Abstract
Conflicting evidence exists regarding the integrity of episodic memory in the behavioral variant of frontotemporal dementia (bvFTD). Recent converging evidence suggests that episodic memory in progressive cases of bvFTD is compromised to the same extent as in Alzheimer's disease (AD). The underlying neural substrates of these episodic memory deficits, however, likely differ contingent on dementia type. In this study we sought to elucidate the neural substrates of episodic memory performance, across recall and recognition tasks, in both patient groups using voxel‐based morphometry (VBM) analyses. We predicted that episodic memory dysfunction would be apparent in both patient groups but would relate to divergent patterns of neural atrophy specific to each dementia type. We assessed episodic memory, across verbal and visual domains, in 19 bvFTD, 18 AD patients, and 19 age‐ and education‐matched controls. Behaviorally, patient groups were indistinguishable for immediate and delayed recall, across verbal and visual domains. Whole‐brain VBM analyses revealed regions commonly implicated in episodic retrieval across groups, namely the right temporal pole, right frontal lobe, left paracingulate gyrus, and right anterior hippocampus. Divergent neural networks specific to each group were also identified. Whereas a widespread network including posterior regions such as the posterior cingulate cortex, parietal and occipital cortices was exclusively implicated in AD, the frontal and anterior temporal lobes underpinned the episodic memory deficits in bvFTD. Our results point to distinct neural changes underlying episodic memory decline specific to each dementia syndrome. Hum Brain Mapp 35:1422–1435, 2014. © 201 Wiley Periodicals, Inc.
Keywords: episodic memory, frontotemporal dementia, Alzheimer's disease, voxel‐based morphometry, medial temporal lobes, posterior cingulate cortex
INTRODUCTION
Episodic memory refers to the ability to consciously encode, store, and retrieve information about previously experienced events, and typically involves the recollection of details about the event embedded within a spatiotemporal framework [Tulving, 1983]. A long standing view holds that the hippocampus and supporting medial temporal lobe (MTL) structures are critical for episodic memory performance [Squire et al., 2004]. Converging evidence from functional neuroimaging studies, however, points to a widely distributed neural network underlying this cognitive function [reviewed by Dickerson and Eichenbaum, 2010]. This network includes the frontal lobes [Rugg et al., 2002; Blumenfeld and Ranganath, 2007], with interactions between prefrontal and temporal regions [Simons and Spiers, 2003], as well as posterior structures including the posterior cingulate cortex/precuneus, lateral parietal and temporal cortices [Buckner et al., 2008]. Importantly, human neurodegenerative disorders are associated with the progressive deterioration of interconnected brain regions, known to support complex cognitive functions, including episodic memory [Irish et al., 2012c]. Here, we investigate the neural correlates of episodic memory performance in Alzheimer's disease (AD) and the behavioral variant of frontotemporal dementia (bvFTD), two neurodegenerative conditions with contrasting clinical features underpinned by distinctive profiles of neural atrophy.
The theoretical prominence of the MTLs, in particular the hippocampus, in episodic memory is supported by studies of patients with probable AD, in which episodic memory decline is the hallmark feature [Nestor et al., 2006; McKhann et al., 2011]. Unsurprisingly, AD patients show episodic memory deficits extending across standard neuropsychological tests of visual and verbal recall [de Toledo‐Morrell et al., 2000], as well as the retrieval of personally relevant episodic autobiographical memories [Piolino et al., 2003; Irish et al., 2011b]. This loss of episodic memory in AD has been interpreted in relation to the characteristic pattern of atrophy beginning in the entorhinal cortex and progressing across the hippocampus to the neocortex [Braak and Braak, 1991], as well as the involvement of more posterior structures including the posterior cingulate cortex [Nestor et al., 2006]. Task‐related fMRI studies have confirmed that the hippocampus is connected functionally to posteromedial regions including the posterior cingulate cortex, precuneus, and lateral parietal regions [Greicius et al., 2004; Kahn et al., 2008; Salmon et al., 2008]. Accordingly, successful episodic memory performance requires coordinated and reciprocal activity between hippocampal/MTL and retrosplenial‐parietal structures [Wagner et al., 2005; Miller et al., 2008; Sperling et al., 2010]. The precuneus and posterior cingulate cortex represent sites of particular interest in episodic memory dysfunction given converging findings pointing to hypometabolism of these structures in the early stages of AD [Buckner et al., 2005; Sperling et al., 2010].
In contrast, patients with bvFTD typically present with behavioral and emotion dysfunction [Neary et al., 1998; Rankin et al., 2005; Kumfor et al., 2011], manifesting in reduced motivation and disinhibition [Piguet et al., 2011]. On a neural level, atrophy emerges first in the frontoinsular and anterior cingulate cortices, as well as the dorsomedial prefrontal cortex, striatum and thalamus [Broe et al., 2003; Schroeter et al., 2008; Seeley et al., 2008]. With disease progression, the pathological process gradually spreads into adjacent frontal and temporal regions [Rabinovici et al., 2007], with right hemisphere structures typically more affected than left. Although severe episodic memory loss represents an exclusion criterion for a diagnosis of bvFTD [Rascovsky et al., 2011], emergent evidence demonstrates that patients with progressive bvFTD can have episodic memory impairments equivalent to that seen in AD across standardized tests [Hornberger et al., 2010; Pennington et al., 2011; Hornberger and Piguet, 2012] and autobiographical memory retrieval [Irish et al., 2011a]. The neural substrates of such deficits remain unknown, however, it has been suggested that more anterior brain regions may be critical in the genesis of episodic memory dysfunction in bvFTD [Simons et al., 2002; Pennington et al., 2011] with possible additional contributions from temporal lobe structures [Söderlund et al., 2008]. Interestingly, while recall‐based processes are vulnerable in bvFTD, item recognition is proposed to remain relatively preserved [Harciarek and Jodzio, 2005].
The study of episodic memory dysfunction in neurodegenerative conditions such as AD and bvFTD represents a critical line of enquiry not only for the differential diagnosis of these dementia syndromes [Giovagnoli et al., 2008; Rascovsky et al., 2011] but also for our understanding of the neural networks responsible for episodic memory in general. Here, we sought to elucidate the neural substrates of episodic memory performance (recall and recognition) in bvFTD and AD using whole‐brain voxel‐based morphometry (VBM) analyses. Specifically, we sought to investigate the neural substrates of episodic memory dysfunction on those tasks typically used in the clinical setting for diagnostic purposes. We predicted that episodic memory dysfunction in both patient groups would relate to divergent patterns of neural atrophy contingent on dementia subtype. Specifically, in AD, we hypothesized that atrophy in the MTLs, posterior cingulate, and posterior parietal cortices would emerge as significant predictors of episodic memory performance. In contrast, we predicted that the orbitofrontal and prefrontal cortices would be more strongly implicated in episodic memory dysfunction in bvFTD.
