Abstract
Patients with striate cortex lesions experience visual perception loss in the contralateral visual field. In few patients, however, stimuli within the blind field can lead to unconscious (blindsight) or even conscious perception when the stimuli are moving (Riddoch syndrome). Using functional magnetic resonance imaging (fMRI), we investigated the neural responses elicited by motion stimulation in the sighted and blind visual fields of eight patients with lesions of the striate cortex. Importantly, repeated testing ensured that none of the patients exhibited blindsight or a Riddoch syndrome. Three patients had additional lesions in the ipsilesional pulvinar. For blind visual field stimulation, great care was given that the moving stimulus was precisely presented within the borders of the scotoma. In six of eight patients, the stimulation within the scotoma elicited hemodynamic activity in area human middle temporal (hMT) while no activity was observed within the ipsilateral lesioned area of the striate cortex. One of the two patients in whom no ipsilesional activity was observed had an extensive lesion including massive subcortical damage. The other patient had an additional focal lesion within the lateral inferior pulvinar. Fiber‐tracking based on anatomical and functional markers (hMT and Pulvinar) on individual diffusion tensor imaging (DTI) data from each patient revealed the structural integrity of subcortical pathways in all but the patient with the extensive subcortical lesion. These results provide clear evidence for the robustness of direct subcortical pathways from the pulvinar to area hMT in patients with striate cortex lesions and demonstrate that ipsilesional activity in area hMT is completely independent of conscious perception. Hum Brain Mapp 36:1585–1594, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: V1 lesion, motion processing, awareness, subcortical pathways
INTRODUCTION
In humans and primates, visual information acquired by the retina is routed to the lateral geniculate nucleus (LGN) and from there to the primary visual cortex via the optical radiation. From the primary visual cortex, the information is processed across extrastriate areas that form two main pathways, the ventral and the dorsal visual streams (Ungerleider and Mishkin, 1982). Visual attributes like color or form are processed along the ventral while spatial information and motion are processed in the dorsal stream. It is important to note that hMT, a specific region in the dorsal stream constituted of motion selective neurons, is particularly well suited to process motion information. Animal studies have shown that this region not only receives projections that are routed over the primary visual cortex but also subcortical ones. The existence of a direct projection from LGN to MT in the brain of macaque monkeys was shown using a retrograde tracing technique (Sincich, et al., 2004; Yukie and Iwai, 1981). A direct connection between MT and pulvinar, which receives input of the superior colliculus has been anatomically described by Standage and Benevento (Standage and Benevento, 1983).
These pathways provide a good explanation for the ability of few patients with lesions of the primary visual cortex, who still perceive motion features (such as speed or direction) in the blind part of the visual field (scotoma) caused by the lesion (ffytche and Zeki, 2011; Riddoch, 1917; Schoenfeld, et al., 2002b; Zeki and ffytche, 1998). This special condition in which motion perception is associated with awareness for moving stimuli presented within the scotoma is known as the Riddoch‐syndome. Most patients with striate cortex lesions, however, experience either a scotoma that depending from the site and size of the lesion encompasses either the contralateral upper or the lower quadrant or the entire half of the visual field. If at all, stimuli presented within the scotoma are not perceived consciously. A small proportion of these patients have the ability to respond to stimuli presented within the scotoma. This clinical condition has been termed blindsight (Weiskrantz, et al., 1974).
In both, blindsight and Riddoch‐syndrome patients hemodynamic imaging studies reported stimulus evoked activity in the extrastriate cortex, especially in hMT, ipsilateral to the lesioned V1, produced by motion stimuli (Barbur, et al., 1993; Goebel, et al., 2001; Sahraie, et al., 1997; Zeki and Ffytche, 1998). A previous study by our group used a combination of fMRI and magnetencephalograpy (Schoenfeld, et al., 2002b) in conjunction with an information theoretic measure (Hinrichs, et al., 2006) and was able to show in a Riddoch patient that the conscious perception of motion presented within the scotoma was associated with contralateral activations in extrastriate areas hMT and V2/V3 of the lesioned hemisphere. The electrophysiological results indicated that the motion‐evoked activity occurred first in the higher tier extrastriate area hMT and later in the lower tier areas V2/V3. This result was compatible with earlier studies in which the existence of a direct pathway to hMT has been postulated in patients with lesions (Barbur, et al., 1993; Blythe, et al., 1987; Goebel, et al., 2001; Zeki and Ffytche, 1998) as well as in healthy subjects (Schoenfeld, et al., 2002a) and was well in line with the clear evidence from animal physiology (Rodman, et al., 1989; Rodman, et al., 1990; Berman and Wurtz, 2008; Berman and Wurtz, 2010; Lyon, et al., 2010; Berman and Wurtz, 2008; Berman and Wurtz, 2010) and anatomical (Stepniewska, et al., 2000) studies.
