Abstract
Although cognitive training usually improves cognitive test performance, the capability to transfer these training gains into respective or functionally related cognitive domains varies significantly. Since most studies demonstrate rather limited transfer effects in older adults, aging might be an important factor in transfer capability differences. This study investigated the transfer capability of logical reasoning training gains to a measure of Fluid Intelligence (Gf) in relation to age, general intelligence, and brain structural integrity as measured by diffusion tensor imaging. In a group of 41 highly educated healthy elderly, 71% demonstrated successful transfer immediately after a 4‐week training session (i.e. short‐term transfer). In a subgroup of 22% of subjects transfer maintained over a 3‐month follow‐up period (i.e. long‐term transfer). While short‐term transfer was not related to structural integrity, long‐term transfer was associated with increased structural integrity in corpus and genu of the corpus callosum. Since callosal structural integrity was also related to age (in the present and foregoing studies), previously observed associations between age and transfer might be moderated by the structural integrity. Surprisingly, age was not directly associated with transfer in this study which could be explained by the multi‐dependency of the structural integrity (modulating factors beside age, e.g. genetics). In this highly educated sample, general intelligence was not related to transfer suggesting that high intelligence is not sufficient for transfer in normal aging. Further studies are needed to reveal the interaction of transfer, age, and structural integrity and delineate mechanisms of age‐dependent transfer capabilities. Hum Brain Mapp 35:309–318, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: cognitive training, transfer, corpus callosum, DTI, normal aging
INTRODUCTION
A long history of cognitive training research on human subjects has shown an increase in task performance after training in the trained task [Dahlin et al., 2008b; Yang et al., 2006]. These training gains occur throughout the life span, even in old age. In addition, task‐limited training gains are not restricted to a certain cognitive domain, but manifest in many different cognitive tasks [Baltes et al., 1982; Bherer et al., 2006; Brehmer et al., 2007; Jones et al., 2006].
Specific cognitive training tasks normally require that putative improvements are not limited to the training task itself. Cognitive training should enhance the performance of other tasks of the same or a different cognitive domain, which leads to improved function of the trained skills in different situations and surroundings (i.e. transfer of training). The nature of transfer has been the subject of considerable empirical and theoretical research for the past 100 years [Blume et al., 2010]. Previous findings are heterogeneous in their results. Some of them demonstrated limited or nonexistent transfer effects [e.g. Ericsson and Delaney, 1998; Healy et al., 2006; Owen et al., 2010; Singley and Anderson, 1989], whereas others provide evidence for successful transfer [e.g. Dahlin et al., 2008a, 2008b; Jaeggi et al., 2008]. Barnett and Ceci 2002 suggest that the controversial results are a consequence of an arbitrary operationalization of transfer across studies. The majority of research demonstrating successful transfer investigated near transfer effects in a temporal context [Barnett and Ceci, 2002]. In addition, Blume et al. 2010 could show stronger predictor and transfer relationships when transfer measures are taken immediately after training. However, if it is to justify the effort invested in training, ideally one would hope for transfer to last for months or years. Despite the relevance of an improved understanding of the mechanisms of transfer capabilities, current explanations lack coherence regarding the necessary conditions under which transfer occurs and persists.
Normal aging is associated with a progressive functional loss in many cognitive domains [Craik and Salthouse, 2000]. Although recent literature provides evidence for successful transfer of training gains even in old age [Ball and Owsley, 2000; Borella et al., 2010; Jennings et al., 2005; Karbach and Kray, 2009; Richmond et al., 2011] most research further indicates rather limited transfer effects in older adults [Ball et al., 2002; Dahlin et al., 2008a, 2008b; Derwinger et al., 2003; Edwards et al., 2002; Rebok et al., 2007]. Therefore, aging or aging‐related decreases in neuroplasticity might be important factors in transfer capability differences. In addition to age, general intelligence commonly impacts transfer of learning and training. Cognitive abilities are strongly related to transfer of training gains, and they are robust predictors of training outcomes [Colquitt et al., 2000].
Multidisciplinary research has shown that aging is associated with many brain‐related alterations that range from genetic to neurochemical and structural neuroanatomical factors [Masoro and Austad, 2010]. These age‐related alterations are accompanied by a decline in cognitive performance [Hofer and Alwin, 2008]. Higher general intelligence is associated with better white matter integrity within the prefrontal cortex (PFC) [Gray et al., 2003] or a higher global network efficacy [Li et al., 2009]. Therefore, the coherence between age, general intelligence and transfer is possibly moderated by the structural integrity, especially in brain networks that underlay age‐sensitive cognitive domains, such as executive functions.