METHODS
Participants
A total of 56 subjects participated in this study: 19 with a clinical diagnosis of behavioral variant frontotemporal dementia (bvFTD), 18 with AD, and 19 age‐ and education‐matched healthy controls, all selected from the FRONTIER database, at Neuroscience Research Australia, Sydney. All dementia patients met clinical diagnostic criteria for bvFTD [Rascovsky et al., 2011] or AD [McKhann et al., 1984; McKhann et al., 2011]. Diagnosis was established by consensus among senior neurologist (JRH), neuropsychologist, and occupational therapist based on extensive clinical investigations, cognitive assessment, carer interviews, and evidence of atrophy on structural neuroimaging. Briefly, bvFTD patients presented with insidious onset, decline in personal conduct and social functioning, displaying emotional blunting, loss of insight, and increased apathy. Only dementia patients with evidence of definite progression over time as reported by the caregivers, and atrophy on structural MRI scans were included in this study. This was to exclude potential phenocopy cases in the FTD group [Hornberger et al., 2008; Kipps et al., 2010] and to confirm the diagnosis for all cases. AD patients displayed significant episodic memory loss, in the context of preserved behavior and personality. Healthy controls were patients' family and friends, and individuals from local community clubs. All controls scored 0 on the Clinical Dementia Rating scale [Morris, 1997], and 85 or above on the Addenbrooke's Cognitive Examination‐Revised [Mioshi et al., 2006]. Exclusion criteria included prior history of mental illness, significant head injury, movement disorders, cerebrovascular disease, alcohol and other drug abuse, and limited English proficiency. Ethical approval for this study was obtained from the Southern Eastern Sydney and Illawarra Area Health Service and the University of New South Wales ethics committees. All participants, or their person responsible, provided informed consent in accordance with the Declaration of Helsinki.
Cognitive testing
Global cognitive functioning was assessed using Addenbrooke's Cognitive Examination Revised [ACE‐R; Mioshi et al., 2006], which is a sensitive and specific tool to detect cognitive impairment and dementia, consisting of subscales measuring attention and orientation, memory, fluency, language and visuospatial function. The Trail Making Test [Reitan, 1958], was administered to all participants as an index of attention, speed, and mental flexibility. Here, we were interested in participants' capacity for set‐switching and divided attention and thus used a Trails B – Trails A difference score [Strauss et al., 2006]. Verbal letter fluency [F,A,S; Strauss et al., 2006] was assessed as an index of strategic search processes. Finally, the Hayling test [Burgess and Shallice, 1997] was used to assess behavioral regulation, specifically response inhibition [Strauss et al., 2006]. Carers rated the behavioral changes of patients on the Cambridge Behavioral Inventory [CBI; Wedderburn et al., 2008], in terms of memory decline and loss of motivation.
Episodic memory testing
Following the procedure of Pennington et al. [2011], we administered a battery of verbal and visual episodic memory tests to all participants. For verbal recall and recognition, we used the Rey Auditory Verbal Learning Test [RAVLT; Schmidt, 1996]. This test consists of 15 nouns that are read aloud (List A), with an interval of 1 second between words, over five consecutive trials, each of which is followed by a free recall test. An interference list (List B) comprising 15 new words is then presented, followed by a free recall test of that list. Immediately following the recall of List B, delayed recall of List A is assessed, without further presentation of those words. Following a 30‐min delay, the participant is required to recall List A, following which a recognition trial is completed containing all items from List A and including lure words from List B and new unrelated words. We extracted the following scores from the RAVLT: immediate recall following the interference trial (maximum score: 15); delayed recall following 30 min (maximum score: 15); and recognition following 30 min (maximum score: 15).
The Rey‐Osterrieth Complex Figure [RCF; Meyers and Meyers, 1995] was administered to obtain visual recall of a complex design following a 3‐min delay. Participants were instructed to copy the complex figure as accurately as possible and, following a 3‐min delay, were required to reproduce the figure from memory. The maximum score for both the copy and recall trials is 36 points. A percentage retained score was calculated by expressing recall performance as a percentage of the initial copy score. A recognition subtest is available for the RCF task, however, this component was not administered in the current study.
Visual recognition was assessed using the Doors and People test [Part A; Baddeley et al., 1994]. The test stimuli for Part A of this task comprise 12 colored photographs of doors taken from various types of buildings (e.g., houses, barns, public buildings, churches). Participants are presented with the images, one at a time, following which the recognition test is administered, consisting of groups of four doors arranged in a 2 × 2 spatial array. The participant must indicate which of the four doors was originally presented. Importantly, the target and distractors belong to the same category, for example if the target image is a barn door, the three distractor photographs are also barn doors. One point was awarded for each successful identification of a target stimulus, leading to a maximum total score of 12 points.
Statistical analyses
Cognitive data were analyzed using PASW Statistics (Version 18.0.0). Kolmogorov‐Smirnov tests were used to determine suitability of variables for parametric analyses. Briefly, all recall and recognition experimental variables were found to be normally distributed (AD, all P values > 0.2; bvFTD, all P values > 0.2; Controls, all P values > 0.1). Multivariate analyses of variance with Sidak post hoc tests were used to explore main effects of group (Controls, bvFTD, AD) for all episodic memory tests. The rationale for using Sidak modification of the traditional Bonferroni post hoc test is that the statistical power of the analyses is not affected, whilst the flexibility of the original Bonferroni method is maintained [discussed by Keppel and Wickens, 2004]. Chi‐squared tests (X 2), based on the frequency patterns of dichotomous variables, were also used. To investigate relationships between patterns of gray matter intensity and episodic memory performance, a Recall composite score (comprising the RAVLT Immediate Recall, RAVLT delayed recall, RCF delayed recall) and a Recognition composite score (comprising RAVLT recognition and Doors Recognition) were created. The two composite scores, derived by averaging the percent correct responses of the relevant tasks, were then used as covariates in the neuroimaging analyses.
Image acquisition
VBM was used to identify gray matter volume changes across groups on a voxel‐by‐voxel basis using structural MRI data. All participants underwent whole‐brain T 1‐weighted imaging using a 3T Philips MRI scanner with standard quadrature head coil (eight channels). The 3D T 1‐weighted images were acquired using the following sequences: coronal orientation, matrix 256 × 256, 200 slices, 1 × 1 mm2 in‐plane resolution, slice thickness 1 mm, echo time/repetition time = 2.6/5.8 ms, flip angle α = 19°. MRI data were analyzed with FSL‐VBM, a VBM analysis [Ashburner and Friston, 2000; Mechelli et al., 2005] using the FSL‐VBM toolbox from the FMRIB software package (http://www.fmrib.ox.ac.uk/fsl/fslvbm/index.html) [Smith et al., 2004]. Briefly, structural images were extracted using the brain extraction tool [Smith, 2002]. Tissue segmentation was then carried out on the brain extracted images using FMRIB's Automatic Segmentation Tool [FAST; Zhang et al., 2001]. The resulting gray matter partial volumes were then aligned to the Montreal Neurological Institute standard space (MNI152) using the FMRIB nonlinear registration approach [FNIRT; Andersson et al., 2007a, b], which uses a b‐spline representation of the registration warp field [Rueckert et al., 1999]. A study‐specific template was created, combining AD, bvFTD and Control images, to which the native gray matter images were re‐registered nonlinearly. The registered partial volume maps were then modulated by dividing by the Jacobian of the warp field. This step was carried out to correct for local expansion or contraction. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm.
