Abstract
Humans vary substantially in their ability to learn new motor skills. Here, we examined inter‐individual differences in learning to play the piano, with the goal of identifying relations to structural properties of white matter fiber tracts relevant to audio‐motor learning. Non‐musicians (n = 18) learned to perform three short melodies on a piano keyboard in a pure audio‐motor training condition (vision of their own fingers was occluded). Initial learning times ranged from 17 to 120 min (mean ± SD: 62 ± 29 min). Diffusion‐weighted magnetic resonance imaging was used to derive the fractional anisotropy (FA), an index of white matter microstructural arrangement. A correlation analysis revealed that higher FA values were associated with faster learning of piano melodies. These effects were observed in the bilateral corticospinal tracts, bundles of axons relevant for the execution of voluntary movements, and the right superior longitudinal fasciculus, a tract important for audio‐motor transformations. These results suggest that the speed with which novel complex audio‐motor skills can be acquired may be determined by variability in structural properties of white matter fiber tracts connecting brain areas functionally relevant for audio‐motor learning. Hum Brain Mapp 35:2483–2497, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: corticospinal tract, fractional anisotropy, inter‐individual differences, piano learning, tract‐based spatial statistics (TBSS)
INTRODUCTION
Learning new motor skills occurs over the entire human life span, but individuals differ substantially in their ability to acquire these skills rapidly [e.g., Ackerman, 1987; Braadbaart et al., 2012; Tubau et al., 2007]. Motor learning is the process of acquiring new movements, or a new sequence of known movements, that can be turned into effortless performance through training. During the process of motor learning, complex interactions take place in movement‐related brain networks, such as cortico‐striatal and the cortico‐cerebellar systems [Coynel et al., 2010; Doyon et al., 2009] and individual learning and task performance are related to individual patterns of brain activation revealed by various neuroimaging techniques [Della‐Maggiore and McIntosh, 2005; Grafton et al., 1994; Jenkins et al., 1994; Schlaug et al., 1994; Toni et al., 1998]. Furthermore, there is growing evidence that structural changes in brain regions specifically relevant for motor learning are linked to specialized motor skills and motor expertise, e.g., in professional musicians [Bangert and Schlaug, 2006; Gaser and Schlaug, 2003; Han et al., 2009; Hutchinson et al., 2003].
The integrity of white matter fiber tracts is decisive for information transfer between gray matter brain areas. White matter structural differences can be investigated in vivo between individuals using diffusion‐weighted magnetic resonance imaging (DWI). This technique is based on the fact that white matter tracts feature a coherent geometrical orientation, which constrains water molecules to diffuse preferentially along the tract rather than perpendicular to it. The attenuation of spin‐echo signals associated with the displacement of water molecules in different directions can be measured using DWI. Using measurements along a sufficient number of directions, a model can be fitted to the data from each voxel to infer the presence of one or more families of fibers. The orientation of the fiber tracts can be deduced from the principal direction of water diffusion, alongside several measures of the microstructural properties of white matter in that voxel [Le Bihan et al., 2001]. One such measure is fractional anisotropy (FA), which quantifies how strongly directional the diffusion of water in each voxel is [Basser et al., 1994]. FA values range from 0 to 1, with values close to 0 indicating voxels in which water diffuses nearly uniformly in every direction, as in the cerebrospinal fluid, while values approaching 1 indicate one strongly preferential direction of diffusion, like in the body of the corpus callosum.
FA is a complex measure that can be influenced by various structural properties of the white matter, including axonal density and size, myelination, as well as fiber complexity [Beaulieu, 2002]. Studies on alterations of integrity and structural properties of white matter fiber tracts suggest that these structural features may influence the speed of neural signal propagation, thereby influencing behavior by leading to more or less efficient information transfer: Variability and alterations in the white matter properties, as reflected by FA values, have been examined in different groups of individuals with different skill levels [Bengtsson et al., 2005; Elmer et al., 2011; Halwani et al., 2011; Han et al., 2009; Oechslin et al., 2009] and deficits [Avery et al., 2012; Bonzano et al., 2011; Ha et al., 2011; Nickl‐Jockschat et al., 2012].
Musicians are often used as a model to examine the effects of extensive motor training on neural mechanisms and structural characteristics of the human brain [Bangert and Schlaug, 2006; Bangert et al., 2006; Baumann et al., 2007; Bengtsson et al., 2005; Han et al., 2009; Münte et al., 2002; Schlaug et al., 2005]. These studies describe training induced changes in white matter properties, which provide insights into fiber tracts that might be important for motor learning associated with playing a musical instrument. For example, Bengtsson et al. [2005] found greater structural coherence in white matter fiber tract organization (reflected in higher FA values) in areas of the corticospinal tract, specifically the posterior branch of the internal capsule, for musicians compared to non‐musicians [see also Han et al., 2009]. The corticospinal tract is a collection of axons that travel from cortical motor areas and terminate on motor neurons in the spinal tract for control of voluntary movements [Martin, 2003]. Additionally, the characteristics of the white mater fiber tracts in the posterior branch of the internal capsule, as well as in the corpus callosum, were related to the amount of hours of piano practice during childhood [Bengtsson et al., 2005].
Another study found that musicians (instrumentalists and singers), compared to non‐musicians, showed larger tract volume and higher FA values in the arcuate fasciculus (a part of the superior longitudinal fasciculus, SLF), which is a white‐matter tract connecting regions that are fundamental to sound perception and production [Halwani et al., 2011]. Abnormality in the right arcuate fasciculus has been related to pitch‐discrimination impairment [Loui et al., 2009]. Consistent with this, significant correlations were observed between performance on a pitch‐based artificial grammar learning task and FA in white matter underlying the supramarginal gyrus, corresponding to the right temporal–parietal junction of the arcuate fasciculus [Loui et al., 2011]. The SLF connects frontal, parietal, and temporal lobes [Makris et al., 2005], which contain motor related areas that are important for perception–action associations [Rizzolatti and Craighero, 2004], including associative links between actions and sounds [Gazzola et al., 2006; Keysers et al., 2003]. These areas are also involved in imitational motor learning [Iacoboni, 2005].
More generally, the variability in white matter structures, characterized by FA, has been related to various performance parameters in different non‐musical tasks. For example, Della‐Maggiore et al. [2009] found that the rate of learning a visuo‐motor task was correlated with properties of white matter (expressed by higher FA for better task performance) in the superior cerebellar peduncle; Wolbers et al. [2006] found that higher proficiency in mental rotation was associated with higher values of FA underlying the inferior parietal cortex; Johansen‐Berg et al. [2007] showed that variations in task performance during a bimanual coordination task are correlated with white matter properties in a specific region in the body of the corpus callosum. Additionally, studies on motor training provide evidence for plasticity in white matter fiber tract architecture by showing microstructural changes in FA after a certain amount of training [Scholz et al., 2009; Taubert et al., 2010].
Such variations of white matter fiber tract properties and their relation to task performance suggest that these inter‐individual differences in the architecture of white matter might reflect potential for motor learning [Sisti et al., 2012; Steele et al., 2012; Tomassini et al., 2011]. In the present study, we investigated whether underlying structural differences in white matter fiber tracts explain inter‐individual differences in progress during sensory‐motor learning. The present study thus examines structural properties that might be prerequisites for swift audio‐motor learning and therefore adds knowledge to the existing literature that shows evidence for the reversed causation, i.e., training‐induced structural changes in gray and white matter [e.g., Bengtsson et al., 2005; Draganski et al., 2004; Gaser and Schlaug, 2003; Scholz et al., 2009; Taubert et al., 2010; Sagi et al., 2012]. We used DWI to characterize structural properties of white‐matter tracts by means of FA. Learning to play simple piano melodies with the right hand via audio‐motor training procedure served as a model task for motor sequence learning. Our main behavioral measure consisted of the time required to master this task, which was novel for the non‐musician participants. White matter tracts connecting areas that are relevant during the initial phases of audio‐motor learning were considered to be of particular interest. Learning times were expected to be associated with structural properties of white matter tracts connecting the cerebellum, basal ganglia, and spinal cord to frontal cortical motor and association areas. Thus, the entire bilateral corticospinal tracts, as well as the tracts of the cerebellar peduncles, were chosen as tracts of interest. Furthermore, the bilateral SLF, which connect premotor areas and the inferior parietal lobe—and thus play a critical role in perception–action associations—were also included as tracts of interest.