This study aims to investigate the relationship between structural integrity measures, age as well as general intelligence and transfer capabilities of training gains. Separate analyses were conducted for immediate transfer (i.e. short‐term transfer) and maintenance of transfer (i.e. long‐term transfer) to compare and specify underlying mechanisms of near and far transfer in a temporal context. We applied a logical reasoning training task and a fluid intelligence (Gf) transfer task given that both are related to executive functions but focus on different skills. The Gf task refers to the ability to identify patterns and relations and convey and implement rules, independent of previous knowledge [Horn & Cattell, 1966]. The logical reasoning task measures the ability to apply a given rule and its precondition to make a conclusion. Beside an increase of alterations of brain‐related factors that come along with normal aging functional imaging studies provide evidence for partial compensation of age‐related deficits [Grady et al., 2006; Reuter‐Lorenz et al., 2000]. Older adults compensate age‐related deficits in part by additional frontal activation. This complex interplay of neurodegeneration and compensation leads to an increased heterogeneity of cognitive capabilities and trainability of cognitive functions in older age. Therefore, separate analyses were planned for a group of younger (60–70 years of age) and a group of older (70–85 years of age) healthy elderly subjects in addition to the analyses of the whole sample (60–85 years of age). We hypothesized that transfer of training gains is associated with the structural integrity within frontal and particularly inferior‐frontal networks in all groups, as these regions are neural substrates of executive functions [Jurado and Rosselli, 2007]. We also expected a coherence between age as well as general intelligence and transfer capabilities.
MATERIAL AND METHODS
Participants and Procedure
The data of 41 healthy elderly subjects [age range 60–85 years, mean age, 69.93 ± 7.90 (SD), 18 male] were analyzed. All participants were recruited by advertisements posted in the Medical University Clinic Mainz and several public institutions and via newspaper announcement. The local Ethics Committee approved the study protocol, and all subjects provided written informed consent. Subjects were excluded if they had any psychiatric (e.g., depression, schizophrenia, alcohol abuse) or cognitive (e.g. dementia, mild cognitive impairment) illness, a history of brain damage, stroke or any central nervous system disorders, or if they were taking cognitive performance‐altering medications.
The study design is illustrated in Figure 1. All participants underwent diffusion tensor imaging (DTI), cognitive training of logical reasoning and an assessment of Gf. DTI was conducted to analyze white matter fractional anisotropy (FA), which quantifies white matter integrity [Basser and Pierpaoli, 1996; Beaulieu, 2002; Mori and Zhang, 2006]. The logical reasoning training phase consisted of 11 sessions over a 4‐week period with three sessions per week. Each session lasted for 60 min and was supervised by a trainer. The assessment of Gf was performed at three time points: (1) immediately prior to the first logical reasoning training session (Pretest), (2) immediately following the last logical reasoning training session (Posttest), and (3) 3 months after the last logical reasoning training session (Follow‐Up). DTI scans were obtained immediately prior to the first logical reasoning training session (Fig. 1). In addition to the trained study group (41 healthy elderly subjects), we examined a separate control group (n = 25, age range 60–76 years, 10 male), who were also assessed longitudinally on Gf but were not trained in logical reasoning, to control the Gf performance alterations of the trained group for mere retest effects.
Figure 1.
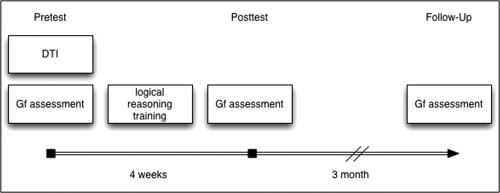
Illustration of the study design. Baseline measurements (Pretest) were comprised of diffusion tensor imaging (DTI) and the assessment of fluid intelligence (Gf) performance. Gf performance was re‐evaluated after 4 weeks (Posttest). Eleven logical reasoning training sessions were applied between Pretest and Posttest. A second re‐evaluation of Gf performance was conducted three months later (Follow‐Up) to determine the maintenance of possible transfer effects.
Neuropsychological Materials
Training task
Logical reasoning was trained using a standardized computer‐based cognitive training task provided by the Cogpack software [Marker, 2008], which is a widely used and well‐established instrument in German‐speaking countries [Rauchensteiner et al., 2011]. Twenty‐four different road signs were presented, and participants were instructed to match the signs to a given logical expression as quickly as possible (e.g., “Mark all signs that are not triangular and red”). In each of the 11 training sessions 20 trials were processed. The performance was rated according to correct responses and response time.
Transfer task
A subtest of a major German intelligence scale [Leistungsprüfsystem; Horn, 1983) that measures nonverbal reasoning (LPS 4) was applied to assess Gf. The task consisted of alternating rows of numbers or letters that were arranged according to a logical system. Each row included a number or letter that did not apply to this logical system, and participants were instructed to identify this outlier. This conceptual formulation is comparable to the Raven's Progressive Advanced Matrices, which is a standard measurement of Gf, which consists of different figures and signs arranged according to a logical system. However, subjects must provide the logical missing part in this system. We applied parallel versions of the task at Pretest and Posttest. Since only two versions of the test are available the “Pretest‐version” was used again at Follow‐Up. Each measurement was timed to 8 min. The performance was rated according to the number of correct responses.
General intelligence
A revised version of the Hamburg Wechsler Intelligence Test [HAWIE‐R; Tewes, 1991], the German version of the Wechsler Adult Intelligence Scale revised [WAIS‐R; Wechsler, 1981], was applied to assess general intelligence.
MRI Data Acquisition
Brain imaging examinations were conducted in a Siemens 3T TrioTim MRI scanner (Siemens, Erlangen, Germany). Apart from the acquisition of routine T1, PD/T2 weighted, fluid attenuated inversion recovery (FLAIR) weighted and Time‐of‐Flight (TOF) sequences, a diffusion‐weighted, single‐shot, spin‐echo, echoplanar‐based sequence (30 directions; b = 1,000 s/mm2; matrix = 128 × 128; section thickness: 3 mm; voxel size: 1.5 × 1.5 × 3 mm3; TR/TE 7,100 ms/102 ms) was applied. Diffusion‐weighted data were processed using FSL 4.1 (FMRIB Analysis Group, Oxford, UK, http://www.fmrib.ox.ac.uk/fsl) and the following procedures: (i) motion and eddy current correction, (ii) adjusting Gradients accordingly by application of the rotational part of the resulting affine transformations, and (iii) removal of the skull and nonbrain tissue using Brain Extraction Tool [Smith, 2002].