Although these findings do not leave any doubt on the existence of such subcortical direct projections to MT, it is much less clear to what extent the activity in the ipsilesional extrastriate cortex of patients with striate cortex lesions is related to conscious perception of moving stimuli. To investigate this question, we sought to carefully present moving stimuli precisely within the scotoma of patients with striate cortex lesions who neither exhibit blindsight nor the Riddoch syndrome. The elicited activity by the moving stimulus would, therefore, be independent of conscious perception. Furthermore, we were able to recruit patients who in addition to the striate cortex lesion also presented focal lesions in the thalamus. Finally, we performed fiber‐tracking based on anatomical and functional markers (Pulvinar and hMT) on individual DTI data from each patient to visualize the corresponding tracks. This enabled us to address a second interesting issue, namely which lesion location in the thalamus might disable activity elicited via direct subcortical projections in area hMT.
PATIENTS AND METHODS
Patients
Eight patients with visual field deficits from ischemic posterior cerebral artery strokes were recruited. The demographic data of the patients is presented in Table 1. A large field perimetry (30 degree) of the visual field confirmed a complete or nearly complete homonymous hemianopia in four cases and a homonymous quadrantanopia in further four cases (see Table 2 and Fig. 1 for detailed information). None of the patients reported any residual perception of motion or other stimuli within the scotoma. No patient had blindsight during repeated tests. In addition, detailed ophthalmological examinations were performed to exclude additional ophthalmological causes for visual field deficits.
Table 1.
The table shows the main demographical data for each patient
| Subject | Sex | Age (years) | Time after stroke (months) |
|---|---|---|---|
| gc23 | male | 70 | 15 |
| gg53 | male | 39 | 30 |
| le43 | male | 67 | 11 |
| fa22 | male | 54 | 31 |
| io15 | male | 68 | 29 |
| rw55 | female | 24 | 22 |
| sp90 | male | 66 | 8 |
| tm63 | female | 50 | 17 |
Table 2.
The table shows the precise descriptions of the lesions and the corresponding scotomas as well as of the locations of hemodynamic activity in response to blind field stimulation
| Subject | Perimetry | T1: Striate cortex lesion | T1: Thalamic lesion | fMRI: Stimulation sighted hemifield | fMRI: Stimulation blind hemifield |
|---|---|---|---|---|---|
| fa22 | Upper left homonymous quadrantanopia, scotoma in the peripheral portion of the lower right visual quadrant | Right striate cortex: lower calcarine bank, occipital gyrus, fusiform and lingual gyrus, parahippocampal gyrus, entorhinal cortex; Left striate cortex: upper calcarine bank, occipital gyrus | Parts of right mediodorsal and ventrolateral thalamic nuclei | Left striate/peristriate region, lateral and middle occipital gyrus just posterior to occipitotemporal junction, precuneus, med. occipital gyrus | Right striate/peristriate region, lateral and middle occipital gyrus |
| gg53 | Left homonymous hemianopia (nearly complete) | Right striate cortex, cuneus, fusiform and lingual gyrus, middle occipital gyrus | Small right lateral pulvinar lesion | Left striate region, lateral and middle occipital gyrus, fusiform gyrus; Right occipial gyrus | Right lateral and middle occipital gyrus, superior parital lobule |
| le43 | Complete right homonymous hemianopia | Left striate cortex, lateral and middle occipital gyrus, cuneus, fusiform and lingual gyrus, parahippocampal gyrus, entorhinal cortex, inferior temporal gyrus | No lesion | Right striate/peristriate region, lateral and middle occipital gyrus | No significant activation of visual cortex areas |
| gc23 | Lower left homonymous quadrantanopia | Right striate cortex: upper calcarine bank | No lesion | Left striate/peristriate region lateral and middle occipital gyrus | Right lateral and middle occipital gyrus |
| io15 | Complete left homonymous hemianopia | Right striate cortex, middle occipital gyrus, cuneus, fusiform and lingual gyrus, entorhinal cortex, subiculum, inferior temporal gyrus | No lesion | Left striate/peristriate region, lateral and middle occipital gyrus, superior parietal lobule; Right lateral and middle occipital gyrus, superior parietal lobule | Right lateral and middle occipital gyrus, superior parietal lobule |
| rw55 | Upper left homonymous quadrantanopia sparing out a small central area and extending to the peripheral part of the lower left visual quadrant | Right striate cortex: lower bank, occipital gyrus | Small right lateral pulvinar/lateral geniculate nucleus lesion | Left striate/peristriate region, lateral and middle occipital gyrus; Right lateral and middle occipital gyrus | Right lateral and middle occipital gyrus |
| sp90 | Right homonymous hemianopia extending to the peripheral parts of left upper and lower visual quadrant | Left striate cortex, occipital gyrus, cuneus, parahippocampal gyrus, entorhinal cortex, subiculum, hippocampus, fusiform and lingual gyrus, inferior temporal gyrus | Left inferolateral pulvinar lesion | Right striate/peristriate region, lateral and middle occipital gyrus, superior parietal lobule and precuneus | No significant activation of visual cortex areas |
| tm63 | Upper right homonymous quadrantanopia (partial) | Left striate cortex: lower bank, occipital gyrus | Small left ventrolateral thalamic lesion | Right striate/peristriate region, lateral and middle occipital gyrus; Left lateral occipital gyrus | Left lateral and middle occipital gyrus, supramarginal gyrus, peristriate region, Right lateral occipital gyrus |
Figure 1.