VBM analysis
A voxel‐wise general linear model was applied to investigate gray matter intensity differences via permutation‐based nonparametric testing [Nichols and Holmes, 2002] with 5000 permutations per contrast. In a first step, differences in cortical gray matter intensities between patients (bvFTD and AD) and Controls were assessed. Next, correlations between performance on experimental episodic memory tests and regions of gray matter atrophy were investigated in bvFTD and AD patients combined with Controls. This procedure serves to increase the statistical power to detect brain‐behavior relationships across the entire brain by achieving greater variance in behavioral scores [see Sollberger et al., 2009; Irish et al., 2012a). For statistical power, a covariate only statistical model with a [1] t‐contrast was used, providing an index of association between gray matter intensity and performance on the experimental measures. Two separate composite models were created to investigate the neural substrates of recall (RAVLT immediate, RAVLT delayed, RCF delayed) and recognition (RAVLT recognition, Doors part A) performance. Anatomical locations of significant results were overlaid on the MNI standard brain, with maximum coordinates provided in MNI stereotaxic space. Anatomical labels were determined with reference to the Harvard‐Oxford probabilistic cortical atlas. For all exploratory comparisons, a threshold of 100 contiguous voxels was used, uncorrected at the P < 0.001 threshold. An overlap analyses was conducted to identify common regions of gray matter intensity decrease implicated in episodic memory disruption. The statistical maps generated from the two contrasts using episodic memory performance as a covariate (i.e., bvFTD and Controls; AD and Controls), were scaled using a threshold of P < 0.001, following which, the two scaled contrasts were multiplied to create an inclusive, or overlap, mask across groups. For the exclusive masks, the same procedure was adopted, however, the scaled images were subsequently divided by each other, to create an exclusive mask for each patient versus control contrast. For these overlap and exclusive masking analyses, we lowered the cluster‐based threshold to 50 contiguous voxels [see Hornberger et al., 2011].
RESULTS
Demographics
The groups were well matched for age, years in education, and sex (all P values > 0.1). Additionally, AD and bvFTD patient groups were matched for disease duration (months elapsed since onset of symptoms (P > 0.1).
Global Cognitive Function
Neuropsychological testing revealed cognitive profiles characteristic of each patient group (Table 1). Briefly, both patient groups were impaired compared to controls on a general cognitive screening test (ACE‐R) (P < 0.0001), but did not differ from each other (P > 0.1). In both patient groups, impairments were observed on the attention, memory and fluency subscales of the ACE‐R (all P values < 0.01). AD patients displayed additional impairments on the language and visuospatial function subscales of the ACE‐R (all P values < 0.01). Compared to controls, patient groups also showed deficits in executive function (Trail Making, Letter fluency, and Hayling) (all P values < 0.05), with AD and bvFTD patient groups scoring at comparable levels on these tests (all P values > 0.1). On the CBI, carer‐rated apathy levels were higher in the bvFTD than the AD group (P = 0.012). No other significant differences were evident between the patient groups for total scores on the CBI (P > 0.4).
Table 1.
Demographic characteristics, clinical, and experimental composite scores for participantsa,b,c
| bvFTD | AD | Controls | Group effect | AD versus bvFTD | |
|---|---|---|---|---|---|
| N | 19 | 18 | 19 | ||
| Sex (m:f) | 11:8 | 11:7 | 8:11 | n/s | n/s |
| Age (years) | 63.6 (7.9) | 65.8 (6.8) | 68.1 (5.3) | n/s | n/s |
| Education (years) | 12.6 (3.3) | 12.1 (3.6) | 13.1 (2.4) | n/s | n/s |
| Disease duration (months) | 47.1 (27.2) | 34.1 (13.6) | n/a | n/s | n/s |
| ACE‐R total (100) | 77.5 (12.0) | 77.2 (12.5) | 94.8 (3.5) | b | n/s |
| Trails (B‐A in s) | 124.8 (100.1) | 127.2 (72.8) | 51.9 (29.6) | a | n/s |
| Letter fluency | 23.9 (12.4) | 30.9 (12.0) | 45.9 (13.7) | b | n/s |
| Hayling scaled score | 2.6 (2.0) | 3.8 (2.0) | 6.2 (0.5) | b | n/s |
| CBI Total (100) | 44.4 (27.9) | 57.5 (31.8) | n/a | n/s | n/s |
| Recall composite score (100) | 24.5 (16.8) | 22.3 (19.1) | 63.9 (13.4) | b | n/s |
| Recognition composite score (100) | 74.5 (17.1) | 70.9 (16.9) | 89.6 (9.0) | b | n/s |
Standard deviations in brackets, maximum score for tests shown in brackets.
bvFTD = behavioral variant frontotemporal dementia; AD = Alzheimer's disease; ACE‐R = Addenbrooke's Cognitive Examination Revised; CBI = Cambridge Behavioral Inventory.
Trail Making test data available for 13 bvFTD and 12 AD cases. CBI information available for 17 AD and 15 bvFTD patients. Hayling data available for 17 bvFTD cases.
P < 0.05;
P < 0.0001; n/s = not significant; n/a = not applicable.
Episodic Memory—Recall
On the RAVLT immediate recall, AD and bvFTD patients showed significantly poorer recall in comparison with Controls [Trial A6; F(2, 52) = 26.537, P < 0.0001] (Fig. 1, Supporting Information Table SI). No significant differences were evident between the AD and bvFTD groups on this measure (P > 0.9). The same result profile was observed on the RAVLT delayed recall (F(2, 52) = 26.183, P < 0.0001). Again, no significant differences emerged between the patient groups (P > 0.9).
Figure 1.
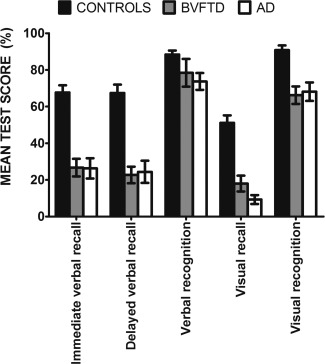
Bar chart showing overall memory performance (percentage correct) across verbal and visual recall and recognition for healthy Controls, behavioral variant FTD and AD participants. Error bars represent standard error of the mean.
Recall of the Rey complex figure was significantly impaired in AD and bvFTD patients compared to Controls [F(2, 51) = 36.490, P < 0.0001] but no significant difference was present between the patient groups (P > 0.2). No significant differences were evident between the patient groups for Copy of the complex figure (P > 0.7). Expressing RCF recall score as a percentage of the encoding Copy scores revealed the same pattern of performance across groups.
Episodic Memory—Recognition
No significant overall group differences emerged on the recognition subscale of the RAVLT (P > 0.05). In contrast, both patient groups showed significant deficits on the Doors Part A compared with Controls (F(2, 53) = 10.482, P < 0.0001), with no significant differences between the patient groups (P > 0.9).
