Abstract
The purpose of this study was to investigate the association between functional connectivity and β‐amyloid depositions in the default mode network (DMN) in Alzheimer's disease (AD), patients with mild cognitive impairment (MCI), and healthy elderly. Twenty‐five patients with AD, 12 patients with MCI, and 18 healthy controls were included in the study. Resting‐state functional magnetic resonance imaging was used to assess functional connectivity in the DMN. In parallel, amyloid burden was measured in the same subjects using positron emission tomography with carbon‐11‐labeled Pittsburgh Compound‐B as amyloid tracer. Functional connectivity of the DMN and amyloid deposition within the DMN were not associated across all subjects or within diagnostic groups. Longitudinal studies are needed to examine if amyloid depositions precede aberrant functional connectivity in the DMN. Hum Brain Mapp 35:779–791, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: amyloid, Alzheimer disease, mild cognitive impairment, positron emission tomography, magnetic resonance imaging, default mode network, resting‐state fMRI
INTRODUCTION
According to the amyloid hypothesis, amyloid pathology is the main factor driving Alzheimer's disease (AD) pathogenesis [Hardy and Selkoe, 2002]. The formation of tangles, inflammation, oxidative stress, synaptic inefficiency, and neuronal loss are thought to be secondary to the deposition of amyloid. By using positron emission tomography (PET) with the tracer carbon‐11‐labeled Pittsburgh Compound‐B ([11C]PIB), it is possible to measure amyloid burden in vivo [Klunk et al., 2004]. [11C]PIB is a sensitive marker of amyloid pathology in patients with AD, patients with mild cognitive impairment (MCI), and healthy controls [Mintun et al., 2006; Rabinovici and Jagust, 2009; Tolboom et al., 2009a]. Although there is ample evidence that amyloid pathology is closely related to AD, its effect on brain function is not completely understood.
Functional connectivity is an analysis of global functioning of the brain. Spatially independent brain areas that show temporally correlated sustained activity are considered to be functionally connected [Biswal et al., 2010]. One method to assess the functional connectivity is by means of resting‐state functional magnetic resonance imaging (rs‐fMRI). Exploring functional connectivity with means of rs‐fMRI has the advantage that it is a noninvasive modality to explore the brain and does not require subject interaction. This situation is especially advantageous when working with patients with AD, as they are often not able to participate in cognitively demanding tasks [Fox and Greicius, 2010; Greicius, 2008]. Previous rs‐fMRI studies have shown that patients with AD have already altered functional connectivity in the early phase of the disease [Greicius et al., 2004; Rombouts et al., 2009; Sanz‐Arigita et al., 2010]. It is believed that abnormal functional connectivity in AD might, at least in part, explain the cognitive decline seen in these patients [Stam et al., 2006; Wang et al., 2007]. One of the most investigated resting‐state networks is the default mode network (DMN). The DMN comprises a set of brain regions that is active during rest and is deactivated when engaged in a cognitively demanding task. Furthermore, its activity has been hypothesized to be implicated in memory function [Fox and Raichle, 2007; Raichle et al., 2001]. Regions belonging to the DMN are the posterior cingulate, precuneus, lateral parietal, lateral temporal, and medial prefrontal cortices [Raichle et al., 2001].
The areas composing the DMN show a striking overlap with regions with high amyloid depositions [Buckner et al., 2005; Hedden et al., 2009; Sheline et al., 2010; Sperling et al., 2009]. Based on this observation, it has been proposed that there might be a direct association between amyloid pathology and decreased functional connectivity in AD [Buckner et al., 2009]. To date, only a few studies have examined this hypothesis and mainly in cognitive healthy subjects. It has been shown that clinically healthy subjects with high amyloid load showed lower functional connectivity in the DMN at rest [Hedden et al., 2009; Sheline et al., 2010] and during a task [Sperling et al., 2009] than those with low amyloid burden. Similar results have been found in patients with MCI [Drzezga et al., 2011]. Based on these results, it has been proposed that “early manifestation of amyloid toxicity” can be detected using functional connectivity measures [Sheline et al., 2010]. However, evidence in favor of a direct relationship between amyloid deposition and functional connectivity across the continuum of AD is not completely clear.
Despite the increasing evidence of the relationship between amyloid accumulation and functional connectivity changes in the DMN, to date, no study has been published examining the association of amyloid depositions and functional connectivity in both patients with AD and patients with MCI and comparing this association with healthy controls.
MATERIALS AND METHODS
Participants
Twenty‐five patients with AD, 12 amnestic patients with MCI, and 18 healthy controls participated in this study. These participants represent a subset of individuals whose fMRI data [Binnewijzend et al., 2012] and PET data [Tolboom et al., 2009a] has been previously reported. However, the combined analysis of these data has not been reported earlier. All subjects received a standard dementia screening, which included medical history, extensive neuropsychological testing, physical and neurologic examination, structural MRI, and screening laboratory tests. The Mini‐Mental State Examination (MMSE) was part of neuropsychological testing. A multidisciplinary team assessed clinical diagnosis at baseline and at 1‐year follow‐up. All patients with AD met the NINCDS‐ADRDA criteria [McKhann et al., 1984] for “probable AD.” The patients with MCI met the Petersen criteria [Petersen et al., 1999] based on subjective and objective cognitive impairment, mostly affecting memory, in the absence of dementia or significant functional loss. The patients with AD and MCI were excluded when they had a history of major psychiatric or neurologic illness (other than AD), used nonsteroidal anti‐inflammatory drugs, moved excessively during MRI scanning, or showed clinically significant abnormalities based on the MRI scan as determined by a neuroradiologist. The same exclusion criteria were applied to healthy controls with the addition of subjective memory complaints. The study was approved by the Medical Ethics Review Committee of the VU University Medical Center. Written informed consent was obtained from subjects after receiving complete written and verbal description of the study.