METHODS
Participants
Twenty‐two healthy volunteer non‐musicians participated in the current study. Four male participants had to be removed from the analysis due to insufficient quality of the acquired diffusion‐weighted (DW) images. The mean age of the remaining 18 participants was 21.8 years (SD = 2.4 years, range 18–26 years; 11 females). None of the participants had received any previous musical training (with the exception of obligatory music classes at school) and none had played the piano before. All participants were right‐handed according to the Edinburgh handedness inventory [Oldfield, 1971] and had normal vision or vision was corrected to normal by contact lenses. They gave their written informed consent to participate, were naïve to the hypotheses, and received monetary compensation in return for participation. The experiment was performed in accordance with ethical standards compliant with the declaration of Helsinki and had been approved by the Medical Ethical Committee of the University Medical Center Groningen, the Netherlands.
Overview of the Study Design
The current analysis is part of a more extensive project, which investigated the cross‐modal transfer of piano sequences trained under audio‐motor and visuo‐motor conditions [see Engel et al., 2012]. The present article provides a detailed description only of procedural information that is relevant to the present research question (i.e., the audio‐motor training condition, especially the first training day and DWI scanning session; more information about the entire procedure can be found in the Supporting Information).
Motor training conditions
Each of the non‐musicians participated in a pure audio‐motor training procedure (scheduled for 2 h per day across three consecutive days) in which they learned to perform three short melodies (each consisting of seven notes) on a piano keyboard with their right hand. The training procedure entailed participants listening to melodies without observing the corresponding finger movements, and subsequently attempting to reproduce these melodies on a piano keyboard with the sight of their own hand occluded by a cover over the keyboard (see Fig. 1A for experimental setup). Thus, learning proceeded in an exploratory fashion through trial‐and‐error. Participants also experienced an equivalent visuo‐motor training procedure (scheduled for 2 h per day across three consecutive days). This procedure entailed learning to perform sequences on a mute piano‐keyboard by observing videos showing a real right hand in a bird's eye view performing silent finger movements on a piano keyboard (i.e., without hearing corresponding tones). In that condition, participants were allowed to see their own fingers playing on the piano keyboard (i.e., the cover over the piano keyboard that occluded sight in the audio‐motor training condition was removed, see Fig. 1A). This visuo‐motor training condition is not of interest for the present analysis, which concerns relations between initial learning abilities in a new complex motor task and properties of the white matter fiber tracts. Pre‐existing visuo‐motor associations related to seeing specific finger movements and actually moving the corresponding fingers exist [Brass et al., 2001, 2000; Iacoboni et al., 1999], due to the experience of participants seeing their own fingers “in action,” for example, when pressing keys while typing on a computer. In contrast, the piano‐naïve participants of the present study lack associations between the sound of particular notes and the finger movements to produce them. Despite the visuo‐motor training condition being not well suited to the purpose of this study, exploratory analyses of visuo‐motor training data can be found in the Supporting Information.
Figure 1.
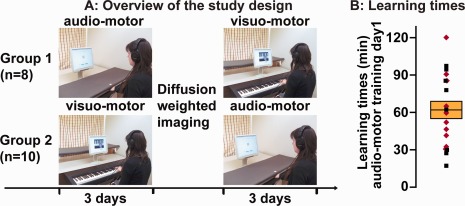
Overview of the study design (A) and learning times (B) for the first audio‐motor training day. A: Eight participants (Group 1) started with the audio‐motor training condition and 10 participants (Group 2) started with the visuo‐motor training condition. Both training conditions lasted three days. DWI scanning was performed the following day. One day (n = 9), two days (n = 4) or three days (n = 5) later participants completed the other motor training condition (lasting three days), which was the visuo‐motor training condition for eight participants and the audio‐motor training condition for 10 participants. Pictures show the experimental setup for the computer‐based audio‐motor and visuo‐motor training procedure for learning melodies on a piano keyboard (vision of own fingers was occluded in the audio‐motor training condition). B: Learning times in minutes for learning to play three melodies at two positions on the first training day in a slow tempo (60 bpm) in the audio‐motor training condition. The middle line of the box plot shows the mean learning time for the whole group. Height of the box plot indicates the standard error of mean of the group. Single dots represent single participants; red rotated dots indicate those participants who received visuo‐motor before audio‐motor training sessions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Design of the training and scanning procedure (Fig. 1A)
Eight of the participants of the analyzed sample of the present study started with the audio‐motor training condition, the other ten participants started with the visuo‐motor training condition (note that four participants were excluded from the present analysis, which resulted in an unequal number of participants in the groups). After the first three days of motor training, a scanning session took place on the following (fourth) day, during which DWI was performed in order to acquire data describing properties of white matter fiber tracts. Thus, DWI images were acquired about (mean ± SD) 24 ± 5 h after the final motor training session of the first motor training condition. The non‐musicians participated in a further three‐day motor training procedure (scheduled for 2 h on each day), in the other modality, one day (n = 9), two days (n = 5), or three days (n = 4) after the scanning session: visuo‐motor training condition for the eight participants that had started with the audio‐motor training sessions, and audio‐motor training condition for the ten which had started with the visuo‐motor training sessions. Comparing behavioral learning measures of the subgroups with different training order, that is, whether participants started with audio‐motor (n = 8; learning time on the first audio‐motor training day, mean ± SD: 61 ± 32 min) or visuo‐motor training condition (n = 10; learning time on the first audio‐motor training day, mean ± SD: 62 ± 29 min), did not reveal significant differences (t(16)<1; P = 0.95, two‐tailed) for performance in the audio‐motor training condition (c.f. other learning measures in the Supporting Information Table S1). Thus, performance in the audio‐motor training condition did not seem to be influenced by the prior visuo‐motor training procedure. Accordingly, learning measures of the audio‐motor training condition of both training order groups can be analyzed as a group.
To take into account whether participants started with the audio‐motor or visuo‐motor training condition, the training order has been included as a covariable in the model for calculating the relations between learning times and FA values (see “DWI data acquisition and analysis” below).
Stimuli for Musical Training
In the audio‐motor training procedure, participants learned to perform three different melodies using their right hand (e.g., Supporting Information Audio S1, Audio S2, and Fig. S1). In the visuo‐motor training procedure, participants learned to perform three different silent finger sequences, corresponding to melodies, on the piano keyboard (Supporting Information Fig. S1). All melodies consisted of seven notes spanning three bars (four eighth notes and two quarter notes in the first two bars, and one half note in the third bar; 2/4 meter; 6 s duration at slow tempo, i.e., 60 beats per minute, bpm). All three melodies were learned in two positions on the keyboard, using five adjacent white keys with each finger assigned to one piano key for each position. One position ranged from C4 to G4 and the other ranged from G4 to D5 (finger assignments position 1/position 2: C4/G4—thumb, D4/A4—index finger, E4/B4—middle finger, F4/C5—ring finger, G5/D5—little finger). This means that performing both melodies required an identical motor pattern (with the same timing, i.e., the same rhythm) but the corresponding sounds were lower or higher and the position of the hand for playing on the keyboard was shifted by five white keys (a musical interval of a fifth). Each of the three melodies had a different rhythm. Each melodic sequence required each of the five white keys on the piano keyboard to be played at least once, and two keys were played twice.
