Abstract
Extremely preterm (EP, <28 weeks) and/or extremely low birth weight (ELBW, <1000 g) infants are at high risk of aberrant neurodevelopment. Sulcogyral folding patterns of the orbitofrontal cortex (OFC) are determined during the third trimester, however little is known about OFC patterning in EP/ELBW cohorts, for whom this gestational period is disturbed. This study investigated whether the distribution of OFC pattern types and frequency of intermediate and/or posterior orbital sulci (IOS/POS) differed between EP/ELBW and control adolescents. This study also investigated whether OFC pattern type was associated with mental illness or executive function outcome in adolescence. Magnetic resonance images of 194 EP/ELBW and 147 full term (>37 completed weeks) and/or normal birth weight (>2500 g) adolescents were acquired, from which the OFC pattern of each hemisphere was classified as Type I, II, or III. Compared with controls, more EP/ELBW adolescents possessed a Type II in the left hemisphere (P = 0.019). The EP/ELBW group had fewer IOS (P = 0.024) and more POS (P = 0.021) in the left hemisphere compared with controls. OFC pattern type was not associated with mental illness, however in terms of executive functioning, Type III in the left hemisphere was associated with better parent‐reported metacognition scores overall (P = 0.008) and better self‐reported behavioral regulation scores in the control group (P = 0.001) compared with Type I. We show, for the first time that EP/ELBW birth is associated with changes in orbitofrontal development, and that specific patterns of OFC folding are associated with executive function at age 18 years in both EP/ELBW and control subjects. Hum Brain Mapp 36:1138–1150, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: orbitofrontal cortex, extremely preterm, extremely low birth weight, sulcogyral folding patterns, mental illness, executive function
Abbreviations
- ADHD
Attention Deficit and Hyperactivity Disorder
- CI
Confidence interval
- ELBW
Extremely low birth weight
- EP
Extremely preterm
- GM
Gray matter
- IOS
Intermediate Orbital Sulcus/Intermediate Orbital Sulci
- IQ
Intelligence quotient
- LH
Left hemisphere
- LOS
Lateral Orbital Sulcus
- LOSc
Caudal region of the Lateral Orbital Sulcus
- LOSr
Rostral Region of the Lateral Orbital Sulcus
- MOS
Medial Orbital Sulcus
- MOSc
Caudal region of the Medial Orbital Sulcus
- MOSr
Rostral region of the Medial Orbital Sulcus
- MRI
Magnetic resonance imaging
- OFC
Orbitofrontal Cortex
- POS
Posterior Orbital Sulcus/Posterior Orbital Sulci
- RH
Right hemisphere
- TOS
Transverse Orbital Sulcus
- VICS
Victorian Infant Collaborative Study
- WM
White matter
INTRODUCTION
Over the past three decades, advances in obstetric and neonatal intensive care have led to a substantial decrease in the mortality rate of infants born preterm (<37 weeks' gestation), particularly those born extremely preterm (EP; <28 weeks' gestation) [Doyle et al., 2010]. Despite these improvements, many survivors of extreme prematurity face adverse neurodevelopmental outcomes that persist through childhood and adolescence [Anderson and Doyle, 2008]. Subsequently, characterising the morphological brain abnormalities and underlying neurobiological mechanisms associated with these deficits has become a topic of active research. Infants born either EP and/or with extremely low birth weight (ELBW; <1000 g) are at the greatest biological risk of complications, and are therefore most vulnerable to postnatal brain injury [Clark et al., 2008]. Indeed, preterm birth has been associated with reduced cerebral white matter (WM) volume as well as whole brain cortical gray matter (GM) volume at term–equivalent age compared with full‐term control participants [Inder et al., 2005]. In addition to reductions in global WM and GM volumes, regional volume reductions in structures such as the hippocampus [Lodygensky et al., 2005; Thompson et al., 2008], cerebellum [Srinivasan et al., 2006], corpus callosum, basal ganglia, and the caudate nucleus [Peterson et al., 2000], are reported in EP/ELBW survivors. In the same EP sample to that of the current study, Cheong et al. [2013] found smaller total brain volume in the EP/ELBW group compared to controls, with the largest differences noted in the thalamus and hippocampus. With this said, abnormalities in cortical gyrification are yet to be explored in this EP cohort.
In light of extensive research showing the widespread volume and WM abnormalities associated with preterm birth, it is likely that gyrification of the preterm brain (which is hypothesized to be influenced by WM connectivity [Van Essen, 1997] and brain volume [Toro et al., 2008]) may also be abnormal, though few studies have systematically examined this. The period from 26 to 36 weeks' gestation has been identified as a crucial stage for the formation and folding of the cortex [Dubois et al., 2008b]. Consequently, some preterm children's brains can appear underdeveloped at term‐equivalent age of 38–42 weeks [Ajayi‐Obe et al., 2000], where the cortex is lacking some of the intricate folding that is apparent in infants born at term. Such aberrant cortical folding could in turn contribute to the biological mechanisms underlying the later emergence of cognitive, emotional, and behavioral deficits that are disproportionately prevalent in preterm children [Anderson et al., 2003a, 2003b]. In particular, children and adolescents born EP show not only widespread deficits in areas of executive function (including behavioral control, working memory, attention, and inhibition) [Anderson et al., 2004] but are also at greater risk of mental illness and attention deficit hyperactivity disorder (ADHD), compared with term‐born children [Treyvaud et al., 2013]. The orbitofrontal cortex (OFC) is heavily implicated in a number of mental illnesses, aspects of executive functioning, and social‐emotional behavior [Bechara et al., 2000]. Consequently, investigating the effect of preterm birth on the OFC may be relevant to mental illness and impaired executive function observed in preterm individuals.
Like most of the cerebral cortex, the OFC is a highly convoluted structure rich in sulci and gyri. The OFC functions as a part of multiple networks, sharing extensive connections with cortical and subcortical regions, including the hypothalamus, amygdala, insula, and the basal ganglia [Kringelbach, 2005]. Although sulcogyral morphology of the OFC shows variability amongst individuals, it has been established that there are four primary orbital sulci: the olfactory sulcus, the medial orbital sulcus (MOS), the lateral orbital sulcus (LOS), and the transverse orbital sulcus (TOS) [Kringelbach and Rolls, 2004]. In accordance with most cortical folding, OFC gyrification occurs early during neurodevelopment in utero and is believed to remain relatively unchanged across the lifespan [Chakirova et al., 2010]. The olfactory sulcus is first to appear, identifiable from 16 weeks of gestation, followed by the MOS by week 28 and the LOS by week 32, with intermediate and posterior orbital sulci (IOS and POS; secondary sulci) forming up until 10 weeks after birth [Kringelbach and Rolls, 2004]. The third trimester is a crucial period for OFC sulcogyral development, and consequently EP/ELBW infants may be at particularly high risk of abnormal OFC gyrification given that they are born before, or during this crucial developmental phase.
To date, few studies have investigated orbitofrontal abnormalities in preterm cohorts, with those that have, focusing on sulcal depth and volume reductions, but not patterns of folding. Previous analyses have identified a reduction in total volume of the OFC of individuals born preterm compared with full‐term controls at term equivalent age [Thompson et al., 2007]. Additionally, Gimenez et al. [2006] found evidence of a reduction in sulcal depth of the MOS, LOS, and TOS in adolescents born preterm compared with term‐born controls. Reduced structural connectivity of the OFC has also been described in children with a history of extreme prematurity/intrauterine growth restriction [Fischi‐Gomez et al., in press]. To date, no study has investigated the orbitofrontal sulcogyral folding pattern, or the particular configuration of OFC sulci, in adolescents born preterm.
