels | |||||||
| Retest‐Training Session: Effect of Age | ||||||||
| 3. Session × group interaction (YN‐ON) | ||||||||
| Left | Occipital | Middle | 136 | −44 | −74 | 36 | 3.78 | 0.000 |
| Right | Occipital | Superior | 82 | 20 | −96 | 26 | 3.67 | 0.000 |
| Left | Occipital | Middle | 41 | −32 | −90 | 18 | 3.61 | 0.000 |
| Left | Occipital | Middle | 4 | −20 | −90 | 40 | 3.23 | 0.001 |
| Left | Parietal | Angular | 2 | −54 | −66 | 32 | 3.15 | 0.001 |
| Right | Cingulate | Anterior | 1 | 6 | 30 | −6 | 3.10 | 0.001 |
| 4. Session × group interaction (ON‐YN) | No significant voxels | |||||||
| Retest‐Training Session: Effect of Sleep | ||||||||
| 5. Session × group interaction (YN‐YNN) | ||||||||
| Left | Parietal | Angular | 25 | −44 | −72 | 34 | 3.33 | 0.000 |
| Left | Hippocampus | 8 | −32 | −28 | −8 | 3.28 | 0.001 | |
| Left | Frontal | Medial Superior | 2 | −10 | 42 | 30 | 3.13 | 0.001 |
| 6. Session × group interaction (ON‐ONN) | No significant voxels | |||||||
Note: Statistics thresholded at p < 0.001 (unc.).
To further decompose the effects of sleep and age from the session by group interaction described above, additional contrast comparisons were made between age groups in the sleep/wake condition (YN‐ON) and between the sleep/wake conditions in young subjects (YN‐YNN). That analysis revealed increased activations in young subjects that slept as compared to older subjects that slept (YN‐ON) bilaterally in the parietal cortex, occipital cortex, and anterior cingulate whereas older subjects that slept had a reduction of activity in these structures from training to retest (Table 4.3; Fig. 5, lower panel). No significant activation was observed for older subjects in the sleep condition (ON‐YN; Table 4.4). Furthermore, young subjects that slept as compared to young subjects that remained awake (YN‐YNN) had increased activation from training to retest in the left frontal cortex, left parietal cortex and the left posterior part of the hippocampus, whereas reduced activity was observed in young subjects in the wake condition for these structures (Table 4.5; Fig. 5, lower panel). No significant differences were observed for older subjects that slept compared to those who stayed awake (ON‐ONN; Table 4.6).
Figure 5.
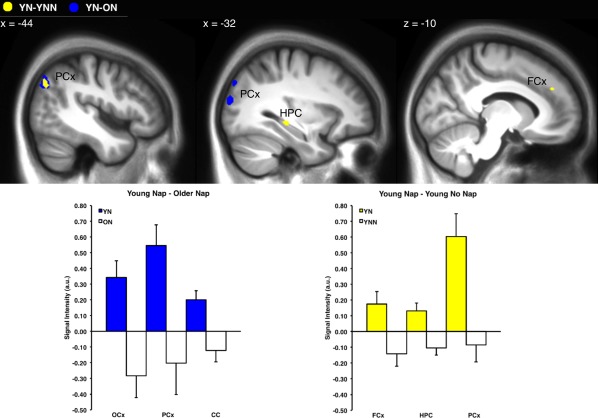
Brain regions with significant differences in activation (P < 0.001, unc.) during motor sequence practice, for the session (retest‐training) by sleep/wake condition interaction in young subjects Young Nap (YN) – Young No Nap (YNN; yellow), and effect of age Young Nap (YN) – Older Nap (ON; blue). Mean voxel signal intensity of significant clusters (arbitrary units; a.u.) for regions (FCx; frontal cortex, HPC; hippocampus, PCx; parietal cortex, OCx; occipital cortex, CC; cingulate cortex) displayed in top panel for the session by group interaction. Error bars represent standard error.
Correlation between sleep spindles and functional brain activity
To investigate whether changes in activation from training to retest were modulated by sleep spindle activity during the nap, separate regression analyses included spindle parameters (density, duration, amplitude, and frequency) as covariates of interest in the GLM. Activations correlating with the duration of non‐REM sleep were masked out as a variable of no interest. Thus, the results reported here represent cerebral activity correlated with sleep spindles over‐and‐above the duration of non‐REM sleep, which makes up the majority of the total sleep time in a daytime nap. Interestingly, this analysis revealed two distinct and dissociable patterns of correlations between YN and ON groups (Fig. 6; Table 5). As predicted, sleep spindle density in YN (but not ON) subjects was correlated significantly with increased activity from training to retest in regions previously reported to be involved in the consolidation of MSL including: the putamen (see for example Fig. 6A; YN: r(11) = 0.77, P =0.002, ON: r(13) = −0.01, P = 0.736), parietal cortex, somatosensory cortex, and the cerebellum (Table 5.1). Conversely, in ON (but not YN) subjects, spindle density (Table 5.2) and duration (Table 5.4) were correlated with increased activity from training to retest in regions typically involved in the early stages of MSL including: the cerebellum (see for example Fig. 6A; ON: r(13) = 0.73, P =0.002, YN: r(11) = −0.19, P = 0.538) and primary motor cortex. Spindle amplitude and frequency were not significantly correlated with cerebral activation in YN or ON subjects.
Figure 6.
