Abstract
Studies of visual‐spatial attention typically use instructional cues to direct attention to a relevant location, but in everyday vision, attention is often focused volitionally, in the absence of external signals. Although investigations of cued attention comprise hundreds of behavioral and physiological studies, remarkably few studies of voluntary attention have addressed the challenging question of how spatial attention is initiated and controlled in the absence of external instructions, which we refer to as willed attention. To explore this question, we employed a trial‐by‐trial spatial attention task using electroencephalography and functional magnetic resonance imaging (fMRI). The fMRI results reveal a unique network of brain regions for willed attention that includes the anterior cingulate cortex, left middle frontal gyrus (MFG), and the left and right anterior insula (AI). We also observed two event‐related potentials (ERPs) associated with willed attention; one with a frontal distribution occurring 250–350 ms postdecision cue onset (EWAC: Early Willed Attention Component), and another occurring between 400 and 800 ms postdecision‐cue onset (WAC: Willed Attention Component). In addition, each ERP component uniquely correlated across subjects with different willed attention‐specific sites of BOLD activation. The EWAC was correlated with the willed attention‐specific left AI and left MFG activations and the later WAC was correlated only with left AI. These results offer a comprehensive and novel view of the electrophysiological and anatomical profile of willed attention and further illustrate the relationship between scalp‐recorded ERPs and the BOLD response. Hum Brain Mapp 36:2443–2454, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: decisions, attention, fMRI, ERPs, vision, EEG
INTRODUCTION
The literature on attention has provided a distinction between top‐down and bottom‐up attentional control. In one well‐represented view, the sources of attentional control and the influence of this control on sensory and motor areas outline the interactions between top‐down and bottom‐up information during selective attention [Asplund et al., 2010; Corbetta and Shulman, 2002; Hopfinger et al., 2000; Mesulam, 1981; Pardo et al., 1991; Petersen and Posner, 2012; Serences et al., 2004]. This interactive concept of attention remains at the core of most current models of voluntary (goal‐directed) attention [Bruce and Tsotsos, 2009; Bundesen et al., 2008].
Empirical studies of voluntary attentional control have utilized trial‐by‐trial attention cuing paradigms that permit control systems to be indexed separately from stimulus selection. In such designs, brain activity related to an attentional cue can be considered to arise from attentional control networks, while activity time‐locked to a subsequent target indexes attention‐related stimulus selection [Corbetta et al., 2000; Harter et al., 1989; Hopf and Mangun, 2000; Hopfinger et al., 2000; Mangun and Hillyard, 1991]. Using variants of this approach, research in animals, patients with neurological disease and damage, and healthy human subjects has shown that the voluntary control of visual attention involves a complex network of widely distributed brain areas, including prefrontal cortex, posterior parietal cortex, posterior‐superior temporal cortex, basal ganglia, and thalamic and midbrain structures [Bisley and Goldberg, 2010; Bressler et al., 2008; Bushnell et al., 1981; Cohen et al., 2009; Corneil et al., 2008; Fecteau et al., 2004; Hung et al., 2001; McAlonan et al., 2006; Mesulam, 1981; Noudoost et al., 2010; Petersen et al., 1994; Posner et al., 1982; Schall, 2004].
The functional anatomy of attentional control in humans has been further refined in recent years by the identification of specific brain networks that interact to support various components of attentional control. Spatial cuing paradigms have been used to isolate activity in a frontal‐parietal attention system that is related to an instruction (e.g., a cue) to selectively direct attention to a location in the visual field while distinguishing this from activity in visual cortex and the motor system [Corbetta et al., 2000; Kastner et al., 1999]. Numerous studies have now supported this conceptualization of a voluntary attentional control network in dorsal frontal‐parietal cortex [Gazzaley and Nobre, 2011; Geng and Mangun, 2011; Slagter et al., 2006; Sylvester et al., 2009; Szczepanski et al., 2010; Walsh et al., 2010; Weissman et al., 2009] and related work has begun to identify additional networks, including the right ventral attention network [Corbetta and Shulman, 2011; Corbetta et al., 2008; Shulman et al., 2010], a lateral prefrontal‐parietal network [Gao and Lin, 2012; Vincent et al., 2008] and a cingulate‐lateral anterior prefrontal (opercular) and anterior cingulate network [Dosenbach et al., 2008].
It is clear from the literature that vast majority of studies of attentional control have used external cues or instructions to direct attention. A simple arrow or other symbolic cue instructs the subject either where to attend, or where the task‐relevant target is most likely to occur [Posner, 1980]. This raises the questions of the meaning of “voluntary” if the instruction is itself external. One may ask instead whether truly voluntary attention when self‐initiated, relies on the same mechanisms that have been identified for voluntary (cued) attention. Although posing significant experimental challenges, it is possible to investigate self‐initiated control of spatial attention, which we will refer to as “willed attention.”
A seminal study by Taylor et al. [Taylor et al., 2008] used fMRI to explore the neural mechanisms of attentional control when participants had to choose where to attend compared with when they were received instructional cues. They found a distinct network of activation in the presupplementary motor area (pre‐SMA), presupplementary eye fields (pre‐SEF), frontal eye fields (FEF), and anterior cingulate cortex (ACC) when subjects had to make a willful decision where to attend. The Taylor et al. findings are in line with evidence that the ACC is active when subjects have to make a voluntary decision [Rushworth et al., 2004; Walton et al., 2004], and with an extensive literature regarding intention and action. In contrast, a related study by Hopfinger and colleagues [Hopfinger et al., 2010] did not observe any significant activation in medial frontal areas when subjects had to initiate a wilful voluntary shift of attention; in their task the subjects began each trial with a motor action that signaled where they would subsequently shift attention. Instead, they observed a generalized difference in brain lateralization for self‐initiated shifts of attention compared with cued trials. However, because the medial frontal regions identified by Taylor et al. were active for both the intention to act and action itself [Passingham et al., 2010], the Hopfinger study may have had reduced sensitivity for observing differences in these structures for willed versus cued attention because in their design both conditions required a motor response that indicated the direction of the attentional shift at trial onset. Thus, the differences in task design between the Taylor et al. [2008] and Hopfinger et al. [2010] studies require further research into the neural anatomy (fMRI) of willed attention. Furthermore, no known study has been conducted on the ERPS associated with willed attentional control, although prior work has explored the frequency‐specific determinants of decisions to attend in the predecision interval [Bengson et al., 2014].