Data Analyses
Short‐ and long‐term transfer
To examine transfer of training gains we only included subjects that demonstrated improvements in the training task after training. After the exclusion of two subjects that did not improve their performance in the training task the study group finally included 41 subjects. We initially investigated short‐term transfer. Successful short‐term transfer was defined as a performance improvement in the training‐ and transfer task from Pre‐ to Posttest (Posttest – Pretest > 0). Transfer task improvements had to be greater than the retest‐effect of the untrained control group. We classified the study participants in subjects that showed successful short‐term transfer (transferring subjects) and subjects that did not show transfer of training gains (nontransferring subjects).
Subsequently, we analyzed long‐term transfer. Successful long‐term transfer was defined as a performance improvement in the training‐ and transfer task from Pre‐ to Posttest (Posttest – Pretest > 0) and maintenance of performance in the transfer task from Posttest to Follow‐Up (Follow‐Up – Posttest ≥ 0). Transfer task performance alterations (Pre‐ to Posttest and Posttest to Follow‐Up) had to be greater than the retest‐effect of the untrained control group. We classified the study participants in subjects that showed successful long‐term transfer (transferring subjects) and subjects that did not show long‐term transfer (nontransferring subjects).
The examinations of short‐ and long‐term transfer included: (i) an explorative voxel‐wise statistical group comparison of the FA data with Tract‐Based Spatial Statistics [TBSS; Smith et al., 2006], and (ii) subsequent region of interest (ROI)‐based group comparisons of regions, that showed significant group differences in the explorative voxel‐wise analyses. Separate explorative TBSS analyses were conducted for a young‐old subgroup (n = 24, age range: 60–70 years), an old‐old subgroup (n = 17, age range: 70–85 years) and the entire group (n = 41, age range: 60–85 years). However, explorative long‐term transfer examinations were restricted to the young‐old group and the entire group due to the small number of subjects with successful long‐term transfer in the old‐old group (n = 3). Statistical ROI‐analyses were principally restricted to the whole sample to achieve adequate statistical power.
TBSS
Tract‐based spatial statistic [TBSS; Smith et al., 2006] analyses included: (i) nonlinear registration to the FMRIB58_FA template of all subjects' FA data using FMRIB's Non‐linear Image Registration Tool [Ruckert et al., 1999], (ii) the creation and thinning of a mean FA image with a threshold of 0.2 to obtain a mean FA skeleton that represented the centers of white matter trajectories, and (iii) projections of each subjects' aligned FA data onto the skeleton. Group‐wise t‐test comparisons between transferring subjects and nontransferring subjects were calculated using the randomize tool, which tested the t‐value at each voxel against a null distribution that was obtained from 5,000 random permutations of group membership. The significant threshold for between‐group differences was set at P < 0.05, corrected for multiple comparisons across voxels, using the threshold‐free cluster‐enhancement option.
ROI‐extraction
ROIs were defined based on the JHU ICBM white matter label atlas [JHU ICBM‐DTI‐81; Mori et al., 2008], which contains hand‐segmented white matter parcellation maps. We selected those labels of the atlas that corresponded to anatomical regions that showed significant differences in voxel‐wise TBSS group comparisons. Subsequently, we registered each subjects FA image to the ICBM‐DTI‐81 template and calculated mean FA‐values of the selected labels for each subject.
Statistical procedures
Neuropsychological performances were evaluated with the Wilcoxon test (in case of two group comparisons) and repeated measures analyses of variance (ANOVAs, in case of multiple comparisons). Post‐hoc analyses were performed with Bonferroni post‐hoc‐tests.
ROI‐based group comparisons to analyze the relationship between structural integrity, age as well as general intelligence and transfer comprised Mann–Whitney U‐tests and analyses of covariance (ANCOVAs). ANCOVAs were used to control statistics for age, general intelligence, gender, and white matter lesion (WML) volume. A linear regression analysis was performed to describe the association between FA values and age, general intelligence, gender, and WML‐volume.
WML‐volumes were defined as bright lesions (>2 mm) of the white matter or basal ganglia and were measured within the FLAIR weighted images using Analyze® Software (Version 8.0; Biomedical‐Imaging‐Software‐System, MayoFoundation for medical education and research, Rochester) by a manually slice‐by‐slice tracing of the WML‐boundaries [Fellgiebel et al., 2009]. WML volume was log‐transformed because the data were not normally distributed.
RESULTS
Short‐term Transfer Analyses
Demographic characteristics of the study group are listed in Table 1. Group‐wise age, general intelligence, education level, gender, and WML volume comparisons demonstrated no significant differences between transferring and nontransferring subjects in the short‐term transfer analyses.
Table 1.
