Abstract
Knowledge about the sensory modality in which a forthcoming event might occur permits anticipatory intersensory attention. Information as to when exactly an event occurs enables temporal orienting. Intersensory and temporal attention mechanisms are often deployed simultaneously, but as yet it is unknown whether these processes operate interactively or in parallel. In this human electroencephalography study, we manipulated intersensory attention and temporal orienting in the same paradigm. A continuous stream of bisensory visuo‐tactile inputs was presented, and a preceding auditory cue indicated to which modality participants should attend (visual or tactile). Temporal orienting was manipulated blockwise by presenting stimuli either at regular or irregular intervals. Using linear beamforming, we examined neural oscillations at virtual channels in sensory and motor cortices. Both attentional processes simultaneously modulated the power of anticipatory delta‐ and beta‐band oscillations, as well as delta‐band phase coherence. Modulations in sensory cortices reflected intersensory attention, indicative of modality‐specific gating mechanisms. Modulations in motor and partly in somatosensory cortex reflected temporal orienting, indicative of a supramodal preparatory mechanism. We found no evidence for interactions between intersensory attention and temporal orienting, suggesting that these two mechanisms act in parallel and largely independent of each other in sensory and motor cortices. Hum Brain Mapp 36:3246–3259, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: beta band, alpha band, oscillations, supramodal, electroencephalography, beamforming
INTRODUCTION
In navigating our environment, we typically confront continuous and varied input streams to our distinct sensory systems. As we cannot reasonably process all these inputs to full elaboration, selective attention mechanisms such as intersensory attention and temporal orienting are deployed to focus our resources on inputs directly relevant to the task at hand. Intersensory attention describes our ability to attend to a specific sensory modality, while disregarding information from other modalities [Spence and Driver, 1997; Talsma et al., 2010]. Temporal orienting of attention facilitates stimulus processing by reducing uncertainties about when an upcoming event will occur [Arnal and Giraud, 2012; Nobre et al., 2007]. Recent studies have demonstrated interactions between other types of attention, such as intersensory and spatial attention [Bauer et al., 2012; van Ede et al., 2010] or temporal orienting and spatial attention [Doherty et al., 2005; Rohenkohl et al., 2014]. While intersensory attention and temporal orienting can also be deployed simultaneously, it is unknown whether they mutually influence each other or operate in an independent manner, and where in the cortical hierarchy they are instantiated.
Studies of intersensory selective attention have found shorter response times (RTs) when a relevant target is presented in a currently attended modality [De Jong et al., 2010; Mattler et al., 2006; Spence and Driver, 1997]. A frequent finding in electrophysiological studies is a decrease of anticipatory occipital alpha‐band activity (ABA, 8–12 Hz) when intersensory attention is deployed to the visual modality [Foxe et al., 1998; Fu et al., 2001]. This decrease is paralleled by a simultaneous increase of ABA over cortical areas of unattended modalities, possibly reflecting an active sensory suppression mechanism [Foxe and Snyder, 2011; Gomez‐Ramirez et al., 2011]. Recent studies investigating intersensory attention using visual‐tactile [Bauer et al., 2012] and auditory‐tactile [van Ede et al., 2010] stimulation have shown similar modulations in beta‐band activity (BBA, 13–30 Hz). Thus, patterns of amplification and suppression of ABA and BBA over sensory areas likely reflect intersensory attention. Like intersensory attention, temporal orienting can facilitate RTs [Miniussi et al., 1999; Rohenkohl et al., 2012; Sanabria and Correa, 2013] and improve perceptual accuracy [Jones et al., 2002; Rohenkohl et al., 2012]. Moreover, recent studies showed that temporal orienting modulates anticipatory BBA over sensory [Arnal et al. 2014; Fujioka et al., 2012] and motor areas [Cravo et al., 2011; Fujioka et al., 2012; Saleh et al., 2010]. In addition, temporal orienting leads to a phase reset of slow‐wave delta‐band activity (DBA, 2–4 Hz) [Besle et al., 2011; Cravo et al., 2013; Saleh et al. 2010]. Hence, temporal orienting is reflected in modulations of BBA and DBA. Taken together, intersensory attention and temporal orienting involve spectrally overlapping modulations of neural activity in sensory and motor cortices.
In this electroencephalography (EEG) study, we examined source localized oscillatory activity in sensory and motor cortices during a visual‐tactile cuing paradigm. Intersensory attention and temporal orienting were manipulated simultaneously, which enabled us (i) to dissociate the neural mechanisms underlying the two types of attention, and (ii) to examine whether they operate interactively or in an independent fashion. We found spatially distinct and modality‐specific modulations of oscillatory power and phase coherence that differed between the two types of attention. Notably, we did not observe interactions between these mechanisms, suggesting that they operate in parallel, largely independently of each other, in spatially distinct sensory and motor cortices.
METHODS
Participants
Twenty right‐handed, paid volunteers participated in the study. Four participants had to be excluded due to extensive muscle or sweat artifacts in the EEG data. The remaining 16 participants (9 female, 23.2 years mean age) had normal or corrected to normal vision and reported no history of neurological or psychiatric illness. The study was conducted in accordance with the local Ethics Committee of the Charité—Universitätsmedizin Berlin as well as with the Declaration of Helsinki, and all participants provided written informed consent.
Setup and Procedure
Participants were seated in a dimly lit, electrically and acoustically shielded chamber, while being presented with visual, tactile, and auditory stimuli (Fig. 1). Prior to the main experiment, participants were presented with a passive localizer task (see below) and then performed two training blocks to familiarize themselves with the experimental paradigm. They were also informed that the stimuli were sometimes presented in a temporally regular and sometimes in an irregular fashion (Fig. 2a). This was done to ensure that all participants were aware of the possible regularity in the stimulus train. The experiment comprised of a two‐by‐two factorial design, with the factors inter–stimulus–interval (ISI) (regular or irregular) and Attention (attend‐visual or attend‐tactile) (Fig. 2b). At the beginning of each trial, an auditory cue was presented that indicated to which sensory modality participants should attend (attend‐visual or attend‐tactile). The auditory cue, which was delivered via in‐ear air‐pressure headphones (E‐A‐R tone Gold, Auditory Systems, Indianapolis), comprised of a sinusoidal 440 or 880 Hz tone with duration of 150 ms (including 5 ms rise and fall time). The mapping between the cue tone frequency and the attentional condition was counterbalanced across participants (e.g., in half of the subjects the 440 Hz tone instructed participants to attend to the visual modality). The cue was followed by an ISI, which blockwise had either a fixed (1,700 ms) or variable (1,000–2,400 ms, mean 1,700 ms) duration. Participants benefited from temporal orienting of attention especially when stimuli were presented with fixed ISIs. Following the ISI, a combined visuo‐tactile stimulus was presented. Irrespective of the experimental condition, this stimulus randomly contained either a visual target (25% of all trials), a tactile target (25%), or no target (50%; i.e., a standard; see below). The participants' task was to perform a speeded button press with the right index finger when a target was presented in the cued modality (e.g., they had to press the button to visual targets when attention was cued to the visual modality). No response was required for standards or for targets in the noncued modality. Overall, participants were required to press a button on 25% of all trials. Following the stimulus, the cue of the next trial was presented either at a fixed interval of 1,700 ms in the blocks with regular ISI or at a variable interval of 1,000–2,400 ms (mean 1,700 ms) in blocks with irregular ISI. The main experiment comprised of 16 blocks lasting about 4 min each. In half of the blocks, stimuli were presented with fixed ISIs and in the other half with variable ISIs. Blocks with regular versus irregular ISIs alternated after every second block. Each block consisted of 80 trials, half of which contained an attend‐visual cue and the other half an attend‐tactile cue (presented in random order). In total, 320 trials were presented for each of the four experimental conditions. Participants received visual feedback about the number of hits and misses, as well as on their mean RTs after each block.