In the present study, we identify the network level activity of willed attention through the computation of choice‐cue specific ERPS from high‐density EEG recordings and analysis of the BOLD response that is specific to willed attention. Our experimental paradigm, and the use of EEG recordings combined with fMRI, represents innovations enabling the isolation of the process‐level electrophysiological dynamics of willed attention, while also offering a powerful event‐related analytical framework to explore the network‐level anatomical architecture associated with the deployment of willed attention.
MATERIALS AND METHODS
Methods
Participants
EEG and fMRI data were recorded in two separate sessions from 19 undergraduate students at the University of California, Davis. Order of data collection (fMRI and EEG), response mapping (i.e., index or middle finger = “thin”), and cue‐meaning (left, right, choose) were counterbalanced across participants. All participants had normal or corrected‐to‐normal vision and were paid for participation. All artifact free trials with correct behavioral performance were entered into statistical analysis of the EEG and fMRI data.
Apparatus and stimuli
For the fMRI session, stimuli were presented on an fMRI‐compatible 24 inch LCD Boldscreen monitor and for the EEG session, stimuli were presented on a 19 inch Viewsonic VX922 color monitor with visual angle of stimulus presentation matched across EEG and fMRI sessions. Stimuli were presented using Presentation® software (Version 14, http://www.neurobs.com). Eye movements were monitored using a Model 504 Applied Science Laboratories eye‐tracking system. Each trial began with the presentation of one of 3 (pseudorandomly selected) possible 200 ms cues (1° × 1° diamond, a cross, or a circle) that instructed participants to attend to the left or right or to choose to attend left or right (See Figure 1). Between a randomly selected Stimulus‐Onset‐Asynchrony (SOA) of 2000–8000 ms after cue presentation, target stimuli (5°x 5° square target gratings) were presented at location markers 11.5° to the left or right of a white dot placed at fixation and 3.5° below the horizontal midline in the left and right hemi‐fields at a ratio of .50 for each hemi‐field for each participant. To hold validity constant across willed‐attention and cued trials, for choose‐cue trials and cued trials, the target appeared at the attended location on 50% of trials. The spatial frequency of each grating varied pseudorandomly within each condition and hemi‐field at a ratio of 0.50 between high (0.53° per cycle) and low (0.59° per cycle) spatial frequencies of alternating black and white square waves. After target presentation, following a pseudorandomly distributed interstimulus interval varying between 2000 and 8000 ms, the word “?SIDE?” (2° × 8°) was presented at fixation to cue participants to report which side they attended to for that trial. Stimuli were presented on a gray background (rgb intensities of 60, 60,60) with an intertrial interval drawn from a pseudorandom distribution varying between 2000 and 8000 ms with each trial proceeding automatically. To isolate the regions of visual cortex sensitive to target presentation, prior to the fMRI session, 18 of the 19 subjects also completed three runs of a functional localizer task. For the functional localizer, stimuli consisted of square wave gratings identical to the targets presented in the main task, presented in alternating blocks of 20 s in each target location. The stimuli appeared for 500 ms with a 500 ms interval. The localizer was presented for 3 runs of 200 s.
Procedure
All participants were told that this was a study of spatial attention and were instructed to attend on a trial‐by‐trial basis in the direction of the cue while sustaining fixation at the center of the screen. If a choice cue was presented, subjects were told to make a spontaneous decision to attend left or right and were explicitly instructed not to use any type of decisional strategy (such as always attending to the side the target appeared on the last trial). Participants were informed to keep attending to the cued hemifield until the target was presented. Upon target presentation, subjects were instructed to make 2‐AFC discrimination ("thick" or "thin") to the thickness of the lines of the gratings by pressing their index or middle finger (the response‐finger mapping was counter‐balanced between subjects). Participants were instructed to respond to the target only if it appeared on the attended side. If it appeared on the unattended side, they were instructed to ignore the target. The response‐relevant perceptual feature of the targets (“thick” or “thin”) varied pseudorandomly and evenly for each of the hemi‐fields and validity conditions and participants. Furthermore, participants were instructed to respond as quickly and as accurately as possible to the targets. Upon the presentation of the word “?SIDE?”, subjects were instructed to accurately respond with where they had been attending for that trial (left or right). For both types of trials (cued and choice), the attended location was determined using both participants' reports and response to targets (whether they responded to the target or not). We only analyzed those trials in which both subjects' report and response to targets were consistent (ie. if the target appeared on the right and the participant responded to the target and reported attending to the right). A Mersene twister algorithm was used to randomize stimulus sequences. This procedure is identical to that reported in Bengson et al. [2014].
EEG recording
The EEG was recorded using an Electro‐cap (64‐channel) from sites: FPZ, FZ, FCZ, CZ, CPZ, PZ, POZ, OZ, INZ, FP1, FP2, F7, F8, F3a, F4a, F3, F4, F7p, F8p, F3i, F4i, C3a, C4a, C3, C4, FC1, FC2, PA1, PA2, C5, C6, C1a, C2a, T3, T4, C1p, C2p, C5p, C6p, P3a, P4a, P1, P2, P3i, P4i, PO1, PO2, O1, O2, T3i, T4i, TO1, TO2, T1i, T2i, O1i, O2i, I1, I2, M1, M2. Scalp channels were referenced to the right mastoid (average mastoid reference was applied after data recording) during online recording and impedances were kept below 5 kΩ. An online band‐pass filter of 0.1–100 Hz was used with a Synamps II amplifier with Scan 4.2 software. Data were recorded in DC at a sampling rate of 1000 Hz. To monitor eye position and blinks, bipolar electrodes were place on the outer left and right canthus and above and below the left eye and referenced to each‐other respectively.
fMRI recording
Imaging data were collected at the Imaging Research Center of the University of California, Davis, using a 3‐T Siemens Skyra Scanner with a 32‐channel head coil. Anatomical images were acquired using an MPRAGE sequence with a sagittal partition direction yielding images with 1‐mm isovoxel resolution (time repetition (TR) = 2500 ms, time echo (TE) = 4.38 ms, flip angle = 7°). Whole‐brain echoplanar functional images were acquired in 34 transverse slices (TR = 2100 ms, TE = 29 ms, matrix = 64 × 64, field of view = 216 mm, slice thickness = 3.4 mm, ascending interleaved slice acquisition order).