PET Acquisition
PET scans were obtained on an ECAT EXACT HR+ scanner (Siemens/CTI, Knoxville, TN), equipped with a neuroinsert to reduce the contribution of scattered photons. This scanner enables the acquisition of 63 transaxial planes over a 15.5‐cm axial field of view, thus allowing the whole brain to be imaged in one bed position. The properties of this scanner have been reported elsewhere [Brix et al., 1997]. To restrict patient movement, a head holder was used. In addition, using laser beams, head movement was checked on a regular basis. First, a 10‐min transmission scan was acquired in two‐dimensional acquisition mode using three retractable rotating line sources. This scan was performed to correct the subsequent emission scan for photon attenuation. Next, a dynamic emission scan in three‐dimensional acquisition mode was acquired. This scan was started at the same time as the start of an intravenous injection of 351 ± 82 MBq [11C]PIB with a specific activity of 41 ± 22 GBq mmol−1. [11C]PIB was synthesized according to a modified procedure proposed by Wilson et al. 2004. The injection process was performed using an infusion pump (Med‐Rad, Beek, The Netherlands) at a rate of 0.8 mL s−1, followed by a flush of 42 mL of saline at 2.0 mL s−1. The emission scan consisted of 23 frames with progressively increasing duration (1 × 15, 3 × 5, 3 × 10, 2 × 30, 3 × 60, 2 × 150, 2 × 300, and 7 × 600 s) for a total scan time of 90 min.
MRI Data Acquisition
All subjects underwent structural MRI using a 1.5‐T Sonata scanner (Siemens Medical Solutions, Erlangen, Germany). Scanning included a coronal T1‐weighted three‐dimensional magnetization‐prepared, rapid‐acquisition gradient echo sequence (echo time = 3.97 ms; repetition time = 2,700 ms; inversion time = 950 ms; flip angle = 8°; 160 coronal slices; voxel size = 1 mm × 1.5 mm × 1 mm), which was used for coregistration, regions of interest (ROI) definition, and anatomical registration of functional data. For functional resting‐state acquisition, participants were instructed to close their eyes, lie as still as possible, and avoid falling asleep, and the lights were switched off. Resting‐state scans included 200 whole‐brain echo‐planar images sensitive to BOLD contrast (echo time = 60 ms; repetition time = 2,850 ms; flip angle = 90°; 36 axial slices; voxel size = 3.3 mm isotropic). This resulted in total scan duration of 9.5 min.
PET Data Analysis
All PET sinograms were corrected for dead time, tissue attenuation using the transmission scan, decay, scatter, and randoms. Images were then reconstructed using a standard filtered backprojection algorithm and a Hanning filter with a cutoff at 0.5 times the Nyquist frequency, resulting in a spatial resolution of 7 mm full width at half maximum (FWHM) at the center of field of view [Boellaard et al., 2001]. A zoom factor of 2 and a matrix size of 256 × 256 × 63 resulted in a voxel size of 1.2 mm × 1.2 mm × 2.4 mm. Structural T1 images were rigidly aligned to the corresponding PET images using a mutual information algorithm. No correction for partial volume effects was applied to the PET data. Partial volume effects could lead to an underestimation of [11C]PIB binding potential (BPND) [Fazio and Perani, 2000] especially in patients with AD. However, we found that correction using standard PVC methods on our [11C]PIB data was highly dependent on the actual method being used and appeared to result in overestimations of [11C]PIB binding. This might have been due to the use of 1.5‐T MRI structural T1 images, as these images have relatively lower resolution when compared with other MRI field strengths that could affect segmentation of gray and white matter. Therefore, PVC correction was not incorporated in this study; however, subject‐specific gray matter masks of the DMN were used to calculate regional [11C]PIB binding (see “Estimation of Average Functional Connectivity and [11C]PIB Binding Within Subject‐Specific Gray Matter DMN Masks” section), and total gray matter volume normalized for head size was used as covariate in all statistical analyses (see “Normalized Gray Matter Volume” section) as an alternative approach to correct for gray matter volume.
Parametric images of BPND of [11C]PIB were generated using a two‐step basis function implementation of the simplified reference tissue model, with cerebellar gray matter as reference tissue (RPM2) [Wu and Carson, 2002]. Cerebellar gray matter lacks Congo Red‐positive and thioflavin‐S‐positive plaques, and therefore, it was chosen as the reference tissue [Joachim et al., 1989; Yamaguchi et al., 1989]. It has been demonstrated that RPM2 is the parametric method of choice with respect to both bias and reproducibility [Tolboom et al., 2009b; Yaqub et al., 2008]. Data were then analyzed using PVE‐lab, a software program that makes use of a probability map based on 35 predefined ROIs [Svarer et al., 2005]. The ROIs were then projected onto [11C]PIB BPND images, and BPND of frontal (volume‐weighted average of orbital, medial inferior, and superior frontal), parietal, and temporal (volume‐weighted average of superior and medial inferior temporal), medial temporal (volume‐weighted average of enthorinal cortex and hippocampus), and posterior cingulate cortex were calculated. A global cortical ROI was defined based on the volume‐weighted average of all regions mentioned above.
To give insight into β‐amyloid load, independent of diagnostic status, the subjects were divided into two groups based on their global [11C]PIB BPND values. When individual mean global [11C]PIB BPND was higher than 0.54, the subjects were assigned to the PIB‐positive (PIB+) group. If values were lower than 0.54, the subjects were assigned to the PIB‐negative (PIB−) group. This cutoff of 0.54 of global [11C]PIB binding was based on a study of Tolboom et al. 2010. The subjects included in this study are a subset of subjects reported by Tolboom et al. 2010; [11C]PIB PET scans were performed and analyzed in a similar manner. It was found that 0.54 was the optimal cutoff that yielded 0.95 sensitivity and specificity for detecting AD, using clinical diagnosis as reference criterion. This differed only slightly from sensitivity (1.00) and specificity (0.85) of visual ratings.