Figure 1.
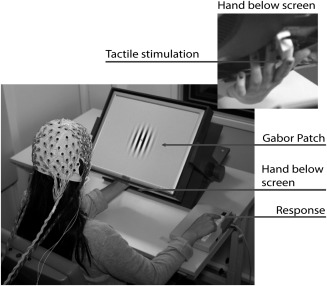
Experimental setup. Visual (Gabor‐patches) and tactile (Braille) stimuli were presented simultaneously and spatially aligned. Participants placed their left‐hand palm upwards on a board beneath a tilted TFT‐monitor on which visual stimuli were presented. They touched the Braille‐display with the tip of their left index finger. Behavioral responses to validly cued targets were made with the right index finger.
Figure 2.
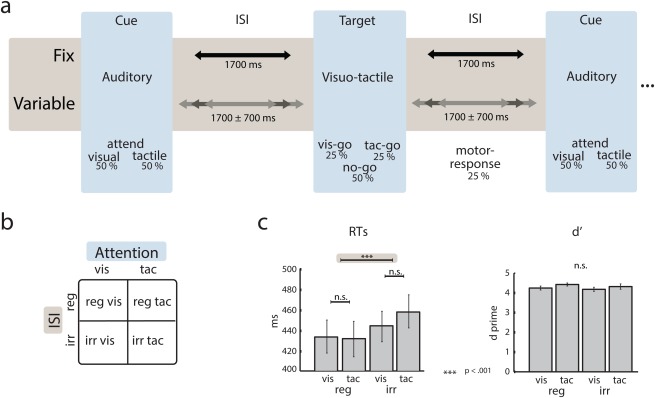
Illustration of the different experimental conditions and behavioral data. (a) Stimulus train in the regular (upper timeline) and irregular (lower timeline) ISI condition. Auditory cues were followed by an ISI of regular or irregular duration. Following the ISI, a visuo‐tactile stimulus was presented. This stimulus was always bisensory but was either constituting a visual target, a tactile target, or a standard. Participants were instructed to respond with a button press only if a target appeared in the cued modality. The focus of the EEG data analysis was on the interval preceding the visuo‐tactile stimulus. (b) Illustration of the 2 × 2 factorial study design. (c) Median reaction times (left barplot) and d′ values (right barplot) for all four conditions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Visuo‐Tactile Stimuli
All combined visuo‐tactile stimuli were presented for 150 ms. Visual inputs were presented on a tilted thin‐film transistor (TFT) monitor at 40 cm distance from the eyes with a monochromatic neutral gray background (mean luminance of 30 cd/m2). Throughout experimental blocks, participants were instructed to focus on a central black fixation cross on the screen. In trials with standards and tactile targets, the visual input comprised of a centrally presented black and white Gabor patch with vertical gratings (diameter: 5.75°, spatial frequency = 1 cycle per degree, Gaussian standard deviation = 2°). In trials with visual targets, the same Gabor patch was presented but it flickered at a frequency of 16.7 Hz. Tactile inputs were delivered to the index finger of the left hand by a 16‐dot piezoelectric Braille display (4 × 4 quadratic matrix, 2.5 mm spacing; QuaeroSys, St. Johann, Germany). The Braille display was attached centrally to the backside of the TFT monitor so that it matched with the location of the visual inputs (Fig. 1). In trials with standards and visual targets, the tactile input comprised of a single elevation that lasted for 150 ms. In trials featuring tactile targets, all pins elevated and contracted at a frequency of 16.7 Hz. To mask the clicking noise by the Braille display during tactile stimulation, auditory white noise (150 ms duration) was presented via in‐ear air‐pressure headphones simultaneously with each visuo‐tactile stimulus. Participants consistently reported that they were unable to hear the noise of the Braille display during stimulation.
Localizer Task
The reason for obtaining EEG data from a localizer task was to select regions of interest (ROIs) in sensory cortices for the analysis of neural oscillations in the main experiment (Fig. 3). The localizer task consisted of 120 unisensory visual and 120 unisensory tactile stimuli, which were presented blockwise at ISIs of 1,300 ms. Visual stimuli were identical to the visual components of the standards in the main experiment. Tactile stimuli were identical to the tactile components of the standards. To mask the noise by the Braille display, continuous white noise was presented via a battery‐driven speaker throughout the entire localizer task. The source‐localized EEG responses to tactile stimuli showed no signs of auditory responses, suggesting that the masking was successful. Participants were instructed to attend to the stimuli and to focus at a central fixation cross, but they did not perform a task. The use of a localizer task for the ROI selection had two major advantages: first, it allowed for ROI selection independent of the experimental manipulations in the main study. Second, stimuli in the main experiment comprised of simultaneously presented visual and tactile components, which hinders the source localization of the separate components.
Figure 3.
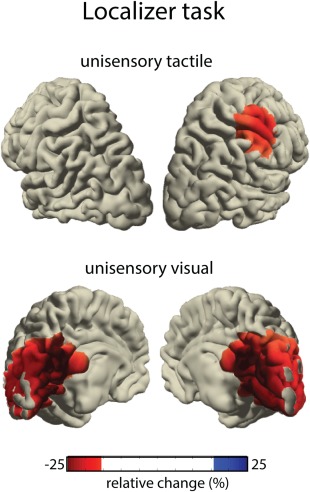
Source localized responses to unisensory tactile (top) and unisensory visual (bottom) stimuli in the localizer task. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
EEG Recordings and Data Preprocessing
EEG data were collected in a sound‐proof electrically shielded chamber using a high‐density 128‐electrodes system (Easycap, Falk Minow services, Herrsching, Germany). To monitor eye movements, two electrodes were placed at the medial upper and lateral border of the right ocular orbit. Recordings were made against nose reference with a passband of 0.016–250 Hz and digitized at a sampling rate of 1,000 Hz. All offline data processing was done using MATLAB (The MathWorks, Natick, MA), EEGLAB [http://www.sccn.ucsd.edu/eeglab; Delorme and Makeig, 2004], and FieldTrip [http://www.ru.nl/fcdonders/fieldtrip; Oostenveld et al., 2011]. Data were offline bandpass filtered (finite impulse response filter) between 0.3 and 125 Hz, downsampled to 500 Hz and re‐referenced to common average. An additional narrow‐band notch filter (49.8–50.2 Hz, fourth‐order two‐pass Butterworth filter) was applied to remove line noise. Trials containing muscle and technical artifacts were rejected by visual inspection. On average, 10.5% of trials were rejected. Electrodes with extremely high‐ and/or low‐frequency artifacts throughout the recording (M = 2.4 SD = 1.1) were linearly interpolated using a model of the amplitude topography at the unit sphere surface based on all nonartifactual electrodes [Perrin et al., 1989]. To reduce artifacts such as eye‐blinks, horizontal eye movements, electrocardiographic activity, as well as artifacts induced by the Braille display, an independent component analysis approach was applied [extended Runica; Lee et al., 1999]. Components representing artifacts were removed from the EEG data by back‐projecting all but these components [mean: 16.7; Schneider et al., 2008].