EEG analysis
Prior to artifact rejection an IRR Butterworth filter between 0.1 and 30 Hz was applied to all channels for all subjects and Independent Component Analysis was used to remove EEG components related to eye‐blinks. The filtered continuous data was then epoched −200 ms to + 2000 ms cue onset and later baseline corrected −200 to 0 ms relative to the cue onset. Channels for which signal was lost (4 participants) during data collection were interpolated (channels F8, F3A, P1, T3L) and artifacts were detected and eliminated using ERPLAB (http://erpinfo.org/erplab) software's moving window peak to peak artifact rejection function with a 100 μV voltage threshold, within any given trial using a moving window of 100 ms in steps of 50 ms. In addition, for each subject, each epoch was inspected and artifacts were also manually rejected prior to averaging.
fMRI analysis
Data were processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). Functional images collected from the scanner were slice‐time corrected, aligned with a representative functional volume and motion corrected using an unwarping procedure, and spatially smoothed using a 4‐mm full‐width at half maximum Gaussian kernel. Structural images were also aligned with the representative functional volume. For the main experiment, separate events representing target position, distractor position and distractor congruency were modeled using a general linear model (GLM). These events were modeled as impulses of activity at the onset of the stimuli and were convolved with the canonical hemodynamic response function (HRF) included with SPM8. For the localizer scans, boxcars representing the duration of each block were convolved with the HRF and similarly modeled using a GLM. Results of the analyses, in the form statistical maps, were overlaid on the structural images collected for each subject. For group‐level analyses, these statistical maps were spatially normalized to the standard Montreal Neurological Institute space and entered into a one‐way t‐test to produce group average statistical maps, which were then overlaid onto an average structural image. To examine which regions of cortex showed a greater blood oxygen‐level dependent (BOLD) response to the various trial types, the group‐level maps were generated using an uncorrected voxel‐level threshold of P < 0.001 and a contiguous‐voxel threshold producing a corrected threshold of P < 0.05. This analysis produced a cluster size threshold of 33 voxels for the explicit vs. choice cue comparison, 34 voxels for the explicit vs. baseline comparison and 37 voxels for the choice cue vs baseline comparison. With respect to the combined ERP‐fMRI analysis, we focus on the sites of activation that are uniquely active in response to the choice cues and linking these areas of activation to the observed choice‐cue specific ERPS. This approach assured direct conclusions concerning the task‐specificity of the relationship between the choice‐specific ERP and fMRI activations and serves as a means to isolate unique networks governing willed attention.
Results
Behavioral Results
We observed no significant bias to choose to attend left or right across our sample with 51% of choice‐trials going to the right hemifield and 49% of trials going to the left hemifield. We aggregated decision direction as a function of prior cue direction and cue type and included the proportion of left vs. right choice trials as a dependent variable in two t‐tests: one with prior explicit cue direction (if that occurred) of the previous trial as the independent variable, and another with prior choice direction (if the previous trial was a choice trial) as an independent variable. Results of this analysis reveal no significant effect of prior trial explicit cue direction on decision outcome, t(19) = 1.585, P = 0.130 and no effect of prior choice direction on current trial, t(19) = 1.609, P = 0.125. Although not definitive, these data suggest that participants were making spontaneous decisions in response to the choice cues in accordance with our instructions.
Attentional Validation Results
The BOLD response within the ROIs contralateral to target presentation was subjected to a 2x2 ANOVA (validity vs cue type). This analysis showed a significant effect of validity [F (1,17) = 6.68, P = 0.02], indicating a larger BOLD response to validly cued targets than invalidly cued targets. Neither the main effect of cue type [F (1,17) = 1.98, P = 0.17] nor the interaction [F (1,17) = 1.62, P = 0.22] were significant. These results suggest that attention was effectively deployed prior to target presentation and that the effect of attention did not differ as a function of cue type. This finding is consistent with a separate behavioral validation study in which participants were instructed to respond to both valid and invalid targets [Bengson et al., 2014]. In that study, significant behavioral cuing effects were observed for both choice and cued trials and these effects did not differ as a function of cue type (P = 0.223). Furthermore, we observed no significant difference in reaction times or accuracy to valid response‐relevant targets between choice and explicit cued trials (P > 0.5). Thus the present fMRI results combined with the behavioral results suggest that significant cueing effects are observed for both choice‐driven attention and explicitly cued attention.
The Electrophysiological Correlates of Willed Attention
To isolate the ERPS associated with willed attention, we compared the averaged cue‐evoked response for choice cues with explicitly cued trials. Figure 2a depicts the averaged evoked response for a representative electrode site (Fz) for the 2 second interval following cue onset and Figure 2b illustrates the topographic distribution of the difference between choice cues and explicit cues for the 250–350ms postcue interval. This choice‐cue specific positivity, which we will refer to as the Early Willed Attention Component (EWAC) has a frontal distribution (Fig. 2b) and manifests as a significant main effect of cue‐type when we conduct a 2 X 2 ANOVA (cue‐direction by cue‐type) for the averaged cue‐evoked ERP response of the 14 frontal electrode sites, F (1,18) = 5.816, P = 0.027, η 2 = 0.244 (Fig. 2c). We did not observe a significant effect of cue‐direction, nor did we observe a cue‐direction by cue‐type interaction for the 250–350ms EWAC interval (both P values > 0.250). We next compared the averaged cue‐evoked activity over the 400–800ms interval following cue onset. Figure 3 depicts the ERPs as a function of cue type for a representative electrode site and Figure 3b depicts the central‐posterior focus of the scalp distribution of the difference between choice‐driven and explicitly‐cued attention for this interval. We conducted a 2 X 2 (cue‐direction by cue‐type) ANOVA for the averaged cue‐evoked ERP response of the 15 central electrode sites (Fig. 3c) and observed a significant effect of cue‐type, F (1,18) = 9.972, P = 0.005, η 2 = 0.356. We refer to this as the willed attention component (WAC). We did not observe an effect of cue‐direction, nor did we observe a cue‐direction by cue‐type interaction for this interval (both p‐values > 0.500). Finally, from viewing Figures 2 and 3 it appears that there is a sustained negativity specific to choice cues from the 1400–1900 ms post cue interval; however, this difference is only significant at 2 electrode sites and does not show a consistent scalp distribution.
Figure 2.
