Abstract
Background
Cognitive dysfunction is considered a core feature of schizophrenia, and impaired performances in episodic memory (EM) and executive function (EF) tasks are consistently reported in schizophrenia patients. Traditional fMRI and EEG studies have helped identifying brain areas, including the prefrontal cortex (PFC), involved in these tasks. However, it is unclear whether intrinsic defects in prefrontal function per se contribute to poor performance in schizophrenia, given the presence of confounds like reduced motivation and psychotic symptoms. TMS/hd‐EEG measurements are obtained without cognitive effort, and can be calculated in any cortical area.
Methods
We performed TMS/hd‐EEG recordings in parietal, motor, premotor, and PFC in healthy individuals (N = 20) and schizophrenia patients (N = 20). Source modeling of TMS‐evoked responses was performed, and measures of cortical activity (significant current density, SCD) and connectivity (significant current scattering, SCS) were computed. Patients with schizophrenia also performed Penn Word memory delayed (CPWd) and Penn Conditional Exclusion Test (PCET). CPWd evaluates EM and involves primarily PFC, whereas PCET reflects EF and implicates PFC with other brain regions.
Findings
We found no difference in SCD and SCS after TMS of parietal/motor cortices, whereas those parameters were reduced in premotor/prefrontal areas in schizophrenia patients. In PFC, where these measures were most defective, SCD was negatively correlated with performance in CPWd whereas higher SCS values were associated with more errors in PCET.
Conclusion
These findings indicate that schizophrenia patients have intrinsic defects in both activity and connectivity of PFC, and that these defects are specifically associated with impairments in cognitive abilities. Hum Brain Mapp 36:4539–4552, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: schizophrenia, prefrontal cortex, TMS, hd‐EEG, source modeling, cognitive function
INTRODUCTION
Cognitive impairments have been consistently reported in patients with schizophrenia [Kalkstein et al., 2010], and are currently considered a core feature of this disorder [Schaefer et al., 2013]. Cognitive ability can predict functional outcome in chronic schizophrenia [Bowie et al., 2006], and interventions aimed at improving cognition may be critical in preventing long‐term disability in these patients[Barlati et al., 2013]. However, one of the main challenges in developing effective interventions is the still limited understanding of the neuronal circuits and mechanisms underlying those cognitive deficits [Keefe and Harvey, 2012].
Traditional functional Magnetic Resonance Imaging (fMRI) and Electroencephalogram (EEG) studies have revealed several brain areas, including the prefrontal cortex (PFC), which are involved in poor cognitive performance in schizophrenia. A recent meta‐analysis of functional neuroimaging studies investigating executive functions in schizophrenia patients and healthy subjects found that both groups activated a similar cortico‐subcortical network, although schizophrenia patients had reduced activation in left dorsolateral PFC, inferior/posterior cortical areas, and the thalamus [Minzenberg et al., 2009]. An EEG study employing a cognitive control paradigm found that enhanced frontal gamma‐band oscillations corresponded to better performance in healthy subjects, whereas schizophrenia patients had reduced frontal gamma activity [Cho et al., 2006]. Another cognitive control study established that both medication‐naive and medicated schizophrenia patients had decreased frontal gamma power compared with controls, and this reduction predicted worse cognitive performance [Minzenberg et al., 2010]. Despite this accumulating evidence, recently reviewed by Sun et al. [2011] and Uhlhaas and Singer [2013], the presence of confounds, like reduced motivation and fluctuation in attention, complicates the assessment of intrinsic defects in cortical neuronal activity and/or connectivity underlying poor cognitive performances in schizophrenia.
Transcranial Magnetic Stimulation (TMS) paradigms, including TMS with fMRI (TMS/fMRI) and TMS with high density (hd)‐EEG (TMS/hd‐EEG), can be utilized to uniquely probe cortical function in schizophrenia, which should likely contribute to reveal the neurobiology of this disorder [McClintock et al., 2011]. Consistent with this prediction, several TMS/hd‐EEG studies demonstrated frontal cortical abnormalities in schizophrenia patients compared to healthy and psychiatric controls. Rhadu et al. showed specific deficits in long‐interval cortical inhibition (LICI), a GABA‐ergic mediated measure of cortical inhibition, in the PFC of schizophrenia patients compared to both healthy controls and obsessive‐compulsive patients, while LICI of motor cortex did not differ between psychiatric groups [Radhu et al., 2015]. Frantseva et al. targeted the motor cortex and found no difference in the initial (75–150 ms) TMS‐evoked activity of schizophrenia patients and healthy controls, whereas these patients had abnormal, longer‐lasting EEG responses in fronto‐temporo‐parietal electrodes bilaterally [Frantseva et al., 2014]. Farzan et al. reported deficits of inhibition of gamma oscillations in the PFC of schizophrenia patients compared to healthy subjects and bipolar disorder patients [Farzan et al., 2010]. The same group also showed that repetitive TMS reduced abnormal gamma oscillations in schizophrenia patients, whereas it increased gamma activity in healthy subjects during a cognitive task; however, no correlation between pre‐ and post‐TMS changes in gamma power and performance improvement was found [Barr et al., 2011]. Furthermore, our group reported a reduction in TMS‐evoked premotor gamma oscillations in schizophrenia patients compared to healthy controls [Ferrarelli et al., 2008], and in a follow‐up study we found a slowing in the main oscillatory activity, or natural frequency, of frontal cortical areas such that the prefrontal natural frequency of schizophrenia patients was slower than in any healthy control, and correlated with both psychotic symptoms and performance in a cognitive task [Ferrarelli et al., 2012].
Despite an increasing number of TMS/hd‐EEG studies in schizophrenia, limited information is currently available about the cortical sources of scalp recorded activity. A recent TMS/hd‐EEG study proposed a data‐driven procedure to characterize cortical responses to TMS by means of two indices: significant current density (SCD) and significant current scattering (SCS) [Casali et al., 2010]. SCD captures the amplitude of TMS‐evoked cortical currents, and therefore measures cortical excitability, whereas SCS reflects the average distance of TMS‐activated cortical sources, thus assessing cortical connectivity. Those indices were employed to characterize changes in neuronal activity and propagation from wakefulness to anesthesia after TMS of the premotor cortex in healthy subjects [Ferrarelli et al., 2010].
Here we performed TMS/hd‐EEG recordings of parietal, motor, premotor, and PFC in healthy individuals (N = 20) and patients with schizophrenia (N = 20). Source modeling analysis of TMS‐evoked responses was performed, and SCD and SCS were computed for both groups. Schizophrenia patients also completed two cognitive tasks, the Computerized Penn Word memory delayed (CPWd) and the Penn Conditional Exclusion Test (PCET). We hypothesized that schizophrenia patients would show deficits in both SCD and SCS of anterior frontal areas compared to healthy controls. We also expected that those impairments would be most prominent in PFC, and tested whether prefrontal SCD and SCS predicted cognitive performance in CPWd and PCET tasks in schizophrenia.
METHODS AND MATERIALS
Participants
Twenty patients with schizophrenia (age = 31.7 ± 7.8; M/F = 13/7) and twenty age‐matched healthy controls (age = 32.8 ± 6.2; M/F = 16/4) were recruited (Table 1). A psychiatrist interviewed all participants and administered the Structured Clinical Interview for DSM‐IV‐TR [American Psychiatric Association and American Psychiatric Association. Task Force on DSM‐IV, 2000] to confirm or exclude psychiatric diagnoses. In schizophrenia patients, the Positive and Negative Symptoms Scores (PANSS) were also collected. Eighteen of twenty patients were taking second‐generation antipsychotics, while two were on first‐generation antipsychotics. All were chronic, stable outpatients with a mean duration of illness of 13 years (SD ± 5). Each participant gave written informed consent, and the study was approved by the University of Wisconsin‐Madison Human Subjects Institutional Review Board.