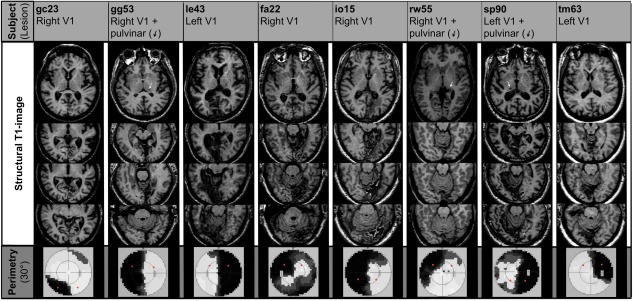
The figure shows the T1 weighted structural MRI of the subjects. All subjects had lesions in the striate cortex. Patient gg53, rw55, and sp90 had additional lesions in the thalamus. Patient le43 had a small pulvinar lesion contralateral to the lesion in the visual cortex. The white arrows point to the thalamic lesion locations. The bottom line shows the results from the large field perimetry. The black regions indicate the locations of the scotomas in the visual field. The red circles indicate the stimulation sites.
All subjects were right‐handed and had cerebral infarctions affecting the striate region located contralateral to the scotoma. The size of the lesions varied between small infarcts limited to parts of the striate cortex and extensive lesions including the striate cortex, the medial occipital gyrus, the cuneus, fusiform and lingual gyri, the parahippocampal gyrus, the subiculum, the hippocampus, the entorhinal cortex and the inferior temporal gyrus. One patient (fa22) had bilateral infarctions of the striate cortex. Additional thalamic lesions ipsilateral to the striate infarction could be observed in six cases, four of them located in the pulvinar. Two subjects had small extrapulvinar thalamic lesions and the remaining two patients had no thalamic lesion. (See Table 2 for detailed information and Fig. 1 for illustration.)
All patients did not have any contraindications for MRI and gave their informed consent to participate in this study that was approved by the ethics committee of the medical faculty of the Otto‐von‐Guericke University Magdeburg.
Stimuli and Experimental Design
Paradigm
Visual stimuli were presented via a projector‐mirror system on a small translucent screen, located close to the subject's chin. The subject viewed the screen via a mirror attached to the head coil.
The subjects were instructed to fixate a white cross (vertical and horizontal bar 1.4° × 0.2° tall) that was presented in middle of the screen's x‐axis (position on the y‐axis was adjusted for each patient individually to ensure stimulus presentation precisely within the scotoma). For stimulation patches consisting of three white vertical bars, each bar 4.5° high and 0.9° wide with a spacing of 2.3°, were presented in alternation in the right and left visual hemifield. The bars moved to either the right or left by 1 degree over a 100‐ms period with an immediate return to the stationary position over further 100 ms (speed 10 degrees per second).
Stimuli were presented in a blocked design with three different conditions (moving bars in the right visual field (RVF), moving bars in the left visual field (LVF), fixation alone). Each block lasted 30 s. The experiment consisted of three scanning sessions (9 min each) separated by short breaks. All stimuli were isoluminant at 200 cd/m2, while the luminance of the background was set to 45 cd/m2. As mentioned before, the vertical position of the fixation cross and position of the moving bars were adjusted individually depending on the individual scotoma. This ensured that the moving bars were presented exactly within the scotoma and in the corresponding location within the sighted hemifield.
The patients were instructed to maintain fixation and ignore moving stimuli. Eye movements were detected with an eye tracking camera and these events were brought to patient's attention. Since the patient were extensively trained to fixate eye movements occurred very rare (<1% of the trials).
Magnetic resonance imaging data acquisition
All subjects were scanned using a 1.5T‐scanner (General Electric Signa Horizon LX, neuro‐optimized, maximum amplitude 40 m Tm−1). A standard quadrature birdcage head coil was used for both the radio‐frequency transmission and nuclear magnetic resonance signal reception.
Functional images were acquired using a gradient echo single‐shot echo planar imaging (EPI) sequence with TE 35 ms, TR 2,000 ms, bandwidth 83.3 kHz, and flip angle 80 degrees. Twenty‐three slices of 5 mm thickness with 1 mm gap (field of view 200 mm, matrix 64 × 64, voxel size 3 × 3 × 6 mm3) parallel to the anterior‐posterior commissure line covering the full brain were collected. In each functional run 275 volumes were collected during a scan time of 9.10 min per run. A T1‐weighted EPI (inversion recovery prepared EPI, TR 12,000 ms; TE 16.4 ms; TI 1,050 ms) volume was collected with slice parameters identical to the functional data.
For anatomical images a T1‐weighted high‐resolution data set was acquired using a three‐dimensional contrast‐optimized spoiled gradient‐echo sequence (SPGR) (TE 8 ms, TR 24 ms, flip angle 30 degrees, field of view 250 mm, 124 sagittal slices, slice thickness 1.5 mm, matrix 256 × 256, voxel size 0.98 × 0.98 × 1.5 mm3).