MRI Data Analysis
Preprocessing of functional images was performed using FSL and included removal of all nonbrain voxels, motion correction, high pass temporal filtering of 100 s, spatial smoothing with a Gaussian kernel of FWHM 5.0 mm, and grand‐mean intensity normalization of the entire four‐dimensional dataset by a single multiplicative factor. Individual T1 images were registered to standard space using a nonlinear registration, with a warp resolution of 10 mm. Next, functional data were linearly registered to the corresponding subject's structural T1. The resulting transformation matrices were combined and used to project all functional data onto standard space (MNI152). For the group analysis, a multisession temporal concatenation ICA approach was applied on the preprocessed data of all subjects [Calhoun et al., 2001], which resulted in 25 components. From these components, the DMN was selected based on the brain regions that are commonly defined as constituents of the DMN [Biswal et al., 1995, Fox and Raichle, 2007]. The DMN map obtained from the group ICA was thresholded, using a z‐score of 3.1, which is representative of a probability value of <0.001 of finding the regions belonging to the DMN was due to chance. This thresholded DMN map was binarized and used as group‐wide DMN mask.
Second, a “dual‐regression” analysis was performed to obtain subject‐specific maps based on the group DMN [Beckmann et al., 2009]. First, the group‐level ICA spatial map was used in a linear regression against the individual fMRI datasets (spatial regression) to find temporal dynamics for each subject associated with the group‐level DMN map. This first spatial regression resulted in matrices describing temporal dynamics for each component and subject. Second, these matrices were used in a linear regression (temporal regression) against the associated fMRI dataset to estimate subject‐specific spatial maps. This second temporal regression resulted in subject‐specific maps, which are still associated with the group‐level spatial ICA maps [Beckmann et al., 2009; Filippini et al., 2009]. These subject‐specific maps are composed of β‐coefficients (regression coefficients), which are representative of the strength of the association of the temporal dynamics corresponding to the ICA spatial map for every voxel. Average functional connectivity within the DMN was calculated for each individual by taking average β‐coefficients within individual gray‐matter DMN masks. Estimation of these individual gray‐matter DMN masks is described later in detail. These average β‐coefficients within the individual gray‐matter DMN masks were used to assess group differences of DMN functional connectivity and the association of functional connectivity with [11C]PIB binding.
A more exploratory analysis was also performed by examining voxelwise differences of functional connectivity of the DMN between diagnostic groups and between PIB+ and PIB− subjects. For this analysis, a nonparametric significance test, that is, permutation, which is part of FSL (function “randomize,” FMRIB's Software Library, version 4.1.5, http://www.fmrib.ox.ac.uk/fsl), was performed. Age, sex, and normalized gray matter volume (NGMV) were used as covariates. Clinical diagnosis was used as extra covariate when examining the differences between PIB+ and PIB− subjects.
Normalized Gray Matter Volume
Gray matter volume, normalized for head size, was estimated using SIENAX (FMRIB's Software Library) [Smith, 2002]. SIENAX first removes nonbrain tissue of the subject's structural T1 image and then uses the brain and skull images to estimate the scaling factor between the T1 image and the standard space. Next, it performs tissue segmentation to estimate the volume of brain tissue and then multiplies this by the estimated scaling factor to correct for head size variability between subjects. NGMV is representative of total gray matter volume of the brain after correction for head size. NGMV was used as covariate in imaging and nonimaging statistics to correct for cortical atrophy.
Estimation of Average Functional Connectivity and [11C]PIB Binding Within Subject‐Specific Gray Matter DMN Masks
To calculate functional connectivity and average [11C]PIB BPND within the DMN for each subject, the group DMN mask was used and adjusted to include only gray matter for all individual subjects. First, individual T1 images were segmented (BET, FMRIB's Software Library) [Jenkinson and Smith, 2001] resulting in gray and white matter and cerebral spinal fluid. Next, only voxels of the group DMN mask corresponding to individual gray matter were selected from the group DMN mask, which resulted in a subject‐specific gray‐matter DMN mask. Finally, functional connectivity and average [11C]PIB BPND binding within the individual gray‐matter DMN masks were obtained.
Voxelwise Multiple Regression of Functional Connectivity Maps of the DMN With Average [11C]PIB Binding in DMN
To investigate the association of [11C]PIB binding in the DMN with functional connectivity of the DMN on a voxel level, a multiple regression analysis in SPM8 (Statistical Parametric Mapping, version 8) was performed. The regression analysis was performed across all subjects and within groups. As PIB− MCI patients may be more susceptible to suffer from different pathology other than AD [Quigley et al., 2011], the regression analysis across all subjects was also performed with the exclusion of PIB− MCI patients to best represent the continuum of AD. Age, sex, and NGMV were used as covariates in the regression analysis. Clinical diagnosis was used as extra covariate when performing regression analysis within PIB+ and PIB− subjects.
Nonimaging Statistics
All statistical analyses were performed using SPSS (version 15.0; SPSS, Chicago, IL). A χ 2 test was performed to examine frequency distributions of sex and PIB status within diagnostic groups. Differences between diagnostic groups on age, MMSE, and NGMV were explored using a one‐way ANOVA. Group differences between both patients with AD and patients with MCI and controls in both average functional connectivity within the DMN as well as in global [11C]PIB BPND and DMN [11C]PIB BPND values were examined using an analysis of covariance, with age, sex, and NGMV as covariates. Group differences between PIB+ and PIB− subjects in both average functional connectivity of the DMN as well as in [11C]PIB BPND values were examined using an analysis of covariance, with age, sex, NGMV, and diagnosis as covariates. Post hoc Bonferroni tests were used to correct for multiple comparisons. The association between functional connectivity of the DMN (dependent variable) with global [11C]PIB BPND, [11C]PIB BPND in the DMN, and MMSE score (explanatory variables) was assessed with a multiple linear regression analysis, both across all groups and within diagnostic groups, using age, sex, and NGMV as covariates. Again, the regression analysis across all subjects was also performed with the exclusion of PIB− MCI patients. Similarly, the association between functional connectivity of the DMN (dependent variable) with global [11C]PIB BPND, [11C]PIB BPND in the DMN, and MMSE score as explanatory variables was assessed with a multiple linear regression analysis within PIB+ and PIB− subjects, with age, sex, NGMV, and diagnosis as covariates. A P‐value of <0.05 was considered significant.