Training Procedure
Audio‐motor (as well as visuo‐motor) training sessions took place on three successive days. On the first training day, participants learned to perform all three melodies in both positions at a slow tempo (60 bpm). On the second day, participants repeated the three melodies in the two positions at the slow tempo and learned to perform them at a fast tempo (120 bpm). On the third day, participants repeated the melodies at the fast tempo to ensure robust sensory‐motor associations and to consolidate learning.
Training procedure was standardized using a computer‐based training method with MIDI‐based software [MaxMSP 5, http://cycling74.com/; see Bangert et al., 2001, for a comparable training procedure]. Participants wore headphones and could navigate through the training program by pressing foot pedals of a digital piano. A visuo‐tactile landmark (a 2 cm × 1 cm × 0.5 cm piece of white foam) attached to the piano key G4, as well as on the corresponding position on the cover of the keyboard (Fig. 1A), helped participants to position their hand (little finger at the marked key = lower position; thumb at the marked key = higher position) and allowed participants to find that key by touch alone. During training sessions, participants were required to go through series of trials until they achieved error‐free performance in terms of the order of tones and the rhythm of the melody through trial‐and‐error learning: Each presentation of the entire model melody could be started with the right foot pedal of the piano. Before trying to reproduce the melody (a trial), participants had to push the left foot pedal and then they had to press seven piano keys in order to perform the model melody. Participants were allowed to repeat model melodies and trials as often as desired. Pushing the foot pedals before presentations of the model melody and trials allowed the number of presentations and trials needed to perform each melody correctly three times to be measured. The pedal press that started the model melody for the first time and the end of the last trial, i.e., when participants played that model melody the third time correctly, defined the learning time for one melody.
The MIDI‐based computer program recorded both the identity and onset times of participants' key presses. A performance was considered to be correct if the identity and rhythm of participant's key presses matched the model melody. A performed rhythm was considered to be correct if the length of a played note (calculated by inter‐onset intervals of key presses) differed by not more than a fourth of the requested length of a note (e.g., a quarter note at the tempo 60 bpm should have a duration of 1000 ms, therefore played lengths between 750 and 1250 ms were considered to be correct). The computer program notified participants of errors (“order and/or rhythm correct/incorrect”) and an experimenter gave verbal feedback at regular intervals (i.e., approximately every 5 min) about the specific errors made by the participant. After playing a melody three times correctly (i.e., the learning criteria) in one position, participants were required to produce the same melody in the other position correctly three times. Whether participants started with the lower or higher position or with which melody they started was counterbalanced over the course of the training sessions.
The amount of time needed to learn to perform the three different melodies at the slow tempo (60 bpm) in two positions (each three times correctly performed) on the first training day was obtained with the described procedure using the computer program (summing the learning times measured for each single melody in each position). These initial learning times were used for the present analyses. Please see the section “Supplementary Results” in the Supporting Information for analyses on the other training parameter of the first audio‐motor training day.
The complete training procedure employed across the three training days included repetitions of the computer‐based training program (described above) across the three days in addition to an over‐learning phase taking place on the second and the third training day (details can be found in the Supporting Information, Supplementary Methods, Motor training procedure over the course of three days). These repetitions of learned sequences served to consolidate motor programs for reaching automaticity in performance [see Engel et al., 2012]. The over‐learning phase aimed at further compensating for initial inter‐individual learning differences (and intra‐individual learning differences for the different melodies and the two different motor training conditions in the entire project; this over‐learning phase was essential for the analyses reported by Engel et al., [2012]). Performance of learned sequences was comparable between participants at the end of the motor training procedure: All participants were able to play all melodies by heart (which was verified by a free recall test). Due to this training procedure aiming at minimizing inter‐individual differences in performance, parameters of other training days were less suitable for addressing the goals of the present study, which was to investigate initial inter‐individual differences in learning abilities. Therefore, analyses reported in the main manuscript focused on the learning times obtained on the first audio‐motor training day (details for results on analyses for the second and third training day are reported in the Supporting Information, Supplementary Results). For further use in control analyses, we have calculated the total training time across the first three days of motor training sessions before DWI scanning. For this purpose, the training times were summed across training days including the repetitions of the computer program and time spent in the over‐learning procedure. Please note that the over‐learning procedure aimed at compensating for initial inter‐individual learning differences. Thus, fast learners had to spend relatively more time in the over‐learning procedure.
A debriefing session took place after each motor training day. Participants were asked about the strategy they adopted for learning to play the piano melodies and what aspect of the task was most difficult for them. Additionally, they were asked to rate the overall difficulty of the task on a four‐point rating scale (4 = very difficult; 3 = difficult; 2 = not that difficult; 1 = easy).
DWI Data Acquisition and Analysis
During DWI data acquisition, participants lay in supine position on the scanner bed while wearing scanner‐compatible headphones (MRconfon GmbH, Magdeburg, Germany; http://www.mr-confon.de/). DW images were acquired using a single‐shot pulsed gradient spin echo echo‐planar imaging (EPI) sequence (repetition time (TR) = 8981 ms, echo time (TE) = 60 ms, sensitivity encoding (SENSE) factor = 2.8) on a 3T Philips Achieva scanner, equipped with an eight‐channel synergy SENSE head coil for excitation and signal collection. Each volume consisted of 50 transverse slices, recorded with a 96 × 96 matrix (field of view (FOV) 224 × 224 mm, voxel size 2.5 × 2.5 mm, slice thickness 2.5 mm, no gap). DW images were acquired along 60 directions optimized using an electrostatic repulsion model [Jones et al., 1999], using a maximum gradient strength of 40 mT/m and a b‐value = 1000 s/mm2. Six non‐DW images (b = 0 s/mm2, i.e., referred to as b0 image) were also obtained for each dataset. Two sets of images were acquired using two opposing phase‐encoded directions (traversing k‐space bottom‐up, denoted as APA, and top‐down, denoted as APP). From these two images, a displacement field was determined and used for image restoration [Andersson et al., 2003].
Images were processed using the FMRIB Diffusion toolbox FDT of FSL 4.1 (The Oxford Centre for Functional Magnetic Resonance Imaging of the Brain; http://www.fmrib.ox.ac.uk/fsl). Images were first corrected for subject movement and eddy‐currents. Next, the two sets of images acquired in two directions (APP, APA) were averaged using the procedure of Andersson et al. [2003]. After that, for each voxel the diffusion tensor was fitted to the data, and the FA measure was derived. Voxelwise statistical analysis of FA data was conducted using tract‐based spatial statistics [TBSS; Smith et al., 2006], which is part of FSL. A mean FA image was created and thinned to generate a mean FA skeleton [threshold was set at an FA value of 0.2, see Smith et al., 2006]. This procedure has been previously shown [Smith et al., 2006] in order to approximate the location of the center of the major tracts common to all participants (see Fig. 2, the FA skeleton is displayed in green color in the brain pictures). FA images were visually inspected after non‐linear registration to check for distortions generated by the registration procedure.
Figure 2.