Diffusion tensor imaging
The protocol for Diffusion‐weighted imaging included a single‐shot diffusion‐weighted spin‐echo EPI sequence (field of view 280 × 280 mm, matrix 128 × 128, 39 axial slices, slice thickness 3 mm, TE 70 ms, TR 10,800 ms, b‐value 1,000 s mm−2). According to the DTI acquisition scheme proposed by Papadakis et al. (Papadakis, et al., 1999) the data were collected with 12 noncollinear gradient orientations, each additionally measured with the opposite diffusion gradient polarity. The total of 24 diffusion‐weighted volumes, each an average of four measurements, were divided into four blocks, with each block preceded by a nondiffusion‐weighted acquisition for later correction of head movements.
Functional magnetic resonance data analysis
The fMRI data were preprocessed and analyzed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK), implemented in MATLAB (The MathWorks, Natick, MA). Functional data were subsampled to 3 × 3 × 3 mm3 isovoxels, realigned, resliced, smoothed (Gaussian kernel; full width at half maximum (FWHM) = 8 × 8 × 8 mm3), and high‐pass‐filtered (128 s). Analysis was performed on non‐normalized images. Single‐subject statistical analysis was performed for the conditions of interest (T‐contrasts estimated for motion in LVF versus fixation and motion RVF vs. fixation). The stimulation conditions (LVF, RVF) were contrasted versus fixation and the results were thresholded at P < 0.01 uncorrected with a cluster size criterion of 10 adjacent voxels (Poline, et al., 1997).
Calculation of diffusion tensor maps
The DT images were eddy‐current‐corrected according to the correction scheme by Bodammer et al. (Bodammer, et al., 2004), followed by a correction for head motion on the basis of the nondiffusion‐weighted images using the AIR software package (Woods, et al., 1998). Brain masks of nondiffusion weighted volumes were created using FSL BET (Behrens, et al., 2003; Smith, 2002).
Fiber tract reconstruction
Fiber tract reconstruction was carried out using a previously described double‐step probabilistic approach (Bodammer, et al., 2009). Briefly, a Monte Carlo simulation algorithm that repeatedly searches for probable paths through the determined diffusion tensor matrix was implemented in Matlab (MathWorks, Natick, MA). Starting from voxels in a predefined start region, each single path propagation step is calculated based on the diffusion tensors of the candidate successor voxels. An estimate of the voxel‐specific probability distribution of axonal connections is used to calculate the probabilities of all allowable propagation steps. Explicitly, drawing randomly from this distribution determinates each step. A full path was terminated if the apparent diffusion coefficient exceeded a threshold of 1.8 × 10−9 m2/s. This ensured that the putative tracks were located within neural tissue.
As seed region the pulvinar was manually outlined as region of interest (ROI) on the nondiffusion weighted DTI images using the FSLVIEW software (http://fsl.fmrib.ox.ac.uk/fsl/fslview/) based on anatomical landmarks from a detailed MRI atlas. On the basis of the motion evoked fMRI activation, we identified the location of area hMT, and used the information from fMRI to outline a target region (in case of missing activation of hMT in the lesioned hemisphere, the location of this region was estimated by mirroring its localization in the intact hemisphere).
Fiber Tracking
Tractographic analyses between seed and target regions were performed in both hemispheres with 10,000 starts per voxel within the start region. The number of start voxels in the seed regions varied from 362 to 753 while the number of the voxels in the target ROIs varied from 200 to 781. Tractography was carried out bidirectionally (from seed and target ROIs) and all detected paths were stored.
Paths with similar properties (i.e., trajectories, length) were grouped into clusters of paths and visualized with MevisLab (Fraunhofer, Bremen).
RESULTS
Stimulation Within the Sighted Visual Field
The contrast between motion stimulation presented to the sighted visual field versus fixation revealed two main foci of hemodynamic activity in the contralateral hemisphere across all subjects. One was located in the striate and peristriate region and another one in the lateral and middle occipital gyrus just posterior to the occipitotemporal junction, which corresponds well to the location of area hMT (Schoenfeld, et al., 2002a; Schoenfeld, et al., 2007; Watson, et al., 1993). Hemodynamic activity could also be observed in the contralateral precuneus, fusiform gyrus, superior parietal lobule and in the lateral and middle occipital gyri of the lesioned hemisphere. (For details, see Table 2 and Fig. 2)
Figure 2.
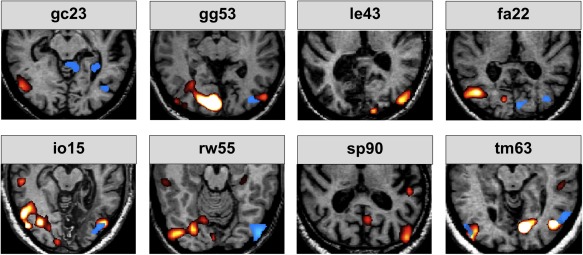
The figure shows the results obtained by stimulation with motion of the sighted (red activations) and blind visual field (blue activations) versus baseline. Note that with the exception of le43 and sp90 all patients exhibited activity in the ipsilesional cortex in area hMT in response to blind field stimulation.