RESULTS
Mean age of all subjects was 65 years, of which 41 subjects were male. Demographic variables of the three diagnostic groups are listed in Table 1. Age and distribution of sex did not differ between the diagnostic groups. The DMN resulting from our group ICA included the posterior cingulate cortex, precuneus, cuneus, bilateral parietal cortex, angular gyrus, anterior cingulate cortex, medial frontal cortex, bilateral middle temporal gyrus, and bilateral thalamus. The group DMN mask is shown in blue in Figure 1. The regions of the DMN, identified in our sample, are consistent with regions normally reported belonging to the DMN [Raichle et al., 2001].
Table 1.
Subject demographics
| Across subjects | AD | MCI | Controls | P‐value | |
|---|---|---|---|---|---|
| N | 55 | 25 | 12 | 18 | |
| Age (years) | 65 ± 7.2 | 63 ± 6 | 68 ± 9 | 67 ± 6 | 0.07 |
| MMSE score | 26 ± 3.2 | 23 ± 2a, b | 28 ± 2 | 29 ± 0.6 | <0.01 |
| Male/female | 41/14 | 17/8 | 10/2 | 14/4 | 0.57 |
| PIB+/PIB− | 29/26 | 23/2a, b | 5/7 | 1/17 | <0.01 |
| Normalized gray matter volume (mm3) | 682,078 ± 63,094 | 672,513 ± 59,159a, b | 669,749 ± 59,823 | 703,051 ± 68,248 | <0.01 |
Data are presented as mean ± standard deviation. If analysis of variance was P < 0.05, a post hoc Bonferroni test was performed.
P < 0.05 when compared with controls.
P < 0.05 when compared with patients with MCI.
Figure 1.
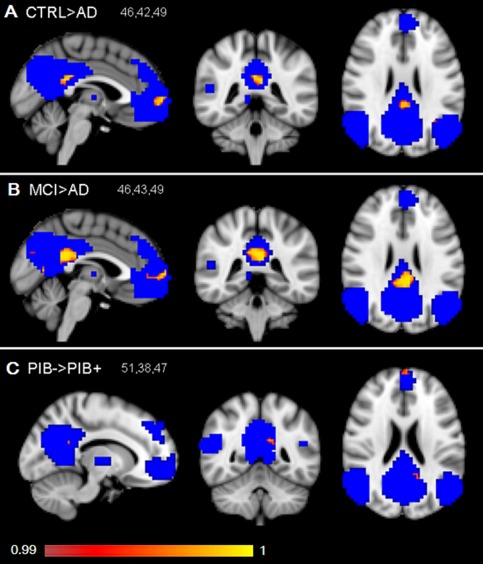
Voxelwise differences of functional connectivity within the DMN between groups were explored (uncorrected P < 0.01). Differences between groups are shown in red and yellow and are overlaid on the group DMN shown in blue. Patients with AD show decreased functional connectivity of the DMN when compared with healthy controls (A). Patients with AD show decreased functional connectivity of the DMN when compared with patients with MCI (B). PIB+ subjects showed decreased functional connectivity of the DMN when compared with PIB− subjects (C). The results are displayed on standard space (MNI152), 2 mm resolution, and radiological orientation. MNI coordinates are reported in the left upper corner of the figures.
[11C]PIB BPND
Global cortical BPND of [11C]PIB was highest in patients with AD, followed by patients with MCI, and lowest in controls. Similar patterns of average BPND of [11C]PIB were seen in the DMN. A statistical difference between diagnostic groups in both global binding and binding within the DMN was found. Post hoc Bonferroni test revealed that patients with AD showed significantly higher [11C]PIB BPND in the DMN (P < 0.01) and on global binding (P < 0.01) when compared with controls. Patients with AD showed significantly higher [11C]PIB BPND in the DMN (P < 0.01) and on global binding (P < 0.01) when compared with patients with MCI. Patients with MCI did not differ from controls on [11C]PIB BPND in the DMN (P = 0.26) and on global binding (P = 0.31).
Functional Connectivity of DMN in Diagnostic Groups
When examining the average β‐coefficients within individual DMN masks, functional connectivity in the DMN was reduced in patients with AD when compared with healthy controls (P < 0.05). No significant difference in average functional connectivity within the DMN between patients with AD and patients with MCI was found (P = 0.06). In addition, no significant difference in average functional connectivity within the DMN between patients with MCI and controls was found (P = 0.98). Voxelwise analysis showed decreased functional connectivity in part of the posterior cingulate cortex and the medial frontal cortex in patients with AD when compared with controls (uncorrected p < 0.01; Fig. 1A). Voxelwise analysis also showed decreased functional connectivity in the posterior cingulate cortex and medial frontal cortex in patients with AD when compared with patients with MCI (uncorrected P < 0.01; Fig. 1B). When corrected for multiple comparisons, no significant voxels remained. No significant differences on a voxel level were found on functional connectivity of the DMN between patients with MCI and controls.
Functional Connectivity of DMN in PIB+ and PIB− Subjects
Based on the average individual global [11C]PIB BPND, 29 subjects were assigned to the PIB+ group and 26 subjects to the PIB− group. PIB+ subjects showed reduced average functional connectivity of the DMN when compared with PIB− subjects (P < 0.05). Voxelwise exploration showed that the decreases in functional connectivity of the DMN in PIB+ subjects when compared with PIB− subjects were mainly present in small regions of the left posterior cingulate cortex and the frontal pole (uncorrected P < 0.01; Fig. 1C). When corrected for multiple comparisons, no significant voxels remained.
Association of Functional Connectivity and [11C]PIB Binding in DMN
No association between average functional connectivity and average [11C]PIB BPND within the DMN was found across subjects or within the diagnostic groups, PIB+ or PIB− subjects, using nonimaging regression analysis (Table 2). The results were not significantly altered when excluding PIB− MCI patients when examining the association across groups. Voxelwise linear regression across subjects revealed that decreased functional connectivity of the DMN within a small part of the posterior cingulate cortex (Fig. 2A) was related to higher levels of [11C]PIB binding in the DMN (uncorrected P < 0.001). When excluding PIB− MCI patients from the voxelwise across subject analysis, results were only slightly altered. We found that a smaller region of significant voxels remained, but within the same part of the precuneus, confirming the original effect (Fig. 2B). When examining the association of [11C]PIB in DMN with functional connectivity in the DMN per voxel in PIB− subjects only (Fig. 2C), it was found that decreased functional connectivity of the DMN in the left superior occipital cortex and a small part of the right precuneus cortex was associated with higher binding of [11C]PIB in the DMN (uncorrected P < 0.001). Voxelwise regression analysis showed no associations of [11C]PIB in the DMN within functional connectivity of the DMN per voxel (for uncorrected P < 0.001), within patients with AD, patients with MCI, controls, or PIB+ subjects only.