Currently, little is known regarding the development of gyrification during adolescence. Nevertheless, it is widely accepted that adolescence is a period of extensive changes in GM, brain volume and thickness and synaptic pruning [Gogtay et al., 2004]. Although the brain's surface morphology of sulci and gyri are largely determined during the third trimester of fetal life, the dynamic phase of adolescence has been hypothesized to potentially further alter the surface curvature of the brain [White et al., 2010].
Three different OFC pattern types have been identified in healthy adult individuals; Type I is most common (found in 56% of hemispheres), Type II is less common (30% of hemispheres), while Type III is uncommon (14% of hemispheres; see Methods section, Figure 1, e.g., of pattern types) [Chiavaras and Petrides, 2000]. In terms of mental illness, the distribution of OFC sulcogyral pattern type has predominantly been investigated in psychosis samples, repeatedly generating results of a decreased incidence of Type I pattern and an increase in occurrence of Type III for cases relative to controls [Chakirova et al., 2010; Nakamura et al., 2007; Takayanagi et al., 2010]. Conversely, two studies found an increase in the Type II pattern in early episode psychosis samples compared with healthy controls [Bartholomeusz et al., 2013; Lavoie et al., 2014]. Additionally, evidence of a decrease in the number of left hemisphere IOS was observed in first‐episode psychosis participants compared with healthy controls, potentially indicating orbitofrontal underdevelopment [Bartholomeusz et al., 2013]. It has been proposed that Type II OFC pattern, which has been associated with 1.5‐ to 5‐fold increased risk of psychosis in an adolescent/early adult sample, might be related to obstetric complications at the time of birth [Bartholomeusz et al., 2013]. However, to date no study has investigated OFC sulcogyral patterns in adolescents born preterm, or whether sulcogyral abnormalities confer risk for the development of mental illness or executive dysfunction in this population.
Figure 1.
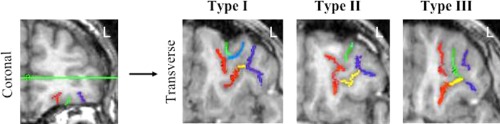
Examples of the three different OFC pattern types. To determine Type, sulci are first traced on the coronal slices (left panel), and then viewed on the transverse images (right panel). Type I: medial orbital rostral and caudal regions (red) are disconnected while the LOS (purple) is continuous. Type II: rostral and caudal regions of the medial and orbital sulci are connected, respectively. Type III: rostral and caudal regions of the medial and orbital sulci are disconnected, respectively. The additional sulci depicted are: TOS (yellow), Intermediate orbital sulci (green and blue). Reproduced with permission from Bartholomeusz et al., 2010.
Despite gyrification being largely fixed from birth, the OFC undergoes extensive maturation during adolescence, and is heavily implicated in various psychopathologies that tend to emerge during these teenage years [Pantelis et al., 2003]. Given the various neurobiological and neuropsychological difficulties that preterm adolescents may experience, it is possible that the developmentally rich period of adolescence is transpiring on a compromised foundation for those born preterm. Thus, characterisation of OFC sulcogyral folding patterns in adolescence will help to identify whether EP/ELBW birth alters this important aspect of early neurodevelopment, and whether this subsequently predisposes adolescents to the emergence of mental illness or executive dysfunction.
The present study aimed to investigate the distribution of orbitofrontal sulcogyral pattern type in a large sample of adolescents who were born EP/ELBW compared with a sample of full term and/or normal birth weight controls. It was hypothesized that the distribution of OFC pattern type would be altered in adolescents who were born EP/ELBW, compared with controls. Second, it was hypothesized that there would be fewer IOS and POS in the EP/ELBW group compared with controls. Finally, this study aimed to investigate whether the distribution of OFC pattern type was associated with mental health and executive function outcomes in adolescence, and whether this relationship differed by group.
METHOD
Participants
The EP/ELBW cohort was derived from the 298 consecutive survivors born prior to 28 weeks' gestation or with a birth weight <1000 g between January 1991 and December 1992 in the state of Victoria. The term‐born/normal birth weight control group (n = 262) were recruited at birth from either the Mercy Hospital for Women, Monash Medical Centre, or the Royal Women's Hospital, in Melbourne, during the same period and were matched with the EP/ELBW group for age, sex, health insurance status (as a proxy for socioeconomic status [SES]), and mother's country of birth (English‐speaking versus not as a proxy for ethnicity). Both groups had neurodevelopmental assessments at ages two, [Doyle et al., 1997a, 1997b] five, [Doyle and Victorian Infant Collaborative Study Group, 2001], and 8 years corrected age [Anderson et al., 2003; Doyle and Anderson, 2005]. Data for the current study were acquired as part of an adolescent follow‐up at 18 years corrected age. Informed consent for the adolescent follow‐up was obtained from all participants (and, where participants were under 18 years of age, their parents/guardians), and the study was approved by the Human Research Ethics Committees of all the participating hospitals.
Neuroimaging
Image acquisition
Structural Magnetic Resonance Imaging (MRI) data were acquired for all participants at two sites in Melbourne, Victoria, Australia; the Children's MRI Centre at the Royal Children's Hospital, Parkville, and the Brain Research Institute, Heidelberg. For each participant, high‐resolution T1‐weighted images were acquired coronally, with nonisotropic voxels (0.7 × 0.7 × 1.2 mm) using the following parameters: repetition time: 1,800 ms; echo time: 2.67 ms; flip angle: 90°; and field of view: 230 mm. At both sites, data were acquired using the same model scanner (a Siemens 3T Trio Magnetom MRI scanner with a 12‐channel matrix coil) and identical imaging parameters. Using the same model equipment and the same acquisition parameters produces the most consistent cross‐site imaging results [Reig et al., 2009].
Image preprocessing
All MRIs were first skull‐stripped using a brain extraction tool [Smith, 2002] and aligned along the anterior commissure‐posterior commissure plane to adjust for head tilt and movement at the time of scanning (using FLIRT rigid body registration to the Montreal Neurological Institute template). This transformation also served to resample images into 1 mm cubic voxels. The images were then used to classify OFC sulcogyral pattern types in each hemisphere on a LINUX workstation using the biomedical imaging software package Analyze 10.0 (Mayo Clinic).
OFC sulcogyral pattern classification
Classification of OFC pattern type was based on the technique devised by Chiavaras and Petrides [2000], which has been revised by Bartholomeusz et al. [2013]. Briefly, visual classification of each hemisphere was based on the continuity/discontinuity of the rostral and caudal regions of the MOS and the LOS (see Fig. 1). In Type I, the caudal and rostral regions of the MOS are disconnected while the LOS is intact. In Type II, both the MOS and LOS are continuous, while in Type III both the MOS and LOS are disconnected.