Analysis of Oscillatory Responses
For the analysis of oscillatory responses in the main experiment, we selected ROIs based on the findings from the localizer task. To this end, evoked responses from the unisensory‐visual and unisensory‐tactile stimuli of the localizer task were projected into source space using a linearly constrained minimum variance (LCMV) beamformer algorithm [Van Veen et al., 1997]. This was done for a poststimulus interval between 100 and 300 ms, with a −300 to −100 ms baseline. To compensate for potential rank reduction during preprocessing, the lambda regularization‐parameter was set to 5%. Using these parameters, responses to unisensory‐visual and unisensory‐tactile stimuli were localized to the visual cortex and to the right somatosensory hand area, respectively, (Fig. 3). Based on these activations, matching ROIs in the visual and right somatosensory hand areas were selected from the BrainMap atlas [Fox et al., 1994]. Additionally, a third ROI comprising the left motor cortex (i.e., contralateral to the response hand) was selected [standard “motor area” from the BrainMap atlas; Fox et al., 1994]. This region was selected because the motor cortex has recently been suggested to play an important role in temporal processing [Arnal, 2012; Coull et al., 2004; Fujioka et al., 2012]. In the next step of the analysis, a set of virtual electrodes [Keil et al., 2014] corresponding to the ROIs was calculated from a three‐dimensional grid covering the entire brain volume (resolution: 1 cm). Due to the different sizes of the ROIs, the visual ROI comprised of 11 virtual electrodes, the right hand ROI of 4 electrodes, and the left motor ROI of 90 electrodes. To project raw data onto the virtual electrodes, they were multiplied with accordant spatial filters. This was done separately for each participant and grid point using a realistic three‐shell boundary‐element volume conduction model based on the MNI standard brain (MNI; http://www.mni.mcgill.ca). Using a LCMV beamformer [Van Veen et al., 1997], a common filter was constructed across all conditions from the covariance matrix of the averaged single trials at sensor level and the respective leadfield. The filter was calculated for an interval ranging from −700 to 700 and from −1,200 to 200 ms around cue and visuo‐tactile stimulus onset, respectively. Note that this interval is longer than the one used for the final statistical analysis. This was done to provide the beamformer with more data for a more accurate source estimation, as well as to avoid zero‐padding and increase the frequency resolution of the time‐frequency transformation. The lambda regularization‐parameter was set to 5%. Next, single trials were projected through these filters separately for each subject, condition, and virtual electrode. For the analysis of oscillatory power, the data at virtual electrodes were transformed into time–frequency domain by applying a sliding window Fourier transform with a single Hanning taper. Power at frequencies from 2 to 35 Hz was computed in 0.5 Hz steps, using a fixed time window (t = 400 ms) and a fixed frequency smoothing (f = 2.5 Hz). Total power in the interval ranging from −1,200 before to 200 ms after visuo‐tactile onset was normalized relative to the interval from −400 to −200 ms before the onset of the cue. Thus, the precue interval served as baseline in the normalization of power:
For the analysis of intertrial phase coherence (ITC), we computed complex Fourier‐spectra using sliding window Fourier transform with a single Hanning taper. In line with results from previous studies [Besle et al., 2011; Cravo et al., 2013; Stefanics et al., 2010], the analysis of phase coherence focused on slow‐wave oscillations in the delta band. Fourier values at frequencies from 2 to 4 Hz were computed in 0.5 Hz steps using a fixed time window (t = 400 ms) and a fixed frequency smoothing (f = 2.5 Hz). Complex Fourier spectra for each time point, frequency, and trial were amplitude‐normalized by dividing them by their absolute values [e.g., Cheron et al., 2007]. ITC was calculated as the mean normalized complex Fourier spectrum across time, frequency, and trials according to the formula
Statistical Analysis of Behavioral Data
Prior to the analysis, outlier trials with RTs above or below three standard deviations of the individual subject and condition mean were removed from the behavioral data. Median RTs were then calculated for each participant and condition. To compare perceptual sensitivity between conditions, d′ values were computed [Green and Swets, 1966] using the formula
with z(P(Y|s)) being the z‐score of the hit rate, and z(P(Y|n)) being the z‐score of the false alarm rate. Because the z transform reaches infinity when percentages are equal to 0 or 100, datasets with values of 0 and 100% were assigned values of 1 and 99%, respectively, [Macmillan and Creelman, 2005]. Finally, RTs and d′ values were compared between the experimental conditions using two‐way repeated measures analysis of variance (ANOVA) with the factors ISI (regular vs. irrregular) and Attention (attend‐visual vs. attend‐tactile).
Statistical Analysis of Neural Oscillations
Time frequency power values were averaged across virtual electrodes separately for each of the three ROIs. In agreement with previous studies on intersensory attention [Bauer et al., 2012; Foxe et al., 1998; Fu et al., 2001; Trenner et al., 2008] and temporal orienting [Cravo et al., 2011, 2013; Fujioka et al., 2009, 2012] our analysis focused on effects in DBA, ABA, and BBA. In the present study, neural oscillations were investigated in an interval ranging from −800 to −200 ms prior to the visuo‐tactile stimulus. This interval was selected to avoid temporal smearing of evoked activity by cue and stimulus onsets during time‐frequency transformation. Across participants DBA, ABA, and BBA were calculated as the mean power during the selected interval separately for each condition and ROI. The power values were then submitted to three‐way repeated measures ANOVAs with the factors ISI (regular vs. irregular), Attention (attend‐visual vs. attend‐tactile), and ROI (visual ROI vs. somatosensory ROI vs. motor ROI). Significant interactions including the factor ROI were followed up by two‐way ANOVAs with the factors ISI and Attention, separately for each ROI. To examine effects in phase coherence between conditions, ITC values were subjected to a three‐way repeated measures ANOVAs with the factors ISI (regular vs. irregular), Attention (attend‐visual vs. attend‐tactile), and ROI (visual vs. somatosensory vs. motor). Significant interactions including the factor ROI were followed up by two‐way ANOVAs with the factors ISI and Attention, separately for each ROI. For both power and ITC, P‐values from first level and follow‐up ANOVAs were Bonferroni adjusted to correct for multiple comparisons (P‐values below 0.0166 were deemed statistically significant).