Stimulation Within the Scotoma
None of the subjects showed any significant hemodynamic activity in the striate region of the lesioned hemisphere in the contrast motion stimulation within the scotoma versus fixation. In six out of eight subjects, however, hemodynamic activity was observed in the lateral and middle occipital gyrus of the lesioned hemisphere, just posterior to the occipitotemporal junction (corresponding well to the location of area hMT). Further activations were observed in the superior parietal lobule, the region of the supramarginal gyrus and in the lateral and middle occipital gyri (see Table 1 and Fig. 2).
Two of the six patients exhibiting hemodynamic activity in the contralateral extrastriate cortex (gg53 and rw55) also had lesions located to the pulvinar. Importantly, two subjects (le43 and sp90) did not show any significant hemodynamic activity of the visual cortex in the lesioned hemisphere. Subject le43 had an extensive infarction including major parts of the inferior occipital and temporal cortex but no thalamic lesion. In the case of subject sp90, there was also a small right pulvinar lesion (contralateral to the V1 lesion) but the V1 ipsilesional cortex around region hMT was intact. However, in addition to the lesion of the striate cortex this patient also had a lesion located in the inferior lateral part of the pulvinar.
Diffusion tensor imaging
Fiber tracts were individually reconstructed for both hemispheres within pulvinar (anatomically outlined) and fMRI‐derived activation peaks corresponding to the ipsilateral hMT region of five representative subjects (see Fig. 3). Subject rw55 had a striate cortex (like all patients) and a contralateral inferior pulvinar lesion. Subject sp90 had a striate cortex lesion and in addition a lateral lesion in the the pulvinar. Subject (le43) had extensive lesions in the striate and also in the medial extrastriate cortex along the border of the inferior occipital and temporal cortex. The ipsilesional pulvinar was intact (the contralateral pulvinar had a small lesion).
Figure 3.
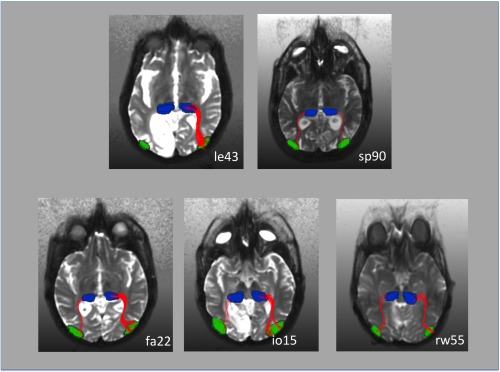
Fiber tracking results in five representative patients. The hMT ROI was obtained from the fMRI results. The pulvinar ROI was determined by hand based on the individual anatomy. In patient le43 who did not exhibit ipsilesional activation in response to blind field stimulation (see also Fig. 2) fiber tracts could be only tracked in the nonlesioned right hemisphere. This patient had an extensive cortical and subcortical lesion in the left visual cortex (see also Fig. 1). Fiber‐tracts between the pulvinar and ipsilateral hMT were tracked in both hemispheres in patient sp90. This patient, however, did not exhibit hMT activation in the lesioned hemisphere in response to contralateral stimulation. In this patient, the disruption of the pathway occurred probably at the level of the pulvinar, in which we observed a lateral inferior lesion (see also Fig. 1). In patient rw55 area hMT was activated by contralateral motion stimulation in both hemispheres although there was a small lesion in the pulvinar contralateral to the V1 lesion. Fiber‐tracts between the pulvinar and ipsilateral hMT nevertheless were tracked in both hemispheres. This was also the case for the patients fa22 and io15, who did not have any damage to the pulvinar.
In subject rw55, who exhibited hemodynamic activity in the hMT region of the lesioned hemisphere and had a contralateral inferior pulvinar lesion, we found connections between the pulvinar and hMT in both hemispheres with over 100 tracked paths in the intact and in the hemisphere with the V1 lesion. We also found over 100 tracked paths between the pulvinar and hMT in both hemispheres of all subjects without pulvinar lesions. Subject sp90 who had a lateral pulvinar lesion and who did not exhibit any activity in area hMT in response to contralateral movement stimulation within the scotoma also showed over 100 connecting tracked paths between pulvinar and the region of hMT in the intact as well as in the lesioned hemisphere. In subject le43, who had extensive damage to cortical and subcortical structures fiber tracks between the pulvinar and hMT were only found in the intact hemisphere (see also Fig. 3).
DISCUSSION
The present study investigated the neural responses elicited by motion stimulation within the scotomas of patients with lesions of the striate cortex. Three patients had also lesions in the ipsilateral pulvinar. None of the patients exhibited blindsight or the Riddoch syndrome. Importantly, the patients were repeatedly trained to maintain fixation during motion stimulation and great care was given that the moving stimulus was always presented within the borders of the scotoma. None of the patients was aware of the moving stimulus that was presented in the blind part of the visual field. All patients displayed hemodynamic activity in the striate and extrastriate cortex, particularly in area hMT (Watson, et al., 1993) located contralateral to the side of the stimulus presentation for sighted visual field stimulation (see red activations in Fig. 2). In six of eight patients, the stimulation within the scotoma elicited hemodynamic activity in the extrastriate cortex, particularly in area hMT while no hemodynamic activity could be observed within the ipsilateral lesioned area of the striate cortex (see blue activations in Fig. 2). These findings are well in line with previous reports in patients with blindsight (Barbur, et al., 1993; Goebel, et al., 2001; Stoerig and Cowey, 1997) or Riddoch syndrome (ffytche and Zeki, 2011; Schoenfeld, et al., 2002b) and provide additional support for the idea that visual information presented within the scotoma is mediated by subcortical pathways (Beckers and Homberg, 1992; Beckers and Zeki, 1995) over the superior colliculus (Rodman, et al., 1990) via the pulvinar (Stepniewska, et al., 2000), over the LGN (Yukie and Iwai, 1981) or directly to hMT (Standage and Benevento, 1983).