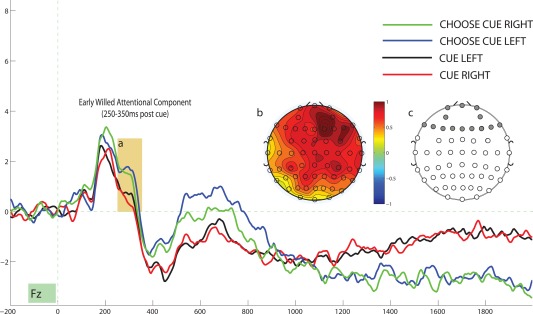
(a) Illustrates the Willed Attention Component (EWAC) at an example electrode site (250–350ms postcue). (b) Illustrates the topographic map of the EWAC (difference between willed attention trials and cued trials in the 250–350 ms postcue interval). (c) Illustrates the 14 frontal electrode sites for which the EWAC was computed. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 3.
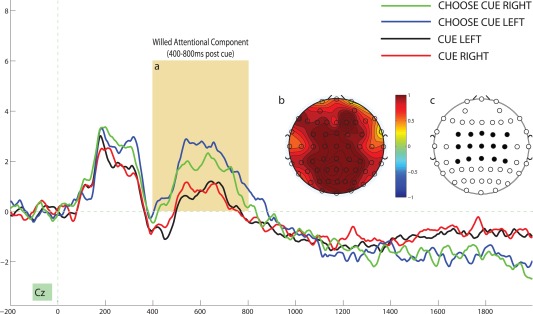
(a) Illustrates the Willed Attention Component (WAC) at an example electrode site (400–800ms postcue). (b) Illustrates the topographic map of the WAC (difference between willed attention trials and cued trials in the 400–800ms postcue interval). (c) Illustrates the 15 central electrode sites for which the EWAC was computed. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 1.
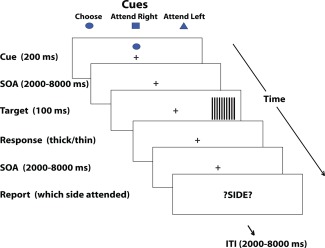
An example trial sequence for the willed attention task.
Willed attention Specific BOLD Response
A comparison between choice and explicit cue activations was also conducted on the BOLD response in the postcue interval across the whole brain (Fig. 4). The results of this analysis reveal an overlapping network for both willed and cued attention that has been previously associated with the orienting of attention [Hopf and Mangun, 2000]. We also isolate a novel network of activation specifically unique to choice cues that includes the middle frontal gyrus (MFG), anterior cingulate, (ACC) and bilateral anterior insula (AI) (Fig. 4). Although we do observe greater activation of the Supplementary Eye Fields (SEF) and Intraparietal sulcus (IPL) for willed attention relative to cued attention, these sites are active in both conditions and hence not unique the willed attention condition. As a result, we focus our combined ERP Bold analysis on the neuroanatomical sites that are specific to willed attention.
Figure 4.
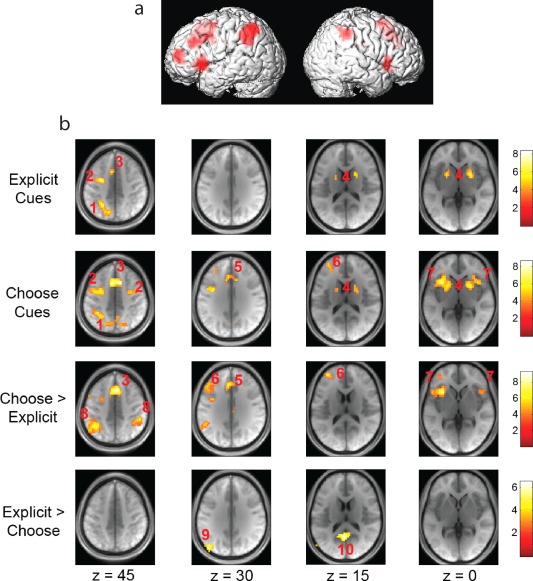
Significant BOLD response differences as a function of condition. (A) Depicts group‐level choice‐cue specific sites of activation projected onto a surface rendering from a typical subject. (B) Depicts regions of the brain showing increased BOLD response to the indicated condition comparisons. Functional data represent significant contrasts from a random effects analysis. Data are overlaid onto an average structural image generated from all study participants. Columns represent different depths along the axial plane. Anatomical region key: (1.) Superior Parietal Lobe/IPS. (2.) FEF. (3.) SEF/SMA. (4.) Basal Ganglia. (5.) Anterior Cingulate. (6.) Middle Frontal Gyrus. (7.) Anterior Insula. (8.) Inferior Parietal Lobule. (9.) Angular Gyrus. (10.) Posterior Cingulate. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Combining EEG, ERP, and fMRI Data
To link our observed patterns of willed attention fMRI activation with the two ERPS that were associated with willed attention (EWAC: 250–350 ms and WAC: 400–800ms postcue), we conducted a set four independent 2 X 2 (cue‐direction by cue‐type) ANOVAs using each of the four willed attention‐specific sites of activation as a covariate in a general linear model. For the EWAC, only the Left Anterior Insula, F (1,18) = 11.352, P = 0.004, η 2 = 0.40, and Left MFG, F (1,18) = 9.793, P = 0.006, η 2 = 0.366 significantly covaried with the choice‐cue specific ERP activation under the Bonferonni‐corrected (4 comparisons) significance level of P > 0.01. When the ACC was included as a covariate, we observed a marginally significant interaction, F (1,18) = 6.121, P = 0.023, η 2 = 0.265 and finally, the Right Anterior Insula did not significantly covary with the EWAC across subjects under our Bonferroni‐corrected significance criterion, F (1,18) = 3,644, P = 0.073, η 2 = 0.177. To illustrate the sign of this significant EWAC covariation with the choice‐cue specific Left AI and Left MFG activations, we also correlated the size of the EWAC effect with the individual BOLD activations specific to these brain regions and observed significant (as expected) correlations that were negative in sign: Left AI: r = −0.633, P = 0.004, Left MFG: r = −0.605, P = 0.006. Specifically, for those subjects who showed an EWAC effect of lesser amplitude, stronger choose‐cue specific BOLD responses were observed in the MFG and left AI. We conducted these same set of analyses on the WAC (400–800 ms postcue) response and observed that only the left AI covaried with the WAC response when entered in the 2 X 2 (cue‐direction by cue‐type) ANOVA as a covariate, F (1,18) = 7.766, P = 0.013, η 2 = 0.314. However, it should be noted that this covariation is marginally significant after Bonferroni correction. As with the EWAC pattern of results, this correlation is negative in sign, r = −0.56, P = 0.013. No other choice‐cue specific brain region significantly covaried with the WAC, and no other interactions were significant (all P > 0.20). In summary, only the Left AI and Left MFG significantly covaried with scalp event‐related electrophysiological activity and this relationship was of an inverse nature; a greater choice‐cue specific BOLD increase was associated with less of an EWAC and WAC across our sample of 19 participants.