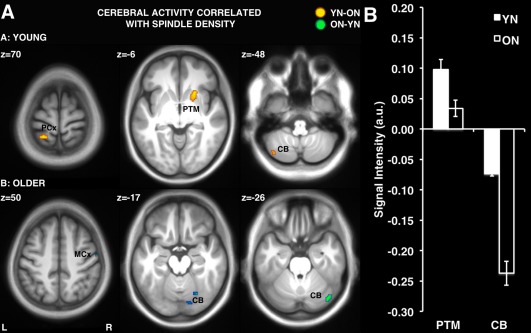
A: Differences in activation (retest – training) correlated with spindle density in the Young Nap (YN‐ON) vs. Older Nap (ON‐YN) groups. B: Mean voxel signal intensity (percent change from training to retest) for significant clusters in the putamen and cerebellum correlated with spindle density. PCx: parietal cortex; MCx: motor cortex; PTM: putamen; CB: cerebellum. Error bars represent standard error.
Table 5.
Functional neuroimaging results from the session (retest ‐ training) by age condition (Young vs. Older) interaction for clusters of voxels correlated with sleep spindle density, duration, amplitude and frequency in the young nap (YN) and older nap (ON) groups
| Hemisphere | Region | Subregion | cluster | X | Y | Z | z | P |
|---|---|---|---|---|---|---|---|---|
| Retest‐Training Session: Effect of Age | ||||||||
| 1. Regression: spindle density (YN‐ON) | ||||||||
| Right | Striatum | Putamen | 76 | 24 | 18 | −8 | 4.39 | 0.000 |
| Left | Parietal | Superior | 56 | −22 | −48 | 72 | 4.14 | 0.000 |
| Left | Thalamus | 11 | −4 | −8 | −2 | 3.67 | 0.000 | |
| Right | Insula | 3 | 28 | 18 | −12 | 3.62 | 0.000 | |
| Left | Temporal | Inferior | 3 | −56 | −10 | −30 | 3.47 | 0.000 |
| Left | Temporal | Inferior | 4 | −50 | −14 | −30 | 3.40 | 0.000 |
| Right | Thalamus | 4 | 6 | −8 | −2 | 3.33 | 0.000 | |
| Left | Cerebellum | VI | 1 | −22 | −52 | −30 | 3.32 | 0.000 |
| Left | Cerebellum | Crus II | 10 | −42 | −70 | −48 | 3.28 | 0.001 |
| Left | Temporal | Inferior | 4 | −46 | −10 | −26 | 3.28 | 0.001 |
| Right | Temporal | Inferior | 1 | 42 | −42 | −14 | 3.21 | 0.001 |
| Left | Temporal | Superior Pole | 2 | −36 | 12 | −24 | 3.18 | 0.001 |
| Left | Temporal | Inferior | 2 | −48 | 8 | −40 | 3.18 | 0.001 |
| Left | Parietal | Somatosensory | 3 | −20 | −30 | 74 | 3.15 | 0.001 |
| Left | Cerebellum | VIIb | 1 | −28 | −66 | −52 | 3.10 | 0.001 |
| 2. Regression: spindle density (ON‐YN) | ||||||||
| Right | Cerebellum | Crus I | 32 | 42 | −74 | −26 | 3.64 | 0.000 |
| Right | Cerebellum | VI | 11 | 24 | −68 | −16 | 3.46 | 0.000 |
| Right | Cerebellum | VI | 10 | 14 | −80 | −18 | 3.30 | 0.000 |
| Right | Frontal | Primary Motor Cortex | 1 | 56 | −10 | 50 | 3.26 | 0.001 |
| Right | Parietal | Calcarine | 2 | 30 | −58 | 6 | 3.21 | 0.001 |
| Right | Cerebellum | Crus I | 1 | 34 | −76 | −20 | 3.10 | 0.001 |
| 3. Regression: spindle duration (YN‐ON) | ||||||||
| No significant voxels | ||||||||
| 4. Regression: spindle duration (ON‐YN) | ||||||||
| Left | Parietal | Postcentral | 5 | −22 | −44 | 54 | 3.31 | 0.000 |
| Right | Occipital | Calcarine | 3 | 28 | −64 | 12 | 3.21 | 0.001 |
| Right | Cerebellum | VIIIb | 2 | 26 | −50 | −48 | 3.14 | 0.001 |
| Right | Cerebellum | VIIb | 1 | 38 | −48 | −50 | 3.11 | 0.001 |
| 5. Regression: spindle amplitude (YN‐ON) | ||||||||
| No significant voxels | ||||||||
| 6. Regression: spindle amplitude (ON‐YN) | ||||||||
| No significant voxels | ||||||||
| 7. Regression: spindle frequency (YN‐ON) | ||||||||
| No significant voxels | ||||||||
| 8. Regression: spindle frequency (ON‐YN) | ||||||||
| No significant voxels | ||||||||
Note: Statistics thresholded at P < 0.001 (unc.).
YN, but not ON, subjects had increased activation from training to retest in the region of the putamen for voxels where activity was correlated with spindle density (Fig. 6B). Conversely, ON subjects had decreased activity in the cerebellum in voxels that correlated with spindle density to a greater extent than YN subjects (Fig. 6B).
DISCUSSION
The goal of this study was to investigate, through fMRI, the neural correlates of the age‐related motor sequence memory consolidation deficit observed in older healthy adults. This was achieved by comparing their performance and pattern of brain activity to that of young control subjects before and after a retention period filled with either a daytime nap or equivalent period of quiet wakefulness. To better delineate the possible physiological mechanisms involved, we also aimed to determine with EEG recordings whether or not this behavioural impairment was associated with an age‐related reduction in spindles detected during non‐REM sleep.