VBM Group Analysis
Patterns of atrophy
Compared to Controls, AD patients showed widespread atrophy involving the medial and lateral temporal lobes including the bilateral hippocampi and parahippocampal gyrus, bilateral temporal fusiform cortex, bilateral inferior frontal gyrus and right frontal pole, and posterior brain regions, notably the posterior cingulate cortex and precuneus bilaterally, and left occipital pole. BvFTD patients showed striking atrophy relative to Controls predominantly in the frontal lobes including bilateral orbitofrontal, medial prefrontal, and insular cortices, as well as the frontal poles and paracingulate gyri bilaterally. Further atrophy was evident in the bilateral temporal lobes including the temporal fusiform cortex, temporal pole, amygdalae, and hippocampi, occipital poles and lateral occipital cortex (Fig. 2).
Figure 2.
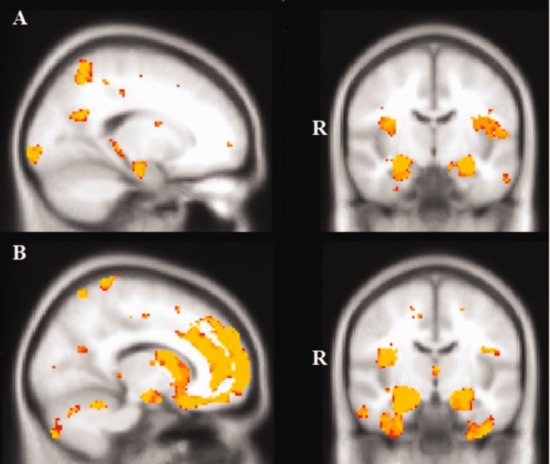
VBM analyses showing brain areas of decreased gray matter intensity in (A) AD patients in comparison with Controls, and (B) behavioral variant FTD patients in comparison with Controls. Colored voxels show regions that were significant in the analysis with P < 0.001 uncorrected for all contrasts, with a cluster threshold of 100 contiguous voxels. All clusters reported t > 3.9. Clusters are overlaid on the MNI standard brain. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Direct comparison of the bvFTD and AD patients revealed greater atrophy in the bilateral orbitofrontal and medial prefrontal cortices, bilateral frontal poles, right inferior temporal gyrus and right temporal pole in bvFTD compared with AD. The reverse contrast revealed greater atrophy in the bilateral intracalcarine, cuneal, and precuneal cortices, in the AD group (see Supporting Information Table SII). These patterns of atrophy are consistent with previous reports in bvFTD [Rosen et al., 2002; Hornberger et al., 2011] and in AD [Karas et al., 2004; Dickerson et al., 2009].
Neural correlates of Recall performance
Recall performance in AD patients combined with Controls was associated with a distributed network involving bilateral frontal lobes, medial temporal regions including the hippocampus bilaterally, bilateral lateral temporal cortices, and bilateral posterior regions including precuneus, posterior cingulate cortex, and the supramarginal gyrus (Fig. 3A, Table 2). A comparatively circumscribed set of regions were implicated for recall performance in the bvFTD group combined with Controls including bilateral paracingulate cortices, anterior cingulate cortex, and orbitofrontal cortices, the right temporal pole and the right hippocampus (Fig. 3B, Table 2). When we analyzed the neural correlates of recall performance in the AD and bvFTD groups combined, no significant clusters were evident at the P < 0.001 threshold.
Figure 3.
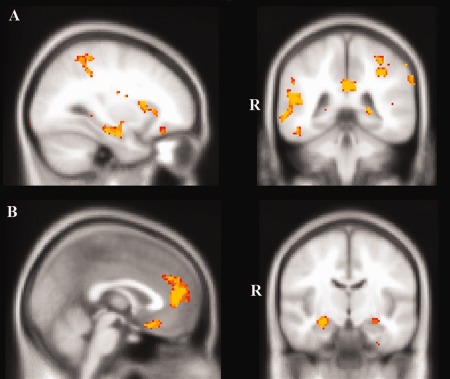
VBM analyses showing brain regions in which gray matter intensity correlates significantly with episodic recall performance in (A) AD in comparison with Controls, and (B) behavioral variant FTD compared with Controls. Colored voxels show regions that were significant in the analysis with P < 0.001 uncorrected with a cluster threshold of 100 contiguous voxels. All clusters reported t > 3.9. Clusters are overlaid on the MNI standard brain. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Voxel‐based morphometry results showing regions of significant grey matter intensity decrease that covary with episodic memory recall performance for the contrasts of AD and behavioral variant frontotemporal dementia (bvFTD) patient groups and Controls
| Contrast | Regions | Hemisphere | MNI coordinates | Number of voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| AD and controls | Frontal pole | Right | 16 | 70 | −4 | 1204 |
| Middle temporal gyrus | Right | 64 | −32 | −8 | 1143 | |
| Precuneus, posterior cingulate cortex | Bilateral | 4 | −54 | 22 | 672 | |
| Supramarginal gyrus | Left | −38 | −34 | 36 | 620 | |
| Hippocampus (anterior and posterior) | Left | −24 | −16 | −24 | 579 | |
| Inferior temporal gyrus | Right | 62 | −58 | −22 | 474 | |
| Superior frontal gyrus | Right | 12 | 20 | 40 | 456 | |
| Inferior temporal gyrus | Left | −60 | −22 | −32 | 380 | |
| Orbitofrontal cortex | Left | −26 | 18 | −12 | 368 | |
| Precuneus cortex | Right | 6 | −76 | 56 | 348 | |
| Parahippocampal gyrus | Right | 26 | −4 | −38 | 312 | |
| Temporal pole | Right | 22 | 6 | −28 | 211 | |
| Postcentral gyrus | Left | −54 | −22 | 24 | 189 | |
| Middle temporal gyrus | Left | −48 | −50 | 6 | 176 | |
| Hippocampus, thalamus | Right | 20 | −34 | −4 | 144 | |
| Precentral gyrus | Right | 30 | 10 | 28 | 112 | |
| bvFTD and controls | Paracingulate gyrus, anterior cingulate cortex | Bilateral | 0 | 46 | 2 | 1173 |
| Temporal pole | Right | 34 | 24 | −34 | 1037 | |
| Orbitofrontal cortex | Left | −10 | 24 | −26 | 247 | |
| Hippocampus (anterior) | Right | 28 | −12 | −20 | 127 | |
All results uncorrected at P < 0.001; only clusters with at least 100 contiguous voxels included. All clusters reported t > 3.9. MNI = Montreal Neurological Institute.
Next, we conducted an overlap analysis to investigate common regions underlying recall performance in AD and bvFTD (Table 3, Fig. 4A). This overlap analysis revealed that atrophy in the right temporal pole, right orbitofrontal cortex, right frontal pole, left paracingulate gyrus and anterior cingulate cortex, and right anterior hippocampus correlated significantly with episodic recall performance in both the AD and the bvFTD groups.
Table 3.
Voxel‐based morphometry results showing common regions of significant grey matter intensity decrease that correlate with recall performance which overlap in behavioral variant frontotemporal dementia (bvFTD) and Alzheimer's disease (AD) patients
| Contrast | Regions | Hemisphere | MNI coordinates | Number of voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Regions of overlap | Temporal pole, orbitofrontal cortex | Right | 26 | 12 | −30 | 120 |
| Frontal pole | Right | 16 | 70 | −4 | 77 | |
| Paracingulate gyrus, anterior cingulate cortex | Left | 0 | 46 | 8 | 73 | |
| Hippocampus (anterior) | Right | 26 | −16 | −20 | 51 | |
All results uncorrected at P < 0.001; only clusters with at least 50 contiguous voxels were used. All clusters reported t > 3.9. MNI = Montreal Neurological Institute.