Table 1.
Participants description
| Clinical variables | Healthy comparison subjects (N = 20) | Patients with schizophrenia (N = 20) | P value |
|---|---|---|---|
| Age | 31.7 ± 7.8 (mean ± SD) | 32.85 ± 6.2 (mean ± SD) | P = 0.6 |
| Male/Female | 16/4 | 13/7 | P = 0.3 |
| Positive and Negative Symptoms Scores (PANSS) | — | ||
| Positive | 17.95 ± 6.3 (mean ± SD) | ||
| Negative | 21.50 ± 5.8 (mean ± SD) | ||
| General | 38.7 ± 10.8 (mean ± SD) | ||
| Antipsychotic medications | |||
| Types | Haldol (2); Clozapine (5); Risperidone (4); Olanzapine (3); Quetiapine (3); Aripiprazole(2); Ziprasidone(1) | ||
| Dosea (Mean ± SD) | 314 ± 128.5 |
Doses are expressed as chlorpromazine equivalents.
TMS/hd‐EEG Setup
The superior parietal, pre‐central, superior frontal, and middle frontal gyri, which corresponded to posterior parietal, motor, premotor, and prefrontal areas respectively were anatomically identified on T1‐weighted individual Magnetic Resonance Imaging (MRIs), acquired using a 3 tesla scanner (GE). Cortical areas were targeted employing a Navigated Brain Stimulation system (NBS, Nexstim, Finland), which displayed the position of the TMS coil relative to participants’ brain. Although differences in the orientation of magnetic and electric fields may lead to stimulation of certain areas with TMS while recording the TMS‐evoked response from other cortical areas, the NBS system enabled targeting a given cortical area taking into account the magnetic field properties of the TMS coil used during the TMS/hd‐EEG sessions. Furthermore, we confirmed that in each TMS/hd‐EEG session the peak of TMS‐evoked cortical currents localized to the cortical areas targeted by the TMS (i.e., PFC during TMS of PFC). The NBS also calculated the distance between TMS scalp position and cortical surface: this scalp‐to‐cortex distance was utilized to estimate the TMS‐evoked electric field, expressed in volts per meter (V/m), on the targeted cortical areas. Each area was stimulated at 120 V/m, which in previous TMS/hd‐EEG studies was effective in eliciting EEG oscillations [Ferrarelli et al., 2008, 2012; Rosanova et al., 2009]. Stimulation intensities were also expressed as percentage of maximal stimulator output (MSO) for each targeted cortical area, and did not differ between groups (Table 2). Recordings were performed with a TMS‐compatible 60‐channel amplifier (Nexstim, Finland) [Virtanen et al., 1999]. EEG signals were high‐pass filtered (0.1 Hz), and sampled at 1,450 Hz. Two additional sensors were applied to record eye movements and a sound, which masked the TMS coil click, was played via earphones throughout TMS/hd‐EEG sessions. For additional details, refer to the work by Ferrarelli et al. [2008] and Massimini et al. [2005].
Table 2.
TMS intensities, expressed as percentage of maximal stimulator output (% MSO), for each targeted cortical area did not differ between groups
| TMS‐intensity (% MSO) | Healthy comparison subjects (mean ± SE) | Patients with schizophrenia (mean ± SE) | Statistics (post hoc t‐test) |
|---|---|---|---|
| Prefrontal cortex | 66.9 ± 4.1 | 67 ± 3.6 | P = 0.96 |
| Premotor cortex | 67.5 ± 3.9 | 66.9 ± 3.5 | P = 0.60 |
| Motor cortex | 66.7 ± 4.5 | 66.2 ± 3.5 | P = 0.72 |
| Parietal cortex | 73.0 ± 2.9 | 72.1 ± 4.6 | P = 0.49 |
Experimental Procedure
During the experiment, each participant was sitting on a reclining armchair with a headrest to ensure a firm, comfortable head position. Each TMS/hd‐EEG session consisted of 200–250 TMS stimuli delivered at 0.4–0.6 Hz, according to international safety guidelines [Rossi et al., 2009]. To ensure wakefulness, subjects had their eyes open throughout the TMS/hd‐EEG sessions and looked at a cross displayed on a computer screen. Electrodes position was digitized using the NBS system, to improve accuracy of source modeling analysis of TMS‐evoked EEG responses.
Cognitive Tasks
Each TMS/EEG session lasted 4–6 h. Thus, in order to acquire high quality data while minimizing the burden on our participants cognitive tasks were collected on a different day. Specifically, the cognitive tasks were performed 2–4 weeks after the TMS/hd‐EEG sessions in each participant who agreed to it. It is conceivable that some long‐lasting effects of TMS on cognitive function may have contributed to task performance. However, most of these effects, including performance changes in Working Memory (WM) short‐term retention [Postle et al., 2006], delayed‐match‐to‐sample [Hamidi et al., 2009], as well as letter discrimination tasks [Cattaneo et al., 2009] have been reported either during or shortly after the TMS procedure. Furthermore, long‐lasting effects of TMS on cognition have been observed primarily using multiple sessions of high‐frequency (≥1 Hz) repetitive TMS, as recently reviewed by Luber and Lisanby [2014], whereas in this study we employed low frequency (<0.5 Hz) stimulation during a single TMS session. Additionally, in a recent review of combined TMS/hd‐EEG studies investigating the aftereffects of a single TMS session on a variety of measures including somatosensory, visual, motor potentials as well as cognitive performance, it was found that these effects tended to be relatively short‐lived (<70 min) [Thut and Pascual‐Leone, 2010].
A subset of schizophrenia patients (N = 15) performed the CPWd and the PCET Tests. Healthy participants were also proposed to perform these tasks, but only a small number (N = 4) agreed to it, due to a number of issues (e.g., lack of time availability, limited financial incentive, etc.). CPWd, which assesses episodic word memory, is lateralized to the left hemisphere (the one targeted by TMS), and involves especially PFC. PCET, which measures abstraction in executive function similarly to the Wisconsin Card Sorting Test [Kurtz et al., 2004], is known to implicate PFC, but also engages several other cortical areas. Of note, patients with schizophrenia performed other tasks included in the Penn Computerized Neuropsychological (CNP) Testing [Gur et al., 2001]. However, these two tasks were selected a priori based on the hypothesis that SCD, a measure of PFC activation, would predict performance in CPWd, whereas SCS, which captured the connectivity between PFC and other brain regions, would predict performance in PCET.
EEG Data Analysis
Data analysis was performed using Matlab (The MathWorks), and the toolbox EEGLAB [Delorme et al., 2011]. EEG trials containing eye and/or muscle artifacts were automatically rejected if the Electrooculogram (EOG) exceeded 70 μV (ocular activity), or if absolute power of an EEG frontal channel (F8) exceeded 0.9 μV2 in the beta range (>25 Hz), thus suggesting activity in eye and fronto‐temporal muscles respectively [van de Velde et al., 1998]. Because the EEG system employed had a sample‐and‐hold circuit timed with TMS discharge [Ilmoniemi et al., 1999], we had virtually no decay artifact following the TMS pulse. We also visually reviewed each session and eliminated EEG trials that showed TMS‐related artifacts. The first 20 milliseconds were excluded to avoid a stereotypical, early (0–20 ms) component observed at each stimulation site, which is likely to reflect a generalized, synchronous discharge of cortical excitatory neurons activated by each TMS pulse. Thus, a 20–300 ms time window was chosen to assess group differences. After trial averaging, channels with poor signal or residual artifacts were excluded from additional analyses. Altogether, ≤15% of channels was rejected across all sessions in each participant, and there were no differences in the number of channels excluded between groups. EEG signals from remaining channels were then band‐pass filtered (2–80 Hz), down‐sampled to 725 Hz, and average‐referenced. Analysis at the scalp level has been described and presented elsewhere [Ferrarelli et al., 2012]; thus, here we focused on source modeling analysis.