In summary, we showed that: (1) offline gains in performance were observed in YN subjects, but not in YNN, ON, or ONN subjects, (2) ON subjects showed reduced sleep spindle duration and had slower spindles than YN subjects, (3) young and older subjects showed an opposite pattern of functional results in dissociable brain regions, whereby the YN group showed increased activity in the hippocampus, cerebellum, cingulate, frontal, temporal, and parietal cortex, whereas the ON group had decreased activity in these regions from training to retest, (4) sleep spindles in YN (but not ON) subjects were positively correlated with BOLD activity from training to retest in the putamen and related brain areas, whereas sleep spindles in ON (but not YN) subjects were correlated with activation in the cerebellum, and (5) activity correlated with spindle density in the putamen was increased in YN subjects as compared with ON subjects.
As predicted, based on previous studies that have investigated the ability of elderly participants to acquire and consolidate a new sequence of movements [Brown et al., 2009; Spencer et al., 2007], older participants improved at the same rate as younger subjects during training on the MSL task, but were slower overall in performing the repeating sequence. Critically, YN but not YNN subjects demonstrated significant gains in performance from training to retest, whereas older subjects did not show spontaneous performance gains. Yet older participants did retain what was learned during training as shown by the maintenance of performance from training to retest. Such findings are consistent with previous studies [Brown et al., 2009; Spencer et al., 2007], which demonstrated that nocturnal sleep during a retention interval does not enhance motor sequence performance in older adults. Finally, our results are also congruent with recent evidence revealing that sleep is beneficial for declarative, but not motor skill learning in older adults [Wilson et al., 2012], hence providing further evidence that sleep‐dependent consolidation is diminished with age. Yet these results are apparently incongruent with one study in which older subjects exhibited improvements in performance similar to those in young subjects over a 24‐h period that included both a full day of wakefulness and a normal night of sleep [Tucker et al., 2011]. Two important methodological differences may explain this inconsistent pattern of behavioral results. First, this incongruence may be due to differences in the way consolidation was measured. More specifically, Tucker et al. [2011] used the last (and not the first) few blocks of practice in the re‐test session to calculate “delayed improvement”. When a conventional measure of consolidation (i.e., the change in performance from the end of training to the beginning of retest) is used, a very similar impairment of offline gains in performance in older subjects was observed compared with young subjects. Second, it is possible that the diverging pattern of results from Tucker et al. may also be due to the fact that they tested their participants after a full night of sleep while we investigated the effects of a daytime nap. Although this suggests that the consolidation deficit reported here might simply be due to the limited amount of sleep that subjects had during the nap, we do not believe that this is the case as Wilson et al. [2012] identified similar consolidation impairment over a full night of sleep in older adults. Furthermore, there is ample evidence that: (1) an afternoon nap is sufficient for significant sleep‐dependent consolidation in young subjects [Albouy et al., 2013a, 2013b; Backhaus and Junghanns, 2006; Doyon et al., 2009a,b; Korman et al., 2007; Mednick et al., 2003; Milner et al., 2006; Nishida and Walker, 2007]; (2) older adults exhibit improved cognitive performance following a similar length of daytime sleep as compared with younger adults [Milner and Cote, 2008]; and (3) older adults have been found to have the same homeostatic response to napping as young adults [Campbell and Feinberg, 2005]. These findings collectively suggests that the deficit observed here in older subjects is specific to the consolidation process, and not likely due to sleep duration alone (e.g., nap vs. a full night's sleep). In addition, our pattern of behavioral results cannot be explained by task difficulty since the sequence was short (5‐item), self‐initiated (un‐cued), and explicitly learned prior to the practice sessions. In fact, accuracy was at ceiling, thus excluding the possibility of a speed‐accuracy trade off. Finally, there was no difference in accuracy between young and older subjects during training; all factors that would minimize age‐related effects on reaction time. In summary, our behavioral findings thus confirm those of previous studies, and strongly suggest that sleep enhances memory consolidation in young subjects, but does not benefit MSL consolidation in older adults.
Unlike previous studies in young subjects that identified a positive correlation between gains in performance and: (1) spindle amplitude at frontal, central and parietal sites [Barakat et al., 2012], (2) fast (but not slow) spindle density [Barakat et al., 2011], and (3) a difference in spindle density between the left and right hemispheres (but not each hemisphere individually) [Nishida and Walker, 2007], this investigation did not reveal any correlation between gains in performance and spindle activity. Although at first glance, the lack of correlation appears at odds with these other reports, it is important to note that links between various spindle characteristics and gains in performance have been found inconsistently from study to study. Indeed, such correlations have been shown to vary depending on the: (1) nature of the spindles (e.g., fast, slow or total bandwidth), (2) localization of the derivations (e.g., frontal and central parietal), (3) hemisphere (left, right, or left‐right), and (4) type of spindle metric used (e.g., density, amplitude, duration, sigma power, etc.). Furthermore, it should be noted that spindle measures were taken from a single time point, and thus may reflect a combination of interindividual differences in sleep spindles and learning‐dependent changes in spindles. Thus, further research is needed to pinpoint precisely what aspects of the spindle are related to gains in MSL.