DISCUSSION
This is the first study to explore the ERP correlates of willed attention and the first to link ERPs associated with willed attention with BOLD activations within the same subjects. Prior studies have explored the anatomical correlates of willed attention using fMRI and have reached differing results [Hopfinger et al., 2010; Taylor et al., 2008]. Because our design was more similar to the Taylor et al., [2008] paper, we will focus on comparing our fMRI results to the Taylor study. Whereas Taylor isolated a network associated with decisions to attend that included the Supplementary Eye Fields (SEF), pre‐Supplementary Motor Area (pre‐SMA), Anterior Cingulate (ACC) and dorsal medial frontal cortex (pre‐SEF), our study isolated a unique network of activation specific to attention decisions that includes the left and right AI, ACC, and MFG. The primary brain area associated with willed attention across both studies is the ACC. Other studies have associated the ACC with decision making [Rushworth et al., 2004]. The consistent principle relating decision making and the ACC is the likely role that the ACC plays in conflict detection [Carter and van Veen, 2007]. Because a decision to attend inherently involves competing representations from hemi‐fields which are otherwise resolved when cued to attend, the ACC activation associated with willed attention across the Taylor et al. [2008] study and our study can be viewed as the detection of the unresolved conflict that is inherently associated with a decision to attend.
Whereas the Taylor et al., [2008] study associated the SEF, lateral FEF and SMA areas with willed attention in addition to the ACC, our study found that the MFG and left and right AI are uniquely associated with willed attention. The Taylor et al., [2008] study interpreted the choice‐specific activation of the ACC, and pre‐SMA as possibly due to the mnemonic reference of a particular choice to previous trial contingencies and further suggested that the lateral and pre‐SEF likely play the critical role in the voluntary decision of where to attend. However, we do not find choice specific activation of the SEF, but instead observe a unique network of choice‐specific activations in the left and right AI and left MFG. Although it is difficult to posit specific reasons for this discrepancy without speculating, a number of distinctions between the Taylor et al., [2008] paradigm and ours are worth noting. The first is that our design uses arbitrary symbols and counterbalances possible stimulus meaning across subjects so that no stimulus differences occur between cue types (instructed vs. chosen) whereas the Taylor design used the highlighting of letters to instruct subjects to attend left or right and a highlighted horizontal line to instruct subjects to choose where to attend (although the overall cue‐shape remained constant). Thus, the instructed condition in the Taylor et al., [2008] study contains an additional differential symbolic interpretation of letters vs. line when transforming the cue representation into appropriate task parameters. Although subtle, this difference in design may have slightly affected the observed areas of activation associated with choice‐driven attention when instructed conditions were subtracted. Another difference is that we instructed participants to make a spontaneous decision and explicitly told subjects to avoid implementing specific strategies, such as consciously choosing where to attend based on prior stimulus contingencies. Thus, instructional differences may also account for the different choice‐cue specific activations observed between the two studies. It is also important to note that whereas the Taylor et al. [2008] study contained an equal number of both cued and choice trials, our design employed less choice trials compared with cued trials and this methodogical difference may also be related to the unique network of activation observed in our study. We should note however that only further research and replication can resolve these discrepancies, and that the possible influence of task differences on choice‐specific BOLD activation are merely speculative.
Of special importance is the observed bilateral anterior insula and left MFG activation specifically in response to willed attention. The anterior insular cortex has been hypothesized to serve a number of different roles in cognition and behavior, including homeostatic regulation [Pacheco‐Lopez et al., 2005], self‐awareness [Karnath et al., 2005], and the feeling of disgust [Wicker et al., 2003]. Directly relevant to our findings is that the anterior insula is specifically involved in generating an internal sense of causal agency [Farrer and Frith, 2002] and the transformation of ventral salience signals into appropriate dorsal attentional control signals [Eckert et al., 2009]. Thus, in our task, the presentation of the choice cue likely involves an interaction between the categorization of the cue as endogenously relevant and the transformation of this categorization into a decisional response that is then executed by the dorsal attention system in the deployment of attention to the left or right hemifield. The work of Eckert et al. [2009] and our finding of comparable activation of the dorsal attention system in response to both explicit and choice cues are consistent with this notion.
In addition to the insular activation, we also observed activation of the left MFG specifically in response to the choice cues. The middle frontal gyrus has been associated with a variety of high level executive functions, including working memory [Barbey et al., 2013], lying [Ito et al., 2012] and social cognition [van Den Bos, 2010]. Most relevant to our finding is that the MFG is active in response to decision‐making tasks [Duncan and Owen, 2000], conflict detection [Mansouri et al., 2007] and spontaneous behavior [Miller, 1999]. Even though the MFG is responsive to a host of diverse cognitive demands, our results are in line with the notion that the MFG's role in our paradigm involves the detection of a conflicting stimulus and the generation of a spontaneous decision to attend. It is important to note that our MFG activation was particular to the left MFG, thus providing evidence of hemispheric specialization that might be particular to willed attention.
One aspect of our data that deserves consideration is the stronger IPL activation for choice cues relative to explicitly cued trials. Prior work has shown that the IPL is involved with computing the spatial coordinates of a to‐be‐attended location, whereas the SPL is involved with a general initiation of attention signal [Green and McDonald, 2008; Green et al., 2011]. In line with this notion is that we observe the same level of activity in the SPL, suggesting that the initiation of spatial attention is equal in both conditions; however, the higher IPL activation for the choice cues might be associated with the need to compute the spatial coordinates of a to‐be‐attended location, because the choice cue does not provide these coordinates which are otherwise provided by the explicit cues. Thus this pattern could highlight an additional distinct aspect of willed attention and provides insight into the fundamental functions of the SPL and IPL.