Source Modeling
The free‐license software SPM was utilized to generate a model of the cortex employing a three‐dimensional grid of 3,004 fixed dipoles, which were oriented normally to the cortical surface. This model, based on the Montreal Neurological Institute (MNI) brain, was adapted to each participant's brain through three steps. First, binary masks of skull and scalp from individual MRIs were warped to corresponding MNI cortical meshes. Second, an inverse transformation from template to individual data was applied to MNI cortical meshes. Finally, EEG sensors and cortical meshes were co‐registered by rigid rotations and translations of digitized anatomical landmarks, which included nasion, left and right tragus. Cortical currents underlying EEG potentials were calculated on single trial applying an “empirical” Bayesian model based on two approaches: Weighted Minimum Norm (WMN) constraint and Gaussian distribution of source covariance along the geodesic distance, with smoothness parameters set to 8 mm [Mattout et al., 2006], in order to enforce correlation among neighboring sources. These parameters were estimated directly from real data by restricted maximum likelihood [Friston et al., 2006; Mattout et al., 2006; Phillips et al., 2005].
Brain sources were calculated at individual cortical meshes (vertices), and corresponding cortical regions (Brodmann areas) were identified using an automatic tool of anatomical classification (WFU Pick Atlas). To assess where and when TMS‐evoked cortical responses were significantly different from pre‐stimulus EEG activity, a statistical procedure was employed. Because a large number (N = 3004) of cortical sources can lead to false positives, a nonparametric, permutation‐based procedure was utilized [Pantazis et al., 2003]. This procedure assumes that, under the null hypothesis of no TMS effects, mean cortical activity calculated by averaging TMS‐evoked EEG responses in the original dataset will not differ from a random permutation of pre‐ and post‐TMS time courses. Thus, for each of those randomly generated datasets, average cortical responses were computed and compared with the average of the original dataset. Responses at each cortical source were normalized by subtracting mean pre‐stimulus current and dividing by the pre‐stimulus variance, thus allowing equal weighting of cortical sources in statistical analysis. Over 1,000 permutations were performed, and significance threshold for multiple comparisons was set at α = 0.01. This allowed the identification of spatial and temporal distribution of TMS‐evoked significant cortical currents. From those currents, SCD and SCS were computed [Casali et al., 2010; Ferrarelli et al., 2010]. SCD was calculated cumulating the absolute amplitude of cortical currents evoked by TMS over a time interval “σ” and for each cortical area “s.” SCS was computed cumulating the geodesic distance between any significant current source and the TMS cortical target over a time range “σ” and for each cortical region “s.” Thus, SCDσ and SCDs reflect, respectively, the topography and the time course of the overall activity evoked by TMS whereas SCSσ and SCSs represent the spatial distribution and temporal modulation of TMS‐evoked connectivity. Finally, SCDsσ is a single value capturing the total currents evoked by TMS in a cortical volume s and time interval σ, and is therefore sensitive to the overall local cortical responsiveness, whereas SCSsσ measures the average distance of significantly activated sources from the site of stimulation. Thus, SCS is more sensitive to the spatial extension of the TMS‐evoked cortical response, while SCD is largely determined by its amplitude, as shown by two recent TMS/hd‐EEG studies in healthy individuals [Casali et al., 2010; Ferrarelli et al., 2010]. SCD is expressed in μA/mm2, while SCS is expressed in millimeters.
Statistical Analysis
The statistical procedure to establish TMS‐evoked significant cortical currents is described above. Group × region repeated measures analyses of variance followed by post hoc, Bonferroni corrected, two‐tailed t‐tests were computed for SCD and SCS values of the four cortical areas. Specifically, given that we had two neurophysiological measures (SCD, SCD) and two cognitive tasks (CPWd, PECT), threshold for significance was set at P < 0.05/4, or P < 0.0125. Furthermore, the magnitude of SCD and SCS deficits in schizophrenia were assessed with the Cohen's d. The Cohen's d, which is calculated as the difference between the means of two groups divided by their pooled standard deviation, provides two types of information: (1) the magnitude (effect size, ES) of the difference between groups (i.e., schizophrenia patients and healthy subjects) in regard to the parameters of interest (i.e., SCD and SCS), and (2) how well these parameters differentiated the two study groups (percent of non‐overlap). We also calculated the Receiver Operating Curves (ROCs) for prefrontal SCD and SCS to assess the sensitivity and specificity of these measures. Finally, Pearson correlation analysis between medication doses, expressed in chlorpromazine equivalents [Andreasen et al., 2010], PANSS scores, PCET, and CPWd performances and the SCS and SCD values after TMS of the PFC were also computed.
RESULTS
TMS‐Related Responsiveness (SCD) of Premotor and Prefrontal Areas is Reduced in Schizophrenia
A group by region repeated measure ANOVA for SCD revealed both a group (F = 4.6, P = 0.04) and a region (F = 14.7, P < 0.0001) effect. Post hoc unpaired t‐test showed that there was no difference in cortical responsiveness, measured with SCD, after TMS of parietal and motor areas between schizophrenia patients and healthy comparison subjects (Table 3, Supporting Information Figs. 1 and 2). By contrast, patients with schizophrenia had decreased SCD values following TMS of premotor area (1.49 ± 0.21.5 vs. 2.41 ± 0.37, P < 0.05), and this reduction was even more dramatic after TMS of the PFC (0.74 ± 0.11 vs. 1.37 ± 0.14, P < 0.005). The topography of evoked cortical currents (SCDσ) showed maximal activation in the frontal areas ipsilateral (left) to the stimulation site in both groups (Fig. 1, top). More specifically, motor, premotor, and prefrontal areas ipsilateral to the stimulation site showed the strongest activation early on (20–50 ms post TMS), whereas afterward (50–100 ms) activity peaked in contralateral (right) frontal/prefrontal areas. Somatosensory, posterior parietal, and temporal bilaterally had the strongest activation in the 100–150 ms interval, while the strongest cortical currents were again in left premotor/prefrontal between 150 and 300 ms in both healthy subjects and patients with schizophrenia. However, the responsiveness of these cortical areas was much diminished in schizophrenia patients, especially in contralateral frontal/prefrontal cortical regions. Similarly, the time course of the evoked cortical activity (SCDs) after TMS of PFC showed that patients with schizophrenia had overall lower amplitude and shorter duration responses compared to normal controls (Fig. 1, middle). Furthermore, SCDσs, a single value index reflecting the overall responsiveness of the PFC was significantly reduced in patients with schizophrenia (Fig. 1, bottom, P < 0.01), and this reduction amounted to a large effect size (ES = 1.21), corresponding to a 63% separation between groups. ROC analysis revealed that prefrontal SCDσs had an area under the curve (AUC) = 0.79, which corresponded to a good sensitivity (75%) and specificity (70%) for patients with schizophrenia compared to healthy controls (Supporting Information Fig. 3).
Table 3.