Figure 4.
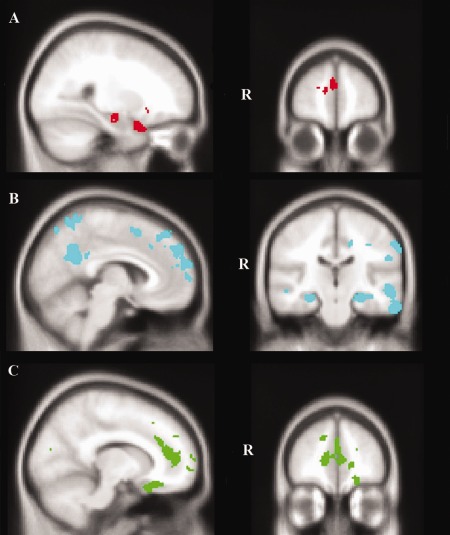
VBM analyses showing brain regions in which gray matter intensity correlates with recall performance in (A) both bvFTD and AD, (B) exclusively in AD, and (C) exclusively in bvFTD. Colored voxels show regions that were significant in the analysis with P < 0.001 uncorrected with a cluster threshold of 100 contiguous voxels for exploratory whole‐brain analyses (B and C) and 50 contiguous voxels for the overlap analysis (A). All clusters reported t > 3.9. Clusters are overlaid on the MNI standard brain. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Exclusive masking was used to determine the regions uniquely contributing to recall performance in each dementia group (Table 4). Bilateral atrophy in posterior regions including the posterior lateral temporal cortices, precuneus and posterior cingulate cortex, posterior hippocampi, left supramarginal gyrus and lateral occipital cortices correlated with recall performance exclusively in AD. Additionally, atrophy in the right frontal pole, and bilateral superior frontal gyri correlated with recall performance in AD (Fig. 4B). In contrast, atrophy in the bilateral frontal poles extending into paracingulate and anterior cingulate cortices, right anterior inferior temporal gyrus and temporal pole, and the left orbitofrontal cortex correlated with recall performance exclusively in the bvFTD group (Fig. 4C).
Table 4.
Voxel‐based morphometry results showing regions of significant grey matter intensity decrease that correlate with recall performance unique to Alzheimer's disease (AD) and behavioral variant frontotemporal dementia (bvFTD) groups
| Group | Regions | Hemisphere | MNI coordinates | Number of voxels | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| AD and controls | Inferior temporal gyrus, middle temporal gyrus | Right | 66 | −38 | −22 | 1143 |
| Frontal pole | Right | 10 | 60 | −6 | 905 | |
| Precuneus, posterior cingulate cortex | Bilateral | 8 | −56 | 20 | 672 | |
| Supramarginal gyrus, superior parietal lobule | Left | −38 | −36 | 36 | 620 | |
| Hippocampus (anterior and posterior) | Left | −22 | −16 | −24 | 551 | |
| Inferior temporal gyrus, lateral occipital cortex | Right | 62 | −58 | −24 | 474 | |
| Paracingulate gyrus, superior frontal gyrus | Right | 14 | 20 | 38 | 456 | |
| Inferior temporal gyrus, middle temporal gyrus | Left | −64 | −16 | −34 | 380 | |
| Orbitofrontal cortex | Left | −24 | 16 | −12 | 368 | |
| Precuneus, lateral occipital cortex | Right | 8 | −74 | 50 | 324 | |
| Temporal fusiform cortex, parahippocampal gyrus, hippocampus | Right | 26 | −6 | −40 | 223 | |
| Supramarginal gyrus | Left | −58 | −26 | 24 | 189 | |
| Middle temporal gyrus | Left | −46 | −50 | 6 | 176 | |
| Hippocampus (posterior), thalamus | Right | 24 | −34 | −4 | 144 | |
| Inferior frontal gyrus | Right | 36 | 10 | 26 | 112 | |
| bvFTD and Controls | Frontal Pole, paracingulate gyrus, anterior cingulate cortex | Bilateral | 16 | 68 | −8 | 847 |
| Inferior temporal gyrus (anterior), temporal pole | Right | 42 | 2 | −44 | 767 | |
| Orbitofrontal cortex, temporal pole | Left | −28 | 16 | −28 | 229 | |
All results uncorrected at P < 0.001; only clusters with at least 100 contiguous voxels were used. All clusters reported t > 3.9. MNI = Montreal Neurological Institute.
Neural correlates of recognition performance
No significant correlations were evident between recognition performance and gray matter intensity in either the AD combined with Controls contrast, or the bvFTD combined with Controls contrast at the statistical threshold of P < 0.001. Again, when we analyzed the neural correlates of recognition performance in the AD and bvFTD groups combined, no significant clusters were evident at the P < 0.001 threshold.
DISCUSSION
The goal of the present study was to advance our understanding of episodic memory retrieval by establishing the neural correlates underlying episodic memory dysfunction in two neurodegenerative conditions. AD and bvFTD patients showed similar profiles of impairment across a range of visual and verbal tests of episodic recall and recognition memory. Whilst AD and bvFTD patients displayed commonalities in terms of the neural structures implicated in recall performance, including the right temporal pole, right frontal lobe, left paracingulate gyrus, and right anterior hippocampus, divergent neural substrates of recall performance were uncovered contingent on dementia subtype. In bvFTD, memory recall performance correlated with an anterior network involving the frontal and anterior temporal lobes. In contrast, a widespread neural network was implicated in the AD group, involving frontal, as well as, posterior structures, including the posterior cingulate cortex, and parietal and occipital cortices. Although the behavioral data highlight the difficulties in the differentiation of these dementia subtypes based on episodic memory performance, our neuroimaging findings suggest that distinctive neural systems underlie the episodic memory deficits in each group.
The present findings dovetail with a growing body of evidence which suggests a significant level of episodic memory impairment in bvFTD [Hornberger et al., 2010; Irish et al., 2011a; Pennington et al., 2011]. This episodic memory loss in bvFTD is not unexpected when we consider the patterns of regional atrophy that characterize this dementia syndrome. It is now well documented that bvFTD patients show profound atrophy of the medial prefrontal cortices and temporal lobes, including the hippocampus [Rabinovici et al., 2007; Seeley, 2008; Whitwell et al., 2009a; Whitwell et al., 2009b]. Our VBM analysis revealed that episodic memory deficits in our bvFTD cohort were underpinned by atrophy in the bilateral paracingulate gyri, right temporal pole, bilateral orbitofrontal cortices, and right anterior hippocampus, key regions associated with episodic memory performance in functional neuroimaging studies of healthy participants [Rugg et al., 2002]. Although some studies have pointed to posterior temporal and MTL pathology as a determinant of memory dysfunction in bvFTD [Söderlund et al., 2008], others have suggested that frontal lobe atrophy is critical to the memory deficits in this cohort [Simons et al., 2002; Pennington et al., 2011]. These inconsistencies across studies may reflect patient group characteristics such as stage of disease, differences in episodic memory tasks employed, or indeed the method used to quantify neural atrophy in patient cohorts. Using a whole‐brain analysis technique, we demonstrated that the integrity of both medial and prefrontal regions is essential for successful general episodic memory retrieval, reflecting current integrative perspectives which emphasize the importance of interactions between frontal and temporal regions in episodic memory performance [Simons and Spiers, 2003].