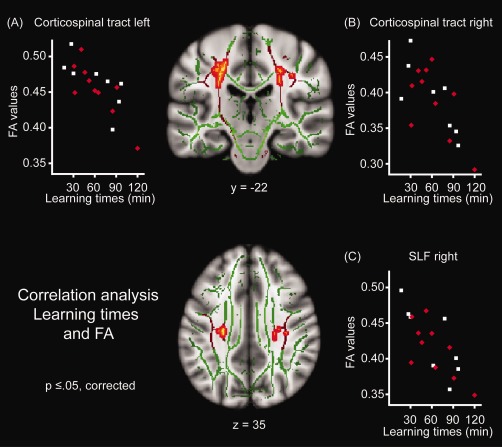
Results for the correlation analysis on learning times of the first audio‐motor training day and FA values. Brains displayed in the center show in a coronal (y = −22; upper brain) and in an axial (z = 35; lower brain) section the localization of the significant clusters (red‐yellow). These clusters are localized on the bilateral cortical spinal tracts and the right SLF comprising voxels that show a significant correlation of faster learning of piano melodies in the audio‐motor training condition and higher FA values (P ≤ 0.05, corrected). The mean FA skeleton, which represents the center of all tracts, common to all participants, is displayed in green. The tracts that belong to the region of interest for the present analysis are indicated in red. The scatter plots show the correlation of the learning times in minutes on the first audio‐motor training day (x‐axis) and the FA values in arbitrary units extracted from the most significant voxel in the (A) left corticospinal tract (MNI coordinates: x = −26, y = −19, z = 30; P = 0.022, corrected); (B) right corticospinal tract (MNI coordinates: x = 25, y = −26, z = 31; P = 0.045, corrected); and (C) in the right SLF (MNI coordinates: x = 36, y = −22, z = 35; P = 0.050, corrected). Single dots represent single participants; red rotated dots indicate those participants who received visuo‐motor before audio‐motor training sessions (see Methods, section “Overview of the study design”). L, left; R, right; SLF, superior longitudinal fasciculus; FA, fractional anisotropy; y and z values for the brain sections refer to MNI coordinates. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Inferential statistics for performing group analyses were carried out by means of nonparametric permutation testing [Holmes et al., 1996; Nichols and Holmes, 2001] implemented in FSL's tool “randomise” (using 5000 permutations). Results are reported at a significance level of P < 0.05, corrected for multiple comparisons using threshold‐free cluster enhancement using the default values implemented in the option “‐‐T2” of FSL's “randomise” tool [Smith and Nichols, 2009]. For exploratory reasons, some results are reported additionally at a more liberal significance level of P < 0.1, corrected for multiple comparisons.
Analyses comprised (1) correlations between learning times and FA values of single participants; (2) correlations between age of participants and FA values, and (3) differences in FA values between participants who started with the audio‐motor training condition (n = 8) or the visuo‐motor training condition (n = 10). The latter two models were calculated in order to examine the influence for possible confounding variables on FA. In all models, a covariate containing the number 1 for participants who started with the audio‐motor training condition or 2 for participants who started with the visuo‐motor training condition before participating in the audio‐motor training sessions was included in order to partial out the influence of training order. Furthermore, for an additional control analysis, the total training time spent in the first three days of motor training sessions before DWI scanning was entered as control variable in model (3) for the group comparison.
All analyses were performed either using a mask containing specific tracts of interest or on the mean FA skeleton mask for whole brain analyses. Masks for tracts of interest, which were selected based on our hypotheses, were created using the atlas toolbox of FSL (http://www.fmrib.ox.ac.uk/fsl/fslview/atlas-descriptions.html). From the Jülich atlas the binarized 50% probability map was used to create masks for the corticospinal tracts. The JHU white matter label atlas (without probability information) was used to create masks for the cerebellar peduncles (superior, middle, and inferior) and the superior longitudinal fasciculi (SLF; Please note that this atlas does not differentiate between different subcomponents of the SLF described by Makris et al. [2005]; Different atlases were used because cerebellar peduncles were not included in the Jülich atlas and the SLF of the Jülich atlas has a more limited extent that does not reflect the gray matter brain connections know from the literature, e.g., Makris et al. [2005]. On the other hand, the corticospinal tract as labeled in the JHU white matter label atlas was more limited, missing some essential parts of the corticospinal tract.). All masks were created in a first step separately for the right and the left hemisphere and then these binary masks were multiplied by the mean FA skeleton mask. In a second step, all single masks were combined to create one region of interest (number of voxels: 9543; see Fig. 2, brain pictures—tracts belonging to the region of interest are displayed in red). There are no overlapping voxels in tracts for different parts of the cerebellar peduncles. The left corticospinal tract contains 49 voxels that are also assigned to the left SLF. The right corticospinal tract contains 83 voxels that are also assigned to the right SLF tract. Inferential statistics were carried within that resulting single region of interest. This procedure minimizes the error associated with finding false positive results since fewer multiple comparisons are performed than if each tract is tested separately as a region of interest.
RESULTS
Behavioral Data
Learning measures
The 18 non‐musician participants showed substantial variance in the initial time needed to learn to play the three melodies in two positions (same motor pattern) at least three times correctly (mean ± SD: 62 ± 29 min; range: 17–120 min; see Fig. 1B) via the audio‐motor training procedure. The correlation between initial learning time and the age of the participants was not significant, but did show a trend for slower learning times in older participants (r = 0.42, P = 0.094, two‐tailed test; training order was included as covariate in order to partial out any effect it may have had). Supporting Information Table S1 2nd column shows the learning times, number of presentations, and number of trials needed to reach the performance criterion (three correct performances of all three melodies in the two positions) in the audio‐motor training condition as a function of training day. Participants needed less time for relearning the melodies on the second day (slow tempo, mean ± SD: 19 ± 11 min, range: 7–39 min; learning the fast tempo, mean ± SD: 18 ± 14 min, range: 3–46 min) and on the third training day, mean ± SD: 6 ± 4 min, range: 3–13 min; c.f. Supporting Information Table S1 2nd column). However, inter‐individual differences in learning times were still existent as evidenced by the correlation of learning times across days (Ps < 0.001; c.f. Supporting Information Table S3, B).
Furthermore, we have calculated the training times summed across the first three training days before DWI scanning including the times for repetitions of the computer program and the over‐learning procedure, regardless of the motor‐training condition (referred to total training time across the first three motor training days before DWI scanning). Participants were engaged in a motor training procedure for 175 ± 62 min (mean ± SD; range: 103–306 min) on the first three training days (before DWI scanning took place). This total training time of the first three training days did not differ significantly (t(16) < 1, P = 0.49) between the participants who first underwent the audio‐motor training procedure (n = 8, mean ± SD: 187 ± 65 min; range: 108–295 min) and those participants who first underwent the visuo‐motor training procedure (n = 10, mean ± SD: 166 ± 61min; range: 103–306 min). Learning times for the initial audio‐motor training procedure (first application of the computer‐based training program) and total learning time across the first three training days were highly correlated: r = 0.92, P < 0.001 (two‐tailed test; training order was included as control variable in order to partial out any effect it may have had). Furthermore, we tested the relation between total training time received in the first three motor training days (before DWI scanning, irrespective of whether this was the audio‐motor or visuo‐motor training condition) and the initial learning times of the first training day after DWI scanning (regardless of whether this was the audio‐motor or visuo‐motor training condition). In this correlation analysis, we included training condition as a control variable and found a significant result: r = 0.83, P < 0.001 (two‐tailed test). Participants who needed more time on the first three days of (auditory or visual) motor training also needed more time to learn the new sequences in the other (visual or auditory) motor training condition.
Debriefing
After the first audio‐motor training day, participants rated the difficulty of the task on a four‐point scale (4 = very difficult; 3 = difficult; 2 = not that difficult; 1 = easy). All participants found the task rather difficult (mean ± SD: 3.2 ± 0.65). Debriefing of participants about what they found most difficult revealed that the majority of participants (12 out of 18) judged hearing the difference between tones and finding the corresponding keys as being most difficult. The remaining six of the 18 participants mentioned other aspects to be most difficult (finding the start of the melody, n = 2; a specific melody, n = 2; process of matching the sounds of the model melody and self‐produced tones, n = 1; keeping the fingers on the same keys throughout a trial, n = 1).
DWI Data
First, we tested whether there are significant differences in FA values in any white matter tracts between participants who started with the audio‐motor training condition (n = 8) and participants who started with the visuo‐motor training condition (n = 10): No significant different FA values could be detected in any tracts neither using the mask for tracts of interest (corticospinal tract, SLF or cerebellar peduncles) nor in a whole brain analysis at significance levels P < 0.05 or P < 0.1, corrected for multiple comparisons. These results hold true also when the total training time received in the first three training days before DWI scanning is entered into the model as a control variable.