TMS‐evoked cortical activity (SCD) and connectivity (SCS) in schizophrenia patients and healthy comparison subjects
| TMS‐evoked source modeling parameters | Healthy comparison subjects (mean ± SE) | Patients with schizophrenia (mean ± SE) | Statistics (post hoc t‐test) |
|---|---|---|---|
| Prefrontal cortex | |||
| TMS‐evoked significant current density (SCD, µA/mm2) | 1.42 ± 0.14 | 0.74 ± 0.11 | P < 0.005 |
| TMS‐evoked significant current scattering (SCS, mm) | 2.90 ± 0.4 | 1.11 ± 0.18 | P < 0.001 |
| Premotor cortex | |||
| TMS‐evoked significant current density (SCD, µA/mm2) | 2.41 ± 0.37 | 1.49 ± 0.21.5 | P < 0.05 |
| TMS‐evoked significant current scattering (SCS, mm) | 4.86 ± 0.71 | 2.44 ± 0.45 | P < 0.01 |
| Motor cortex | |||
| TMS‐evoked significant current density (SCD, µA/mm2) | 2.80 ± 0.46 | 2.27 ± 0.45 | P = 0.38 |
| TMS‐evoked significant current scattering (SCS, mm) | 4.92 ± 0.77 | 3.61 ± 1.02 | P = 0.31 |
| Parietal cortex | |||
| TMS‐evoked significant current density (SCD, µA/mm2) | 1.17 ± 0.21 | 0.9 ± 0.17 | P = 0.3 |
| TMS‐evoked significant current scattering (SCS, mm) | 2.29 ± 0.43 | 1.46 ± 0.3 | P = 0.1 |
Figure 1.
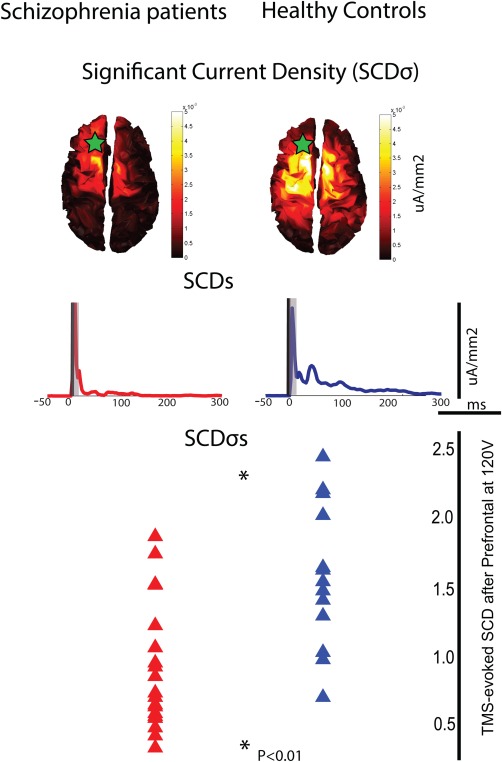
Cortical activity (SCD) evoked by TMS of the PFC is reduced in schizophrenia patients compared to healthy control. Top: Spatial distribution of TMS‐evoked cortical currents cumulated over time (SCDσ). Green star reflects the area targeted with TMS. Middle: Time course of the evoked cortical currents (SCDs). Bottom: Average SCD values were reduced in schizophrenia patients (red) relative to healthy subjects (blue, P = 0.001). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Cortical Connectivity (SCS) of Premotor and Prefrontal Areas is Defective in Schizophrenia
A group by region repeated measure ANOVA for SCS demonstrated both a group (F = 7.6, P = 0.01) and a region (F = 10.8, P < 0.0001) effect. Post hoc t‐test indicated that SCS, a measure of cortical current propagation accounting for the distance of TMS‐evoked cortical activity, was not different between schizophrenia patients and healthy controls after TMS of parietal and motor areas (Table 3, Supporting Information Figs. 1 and 2). Patients with schizophrenia, however, showed reduced connectivity in premotor cortex (4.86 ± 0.71 vs. 2.44 ± 0.45, P < 0.01) as well as in PFC (2.90 ± 0.4 vs. 1.11 ± 0.18, P < 0.001). In healthy subjects, spatial distribution of TMS‐evoked prefrontal currents (SCSσ) consisted of a significant activation in temporal, parietal, frontal, and prefrontal regions, especially on the side contralateral to the stimulation site. By contrast, in schizophrenia patients SCSσ was decreased, especially in contralateral (right) prefrontal, parietal, and temporal areas (Fig. 2, top). SCS reduction in these areas was significant in the 50–300 ms post‐TMS interval. Additionally, the time course of cortical scattering (SCSs) revealed that patients with schizophrenia had reduced connectivity throughout the entire post‐TMS period and that SCSs returned to baseline values within 150 ms, whereas in healthy subjects it lasted at least 300 ms after TMS (Fig. 2, middle). Furthermore SCSσs, a single parameter capturing that spatio‐temporal characteristics of prefrontal cortical connectivity, was markedly decreased in schizophrenia patients compared to healthy controls (Fig. 2, bottom, P < 0.001), with an ES = 1.4, corresponding to 68% separation between the two groups. Prefrontal SCDσs also had an AUC = 0.86, which corresponded to good sensitivity (70%) and excellent specificity (95%) for patients with schizophrenia compared to healthy controls (Supporting Information Fig. 4).
Figure 2.
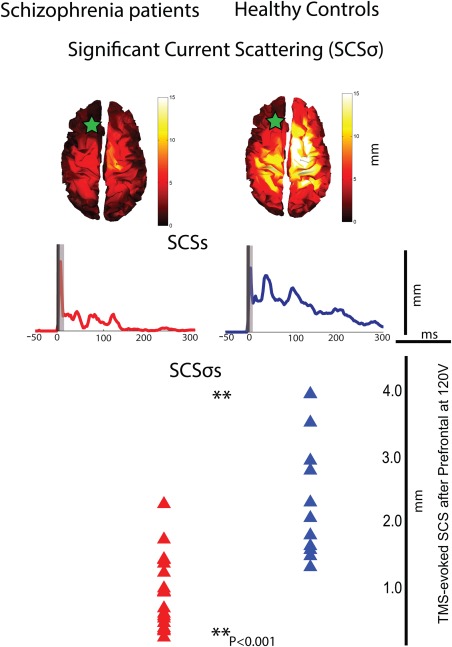
Cortical connectivity (SCS) after TMS of the PFC is decreased in schizophrenia patients compared to healthy control. Top: topographic maps of TMS‐evoked cortical current propagation cumulated over time (SCSσ). Green star reflects the area targeted with TMS. Middle: Time course of the TMS‐evoked cortical current scattering (SCSs). Bottom: Mean SCS values, a synthetic connectivity measure, were markedly reduced in schizophrenia patients (red) compared to healthy subjects (blue, P = 0.0003). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Prefrontal SCD and SCS Deficits Are Not Due to Cortical Gray Matter Atrophy in Schizophrenia
To assess whether SCD and SCS deficits were related to cortical gray matter atrophy, we performed a structural Gray Matter (GM) volume analysis utilizing Voxel‐based Morphometry (VBM), a toolbox available within SPM 8, for both healthy subjects and patients with schizophrenia and found that there were no differences between groups in either whole cortical GM volumes (healthy subjects GM = 626.9 ± 60.3, schizophrenia patients GM = 620.8 ± 70.0; P = 0.8) or left prefrontal GM volumes (healthy subjects GM = 186.5 ± 30, schizophrenia patients GM = 185 ± 20.5; P = 0.85). Furthermore, neither SCS nor SCD measures correlated with GM volumes in patients with schizophrenia (r ≤ 0.45, P ≥ 0.2).
Prefrontal SCDσs and SCSσs Predict Poor Performance in Different Cognitive Tasks in Schizophrenia
We found that prefrontal SCDσs, an index of intrinsic cortical responsiveness, inversely correlated with performance scores in the CPWd, a WM test that implicates primarily the PFC, in schizophrenia patients. Higher SCDσs values were associated with fewer correct trials (r = −0.55, P = 0.03) and increased reaction time (r = −0.5, P = 0.06) in patients with schizophrenia (Fig. 3, top), whereas higher SCSσs, a measure of prefrontal connectivity, predicted poorer performance in the Penn Conditional Exclusion Test. The PCET is an EF task thought to involve the PFC in coordination with other cortical and subcortical structures, and in patients with schizophrenia the numbers of correct trials were inversely related to SCSσs values (r = −0.56, P = 0.03), whereas higher SCSσs values were associated with quicker reaction time (r = −0.47, P = 0.09, Fig. 3, bottom). We also performed correlation analyses within and between the two cognitive tasks as well as between prefrontal SCS/SCD parameters in patients with schizophrenia. We found that performance and reaction time strongly correlated within each cognitive task (r ≥ 0.86), whereas this correlation was moderate (r ≥ 0.4) between tasks. Furthermore, a similar level of correlation (r = 0.53) was present between SCD and SCS values.