Demographic characteristics and ROI‐based FA findings of the short‐ and long‐term transfer analyses of the entire group
| Short‐term transfer analyses | P a | Long‐term transfer analyses | P a | |||
|---|---|---|---|---|---|---|
| Transferring subjects | Nontransferring subjects | Transferring subjects | Nontransferring subjects | |||
| N | 29 | 12 | 9 | 32 | ||
| Age | 69.31 ± 7.61 | 71.42 ± 8.74 | n.s. | 66.89 ± 6.90 | 70.78 ± 8.06 | n.s. |
| Gender | ||||||
| Male | 45% | 33% | 33% | 44% | ||
| Female | 55% | 66% | n.s. | 67% | 56% | n.s. |
| Education years | 12.34 ± 3.34 | 12.33 ± 3.17 | n.s. | 12.56 ± 3.50 | 12.28 ± 3.24 | n.s. |
| HAWIE‐R | 140 ± 16 | 135 ± 14 | n.s. | 135 ± 14 | 139 ± 16 | n.s. |
| WML volume (ml) | 1.9 ± 3.7 | 0.8 ± 1.8 | n.s. | 0.8 ± 1.2 | 1.7 ± 3.6 | n.s. |
| FA ROI corpus and genu CC | 0.50 ± 0.04 | 0.50 ± 0.03 | n.s. | 0.52 ± 0.04 | 0.50 ± 0.03 | 0.038 |
HAWIE‐R: Hamburger–Wechsler intelligence scale; WML: White matter lesion; FA: fractional anisotropy; ROI: region‐of‐interest; CC: corpus callosum.
Short‐term transfer analyses: investigation of transfer measured immediately after training; transferring subjects: participants showing short‐term performance improvements (Pretest to Posttest) in the training and transfer task; nontransferring subjects: participants showing short‐term performance improvements (Pretest to Posttest) in the training but not the transfer task. Long‐term transfer analyses: investigation of maintenance of transfer; transferring subjects: participants showing short‐term performance improvements in the training and transfer task and maintenance of transfer task improvements (Posttest to Follow‐Up); nontransferring subjects: participants showing short‐term performance improvements in the training task (Pretest to Follow‐Up) but no short‐term improvements and maintenance of performance in the transfer task (Posttest to Follow‐Up).
Group comparisons: Mann–Whitney U‐tests were used for continuous data; chi‐square tests were used for categorical data (two‐tailed p values).
Performance data of the logical reasoning training task and the Gf transfer task of the short‐term analyses are given in Table 2 (entire group). Transferring subjects (n = 29) demonstrated significant improvements in the logical reasoning training task (P < 0.001, Wilcoxon test) and the Gf transfer task (P < 0.001, Wilcoxon test) between Pre‐ and Posttest indicating successful short‐term transfer of training gains. Nontransferring subjects (n = 12) showed significant improvements in the training task (P = 0.002, Wilcoxon test); however, significant decreases in the transfer task (P = 0.027, Wilcoxon test). Controls (n = 25) did not differ in their Gf performance.
Table 2.
Short‐term transfer (performance data of the study‐ and control group)
| Pretest | Posttest | P a | |
|---|---|---|---|
| Transferring subjects (n = 29) | |||
| Logical reasoning training task | 17.08 ± 1.21 | 18.44 ± 0.66 | <0.001 |
| Fluid intelligence transfer task | 24.04 ± 5.47 | 28.24 ± 3.69 | <0.001 |
| Nontransferring subjects (n = 12) | |||
| Logical reasoning training task | 16.96 ± 1.04 | 18.05 ± 0.95 | 0.002 |
| Fluid intelligence transfer task | 24.67 ± 3.00 | 23.50 ± 2.84 | 0.027 |
| Controls (n = 25) | |||
| Fluid intelligence transfer task | 23.60 ± 5.51 | 24.00 ± 4.56 | n.s. |
Logical reasoning training task: Cogpack Logic, part of the Cogpack software (Marker, 2008); Fluid intelligence transfer task: LPS4, part of a major German intelligence scale (Leistungsprüfsystem; Horn, 1983); transferring subjects: participants showing short‐term performance improvements (Pretest to Posttest) in training and transfer task; nontransferring subjects: participants showing short‐term performance improvements (Pretest to Posttest) in the training but not the transfer task.
Wilcoxon tests were used for group comparisons between Pre‐ and Posttest.
Explorative voxel‐wise TBSS analyses of short‐term transfer demonstrated no significant differences in the FA between transferring‐ and nontransferring subjects, neither in the young–old or old–old subgroup nor in the entire sample. Thus, no further ROI analyses were performed.
Long‐term Transfer Analyses
Group‐wise comparisons of the demographic variables age, general intelligence, education level, gender, and WML volume demonstrated no significant differences between transferring and nontransferring subjects in the long‐term transfer analyses (Table 1).
Performance data of the training‐ and transfer task of the analyses of long‐term transfer are given in Table 3 (entire group). Transferring subjects (n = 9) demonstrated significant improvements in the logical reasoning training task (P = 0.002, Wilcoxon test). Furthermore, we could observe significant improvements in the transfer task from Pretest to Posttest (P < 0.001, Bonferroni post‐hoc test), Pretest to Follow‐Up (P < 0.001, Bonferroni post‐hoc test) and Posttest to Follow‐Up (P = 0.005, Bonferroni post‐hoc test) indicating long‐term training gain transfer. Nontransferring subjects (n = 32) demonstrated performance improvements in the training task (P < 0.001, Wilcoxon test) and short‐term improvements (Pretest to Posttest) in the transfer task (P = 0.001, Bonferroni post‐hoc test). Long‐term transfer could not be observed, as nontransferring subjects demonstrated a performance decrease from Posttest to Follow‐Up (P = 0.006, Bonferroni post‐hoc test) and no significant performance differences between Pretest and Follow‐Up. Controls (n = 25) did not differ in their Gf performance between Pretest, Posttest and Follow‐Up.