RESULTS
Behavioral Data
Figure 2c illustrates RTs and d′ values for the four experimental conditions. The two‐way ANOVA for RTs with the factors Attention (attend‐visual vs. attend‐tactile) and ISI (regular vs. irregular) revealed a significant main effect of ISI (F (1,15) = 21.19, P < 0.001). Irrespective of the cued modality, RTs were shorter when stimuli were presented at regular compared with irregular ISIs. This shows that participants had a response advantage when stimuli were presented in a rhythmic fashion (i.e., with regular ISIs). Additionally, a significant interaction between the factors Attention × ISI was found (F (1,15) = 5.41, P = 0.034), indicating that the effect of temporal orienting was slightly larger for tactile compared to visual targets. The two‐way ANOVA for d′ values did not reveal any significant main effects or interactions (all P‐values > 0.11). This suggests that the task difficulty was comparable across conditions.
Effects of Intersensory Attention and Temporal Orienting on Oscillatory Power
In line with previous studies [Bauer et al., 2012; Besle et al., 2011], our time‐frequency analyses revealed activity modulations by anticipatory attention in all three ROIs (Figs. 4, 5, 6, 7). In the visual ROI (Fig. 4), an attend‐tactile cue led to an increase in ABA, whereas an attend‐visual cue caused a robust suppression in ABA. A similar pattern emerged in the delta and beta band, although there was no increase in DBA or BBA following the attend‐visual cue. In the somatosensory ROI (i.e., contralateral to the tactile stimulation site), a robust suppression of BBA was observed (Fig. 5). The BBA suppression was stronger in the attend‐tactile compared with the attend‐visual condition. In addition, the BBA suppression was stronger in the irregular ISI compared with the regular ISI condition. Finally, in the motor ROI (i.e., contralateral to the response hand), a robust suppression of BBA was found (Fig. 6). The suppression was stronger in the irregular compared with the regular ISI condition. For the statistical analysis, Bonferroni‐corrected three‐way ANOVAs with the factors ISI (regular vs. irregular), Attention (attend‐visual vs. attend‐tactile), and ROI (visual vs. somatosensory vs. motor) were calculated for each frequency band. Figure 8 provides an overview of the main statistical findings, which are described in detail in what follows.
Figure 4.
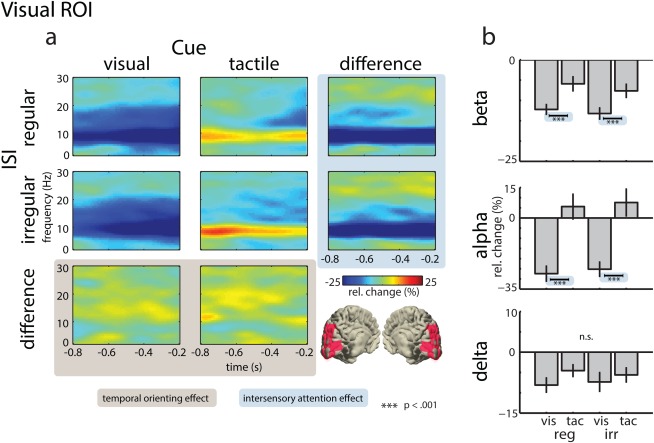
Power of neural oscillations in the visual ROI. (a) Time‐frequency representations of virtual electrodes for the visual (left panel) and tactile attention conditions (middle panel), and their difference (right panel), for the regular ISI condition (top row), the irregular ISI condition (middle row), and their difference (bottom row). The bottom right panel illustrates the location of the visual ROI in source space. (b) Mean power change (−800 to −200 ms, relative to baseline) for BBA (13–30 Hz), ABA (8–12 Hz), and DBA (2–4 Hz). Effects of temporal orienting are highlighted in ocher and effects of intersensory attention are highlighted in blue. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 5.
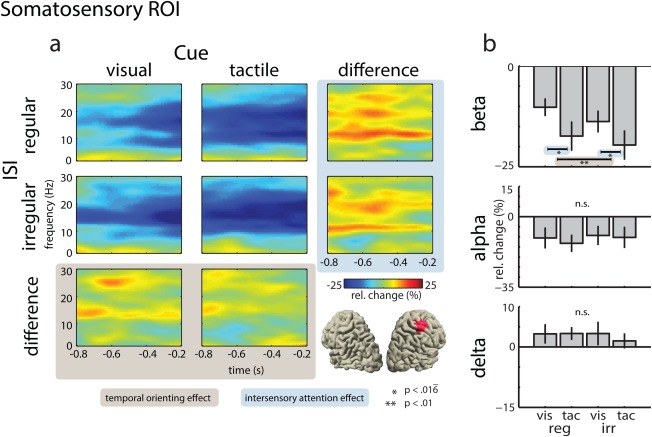
Power of neural oscillations in the somatosensory ROI. (a) Time‐frequency representations of virtual electrodes for the visual (left panel) and tactile attention conditions (middle panel), and their difference (right panel), for the regular ISI condition (top row), the irregular ISI condition (middle row), and their difference (bottom row). The bottom right panel illustrates the location of the somatosensory ROI in source space. (b) Mean power change for BBA, ABA, and DBA. Effects of temporal orienting are highlighted in ocher and effects of intersensory attention are highlighted in blue. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 6.
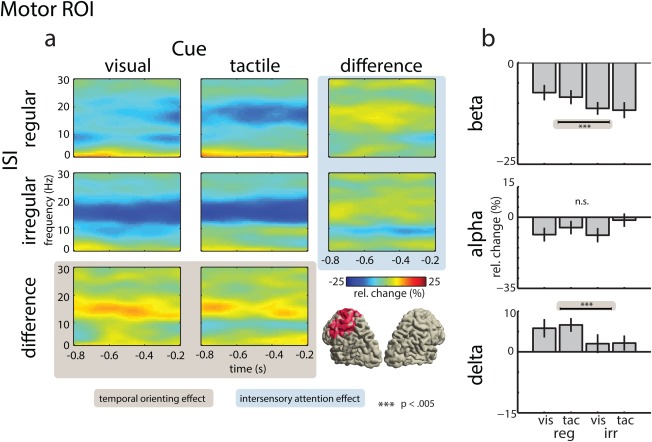
Power of neural oscillations in the motor ROI. (a) Time‐frequency representations of virtual electrodes for the visual (left panel) and tactile attention conditions (middle panel), and their difference (right panel), for the regular ISI condition (top row), the irregular ISI condition (middle row), and their difference (bottom row). The bottom right panel illustrates the location of the motor ROI in source space. (b) Mean power change for BBA, ABA, and DBA. Effects of temporal orienting are highlighted in ocher and effects of intersensory attention are highlighted in blue. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 7.
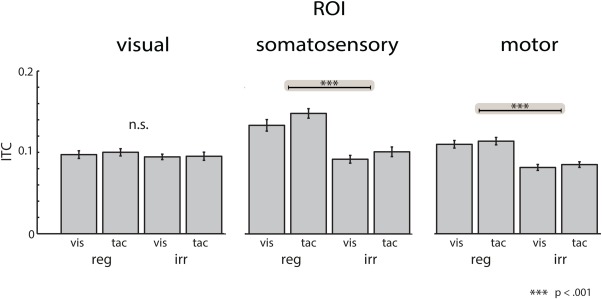
Experimental effects on intertrial coherence. Delta‐band (2–4 Hz) intertrial coherence during the prestimulus interval at the visual (left column), somatosensory (middle column) and motor ROI (right column).