Figure 3.
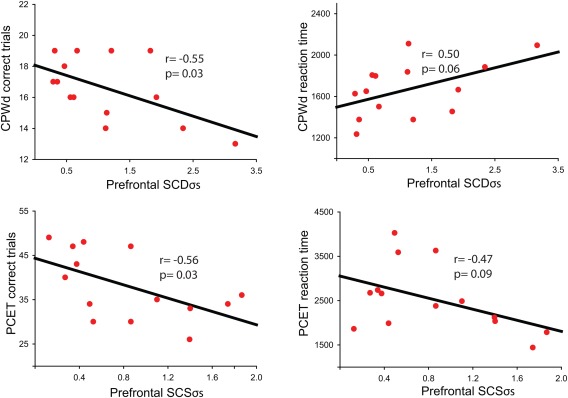
Prefrontal SCDσs and SCSσs predict poor performance in different cognitive tasks in patients with schizophrenia. Top: SCDσs values showed a significant correlation with correct trials and reaction time with a delay working memory task (CPWd) known to engage primarily the PFC. Bottom: SCSσs values correlated with reaction time and performance in an executive function task (PCET) thought to implicate the PFC with other cortical as well as subcortical regions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
SCSσs, But Not SCDσs, is Associated With the Positive Symptoms of Schizophrenia
Correlation analysis between SCSσs, SCDσs, PANSS scores, and antipsychotic medication doses were also performed in patients with schizophrenia. None of these clinical parameters significantly correlated with SCDσs (Supporting Information Table 1). By contrast, SCSσs showed a significant correlation with PANSS positive symptom scores (r = −0.44, P = 0.05).
DISCUSSION
Several neuroimaging studies have examined frontal activity in schizophrenia. Reduced resting cerebral blood flow and regional glucose metabolism, measured by PET and SPECT respectively, was reported in the PFC of schizophrenia patients [Li et al., 2005; Zakzanis and Heinrichs, 1999]. However, recent meta‐analyses found that those deficits amounted to a small‐to‐medium effect size (ES = 0.25–0.55), corresponding to 67–80% overlap between schizophrenia patients and healthy controls [Davidson and Heinrichs, 2003; Hill et al., 2004]. Resting hypofrontality was also reported in a study employing near‐infrared spectroscopy (NIRS), which quantified hemoglobin (Hb) concentrations, but again reduced Hb activity was observed only in a subset (7 of 14) of schizophrenia patients [Hoshi et al., 2006]. Frontal metabolic and blood flow deficiencies were more consistently observed during cognitive tasks in schizophrenia, including Wisconsin Card Sorting Test (WCST) and Continuous Performance Test (CPT), to the extent that average ES of “task‐related” hypofrontality was higher (ES = 0.81) than in resting conditions, yielding a 53% overlap between schizophrenia and healthy subjects [Davidson and Heinrichs, 2003].
Here we found that TMS‐evoked prefrontal cortical currents, assessed with SCD, were markedly reduced in schizophrenia patients compared to controls, and this reduction corresponded to an ES = 1.21, or 63% separation between groups. Compared to traditional event‐related studies employing sensory stimuli, TMS/hd‐EEG allows directly probing frontal regions while measuring TMS‐evoked responses with exquisite temporal and good spatial resolution [Ilmoniemi and Kicic, 2010]. Auditory event‐related studies have shown reduced frontal EEG responses in schizophrenia patients compared to healthy controls [Gallinat et al., 2004; Slewa‐Younan et al., 2004; Uhlhaas et al., 2006]; however, those studies could not determine where those deficits occurred along the neuronal pathway engaged by sensory stimuli, thus providing an ancillary measure of activity in frontal areas. Furthermore, advancements in source modeling analysis enabled a more accurate localization of cortical sources underlying scalp‐recorded EEG potentials. We recently performed a group source modeling analysis of average cortical responses after TMS of premotor cortex, which identified current maxima shifting between premotor and sensorimotor areas [Ferrarelli et al., 2008]. Following the development of an in‐house source modeling algorithm and an improvement in TMS/hd‐EEG procedures, here we performed single‐subject source modeling of EEG responses after TMS of PFC. Patients with schizophrenia had reduced TMS‐evoked cortical currents (SCD) in anterior frontal areas, which were maximal in PFC, thus suggesting intrinsic defects in the activity of prefrontal neuronal networks in schizophrenia. While confounds like psychotic symptoms or fluctuation in attention, which commonly occur in schizophrenia patients at rest, may not be completely discounted during TMS/hd‐EEG, their contribution to SCD deficits is likely not significant, since those events tend to randomly occur in relation to TMS stimuli, thus making their impact on TMS‐evoked responses minimal. Finally, by performing TMS/hd‐EEG recordings of four cortical areas and calculating their SCD, we established that schizophrenia patients had a relatively intact response in parietal and motor cortices, whereas premotor/prefrontal SCD were significantly reduced, consistent with the implication of anterior frontal regions in the neurobiology of schizophrenia [Saugstad, 2008; Woo, 2014].
We also established that schizophrenia patients had marked reductions in SCS, a single value connectivity measure after TMS of premotor and prefrontal areas. Prefrontal SCSσs, an index capturing the spatio‐temporal propagation of TMS‐evoked cortical currents, was the parameter showing the largest reduction in patients with schizophrenia, with an ES of 1.4, or 68% separation between groups. SCSσs also had good sensitivity and excellent specificity for patients with schizophrenia compared to healthy controls, as assessed by ROC analysis (Supporting Information Fig. 4). Schizophrenia has been conceptualized as a disorder of altered brain communication since this term was introduced by Bleuler [Heckers, 2011]. Converging evidence from neuroimaging and neurophysiological studies suggest abnormalities in neuronal networks implicating PFC in schizophrenia [Stephan et al., 2009]. Resting‐state fMRI studies showed altered connectivity within prefrontal‐parietal networks in schizophrenia [Rotarska‐Jagiela et al., 2010; Skudlarski et al., 2010; Zhou et al., 2007], which correlated with clinical symptoms severity [Rotarska‐Jagiela et al., 2010; Skudlarski et al., 2010]. Neurophysiologic studies found that schizophrenia patients had shorter lasting fronto‐parietal EEG microstates [Nishida et al., 2013] and reduced fronto‐parietal resting‐state MEG connectivity [Hinkley et al., 2011]. All those findings indicate abnormalities in the functional connectivity of frontal areas in schizophrenia, since they were based on temporal correlations of vascular (Blood‐oxygen‐level dependent, BOLD) or neuronal (EEG/MEG) activities across different brain regions. Employing TMS/hd‐EEG allows measuring effective connectivity, which is the ability of a cortical area to causally affect the activity of other brain regions [Lee et al., 2003], thus assessing the directionality and causality of changes in activation. We can therefore assume that deficits in TMS‐evoked connectivity reflect a reduced ability of frontal areas, and especially PFC, to effectively communicate with other cortical areas. Nonetheless, additional studies are necessary to better establish the biological underpinnings of SCS, including the specific connectivity patterns that are defective in patients with schizophrenia.