Table 3.
Long‐term transfer (performance data of the study‐ and control group)
| Pretest | Posttest | Follow‐up | P b | |
|---|---|---|---|---|
| Transferring subjects (n = 9) | ||||
| Logical reasoning training task | 16.58 ± 0.98 | 18.37 ± 0.51 | 0.002 | |
| Fluid intelligence transfer task | 22.78 ± 2.44a | 26.89 ± 2.89a | 28.22 ± 2.64a | <0.001 |
| Nontransferring subjects (n = 32) | ||||
| Logical reasoning training task | 17.18 ± 1.17 | 18.32 ± 0.83 | <0.001 | |
| Fluid intelligence transfer task | 24.59 ± 3.77a | 26.84 ± 4.38a | 25.38 ± 3.87a | <0.001 |
| Controls (n = 25) | ||||
| Fluid intelligence transfer task | 23.60 ± 5.51 | 24.00 ± 4.56 | 23.08 ± 4.89 | n.s. |
Logical reasoning training task: Cogpack Logic, part of the Cogpack software (Marker, 2008); Fluid intelligence transfer task: LPS4, part of a major German intelligence scale (Leistungsprüfsystem; Horn, 1983); transferring subjects: participants showing short‐term performance improvements in the training and transfer task (Pretest to Posttest) and maintenance of transfer task improvements (Posttest to Follow‐Up); nontransferring subjects: participants showing short‐term performance improvements in the training task (Pretest to Follow‐Up) but no short‐term improvements and maintenance of performance in the transfer task (Posttest to Follow‐Up).
Numbers with different superscripts (a–c) demonstrate significant differences in post‐hoc analyses (Bonferroni).
Group comparisons: Wilcoxon tests were used for two‐group comparisons; repeated measures ANOVA were used for multiple comparisons.
Explorative voxel‐wise TBSS analyses of long‐term transfer revealed significantly higher FA values within the corpus and genu of the corpus callosum (CC) in transferring subjects compared to nontransferring subjects in the young‐old subgroup (Fig. 2). This finding remained significant after controlling for age, education, and general intelligence. A similar explorative analysis in the entire sample indicated the same tendencies but did not reach significance.
Figure 2.
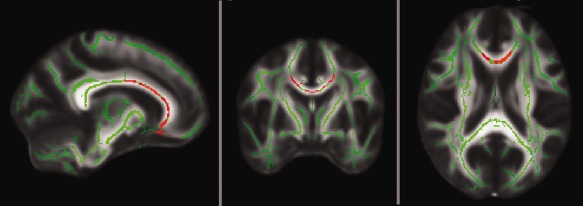
White matter integrity in transferring subjects versus nontransferring subjects of the long‐term transfer analysis. TBSS analysis on cerebral white matter skeleton (green) demonstrates areas with significantly higher FA values (red) in subjects with successful transfer of training gains (transferring subjects) compared to subjects without successful transfer of training gains (nontransferring subjects) in the young‐old group (age range 60–70 years).
ROI‐based analyses of the entire sample confirmed these results and demonstrated significantly higher FA values in corpus and genu of the CC in transferring subjects compared to nontransferring subjects (P = 0.038, MWU test, Table 1 and Fig. 3). The splenium was used as reference and exhibited no group differences. The observed group differences in the FA of corpus and genu of the CC persisted after controlling the statistic for age, general intelligence, gender and WML volume (ANCOVA, F = 4.833, P = 0.002). The covariate age was a significant predictor of FA values (F = 8.207, P = 0.007), but general intelligence (F = 2.320, P = 0.137), gender (F = 0.210, P = 0.650) and WML volume (F = 2.357, P = 0.134) were not significant predictors. An additional linear Regression analysis to predict FA‐values with age, general intelligence, gender and WML volume showed an overall significant effect (F = 5.335, P = 0.002). In accordance to the ANCOVA result FA was predicted by age (t = −3.224, P = 0.003), however, not by general intelligence (t = 1.447, P = 0.157), gender (t = 0.704, P = 0.486), and WML volume (t = −1.612, P = 0.116).
Figure 3.
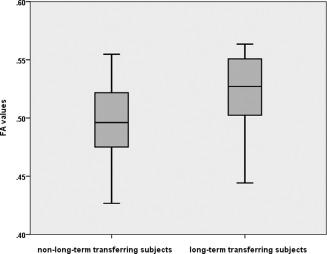
Boxplot diagrams of mean FA of genu and corpus of the corpus callosum (region‐of‐interest analysis) of the long‐term transfer analysis. Group comparisons of subjects with successful transfer of training gains (transferring subjects) and subjects without successful transfer of training gains (nontransferring subjects) in the whole study group.