Consistent with the behavioral pattern observed, the fMRI data revealed that YN subjects had increased activity from training to retest in the parietal cortex and the hippocampus (among other structures related to MSL) as compared to YNN subjects during the retention interval. Interestingly, recent evidence from animal studies [Lansink et al., 2009] has shown joint reactivations in both hippocampus and striatum during post‐training sleep. These reactivations are known to be time‐locked to hippocampal sharp wave ripple complexes, which are driven by the occurrence of sleep spindles [for review see: Girardeau and Zugaro, 2011]. Furthermore, sleep‐dependent hippocampal activations have been observed during initial sequence learning [Walker et al., 2005], and have been found to be predictive of subsequent overnight gains in performance [Albouy et al., 2008, 2013a,b]. Thus, these results suggest that the hippocampus and related areas such as the parietal cortex not only play a role in the early acquisition phase, but also in the consolidation of MSL after sleep in young healthy adults. Indeed, during non‐REM sleep, it has previously been shown in rats, that spatial memory traces are repeatedly reactivated through hippocampal sharp‐wave ripples [Wilson and McNaughton, 1994], which are synchronized with sleep spindles by the action of slow oscillations [Clemens et al., 2007]. Consistent with such findings in rodents, an increase in spindles time‐locked to hippocampal ripples has also been reported following declarative learning in humans [Mölle et al., 2009]. In addition, human neuroimaging studies have demonstrated that motor memory traces are also reactivated during the night [Maquet et al., 2000; Peigneux et al., 2004], and that spindles during non‐REM sleep are associated with increased BOLD activity in the hippocampus as well as in the mesial prefrontal cortex, precentral gyrus, and postcentral gyrus [Schabus et al., 2007]. Taken together, these studies suggest that non‐REM sleep, and spindles in particular, are involved in the hippocampal‐neocortical dialogue, which are thought, in turn, to contribute to the consolidation of MSL. Our results also suggest that the hippocampus may be part of a larger network of structures involved in the sleep‐dependent consolidation of MSL including the frontal cortex, parietal cortex and the cerebellum. Future studies employing functional connectivity techniques would be best suited to investigate this hypothesis further.
Only a handful of studies have investigated the EEG characteristics of a daytime nap in elderly populations [Campbell and Feinberg, 2005; Knoblauch et al., 2005; Milner and Cote, 2008; Tamaki et al., 2000, 1999]. Here, we observed slower frequency spindles in ON subjects. This is consistent with previous research [Knoblauch et al., 2005], and is similar to Martin et al. [2012] who reported slower frequency spindles at frontal sites (Fp1, F3) in the first non‐REM period of overnight sleep. Also consistent with previous overnight studies [Carrier et al., 2001; Landolt et al., 1996; Martin et al., 2012; Nicolas et al., 2001], our results revealed that ON participants had reduced spindle duration during the nap compared to YN participants. In addition, we found no significant difference in spindle density between YN and ON subjects, which is also in line with Martin et al. [2012] who did not find any difference between young and older subjects in the first non‐REM period of the night. Thus, our present findings suggest that there are age‐related changes in the characteristics of spindles, but no change in spindle density during a daytime nap. Although seemingly incongruent with previously reported age differences in spindle density [Carrier et al., 2001; Landolt et al., 1996; Martin et al., 2012; Nicolas et al., 2001], these studies employed detection methods that use a minimum duration criteria. Unlike the current study, these detection methods do not include short duration spindles (e.g., < 0.5 s), thus this may partly account for the different pattern of results observed here in older subjects. In fact, inspection of the results presented in Table 2 indicates that minimum criteria of 0.5 s would have rejected a greater number of spindles in older subjects than in young subjects. To counteract such bias, no minimum duration criteria was used here, hence allowing spindles as short as 0.2 s to be accurately identified.
The lack of a significant difference in spindle density does not preclude, however, the possibility that there may be a systematic relationship between spindle density and cerebral activation in one age group, but not the other. Thus, when entered into the GLM as covariates of interest, our functional data revealed that spindle density was associated with activation in the putamen in young (but not older) subjects (YN‐ON). In addition, activation was reduced in ON subjects as compared with YN subjects for the voxels in the putamen that were significantly correlated with spindle density, hence suggesting that sleep spindles modulate activity in the cortico‐striatal network in young subjects. The latter results are in line with recent investigations, which have reported that, at baseline, inter‐individual differences in sleep spindles are associated with activity in the putamen [Caporro et al., 2011; Tyvaert et al., 2008]. Moreover, motor sequence related activity in the putamen has been found to be increased following sleep, but not following a retention period filled with wake in young, health adults [Debas et al., 2010]. Finally, another study identified that sleep spindles were correlated with increased activity in the putamen [Barakat et al., 2012]. Thus, this study demonstrates that activation of the putamen is correlated with sleep spindles in young (but not older) subjects and that this activity is reduced in older as compared with young subjects, possibly explaining the age‐related, sleep‐dependent deficit in MSL consolidation. Several pieces of physiological and structural evidence are consistent with this interpretation: (1) aging is associated with decreased dopamine in the basal ganglia, a neurotransmitter involved in motor learning [Bäckman et al., 2006, 2010; Kaasinen and Rinne, 2002], (2) age‐related reductions in the volume of the caudate nucleus and the putamen have been observed [Gunning‐Dixon et al., 1998; Hedden and Gabrieli, 2004; Kennedy and Raz, 2005], and (3) degradation of white matter tracts connecting the caudate nucleus and the dorsolateral prefrontal cortex are observed with age [Bennett et al., 2011]; a circuit thought to be involved in forming associations between repeated elements of a motor sequence [Jueptner et al., 1997; Schendan et al., 2003].