Figure 8.
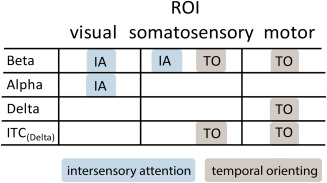
Overview of statistically significant findings. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Delta‐Band Effects
A three‐way ANOVA with the factors ISI (regular vs. irregular), Attention (attend‐visual vs. attend‐tactile), and ROI (visual vs. somatosensory vs. motor) was calculated to investigate the effects of anticipatory attention on delta‐band power. The ANOVA revealed a main effect for the factor ROI (F (1,15) = 46.94, P < 0.0001). Although there was no significant interaction including the factor ROI, for exploratory purposes we conducted follow‐up ANOVAs using the factors ISI and Attention, separately for each ROI. For the motor ROI, the ANOVA yielded a robust main effect of ISI (F (1,15) = 16.72, P < 0.001), due to larger DBA in the regular compared with the irregular condition. No significant effects were found at the visual and somatosensory ROI. Thus, in agreement with previous studies [Besle et al., 2011; Saleh et al., 2010], this analysis showed that temporal orienting does modulate DBA in motor areas.
Alpha‐Band Effects
A three‐way ANOVA with the factors ISI (regular vs. irregular), Attention (attend‐visual vs. attend‐tactile), and ROI (visual vs. somatosensory vs. motor) was calculated to investigate the effects of anticipatory attention on alpha‐band power. The ANOVA revealed a significant main effect of Attention (F (1,15) = 9.09, P < 0.009), due to stronger suppression of ABA in the attend‐visual compared with the attend‐tactile condition. Moreover, a significant interaction between Attention × ROI was found (F (2,30) = 22.51, P < 0.0001). To further disentangle the effects of attention and ISI, planned follow‐up two‐way ANOVAs with the factors ISI and Attention were calculated separately for the three ROIs. The ANOVA for the visual ROI yielded a significant main effect of Attention (F (1,15) = 28.8, P < 0.0001), indicating that the suppression of ABA was larger in the attend‐visual than in the attend‐tactile condition. No other significant main effects or interactions were found for the other ROIs and in relation to the factor ISI.
Beta‐Band Effects
Finally, a three‐way ANOVA with the factors ISI (regular vs. irregular), Attention (attend‐visual vs. attend‐tactile), and ROI (visual vs. somatosensory vs. motor) was calculated to investigate the effects of anticipatory attention on beta‐band power. The ANOVA revealed significant main effects of ROI (F (2,30) = 19.30, P < 0.0005) and ISI (F (1,15) = 6.78, P < 0.0037), indicating stronger BBA suppression in the irregular compared with the regular ISI condition. Furthermore, a significant interaction between Attention and ROI was found (F (2,30) = 16.64, P < 0.0001). The follow‐up two‐way ANOVAs for the visual ROI revealed a significant main effect of Attention (F (1,15) < 26.01, P < 0.0001), due to a stronger suppression in the attend‐visual compared with the attend‐tactile condition. The follow‐up ANOVA for the somatosensory ROI revealed a significant main effect of Attention (F (1,15) = 7.66, P < 0.014), due to stronger suppression in the attend‐tactile compared with the attend‐visual condition, that is, an effect in the opposite direction from the one found at the visual ROI. Furthermore, a significant main effect of ISI was observed (F (1,15) = 9.25, P < 0.0083). In the somatosensory ROI, the suppression of BBA was stronger in the irregular compared with the regular ISI condition. Finally, the follow‐up ANOVA for the motor ROI yielded a significant main effect of ISI (F (1,15) = 14.59, P < 0.0017), indicating a stronger suppression of BBA power in the irregular compared with the regular ISI condition. Interestingly, no significant effects of intersensory attention were found in the motor ROI, suggesting that oscillatory activity in this region primarily reflects target anticipation due to stimulus regularity.
As the regular and irregular conditions also differed with regard to the temporal predictability of the cue, differences in oscillatory power might already be present in the precue interval. As the precue interval served as the baseline for the present analyses, possible differences in this interval could have contributed to the present findings. Therefore, we compared the power in the nonbaseline corrected interval from −400 to −200 ms before cue onset (see Supporting Information Fig. 1), for each ROI and each frequency, using dependent t‐tests (regular vs. irregular conditions). These tests did not yield any significant differences (all P‐values > 0.19, uncorrected). Thus, it is likely that precue baseline differences did not substantially contribute to the present findings.
Effects of Intersensory Attention and Temporal Orienting on Intertrial Coherence
As the phase of low‐frequency oscillations has previously been related to attentional selection [Besle et al., 2011; Schroeder and Lakatos, 2009], we investigated the effect of intersensory attention and temporal orienting on delta‐band ITC. Figure 7 illustrates the prestimulus ITC at the three ROIs. At the motor and the somatosensory ROI, ITC was stronger for the regular compared with the irregular ISI condition. By contrast, no such difference was found at the visual ROI. Importantly, the timecourse of delta‐band ITC differed from the timecourse of delta‐band power (Supporting Information Figs. 2 and 3). This shows that delta‐band ITC reflects, at least to some extent, different processing mechanisms than delta‐band power. The three‐way ANOVA for delta‐band ITC with the factors ISI, Attention, and ROI revealed a significant main effect for ISI (F (1,15) = 32.42, P < 0.001). Across ROIs, the ITC was stronger in the regular ISI compared with the irregular ISI condition. An additional main effect of ROI was found (F (1,30) = 17.29, P < 0.001), indicating stronger ITC in the somatosensory and motor ROIs compared with the visual ROI. Although no significant interactions in relation to the factor ROI were observed, Figure 7 indicates clear differences in ITC between the regular and irregular ISI conditions in the somatosensory and motor ROI but not in the visual ROI (see also Supporting Information Fig. 2). For exploratory purposes, we calculated follow‐up ANOVAs separately for the three ROIs. The ANOVAs for the somatosensory ROI yielded a significant main effect of ISI (F (1,15) = 18.42, P < 0.0007), due to stronger phase coherence in the regular compared with the irregular ISI condition. The follow‐up ANOVA at the motor ROI yielded a significant main effect of ISI (F (1,15) = 24.47, P < 0.0002), due to stronger ITC in the regular compared with the irregular ISI condition. No other effects were found and no significant main effects or interactions were observed in either ANOVA for the visual ROI.