The most novel finding of this study is that we highlight the ERPs associated with willed attention. Although from viewing Figure 2 it appears that there is a significant choice‐cue ERP difference in the sustained 1100 ms to 2000 ms post cue interval, this difference is not statistically significant. However, the absence of a significant difference in this interval might be due to the high degree of variability in the cue‐target interval (2–8 s). Prior work [Green and McDonald, 2010] has shown that another sustained component, the LDAP, depends on the predictability of the target onset. Only further research can clarify if the lack of choice‐cued specific sustained activity is due to the variability in target onsets or if a more stable cue‐target interval would induced a sustained choice cue specific ERP. In any case, we do observe two unique ERP components that are specific to willed attention. The first component, what we have termed the EWAC, occurs between 250 and 350 ms post decision cue and has a frontal topographic distribution. A possible interpretation of the EWAC is that it represents a variant of a P300. The P300 is a classic ERP associated with the onset of an unexpected stimulus and has been divided into 2 distinct subcomponents: the P3a and P3b. The P3a is thought to index the presentation of a rare nontarget stimulus and the P3b is thought to index a task‐relevant rare stimulus [Comerchero and Polich, 1999; Donchin, 1981]. Usually the P300 is induced by presenting a rare stimulus in a sequence of common stimuli [Polich, 2003]. However, because our design equates the probability of stimulus presentation by presenting an equal number of stimuli for each condition across attend left, attend right, and choose conditions, the EWAC is not a stimulus induced variant of P300. Essentially, there is no rare stimulus in our design. However, because the general semantic category of stimulus cue (cued vs. choice) is not equated (there are 2 explicit cues [left vs. right] for every choice cue), the EWAC could possibly be due to the effect of a rare semantic category (choice attention), even though the three cuing stimuli are presented equally. However, when we observe the topographic distribution of the EWAC, it seems quite distinct from the distribution normally observed for P3 related effects, which are much more centrally distributed [Polich, 2003] We can see from viewing Figure 2b that the EWAC is much more anteriorly distributed than the classic P300 effect. However, research has shown that shorter latency P300s (P3a) are more anteriorly distributed [Polich, 1997] and recent research using simultaneous EEG/fMRI to study the neural locus of the P300 effect has demonstrated that the left insula and MFG are correlated on a trial by trial basis with P300 activation [Warbrick et al., 2009]. We observe a similar result as Warbrick et al. [2009] by showing that specifically the MFG and Left AI significantly covary with the EWAC across subjects. Thus it is possible that the EWAC is a specialized task‐specific variant of a P300. A related possibility is that the EWAC is a variant of the frontal P2a [Potts et al., 2004]. Potts et al. [2004] observed that the P2a is sensitive to task relevance, whether covert or overt tasks are conducted. Relevant to this notion is that the presentation of a choice‐cue signals an additional act of cognition (deciding) prior to the deployment of attention and this additional task activation may be the cognitive source of the EWAC observed here. Only further research can establish the exact functional role of the EWAC in decision making to elucidate if it serves a unique function separate from the P300 or is a specialized variant of a P300‐related process.
With respect to the observed broadly distributed positivity in the 400–800ms post cue interval that we have termed the WAC, our initial inclination is that this component indexes the decisional process itself, whereas the EWAC may index the categorization of the stimulus as a choice cue. Given the refractory period between the EWAC and WAC, each is likely a distinct modular process involved with willful decisions to attend. Because of the onset of around 400ms, the first point of comparison of the WAC to other known components would begin with the N400 or FN400, which each respectively index semantic updating and familiarity (although not exclusively). Despite the cognitive difference between those components and our paradigm, it is possible that the WAC is indexing some cognitive construct that is shared with N400 studies. This seems unlikely however, given that the polarity for the WAC is opposite that which is commonly observed in N400 studies. The WAC is characterized by a sustained positivity to choice cues whereas N400 related effects are indexed be a negativity over this interval. Furthermore the N400 is thought to originate in the temporal lobe [Kutas and Federmeier, 2011], whereas the WAC is anticorrelated with BOLD activation in the left AI across subjects.
Of particular relevance to other research that has explored the relationship between electrophysiological activity and the BOLD response is the sign of the inverse correlation between our evoked willed attention components and the associated willed attention specific BOLD responses. While it is natural to hypothesize that a greater ERP should correspond with a larger BOLD response, the true nature of the relationship between electrophysiological activity and the BOLD response is not clear [Nunez and Silberstein, 2000]. Prior work has shown a negative relationship between the BOLD response and ERP amplitude [Foucher et al., 2003; Ngyuen and Cunnington, 2013] while other work has shown a positive relationship between the two measures [Nagai et al. 2004]. Some studies have suggested that the relationship differs in sign depending on the brain region of the observed BOLD response [Huettel et al., 2004] and the ERP component of interest [Whittingstall et al., 2006]. Other work has shown that the relationship between particular frequencies and the BOLD response differs depending on the frequency of interest, with positive gamma‐BOLD [Magri et al., 2012] coupling and negative alpha‐BOLD coupling [Laufs et al., 2003; Liu et al., [Link]]. Thus, slow cortical ERPs that are more likely to consist of low‐frequency activity may have a different relationship with the BOLD response than high frequency components. In general, because the BOLD response can increase for both excitatory [Lauritzen, 2005] and inhibitory neural activity [Kobayashi et al., 2006], conclusions based on the sign of EEG–BOLD coupling should be drawn cautiously. Our present data suggest that the EWAC and WAC components are specifically related to the left MFG and Insula BOLD changes and the exact nature of this relationship requires further research.
In conclusion, we view the EWAC and WAC components as ERP signatures of the general construct of “willed attentional control”. However, it is important to note that these components were isolated by comparing choice cues with explicit cues, and that therefore, the WAC and EWAC reflect all mental operations involved with allocating willed attention versus cued attention [Donchin, 1984]. Willed attentional control as isolated in our paradigm involves a series of cognitive operations including stimulus categorization (choice cue versus instructional cue), conflict perception (decisions entail more conflict than cued trials), and the actual willful decision‐making process. This conglomerate of constructs can be thought of as volitional attentional control in general, but only further research can isolate the subcomponentry involving the EWAC and WAC responses and the associated willed attention specific BOLD response. Nonetheless, our findings are the first to demonstrate the ERP componentry of truly endogenous attentional control and the first intermodal study of willed attention that links electrophysiology with the anatomy of willed attentional control using combined ERP and fMRI methods.