The neurocognitive mechanisms underlying the episodic memory impairment in bvFTD remain unknown, although it has been suggested that such memory deficits reflect executive dysfunction [Mendez and Cummings, 2003; Piolino et al., 2003], most likely due to the overwhelming burden of atrophy in the prefrontal cortices. Difficulties in selecting, comparing and deciding on information may stem from orbitofrontal cortex damage in this cohort [Collette and Van der Linden, 2002; Piolino et al., 2007]. Disturbances in remembering contextual details such as the source or recency of the test information have also been documented in bvFTD [Simons et al., 2002; Söderlund et al., 2008; Irish et al., 2012b] in the context of relatively preserved item memory [Simons et al., 2002; Harciarek and Jodzio, 2005]. Such source memory deficits have been interpreted as not only reflecting frontal lobe damage [Simons et al., 2002], but also point to the role of the medial and posterior temporal lobe structures [Söderlund et al., 2008] in bvFTD. On visual item recognition, however, we found that bvFTD patients were impaired to the same extent as AD patients, possibly reflecting the additional involvement of the hippocampus, although our imaging analysis did not reveal any significant neural correlates for the recognition composite.
Our findings in AD mesh well with a large body of evidence implicating a widespread neural network underlying episodic memory disturbances in this patient cohort [Buckner et al., 2005]. Unsurprisingly, AD patients showed marked episodic memory deficits irrespective of task (recall, recognition) or modality (visual, verbal). Recall performance in AD correlated with a distributed neural network including the hippocampus, posterior cingulate cortex, temporoparietal, occipital, and prefrontal regions. These structures are known to participate in episodic memory [Buckner et al., 2005] and visuospatial imagery [Cavanna and Trimble, 2006], the prototypical functions vulnerable in the early stages of AD [McKhann et al., 1984]. Interestingly, the posterior cingulate cortex has been posited to underlie the capacity for old/new detection, and may play a particular role in the recollective aspect of episodic memory retrieval [Wagner et al., 2005].
Our findings have potential clinical implications. We have confirmed that patients with bvFTD are indistinguishable from AD patients across a battery of standardized tests of verbal and visual episodic memory, typically used in the differential diagnosis of these cohorts. This raises concerns regarding the sensitivity of the current diagnostic criteria for bvFTD given that severe amnesia remains an exclusion criterion for bvFTD [Piguet et al., 2009; Rascovsky et al., 2011]. The difficulty in accurate diagnosis of bvFTD is reinforced by our finding of commonalities in the patterns of neural atrophy underlying the episodic memory deficits in bvFTD and AD. Our VBM analyses revealed common regions of neural atrophy that were significantly related to general episodic memory dysfunction, irrespective of dementia type. Specifically, these regions included the frontal and temporal poles, paracingulate gyrus, and the hippocampus. The discovery of significant hippocampal involvement mediating the episodic memory impairment in bvFTD, coupled with considerable frontal lobe involvement in episodic memory deficits in AD, appears to run counter to the classic conceptualization of these dementia syndromes. Hippocampal atrophy is well documented in bvFTD [Rabinovici et al., 2007; Whitwell et al., 2009b], yet paradoxically, episodic memory is assumed to be relatively intact in the early stages of the disease [Neary et al., 1998; Rascovsky et al., 2011]. This dictum represents something of a conundrum for the accurate diagnosis of bvFTD, when MTL atrophy and episodic memory deficits continue to represent the hallmark features of AD. Episodic memory is a complex construct, relying on the integrity of dissociable underlying processes [Easton and Eacott, 2010]. Using a general episodic memory composite, our results reinforce the inherent difficulty in the differential diagnosis of bvFTD from AD, and further corroborate the growing opinion that the presence of severe episodic memory deficits should not preclude a diagnosis of bvFTD [Hornberger et al., 2012].
Of particular interest, however, is evidence pointing to divergent neuroanatomical structures which are differentially implicated in episodic memory dysfunction contingent on dementia syndrome. Specifically, posterior regions, including the bilateral posterior cingulate cortices, were exclusively implicated in the AD group. This finding supports converging evidence pointing toward the posterior cingulate cortex as a crucial region in the genesis of episodic memory deficits in AD [Nestor et al., 2006], and stands in contrast with the involvement of anterior regions, including the bilateral anterior temporal poles and bilateral frontal poles and paracingulate gyri, in the bvFTD group. The precuneus has been implicated in successful performance of tasks drawing on mental imagery processes, leading to the proposal that visuospatial aspects of episodic memory rely on the integrity of this posterior region [Cavanna and Trimble, 2006]. Accordingly, poor performance on the RCF task may be anticipated in AD patients given its visuospatial loading and the pathology burden in more posterior brain regions in this disease. Although we did not find significant differences between AD and bvFTD patients on this task, our behavioral results suggest that this task may be useful to differentiate between these groups. Teasing apart the differential contributing factors underlying poor performance in each patient group is imperative: visuospatial dysfunction may impair AD performance, whereas impaired organization and planning of the figure may underlie memory difficulties in bvFTD. Further exploration of the divergent neural contributions to episodic memory dysfunction in bvFTD and AD represents a potentially critical avenue in refining accurate diagnosis of these dementia syndromes. In particular, it may be possible to develop specific neuropsychological tasks which rely on posterior parietal regions, and thus visuospatial aspects of memory, versus those which emphasize frontal processes, such as planning and organization, to aid in the differential diagnosis of AD and bvFTD patients, respectively.
A number of methodological issues warrant discussion. Firstly, given that the majority of our sample had not yet come to autopsy, we did not have access to neuropathological data to definitively confirm the underlying disease pathology in each group. As such, we cannot exclude the possibility that some of the bvFTD cases, in particular those who performed very poorly on the experimental memory measures, may have underlying AD pathology. Importantly, 6 patients (4 AD, 2 bvFTD) whose initial clinical diagnosis was uncertain, underwent Pittsburgh Compound B (PiB) PET imaging which confirmed the AD diagnosis in all four suspected AD cases (PiB positive scans) and corroborated the bvFTD diagnosis in the remaining two cases (PiB negative scans). Our findings converge well with previous reports of severe memory disturbance in pathologically confirmed cases of bvFTD [Graham et al., 2005; Hornberger et al., 2010; Hornberger et al., 2012]. Nevertheless, replication of our results in patient cohorts with a combination of in vivo PiB‐PET imaging and neuropathological data represents an important area of future enquiry.