Unlike the pattern of results found in young subjects, spindle density was correlated with activation in the cerebellum between the training and retest sessions in older (but not young) subjects (ON‐YN), suggesting that spindles modulated activity in the cortico‐cerebellar network in older subjects. Such a correlation between spindles and activity in the cerebellum in older subjects may represent a reliance on structures involved in the early stages of the consolidation process, suggesting that this group may require additional practice and subsequent opportunities for memory consolidation. Alternatively, this may reflect a compensatory mechanism whereby during sleep there is reliance on only part of the memory system (including the cerebellum and motor cortex) as a result of reduced age‐related functioning of the putamen. Yet the latter interpretation is only speculative as there is little evidence in the extant literature linking sleep spindles to activity in the cerebellum. For example, electrical stimulation of the medial cerebellum in monkeys has been found to excite thalamocortical pathways and primary motor cortex (BA4) responses [Sasaki et al., 1976], the same neural circuitry involved in the production of sleep spindles. A recent neuroimaging study has also revealed that slow oscillations are associated with activation of both the cerebellum and hippocampus, suggesting that the cerebellum and hippocampus may be part of a functional network of structures during non‐REM sleep and that they may play a role in memory consolidation [Ji and Wilson, 2007; Marshall et al., 2006; Peigneux et al., 2004; Rasch et al., 2007].
Thus, taken together, these findings suggest that age‐related degradations in the striatum contribute to deficits in motor sequence memory consolidation. Given that sleep‐dependent activation of the putamen during motor sequence performance [Debas et al., 2010] is correlated with sleep spindles [Barakat et al., 2012], and that spindles are increased following MSL [Morin et al., 2008; Nishida and Walker, 2007], age‐related changes in sleep spindles would be expected to reduce the benefit to consolidation that sleep affords. The mechanisms mediating this behavioral impairment may be related to a deficiency in the normal function of cortically driven [Bonjean et al., 2011] sleep spindles involved in the reactivation of cortico‐striatal networks via the densely interconnected cortico‐striato‐thalamo‐cortical loops. Yet at present, this remains a working hypothesis, awaiting further experimental investigation.
CONCLUSIONS
This study confirms that while sleep in a daytime nap facilitates offline improvements motor sequence performance in younger subjects, it serves little benefit in this regard for older subjects. Our findings also reveal age‐related changes in both sleep characteristics and neural correlates responsible for the MSL consolidation impairment. They show that in young subjects, sleep spindles are related to sleep‐dependent changes in cerebral activation by modulating the cortico‐striatal network known to be involved in the process of motor memory consolidation. Conversely, we demonstrate that in older subjects, sleep spindles modulate activity in the cortico‐cerebellar network, which had no beneficial effect on post‐sleep performance. These results thus suggest that the activity of brain regions related to motor sequence performance like the cerebellum, even when modulated by sleep spindles, does not produce significant performance gains (i.e., consolidation). Rather, the over‐nap increase in activity related to sleep spindles in structures such as the putamen appears to be required for off‐line improvements. Thus, these findings indicate that the age‐related changes in sleep spindles and reduced activity in the striatum in older subjects underlie the deficit in MSL consolidation seen with age.
ACKNOWLEDGMENTS
The authors would like to acknowledge the technical support of Vo An Nguyen, Laura Ray, Andre Cyr, Carollyn Hurst, Frederic Jeay, and Amel Bouyoucef. J.D., S.F., and J.C. jointly conceived the study design, analysis techniques, and analysis strategy. J.D. and J.C. supervised the project and revised the manuscript. S.F. and G.A. jointly implemented the study design and analysis techniques. In addition, S.F. performed the experiment, analyzed the data and wrote the manuscript. G.A. developed the analysis tools, helped perform the experiment, and made comments to previous versions of the manuscript. C.V. and R.P. assisted with data collection. B.K. helped model the behavioral data and revise the manuscript. R.H., S.J., H.B., A.K., and P.M. contributed equally to the study design and analysis techniques.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Julie Carrier and Julien Doyon are co‐senior authors.
Conflicts of Interest: The authors declare no actual or potential conflicts of interest.