Correlation analysis of FA values and practicing times needed for learning to play the three piano melodies on the first audio‐motor training day identified significant results in clusters assigned to the left corticospinal tract (cluster extend: 290 voxels) and the right corticospinal tract (cluster extend: 87 voxels; see Table 1, Fig. 2): Individuals with shorter learning times had higher FA values. Both clusters extended into parts of the cortical spinal tract that overlap with the SLF (left hemisphere: 13 of 290 voxels; right hemisphere: 4 of 87 voxels). Lowering the threshold revealed that the cluster in the right hemisphere reached into the SLF (in total 82 voxels belong to parts of the SLF that does not intersect with the corticospinal tract at P < 0.1, corrected). In the left hemisphere, four voxels were detected in parts of the SLF that does not intersect with the corticospinal tract at P < 0.05, corrected (in total six voxels belong to parts of the SLF that does not intersect with the corticospinal tract at P < 0.1, corrected). All clusters in both hemispheres were located in close proximity of the primary motor and somatosensory cortices. No significant correlations in any tracts of the cerebellar peduncles were identified at the P < 0.05 level or P < 0.1 (corrected). Including age of the participants as a covariate in the model in addition to training order leads to similar results, still evidencing correlations of FA values with learning time in the audio‐motor training condition in the corticospinal tracts and the SLF. Furthermore, relations between higher FA values in the left corticospinal tract and faster relearning on the second and third training day remained significant (c.f. Supporting Information, Supplementary Results, section “Other audio‐motor training days”).
Table 1.
Localization and characteristics for results of the FA analyses
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Anatomical region | Hemisphere | x | y | z | P‐value (corr) | Cluster size |
| A) Analysis for ROI | ||||||
| Corticospinal tract | L | −26 | −19 | 30 | 0.022 | 290a |
| Corticospinal tract | R | 25 | −26 | 31 | 0.045 | 87a |
| Superior longitudinal fasciculus | R | 36 | −22 | 35 | 0.050 | 82b |
| Superior longitudinal fasciculus | L | −30 | −24 | 40 | 0.047 | 6b |
| B) Analysis for the whole brain | ||||||
| Cluster I | 281b | |||||
| Corticospinal tract | R | 25 | −26 | 31 | 0.096 | |
| Superior Corona radiata | R | 24 | −25 | 34 | 0.094 | |
| Posterior Corana radiata | R | 24 | −27 | 33 | 0.094 | |
| Superior longitudinal fasciculus | R | 29 | −24 | 40 | 0.097 | |
| Cluster II | ||||||
| Corpus callosum | R | 17 | −22 | 35 | 0.090 | 21b |
The values shown are Montreal Neurological Institute (MNI) coordinates and the corrected P‐value for the most significant voxel (as expressed by the lowest P‐value) in a cluster that belongs to a specific white matter fiber tract. Cluster size refers to the number of voxels (voxel size 1 mm × 1 mm × 1 mm) within a labeled tract. The cluster on the corticospinal tract in (A) comprises voxels that were also assigned to the superior longitudinal fasciculus: 13 of the 290 voxels in the left and 4 of the 87 voxels in the right hemisphere.
R, right; L, left; ROI, region of interest (comprising the corticospinal tract, the superior longitudinal fasciculus and the cerebellar peduncles).
Number of voxels in the cluster that have a significance level of P < 0.05, corrected.
Number of voxels in the cluster that have a significance level of P < 0.1, corrected.
In order to explore whether the identified significant correlations in the bilateral corticospinal tract and the right SLF are still present when groups were divided according to their training order, we correlated the FA values of the maximum voxels of the bilateral corticospinal tracts and the right SLF with the learning times from the initial audio‐motor training times separately for the subgroup who started with the audio‐motor training condition (n = 8) and the subgroup who had the visuo‐motor training condition before the audio‐motor training sessions (n = 10). All correlations remained significant in both groups (Ps < 0.05, two tailed testing; see correlation coefficients in Table S4 in the Supporting Information and for illustration the scatter plots in Fig. 2A–C).
In addition, we explored whether identified significant correlations in the bilateral corticospinal tract and the right SLF remained if the total training time on the first three days of motor training (before DWI scanning) is partialed out. To this end, we correlated the FA values of the maximum voxels of the bilateral corticospinal tracts and the right SLF with the learning times from the initial audio‐motor training times again, using the total training time of the first three motor training days as a control variable. The correlations in the left corticospinal tract (r = −0.50, P < 0.05) but not in the right corticospinal tract (r = −0.11, P = 0.68) and the right SLF (r = −0.39, P = 0.13) remained significant (using two‐tailed tests).
A whole brain analysis did not reveal any significant correlations between learning time in the audio‐motor training condition and FA values in any other white matter fiber tracts at a significance level P < 0.05, corrected. However, at a marginal significance level of P < 0.1 (corrected), the whole brain analysis showed a cluster (281 voxels) in the right hemisphere extending from the corticospinal tract into the SLF, the superior and posterior corona radiata and a further cluster (21 voxels) in the body of the corpus callosum. Furthermore, no positive correlations between learning times and FA, and no significant correlations between the participant age and FA, were found in any tract belonging to the region of interest or in whole brain analyses (at the P < 0.05 or P < 0.1 level, corrected).
DISCUSSION
The present study investigated relations between learning of a new complex audio‐motor task—piano playing—and properties of white matter fiber tracts. We found substantial individual differences in the speed with which non‐musicians learned to play three different melodies on a piano keyboard under purely audio‐motor training conditions. A correlation analysis revealed that faster learning was associated with higher FA values in the bilateral corticospinal tracts and the right SLF. No significant correlations were found in the cerebellar peduncles. In the following, we will first discuss the cognitive and audio‐motor demands of our task, and the relevance of the aforementioned white matter fiber tracts for audio‐motor learning of piano melodies.
Participants showed substantial variability in their ability to learn to perform three different piano melodies, expressed by time required as well as other parameters (number of presentations and trials needed). However, all participants managed to play these three melodies three times correctly on the first training day. Differences between participants in the learning parameters were smaller but still evident on subsequent training days (Supporting Information Tables S1, S3), and participants who were faster on the first three days of motor training before DWI scanning (no matter which training condition) were also faster in the second training condition after DWI scanning. These results suggest that inter‐individual differences in learning abilities are stable across days and training conditions, and could therefore reflect a relatively stable difference in the brain of the participants. The decrease in learning times on subsequent training days might be a result of repetitions and over‐learning of the training procedure. Learning took place via an audio‐motor training procedure, during which the vision of the participants' fingers was occluded and they likewise never saw the finger movements associated with the model melodies. Thus, the non‐musicians not only had to learn new sequences of finger movements, but were also required to learn to map specific sounds to specific finger movements. Our task therefore required the formation of novel audio‐motor associations. Furthermore, all melodies were characterized by a specific rhythm, making timing accuracy an inherent demand of our task. Taken together, the task of learning to play piano melodies was challenging enough to reveal stable inter‐individual differences in audio‐motor learning speed.
We found that faster learning times in the audio‐motor training condition were associated with higher FA values in the bilateral corticospinal tracts, while the significant cluster had a greater extent in the left hemisphere which is contralateral to the right hand used in the motor task. The corticospinal tract contains fibers originating from cortical motor areas (mainly from the primary motor cortex, but also from the somatosensory cortex, supplementary motor areas, and premotor cortex) terminating on neurons in the spinal tract, and is important for the control of voluntary movements of the contralateral side of body [Martin, 2003]. As mentioned in the results, the clusters showing higher FA values in faster learners were located close to the primary motor and somatosensory cortex. Thus, higher FA values could be related to the finer control of body movements in participants who mastered the task more swiftly. This finer corticospinal control might directly benefit our task and/or might be an indicator for a more elaborate representation of the body in sensorimotor cortices, which in turn would benefit sensorimotor learning.