The relationship between activity in early visual areas and awareness is a hot topic in the cognitive neuroscience literature. A frequently asked question is whether area V1 is necessary for visual awareness. Although many studies have shown that neural activity in area V1 typically correlates with some aspects of perception, the responses of V1 neurons seem to be more closely tied to the sensory afferents arriving from the LGN and less to perception‐sensitive responses characteristic of extrastriate visual areas [reviewed in (Tong, 2003)]. The currently dominant view is that the critical role of V1 in vision rather follows naturally from its position as a bottleneck for visual signals. Awareness is currently thought to be generated by feed‐back processes (Pollen, 1999; Block, 2005; Boehler, et al., 2008; Roelfsema, et al., 1998) through crosstalk across higher‐tier cortical areas and lower‐tier visual areas such as V1 (Silvanto, 2014). Importantly, awareness can also be generated by feed‐back to V2 or V3 when V1 is lesioned (Schoenfeld, et al., 2002b). Since in the current study none of the patients was aware of the stimuli presented within the scotoma it is reasonable to assume that the activity in area hMT is independent of awareness.
Activity in ipsilesional area hMT was observed in more than the half (six out of eight) of our patients although some also had focal lesions of the pulvinar. This finding indicates a certain robustness of the subcortical pathways that project to hMT (Schoenfeld, et al., 2002a). This gives rise to the question which lesion location might knock out the subcortical mediation of visual information from the blind parts of the visual field? To answer this question, we need to look at the two patients who did not exhibit any activity in the extrastriate cortex contralateral to the stimulus presentation within the scotoma.
Patient le43 (see also Fig. 1) had an extensive lesion in the left hemisphere that included cortical and subcortical medial inferior occipital and inferior temporal regions. It is most likely that the lesion also included subcortical pathways from the pulvinar to hMT. The second patient (sp90) who did not exhibit extrastriate activity in response to contralateral scotoma stimulation had a focal lateral lesion of the ipsilateral pulvinar in addition to the striate cortex lesion. Based on the present knowledge on the functional anatomy of the pulvinar (Morel, et al., 1997), this focal lateral inferior lesion might well interrupt the mediation of information over subcortical pathways to hMT. Recently, the viability of the V1 bypassing pathways to area MT was estimated from early in postnatal life to adulthood in the marmoset (Warner, et al., 2012). This study used a combination of neural tracing and immunhistochemestry and found that the early maturation of area MT is likely due to the disynaptic retinopulvinar input and not the retinogeniculate input or the direct projection from V1. From after birth to adulthood, the authors observed a dynamic shift in the ratio of input from these three structures to area MT, with an increasing dominance of the direct V1 afference. A disynaptic pathway from the colliculus superior to MT via the pulvinar was found in the adult macaque (Berman and Wurtz, 2008; Berman and Wurtz, 2010; Lyon, et al., 2010). The interruption of similar pathways in the human would provide a valid explanation for the absent response of area hMT to contralateral stimulation within the scotoma of the patient.
To further strengthen this idea, we performed fiber tracking based on DTI data in representative patients (see Fig. 3). The start and end points for the algorithm were the pulvinar and area hMT that were defined by anatomical (pulvinar) and functional (hMT) means. Patient rw55 had a right striate cortex and left inferior pulvinar lesion. Nevertheless, his right hMT was responsive to blind field stimulation. In this patient, we were able to reconstruct almost symmetric fiber tracks corresponding to subcortical pathways between the pulvinar and hMT for both hemispheres with over 100 tracks in each hemisphere. The focal inferior lesion in the pulvinar did not interrupt the mediation of visual information over subcortical pathways. Patient sp90 had a left striate and a focal lesion in the inferior lateral pulvinar. His hMT was unresponsive to contralateral blind field stimulation. In this patient, the algorithm also was able to reconstruct symmetrical fiber tracks with over 100 tracks per hemisphere. Presumably, in this patient the pathway between pulvinar and hMT is intact but the focal lesion in the lateral pulvinar disrupts the mediation of visual information. Patient le43 had an extensive lesion that included cortical and subcortical structures of the left medial inferior occipital and temporal lobe. The left hMT was unresponsive to contralateral blind field stimulation (see Fig. 2). In this patient, the fiber tracking algorithm was able to reconstruct tracks between the pulvinar and hMT, but only in the intact right hemisphere. In this patient, the extensive lesion appears to have also damaged enough subcortical tissue to disrupt the mediation of information from the ipsilesional pulvinar to hMT.