DISCUSSION
This study investigated whether the transfer capability of Gf‐related training gains was associated with the structural integrity of the brain. The relationship of transfer to age and general intelligence was also examined. Separate analyses were conducted for short‐term‐ and long‐term transfer. 71% (29 of 41) of subjects demonstrated successful short‐term transfer. Only 22% (9 of 41) of subjects demonstrated successful long‐term transfer. Furthermore, long‐term transfer was more prevalent in younger subjects of our sample. This result is consistent with previous studies demonstrating limited transfer effects in older adults [Ball et al., 2002; Dahlin et al., 2008a, 2008b; Derwinger et al., 2003; Edwards et al., 2002; Rebok et al., 2007].
Importantly, transfer of training gains was associated with a higher degree of structural integrity within corpus and genu of the CC as measured by FA values using DTI. However, this association was restricted to long‐term transfer. The exclusive relationship of callosal integrity with long‐term transfer may be based on an imprecise measurement of transfer if transfer is operationalized by an immediate measurement after training. Immediate transfer effects may partially be an artifact of temporary factors such as improvements in motivation, habituation to the neuropsychological testing situation caused by cognitive training, or an increase in cognitive effort to compensate increased cognitive demands. A consideration of maintenance of short‐term transfer prevents a partial investigation of temporary compensatory mechanisms, which leads to a more precise investigation of transfer.
The CC is a major neural pathway that connects homologous cortical areas of the two cerebral hemispheres [Bloom and Hynd, 2005]. The corpus and genu of the CC connect beside homologue pre‐motor and ventral‐frontal areas the bilateral dorsolateral prefrontal cortex (DLPFC) [Barbas and Pandya, 1984, Bloom and Hynd, 2005], which includes the neural networks underlying executive functions and other cognitive abilities, such as working memory [Smith, 1999, Baddeley, 2003]. Therefore, the assumable underlying mechanism of successful long‐term training gain transfer is a better interhemispheric information transfer within relevant networks through an enhanced structural integrity within the corpus and genu of the CC. The CC may be important to training gain transfer in all domains that rely on the DLPFC because this area is associated with many cognitive domains.
In addition to the association between structural integrity and transfer, a relationship between age as well as general intelligence and transfer was also expected. Successful transfer in younger adults has been observed previously [Dahlin et al., 2008a, 2008b; Jaeggi et al., 2008]. Training gain transfer in older adults primarily yields rather limited transfer effects [Ball et al., 2002; Dahlin et al., 2008a, 2008b; Derwinger et al., 2003; Edwards et al., 2002; Rebok et al., 2007]. These results suggest that transfer is age related. Limited transfer capabilities in old age may partially be associated with the decline in white matter integrity that accompanies normal aging [Masoro and Austad, 2010]. The present results support this assumption. Structural integrity of corpus and genu of the CC appears to be fundamental factor in this age‐related decrease of transfer capabilities, as integrity measures of these regions were associated with both, transfer capability and age. Surprisingly, a direct relationship between age and transfer was not observed; transferring subjects and nontransferring subjects did not differ in age. We further demonstrated that transfer occurred in old age. In addition to age, structural integrity depends on other factors, such as genetics [Heise et al., 2011], general intelligence [Li et al., 2009], and cerebrovascular factors [Kennedy and Raz, 2009]. This multidependency might explain the absence of a direct association between age and transfer in our study. However, a greater sample size could have shown a statistically relevant relationship because nontransferring subjects demonstrated a tendency to higher age (Table 1).
A comprehensive meta‐analysis [Colquitt et al., 2000] of 20 years of training research reported an association between general intelligence and transfer of training gains. However, in our sample, subjects with successful transfer did not differ in general intelligence from subjects without successful transfer, neither in the short‐term‐ nor in the long‐term transfer analyses (Table 1). This discrepancy may be due to our homogeneous highly intelligent study sample (mean HAWIE IQ = 138). Intelligence is likely to facilitate Gf transfer, but higher intelligence is not sufficient for Gf transfer. Higher intelligence could be related to a better network efficacy of anatomical structures over the whole brain, including the genu and truncus of the CC [Li et al., 2009], which is additionally important for Gf transfer in highly intelligent aged subjects.
This study has several limitations. The number of transferring subjects in the long‐term transfer analyses in our study group is quite small. Group comparisons and regression analyses would be more powerful in a larger sample. Although it is remarkable that we observed significant group differences in this small sample, the findings have to be replicated in a larger study group. The number of male (n = 17) and female (n = 24) subjects differs in our study sample. Even though this difference does not reach statistical significance and a recent training review indicated only a small association between gender and transfer of training [Blume et al., 2010], the present results should be verified in a more homogenous sample. Despite of evidence that DTI indices like FA values are largely independent of brain volume, a partial overlap of CC integrity measurements and volume changes (that have not been determined for the individual subjects) cannot be entirely excluded [Hugenschmidt et al., 2008]. Another limitation relates to the choice of the training task. The principle cognitive ability that is measured or trained by this task is logical reasoning. A successful processing may also require some aspects of memory and attention functions. Thus, besides logical reasoning, the training task could have trained memory and attention abilities.