ACKNOWLEDGMENT
The authors are grateful to Sharon Corina and Dennis Thompson for their assistance.
REFERENCES
- Asplund CL, Todd JJ, Snyder AP, Marois R (2010): A central role for the lateral prefrontal cortex in goal‐directed and stimulus‐driven attention. Nat Neurosci 13:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Koenigs M, Grafman J (2013): Dorsolateral prefrontal contributions to human working memory. Cortex 49:1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengson JJ, Kelley TA, Zhang X, Wang JL, Mangun GR (2014): Spontaneous neural fluctuations predict decisions to attend. J Cogn Neurosci 26:2578–2584. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME (2010): Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci 33:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M (2008): Top‐down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci 28:10056–10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundesen C, Habekost T, Kyllingsbaek S (2008): A neural theory of visual attention: Bridging cognition and neurophysiology. Psychol Rev 112:291–328. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Goldberg ME, Robinson DL (1981): Behavioral enhancement of visual responses in monkey cerebral cortex. I. Modulation in posterior parietal cortex related to selective visual attention. J Neurophysiol 46:755–772. [DOI] [PubMed] [Google Scholar]
- Bruce ND, Tsotsos JK (2009): Saliency, attention, and visual search: An information theoretic approach. J Vis 9:1–24. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V (2007): Anterior cingulate cortex and conflict detection: An update of theory and data. Cogn Affect Behav Neurosci 7:367–379. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J (1999): P3a and P3b from typical auditory and visual stimuli. Clin Neurophys 110:24–30. [DOI] [PubMed] [Google Scholar]
- Cohen JY, Heitz RP, Schall JD, Woodman GF (2009): On the origin of event‐related potentials indexing covert attentional selection during visual search. J Neurophysiol 102:2375–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL (2000): Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3:292–297. [DOI] [PubMed] [Google Scholar]
- Corbetta, M , Shulman GL (2011): Spatial neglect and attention networks. Ann Rev Neurosci 34:569–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL (2008): The reorienting system of the human brain: From environment to theory of mind. Neuron 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corneil BD, Munoz DP, Chapman BB, Admans T, Cushing SL (2008): Neuromuscular consequences of reflexive covert orienting. Nat Neurosci 11:13–5. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen BE (2008): A dual‐networks architecture of top‐down control. Trends Cogn Sci 12:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E (1981): Surprise!.Surprise? Psychophysiology 18:493–513. [DOI] [PubMed] [Google Scholar]
- Donchin E (1984): Cognitive Psychophysiology: Event‐Related Potentials and the Study of Cognition. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Duncan J, Owen AM (2000): Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 23 :475–483. [DOI] [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR (2009): At the heart of the ventral attention system: The right anterior insula. Hum Brain Mapp 30:2530–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer C, Frith CD (2002): Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage 15:596–603. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Bell AH, Munoz DP (2004): Neural correlates of the automatic and goal‐driven biases in orienting spatial attention. J Neurophysiol 92: 1728–1737. [DOI] [PubMed] [Google Scholar]
- Foucher JR, Otzenberger H, Gounot D (2003): The BOLD response and the gamma oscillations respond differently than evoked potentials: An interleaved EEG‐fMRI study. BMC Neurosci 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Lin W (2012): Frontal parietal control network regulates the anti‐correlated default and dorsal attention networks. Hum Brain Mapp 33:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC (2011): Top‐down modulation: Bridging selective attention and working memory. Trends Cogn Sci 16:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng JJ, Mangun GR (2011): Right temporoparietal junction activation by a salient contextual cue facilitates target discrimination. Neuroimage 54:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JJ, McDonald JJ (2008): Electrical neuroimaging reveals timing of attentional control activity in human brain. PLOS Bio 6:e81. doi:10.1371/journal.pbio.0060081 [Google Scholar]
- Green JJ, Doesburg SM, Ward LM, McDonald JJ (2011): Electrical neuroimaging of voluntary audiospatial attention: evidence for a supramodal attention control network. J Neurosci 10:3560–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JJ, McDonald JJ (2010): The role of temporal predictability in the anticipatory biasing of sensory cortex during visuospatial shifts of attention. Psychophysiology 47:1057–1065. [DOI] [PubMed] [Google Scholar]
- Harter MR, Miller SL, Price NJ, Lalonde ME, Keyes AL (1989): Neural processes involved in directing attention. J Cogn Neurosci 1:223–237. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR (2000): The neural mechanisms of top‐down attentional control. Nat Neurosci 3:284–291. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Camblin CC, Parks EL (2010): Isolating the internal in endogenous attention. Psychophysiology 47:739–747. [DOI] [PubMed] [Google Scholar]
- Hopf JM, Mangun GR (2000): Shifting visual attention in space: An electrophysiological analysis using high spatial resolution mapping. Clin Neurophysiol 111:1241–1257. [DOI] [PubMed] [Google Scholar]
- Huettel SA, McKeown MJ, Song AW, Hart S, Spencer DD, Allison T, McCarthy G (2004): Linking hemodynamic and electrophysiological measures of brain activity: Evidence from functional MRI and intracranial field potentials. Cerebral Cortex 14:165–173. [DOI] [PubMed] [Google Scholar]
- Hung J, Driver J, Walsh V (2011): Visual selection and the human frontal eye fields: Effects of frontal transcranial magnetic stimulation on partial report analyzed by Bundesen's theory of visual attention. J Neurosci 31:15904–15913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Abe N, Fujii T, Hayashi A, Ueno A, Mugikura S, Takahashi S, Mori E (2012): The contribution of the dorsolateral prefrontal cortex to the preparation for deception and truth‐telling. Brain Res 1464:43–52. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nägele T (2005): Awareness of the functioning of one's own limbs mediated by the insular cortex? J Neurosci 25:7134–7138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider, LG (1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22:751–761. [DOI] [PubMed] [Google Scholar]
- Kutas, M, Federmeier, KD (2011): Thirty years and counting: finding meaning in the N400 component of the event‐related potential (ERP). Annu Rev Psychol 62:621–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E, Bagshaw AP, Grova C, Dubeau F, Gotman J (2006): Negative BOLD responses to epileptic spikes. Hum Brain Mapp 27:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen, M (2005): Reading vascular changes in brain imaging: Is dendritic calcium the key? Nat Rev Neurosci 6: 77–85. [DOI] [PubMed] [Google Scholar]