Table 2.
Association of functional connectivity in DMN with global [11C]PIB BPND, [11C]PIB BPND in the DMN, and global cognition (MMSE score) across subjects and within groups
| Across subjects | Across subjects (excluding PIB− MCI patients) | AD | MCI | Controls | PIB+ | PIB− | |
|---|---|---|---|---|---|---|---|
| Global [11C]PIB BPND | −0.21 (P = 0.11) | −0.24 (P = 0.09) | −0.27 (P = 0.24) | −0.01 (P = 0.98) | −0.22 (P = 0.43) | −0.18 (P = 0.32) | −0.15 (P = 0.52) |
| [11C]PIB BPND DMN | −0.19 (P = 0.15) | −0.20 (P = 0.16) | −0.17 (P = 0.49) | −0.07 (P = 0.87) | −0.07 (P = 0.80) | −0.12 (P = 0.50) | −0.14 (P = 0.54) |
| MMSE score | 0.16 (P = 0.26) | 0.14 (P = 0.37) | 0.13 (P = 0.60) | 0.04 (P = 0.94) | 0.09 (P = 0.80) | 0.05 (P = 0.85) | 0.62 (P = 0.12) |
Regression analysis‐standardized β‐coefficient values (with corresponding P‐values) are reported, explaining the association of functional connectivity in the DMN (dependent variable) with global [11C]PIB BPND, [11C]PIB BPND in the DMN, and MMSE score as explanatory variables. Age, sex, and total gray matter volume normalized for head size (NGMV) were used as covariates. To assess the association within PIB+ and PIB− subjects, clinical diagnosis was used as extra covariate besides age, sex, and NGMV.
Figure 2.
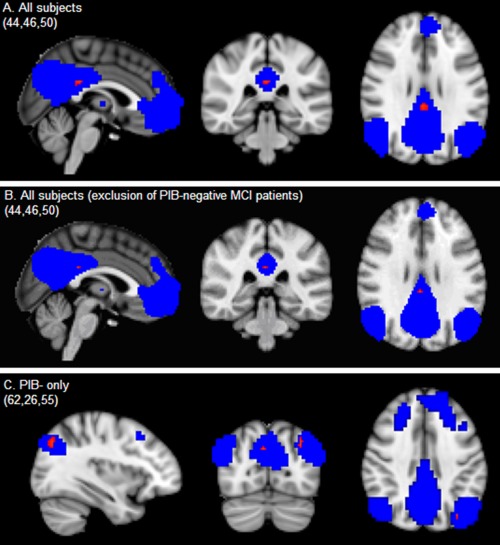
Multiple regression analysis of average [11C]PIB binding in the default mode network (DMN) with voxelwise functional connectivity of the DMN (uncorrected P < 0.001) was examined across subjects (A), across subjects with exclusion of PIB− MCI patients (B), and within PIB− subjects (C), correcting for age, sex, and normalized gray matter volume (extra correction for clinical diagnosis in PIB subjects). Significant associations are plotted in red (uncorrected P < 0.001) on top of the blue group DMN mask on 2‐mm MNI152 space. MNI coordinates are reported in the left upper corner of the figures.
The linear relationship between functional connectivity and [11C]PIB BPND in the DMN across subjects (not correcting for age, sex, and NGMV) is shown in Figure 3. Of the 12 patients with MCI, five patients converted to AD after a follow‐up period of 1 year. These MCI converters are marked in Figure 3 by a box. Three of these converted patients with MCI were in the range of patients with AD on both variables, showing low functional connectivity as well as high [11C]PIB BPND values at baseline. One of the converted patients with MCI showed increased [11C]PIB binding in the DMN; however, functional connectivity still appeared in the normal range. One MCI converter displayed neither increased [11C]PIB binding nor decreased functional connectivity.
Figure 3.
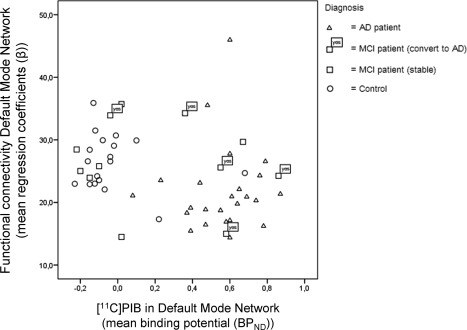
This scatter plot represents the association of functional connectivity of the DMN (y‐axis) with amyloid load in the DMN (x‐axis) for each subject. No correction for age, gender, or NGMV is performed in this graph. Patients with MCI who converted to AD after 1 year are represented by a box with the text “yes.”
Association of Functional Connectivity and [11C]PIB BPND in the DMN With MMSE Score
No association between functional connectivity of the DMN and MMSE score across all groups and within diagnostic groups was found (Table 2). Reduced functional connectivity of the DMN appeared to be associated with lower MMSE score in PIB− subjects only; however, this association was not significant (ρ = 0.62, P = 0.12).
DISCUSSION
To summarize, amyloid load in the DMN was highest in patients with AD, intermediate in patients with MCI and lowest in controls. Reduced functional connectivity of the DMN was found in patients with AD when compared with controls, which seemed to be most pronounced in the posterior cingulate and medial frontal cortex. Similarly, reduced functional connectivity of the DMN was found in PIB+ subjects when compared with PIB− subjects, independent of clinical diagnosis, which was mainly expressed in the left posterior cingulate cortex and the frontal pole. With respect to our main research question, no direct association of functional connectivity of the DMN with amyloid depositions within the DMN across the spectrum of AD was found. Our more exploratory, voxelwise regression analysis did show reduced functional connectivity in parts of the DMN associated with lower levels of amyloid in the DMN in PIB− subjects only. Combining both techniques in early stages of the disease might still provide complementary information about conversion to AD. To the best of our knowledge, this is the first study to examine functional connectivity and its association with amyloid in both patients with AD and patients with MCI and to compare this association with healthy controls.