CONCLUSIONS
To date, current explanations lack coherence regarding the mechanism and brain structural basis of training gain transfer. We could demonstrate that the structural integrity of corpus and genu of the CC predict transfer of logical reasoning training gains to a measure of Gf in healthy elderly. Thus, a better interhemispheric information transfer between frontal areas underlying executive and working memory functions may facilitate training gain transfer within tasks related to these cognitive domains. Since callosal structural integrity was also related to age (in the present and in foregoing studies), our results suggest that previously observed direct associations between age and transfer might be mediated by callosal structural integrity. The lack of a direct association in this study might be due to the multidependency of the structural integrity. Beside age, general intelligence could be shown to be related to transfer capabilities. In this highly educated sample, general intelligence was not related to transfer suggesting that high intelligence is not sufficient for transfer in normal aging. Interestingly, the association between callosal structural integrity and transfer was restricted to long‐term transfer. Short‐term transfer measurements may partly quantify temporary factors like habituation to the neuropsychological testing situation, which results in an imprecise measurement of transfer. This result illustrates the necessity for a separate consideration of short‐and long‐term transfer.
More studies are required to replicate these findings and improve our understanding of the interactions between short‐ and long‐term transfer, age, and structural integrity to delineate the relevant mechanisms of age‐dependent transfer capability. The present data enhance our understanding of the mechanism and brain structural basis of training transfer. Since transfer is an essential feature of successful training our findings offer a promising tool to predict or to monitor significant training effects in the elderly.
ACKNOWLEDGMENTS
The presented data are part of the doctoral thesis of Johanna Fesenbeckh. We thank Christine Ploch, Johanna Fesenbeckh, and Lisa Zschutschke for their efforts with recruitment, training, and investigation of participants during the study.
REFERENCES
- Baddeley A (2003): Working memory: Looking back and looking forward. Nat Rev Neurosci 4:829–839. [DOI] [PubMed] [Google Scholar]
- Ball K, Owsley C (2000): Increasing mobility and reducing accidents of older drivers In: Schaie KW, Pietrucha M, editors. Mobility and Transportation in the Elderly.New York:Springer; pp213–251. [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, Morris JN, Rebok GW, Smith DM, Tennstedt SL, Unverzagt FW, Willis SL (2002): Effects of cognitive training interventions with older adults: A randomized controlled trial. JAMA 288:2271–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltes, PB , Willis SL (1982): Plasticity and enhancement of intellectual functioning in old age: Penn State's Adult Development and Enrichment Project (ADEPT) In: Craik F, Trehub S, editors. Aging and Cognitive Processes.New York:Plenum Press; pp353–390. [Google Scholar]
- Barbas H, Pandya DN (1984): Topography of commissural fibers of the prefrontal cortex in the rhesus monkey. Exp Brain Res 55:187–191. [DOI] [PubMed] [Google Scholar]
- Barnett SM, Ceci J (2002): When and where do we apply what we learn? A taxonomy for far transfer. Psychol Bull 128:612–637. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 111:209–19. [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system: A technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E (2006): Testing the limits of cognitive plasticity in older adults: Application to attentional control. Acta Psychologica 123:261–278. [DOI] [PubMed] [Google Scholar]
- Bloom JS, Hynd GW (2005): The role of the corpus callosum in interhemispheric transfer of information: excitation or inhibition? Neuropsychol Rev 15:59–71. [DOI] [PubMed] [Google Scholar]
- Blume BD, Ford JK, Baldwin TT, Huang JL (2010): Transfer of training: A meta‐analytic review. J Management 36:1065–1105. [Google Scholar]
- Brehmer Y, Li SC, Muller V, Oertzen T, Lindenberger U (2007): Memory plasticity across the life span: Uncovering children's latent potential. Dev Psychol 43:465–478. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, Riboldi F, De Beni R (2010): Working memory training in older adults: Evidence of transfer and maintenance effects. Psychol Aging 25:767–778. [DOI] [PubMed] [Google Scholar]
- Colquitt JA, LePine JA, Noe RA (2000): Toward an integrative theory of training motivation: A meta‐analytic path analysis of 20 years of research. J Appl Psychol 85:678–707. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Salthouse TA (2000): The Handbook of Aging and Cognition.Hillsdale, NJ:Erlbaum. [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L (2008a): Transfer of learning after updating training mediated by the striatum. Science 320:1510–1512. [DOI] [PubMed] [Google Scholar]
- Dahlin E, Nyberg L, Bäckman L, Neely AS (2008b): Plasticity of executive functioning in young and older adults: Immediate training gains, transfer, and long‐term maintenance. Psychol Aging 23:720–730. [DOI] [PubMed] [Google Scholar]
- Derwinger A, Stigsdotter Neely A, Persson M., Hill RD, Bäckman L (2003): Remembering numbers in old age: Mnemonic training versus self‐generated strategy training. Aging Neuropsychol Cogn 10:202–214. [Google Scholar]
- Edwards JD, Wadley VG, Myers RS, Roenker DL, Cissell GM, Ball K (2002): Transfer of a speed of processing intervention to near and far cognitive functions. Gerontology 48:329–340. [DOI] [PubMed] [Google Scholar]