REFERENCES
- Aeschbach D, Borbely AA (1993): All‐night dynamics of the human sleep EEG. J Sleep Res 2:70–81. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang‐Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, Peigneux P, Luxen A, Maquet P (2008): Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron 58:261–272. [DOI] [PubMed] [Google Scholar]
- Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, Carrier J, Robertson E, Doyon J (2013a): Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One 8:e52805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Vandewalle G, Darsaud A, Gais S, Rauchs G, Desseilles M, Boly M, Dang‐Vu T, Balteau E, Degueldre C, Phillips C, Luxen A, Maquet P (2013b): Interaction between hippocampal and striatal systems predicts subsequent consolidation of motor sequence memory. PLoS One 8:e59490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K (2006): Daytime naps improve procedural motor memory. Sleep Med 7:508–512. [DOI] [PubMed] [Google Scholar]
- Barakat M, Carrier J, Debas K, Lungu O, Fogel S, Vandewalle G, Hoge RD, Bellec P, Karni A, Ungerleider LG, Benali H, Doyon J (2012): Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Hum Brain Mapp 34(11):2918–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, Benali H, Carrier J (2011): Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav Brain Res 217:117–121. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L (2006): The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev 30:791–807. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Lindenberger U, Li SC, Nyberg L (2010): Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neurosci Biobehav Rev 34:670–677. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rial WY, Rickels K (1974): Short form of depression inventory: Cross‐validation. Psychol Rep 34:1184–1186. [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA (1988): An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol 56:893–897. [DOI] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Howard DV (2011): White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiol Aging 32:2317e1–2317.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann TO, Mölle M, Marshall L, Kaya‐Yildiz L, Born J, Roman Siebner H (2008): A local signature of LTP‐ and LTD‐like plasticity in human NREM sleep. Eur J Neurosci 27:2241–2249. [DOI] [PubMed] [Google Scholar]
- Bonjean M, Baker T, Lemieux M, Timofeev I, Sejnowski T, Bazhenov M (2011): Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci 31:9124–9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brashers‐Krug T, Shadmehr R, Bizzi E (1996): Consolidation in human motor memory. Nature 382:252–255. [DOI] [PubMed] [Google Scholar]
- Brown RM, Robertson EM, Press DZ (2009): Sequence skill acquisition and off‐line learning in normal aging. PLoS One 4:e6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Feinberg I (2005): Homeostatic sleep response to naps is similar in normal elderly and young adults. Neurobiol Aging 26:135–144. [DOI] [PubMed] [Google Scholar]
- Campbell K, Kumar A, Hofman W (1980): Human and automatic validation of a phase‐locked loop spindle detection system. Electroencephalogr Clin Neurophysiol 48:602–605. [DOI] [PubMed] [Google Scholar]
- Caporro M, Haneef Z, Yeh HJ, Lenartowicz A, Buttinelli C, Parvizi J, Stern JM (2011): Functional MRI of sleep spindles and K‐complexes. Clin Neurophysiol 123:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier J, Land S, Buysse DJ, Kupfer DJ, Monk TH (2001): The effects of age and gender on sleep EEG power spectral density in the middle years of life (ages 20‐60 years old). Psychophysiology 38:232–242. [PubMed] [Google Scholar]
- Chapman CA, Trepel C, Ivanco TL, Froc DJ, Wilson K, Racine RJ (1998): Changes in field potentials and membrane currents in rat sensorimotor cortex following repeated tetanization of the corpus callosum in vivo. Cereb Cortex 8:730. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Mölle M, Eross L, Barsi P, Halász P, Born J (2007): Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain 130:2868–2878. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Mölle M, Eross L, Jakus R, Rásonyi G, Halász P, Born J (2011): Fine‐tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci 33:511–520. [DOI] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF (1988): Mini‐Mental State Examination (MMSE). Psychopharmacol Bull 24:689–692. [PubMed] [Google Scholar]
- Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM (2002): The effects of normal aging on sleep spindle and K‐complex production. Clin Neurophysiol 113:1615–1622. [DOI] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Hadj Tahar A, Bellec P, Karni A, Ungerleider LG, Benali H, Doyon J (2010): Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc Natl Acad Sci USA 107:17839–17844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J (2010): The memory function of sleep. Nat Rev Neurosci 11:114–126. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Powell JW (1985): Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods 17:652–655. [Google Scholar]
- Doyon J, Benali H (2005): Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15:161–167. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG (2003): Distinct contribution of the cortico‐striatal and cortico‐cerebellar systems to motor skill learning. Neuropsychologia 41:252–262. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H (2009a): Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199:61–75. [DOI] [PubMed] [Google Scholar]
- Doyon J, Korman M, Morin A, Dostie V, Hadj Tahar A, Benali H, Karni A, Ungerleider LG, Carrier J (2009b): Contribution of night and day sleep vs. simple passage of time to the consolidation of motor sequence and visuomotor adaptation learning. Exp Brain Res 195:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y (2004): The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55:51–86. [DOI] [PubMed] [Google Scholar]
- Fischer S, Born J (2009): Anticipated reward enhances offline learning during sleep. J Exp Psychol Learn Mem Cogn 35:1586–1593. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT (2006): Learning‐dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res 15:250–255. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT (2011): The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep‐dependent memory consolidation. Neurosci Biobehav Rev 35:1154–1165. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT, Cote KA (2007): Dissociable learning‐dependent changes in REM and non‐REM sleep in declarative and procedural memory systems. Behav Brain Res 180:48–61. [DOI] [PubMed] [Google Scholar]