The DMN was chosen as target of our study because of its involvement in memory function and its overlap with regions that have high amyloid buildup [Buckner et al., 2005]. The brain regions found to be part of the DMN in our dataset are consistent with earlier reports [Raichle et al., 2001]. The group DMN in our study was identified using ICA based on our entire sample, including both healthy controls and patients. To identify subject‐specific representations of the DMN, the ICA was followed by a dual‐regression analysis. Other studies perform ICA on an independent group of healthy controls in order to identify the DMN and to exclude possible influence of patients on the shape and extent of the DMN [Sorg et al., 2007]. The dual‐regression analysis performed in this study is designed to find subject‐specific maps based on individual time activity patterns corresponding to the areas of the group DMN [Beckmann et al., 2009]. Using this methodology, we were still able to identify the DMN with an anatomical pattern similar to the one found in controls. We found that functional connectivity of the DMN was decreased in patients with AD when compared with controls. By combining all subjects in our ICA, we might end up with more conservative results when describing decreased functional connectivity in patients with AD.
Highest [11C]PIB BPND values in the DMN were found in patients with AD and lowest values in controls, with intermediate values in patients with MCI, which is in agreement with the studies examining global [11C]PIB BPND values [Rabinovici and Jagust, 2009]. With respect to functional connectivity, we confirm earlier results describing reduced functional connectivity of the DMN in patients with AD when compared with controls [Binnewijzend et al., 2012; Rombouts et al., 2009]. Voxelwise analysis revealed that decreases in functional connectivity of the DMN in patients with AD when compared with controls were present in posterior part of the cingulate cortex and the medial frontal cortex. These findings on a voxel level should be made with caution; after correction for multiple comparisons, no significant voxels remained. These results overlap partly with the findings of Binnewijzend et al. 1995 and Sanz‐Arigita et al. 2006; functional connectivity decreases of the DMN in patients with AD when compared with controls were found in the posterior cingulate cortex and precuneus.
No association of functional connectivity in the DMN with amyloid load in the DMN was found. A similar lack in association with functional connectivity of the DMN was found with global cortical amyloid load. Reduced average DMN functional connectivity was not associated with increased [11C]PIB binding across all subjects or within diagnostic groups. The more exploratory, voxelwise regression analysis showed that increased [11C]PIB binding in the DMN was related to decreased functional connectivity of the DMN in the posterior cingulate cortex across all subjects, which was probably averaged out by our nonimaging regression analysis. The association across groups found on a voxel level might reflect a group effect; patients with AD show decreased connectivity and increased amyloid load in the DMN when compared with controls. Other studies did report a relationship of global functional connectivity with amyloid depositions in the precuneus/posterior cingulate across groups, which remained significant after correction for gray matter volume [Drzezga et al., 2011]. This difference could be due to the fact that a global measure of functional connectivity might be more sensitive in detecting changes in overall organization in relation to amyloid load. This is further discussed in more detail.
No association between functional connectivity of the DMN and amyloid depositions was found within diagnostic groups. Combining both [11C]PET and fMRI in certain subjects at risk for AD (patients with MCI) might still provide insight in progression to AD, even though we did not find a direct relationship between both measures. Five of the 12 patients with MCI in our study converted to AD after 1 year; of which one subject was not PIB+ at baseline. It is important to note that not all patients with MCI convert to AD and might also reflect different underlying pathologies. Three of the five converted patients with MCI were in the range of patients with AD on both variables, showing low functional connectivity as well as high [11C]PIB BPND values at baseline. However, the other two patients with MCI who converted to AD showed different patterns. Larger samples of patients with MCI that either convert to AD or remain stable are necessary to explore the additional value of combining [11C]PIB PET and resting‐state fMRI for progression to AD.
As mentioned above, the fact that no associations between functional connectivity of the DMN and amyloid depositions were found within diagnostic groups could be due to the small number of subjects or the low variance in [11C]PIB binding seen in patients with AD and in controls. Importantly, amyloid accumulation in patients with AD reaches a plateau, and neuronal dysfunction still worsens [Jack et al., 2010]. Therefore, in patients with AD, a direct association of β‐amyloid with functional connectivity might not be expected. This makes amyloid load perhaps more suitable as an “on–off” variable and not as continuous variable, especially when examining patients with AD. Therefore, subjects were either classified as PIB+ or PIB−, independent of clinical diagnosis. In small areas in the right posterior cingulate cortex and the medial frontal cortex, functional connectivity of the DMN was found to be decreased in amyloid‐positive subjects when compared with amyloid‐negative subjects. This agrees with the previous findings that decreases in DMN connectivity were observed in healthy elderly with high amyloid load when compared with healthy elderly with low amyloid [Hedden et al., 2009; Sheline et al., 2010; Sperling et al., 2009]. In our study, patients with MCI and AD were also included in the PIB+ group, and therefore, comparisons with other studies focusing only on healthy elderly should be made with caution. The general trend seems to be similar; subjects with increased amyloid load show decreased functional connectivity of the DMN, where the posterior cingulate cortex, precuneus, and medial prefrontal cortex are most affected. Our voxelwise regression analysis showed that in PIB− subjects, amyloid burden in the DMN seemed to be related to decreased functional connectivity in the left occipital cortex and in the precuneus. This association in PIB− subjects was not confirmed by our nonimaging regression; however, this could be because the effect was averaged out. In PIB+ subjects, no association was found. As mentioned earlier, this lack of association is probably due to the fact that the PIB+ subjects have already reached their “plateau” with respect to amyloid binding.