A global reduction in gray matter (GM), including the PFC, has been reported by several neuroanatomical studies in schizophrenia [Ellison‐Wright and Bullmore, 2010; Glahn et al., 2008]. We therefore performed a VBM analysis to establish whether patients with schizophrenia had smaller cortical volumes, especially in the left PFC. We found that schizophrenia patients had reduced whole brain as well as PFC GM volumes, suggesting a degree of atrophy. However, this decrease failed to reach significance, and prefrontal GM volume did not correlate with either SCS or SCD values, thus suggesting that cortical atrophy is unlikely to account for the SCD/SCS deficits in schizophrenia.
In healthy humans, PFC contributes to several aspects of cognition, including abstraction [Gray et al., 2003], problem solving [Colom et al., 2010], and manipulation of verbal material [Murphy et al., 2007]. Deficits in these cognitive functions have been consistently reported in schizophrenia [Lett et al., 2014]. Furthermore, a recent meta‐analysis of 41 neuroimaging studies found that patients with schizophrenia had decreased activation in PFC and posterior parietal areas compared to healthy controls across several cognitive domains [Minzenberg et al., 2009]. Here we established that TMS‐evoked prefrontal activity (SCD) and connectivity (SCS) specifically predicted impaired performances in two different tasks, CPWd and PCET, in schizophrenia patients. CPWd is a delayed word memory task, and an fMRI study reported that schizophrenia patients had decreased activation in left PFC during the recognition phase of this task, whereas contralateral prefrontal activation was preserved [Ragland et al., 2004]. PCET is a measure of abstraction in EF closely related to the Wisconsin Card Sorting Test (WCST) [Kurtz et al., 2004]. Reduced performance on WCST, associated with poor illness insight, has been consistently reported in schizophrenia, and neuroimaging studies employing PET, MRS, and fMRI demonstrated abnormal activation in a brain network, including PFC, parietal, and temporal cortices during WCST in schizophrenia patients compared to healthy controls [Bertolino et al., 2000; Ragland et al., 2007]. Here we found a similar reduction in prefrontal‐parietal and prefrontal‐temporal connectivity (SCS) following TMS of PFC. We also established that SCS, rather than SCD, predicted an impaired performance in schizophrenia patients in PCET, a task showing convergent validity with WCST [Kurtz et al., 2004].
We found a highly counterintuitive inverse relationship between SCD and SCS with performance in these cognitive tasks in schizophrenia patients. The direction of the correlations between cognitive measures and neural indices was exactly in the opposite direction of what it was expected. Specifically, if reduced TMS‐evoked activity (SCD) and connectivity (SCS) of PFC in patients with schizophrenia compared to healthy controls suggest intrinsic defects in prefrontal cortical neurons, it was anticipated that higher, rather than lower SCD and SCS values would predict better cognitive performance. One possible, although speculative, explanation for these contradictory findings is that not only do patients with schizophrenia have an overall reduced ability to engage PFC, but this activation is also abnormal and leads to poorer cognitive function. Intriguingly, a recent neuroimaging study reported that higher fractional amplitude of low‐frequency fluctuations (fALFF) reflected more intense regional spontaneous brain activity, that healthy controls had higher fALFF values compared to patients with schizophrenia, and that higher fALFF of DLPFC were associated with worse cognitive performance in schizophrenia patients [Sui et al., 2015]. Although these data would appear to be consistent with our findings, at this stage this interpretation is clearly tentative, and the results will have to be validated and any interpretation will have to be properly tested in future studies.
Only a small group (N = 4) of healthy controls agreed to perform the cognitive tasks, due to a number of issues (e.g., lack of time availability, limited financial incentive, etc.). Thus, cognitive performance in patients with schizophrenia was compared with normative data from healthy comparison subjects using a large cohort available at Penn Computerized Neuropsychological (CNP) Testing [Gur et al., 2001]. Although this choice allowed establishing the presence of an impaired performance in these tasks in patients with schizophrenia, we acknowledge that investigating such correlations in healthy subjects could help clarify the link between these neurophysiological measures and cognitive performance. Specifically, we anticipate a direct, positive correlation between SCD/SCS values and performance in these cognitive tasks in the general population, as suggested by several fMRI studies showing that for both word memory and executive function tasks higher level of PFC activity and/or connectivity are associated with better performance in healthy individuals [Ragland et al., 2004; Wolf et al., 2007]. Furthermore, future studies should balance pre‐ and post‐TMS cognitive testing across healthy subjects and psychiatric patients to further minimize any TMS effect on cognitive performance.
The presence of confounds like levels of attention or lack of motivation often makes it difficult to establish whether an impaired performance in a cognitive task actually reflect a deficit in underlying neuronal circuits. We think that the neurophysiological measures presented in this study, SCD and SCS, were minimally affected by these confounds for two reasons. First, SCD and SCS were collected at rest, without requiring any conscious effort from the participant, and therefore they were unlikely to be significantly affected by the level of cognitive engagement or by motivation. Second, even if patients with schizophrenia were experiencing fluctuation in levels of attention or lack of motivation while performing their cognitive tasks, this should have resulted in a generalized deficit in performance, which would not explain why SCS and SCD would specifically predict performance in the PCET and CPWd task respectively.
We also established strong correlations (r ≥ 0.86) between performance and reaction time for both CPWd and PCET, whereas the between tasks correlations were moderate (r ≥ 0.4). Notably, a similar level of correlation (r = 0.53) was found between SCD and SCS values, thus suggesting that these cognitive and neurophysiological measures share some characteristics (i.e., both SCD and SCS involve PFC), but also partially differ in their mechanisms and underlying neuronal circuits. Future work is needed to further investigate these aspects, including correlating SCS and SCD values with fMRI activation during these tasks as well as observing whether prefrontal SCD/SCD specifically predict performance scores in cognitive tests involving PFC.
Reduced prefrontal SCS values were also associated with positive symptoms in these patients. Notably, a recent resting state fMRI study in schizophrenia patients, bipolar patients, and healthy comparisons reported functional connectivity abnormalities in a prefronto‐parietal network, which were specific for schizophrenia patients and correlated with their PANSS positive symptoms [Khadka et al., 2013]. The present results confirm these findings, and also suggest that an ineffective communication of PFC with temporal/parietal areas may underlie some of the symptoms commonly experienced by these patients.
Schizophrenia patients were medicated at the time of the recordings, and it has been shown that changes in cortical excitability may be related to antipsychotic treatment in schizophrenia [Daskalakis et al., 2002]. However, these studies assessed motor cortex activity, whereas we found no differences in the motor SCS or SCD in schizophrenia patients. Furthermore, neuroimaging studies establishing abnormal prefrontal activity in schizophrenia during cognitive tasks reported comparable deficits when patients were on and off medications [Ragland et al., 2007]. We also found no correlation between medication doses and SCD or SCS in schizophrenia patients. Nonetheless, additional studies are needed to confirm the present results in larger groups of patients, including medication‐free patients with schizophrenia. Another issue that should be addressed in future work concerns illness specificity. For example, are these neurophysiological and cognitive deficits present exclusively in patients with schizophrenia, or do they rather reflect a functional impairment observable in other severe psychiatric disorders? Collecting these measures in other psychiatric populations, such as patients with bipolar disorder, will help answer this question. Nonetheless, in the present study we found that prefrontal SCD had excellent specificity regarding the presence of absence of schizophrenia, with about a 95% chance of correctly identifying healthy subjects when compared to patients with schizophrenia.