In addition, faster learners had higher FA values in the right corticospinal tract, that is, ipsilateral to the hand used to perform the task. Activation of the ipsilateral motor cortex has been observed for unimanual actions (e.g., movement sequences with multiple fingers or repetitive “chords” composed of three simultaneous key presses) and seems to be dependent on the complexity of the given motor task [Verstynen et al., 2005]. As described above, our task was challenging and complex (as indicated by the difficulty ratings of the participants)—and might therefore recruit ipsilateral motor‐related brain areas. More efficient ipsilateral connections, as reflected by higher FA values in the right corticospinal tract, might make this ipsilateral involvement more effective.
Previous studies have reported motor training related changes in the properties of white matter in parts of the corticospinal tract—namely the posterior internal capsule [Bengtsson et al., 2005; Han et al., 2009]. However, our analysis did not reveal significant results in this part of the corticospinal tract. In our study, we investigated differences in initial learning abilities and properties of white matter fiber tracts in non‐musicians (i.e., non‐motor experts for piano playing), whereas Bengtsson et al. [2005] and Han et al. [2009] investigated professional musicians (i.e., motor experts for piano playing who received a huge amount of training in piano playing). When investigating professional musicians, it is difficult to determine whether group differences in the posterior part of the internal capsule are the result of the extensive training received by the pianists, or the reason why they were drawn to or selected for extensive piano practice in the first place. Our finding that FA values predict initial learning times, in the absence of extensive piano training experience, sheds new light in this issue by showing that FA differences seem to influence the initial piano‐learning aptitude of individuals.
The right SLF also showed higher FA values for participants who learned more swiftly to play the piano melodies in the audio‐motor training condition. The SLF interconnects the temporal and parietal lobe with the frontal lobes [Makris et al., 2005]. Parts of the SLF (the arcuate fasciculus) have been identified as relevant to pitch‐discrimination impairments and individual differences in pitch learning [Loui et al., 2009, 2011]. The SLF provides connections between premotor areas and the inferior parietal lobe—areas known to be involved in perception–action matching mechanisms in general [Rizzolatti and Craighero, 2004], and the transformation of sounds into actions in particular [Gazzola et al., 2006; Keysers et al., 2003]. This fronto‐parietal network has been shown to be involved in auditory piano learning [Lahav et al., 2007] and piano expertise [Bangert et al., 2006]. Discriminating tones and translating the perceived sound to a corresponding action was judged to be the most difficult aspect of our piano‐learning task by the majority of the non‐musicians. Thus, connections provided by the SLF may play an essential role for mastering the audio‐motor component of the audio‐motor piano‐learning procedure efficiently. Our results suggest that certain properties of white matter fiber tract organization, as reflected by higher FA values in the SLF, facilitate the acquisition of the audio‐motor associations necessary for mastering a musical instrument, and may thus reflect a predisposition to instrumental musical ability.
In our study, we did not detect relations between properties of white matter fiber tracts in the cerebellar peduncles and piano‐learning abilities in non‐musicians. We had hypothesized such relations due to the involvement of the cortico‐cerebellar loop in motor skill learning [Kelly and Strick, 2003]. The cerebrocerebellum is strongly implicated in planning the timing of movements [for review see Ivry et al., 2002; Molinari et al., 2007; O'Hearn and Molliver, 2001]. However, during debriefing, none of the participants mentioned the learning of rhythm or timing to be particularly difficult (on the first audio‐motor training day); it was reportedly most difficult for the majority to find the piano key corresponding to a heard tone (i.e., the audio‐motor mapping) on the first audio‐motor training day. Participants learned the melodies at a slow tempo, which allowed for some degree of temporal imprecision (see Methods section). Thus, highly precise timing abilities (as needed, e.g., in professional piano performance or playing the melodies at a fast tempo) were not critical for learning piano melodies at the slow tempo in our task on the first training day given the allowance for a certain degree of timing error. Consequently, inter‐individual differences in such timing abilities may not have contributed substantially to differences in learning times on the first training day. This may explain why we failed to find a relation between learning times in our specific motor task and properties of white matter fiber tracts in the cerebellum.
Although we found no significant correlation between participant age and learning times, there was a trend for faster learning in younger participants. In general, the age range of our participants was limited (18–26 years). Age‐related changes in micro‐structural properties of white matter fiber tracts have been found previously, showing an increase of FA during development in childhood and adolescence and decrease of FA during aging in adulthood [e.g., Hsu et al., 2010; Lebel et al., in 2012]. However highest values of FA in the corticospinal tract and the SLF before decreasing during aging were reported for ages older than 26 years [Lebel et al., 2012]. In light of this, the relatively young and restricted age of our participants may be the reason why we did not find significant correlations between FA values and age in fiber tracts in which correlations of FA with learning time were observed (or in any other white matter fiber tracts). Thus, age is unlikely to be a causal variable driving variability in properties of the white matter fiber tracts and learning times in our sample of participants.
So far, we have interpreted the relation between FA values and initial training time as reflecting causality in a specific direction: differences in FA reflect pre‐existing differences in the structure of white matter, and thereby predispose participants to learn our audio‐motor task more or less rapidly. It should be noted, however, that three days of motor training (be it audio‐motor or visuo‐motor, depending on the group) preceded the acquisition of our DWI data. This raises, at least in principle, the possibility that the opposite direction of causality may also have contributed to the correlation: individual differences in learning time influenced the total amount of training received by the participants prior to DWI scanning, and may have altered the structure of white matter and thus FA. A number of considerations, however, suggest that structural differences (measured using FA as a proxy) influenced initial learning times more than the amount of training influenced structural differences:
First, evidence for motor learning and training influencing white matter fiber tract architecture stems from studies on motor training that show microstructural changes in FA after substantially longer time periods of motor training procedures [after 6 weeks training for learning a new task, i.e. juggling, totaling about 30 h practice time across 30 days, Scholz et al., 2009; after 2–6 weeks following a weekly 45 min training in a whole body balancing task, Taubert et al., 2010; or after years of training for comparisons between musicians vs. non‐musicians, e.g., Bengtsson et al., 2005; Han et al., 2009; Imfeld et al., 2009; Schmithorst and Wilke, 2002; see also Takeuchi et al., 2010, for changes in FA after 2 months training in a working memory task, and Keller and Just, 2009 for changes in microstructural properties after 100 h training in explicit remedial reading instruction for poor readers]. Hence one would consider that the short amount of motor training (ranging from about 2–5 h, across three days) received by our participants prior to DWI should not have altered the DWI measurements significantly and differentially. However, a very recent study by Sagi et al. [2012] suggests that under certain conditions significant changes in DWI measures can be induced by shorter training times (1.5 h followed by a DWI scan 30 min after training) in structures known to show rapid plasticity (the hippocampus and the parahippocampus). Please note, that in this study spatial learning and memorizing a path in a computer car race game was assumed to have caused changes in DWI measures, but not motor learning of a new skill or motor training as in our study [which presumably takes longer durations of training as suggested by results of motor training studies, Scholz et al., 2009; Taubert et al., 2010]. Furthermore, substantial training‐related microstructural changes in white matter fiber tracts (such as dendritic sprouting or neurogenesis) measurable with DWI need longer timescales (rather days to weeks) than a few hours [Johansen‐Berg et al., 2012]. Although, the study of Taubert et al. [2010] might suggest that motor training induced changes in DWI measures are possible after only two motor training sessions totaling 1.5 h practice time, it should be noted that in this experimental design a time factor could have influenced the results: the training sessions did not comprise daily practice, but were performed only once a week and DWI scans were acquired about 7 days after the second training session (i.e., 14 days after the first training session). Thus such an experimental design allowed more time than, e.g. in our study, to develop changes in properties of white matter fiber tracts caused by motor training procedures. DWI scanning in our study was done on the following (fourth) day after the first three days of motor training sessions (on average, mean ± SD, 24 ± 5 h after the last motor training session had finished).