To make a classification, sulci were highlighted in the coronal plane slice‐by‐slice using the Analyze tracing tool, and then viewed in transverse and sagittal planes to aid in the visual classification of OFC pattern type (see Fig. 1). Each sulcus that appeared on the orbital surface was traced, and the number of IOS and POS were recorded. In accordance with our previous research, a fissure was considered to be a sulcus if it was at least 4 mm long and 4 mm deep (i.e., visible in four coronal and four transverse slices). For a sulcus to be continuous with another sulcus it had to be clearly connected in at least three slices.
OFC pattern classification for each hemisphere was performed by E.G., who was blinded to sex and group. Intra and inter‐rater reliability was performed by E.G. and C.B. on 30 randomly selected brains from the Melbourne Neuropsychiatry Centre database, independent of the present study. Interclass correlation coefficients (ICC) were 0.90 and 1.00 for inter‐ and intra‐rater reliability, respectively. Given the precise nature of the classification method, C.B. reviewed 22% of hemispheres from the current sample, which were deemed most difficult to classify. Reliability between raters remained high for the overall sample (ICC 0.87), and for the two individual groups (EP/ELBW; Right hemisphere ICC‐0.85, Left hemisphere ICC‐0.82), (control group; Right hemisphere ICC‐0.80, Left hemisphere ICC‐0.84).
Mental Health Assessment
Structured clinical interview for DSM‐IV disorders
The Structured Clinical Interview for DSM‐IV Disorders (SCID‐IV) research version, nonpatient edition was used to identify a range of Axis I psychiatric disorders (mood, anxiety, substance use, psychotic, eating, and adjustment disorders; past and/or current), consistent with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM‐IV‐TR) criteria [First et al., 2002]. Assessments were administered by five trained interviewers blinded to group and consensus diagnoses were reached for all participants [Burnett et al., 2014]. The ADHD module of the Children's Interview for Psychiatric Syndromes [Weller et al., 2000] was used to assess ADHD (inattentive, hyperactive/impulsive, and combined subtypes). We have previously reported these outcomes in this cohort [Burnett et al., 2014] and diagnoses identified were primarily ADHD, mood disorders, and anxiety disorders.
Executive Function Assessment Measures
To investigate executive function outcome, we used data from a range of measures (self‐report, parent‐report, and direct assessments) collected at the 18‐year follow‐up. The Behavior Rating Inventory of Executive Function Questionnaire (BRIEF) [Gioia et al., 2000] was used to assess behavioral manifestations of executive functioning. A parent‐report (BRIEF‐PR) and self‐report (BRIEF‐SR) were administered, both of which consist of two primary indexes; the Behavioral Regulation Index (including behavioral and emotional regulation, shifting between activities), and the Metacognition Index (including initiation, working memory, planning, organization, and self‐monitoring) [Gioia et al., 2000]. Scores for each index were standardized based on age and sex to a mean of 50 and standard deviation of 10, with higher scores indicating greater difficulties.
We also collected two direct measures of executive function. Participants completed the Hayling Sentence Completion Test which assessed inhibitory control, [Burgess and Shallice, 1997]. The overall score reflects both speed and errors, with higher scores indicating better performance. Additionally, participants completed the Contingency Naming Test (CNT) which assesses working memory and cognitive flexibility. The efficiency score (a ratio of time and errors) was the outcome measure for each CNT trial. We controlled for IQ for both the Hayling test and the CNT.
IQ Measures and SES
IQ and SES were collected at the 18 year follow‐up assessment. IQ was estimated using the 2‐subtest version (consisting of the Matrix Reasoning and Vocabulary subtests) of the Wechsler Abbreviated Scale of Intelligence [Wechsler, 1999]. Parental occupation was used as a measure of SES, assessed using the Congalton scale [Congalton, 1969] and dichotomised into lower and higher SES.
Data Analysis
All data analyses were performed using SPSS statistical software (IBM SPSS 20.0 for Windows, SPSS, Chicago, Illinois). Group differences for continuous variables (e.g., IQ) were assessed using independent samples t‐tests, while categorical demographic variables (e.g., sex, history of a mental disorder) were compared using Pearson's chi‐square statistic. Pearson's chi‐square statistics were used to assess whether there were group differences in the distribution of pattern type, with analyses carried out separately in the two hemispheres. For primary analyses, the comparison of OFC pattern type between the EP/ELBW and control groups, and the assessment of the relationship between OFC pattern type and psychopathology, α was set at 0.05, in accordance with the method adopted by previous studies in this area [Chakirova et al., 2010, Nakamura et al., 2007; Takayanagi et al., 2010]. For the additional exploratory analyses (e.g., investigating whether the distribution of pattern Type II differed between the two groups), post hoc tests were adjusted for multiple testing using a Bonferroni correction. Given there were three comparisons (pattern Type I, II, and III), when interpreting P‐values a threshold of 0.05/3 = 0.017 was used to identify evidence of a relationship. We chose to only correct post hoc analyses given that the Bonferroni method is conservative and it was considered important to also protect against Type II errors.
To compare the frequency of IOS and POS between groups, Mann–Whitney U tests were performed separately for each hemisphere. To assess whether OFC pattern type could predict mental health outcomes (presence/history of any disorder vs. no disorder), logistic regression models were constructed separately for each hemisphere. Models first included only pattern type as a predictor (Type I as reference), then included an adjustment for group (control group as reference), and finally screened for an interaction between group and pattern type. Similar analyses were used to investigate whether pattern type predicted mood disorder, anxiety disorder or ADHD separately, and whether this relationship differed by group.
Finally, to investigate whether OFC pattern type was associated with measures of executive function, univariable analyses of variance were used for each hemisphere. For the BRIEF parent‐reported and self‐reported measures, Behavioral Regulation and Metacognition indices were analysed separately, and a group by pattern type interaction term was also included. The Bonferroni method was used to correct for multiple testing during post hoc pairwise comparisons between the three pattern types. Similar univariable analyses of variance were also used to investigate the relationship between pattern type and direct measures of executive function (the CNT and Hayling test analysed separately), while controlling for IQ (for both the CNT and Hayling test) and Vocabulary (for the Hayling test).
RESULTS
Demographics and Mental Illness
Of the 205 EP/ELBW and 149 control participants imaged at 18 years, data from 194 EP/ELBW and 147 control participants were suitable for analysis (see Table 1 for details on demographic sample characteristics). MRI data from the remaining 11 EP/ELBW and two control participants could not be assessed due to imaging artefact and movement, however, the excluded participants were similar in key baseline characteristics (birth weight, gender, gestational age) to those that were included (data not shown).
Table 1.