A second limitation of this study concerns the heterogeneity of the experimental measures used to probe episodic memory recall and recognition. Although the aim of this study was to elucidate the neural correlates of general episodic memory dysfunction in neurodegenerative disorders, the composite scores we created for recall and recognition performance do not permit the dissection of specific aspects of retrieval or recognition. Differences in test administration, such as incremental learning on the RAVLT versus the one trial learning of the RCF and Doors test, also need to be taken into consideration when discussing the neural substrates of specific memory processes. In spite of this limitation, our findings point to important commonalities and differences in the neural networks that underpin episodic disturbances in different neurodegenerative conditions. Finally, our neuroimaging results did not survive conservative corrections for multiple comparisons (i.e., Family‐Wise Error) and were therefore reported uncorrected at P < 0.001. We reduced the potential for false positive results, however, by applying cluster extent thresholds of 100 and 50 contiguous voxels for the exploratory and overlap analyses, respectively. Importantly, Monte Carlo simulations and experimental data demonstrate that cluster thresholding is an effective tool to reduce the probability of false positive findings without compromising the statistical power of the study [Forman et al., 1995]. Given our sample size, the application of stringent cluster extent thresholds, and our a priori assumptions, we are confident that our results do not represent false positive findings, however, it will be important to replicate these findings in a larger patient cohort using corrected neuroimaging results.
A number of avenues warrant future investigation, one of which relates to the finding of significant frontal lobe involvement in episodic memory dysfunction in AD. Recent studies have demonstrated executive dysfunction even in the early stages of AD [Gleichgerrcht et al., 2011]. The extent to which frontal pathology contributes to episodic memory deficits in this group remains, to date, underexplored. Although we reported on the aggregate performance of our patient groups, it is possible that the neural underpinnings of episodic memory deficits may differ contingent on disease stage. Although the patient cohorts in this study were matched for disease severity and disease duration, future studies using larger patient samples, stratified by disease stage, will be necessary to clarify at what point during the pathological process structures such as the hippocampus are implicated in episodic memory disruption in bvFTD.
In conclusion, we have demonstrated that episodic memory impairments in progressive cases of bvFTD are as pronounced as in AD. Critically, while the neural regions responsible for such general memory deficits were found to overlap significantly in each patient group, including the right temporal and frontal poles, left paracingulate gyrus, and right hippocampus, divergent anterior versus posterior neural networks were implicated exclusively in bvFTD and AD, respectively. We suggest here that the next challenge lies in the creation of suitably specific tasks which differentially stress anterior versus posterior aspects of episodic memory retrieval.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The authors are grateful to the patients and their families for supporting our research.
REFERENCES
- Andersson JLR, Jenkinson M, Smith S (2007a):Non‐linear optimisation. FMRIB Technical Report TR07JA1. Oxford:University of Oxford FMRIB Centre. [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S (2007b):Non‐linear registration, aka Spatial normalisation. FMRIB Technical Report TR07JA2. Oxford:University of Oxford FMRIB Centre. [Google Scholar]
- Ashburner J, Friston KJ (2000): Voxel‐based morphometry—The methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Emslie H, Nimmo‐Smith I (1994):The Doors and People Test: a test of visual and verbal recall and recognition. Bury St. Edmunds:Thames Valley Test Company. [Google Scholar]
- Blumenfeld RS, Ranganath C (2007): Prefrontal cortex and long‐term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13:280–291. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E (1991): Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol 82:239–249. [DOI] [PubMed] [Google Scholar]
- Broe M, Hodges JR, Schofield E, Shepherd CE, Kril JJ, Halliday GM (2003): Staging disease severity in pathologically confirmed cases of frontotemporal dementia. Neurology 60:1005–1011. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA (2005): Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25:7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P, Shallice T, editors.1997. The Hayling and Brixton Tests. Thurston Suffolk:Thames Valley Test Company. [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129(Pt 3):564–583. [DOI] [PubMed] [Google Scholar]
- Collette F, Van der Linden M (2002): Brain imaging of the central executive component of working memory. Neurosci Biobehav Rev 26:105–125. [DOI] [PubMed] [Google Scholar]
- de Toledo‐Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, Bennett DA (2000): Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer's disease. Hippocampus 10:136–142. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD and others (2009): The cortical signature of Alzheimer's disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid‐positive individuals. Cereb Cortex 19:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H (2010): The episodic memory system: Neurocircuitry and disorders. Neuropsychopharmacology 35:86–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Eacott MJ (2010): Recollection of episodic memory within the medial temporal lobe: Behavioural dissociations from other types of memory. Behav Brain Res 215:310–317. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33:636–647. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Erbetta A, Reati F, Bugiani O (2008): Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer's disease in a study of diagnostic concordance. Neuropsychologia 46:1495–1504. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E, Torralva T, Martinez D, Roca M, Manes F (2011): Impact of executive dysfunction on verbal memory performance in patients with Alzheimer's disease. J Alzheimers Dis 23:79–85. [DOI] [PubMed] [Google Scholar]
- Graham A, Davies RR, Xuereb JH, Halliday GM, Kril JJ, Creasey H, Graham KS, Hodges JR (2005): Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain 128:597–605. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harciarek M, Jodzio K (2005): Neuropsychological differences between frontotemporal dementia and Alzheimer's disease: A review. Neuropsychol Rev 15:131–145. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Geng J, Hodges JR (2011): Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioural variant frontotemporal dementia. Brain 134(Pt 9):2502–2512. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Piguet O (2012): Episodic memory in frontotemporal dementia: A critical review. Brain 135:678–692. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Piguet O, Graham AJ, Nestor PJ, Hodges JR (2010): How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology 74:472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornberger M, Piguet O, Kipps CM, Hodges JR (2008): Executive function in progressive and nonprogressive behavioral variant frontotemporal dementia. Neurology 71:1481–1488. [DOI] [PubMed] [Google Scholar]
- Hornberger M, Wong S, Tan R, Irish M, Piguet O, Kril J, Hodges JR, Halliday G (2012): In vivo and post‐mortem memory circuit integrity in frontotemporal dementia and Alzheimer's disease. Brain 135(Pt 10):3015–3025. [DOI] [PubMed] [Google Scholar]
- Irish M, Addis DR, Hodges JR, Piguet O (2012a): Considering the role of semantic memory in episodic future thinking: evidence from semantic dementia. Brain 135(Pt 7):2178–2191. [DOI] [PubMed] [Google Scholar]
- Irish M, Graham A, Graham KS, Hodges JR, Hornberger M (2012b): Differential impairment of source memory in progressive versus non‐progressive behavioral variant frontotemporal dementia. Arch Clin Neuropsychol 27:338–347. [DOI] [PubMed] [Google Scholar]
- Irish M, Hornberger M, Lah S, Miller L, Pengas G, Nestor PJ, Hodges JR, Piguet O (2011a): Profiles of recent autobiographical memory retrieval in semantic dementia, behavioural‐variant frontotemporal dementia, and Alzheimer's disease. Neuropsychologia 49:2694–2702. [DOI] [PubMed] [Google Scholar]