Several studies in patients with blindsight or Riddoch syndrome suggested that direct subcortical connections from superior colliculus, pulvinar or LGN to hMT mediate residual visual motion perception (Stoerig and Cowey, 1997). However, only a minority of patients with loss of striate cortex preserves residual perception (Barton and Sharpe, 1997; Lanyon, et al., 2009; Scharli, et al., 1999).
Another study detected connections between hMT and pulvinar in 4 out of 10 healthy subjects using DTI tractography. In two of these cases, additional fiber tracts between hMT and the superior colliculus and in one case an additional hMT‐LGN connection was found (Lanyon, et al., 2009). The authors suggest that the observed interindividual variability in normal subjects could explain why only few patients with striate cortex lesions have residual visual motion perception. In contrast, another study found connections between pulvinar and visual cortical areas including hMT in six healthy subjects performing DTI tractography from the pulvinar as ROI (Leh, et al., 2008).
In the present study, we deliberately chose patients without any residual perception for stimuli presented within the scotoma and found hemodynamic responses in the contralateral hMT in six out of eight patients for motion stimulation. One of the patients with an unresponsive hMT had an extensive lesion that most likely disrupted the subcortical connections, which is backed up by the finding that no fiber tracks could be reconstructed between pulvinar and hMT in that hemisphere. The other patient had a focal lateral pulvinar lesion most likely directly disrupting the mediation of information within the pulvinar (Morel, et al., 1997). Fiber tracks could be reconstructed between pulvinar and hMT in the lesioned hemisphere indicating that the disruption must have occurred before. Hence, the missing hMT response to contralateral blind field stimulation can be attributed to the intrapulvinar lesion.
The current data is well in line with previous findings (Schoenfeld, et al., 2002a) arguing that direct subcortical pathways bypassing the striate cortex and mediating motion information (Schoenfeld, et al., 2002b) are in place in the human brain. Our findings that in six out of eight patients motion stimulation within the scotoma elicited activity in the contralateral hMT further indicate that these subcortical pathways are rather robust and can only be disrupted by extensive subcortical or specific focal (lateral) pulvinar lesions. Again, none of our patients had residual vision within the scotoma. Therefore, we can conclude that mere activation of area hMT is not sufficient to elicit conscious perception. The recurrent activation of lower‐tier regions (Boehler, et al., 2008; Roelfsema, et al., 1998; Schoenfeld, et al., 2002b) might be the key toward the understanding of processes that lead to awareness.
REFERENCES
- Barbur JL, Watson JD, Frackowiak RS, Zeki S (1993): Conscious visual perception without V1. Brain 116(Pt 6):1293–302. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Sharpe JA (1997): Smooth pursuit and saccades to moving targets in blind hemifields. A comparison of medial occipital, lateral occipital and optic radiation lesions. Brain 120(Pt 4):681–699. [DOI] [PubMed] [Google Scholar]
- Beckers G, Homberg V (1992): Cerebral visual motion blindness: Transitory akinetopsia induced by transcranial magnetic stimulation of human area V5. Proc Biol Sci 249:173–178. [DOI] [PubMed] [Google Scholar]
- Beckers G, Zeki S (1995): The consequences of inactivating areas V1 and V5 on visual motion perception. Brain 118(Pt 1):49–60. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50:1077–1088. [DOI] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH (2008): Exploring the pulvinar path to visual cortex. Prog Brain Res 171:467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RA, Wurtz RH (2010): Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci 30:6342–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block N (2005): Two neural correlates of consciousness. Trends Cogn Sci 9:46–52. [DOI] [PubMed] [Google Scholar]
- Blythe IM, Kennard C, Ruddock KH (1987): Residual vision in patients with retrogeniculate lesions of the visual pathways. Brain 110(Pt 4):887–905. [DOI] [PubMed] [Google Scholar]
- Bodammer N, Kaufmann J, Kanowski M, Tempelmann C (2004): Eddy current correction in diffusion‐weighted imaging using pairs of images acquired with opposite diffusion gradient polarity. Magn Reson Med 51:188–193. [DOI] [PubMed] [Google Scholar]
- Bodammer NC, Kaufmann J, Kanowski M, Tempelmann C (2009): Monte Carlo‐based diffusion tensor tractography with a geometrically corrected voxel‐centre connecting method. Phys Med Biol 54:1009–1033. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Schoenfeld MA, Heinze HJ, Hopf JM (2008): Rapid recurrent processing gates awareness in primary visual cortex. Proc Natl Acad Sci USA 105:8742–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffytche DH, Zeki S (2011): The primary visual cortex, and feedback to it, are not necessary for conscious vision. Brain 134:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel R, Muckli L, Zanella FE, Singer W, Stoerig P (2001): Sustained extrastriate cortical activation without visual awareness revealed by fMRI studies of hemianopic patients. Vision Res 41:1459–1474. [DOI] [PubMed] [Google Scholar]