Demographic and mental illness data
| EP/ELBW (n = 194) | Data missing (n) | Controls (n = 147) | Data missing (n) | χ2 Statistic/MD(±95%CI) | P value | |
|---|---|---|---|---|---|---|
| Age at scan (yrs) | 17.9 ± 0.8 | 2 | 18.1 ± 0.8 | 3 | 0.14 (−0.03, 0.31) | 0.11 |
| Male ‐ n (%) | 87 (45%) | 0 | 62 (42%) | 0 | χ2 = 0.24 | 0.62 |
| Gestational age at birth (weeks) | 26.6 ± 1.9 | 0 | 39.2 ± 1.5 | 0 | 12.6 (−12.96, −12.21) | <0.001 |
| Birth weight (grams) | 890 ± 160 | 0 | 3431 ± 456 | 0 | 2541 (−2618, −2463) | <0.001 |
| Higher SES ‐ n (%) | 111/75 (52%) | 8 | 104/39 (48%) | 4 | χ2 = 6.08 | 0.014 |
| IQ | 96.8 ± 16.2 | 8 | 107.8 ± 12.9 | 6 | 11.1 (−14.33, −7.82) | <0.001 |
| BRIEF‐BR‐PR | 43.3 ± 2.4 | 11 | 45.2 ± 2.8 | 19 | 1.9 (−5.3, 9.1) | 0.61 |
| BRIEF‐BR‐SR | 37.9 ± 2.5 | 26 | 45.5 ± 2.8 | 16 | 7.5 (.16, 14.9) | 0.045 |
| BRIEF‐MCGN‐PR | 45.7 ± 2.4 | 11 | 47.7 ± 2.9 | 19 | 1.9 (−5.5, 9.4) | 0.61 |
| BRIEF‐MCGN‐SR | 40.8 ± 2.5 | 26 | 48.1 ± 2.9 | 18 | 7.3 (−.23, 14.9) | 0.06 |
| Hayling Test | −0.20 ± 1.4 | 0 | 4.7 ± 1.7 | 2 | 4.9 (.58, 9.2) | 0.03 |
| CNT | 14.1 ± 3.6 | 119 | 4.2 ± 4.8 | 105 | 10.0 (−1.9, 21.9) | 0.1 |
| Any psychiatric diagnosis ‐ past or current ‐ n (%) | 58 (30%) | 3 | 32 (22%) | 3 | χ2 = 2.68 | 0.10 |
| Any mood disorder | 32 (16%) | 19 (13%) | χ2 = 1.99 | 0.20 | ||
| Any anxiety disorder | 22 (11%) | 15 (10%) | χ2 = 0.74 | 0.47 | ||
| Any ADHD diagnosis | 29 (15%) | 11 (7%) | χ2 = 6.90 | 0.01 |
Note: Results are mean ± standard deviation unless otherwise stated; EP/ELBW – Extremely preterm/extremely low birth weight; SES – socio‐economic status; BRIEF – Behavior rating inventory of executive function; BR – Behavioral regulation; MCGN – Metacognition; PR – Parent report; SR – Self report; CNT – Contingency naming task; ADHD – Attention deficit hyperactivity disorder; MD – difference in means; CI – confidence interval; SES – Socioeconomic status.
As we have previously reported, EP/ELBW participants had a lower IQ than control participants [Cheong et al., 2013]. The groups were similar in terms of sex and history of most psychiatric disorders, although ADHD diagnoses were more prevalent in the EP/ELBW group than controls [Burnett et al., 2014].
OFC Sulcogyral Pattern
There was some evidence for group differences in distribution of the three OFC pattern types in the left hemisphere (P = 0.057) but not the right (see Table 2). For the left hemisphere, post hoc analyses revealed Type II was more frequent in the EP/ELBW group than in the control group (P = 0.019). There was little evidence of a difference between groups in the incidence of pattern Types I or III (see Table 2).
Table 2.
Distribution of orbitofrontal sulcogyral pattern types in participants born EP/ELBW and controls
| EP/ELBW (n = 194) | Controls (n = 147) | χ2 | P value | |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Right hemisphere | 0.139 | 0.933 | ||
| Type I | 99 (51) | 78 (53) | ||
| Type II | 36 (19) | 26 (18) | ||
| Type III | 59 (30) | 43 (29) | ||
| Left hemisphere | 5.87 | 0.057 | ||
| Type I | 94 (49) | 76 (52) | ||
| Type II | 46 (24) | 20 (14) | ||
| Type III | 54 (28) | 51 (35) |
Note: EP/ELBW –extremely preterm/extremely low birth weight.
IOS and POS
The proportion of participants with each number of IOS and POS in each hemisphere is shown in Table 3. The median, range, and interquartile range of number of IOS and POS for each group (hemispheres analysed separately) are also shown in Table 3. The EP/ELBW group had fewer IOS than the controls in the left hemisphere (P = 0.01), but showed little evidence of a difference in the right (P = 0.72). In contrast with the IOS, the EP/ELBW group had more POS than the controls in the left hemisphere (P = 0.005), but again showed little evidence of a difference in the right (P = 0.53).
Table 3.
Proportion and frequency of IOS and POS in the right and left hemispheres in participants born EP/ELBW and controls
| Right hemisphere | Left hemisphere | |||
|---|---|---|---|---|
| EP/ELBW (n = 194) n (%) | Controls (n = 147) n (%) | EP/ELBW (n = 194) n (%) | Controls (n = 147) n (%) | |
| Number of IOS | ||||
| 0 | 3 (1) | 5 (3) | 4 (2) | 3 (2) |
| 1 | 101 (53) | 70 (48) | 101 (52) | 60 (40) |
| 2 | 82 (42) | 64 (44) | 82 (42) | 66 (45) |
| 3 | 8 (4) | 8 (5) | 7 (4) | 17 (12) |
| 4 | 0 (0) | 0 (0) | 0 (0) | 1 (1) |
| MD, R, IQR | (1,3,1) | (1,3,1) | (1,3,1) | (2,4,1) |
| Mann–Whitney U | U = 0.37 | P = 0.72 | U = 2.57 | P = 0.010 |
| Number of POS | ||||
| 0 | 63 (32) | 54 (37) | 56 (29) | 60 (41) |
| 1 | 103 (53) | 72 (49) | 104 (54) | 74 (50) |
| 2 | 27 (14) | 19 (13) | 32 (16) | 12 (8) |
| 3 | 1 (1) | 2 (1) | 2 (1.0) | 1 (1) |
| MD, R, IQR | (1,3,1) | (1,3,1) | (1,3,1) | (1,3,1) |
| Mann–Whitney U | U = −0.63 | P = 0.53 | U = −2.80 | P = 0.005 |
Note: EP/ELBW – extremely preterm/extremely low birth weight; IOS – intermediate orbital sulci; POS – posterior orbital sulci; MD – median; R – range; IQR; interquartile range.
Orbitofrontal Pattern Type as a Predictor of Mental Health Disorder
Table 4 shows the odds ratios for meeting criteria for a DSM‐IV disorder (predominantly ADHD or mood/anxiety disorders) for each pattern type. There was no evidence of a relationship between pattern type and diagnosis of mental health outcomes either on univariable analysis (see Table 4), or after controlling for group (right hemisphere: P = 0.26; left hemisphere: P = 0.45), and no interaction between group and pattern type (data not shown). Furthermore, analyses of individual diagnosis classes did not reveal relationships between pattern type and any of the outcomes (data not shown).
Table 4.
Associations between orbitofrontal pattern type and mental illness on DSM‐IV criteria in EP/ELBW and control adolescents
| OR (95% CI) (univariate) | P value | OR (95% CI) (adjusted for group) | P value | |
|---|---|---|---|---|
| Right hemisphere | ||||
| Group | 1.51 (0.92‐2.50) | 0.10 | ||
| Type I | Reference | 0.44 | Reference | 0.44 |
| Type II | 1.11 (0.57‐2.16) | 0.76 | 1.11 (0.57‐2.16) | 0.77 |
| Type III | 1.43 (0.83‐2.46) | 0.20 | 1.42 (0.82‐2.46) | 0.20 |
| Left hemisphere | ||||
| Group | 1.53 (0.92–2.53) | 0.10 | ||
| Type I | Reference | 0.95 | Reference | 0.92 |
| Type II | 0.95 (0.50‐1.82) | 0.88 | 0.89 (0.46‐1.71) | 0.72 |
| Type III | 0.91 (0.52‐1.58) | 0.74 | 0.92 (0.53‐1.61) | 0.78 |
Note: EP/ELBW –extremely preterm/extremely low birth weight; OR – odds ratio; CI – confidence interval.