- Fogel SM, Martin N, Lafortune M, Barakat M, Debas K, Laventure S, Latreille V, Gagnon JF, Doyon J, Carrier J (2012): NREM sleep oscillations and brain plasticity in aging. Front Neurol 3:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gennaro L, Ferrara M, Vecchio F, Curcio G, Bertini M (2005): An electroencephalographic fingerprint of human sleep. Neuroimage 26:114–122. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Zugaro M (2011): Hippocampal ripples and memory consolidation. Curr Opin Neurobiol 21:452–459. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Head D, McQuain J, Acker JD, Raz N (1998): Differential aging of the human striatum: A prospective MR imaging study. Am J Neuroradiol 19:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD (2004): Insights into the ageing mind: A view from cognitive neuroscience. Nat Rev Neurosci 5:87–96. [DOI] [PubMed] [Google Scholar]
- Himanen SL, Virkkala J, Huhtala H, Hasan J (2002): Spindle frequencies in sleep EEG show U‐shape within first four NREM sleep episodes. J Sleep Res 11:35–42. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O (1976): A self‐assessment questionnaire to determine morningness‐eveningness in human circadian rhythms. Int J Chronobiol 4:97–110. [PubMed] [Google Scholar]
- Iber C (2007): The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine. [Google Scholar]
- Ivanco TL, Racine RJ (2000): Long‐term potentiation in the reciprocal corticohippocampal and corticocortical pathways in the chronically implanted, freely moving rat. Hippocampus 10:143–152. [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson M (2007): Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10:100–107. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Ores Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, Oakes TR (2006): Motion correction and the use of motion covariates in multiple‐subject fMRI analysis. Hum Brain Mapp 27:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE (1997): Anatomy of motor learning. II. Subcortical structures and learning by trial and error. J Neurophysiol 77:1325–1337. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Rinne JO (2002): Functional imaging studies of dopamine system and cognition in normal aging and Parkinson's disease. Neurosci Biobehav Rev 26:785–793. [DOI] [PubMed] [Google Scholar]
- Karni A, Sagi D (1993): The time course of learning a visual skill. Nature 365:250–252. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377:155–158. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N (2005): Age, sex and regional brain volumes predict perceptual‐motor skill acquisition. Cortex 41:560–569. [DOI] [PubMed] [Google Scholar]
- Knoblauch V, Münch M, Blatter K, Martens WL, Schröder C, Schnitzler C, Wirz‐Justice A, Cajochen C (2005): Age‐related changes in the circadian modulation of sleep‐spindle frequency during nap sleep. Sleep 28:1093–1101. [DOI] [PubMed] [Google Scholar]
- Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A (2007): Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci 10:1206–1213. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Achermann P, Borbély AA (1996): Effect of age on the sleep EEG: Slow‐wave activity and spindle frequency activity in young and middle‐aged men. Brain Res 738:205–212. [DOI] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM (2009): Hippocampus leads ventral striatum in replay of place‐reward information. PLoS Biol 7:e1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq Y, Schrouff J, Noirhomme Q, Maquet P, Phillips C (2011): fMRI artefact rejection and sleep scoring toolbox. Comput Intell Neurosci 2011:598206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean AW, Fekken GC, Saskin P, Knowles JB (1992): Psychometric evaluation of the Stanford Sleepiness Scale. J Sleep Res 1:35–39. [DOI] [PubMed] [Google Scholar]
- Maquet P (2001): The role of sleep in learning and memory. Science 294:1048. [DOI] [PubMed] [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T (2000): Experience‐dependent changes in cerebral activation during human REM sleep. Nat Neurosci 3:831–836. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadóttir H, Mölle M, Born, J (2006): Boosting slow oscillations during sleep potentiates memory. Nature 444:610–613. [DOI] [PubMed] [Google Scholar]
- Martin N, Lafortune M, Godbout J, Barakat M, Robillard R, Poirier G, Bastien C, Carrier J (2012): Topography of age‐related changes in sleep spindles. Neurobiol Aging 34:468–476. [DOI] [PubMed] [Google Scholar]
- Mednick S, Nakayama K, Stickgold R (2003): Sleep‐dependent learning: a nap is as good as a night. Nat Neurosci 6:697–698. [DOI] [PubMed] [Google Scholar]
- Milner CE, Cote KA (2008): A dose‐response investigation of the benefits of napping in healthy young, middle‐aged and older adults. Sleep Biol Rhythms 6:2–15. [Google Scholar]
- Milner CE, Fogel SM, Cote KA (2006): Habitual napping moderates motor performance improvements following a short daytime nap. Biol Psychol 73:141–156. [DOI] [PubMed] [Google Scholar]
- Morin A, Doyon J, Dostie V, Barakat M, Hadj Tahar A, Korman M, Benali H, Karni A, Ungerleider LG, Carrier J (2008): Motor sequence learning increases sleep spindles and fast frequencies in post‐training sleep. Sleep 31:1149–1156. [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Marshall L, Gais S, Born J (2002): Grouping of spindle activity during slow oscillations in human non‐rapid eye movement sleep. J Neurosci 22:10941–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mölle M, Eschenko O, Gais S, Sara SJ, Born J (2009): The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci 29:1071–1081. [DOI] [PubMed] [Google Scholar]
- Nemeth D, Janacsek K (2011): The dynamics of implicit skill consolidation in young and elderly adults. J Gerontol B Psychol Sci Soc Sci 66:15–22. [DOI] [PubMed] [Google Scholar]
- Nemeth D, Janacsek K, Londe Z, Ullman MT, Howard DV, Howard JH (2010): Sleep has no critical role in implicit motor sequence learning in young and old adults. Exp Brain Res 201:351–358. [DOI] [PubMed] [Google Scholar]