It could be argued that the observed differences in ITC between the regular and irregular conditions are due to differences in cue processing, as not only targets but also cues differed with respect to predictability. To investigate this issue, we calculated additional ITC values for the postcue interval (200–800 ms after cue onset). In this interval, we found no significant differences in ITC between the regular and irregular conditions (all P‐values > 0.21). This suggests that the observed ITC effects develop later and are functionally related to the expectancy of an upcoming target. Furthermore, it is possible that the ITC in the irregular condition could be affected by cue evoked activity (at least in trials with short ISI). However, we consider this is unlikely for several reasons: first, the preceding auditory cue elicits a response that should be localized in bilateral auditory areas. Our findings in somatosensory and motor areas are strongly lateralized, with larger absolute ITC values and larger effects in the right (i.e., somatosensory) compared to the left (i.e., motor) ROI. Second, if cue‐evoked responses would have influenced ITC in the irregular condition, this influence should be strongest at the beginning of the analysis window, and should then decline over time. In our data, the ITC differences remained constant throughout the prestimulus interval (Supporting Information Fig. 2). Third, if ITC would have been influenced by evoked activity in the irregular ISI condition, ITC values would be expected to also differ in response to the cue. This is because cue‐locked responses would also be distorted by overlapping activity from the preceding target, at least in short ISI trials in the irregular condition. However, this was not the case (Supporting Information Fig. 4). Therefore, it is unlikely that cue‐evoked activity affected the observed ITC effects. Taken together, our data show that temporal orienting enhances delta‐band ITC in the somatosensory cortex and motor cortex.
DISCUSSION
We investigated, for the first time, the simultaneous deployment of intersensory attention and temporal orienting within the same task. Temporal orienting during regular compared with irregular stimulation significantly reduced RTs. At the neuronal level, we observed distinct patterns of anticipatory DBA and BBA modulations in sensory and motor cortices, which differentially reflected the two attentional mechanisms. Notably, we found no evidence for interactions between the two processes, suggesting that they operate in parallel and in spatially distinct sensory and motor cortices.
Temporal Orienting Facilitates Behavioral Responses to Visual and Tactile Targets
RTs were faster when stimuli were presented in a regular compared with an irregular fashion. This finding is in line with previous studies, which showed that temporal predictability in stimulus trains facilitates target processing [Coull and Nobre, 1998; Cravo et al., 2011; Zahn and Rosenthal, 1966]. The interaction between ISI and attended modality also suggests that temporal orienting was more effective in the tactile compared to the visual modality. This might be due to the higher temporal resolution of the somatosensory system [van Erp and Werkhoven, 2004]. While participants in our study were not explicitly instructed to pay attention to the temporal regularities in the stimulus trains, our behavioral results demonstrate that they automatically oriented their attention to the expected target onset.
Alpha‐ and Beta‐Band Oscillations in Sensory Cortices Reflect Intersensory Attention
In the visual cortex, we found a decrease of ABA when the visual modality was attended and an increase of ABA when the tactile modality was attended. Our source‐space findings are in line with and extend previous reports of anticipatory ABA modulations by attention at the scalp level [Bauer et al., 2012; Foxe and Snyder, 2011; Foxe et al., 1998]. The modulation in ABA has been attributed to a sensory gating mechanism, by which task irrelevant inputs are suppressed and task relevant inputs are enhanced [Kelly et al., 2006; Lopes da Silva, 1991]. Interestingly, we also found a stronger suppression of BBA in visual regions when attention was directed to the visual modality. Comparable intersensory attention effects have been found for tactile [Bauer et al., 2012; van Ede et al., 2014] and auditory stimuli [Leske et al., 2014; Mazaheri et al., 2014]. Hence, our findings suggest that anticipatory BBA modulations may serve a similar sensory gating mechanism as proposed for ABA. Thus, the effects of intersensory attention in visual cortex were reflected by a stronger anticipatory reduction in ABA and BBA when participants attended to the visual compared to the tactile modality (Fig. 4). In the somatosensory cortex, effects of intersensory attention were observed specifically in BBA. The suppression of BBA was stronger when participants attended to the tactile compared to the visual modality. Our source‐space data fit with a number of recent magnetoencephalographic studies examining BBA at the sensor level [Bauer et al., 2012; van Ede et al., 2010, 2014]. Bauer et al. [2012] reported a suppression of BBA over somatosensory areas when participants were cued to the tactile compared to the visual modality. Thus, our findings of anticipatory BBA modulations in the somatosensory cortex provide further evidence that BBA may reflect an intersensory gating mechanism. In contrast to the sensory cortices, we did not find effects of intersensory attention in the motor cortex. This observation likely relates to the fact that go‐targets in both modalities required the same motor response.
DBA and BBA in Motor and Somatosensory Cortex Reflect Temporal Orienting
Unlike intersensory attention, temporal orienting did not modulate anticipatory activity in the visual cortex. At first glance, this finding is in contrast with recent studies that showed entrainment effects of DBA [Cravo et al., 2013] and modulations of ABA [Rohenkohl and Nobre, 2011] in visual areas during temporal orienting. A reason for this discrepancy could be that our task was relatively easy (d′ > 4). Previous studies reporting temporal orienting effects in the visual cortex likely called for more elaborate sensory processing [Correa et al., 2006; Rohenkohl and Nobre, 2011; Cravo et al., 2013] rather than the speeded response required in the present experiment, which presumably led to a stronger attentional modulation in visual areas. Alternatively, it might be that stimulus timing is a more important parameter for the somatosensory and motor systems than for the visual one. This could be related to the much higher temporal resolution of the motor and somatosensory systems compared to the visual system [van Erp and Werkhoven, 2004].
In the somatosensory cortex, we observed a stronger suppression of BBA when stimuli were presented in an irregular compared with a regular fashion. This finding relates to recent electrophysiological studies that showed anticipatory modulations of BBA over sensory and motor areas during temporal orienting [Arnal et al., 2014; Cravo et al., 2011; Fujioka et al., 2012; Saleh et al., 2010; van Ede et al., 2011; see Arnal, 2012 for a review]. For instance, van Ede et al. [2011] reported a temporally specific suppression of ABA and BBA in sensorimotor cortex during anticipation of a tactile event. This suppression was strongest before the expected onset of the stimulation. Accordingly, our finding of stronger BBA suppression during temporal uncertainty in the irregular compared with the regular condition might indicate a less timed but more sustained deployment of attention.
Another main finding was that the ITC of DBA was stronger during the regular compared with the irregular condition. Increased ITC could be a marker for an attention selection mechanism that entrains the phase of ongoing slow‐wave oscillations so that the expected stimulus arrives at a state of high neuronal excitability [Lakatos et al., 2008; Schroeder and Lakatos, 2009]. Electrophysiological studies in humans have provided evidence for such a mechanism in sensory [Besle et al., 2011; Cravo et al., 2013; Gomez‐Ramirez et al., 2011; Gray et al., 2015] and motor areas [Besle et al., 2011; Saleh et al., 2010]. In the motor cortex, temporal orienting was reflected by a similar pattern as in the somatosensory cortex: BBA was more strongly suppressed when stimuli were presented in an irregular compared with a regular fashion. It is well known that suppression of BBA relates to response preparation [Kilavik et al., 2013; Pfurtscheller, 1981]. Accordingly, in our study, the stronger BBA suppression in the irregular condition likely reflects enhanced preparatory engagement of motor areas due to temporal uncertainty. By contrast, knowledge about the temporal onset of stimuli in the regular condition results in a less energy‐demanding state, as reflected by an overall reduced suppression of BBA. Finally, we observed an increase in power and ITC of DBA in motor cortex. As in the somatosensory cortex, we suggest that this reflects entrainment of DBA to the temporal properties of the stimulation. We would like to emphasize that the difference between regular and irregular conditions was much stronger in the motor than in the somatosensory cortex. This supports the recently proposed crucial role of neural oscillations in motor areas for temporal orienting [Arnal, 2012; Arnal et al., 2014]. It could be argued that the finding of temporal orienting effects in right somatosensory areas is, at least partially, explained by volume conduction from left motor cortex activity. However, there are reasons why it is unlikely that volume conduction substantially contributed to the present finding. First, while the effect of temporal orienting on BBA was more pronounced in motor cortex, the overall suppression of BBA was stronger in somatosensory cortex. Contrary to this finding, previous studies reported stronger BBA suppression during motor preparation in the contralateral hemisphere [Doyle et al., 2005; Kilner et al., 2005]. Second, if volume conduction would have contributed to the present results, it should also cause the intersensory attention effect, which was found in the right somatosensory ROI, to be additionally found in left motor ROI. As this was not the case, we argue that volume conduction does not explain the current results. Finally, the pattern of DBA modulation was clearly different in the motor compared to the somatosensory cortex. While temporal orienting led to a power increase in motor cortex, no differences between regular and irregular conditions were found in somatosensory cortex.