Drzezga et al. 2009 not only described the direct correlation between whole‐brain functional connectivity and amyloid depositions but also included [18F]FDG PET as a measure of metabolism. An important difference is that in the study of Drzezga et al. 2009, global functional connectivity of the brain was examined, whereas in our study, only connectivity of a single resting‐state network was examined. Whole‐brain connectivity was negatively correlated with amyloid load as measured with [11C]PIB in both healthy controls and patients with MCI [Drzezga et al., 2011]. In addition, decreased whole‐brain functional connectivity showed a high overlap (42%) with decreased metabolism as measured with [18F]FDG PET. Decreased metabolism in the posterior cingulate cortex was related to increased [11C]PIB binding within this same region, pointing toward neuronal dysfunction leading related to the presence of amyloid [Drzezga et al., 2011]. The authors hypothesized that emerging amyloid load may initiate the synaptic dysfunction and disrupted functional connectivity in these important hub regions.
Several studies have described that accumulation of amyloid is followed by synaptic dysfunction, neuronal loss, and finally resulting in cognitive dysfunction [Hardy and Selkoe, 2002; Jack et al., 2010]. In contrast, others have suggested that increased neuronal activity might lead to amyloid accumulation [Buckner et al., 2005]. Using transgenic mouse models, it has been shown that neuronal activity will increase the presence of amyloid in the extracellular space and the synaptic transmission enhances amyloid precursor protein endocytosis, which in turn results in increased amyloid depositions [Cirrito et al., 2008]. This would explain why the regions of the DMN are especially vulnerable to amyloid depositions; the DMN is composed of very active and highly connected brain regions that continuously mediate information throughout the entire brain [Buckner et al., 2009]. It has been reasoned that most changes in amyloid accumulation and neuronal dysfunction occur in pre‐AD stages [Jack et al., 2010]. The investigation of longitudinal changes on functional connectivity and amyloid load in healthy elderly who are amyloid‐positive and patients with MCI would therefore provide best insight into the order of events. This study was not designed to make any statements concerning the order of events. Larger samples and follow‐up measurements including both imaging methods are needed.
LIMITATIONS AND FUTURE RECOMMENDATIONS
The added difficulty of combining both MRI and PET techniques limits the number of subjects under study. Second, NGMV was used as covariate in statistical analysis to correct for atrophy, which is especially important in the AD population. Gray matter loss will probably affect [11C]PIB binding, but only by diminishing the effect in patients with AD. The opposite is true for functional connectivity, which could result in making the correlations reported in this study to seem stronger than they truly are. It should be noted that NGMV as covariate does not directly correct for regional variance in gray matter volume. PVC is often applied to PET data to address the problem of partial volume effects [Fazio and Perani, 2000]. However, this correction was not performed in the current study as we found that PVC could result in overestimations of the [11C]PIB binding. This could have been due to the native resolution of the 1.5‐T MRI structural images used for segmentation purposes. To avoid this effect, we would recommend to perform future studies on a MRI scanner with a higher field strength. For this study, only functional connectivity of the DMN was examined, not taking into account functional connectivity in other networks. It has been proposed that enhanced activity might result in an increase in amyloid burden, which in turn could result in reduced functional connectivity through neuronal cell death on a later stage. To explore this last hypothesis, a longitudinal analysis in an MCI population (taking into account AD converters and nonconverters) or healthy elderly with high amyloid load will be necessary. For the across‐group analyses described in this article, PIB− MCI patients were included which might have influenced the results. PIB− MCI patients may be more susceptible to suffer from different pathology other than AD [Quigley et al., 2011], and the across‐group results should therefore be interpreted with caution. However, exclusion of PIB− MCI patients did not alter the results. Finally, other measures of AD pathology, for example, measure of metabolism, might provide additional information on the changes in functional connectivity.
CONCLUSIONS
In this study, decreased functional connectivity of the DMN and amyloid buildup in the DMN were not directly associated along the continuum of AD pathology.
Disclosure: Dr. S.A.R.B. Rombouts is supported by a grant from The Netherlands Organization for Scientific Research (NWO). Dr. B.N.M. van Berckel received research support from the American Health Assistance Foundation, Alzheimer Association, Internationale Stichting Alzheimer Onderzoek, Center of Translational Molecular Medicine, and the Dutch Organization for Scientific Research. Dr. F. Barkhof serves on the editorial boards of Brain, European Radiology, Journal of Neurology, Neurosurgery, and Psychiatry, Journal of Neurology, Multiple Sclerosis, and Neuroradiology and serves as a consultant for Bayer‐Shering Pharma, Sanofi‐Aventis, Biogen‐Idec, UCB, Merck‐Serono, Jansen Alzheimer Immunotherapy, Baxter, Novartis, and Roche. There are no other actual or potential conflicts of interest to disclose. All authors have read and agreed with the contents of the manuscript. The results of the study have not been published before and they are not under consideration to be published by another journal.