- Hinrichs H, Heinze HJ, Schoenfeld MA (2006): Causal visual interactions as revealed by an information theoretic measure and fMRI. Neuroimage 31:1051–1060. [DOI] [PubMed] [Google Scholar]
- Lanyon LJ, Giaschi D, Young SA, Fitzpatrick K, Diao L, Bjornson BH, Barton JJ (2009): Combined functional MRI and diffusion tensor imaging analysis of visual motion pathways. J Neuroophthalmol 29:96–103. [DOI] [PubMed] [Google Scholar]
- Leh SE, Chakravarty MM, Ptito A (2008): The connectivity of the human pulvinar: A diffusion tensor imaging tractography study. Int J Biomed Imaging 2008:789539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon DC, Nassi JJ, Callaway EM (2010): A disynaptic relay from superior colliculus to dorsal stream visual cortex in macaque monkey. Neuron 65:270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A, Magnin M, Jeanmonod D (1997): Multiarchitectonic and stereotactic atlas of the human thalamus. J Comp Neurol 387:588–630. [DOI] [PubMed] [Google Scholar]
- Papadakis NG, Xing D, Huang CL, Hall LD, Carpenter TA (1999): A comparative study of acquisition schemes for diffusion tensor imaging using MRI. J Magn Reson 137:67–82. [DOI] [PubMed] [Google Scholar]
- Poline JB, Worsley KJ, Evans AC, Friston KJ (1997): Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 5:83–96. [DOI] [PubMed] [Google Scholar]
- Pollen DA (1999): On the neural correlates of visual perception. Cereb Cortex 9:4–19. [DOI] [PubMed] [Google Scholar]
- Riddoch G (1917): On the relative perceptions of movement and a stationary object in certain visual disturbances due to occipital injuries. Proc R Soc Med 10:13–34. [PMC free article] [PubMed] [Google Scholar]
- Rodman HR, Gross CG, Albright TD (1989): Afferent basis of visual response properties in area MT of the macaque. I. Effects of striate cortex removal. J Neurosci 9:2033–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodman HR, Gross CG, Albright TD (1990): Afferent basis of visual response properties in area MT of the macaque. II. Effects of superior colliculus removal. J Neurosci 10:1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H (1998): Object‐based attention in the primary visual cortex of the macaque monkey. Nature 395:376–381. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Weiskrantz L, Barbur JL, Simmons A, Williams SC, Brammer MJ (1997): Pattern of neuronal activity associated with conscious and unconscious processing of visual signals. Proc Natl Acad Sci USA 94:9406–9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharli H, Harman AM, Hogben JH (1999): Blindsight in subjects with homonymous visual field defects. J Cogn Neurosci 11:52–66. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Heinze HJ, Woldorff MG (2002a): Unmasking motion‐processing activity in human brain area V5/MT+ mediated by pathways that bypass primary visual cortex. Neuroimage 17:769–779. [PubMed] [Google Scholar]
- Schoenfeld MA, Noesselt T, Poggel D, Tempelmann C, Hopf JM, Woldorff MG, Heinze HJ, Hillyard SA (2002b): Analysis of pathways mediating preserved vision after striate cortex lesions. Ann Neurol 52:814–824. [DOI] [PubMed] [Google Scholar]
- Schoenfeld MA, Hopf JM, Martinez A, Mai HM, Sattler C, Gasde A, Heinze HJ, Hillyard SA (2007): Spatio‐temporal analysis of feature‐based attention. Cereb Cortex 17:2468–2477. [DOI] [PubMed] [Google Scholar]
- Silvanto J: Why is “blindsight” blind? A new perspective on primary visual cortex, recurrent activity and visual awareness. Conscious Cogn 2014. Sep 25. pii: S1053‐8100(14)00132‐9. [DOI] [PubMed] [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC (2004): Bypassing V1: A direct geniculate input to area MT. Nat Neurosci 7:1123–1128. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standage GP, Benevento LA (1983): The organization of connections between the pulvinar and visual area MT in the macaque monkey. Brain Res 262:288–294. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Ql HX, Kaas JH (2000): Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis Neurosci 17:529–549. [DOI] [PubMed] [Google Scholar]
- Stoerig P, Cowey A (1997): Blindsight in man and monkey. Brain 120(Pt 3):535–559. [DOI] [PubMed] [Google Scholar]
- Tong F (2003): Primary visual cortex and visual awareness. Nature reviews. Neuroscience 4:219–229. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M (1982): Two cortical visual systems In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge: MIT Press; pp 549–586. [Google Scholar]
- Warner CE, Kwan WC, Bourne JA (2012): The early maturation of visual cortical area MT is dependent on input from the retinorecipient medial portion of the inferior pulvinar. J Neurosci 32:17073–17085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S (1993): Area V5 of the human brain: Evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex 3:79–94. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Warrington EK, Sanders MD, Marshall J (1974): Visual capacity in the hemianopic field following a restricted occipital ablation. Brain 97:709–728. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC (1998): Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr 22:139–152. [DOI] [PubMed] [Google Scholar]
- Yukie M, Iwai E (1981): Direct projection from the dorsal lateral geniculate nucleus to the prestriate cortex in macaque monkeys. J Comp Neurol 201:81–97. [DOI] [PubMed] [Google Scholar]
- Zeki S, ffytche DH (1998): The Riddoch syndrome: insights into the neurobiology of conscious vision. Brain 121(Pt 1):25–45. [DOI] [PubMed] [Google Scholar]