Intersensory Attention and Temporal Orienting Operate in Parallel in Sensory and Motor Cortex
The analysis of neural oscillations in the different ROIs and frequency bands did not reveal significant interactions between the two types of attention. Although null results should be interpreted cautiously, we suggest that the complete absence of interactions indicates that intersensory attention and temporal orienting operate, to a substantial degree, independently of each other in separate sensory and motor cortices. Moreover, the lack of interactions is in line with studies that suggest the existence of a supramodal temporal orienting mechanism [Bolger et al., 2013; Lange and Röder, 2006; Lunghi et al., 2014] which facilitates behavioral responses independent of the sensory modality. Interestingly, recent studies revealed that another attentional mechanism, namely spatial attention, interacts with both intersensory attention [Banerjee et al., 2011; Bauer et al., 2012; Rohenkohl et al., 2014; van Ede et al., 2010] and temporal orienting [Doherty et al., 2005; van Ede et al., 2011]. For example, Banerjee et al. [2011] showed that, while both auditory and visual spatial attention modulate ABA, this modulation shows a clear modality‐specific topographical distribution. As spatial attention and intersensory attention both involve modulations of ABA and BBA in sensory areas [Bauer et al., 2012], this might facilitate interactions between the two mechanisms. By contrast, temporal orienting also involves modulations of slow‐wave DBA [Besle et al., 2011; Cravo et al., 2013; Saleh et al., 2010], which often operates on a global brain‐wide rather than local scale [Buzsáki, 2006]. Whether these two mechanisms of attention interact in other cortical areas, such as prefrontal cortex, remains to be elucidated.
CONCLUSION
We investigated intersensory visuo‐tactile attention and temporal orienting in the same experimental paradigm. We found that a combination of anticipatory BBA power and DBA power and phase modulations reflects both attention mechanisms. Effects of intersensory attention were observed in visual and somatosensory cortex, whereas effects of temporal orienting were found in motor areas and somatosensory cortex. Our data show that oscillatory responses in the delta and alpha band simultaneously encode separate attentional task demands. Despite their similar function and partially overlapping neural signatures, we found no interactions between intersensory attention and temporal orienting. This study provides compelling evidence that spectrally and anatomically distinct patterns of neuronal activity encode intersensory attention and temporal orienting in a largely independent manner.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors would like to thank Ivan Prikhodko for his help in implementing the experimental setup. The authors are also thankful to two anonymous reviewers for their helpful input.
REFERENCES
- Arnal LH (2012): Predicting “when” using the motor system's beta‐band oscillations. Front Hum Neurosci 6:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal LH, Giraud A‐L (2012): Cortical oscillations and sensory predictions. Trends Cogn Sci 16:390–398. [DOI] [PubMed] [Google Scholar]
- Arnal LH, Doelling KB, Poeppel D (2014): Delta‐beta coupled oscillations underlie temporal prediction accuracy. Cereb Cortex. doi: 10.1093/cercor/bhu103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Snyder AC, Molholm S, Foxe JJ (2011): Oscillatory alpha‐band mechanisms and the deployment of spatial attention to anticipated auditory and visual target locations: Supramodal or sensory‐specific control mechanisms? J Neurosci 31:9923–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Kennett S, Driver J (2012): Attentional selection of location and modality in vision and touch modulates low‐frequency activity in associated sensory cortices. J Neurophysiol 107:2342–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besle J, Schevon CA, Mehta AD, Lakatos P, Goodman RR, Mckhann GM, Emerson RG, Schroeder CE (2011): Tuning of the human neocortex to the temporal dynamics of attended events. J Neurosci 31:3176–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger D, Trost W, Schön D (2013): Rhythm implicitly affects temporal orienting of attention across modalities. Acta Psychol 142:238–244. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2006): Rhythms of the Brain. New York, New York: Oxford University Press. [Google Scholar]
- Cheron G, Cebolla A, De Saedeleer C, Bengoetxea A, Leurs F, Leroy A, Dan B (2007): Pure phase‐locking of beta/gamma oscillation contributes to the n30 frontal component of somatosensory evoked potentials. BMC Neurosci 8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa Á, Lupiáñez J, Madrid E, Tudela P (2006): Temporal attention enhances early visual processing: A review and new evidence from event‐related potentials. Brain Res 1076:116–128. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC (1998): Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. J Neurosci 18:7426–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, Macar F (2004): Functional anatomy of the attentional modulation of time estimation. Science 303:1506–1508. [DOI] [PubMed] [Google Scholar]
- Cravo AM, Rohenkohl G, Wyart V, Nobre AC (2011): Endogenous modulation of low frequency oscillations by temporal expectations. J Neurophysiol 106:2964–2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravo AM, Rohenkohl G, Wyart V, Nobre AC (2013): Temporal expectation enhances contrast sensitivity by phase entrainment of low‐frequency oscillations in visual cortex. J Neurosci 33:4002–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong R, Toffanin P, Harbers M (2010): Dynamic crossmodal links revealed by steady‐state responses in auditory‐visual divided attention. Int J Psychophysiol 75:3–15. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S (2004): EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. [DOI] [PubMed] [Google Scholar]
- Doherty JR, Rao A, Mesulam MM, Nobre AC (2005): Synergistic effect of combined temporal and spatial expectations on visual attention. J Neurosci 25:8259–8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LMF, Yarrow K, Brown P (2005): Lateralization of event‐related beta desynchronization in the EEG during pre‐cued reaction time tasks. Clin Neurophysiol 116:1879–1888. [DOI] [PubMed] [Google Scholar]
- Fox P, Mikiten S, Davis G, Lancaster J (1994): BrainMap: A database of human function brain mapping Functional Neuroimaging: Technical Foundations. Waltham, Massachusetts: Academic Press. [Google Scholar]
- Foxe JJ, Snyder AC (2011): The role of alpha‐band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol 2:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP (1998): Parieto‐occipital approximately 10 hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport 9:3929–3933. [DOI] [PubMed] [Google Scholar]