It would also be important to establish whether prefrontal SCD and SCS impairments are observed in first‐break and in individuals at high‐risk for psychosis. The presence of these abnormalities at illness onset would further support their involvement in the neurobiology of schizophrenia and may contribute to the identification of individuals who will eventually experience a psychotic break. Additionally, performing TMS/hd‐EEG and TMS/fMRI in the same group of patients would help to clarify the involvement of subcortical structures in the deficits observed here. Source modeling analysis allows localization of cortical sources of scalp‐recorded EEG potentials, but it is unable to establish the contribution of subcortical regions, including the thalamus, to those EEG responses. Notably, we recently employed TMS/fMRI in schizophrenia patients and found that they had reduced thalamic and anterior frontal activation following direct perturbation with TMS of the frontal cortex, which was combined with weaker thalamus–superior frontal cortex connectivity [Guller et al., 2012]. Finally, improvement in SCD or SCS values may be associated to effective clinical interventions targeting cognitive deficits in schizophrenia, including cognitive remediation therapy [Wykes et al., 2011], which could contribute to identify the neuronal mechanisms differentiating treatment responders from non‐responders.
CONCLUSION
In this study, we performed source modeling analysis of TMS‐evoked brain responses in the parietal, motor, premotor, and prefrontal areas of healthy subjects and schizophrenia patients and computed several measures of evoked cortical activity (SCD) and connectivity (SCS). Patients with schizophrenia also performed two episodic verbal memory/executive function tasks, the CPWd and the PCET. We found no difference in SCD and SCS values after TMS of parietal and motor cortex between the two groups, whereas both parameters were significantly reduced in both premotor and prefrontal areas of schizophrenia patients. Additionally in the PFC, where those indices were most defective, SCD predicted an impaired performance in the CPWd task whereas SCS was associated with shorter reaction time and more errors in the PCET. Altogether, these findings point to intrinsic defects in the activity and connectivity of the PFC, which are associated with impairments in distinct, specific cognitive abilities in patients with schizophrenia.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
Authors would like to thank Dr. Casali for his contribution in developing the source modeling analysis here employed as well as Drs. Massimini and Sarasso for their helpful feedback during the preparation of the manuscript. They also thank the patients who agreed to participate to the study.
Conflict of interest: Dr. Peterson has received research grant support from Sanofi‐Aventis. Dr. Tononi has served as a consultant for Tikvah Therapeutics and Respironics; he has received speaker's honoraria from Sanofi‐Aventis and Respironics; he has received research support from Sanofi‐Aventis as well as from Respironics (Philips). Drs. Riedner, and Ferrarelli report no financial relationships with commercial interests.
REFERENCES
- American Psychiatric Association, American Psychiatric Association. Task Force on DSM‐IV (2000): Diagnostic and Statistical Manual of Mental Disorders: DSM‐IV‐TR. Washington, DC: American Psychiatric Association; xxxvii, 943 p. [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC (2010): Antipsychotic dose equivalents and dose‐years: A standardized method for comparing exposure to different drugs. Biol Psychiatry 67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlati S, Deste G, De Peri L, Ariu C, Vita A (2013): Cognitive remediation in schizophrenia: Current status and future perspectives. Schizophr Res Treatment 2013:156084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MS, Farzan F, Arenovich T, Chen R, Fitzgerald PB, Daskalakis ZJ (2011): The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PLoS One 6:e22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Esposito G, Callicott JH, Mattay VS, Van Horn JD, Frank JA, Berman KF, Weinberger DR (2000): Specific relationship between prefrontal neuronal N‐acetylaspartate and activation of the working memory cortical network in schizophrenia. Am J Psychiatry 157:26–33. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD (2006): Determinants of real‐world functional performance in schizophrenia subjects: Correlations with cognition, functional capacity, and symptoms. Am J Psychiatry 163:418–425. [DOI] [PubMed] [Google Scholar]
- Casali AG, Casarotto S, Rosanova M, Mariotti M, Massimini M (2010): General indices to characterize the electrical response of the cerebral cortex to TMS. Neuroimage 49:1459–1468. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Rota F, Walsh V, Vecchi T, Silvanto J (2009): TMS‐adaptation reveals abstract letter selectivity in the left posterior parietal cortex. Cereb Cortex 19:2321–2325. [DOI] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS (2006): Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA 103:19878–19883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Karama S, Jung RE, Haier RJ (2010): Human intelligence and brain networks. Dialogues Clin Neurosci 12:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S (2002): Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry 59:347–354. [DOI] [PubMed] [Google Scholar]
- Davidson LL, Heinrichs RW (2003): Quantification of frontal and temporal lobe brain‐imaging findings in schizophrenia: A meta‐analysis. Psychiatry Res 122:69–87. [DOI] [PubMed] [Google Scholar]
- Delorme A, Mullen T, Kothe C, Akalin Acar Z, Bigdely‐Shamlo N, Vankov A, Makeig S (2011): EEGLAB, SIFT, NFT, BCILAB, and ERICA: New tools for advanced EEG processing. Comput Intell Neurosci 2011:130714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison‐Wright I, Bullmore E (2010): Anatomy of bipolar disorder and schizophrenia: A meta‐analysis. Schizophr Res 117:1–12. [DOI] [PubMed] [Google Scholar]
- Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, Daskalakis ZJ (2010): Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain 133:1505–1514. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, Riedner BA, Lazar M, Murphy MJ, Huber R, Rosanova M, Alexander AL, Kalin N, Tononi G (2008): Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: A TMS/EEG study. Am J Psychiatry 165:996–1005. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, Tononi G, Pearce RA (2010): Breakdown in cortical effective connectivity during midazolam‐induced loss of consciousness. Proc Natl Acad Sci USA 107:2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Sarasso S, Guller Y, Riedner BA, Peterson MJ, Bellesi M, Massimini M, Postle BR, Tononi G (2012): Reduced natural oscillatory frequency of frontal thalamocortical circuits in schizophrenia. Arch Gen Psychiatry 69:766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantseva M, Cui J, Farzan F, Chinta LV, Perez Velazquez JL, Daskalakis ZJ (2014): Disrupted cortical conductivity in schizophrenia: TMS‐EEG study. Cereb Cortex 24:211–221. [DOI] [PubMed] [Google Scholar]
- Friston K, Henson R, Phillips C, Mattout J (2006): Bayesian estimation of evoked and induced responses. Hum Brain Mapp 27:722–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D (2004): Reduced oscillatory gamma‐band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol 115:1863–1874. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison‐Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT (2008): Meta‐analysis of gray matter anomalies in schizophrenia: Application of anatomic likelihood estimation and network analysis. Biol Psychiatry 64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS (2003): Neural mechanisms of general fluid intelligence. Nat Neurosci 6:316–322. [DOI] [PubMed] [Google Scholar]
- Guller Y, Ferrarelli F, Shackman AJ, Sarasso S, Peterson MJ, Langheim FJ, Meyerand ME, Tononi G, Postle BR (2012): Probing thalamic integrity in schizophrenia using concurrent transcranial magnetic stimulation and functional magnetic resonance imaging. Arch Gen Psychiatry 69:662–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE (2001): Computerized neurocognitive scanning. I. Methodology and validation in healthy people. Neuropsychopharmacology 25:766–776. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Tononi G, Postle BR (2009): Evaluating the role of prefrontal and parietal cortices in memory‐guided response with repetitive transcranial magnetic stimulation. Neuropsychologia 47:295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S (2011): Bleuler and the neurobiology of schizophrenia. Schizophr Bull 37:1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Mann L, Laws KR, Stephenson CM, Nimmo‐Smith I, McKenna PJ (2004): Hypofrontality in schizophrenia: A meta‐analysis of functional imaging studies. Acta Psychiatr Scand 110:243–256. [DOI] [PubMed] [Google Scholar]