REFERENCES
- Beckmann CF, Mackay CE, Filippini N, Smith SM (2009): Group comparison of resting‐state fMRI data using multi‐subject ICA and dual regression. Neuroimage 47 ( Suppl 1):S148. [Google Scholar]
- Binnewijzend MAA, Schoonheim MM, Sanz‐Arigita E, Wink AM, van der Flier WM, Tolboom N, Adriaanse SM, Damoiseaux JS, Scheltens P, van Berckel BNM, Barkhof F (2012): Resting‐state fMRI changes in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging 33:2018–2028. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP (2010): Toward discovery science of human brain function. Proc Natl Acad Sci USA 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boellaard R, van Lingen A, Lammertsma AA (2001): Experimental and clinical evaluation of iterative reconstruction (OSEM) in dynamic PET: Quantitative characteristics and effects on kinetic modelling. J Nucl Med 42:808–817. [PubMed] [Google Scholar]
- Brix G, Zaers J, Adam LE, Bellemann ME, Ostertag H, Trojan H, Haberkorn U, Doll J, Oberdorfer F, Lorenz WJ (1997): Performance evaluation of a whole‐body PET scanner using the NEMA protocol. National Electrical Manufacturers Association. J Nucl Med 38:1614–1623. [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA (2005): Molecular, structural, and functional characterization of Alzheimer's disease: Evidence for a relationship between default activity, amyloid and memory. Neurobiol Dis 25:7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews‐Hanna JR, Sperling RA, Johnson KA (2009): Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability and relation to Alzheimer's disease. Neurobiol Dis 29:1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM (2008): Endocytosis is required for synaptic activity‐dependent release of amyloid‐β in vivo. Neuron 58:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga AJ, Becker A, van Dijk KRA, Sreenivasan A, Talukdar T, Sullivan C, Schultz AP, Sepulcre J, Putcha D, Greve D, Johnson KA, Sperling RA (2011): Neuronal dysfunction and disconnection of cortical hubs in non‐demented subjects with elevated amyloid burden. Brain 134 ( Part 6):1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio F, Perani D (2000): Importance of partial‐volume correction in brain PET studies. J Nucl Med 41:1849–1850. [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE (2009): Distinct patterns of brain activity in young carriers of the APOE‐ε4 allele. Proc Natl Acad Sci USA 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Greicius M (2010): Clinical application of resting state functional connectivity. Front Syst Neurosci 17:4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Greicius MD (2008): Resting‐state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 21:424–430. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V (2004): Default‐mode network activity distinguishes Alzheimer's disease from healthy aging: Evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ (2002): The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science 297:353–357. [DOI] [PubMed] [Google Scholar]
- Hedden T, van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL (2009): Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci 29:12686–12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen PC, Trojanowski JQ (2010): Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Joachim CL, Morris JH, Selkoe DJ (1989): Diffuse senile plaques occur commonly in the cerebellum in Alzheimer's disease. Am J Pathol 135:309–319. [PMC free article] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B (2004): Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound‐B. Ann Neurol 55:306–319. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 34:939–944. [DOI] [PubMed] [Google Scholar]
- Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC (2006): [11C]PIB in a nondemented population. Potential antecedent marker of Alzheimer disease. Neurology 67:446–452. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E (1999): Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol 56:303–308. [DOI] [PubMed] [Google Scholar]
- Quigley H, Colloby SJ, O'Brien JT (2011): PET imaging of brain amyloid in dementia: A review. Int J Geriatr Psychiatry 26:991–999. [DOI] [PubMed] [Google Scholar]
- Rabinovici GD, Jagust WJ (2009): Amyloid imaging in aging and dementia: Testing the amyloid hypothesis in vivo. Behav Neurol 21:117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, Damoiseaux JS, Goekoop R, Barkhof F, Scheltens P, Smith SM, Beckmann CF (2009): Model‐free group analysis shows altered BOLD fMRI networks in dementia. Hum Brain Mapp 30:256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz‐Arigita EJ, Schoonheim MM, Damoiseaux JS, Rombouts SARB, Maris E, Barkhof F, Scheltens P, Stam CJ (2010): Loss of ‘small‐world’ networks in Alzheimer's disease: Graph analysis of fMRI resting‐state functional connectivity. PLoS One 5:e13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA (2010): Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 67:584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Mühlau M, Calhoun VD, Eichele T, Läer L, Drzezga A, Förstl H, Kurz A, Zimmer C, Wohlschläger AM (2007): Selective changes of resting‐state networks in individuals at risk for Alzheimer's disease. Proc Natl Acad Sci USA 104:18760–18765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O'Keefe K, O'Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA (2009): Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ, Jones BF, Manshanden I, van Cappellen van Walsum AM, Montez T, Verbunt JPA, de Munck JC, van Dijk BW, Berendse HW, Scheltens P (2006): Magnetoencephalographic evaluation of resting‐state functional connectivity in Alzheimer's disease. Neuroimage 32:1335–1344. [DOI] [PubMed] [Google Scholar]
- Svarer C, Madsen K, Hasselbalch SG, Pinborg LH, Haugbol S, Frokjaer VG, Holm S, Paulson OB, Knudsen GM (2005): MR‐based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage 24:969–979. [DOI] [PubMed] [Google Scholar]
- Tolboom N, Yaqub M, van der Flier WM, Boellaard R, Luurtsema G, Windhorst AD, Barkhof F, Scheltens P, Lammertsma AA, van Berckel BNM (2009a)Detection of Alzheimer pathology in vivo using both [11C]PIB and [18F]FFDNP PET. J Nucl Med 50:191–197. [DOI] [PubMed] [Google Scholar]
- Tolboom N, Yaqub M, Boellaard R, Luurtsema G, Windhorst AD, Scheltens P, Lammertsma AA, van Berckel BNM (2009b)Test–retest variability of quantitative [11C]PIB studies in Alzheimer's disease. Eur J Nucl Med Mol Imaging 36:1629–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolboom N, van der Flier WM, Boverhoff J, Yaqub M, Wattjes M, Raijmakers PG, Barkhof F, Scheltens P, Herholz K, Lammertsma AA, van Berckel BNM (2010): Molecular imaging in the diagnosis of Alzheimer's disease: Visual interpretation of [11C]PIB and [18F]FDDNP PET images. J Neurol Neurosurg Psychiatry 81:882–884. [DOI] [PubMed] [Google Scholar]
- Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T (2007): Altered functional connectivity in early Alzheimer's disease: A resting‐state fMRI study. Hum Brain Mapp 28:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AA, Garcia A, Chestakova A, Kung HF, Houle S (2004): A rapid one‐step radiosynthesis of the β‐amyloid imaging radiotracer N‐methyl‐[11C]2‐(4′‐methylaminophenyl)‐6‐hydroxybenzothiazole ([C‐11]‐6‐OH‐BTA‐1). J Labelled Comp Radiopharm 47:679–682. [Google Scholar]
- Wu Y, Carson RE (2002): Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J Cereb Blood Flow Metab 22:1440–1452. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Hirai S, Morimatsu M, Shoji M, Nakazato Y (1989): Diffuse type of senile plaques in the cerebellum of Alzheimer‐type dementia demonstrated by β‐protein immunostain. Acta Neuropathol 77:314–319. [DOI] [PubMed] [Google Scholar]
- Yaqub M, Tolboom N, Boellaard R, van Berckel BN, van Tilburg EW, Luurtsema G, Scheltens P, Lammertsma AA (2008): Simplified parametric methods for [11C]PIB studies. Neuroimage 42:76–86. [DOI] [PubMed] [Google Scholar]