Second, if training had influenced measures of the white matter microstructural properties in significant and specific ways, we would expect that different types of training occurring before DWI should lead to different DWI results. Comparing the ten participants that underwent visuo‐motor training condition prior to DWI with the eight that underwent audio‐motor training condition, however, did not reveal any differences in FA values, not even if the total training time received before scanning is taken into account.
Third, for the 10 participants that underwent the audio‐motor training condition after DWI acquisition, audio‐motor learning time could not have be causing differences in DWI because the latter were acquired before the former. However, exploratory correlation analyses showed that the relations between initial audio‐motor learning time and FA holds in the identified peak voxel in the bilateral corticospinal tracts and the right SLF even when restricting the analysis to this group (Supporting Information Table S4, C; see red dots in Fig. 2A–C that represent the group who received the DWI scan before the audio‐motor training procedure).
Fourth, it still could be argued that the total time of motor training sessions before DWI (be it visuo‐motor or audio‐motor training condition) might have caused unusually rapid training dependent plasticity. However, a further control analysis showed that the correlation between the initial audio‐motor training time and FA in the left corticospinal tract remained significant even when total training time prior to DWI scanning (which was highly correlated with the initial audio‐motor training time) was included as a covariate.
Taken together, these control analyses speak against an alternative explanation of our correlation that suggests that differences in training prior to DWI caused differences in FA. Accordingly, the most likely explanation of our data is that individual differences in microstructural properties in the bilateral corticospinal tract and the right SLF predisposed our participants to learn the task in the audio‐motor training condition at different speeds, with those showing higher FA values acquiring the audio‐motor associations more swiftly. However, it is important to acknowledge that the interpretation of variability of FA in terms of specific microstructural properties in the white matter must remain tentative. There is currently incomplete understanding of how changes in white‐matter microstructure affect FA, and how these differences transform into more or less efficient information transfer. Hence, we prefer to interpret performance related variability in FA as a tool for identifying fiber tracts that might play a role in a particular task, without favoring specific interpretations of how underlying microstructure caused the FA measurements and behavior to change.
CONCLUSIONS
A growing body of literature shows that motor skill learning can change the structure of gray and white matter [e.g., Bengtsson et al., 2005; Draganski et al., 2004; Gaser and Schlaug, 2003; Sagi et al., 2012; Scholz et al., 2009; Taubert et al., 2010]. The present study examined the reverse causation: how differences in brain structure determine the swiftness with which a new sensorimotor skill can be acquired. Our results suggest that higher FA values in the white matter connecting brain areas relevant for audio‐motor learning predict faster learning in an audio‐motor piano training task. Factors that influence FA include myelination of axons, axonal diameter, and axonal membrane integrity [Beaulieu, 2002]. These axonal features may have an influence on speed or efficiency of neural signal propagation, thereby influencing behavioral outcomes. Exactly how they do so remains to be determined. Our findings are consistent with the assumption that properties of white matter tracts connecting functionally relevant areas of the human brain underlie the predisposition for how fast one can learn complex new audio‐motor skills such as playing the piano.
Author Contributions
AE, MB, PK and CK designed the training procedure and stimuli; AE, BH, LN acquired the behavioral and DWI data; AE, BH and LC analyzed the behavioral and the DWI data with advices from CK, MB and PK. The manuscript was written by AE and BH with contributions from all authors.
ACKNOWLEDGMENTS
Authors thank Anita Kuiper, Judith Streurman, and Luca Nanetti for MRI scanning, David Horbank for preparing the stimuli and developing the computer training program in cooperation with AE and MB, Katharina Wilkens, and David Horbank for acquiring pilot data for the motor training procedure, and participants for their patience in taking part in extensive training sessions and scanning procedures.
Supporting information
Supporting Information
Supporting Figure 1.
Supporting Audio 1.
Supporting Audio 2.
REFERENCES
- Ackerman PL (1987): Individual differences in skill learning: An integration of psychometric and information processing perspectives. Psychol Bulletin. 102:3–27. [Google Scholar]
- Andersson JL, Skare S, Ashburner J (2003): How to correct susceptibility distortions in spin‐echo echo‐planar images: Application to diffusion tensor imaging. NeuroImage 20:870–888. [DOI] [PubMed] [Google Scholar]
- Avery SN, Thornton‐Wells TA, Anderson AW, Blackford JU (2012): White matter integrity deficits in prefrontal‐amygdala pathways in Williams syndrome. NeuroImage 59:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangert M, Haeusler U, Altenmüller E (2001): On practice: How the brain connects piano keys and piano sounds. Ann NY Acad Sci 930:425–428. [DOI] [PubMed] [Google Scholar]
- Bangert M, Peschel T, Schlaug G, Rotte M, Drescher D, Hinrichs H, Heinze HJ, Altenmüller E (2006): Shared networks for auditory and motor processing in professional pianists: Evidence from fMRI conjunction. Neuroimage 30:917–926. [DOI] [PubMed] [Google Scholar]
- Bangert M, Schlaug G (2006): Specialization of the specialized in features of external human brain morphology. Eur J Neurosci 24:1832–1834. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann S, Koeneke S, Schmidt CF, Meyer M, Lutz K, Jäncke L (2007): A network for audio‐motor coordination in skilled pianists and non‐musicians. Brain Res 1161:65–78. [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullén F (2005): Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci 8:1148–1150. [DOI] [PubMed] [Google Scholar]
- Bonzano L, Tacchino A, Roccatagliata L, Sormani MP, Mancardi GL, Bove M (2011): Impairment in explicit visuomotor sequence learning is related to loss of microstructural integrity of the corpus callosum in multiple sclerosis patients with minimal disability. NeuroImage 57:495–501. [DOI] [PubMed] [Google Scholar]
- Braadbaart L, Waiter GD, Williams JH (2012): Neural correlates of individual differences in manual imitation fidelity. Front Integr Neurosci 6:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Prinz W (2001): Movement observation affects movement execution in a simple response task. Acta Psychol (Amst) 106:3–22. [DOI] [PubMed] [Google Scholar]
- Brass M, Bekkering H, Wohlschläger A, Prinz W (2000): Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cogn 44:124–143. [DOI] [PubMed] [Google Scholar]
- Coynel D, Marrelec G, Perlbarg V, Pelegrini‐Issac M, Van de Moortele PF, Ugurbil K, Doyon J, Benali H, Lehericy S (2010): Dynamics of motor‐related functional integration during motor sequence learning. NeuroImage 49:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della‐Maggiore V, McIntosh AR (2005): Time course of changes in brain activity and functional connectivity associated with long‐term adaptation to a rotational transformation. J Neurophysiol 93:2254–2262. [DOI] [PubMed] [Google Scholar]
- Della‐Maggiore V, Scholz J, Johansen‐Berg H, Paus T (2009): The rate of visuomotor adaptation correlates with cerebellar white‐matter microstructure. Hum Brain Mapp 30:4048–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehericy S, Benali H (2009): Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199:61–75. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A (2004): Neuroplasticity: Changes in grey matter induced by training. Nature 427:311–312. [DOI] [PubMed] [Google Scholar]
- Elmer S, Hanggi J, Meyer M, Jäncke L (2011): Differential language expertise related to white matter architecture in regions subserving sensory‐motor coupling, articulation, and interhemispheric transfer. Hum Brain Mapp 32:2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A, Bangert M, Horbank D, Hijmans BS, Wilkens K, Keller PE, Keysers C (2012): Learning piano melodies in visuo‐motor or audio‐motor training conditions and the neural correlates of their cross‐modal transfer. NeuroImage 63:966–978. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G (2003): Brain structures differ between musicians and non‐musicians. J Neurosci 23:9240–9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzola V, Aziz‐Zadeh L, Keysers C (2006): Empathy and the somatotopic auditory mirror system in humans. Curr Biol 16:1824–1829. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Tyszka JM (1994): Functional imaging of procedural motor learning: Relating cerebral blood flow with individual subject performance. Hum Brain Mapp 1:221–234. [DOI] [PubMed] [Google Scholar]