- Ericsson AK, Delaney PF (1998): Working memory and expert performance In: Logie R, Gilhooly KJ, editors. Working Memory and Thinking.Hillsdale, NJ:Erlbaum; pp93–114. [Google Scholar]
- Fellgiebel A, Keller I, Marin D, Müller MJ, Schermuly I, Yakushev I, Albrecht J, Bellhäuser H, Kinateder M, Beck M, Stoeter P (2009): Diagnostic utility of different MRI and MR angiography measures in Fabry disease. Neurol 72:63–68. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G (2006): Age‐related changes in brain activity across the adult lifespan. J Cogn Neurosci,18:227–241. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS (2003): Neural mechanisms of general fluid intelligence. Nat Neurosci 6:316–322. [DOI] [PubMed] [Google Scholar]
- Healy AF, Wohldmann EL, Sutton EM, Bourne LE,JR (2006): Specificity effects in training and transfer of speeded responses. J Exp Psychol: Learn Mem Cogn 32:534–546. [DOI] [PubMed] [Google Scholar]
- Heise V, Filippini N, Ebmeier KP, Mackay CE (2011): The APOE ε4 allele modulates brain white matter integrity in healthy adults. Mol Psych 16:908–916. [DOI] [PubMed] [Google Scholar]
- Hofer SM, Alwin DF (2008): Handbook of Cognitive Aging. Interdisciplinary Perspectives.Los Angeles:Sage Publications. [Google Scholar]
- Horn W (1983): Leistungsprüfsystem (L‐P‐S).Göttingen:Hogrefe. [Google Scholar]
- Horn JL, Cattell RB (1966): Refinement and test of the theory of fluid and crystllized general intellegences. J Educ Psychol 57:253–270. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Pfeiffer AM, Kraft RA, Casanova R, Dreibler AR, Burdette JH, Maldijan JA, Laurenti PJ (2008): Relating imaging indices of white matter integrity and volume in healthy older adults. Cereb Cortex 18:433–442. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ (2008): Improving fluid intelligence with training on working memory. Proc Nat Acad Sci USA 105:6829–6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JM, Webster LM, Kleykamp BA, Dagenbach D (2005): Recollection training and transfer effects in older adults: Successful use of a repetition‐lag procedure. Aging, Neuropsychol Cognit 12:278–298. [DOI] [PubMed] [Google Scholar]
- Jones S, Nyberg L., Sandblom J., Stigsdotter NA, Ingvar M, Petersson KM, Bäckman L (2006): Cognitive and neural plasticity in aging: General and task‐specific limitations. Neurosci Biobehav Rev 30:864–871. [DOI] [PubMed] [Google Scholar]
- Jurado MB, Rosselli M (2007): The elusive nature of executive functions: A review of our current understanding. Neuropsychol Rev 17:213–233. [DOI] [PubMed] [Google Scholar]
- Karbach J, Kray J (2009): How useful is executive control training? Age differences in near and far transfer of task‐switching training. Dec Sci 12:978–990. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Raz N (2009): Pattern of normal age‐related regional differences in white matter microstructure is modified by vascular risk. Brain Res 1297:41–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T (2009): Brain anatomical network and intelligence. PLoS Comput Biol 5:e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marker KR (2008): Cogpack 8.19.Ladenburg:Markersoftware. [Google Scholar]
- Masoro EJ, Austad SN (2010): Handbook of the Biology of Aging.London:Academic Press. [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, Zijl P, Mazziotta J (2008): Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40:570–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Zhang JY (2006): Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539. [DOI] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS, Howard RJ, Ballard CG (2010): Putting brain training to the test. Nature 465:775–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchensteiner S, Kawohl W, Ozgurdal S, Littmann E, Gudlowski Y, Witthaus H, Heinz A, Juckel G (2011): Test‐performance after cognitive training in persons at risk mental state of schizophrenia and patients with schizophrenia. Psychiat Res 185:334–339. [DOI] [PubMed] [Google Scholar]
- Rebok GW, Carlson MC, Langbaum JBS (2007): Training and maintaining memory abilities in healthy older adults: Traditional and novel approaches. J Gerontol B Psychol Sci Soc Sci 62:53–61. [DOI] [PubMed] [Google Scholar]
- Reuter‐Lorenz PA, Park DC (2010): Human neuroscience and the aging mind: A new look at old problems. J Gerontol B Psychol Sci Soc Sci 65:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond LL, Morrison AB, Chein JM, Olson IR (2011): Working memory training and transfer in older adults. Psychol Aging 26:813–822. [DOI] [PubMed] [Google Scholar]
- Ruckert D, Sonoda LI, Hayes C, Hill DLG, Leach MO, Hawkes DJ (1999): Nonrigid registration using free‐form deformations: Application to breast MR images. IEEE Trans Med Imaging 18:712–721. [DOI] [PubMed] [Google Scholar]
- Singley MK, Anderson JR (1989): The Transfer of Cognitive Skill.Cambridge, MA:Harvard Univ Press. [Google Scholar]
- Smith EE (1999): Storage and executive processes in the frontal lobes. Science 283:1657–1661. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ (2006): Tract‐based spatial statistics: Voxelwise analyses of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Tewes U (1991): Hamburg Wechsler Intelligenztest für Erwachsene, Revision 1991 (HAWIE‐R). Handbuch und Testanweisung.Göttingen:Hans Huber. [Google Scholar]
- Wechsler D (1981): Wechsler Adult Intelligence Scale‐Revised (WAIS‐R).San Antonio:The Psychological Corporation. [Google Scholar]
- Yang L, Krampe RT, Baltes PB (2006): Basic forms of cognitive plasticity extended into the oldest‐old: Retest learning, age, and cognitive functioning. Psychol Aging 21:372–378. [DOI] [PubMed] [Google Scholar]