Orbitofrontal Pattern Type as a Predictor of Executive Function
BRIEF parent‐report
Table 5 shows summaries of the BRIEF index scores for each pattern type combining data from the EP/ELBW and control group (n = 341). There was little evidence that pattern type was associated with parent‐reported Behavioral Regulation scores in either hemisphere (right hemisphere: P = 0.31; left hemisphere; P = 0.22). There was also little evidence of a relationship between pattern type and parent‐reported Metacognition scores in the right hemisphere (P = 0.90), although there was some evidence of a relationship in the left hemisphere, (F(2,297) = 3.73, P = 0.025). Post hoc pairwise comparisons between the three pattern types revealed evidence that parent‐reported Metacognition scores were different between individuals with a Type I and Type III. Specifically, pattern Type III was associated with better parent‐reported metacognition scores (i.e., lower scores) than Type I (mean difference = 4.1, 95% CI 1.1, 7.2 P = 0.008). There was little evidence that group was associated with parent‐reported Behavioral Regulation (right hemisphere: P = 0.90; left hemisphere: P = 0.06) or Metacognition scores (right hemisphere: P = 0.91; left hemisphere: P = 0.51).
Table 5.
Associations between orbitofrontal pattern type and Behavior Rating Inventory of Executive Function (BRIEF) in EP/ELBW and control adolescents combined
| Right hemisphere | Left hemisphere | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| Type I | Type II | Type III | Type I | Type II | Type III | |
| BRIEF (parent‐report) | ||||||
| Metacognition | 52.7 ± 11.8 | 52.4 ± 11.9 | 53.4 ± 12.1 | 54.1 ± 11.8 | 54.7 ± 12.8 | 49.9 ± 11.2 |
| Behavioral regulation | 50.4 ± 11.5 | 51.8 ± 12.0 | 49.1 ± 9.3 | 50.9 ± 11.2 | 52.0 ± 10.9 | 48.4 ± 10.5 |
| BRIEF (self‐report) | ||||||
| Metacognition | 51.1 ± 11.9 | 49.6 ± 11.8 | 50.0 ± 13.8 | 51.5 ± 12.7 | 51.4 ± 13.1 | 48.3 ± 11.2 |
| Behavioral regulation | 48.3 ± 11.1 | 47.2 ± 10.9 | 46.8 ± 12.4 | 48.6 ± 12.0 | 48.4 ± 11.8 | 45.7 ± 10.1 |
Note: BRIEF – Behavior Rating Inventory of Executive Function; SD – Standard deviation; higher scores indicate more difficulty.
BRIEF self‐report
There was no evidence that pattern type was associated with self‐reported Behavioral Regulation (right: P = 0.55; left: P = 0.15) or Metacognition scores (right: P = 0.68; left: P = 0.13) in either hemisphere. We also found no evidence that group was associated with Metacognition scores in either hemisphere (right hemisphere: P = 0.81; left hemisphere: P = 0.31), or Behavioral Regulation in the right hemisphere (P = 0.65). There was, however, evidence of an interaction effect between group and pattern type for self‐reported Behavioral Regulation in the left hemisphere (F(2,283) = 5.14, P = 0.006). Post hoc pairwise comparisons between each of the three pattern types within each of the birth groups revealed evidence of a difference in mean self‐reported Behavioral Regulation in the control group between individuals with a Type I and Type III (F(2,126) = 6.5, P = 0.002). Specifically, pattern Type III was associated with better average self‐reported Behavioral Regulation scores than Type I (mean difference = 7.5 95% CI 3.3, 11.6, P = 0.001). There was no evidence of a relationship in the EP/ELBW group.
The Contingency Naming Task (CNT) and Hayling Test
Table 6 shows summaries of the CNT and Hayling test scores for each pattern type. There was no evidence that pattern type was associated with the CNT score (right: P = 0.40; left: P = 0.19) or the score on the Hayling Test (right: P = 0.63; left: P = 0.59) in either hemisphere. Covarying for Vocabulary did not alter the pattern of results (data not shown).
Table 6.
Associations between orbitofrontal pattern type and scores on the Contingency Naming Task (CNT) and Hayling test in EP/ELBW and control adolescents
| Right hemisphere | Left hemisphere | |||||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||||
| Type I | Type II | Type III | Type I | Type II | Type III | |
| CNT (overall score) | 0.52 ± 0.27 | 0.48 ± 0.21 | 0.47 ± 0.21 | 0.51 ± 0.26 | 0.45 ± 0.22 | 0.52 ± 0.25 |
| Hayling test (overall score) | 5.96 ± 1.19 | 5.65 ± 1.27 | 5.89 ± 1.24 | 5.97 ± 1.20 | 5.60 ± 1.44 | 5.91 ± 1.11 |
Note: CNT – contingency naming task; SD – Standard deviation.
Additionally, there was no evidence of an interaction effect between group and pattern type in either hemiphere for the CNT (right hemsiphere: P = 0.30; left hemisphere: P = 0.08) or the Hayling test (right hemisphere: P = 0.30; left hemisphere: P = 0.96).
DISCUSSION
This study found that the distribution of orbitofrontal sulcogyral folding pattern was altered in a geographical cohort of EP/ELBW adolescents compared with a group of term‐born/normal birth weight controls. Specifically, EP/ELBW adolescents had a higher prevalence of OFC pattern Type II in the left hemisphere. We also found that in the left hemisphere, the EP/ELBW group had fewer IOS, yet more POS than the control group. Although OFC pattern type was not associated with mental illness, pattern Type III was associated with better parent‐reported Metacognition scores across all participants, and self‐reported Behavioral Regulation scores in the control group. This study provides novel evidence that, in addition to quantitatively smaller OFC volumes at term equivalent age [Thompson et al., 2007], qualitative morphological variations are also present in the OFC of adolescents born EP/ELBW.
Interestingly, the distribution of pattern types observed in our control group did not parallel that of Chiavaras and Petrides [2000] original study in 50 healthy adult Canadian participants, where they found Type I – 56%; Type II – 30%; and Type III – 14%. Despite this, our result of Type I being most common, followed by Type III, with Type II being least common is consistent with a previous report in a control group of another Australian study, indicating that differences in pattern type distributions between control groups may reflect geographic differences between cohorts [Bartholomeusz et al., 2013]. It is also worth mentioning, that aside from our finding of an increased prevalence of left pattern Type II in the EP/ELBW group, the overall distribution of pattern types (Type I > Type II > Type III) were similar in both groups. Previous research has indeed identified decreased connectivity of the orbital network in EP and intrauterine growth restricted children [Fischi‐Gomez et al., in press]. Therefore, although the EP/ELBW group possess the same previously defined pattern types (Type I, II, and III) that are seen in the controls, they might differ in terms of orbitofrontal WM connectivity. Abnormal WM projections to and from other prefrontal and subcortical regions in the EP/ELBW group may further explain the orbitofrontal specific cognitive and executive functioning deficits that are more prevalent in the preterm population. However, this is speculative and is a subject that warrants further research.