- Hinkley LB, Vinogradov S, Guggisberg AG, Fisher M, Findlay AM, Nagarajan SS (2011): Clinical symptoms and alpha band resting‐state functional connectivity imaging in patients with schizophrenia: Implications for novel approaches to treatment. Biol Psychiatry 70:1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi Y, Shinba T, Sato C, Doi N (2006): Resting hypofrontality in schizophrenia: A study using near‐infrared time‐resolved spectroscopy. Schizophr Res 84:411–420. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Kicic D (2010): Methodology for combined TMS and EEG. Brain Topogr 22:233–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Ruohonen J, Karhu J (1999): Transcranial magnetic stimulation—A new tool for functional imaging of the brain. Crit Rev Biomed Eng 27:241–284. [PubMed] [Google Scholar]
- Kalkstein S, Hurford I, Gur RC (2010): Neurocognition in schizophrenia. Curr Top Behav Neurosci 4:373–390. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Harvey PD (2012): Cognitive impairment in schizophrenia. Handb Exp Pharmacol 213:11–37. [DOI] [PubMed] [Google Scholar]
- Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA, Tamminga CA, Keshavan MS, O'Neil K, Schretlen D, Pearlson GD (2013): Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry 74:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Wexler BE, Bell MD (2004): The Penn Conditional Exclusion Test (PCET): Relationship to the Wisconsin Card Sorting Test and work function in patients with schizophrenia. Schizophr Res 68:95–102. [DOI] [PubMed] [Google Scholar]
- Lee L, Harrison LM, Mechelli A (2003): A report of the functional connectivity workshop, Dusseldorf 2002. Neuroimage 19:457–465. [DOI] [PubMed] [Google Scholar]
- Lett TA, Voineskos AN, Kennedy JL, Levine B, Daskalakis ZJ (2014): Treating working memory deficits in schizophrenia: A review of the neurobiology. Biol Psychiatry 75:361–370. [DOI] [PubMed] [Google Scholar]
- Li X, Tang J, Wu Z, Zhao G, Liu C, George MS (2005): SPECT study of Chinese schizophrenic patients suggests that cerebral hypoperfusion and laterality exist in different ethnic groups. World J Biol Psychiatry 6:98–106. [DOI] [PubMed] [Google Scholar]
- Luber B, Lisanby SH (2014): Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS). Neuroimage 85 (Part 3):961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G (2005): Breakdown of cortical effective connectivity during sleep. Science 309:2228–2232. [DOI] [PubMed] [Google Scholar]
- Mattout J, Phillips C, Penny WD, Rugg MD, Friston KJ (2006): MEG source localization under multiple constraints: An extended Bayesian framework. Neuroimage 30:753–767. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Freitas C, Oberman L, Lisanby SH, Pascual‐Leone A (2011): Transcranial magnetic stimulation: A neuroscientific probe of cortical function in schizophrenia. Biol Psychiatry 70:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009): Meta‐analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Firl AJ, Yoon JH, Gomes GC, Reinking C, Carter CS (2010): Gamma oscillatory power is impaired during cognitive control independent of medication status in first‐episode schizophrenia. Neuropsychopharmacology 35:2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KJ, West R, Armilio ML, Craik FI, Stuss DT (2007): Word‐list‐learning performance in younger and older adults: Intra‐individual performance variability and false memory. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14:70–94. [DOI] [PubMed] [Google Scholar]
- Nishida K, Morishima Y, Yoshimura M, Isotani T, Irisawa S, Jann K, Dierks T, Strik W, Kinoshita T, Koenig T (2013): EEG microstates associated with salience and frontoparietal networks in frontotemporal dementia, schizophrenia and Alzheimer's disease. Clin Neurophysiol 124:1106–1114. [DOI] [PubMed] [Google Scholar]
- Pantazis D, Nichols TE, Baillet S, Leahy RM (2003): Spatiotemporal localization of significant activation in MEG using permutation tests. Inf Process Med Imaging, 18:512–523. [DOI] [PubMed] [Google Scholar]
- Phillips C, Mattout J, Rugg MD, Maquet P, Friston KJ (2005): An empirical Bayesian solution to the source reconstruction problem in EEG. Neuroimage 24:997–1011. [DOI] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson M, Alexander A, Tononi G (2006): Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in the prefrontal, but not posterior parietal, cortex. J Cogn Neurosci 18:1712–1722. [DOI] [PubMed] [Google Scholar]
- Radhu, N , Garcia Dominguez, L , Farzan, F , Richter, MA , Semeralul, MO , Chen, R , Fitzgerald, PB , Daskalakis, ZJ (2015): Evidence for inhibitory deficits in the prefrontal cortex in schizophrenia. Brain 138(Pt 2):483–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE (2004): Event‐related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry 161:1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Yoon J, Minzenberg MJ, Carter CS (2007): Neuroimaging of cognitive disability in schizophrenia: Search for a pathophysiological mechanism. Int Rev Psychiatry 19:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M (2009): Natural frequencies of human corticothalamic circuits. J Neurosci 29:7679–7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual‐Leone A (2009): Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotarska‐Jagiela A, van de Ven V, Oertel‐Knochel V, Uhlhaas PJ, Vogeley K, Linden DE (2010): Resting‐state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res 117:21–30. [DOI] [PubMed] [Google Scholar]
- Saugstad LF (2008): What is a psychosis and where is it located? Eur Arch Psychiatr Clin Neurosci 258 (Suppl 2):111–117. [DOI] [PubMed] [Google Scholar]
- Schaefer J, Giangrande E, Weinberger DR, Dickinson D (2013): The global cognitive impairment in schizophrenia: Consistent over decades and around the world. Schizophr Res 150:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, Pearlson G (2010): Brain connectivity is not only lower but different in schizophrenia: A combined anatomical and functional approach. Biol Psychiatry 68:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slewa‐Younan S, Gordon E, Harris AW, Haig AR, Brown KJ, Flor‐Henry P, Williams LM (2004): Sex differences in functional connectivity in first‐episode and chronic schizophrenia patients. Am J Psychiatry 161:1595–1602. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD (2009): Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self‐monitoring. Schizophr Bull 35:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Pearlson GD, Du Y, Yu Q, Jones TR, Chen J, Jiang T, Bustillo J, Calhoun VD: In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia Biol Psychiatry 2015 Feb. 24. [DOI] [PMC free article] [PubMed]
- Sun Y, Farzan F, Barr MS, Kirihara K, Fitzgerald PB, Light GA, Daskalakis ZJ (2011): Gamma oscillations in schizophrenia: Mechanisms and clinical significance. Brain Res 1413:98–114. [DOI] [PubMed] [Google Scholar]
- Thut G, Pascual‐Leone A (2010): A review of combined TMS‐EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr 22:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W (2013): High‐frequency oscillations and the neurobiology of schizophrenia. Dialogues Clin Neurosci 15:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Linden DE, Singer W, Haenschel C, Lindner M, Maurer K, Rodriguez E (2006): Dysfunctional long‐range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci 26:8168–8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Velde M, van Erp G, Cluitmans PJ (1998): Detection of muscle artefact in the normal human awake EEG. Electroencephalogr Clin Neurophysiol 107:149–158. [DOI] [PubMed] [Google Scholar]
- Virtanen J, Ruohonen J, Naatanen R, Ilmoniemi RJ (1999): Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation. Med Biol Eng Comput 37:322–326. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Gur RC, Valdez JN, Loughead J, Elliott MA, Gur RE, Ragland JD (2007): Alterations of fronto‐temporal connectivity during word encoding in schizophrenia. Psychiatr Res 154:221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU (2014): Neurobiology of schizophrenia onset. Curr Top Behav Neurosci 16:267–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P (2011): A meta‐analysis of cognitive remediation for schizophrenia: Methodology and effect sizes. Am J Psychiatry 168:472–485. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Heinrichs RW (1999): Schizophrenia and the frontal brain: A quantitative review. J Int Neuropsychol Soc 5:556–566. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F (2007): Functional dysconnectivity of the dorsolateral prefrontal cortex in first‐episode schizophrenia using resting‐state fMRI. Neurosci Lett 417:297–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