- Fu KG, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE (2001): Attention‐dependent suppression of distracter visual input can be cross‐modally cued as indexed by anticipatory parieto‐occipital alpha‐band oscillations. Cogn Brain Res 12:145–152. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Trainor LJ, Large EW, Ross B (2009): Beta and gamma rhythms in human auditory cortex during musical beat processing. Ann N Y Acad Sci 1169:89–92. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Trainor LJ, Large EW, Ross B (2012): Internalized timing of isochronous sounds is represented in neuromagnetic beta oscillations. J Neurosci 32:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez‐Ramirez M, Kelly SP, Molholm S, Sehatpour P, Schwartz TH, Foxe JJ (2011): Oscillatory sensory selection mechanisms during intersensory attention to rhythmic auditory and visual inputs: A human electrocorticographic investigation. J Neurosci 31:18556–18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Frey HP, Wilson TJ, Foxe JJ (2015): Oscillatory recruitment of bilateral visual cortex during spatial attention to competing rhythmic inputs. J Neurosci 35:5489–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA (1966): Signal Detection Theory and Psychophysics. New York: Wiley. [Google Scholar]
- Jones MR, Moynihan H, Mackenzie N, Puente J (2002): Temporal aspects of stimulus‐driven attending in dynamic arrays. Psychological science. 13:313–319. [DOI] [PubMed] [Google Scholar]
- Keil J, Müller N, Hartmann T, Weisz N (2014): Prestimulus beta power and phase synchrony influence the sound‐induced flash illusion. Cereb Cortex 24:1278–1288. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ (2006): Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol 95:3844–3851. [DOI] [PubMed] [Google Scholar]
- Kilavik E, Zaepffel M, Brovelli A, Mackay WA, Riehle A (2013): The ups and downs of beta oscillations in sensorimotor cortex. Exp Neurol 245:15–26. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Bott L, Posada A (2005): Modulations in the degree of synchronization during ongoing oscillatory activity in the human brain. Eur J Neurosci 21:2547–2554. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE (2008): Entrainment of neuronal oscillations as a mechanism of attentional selection. Science 320:110 [DOI] [PubMed] [Google Scholar]
- Lange K, Röder B (2006): Orienting attention to points in time improves stimulus processing both within and across modalities. J Cogn Neurosci 18:715–729. [DOI] [PubMed] [Google Scholar]
- Lee TW, Girolami M, Sejnowski TJ (1999): Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput 11:417–441. [DOI] [PubMed] [Google Scholar]
- Leske S, Tse A, Oosterhof NN, Hartmann T, Müller N, Keil J, Weisz N (2014): The strength of alpha and beta oscillations parametrically scale with the strength of an illusory auditory percept. Neuroimage 88:69–78. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F (1991): Neural mechanisms underlying brain waves: From neural membranes to networks. Electroencephalogr Clin Neurophysiol 79:81–93. [DOI] [PubMed] [Google Scholar]
- Lunghi C, Morrone MC, Alais D (2014): Auditory and tactile signals combine to influence vision during binocular rivalry. J Neurosci 34:784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD (2005): Detection Theory: A User's Guide, 2nd ed Mahwah, New Jersey: Psychology Press. [Google Scholar]
- Mattler U, Lugt A, Van Der Münte TF (2006): Combined expectancies: Electrophysiological evidence for the adjustment of expectancy effects. BMC Neurosci 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Schouwenburg MR, Van Dimitrijevic A, Denys D, Cools R Jensen O (2014): Region‐specific modulations in oscillatory alpha activity serve to facilitate processing in the visual and auditory modalities. Neuroimage 87:356–362. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Wilding EL, Coull JT, Nobre AC (1999): Orienting attention in time modulation of brain potentials. Brain 122:1507–1518. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Correa A, Coull JT (2007): The hazards of time. Curr Opin Neurobiol 17:465–470. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J‐M (2011. ): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF (1989): Spherical splines for scalp potential and current density mapping. Electroencephalogr Clin Neurophysiol 72:184–187. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G (1981): Central beta rhythm during sensorimotor activities in man. Electroencephalogr Clin Neurophysiol 51:253–264. [DOI] [PubMed] [Google Scholar]
- Rohenkohl G, Nobre AC (2011): α oscillations related to anticipatory attention follow temporal expectations. J Neurosci 31:14076–14084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohenkohl G, Cravo AM, Wyart V, Nobre AC (2012): Temporal expectation improves the quality of sensory information. J Neurosci 32:8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohenkohl G, Gould IC, Nobre AC (2014): Combining spatial and temporal expectations to improve visual perception. J Vision 14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Reimer J, Penn R, Ojakangas CL, Hatsopoulos NG (2010): Fast and slow oscillations in human primary motor cortex predict oncoming behaviorally relevant cues. Neuron 65:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria D, Correa Á (2013): Electrophysiological evidence of temporal preparation driven by rhythms in audition. Biol Psychol 92:98–105. [DOI] [PubMed] [Google Scholar]
- Schneider TR, Debener S, Oostenveld R, Engel AK (2008): Enhanced EEG gamma‐band activity reflects multisensory semantic matching in visual‐to‐auditory object priming. Neuroimage 42:1244–1254. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P (2009): Low‐frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence C, Driver J (1997): On measuring selective attention to an expected sensory modality. Percept Psychophys 59:389–403. [DOI] [PubMed] [Google Scholar]
- Stefanics G, Hangya B, Hernádi I, Winkler I, Lakatos P, Ulbert I (2010): Phase entrainment of human delta oscillations can mediate the effects of expectation on reaction speed. J Neurosci 30:13578–13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsma D, Senkowski D, Soto‐Faraco S, Woldorff MG (2010): The multifaceted interplay between attention and multisensory integration. Trends Cogn Sci 14:400–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenner MU, Heekeren HR, Bauer M, Rössner K, Wenzel R, Villringer A, Fahle M (2008): What happens in between? Human oscillatory brain activity related to crossmodal spatial cueing. PLoS One 3:e1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, Jensen O, Maris E (2010): Tactile expectation modulates pre‐stimulus β‐band oscillations in human sensorimotor cortex. Neuroimage 51:867–876. [DOI] [PubMed] [Google Scholar]
- van Ede F, de Lange F, Jensen o, Maris E (2011): Orienting attention to an upcoming tactile event involves a spatially and temporally specific modulation of sensorimotor Alpha‐ and Beta‐band oscillations. J Neurosci 31:2016–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ede F, de Lange FP, Maris E (2014): Anticipation increases tactile stimulus processing in the ipsilateral primary somatosensory cortex. Cereb Cortex 24:2562–2571. [DOI] [PubMed] [Google Scholar]
- van Erp JBF, Werkhoven PJ (2004): Vibro‐tactile and visual asynchronies: Sensitivity and consistency. Perception 33:103–111. [DOI] [PubMed] [Google Scholar]
- Van Veen BD, van Drongelen W, Yuchtman M, Suzuki A (1997): Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans Biomed Eng 44:867–880. [DOI] [PubMed] [Google Scholar]
- Zahn TP, Rosenthal D (1966): Simple reaction time as a function of the relative frequency of the preparatory intervale. J Exp Psychol 72:15–19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