In line with our second hypothesis, the EP/ELBW group had fewer left IOS than the control group. The observed reduction in number of IOS in the EP/ELBW group may be suggestive of underdevelopment of the OFC neural system in EP/ELBW individuals, and could in turn help to explain the neurobiological mechanism underlying orbitofrontal specific deficits that are disproportionally prevalent in the preterm population, such as inhibition, attention, and emotional control [Ritter et al., 2014]. In contrast, we found more POS in the left hemisphere in EP/ELBW compared with control adolescents. The IOS (more anterior) and POS (more posterior) form at a later stage of brain development in a posterior–anterior trend continuing until around 5 to 10 weeks after birth [Kringelbach and Rolls, 2004]. This developmental sequence could potentially explain the present findings, and is also consistent with the notion that the sulci most immature at birth (sulci that develop later) will be more adversely affected by prematurity [Gimenez et al., 2006].
Although the posterior–anterior sequence of sulcal maturation assists in explaining why the number of POS was not reduced in the EP/ELBW compared with control adolescents, the reason for an increase in POS frequency remains unclear. A possible explanation for this phenomenon could be that the posterior region may have been subject to over‐gyrification, as has been reported in other brain regions (temporal lobe) in preterm children [Kesler et al., 2006] and intrauterine growth restricted infants [Dubois et al., 2008a]. While the neural mechanisms behind increased gyrification are not fully understood, contributing factors could include reductions of temporal and subcortical volumes, as well as disproportionate development of cortical strata volumes, which is often observed in preterm populations [Kesler et al., 2006]. Interestingly, a previous study has found an association between the absence of POS and lower depressive symptoms in adolescents [Whittle et al., 2014]. This finding is somewhat supported by evidence of an association between the absence of POS and positive emotionality and higher openness [Roppongi et al., 2010]. Our finding, in conjunction with past research implies that fewer POS could be predictive of a healthier outcome, though further research is needed to examine this.
The third aim of the current study involved investigating whether OFC pattern type predicted functional outcomes, including mental illness and executive functioning. Previous research has identified that those born preterm are three times more likely to be hospitalized with a psychiatric illness than those born at term [Nosarti et al., 2012]. Furthermore, abnormalities in OFC pattern type distribution have previously been associated with psychotic illnesses, yet no previous research had investigated OFC pattern type in other mental health disorders. In the current study, pattern type was not associated with mental illness, and this was not influenced by group, despite previous research showing a higher incidence of mental illness in EP cohorts [Johnson et al., 2010]. Additionally, analyses of individual diagnosis classes did not reveal any relationships between pattern type and outcome. With this said, we acknowledge that by attempting to maximise power, the psychiatric diagnoses in our sample were of necessity heterogeneous, and mostly comprised ADHD, anxiety disorders, and mood disorders. Therefore, future research should investigate the relationship between OFC pattern type and larger samples of specific mental illnesses in preterm populations, as opposed to the genarlised category of “any mental illness.”
In terms of executive function, there was little evidence of a relationship between pattern type and direct measures of executive function (the CNT and Hayling test). However, when investigating pattern type in relation to BRIEF indices, results indicated that pattern Type III was associated with better parent‐reported metacognition scores, compared with Type I across all participants. Additionally, Type III was also found to be associated with better self‐reported behavioral regulation scores than Type I in the control group.
Interestingly, previous research indicates that Type I is most common in the general healthy adult population [Chiavaras and Petrides, 2000] and associated with better cognitive performance [Nakamura et al., 2007]. Our adolescent cohort (mean age 18 years), however, is younger than the Chiavaras and Petrides [2000] cohort (mean age 25 years) and the Nakamura et al. [2007] sample (mean age 40 years). Adolescence is a period of heightened vulnerability, when self‐regulating behavior and higher order thinking processes are still developing and may, therefore, be atypical and volatile [Steinberg, 2005]. The finding that the most commonly observed Type I pattern is associated with poor behavioral regulation and metacogntion may be unique to the adolescent period. Given that executive function is expected to develop across adolescence to adulthood, Type I may remain the most commonly observed pattern type, however behavioral regulation and metacognition is considered likely to improve across this period. With this said, Type I pattern being associated with poorer self‐reported behavioral regulation in the control group only, is harder to explain. It is possible that this finding may reflect reduced insight in the preterm group that could affect their self‐ratings. As seen in Table 1, the preterm group report themselves as having better behavioral regulation than the control group (and better than the evaluation of their parents). This is not a novel phenomenon, with adolescents experiencing difficulty in self‐appraisal often have a tendency to over‐self‐report their abilities in comparison to their parent‐report [Wilson et al., 2011].
It is also interesting that the executive function findings, as well as the sulcogyral patterning and frequency differences, were all lateralised to the left hemisphere. The precise reason for this laterality is not fully understood and warrants further investigation. However, previous research has found that development of the right hemisphere occurs earlier than that of the left. According to this long‐standing theory, there would, therefore, be a prolonged period of vulnerability for the left hemisphere, thus increasing the chance of abnormal development relative to the right hemisphere [Chiron et al., 1997; Huttenlocher, 1990]. Although speculative, this could potentially explain why the observed differences in the EP/ELBW group in this study were lateralised to the left hemisphere.
It is worth noting that the BRIEF did not tap any discrete orbitofrontal processes; it instead served as an overall measure of OFC functioning based on the perceptions of the parent and the adolescent. It is also worth noting that the parent and self‐report BRIEF scores appeared to differ between raters in terms of Metacognition and Behavioral Regulation, with adolescents self‐reporting better executive functioning scores than the parent‐report. These discrepencies should be regarded as realistically reflecting each respondent's perspective [White‐Koning et al., 2005], and the two different assessments analysed and interpreted separately, allow for a broader scope of exploration into the executive functioning of this adolescent cohort.
The OFC classification method captures a specific and unique aspect that has been largely overlooked by previous research, with this study being the first to investigate whether OFC sulcogyral patterning is influenced by extreme prematurity at birth. This study provides important and novel information around the consequences of prematurity for the developing brain. Nonetheless, the highly heterogeneous nature of the OFC makes the task of classification challenging. Only a few research groups have applied the technique, and given the complexity of the method, some variability in pattern type distributions may be partly affected by differences in research groups' classification techniques. Therefore, to truly address this issue, reliability and consistency in OFC classification techniques should be achieved across laboratories.
Given that we have found evidence of differences in OFC structure in EP/ELBW adolescents compared with controls, to further characterise the scope of preterm neurodevelopmental consequences, it is proposed that future research should endeavour to look at orbitofrontal sulcal depth and length [Gimenez et al., 2006], in addition to whole brain gyrification analyses. These measures will help to elucidate which other cortical areas show sulcal variation associated with prematurity. Additionally, future research should endeavour to investigate any existing relationship between OFC gyrification and regional volume of the OFC. Given that volumetric reductions have previously been identified in preterm populations [Thompson et al., 2007], such differences could potentially influence OFC pattern type.