- Nicolas A, Petit D, Rompré S, Montplaisir J (2001): Sleep spindle characteristics in healthy subjects of different age groups. Clin Neurophysiol 112:521–527. [DOI] [PubMed] [Google Scholar]
- Nishida M, Walker MP (2007): Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One 2:e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV (2004): Meta‐analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan. Sleep 27:1255–1273. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Pace‐Schott EF, Spencer RM (2011): Age‐related changes in the cognitive function of sleep. Prog Brain Res 191:75–89. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, Reggers J, Phillips C, Degueldre C, Del Fiore G, Aerts J, Luxen A, Maquet P (2004): Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron 44:535–545. [DOI] [PubMed] [Google Scholar]
- Peters KR, Ray L, Smith V, Smith C (2008): Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res 17:23–33. [DOI] [PubMed] [Google Scholar]
- Peters KR, Smith V, Smith CT (2007): Changes in sleep architecture following motor learning depend on initial skill level. J Cogn Neurosci 19:817–829. [DOI] [PubMed] [Google Scholar]
- Principe JC, Smith JR (1982): Sleep spindle characteristics as a function of age. Sleep 5:73. [PubMed] [Google Scholar]
- Rasch B, Büchel C, Gais S, Born J (2007): Odor cues during slow‐wave sleep prompt declarative memory consolidation. Science 315:1426–29. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, Hot P, Denise P, Desgranges B, Eustache F, Gruber G, Anderer P (2008): Is there a link between sleep changes and memory in Alzheimer's disease? Neuroreport 19:1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LB, Fogel SM, Smith CT, Peters KR (2010): Validating an automated sleep spindle detection algorithm using an individualized approach. J Sleep Res 19:374–378. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Ulrich D (2005): Pattern‐specific associative long‐term potentiation induced by a sleep spindle‐related spike train. J Neurosci 25:9398–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Kawaguchi S, Oka H, Sakai M, Mizuno N (1976): Electrophysiological studies on the cerebellocerebral projections in monkeys. Exp Brain Res 24:495–507. [DOI] [PubMed] [Google Scholar]
- Schabus M, Dang‐Vu TT, Albouy G, Balteau E, Boly M, Carrier J, Darsaud A, Degueldre C, Desseilles M, Gais S, Phillips C, Rauchs G, Schnakers C, Sterpenich V, Vandewalle G, Luxen A, Maquet P (2007): Hemodynamic cerebral correlates of sleep spindles during human non‐rapid eye movement sleep. Proc Natl Acad Sci USA 104:13164–13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabus M, Hoedlmoser K, Pecherstorfer T, Anderer P, Gruber G, Parapatics S, Sauter C, Kloesch G, Klimesch W, Saletu B, Zeitlhofer J (2008): Interindividual sleep spindle differences and their relation to learning‐related enhancements. Brain Res 1191:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE (2003): An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron 37:1013–1025. [DOI] [PubMed] [Google Scholar]
- Shea CH, Park JH, Braden HW (2006): Age‐related effects in sequential motor learning. Phys Ther 86:478–488. [PubMed] [Google Scholar]
- Siapas AG, Wilson MA (1998): Coordinated interactions between hippocampal ripples and cortical spindles during slow‐wave sleep. Neuron 21:1123–1128. [DOI] [PubMed] [Google Scholar]
- Silverstein LD, Levy CM (1976): The stability of the sigma sleep spindle. Electroencephalogr Clin Neurophysiol 40:666–670. [DOI] [PubMed] [Google Scholar]
- Sirota A, Buzsáki G (2005): Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat Syst 3:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Csicsvari J, Buhl D, Buzsáki G (2003): Communication between neocortex and hippocampus during sleep in rodents. Proc Natl Acad Sci USA 100:2065–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RM, Gouw AM, Ivry RB (2007): Age‐related decline of sleep‐dependent consolidation. Learn Mem 14:480–484. [DOI] [PubMed] [Google Scholar]
- Tamaki M, Shirota A, Hayashi M, Hori T (2000): Restorative effects of a short afternoon nap (<30 min) in the elderly on subjective mood, performance and eeg activity. Sleep Res Online 3:131–139. [PubMed] [Google Scholar]
- Tamaki M, Shirota A, Tanaka H, Hayashi M, Hori T (1999): Effects of a daytime nap in the aged. Psychiatry Clin Neurosci 53:273–275. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ (1992): The mini‐mental state examination: A comprehensive review. J Am Geriatr Soc 40(9):922–35. [DOI] [PubMed] [Google Scholar]
- Trepel C, Racine RJ (1998): Long‐term potentiation in the neocortex of the adult, freely moving rat. Cereb Cortex 8:719–729. [DOI] [PubMed] [Google Scholar]
- Tucker M, McKinley S, Stickgold R (2011): Sleep optimizes motor skill in older adults. J Am Geriatr Soc 59:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyvaert L, Levan P, Grova C, Dubeau F, Gotman J (2008): Effects of fluctuating physiological rhythms during prolonged EEG‐fMRI studies. Clin Neurophysiol 119:2762–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G (2005): Sleep‐dependent motor memory plasticity in the human brain. Neuroscience 133:911–917. [DOI] [PubMed] [Google Scholar]
- Walter DO (1968): The method of complex demodulation. Electroencephalogr Clin Neurophysiol:Suppl 27:53‐Suppl 27:57. [PubMed] [Google Scholar]
- Wei HG, Riel E, Czeisler CA, Dijk DJ (1999): Attenuated amplitude of circadian and sleep‐dependent modulation of electroencephalographic sleep spindle characteristics in elderly human subjects. Neurosci Lett 260:29–32. [DOI] [PubMed] [Google Scholar]
- Werk CM, Harbour VL, Chapman CA (2005): Induction of long‐term potentiation leads to increased reliability of evoked neocortical spindles in vivo. Neuroscience 131:793–800. [DOI] [PubMed] [Google Scholar]
- Werth E, Achermann P, Dijk DJ, Borbely AA (1997): Spindle frequency activity in the sleep EEG: Individual differences and topographic distribution. Electroencephalogr Clin Neurophysiol 103:535–542. [DOI] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace‐Schott EF, Ivry RB, Spencer RM (2012): Sleep modulates word‐pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging 33:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL (1994): Reactivation of hippocampal ensemble memories during sleep. Science 265:676–679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