- Laufs H, Kleinshcmidt A, Beyerle A, Eger E, Salek‐Haddadi AS, Preibisch C, Krakow K (2003): EEG‐correlated fMRI of human alpha acitivity. Neuroimage 19:1463–1476. [DOI] [PubMed] [Google Scholar]
- Liu Y, Bengson JJ, Huang H, Mangun GR, Ding M: Top‐down modulation of neural activity in anticipatory visual attention: Control mechanisms revealed by simultaneous EEG‐fMRI. Cereb Cortex (2014). doi: 10.1093/cercor/bhu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis N (2012): The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J Neurosci 32:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA (1991): Modulations of sensory‐evoked brain potentials indicate changes in perceptual processing during visual‐spatial priming. J Exp Psychol Hum Percept Perform 17:1057–1074. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Buckley MJ, Tanaka K (2007): Mnemonic function of the dorsolateral prefrontal cortex in conflict‐induced behavioral adjustment. Science 318:987–990. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Cavanaugh J, Wurtz RH (2006): Attentional modulation of thalamic reticular neurons. J Neurosci 26:4444–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1981): A cortical network for directed attention and unilateral neglect. Ann Neurol 10:309–325. [DOI] [PubMed] [Google Scholar]
- Miller BL (1999): The Human Frontal Lobes. New York: The Guilford Press. [Google Scholar]
- Nunez PL, Silberstein RB (2000): ON the relationship of synaptic activity to macroscopic measurements: Does co‐registration of EEG with fMRI make sense? Brain Topogr 13:79–96. [DOI] [PubMed] [Google Scholar]
- Pardo J, Fox P, Raichle M (1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349:61–64. [DOI] [PubMed] [Google Scholar]
- Pacheco‐Lopez G, Niemi MB, Kou W., Harting M, Fandrey J, Schedlowski M (2005): Neural substrates for behaviorally conditioned immunosuppresssion in the rat. J Neurosci 25:23330–23337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Bengtsson SL, Lau HC (2010): Medial frontal cortex: From self‐generated action to reflection on one's own performance. Trends Cogn Sci 14:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, Posner, MI (2012): Medial frontal cortex: From self‐generated action to reflection on one's own performance. Trends Cogn Sci 35:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J (2003): Overview of the P3a andP3b. In: Polich J, editor. Detection of Change: Event‐Related Potential and fMRI Findings, Boston: Kluwer Academic Press; pp 83–98. [Google Scholar]
- Polich J (1997): On the relationship between EEG and P300: Individual difference, aging and ultradian rhythms. Int J Psychophysiol 26:299–317. [DOI] [PubMed] [Google Scholar]
- Posner MI, Cohen Y Rafal RD (1982): Neural systems control of spatial orienting. Philos Trans R Soc Lond B Biol Sci 298:187–198. [DOI] [PubMed] [Google Scholar]
- Posner MI (1980): Orienting of attention. Q J Exp Psychol 32:3–25. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Corbetta M, Miezin FM, Shulman GL (1994): PET studies of parietal involvement in spatial attention: Comparison of different task types. Can J Exp Psychol 48:319–338. [DOI] [PubMed] [Google Scholar]
- Potts GF, Patel SH, Azzam PN (2004): Impact of instructed relevance on the visual ERP. Int J Psychophysiol 2:197–209. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Fenwick PB, Trimble MR, Dolan RJ (2004): Brain activity relating to the contingent negative variation: An fMRI Investigation. Neuroimage 21:1232–1241. [DOI] [PubMed] [Google Scholar]
- Ngyuen VT, Cunnington R (2013): The superior temporal sulcus and the N170 during face processing: Single trial analysis of concurrent EEG‐fMRI. Neuroimage 86:492–502. [DOI] [PubMed] [Google Scholar]
- Noudoost B, Chang MH, Steinmetz NA, Moore T (2010): Top‐down control of visual attention. Curr Opin Neurobiol 20:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM (2004): Action sets and decisions in the medial frontal cortex. Trends Cogn Sci 8:410–417. [DOI] [PubMed] [Google Scholar]
- Schall JD (2004): On the role of frontal eye field in guiding attention and saccades. Vision Res 44:1453–1467. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S, Culberson A, Awh E (2004): Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. J Neurophysiol 92:3538–3545. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Pope DL, Asafiev SV, Mcavoy MP, Snyder AZ, Corbetta M (2010): McRight hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J Neurosci 30:3640–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Konen CS, Kastner S (2010): Mechanisms of spatial attention control in frontal and parietal cortex. J Neurosci 30:148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester CM, Shulman GL, Jack AI, Corbetta M (2009): Anticipatory and stimulus‐evoked blood oxygenation level‐dependent modulations related to spatial attention reflect a common additive signal. J Neurosci 29:10671–10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Weissman DH, Giesbrecht B, Kenemans JL, Mangun GR, Kok A, Woldorff MG(2006): Brain regions activated by endogenous preparatory set shifting as revealed by fMRI. Cogn Affect Behav Neurosci 6:175–189. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Rushworth MF, Nobre AC (2008): Choosing where to attend and the medial frontal cortex: An FMRI study. J Neurophysiol 100:1397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, Van Dijk E, Westenberg M, Rombouts SA, Crone EA (2010): Changing brains, changing perspectives: The neurocognitive development of reciprocity. Psychol Sci 22:60–70. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL (2008): Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol 100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BJ, Buonocore MH, Carter CS, Mangun GR (2010): Integrating conflict detection and attentional control mechanisms. J Cogn Neurosci 23:2211–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Warner LM, Woldorff MG (2009): Momentary reductions of attention permit greater processing of irrelevant stimuli. Neuroimage 48:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Devlin JT, Rushworth MF (2004): Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci 7:1259–1265. [DOI] [PubMed] [Google Scholar]
- Warbrick T, Mobascher A, Brinkmeyer J, Musso F, Richter N, Stoecker T, Fink GR, Shah NJ, Winterer G (2009): Single‐trial amplitude and latency informed event‐related fMRI models yield different BOLD response patterns to a target detection task. Neuroimage 4:1532–1544. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G (2003): Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron 40:655–664. [DOI] [PubMed] [Google Scholar]
- Whittingstall K, Stroink G, Schmidt M (2006): Evaluating the relationship of event‐related potential and functional MRI sources in the primary visual cortex. Hum Brain Mapp 28:134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]