Further research is also needed to determine whether OFC pattern type represents a specific risk factor for particular classes of psychiatric disorder or psychiatric symptoms. Importantly, longitudinal studies are required in clinical and nonclinical populations to verify the stability of OFC sulcogyral morphology over the lifespan. Although established early during neurodevelopment, and expected to be a stable characteristic, it could be argued that synaptic pruning and cortical thinning (as seen during adolescence), may potentially alter sulcal morphology. For example, following significant neuronal loss, sulci that appear connected to other sulci (as seen in Types I and II), may subsequently appear disconnected.
In conclusion, the present study indicates that left hemisphere OFC sulcogyral patterning is altered in EP/ELBW adolescents compared with controls. Differences were identified in both primary (i.e., MOS, TOS, and LOS) and secondary (i.e., IOS and POS) sulci. This study also suggests that Type III is associated with better performance in some areas of adolescent executive function relative to Type I. However, this study found no evidence to link OFC pattern type with the presence of mental illness, such as ADHD, mood disorders, or anxiety disorders. The relevance of OFC sulcogyral anomalies for specific mental illnesses merits further exploration in preterm samples.
ACKNOWLEDGMENTS
This work is published on behalf of the Victorian Infant Collaborative Study Group, the following members of which were involved in the study design or data collection at the various study sites:
Convenor; Lex W Doyle, MD, FRACP.1,2,3,4. Collaborators (in alphabetical order): Peter Anderson, PhD,3,4 Alice Burnett, PhD, 1, 4 Catherine Callanan, RN,1 Elizabeth Carse, FRACP,6 Margaret P Charlton, M Ed Psych,6 Jeanie Cheong, MD, FRACP,2,4 Noni Davis, FRACP,1 Katherine J Lee, PhD, 3,4 Cinzia R De Luca, BSc, PhD,1,2 Julianne Duff, FRACP,1 Marie Hayes, RN,6 Esther Hutchinson, BSc (Hons)1 Elaine Kelly, MA,1,5 Marion McDonald, RN,1 Gillian Opie, FRACP,5 Gehan Roberts, MPH, PhD, FRACP,1,3,4 Karissa Searle,1 Andrew Watkins, FRACP,5 Amanda Williamson,5 Heather Woods, RN.5
From: the 1Premature Infant Follow‐up Program at the Royal Women's Hospital, the Departments of 2Obstetrics and Gynaecology, and 3Paediatrics at The University of Melbourne, 4Murdoch Childrens Research Institute, 5Mercy Hospital for Women, and 6Monash Medical Centre, Melbourne, Australia.
REFERENCES
- Ajayi‐Obe M, Saeed N, Cowan FM, Rutherford MA, Edwards AD (2000): Reduced development of cerebral cortex in extremely preterm infants. Lancet 356:1162–1163. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW (2008): Cognitive and educational deficits in children born extremely preterm. Semin Perinatol 32, 51–58. [DOI] [PubMed] [Google Scholar]
- Anderson P, Doyle LW, Victorian Infant Collaborative Study Group (2003a): Neurobehavioral outcomes of school‐age children born extremely low birth weight or very preterm in the 1990s. JAMA 289:3264–3272. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group (2003b): Neurobehavioral outcomes of school‐age children born extremely low birth weight or very preterm in the 1990s. JAMA 289:3264–3272. [DOI] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW, Victorian Infant Collaborative Study Group (2004): Executive functioning in school‐aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics 114:50–57. [DOI] [PubMed] [Google Scholar]
- Bartholomeusz CF, Whittle SL, Montague A, Ansell B, McGorry PD, Velakoulis D, Pantelis C, Wood SJ (2013): Sulcogyral patterns and morphological abnormalities of the orbitofrontal cortex in psychosis. Prog Neuropsychopharmacol Biol Psychiatry 44:168–177. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR (2000): Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 10:295–307. [DOI] [PubMed] [Google Scholar]
- Burnett A, Davey CG., Wood SJ, Wilson‐Ching M, Molloy C, Cheong JLY, Doyle LW, Anderson PJ (2014): Extremely preterm birth and adolescent mental health in a geographical cohort born in the 1990s. Psychol Med 44:1533–1544. [DOI] [PubMed] [Google Scholar]
- PW Burgess, T Shallice (1997): The Hayling and Brixton Tests Thames Valley Test Company, Thurston, Suffolk. [Google Scholar]
- Chakirova G, Welch KA, Moorhead TW, Stanfield AC, Hall J, Skehel P, Brown VJ, Johnstone EC, Owens DG, Lawrie SM, McIntosh AM (2010): Orbitofrontal morphology in people at high risk of developing schizophrenia. Eur Psychiatry 25:366–372. [DOI] [PubMed] [Google Scholar]
- Cheong JL, Anderson PJ, Roberts G, Burnett AC, Lee KJ, Thompson DK, C Molloy, M Wilson‐Ching, A Connelly, ML Seal, SJ Wood, Doyle LW (2013): Contribution of brain size to IQ and educational underperformance in extremely preterm adolescents. PLoS One 8:e77475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiavaras MM, Petrides M, (2000): Orbitofrontal sulci of the human and macaque monkey brain. Journal of Comparative Neurology 422:35–54. [PubMed] [Google Scholar]
- Chiron C, Jambaque I, Nabbout R, Lounes R, Syrota A, Dulac O (1997): The right brain hemisphere is dominant in human infants. Brain 120:1057–1065. [DOI] [PubMed] [Google Scholar]
- Clark CA, Woodward LJ, Horwood LJ, Moor S (2008): Development of emotional and behavioral regulation in children born extremely preterm and very preterm: Biological and social influences. Child Dev 79:444–1462. [DOI] [PubMed] [Google Scholar]
- Congalton AA (1969): Status and Prestige in Australia. Melbourne: Cheshire. [Google Scholar]
- Doyle LW, Anderson PJ (2005): Improved neurosensory outcome at 8 years of age of extremely low birthweight children born in Victoria over three distinct eras. Arch Dis Child Fetal Neonatal Ed 90:F484–F488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LW, Victorian Infant Collaborative Study Group (2001): Outcome at 5 years of age of children 23 to 27 weeks' gestation: Refining the prognosis. Pediatrics 108:134–141. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Bowman E, Callanan C, Carse E, Charlton MP, Drew J, Yu V (1997a): Improved outcome into the 1990s for infants weighing 500–999 g at birth. Arch Dis Child Fetal Neonatal Ed 77:F91–F94. [PMC free article] [PubMed] [Google Scholar]
- Doyle LW, Bowman E, Callanan C, Carse E, Charlton MP, Drew J, G Ford, S Fraser, J Halliday, M Hayes, E Kelly, P McDougall, A Rickards, A Watkins, H Woods, Yu V (1997b): Outcome at 2 years of children 23–27 weeks' gestation born in Victoria in 1991‐92. J Paediatr Child Health 33:161–165. [PubMed] [Google Scholar]
- Doyle LW, Roberts G, Anderson PJ (2010): Outcomes at age 2 years of infants < 28 weeks' gestational age born in Victoria in 2005. J Pediatr 156:U49–U84. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Borradori‐Tolsa C, Cachia A, Lazeyras F, Ha‐Vinh Leuchter R, SV Sizonenko, SK Warfield, JF Mangin, Huppi PS (2008a): Primary cortical folding in the human newborn: An early marker of later functional development. Brain 131:2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Benders M, Cachia A, Lazeyras F, Ha‐Vinh Leuchter R, Sizonenko SV, C Borradori‐Tolsa, JF Mangin, Huppi PS (2008b): Mapping the early cortical folding process in the preterm newborn brain. Cereb Cortex 18:1444–1454. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002): Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Non‐patient Edition (SCID‐I/NP). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fischi‐Gomez E, Vasung L, Meskaldji DE, Lazeyras F, Borradori‐Tolsa C, Hagmann P, K Barisnikov, JP Thiran, Huppi PS: Structural brain connectivity in school‐age preterm infants provides evidence for impaired networks relevant for higher order cognitive skills and social cognition (in press). DOI: 10.1093/cercor/bhu073. [DOI] [PubMed]