- Ha TH, Her JY, Kim JH, Chang JS, Cho HS, Ha K (2011): Similarities and differences of white matter connectivity and water diffusivity in bipolar I and II disorder. Neurosci Lett 505:150–154. [DOI] [PubMed] [Google Scholar]
- Halwani GF, Loui P, Ruber T, Schlaug G (2011): Effects of practice and experience on the arcuate fasciculus: Comparing singers, instrumentalists, and non‐musicians. Front Psychol 2:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Yang H, Lv YT, Zhu CZ, He Y, Tang HH, Gong QY, Luo YJ, Zang YF, Dong Q (2009): Gray matter density and white matter integrity in pianists' brain: A combined structural and diffusion tensor MRI study. Neurosci Lett 459:3–6. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I (1996): Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab 16:7–22. [DOI] [PubMed] [Google Scholar]
- Hsu JL, Van Hecke W, Bai CH, Lee CH, Tsai YF, Chiu HC, Jaw FS, Hsu CY, Leu JG, Chen WH, Leemans A (2010): Microstructural white matter changes in normal aging: A diffusion tensor imaging study with higher‐order polynomial regression models. NeuroImage 49:32–43. [DOI] [PubMed] [Google Scholar]
- Hutchinson S, Lee LH, Gaab N, Schlaug G (2003): Cerebellar volume of musicians. Cereb Cortex 13:943–949. [DOI] [PubMed] [Google Scholar]
- Iacoboni M (2005): Neural mechanisms of imitation. Curr Opin Neurobiol 15:632–637. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G (1999): Cortical mechanisms of human imitation. Science 286:2526–2528. [DOI] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jäncke L (2009): White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. NeuroImage 46:600–607. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J (2002): The cerebellum and event timing. Ann NY Acad Sci 978:302–317. [DOI] [PubMed] [Google Scholar]
- Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE (1994): Motor sequence learning: A study with positron emission tomography. J Neurosci 14:3775–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H, Baptista CS, Thomas AG (2012): Human structural plasticity at record speed. Neuron 73:1058–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen‐Berg H, Della‐Maggiore V, Behrens TE, Smith SM, Paus T (2007): Integrity of white matter in the corpus callosum correlates with bimanual co‐ordination skills. NeuroImage 36 (Suppl 2): T16–T21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A (1999): Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn Reson Med 42:515–525. [PubMed] [Google Scholar]
- Keller TA, Just MA (2009): Altering cortical connectivity: Remediation‐induced changes in the white matter of poor readers. Neuron 64:624–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL (2003): Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Kohler E, Umilta MA, Nanetti L, Fogassi L, Gallese V (2003): Audiovisual mirror neurons and action recognition. Exp Brain Res 153:628–636. [DOI] [PubMed] [Google Scholar]
- Lahav A, Saltzman E, Schlaug G (2007): Action representation of sound: Audiomotor recognition network while listening to newly acquired actions. J Neurosci 27:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H (2001): Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging 13:534–546. [DOI] [PubMed] [Google Scholar]
- Lebel C, Gee M, Camicioli R, Wieler M, Martin W, Beaulieu C (2012): Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage 60:340–352. [DOI] [PubMed] [Google Scholar]
- Loui P, Alsop D, Schlaug G (2009): Tone deafness: A new disconnection syndrome? J Neurosci 29:10215–10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loui P, Li HC, Schlaug G (2011): White matter integrity in right hemisphere predicts pitch‐related grammar learning. NeuroImage 55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN (2005): Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15:854–869. [DOI] [PubMed] [Google Scholar]
- Martin J (2003): Descending motor pathways and the motor function of the spinal cord In: Foltin JLH, Fernando N, editors. Neuroanatomy Text and Atlas, 3rd ed. Columbia: McGraw‐Hill Companies Inc; pp 229–325. [Google Scholar]
- Molinari M, Leggio MG, Thaut MH (2007): The cerebellum and neural networks for rhythmic sensorimotor synchronization in the human brain. Cerebellum 6:18–23. [DOI] [PubMed] [Google Scholar]
- Münte TF, Altenmüller E, Jäncke L (2002): The musician's brain as a model of neuroplasticity. Nat Rev Neurosci 3:473–478. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2001): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl‐Jockschat T, Stocker T, Markov V, Krug A, Huang R, Schneider F, Habel U, Zerres K, Nothen MM, Treutlein J, Rietschel M, Shah NJ, Kircher T (2012): The impact of a Dysbindin schizophrenia susceptibility variant on fiber tract integrity in healthy individuals: A TBSS‐based diffusion tensor imaging study. NeuroImage 60:847–853. [DOI] [PubMed] [Google Scholar]
- O'Hearn E, Molliver ME (2001): Organizational principles and microcircuitry of the cerebellum. Int Rev Psychiatry 13:232–246. [Google Scholar]
- Oechslin MS, Imfeld A, Loenneker T, Meyer M, Jäncke L (2009): The plasticity of the superior longitudinal fasciculus as a function of musical expertise: A diffusion tensor imaging study. Front Hum Neurosci 3:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L (2004): The mirror‐neuron system. Annu Rev Neurosci 27:169–192. [DOI] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur‐Moryosef S, Blumenfeld‐Katzir T, Assaf Y (2012): Learning in the fast lane: New insights into neuroplasticity. Neuron 73:1195–1203. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Knorr U, Seitz R (1994): Inter‐subject variability of cerebral activations in acquiring a motor skill: A study with positron emission tomography. Exp Brain Res 98:523–534. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Norton A, Overy K, Winner E (2005): Effects of music training on the child's brain and cognitive development. Ann NY Acad Sci 1060:219–230. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M (2002): Differences in white matter architecture between musicians and non‐musicians: A diffusion tensor imaging study. Neurosci Lett 321:57–60. [DOI] [PubMed] [Google Scholar]
- Scholz J, Klein MC, Behrens TE, Johansen‐Berg H (2009): Training induces changes in white‐matter architecture. Nat Neurosci 12:1370–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti HM, Geurts M, Gooijers J, Heitger MH, Caeyenberghs K, Beets IAM, Serbruyns L, Leemans A, Swinnen SP (2012): Microstructural organization of corpus callosum projections to prefrontal cortex predicts bimanual motor learning. Learn Mem 19:351–357. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens T (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Steele CJ, Scholz J, Douaud G, Johansen‐Berg H, Penhune VB (2012): Structural correlates of skilled performance on a motor sequence task. Front Hum Neurosci 6:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, Yamanouchi T, Suzuki S, Kawashima R (2010): Training of working memory impacts structural connectivity. J Neurosci 30:3297–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubert M, Draganski B, Anwander A, Müller K, Horstmann A, Villringer A, Ragert P (2010): Dynamic properties of human brain structure: Learning‐related changes in cortical areas and associated fiber connections. J Neurosci 30:11670–11677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassini V, Jbabdi S, Kincses ZT, Bosnell R, Douaud G, Pozzilli C, Matthews PM, Johansen‐Berg H. (2011): Structural and functional bases for individual differences in motor learning. Hum Brain Mapp 32:494–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toni I, Krams M, Turner R, Passingham RE (1998): The time course of changes during motor sequence learning: A whole‐brain fMRI study. NeuroImage 8:50–61. [DOI] [PubMed] [Google Scholar]
- Tubau E, Escera C, Carral V, Corral MJ (2007): Individual differences in sequence learning and auditory pattern sensitivity as revealed with evoked potentials. Eur J Neurosci 26:261–264. [DOI] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB (2005): Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol 93:1209–1222. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Schoell ED, Büchel C (2006): The predictive value of white matter organization in posterior parietal cortex for spatial visualization ability. NeuroImage 32:1450–1455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Figure 1.
Supporting Audio 1.
Supporting Audio 2.