- Irish M, Lawlor BA, O'Mara SM, Coen RF (2011b): Impaired capacity for autonoetic reliving during autobiographical event recall in mild Alzheimer's disease. Cortex 47:236–249. [DOI] [PubMed] [Google Scholar]
- Irish M, Piguet O, Hodges JR (2012c): Self‐projection and the default network in frontotemporal dementia. Nat Rev Neurol 8:152–161. [DOI] [PubMed] [Google Scholar]
- Kahn I, Andrews‐Hanna JR, Vincent JL, Snyder AZ, Buckner RL (2008): Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol 100:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karas GB, Scheltens P, Rombouts SA, Visser PJ, van Schijndel RA, Fox NC, Barkhof F (2004): Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. Neuroimage 23:708–716. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD (2004):Design and analysis. A researcher's handbook. Englewood Cliffs (NJ):Prentice Hall. [Google Scholar]
- Kipps CM, Hodges JR, Hornberger M (2010): Nonprogressive behavioural frontotemporal dementia: recent developments and clinical implications of the ‘bvFTD phenocopy syndrome’. Curr Opin Neurol 23:628–632. [DOI] [PubMed] [Google Scholar]
- Kumfor F, Miller L, Lah S, Hsieh S, Savage S, Hodges JR, Piguet O (2011): Are you really angry? The effect of intensity on facial emotion recognition in frontotemporal dementia. Soc Neurosci 6:502–514. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Drachman D, Folstein M, Katzman R, Price D, Stadlan E (1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34:939–944. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R and others (2011): The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ, Ashburner J (2005): Voxel‐based morphometry of the human brain: methods and applications. Curr Med Imaging Rev 1:1–9. [Google Scholar]
- Mendez M, Cummings JL.2003. Dementia—A Clinical Approach. Philadelphia:Butterworth‐Heinemann (Elsevier). [Google Scholar]
- Meyers J, Meyers K.1995. The Meyers Scoring System for the Rey Complex Figure and the Recognition Trial: professional manual. Odessa, FL.:Psychological Assessment Resources. [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC (2008): Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry 79:630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR (2006): The Addenbrooke's Cognitive Examination Revised (ACE R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 21:1078–1085. [DOI] [PubMed] [Google Scholar]
- Morris J (1997): Clinical dementia rating: A reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 9(S1):173–176. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden J, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert P, Albert M (1998): Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Hodges JR (2006): Declarative memory impairments in Alzheimer's disease and semantic dementia. Neuroimage 30:1010–1020. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2002): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington C, Hodges JR, Hornberger M (2011): Neural correlates of episodic memory in behavioural variant frontotemporal dementia. J Alzheimer's Dis 24:261–268. [DOI] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Mioshi E, Hodges JR (2011): Behavioural‐variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol 10:162–172. [DOI] [PubMed] [Google Scholar]
- Piguet O, Hornberger M, Shelley BP, Kipps CM, Hodges JR (2009): Sensitivity of current criteria for the diagnosis of behavioral variant frontotemporal dementia. Neurology 72:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piolino P, Chételat G, Matuszewski V, Landeau B, Mézenge F, Viader F, De La Sayette V, Eustache F, Desgranges B (2007): In search of autobiographical memories: A PET study in the frontal variant of frontotemporal dementia. Neuropsychologia 45:2730–2743. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Belliard S, Matuszewski V, Lalevee C, De La Sayette V, Eustache F (2003): Autobiographical memory and autonoetic consciousness: Triple dissociation in neurodegenerative diseases. Brain 126:2203–2219. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Seeley WW, Kim EJ, Gorno‐Tempini ML, Rascovsky K, Pagliaro TA, Allison SC, Halabi C, Kramer JH, Johnson JK and others (2007): Distinct MRI atrophy patterns in autopsy‐proven Alzheimer's disease and frontotemporal lobar degeneration. Am J Alzheimers Dis Other Demen 22:474–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Baldwin E, Pace‐Savitsky C, Kramer JH, Miller BL (2005): Self awareness and personality change in dementia. J Neurol Neurosurg Psychiatry 76:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU and others (2011): Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134(Pt 9):2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R (1958): Validity of the Trail Making Test as an indicator of organic brain damage. Percep Motor Skills 8:271–276. [Google Scholar]
- Rosen HJ, Gorno‐Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, Feiwell R, Kramer JH, Miller BL (2002): Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology 58:198–208. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ (1999): Nonrigid registration using free‐form deformations: application to breast MR images. IEEE Trans Med Imaging 18:712–721. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN (2002): The neural basis of episodic memory: Evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci 357:1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon E, Lekeu F, Bastin C, Garraux G, Collette F (2008): Functional imaging of cognition in Alzheimer's disease using positron emission tomography. Neuropsychologia 46:1613–1623. [DOI] [PubMed] [Google Scholar]
- Schmidt M.1996. Rey Auditory and Verbal Learning Test: A Handbook. Los Angeles:Western Psychological Services. [Google Scholar]
- Schroeter ML, Raczka K, Neumann J, von Cramon DY (2008): Neural networks in frontotemporal dementia‐‐a meta‐analysis. Neurobiol Aging 29:418–426. [DOI] [PubMed] [Google Scholar]
- Seeley WW (2008): Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr Opin Neurol 21:701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno‐Tempini ML (2008): Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 65:249–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ (2003): Prefrontal and medial temporal lobe interactions in long‐term memory. Nat Rev Neurosci 4:637–648. [DOI] [PubMed] [Google Scholar]
- Simons JS, Verfaellie M, Galton CJ, Miller BL, Hodges JR, Graham KS (2002): Recollection‐based memory in frontotemporal dementia: Implications for theories of long‐term memory. Brain 125:2523–2536. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE and others (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund H, Black SE, Miller BL, Freedman M, Levine B (2008): Episodic memory and regional atrophy in frontotemporal lobar degeneration. Neuropsychologia 46:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollberger M, Stanley CM, Wilson SM, Gyurak A, Beckman V, Growdon M, Jang J, Weiner MW, Miller BL, Rankin KP (2009): Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia 47:2812–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, LaViolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ and others (2010): Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular Med 12:27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE (2004): The medial temporal lobe. Annu Rev Neurosci 27:279–306. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O.2006. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. USA:Oxford University Press,. [Google Scholar]
- Tulving E.1983. Elements of Episodic Memory. London:Oxford University Press. [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL (2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9:445–453. [DOI] [PubMed] [Google Scholar]
- Wedderburn C, Wear H, Brown J, Mason SJ, Barker RA, Hodges JR, Williams‐Gray C (2008): The utility of the Cambridge Behavioural Inventory in neurodegenerative disease. J Neurol Neurosurg Psychiatry 79:500–503. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR Jr, Boeve BF, Senjem ML, Baker M, Rademakers R, Ivnik RJ, Knopman DS, Wszolek ZK, Petersen RC, Josephs KA (2009a): Voxel‐based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology 72:813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Ivnik RJ, Vemuri P, Gunter JL, Senjem ML, Shiung MM, Boeve BF, Knopman DS, Parisi JE, Dickson DW, Petersen RC, Jack CR Jr, Josephs KA (2009b): Distinct anatomical subtypes of the behavioural variant of frontotemopral dementia: A cluster analysis study. Brain 132:2932–2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S (2001): Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging 20:45–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