- Gimenez M, Junque C, Vendrell P, Narberhaus A, Bargallo N, Botet F, Mercader JM (2006): Abnormal orbitofrontal development due to prematurity. Neurology 67:1818–1822. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L (2000): Behavior rating inventory of executive function. Child Neuropsychol 6:235–238. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, TF Nugent, DH Herman, LS Clasen, AW Toga, JL Rapoport, Thompson PM (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR (1990): Morphometric study of human cerebral cortex development. Neuropsychologia 28:517–527. [DOI] [PubMed] [Google Scholar]
- Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ (2005): Abnormal cerebral structure is present at term in premature infants. Pediatrics 115:286–294. [DOI] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N (2010): Psychiatric disorders in extremely preterm children: Longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry 49:453–463 e451. [PubMed] [Google Scholar]
- Kesler SR, Vohr B, Schneider KC, Katz KH, Makuch RW, Reiss AL, Ment LR (2006): Increased temporal lobe gyrification in preterm children. Neuropsychologia 44:445–453. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML (2005): The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci 6:691–702. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET (2004) The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol 72:341–372. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Bartholomeuz CF, Nelson B, Lin A, McGorry PD, Velakoulis D, SL Whittle, AR Yung, C Pantelis, Wood SJ (2014): Sulcogyral pattern and sulcal count of the orbitofrontal cortex in individuals at ultra high risk for psychosis. Schizophr Res 154:93–99. [DOI] [PubMed] [Google Scholar]
- Lodygensky GA, Rademaker K, Zimine S, Gex‐Fabry M, Lieftink AF, Lazeyras F, F Groenendaal, LS De Vries, Huppi PS (2005): Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 116:1–7. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Salisbury DF, Hirayasu Y, Bouix S, Pohl KM, Yoshida T, MS Koo, ME Shenton, McCarley RW (2007): Neocortical gray matter volume in first‐episode schizophrenia and first‐episode affective psychosis: A cross‐sectional and longitudinal MRI study. Biol Psychiatry 62:773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, J MacCabe, L Rifkin, Hultman CM (2012): Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry 69:E1–E8. [DOI] [PubMed] [Google Scholar]
- Pantelis C, Yucel M, Wood SJ, McGorry PD, Velakoulis D (2003): Early and late neurodevelopmental disturbances in schizophrenia and their functional consequences. Aust N Z J Psychiatry 37:399–406. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, KH Katz, M Westerveld, S Sparrow, AW Anderson, CC Duncan, RW Makuch, JC Gore, Ment LR (2000): Regional brain volume abnormalities and long‐term cognitive outcome in preterm infants. JAMA 284:1939–1947. [DOI] [PubMed] [Google Scholar]
- Reig S, Sánchez‐González J, Arango C, Castro J, González‐Pinto A, Ortuño F, B Crespo‐Facorro, N Bargallo, Desco M (2009): Assessment of the increase in variability when combining volumetric data from different scanners. Hum Brain Mapp 30:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter BC, Perrig W, Steinlin M, Everts R (2014): Cognitive and behavioral aspects of executive functions in children born very preterm. Child Neuropsychol 20:129–144. [DOI] [PubMed] [Google Scholar]
- Roppongi T, Nakamura M, Asami T, Hayano F, Otsuka T, Uehara K, A Fujiwara, T Saeki, S Hayasaka, T Yoshida, R Shimizu, T Inoue, Hirayasu Y (2010): Posterior orbitofrontal sulcogyral pattern associated with orbitofrontal cortex volume reduction and anxiety trait in panic disorder. Psychiatry Clin Neurosci 64:318–326. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan L, Allsop J, Counsell SJ, Boardman JP, Edwards AD, Rutherford M (2006): Smaller cerebellar volumes in very preterm infants at term‐equivalent age are associated with the presence of supratentorial lesions. AJNR Am J Neuroradiol 27:573–579. [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2005): Cognitive and affective development in adolescence. Trends Cogn Sci 9:69–74. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Takahashi T, Orikabe L, Masuda N, Mozue Y, Nakamura K, Y Kawasaki, M Itokawa, Y Sato, H Yamasue, K Kasai, Y Okazaki, Suzuki M (2010): Volume reduction and altered sulco‐gyral pattern of the orbitofrontal cortex in first‐episode schizophrenia. Schizophr Res 121:55–65. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, MJ Kean, LW Doyle, GF Egan, Inder TE (2007): Perinatal risk factors altering regional brain structure in the preterm infant. Brain 130:667–677. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Wood SJ, Doyle LW, Warfield SK, Lodygensky GA, Anderson PJ, GF Egan, Inder TE (2008): Neonate hippocampal volumes: Prematurity, perinatal predictors, and 2‐year outcome. Ann Neurol 63:642–651. [DOI] [PubMed] [Google Scholar]
- Toro R, Perron M, Pike B, Richer L, Veillette S, Pausova Z, Paus T (2008): Brain size and folding of the human cerebral cortex. Cereb Cortex 18:2352–2357. [DOI] [PubMed] [Google Scholar]
- Treyvaud K, Ure A, Doyle LW, Lee KJ, Rogers CE, Kidokoro H, Anderson PJ (2013): Psychiatric outcomes at age seven for very preterm children: Rates and predictors. J Child Psychol Psychiatry 54:772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC (1997): A tension‐based theory of morphogenesis and compact wiring in the central nervous system. Nature 385:313–318. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1999): Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Harcourt Assessment. [Google Scholar]
- Weller EB, Weller RA, Fristad MA, Rooney MT, Schecter J (2000): Children's interview for psychiatric syndromes (ChIPS). J Am Acad Child Adolesc Psychiatry 39:76–84. [DOI] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao CY, Sapiro G (2010): The development of gyrification in childhood and adolescence. Brain Cogn 72:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White‐Koning M, Arnaud C, Bourdet‐Loubere S, Bazex H, Colver A, Grandjean H (2005): Subjective quality of life in children with intellectual impairment—How can it be assessed? Dev Med Child Neurol 47:281–285. [DOI] [PubMed] [Google Scholar]
- Whittle S, Bartholomeusz C, Yucel M, Dennison M, Vijayakumar N, Allen NB (2014): Orbitofrontal sulcogyral patterns are related to temperamental risk for psychopathology. Soc Cogn Affect Neurosci 9:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KR, Donders J, Nguyen L (2011): Self and parent ratings of executive functioning after adolescent traumatic brain injury. Rehabil Psychol 56:100–106. [DOI] [PubMed] [Google Scholar]


