Abstract
The feeling of illusory movement is considered important in the study of human behavior because it is deeply related to motor consciousness. However, the neural basis underlying the illusion of movement remains to be understood. Following optimal vibratory stimulation of muscle tendon, certain subjects experience illusory movements while others do not. In the present fMRI study, we sought to uncover the neural basis of illusory movement awareness by contrasting a posteriori these two types of subjects. Examining fMRI data using leave‐one‐subject‐out general linear models and region of interest analyses, we found that a non‐limb‐specific associative network, including the opercular part of the right inferior frontal gyrus and the right inferior parietal lobule, was more active in subjects with illusions. On the other hand, levels of activation in other brain areas involved in kinaesthetic processing were rather similar between the two subsamples of subjects. These results suggest that activation of the right inferior frontoparietal areas, once passed a certain threshold, forms the basis of illusory movements. This is consistent with the global neuronal workspace hypothesis that associates conscious processing with surges of frontoparietal activity. Hum Brain Mapp 35:5166–5178, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: kinaesthesia, illusory movements, consciousness, fMRI, leave one subject out procedure
INTRODUCTION
Stimulus integration gives rise to a complex pattern of brain activity, reflecting different stages of information processing. While some of these stages involve conscious processing, meaning that information is consciously accessed and forms a perceptual representation that is reportable, others do not elicit consciousness. Using visual, auditory or tactile paradigms contrasting conscious and nonconscious stimuli, functional magnetic resonance imaging (fMRI) studies have identified a core neural correlate that contributes to conscious processing [Boly et al., 2007; Dehaene et al., 2006, 2001; Sadaghiani et al., 2009]. These studies reported that activation is first amplified in task‐specific sensory areas responsible for nonconscious perception and then spreads to a higher‐level large‐scale prefrontal‐parietal network. Moreover, it was demonstrated that this correlate, together with other mechanisms of the emergence of consciousness revealed using time‐resolved imaging methods [see Dehaene and Changeux, 2011, for review], originate in a set of cortical “workspace” neurons with long‐range axons, which allows parietal and prefrontal cortices to exchange information [Dehaene and Changeux, 2005].
Such a large‐scale prefrontal‐parietal network may also mediate illusory limb movements, thus subtending motor consciousness. Previous studies that used tendon vibration paradigm to generate movement illusion through activation of muscle spindle afferents reported activation of limb‐specific regions of the somatosensory and motor cortices contralateral to the site of stimulation, as well as non‐limb‐specific right‐sided frontoparietal brain regions, especially Brodmann areas (BAs) 44 and 45 and the intraparietal and parietal opercular regions [Kavounoudias et al., 2008; Naito et al., 1999, 2002, 2005, 2007; Radovanovic et al., 2002; Romaiguère et al., 2003]. An explanation for the activation of right‐sided frontoparietal sites irrespective of illusory movements of right or left limbs may be that they serve as a gateway to the feeling of movement. Data from an experiment using electrical stimulation in patients undergoing awake brain surgery are in favor of this hypothesis [Desmurget and Sirigu, 2009; Desmurget et al., 2009]. While stimulation of the posterior parietal cortex (BAs 39 and 40) caused conscious limb movement intention, increasing the intensity of the stimulation triggered illusory movements. Therefore, motor consciousness arises from stimulation of the posterior parietal cortex. Furthermore, an amplification of activation in this region replaces conscious intention to produce a movement by illusory movement awareness, likely recruiting the executive network responsible for forward computations located in the posterior parietal cortex [Blakemore and Frith, 2003; Daprati et al., 2010]. However, there is no clue yet that the aforementioned non‐limb‐specific right‐lateralised frontal regions related to illusory movements (e.g., right hemisphere BAs 44 and 45) also mediates motor consciousness.
Therefore, the aim of the present study was to examine whether a main neural correlate of conscious processing evidenced in studies on (un)conscious perception, namely a surge of activation in parietal and frontal areas, is also associated with illusory movement awareness. A few studies on proprioceptive integration evoked by tendon vibration reported that not all individuals experience illusory movements despite optimal stimulation of muscle spindle receptors [Goble et al., 2011, 2012]. Accordingly, we divided subjects based on whether they experienced kinaesthetic illusory movement during a tendon vibration paradigm. Given the dominance of the right hemisphere in the awareness of illusory limb movement, we expected that a right‐sided frontoparietal network, i.e. right posterior parietal and inferior frontal cortices, would be more active in subjects having experienced illusory movements as compared to those who did not.
MATERIALS AND METHODS
Subjects, Paradigm, and fMRI Data Acquisition
Eighteen healthy right‐handed normal‐hearing subjects took part in the experiment (mean age ± SD: 32 ± 4 years; nine females). All the subjects were naive as to the purpose of the study and gave informed consent. The study was approved by the research ethics committee CPP Sud‐Méditerranée 1.
Subjects underwent a muscle tendon vibration paradigm, as previously used in fMRI studies on kinaesthetic processing [Goble et al., 2011a,b; Naito et al., 1999, 2002, 2005, 2007; Romaiguère et al., 2003]. Vibrating the tendon of a limb's muscle excites mainly the muscle spindle primary endings, whose information is processed by the brain so that individuals may experience kinaesthetic illusory movement in the absence of actual movement [Roll and Vedel, 1982; Roll et al., 1989]. Specifically, the subjects were placed head first and supine into a 3‐T fMRI scanner (Medspec 30/80 AVANCE, Bruker, Ettlingen, Germany) with arms resting at the body sides and were asked to keep their eyes closed throughout testing. A soft strap was attached around the head to minimize head movement and the lower limbs were supported in a bent position at the knee, so that feet did not rest on the bed. The subjects also removed their socks and turned up the bottom of their pants. Custom‐made pneumatic vibration devices, driven by constant air pressure, were placed perpendicular to the right and left tendons of the tibialis anterior muscles using elastic straps (contact area = ∼6 cm2). The amplitude of the vibration stimulus was about 0.5 mm and the vibration frequency of either 30 Hz or 100 Hz, leading to four vibration conditions: right and left tendon vibration at 30 Hz (R30 and L30) and 100 Hz (R100 and L100). These stimulation parameters were selected based on the fact that (i) 20–40 Hz frequencies drive weak discharges of the primary endings, which are not likely to elicit kinaesthetic illusions, and (ii) ∼100 Hz frequency optimally activates primary endings, generally providing consistent illusory movements [Naito et al., 1999; Radovanovic et al., 2002; Roll and Vedel, 1982; Roll et al., 1989].
Importantly, the perceptual effects of the vibration conditions were assessed just before the subject was entered into the scanner. In this pre‐scanning session, the subject experienced the R30, L30, R100, and L100 conditions with the eyes closed. The vibration conditions were followed by rest periods. Subjects experienced 12 vibratory stimulations in total (each condition having been repeated twice), presented in a randomized order. During each rest period, the subjects were questioned as to whether the stimulation generated illusory movements and had to verbally describe them. Among the fifteen subjects included at first, nine felt systematic illusory movement in the L100 and R100 conditions, reporting ankle plantar‐flexion movement, whereas the six other subjects did not experience illusions in any of the conditions. However, given the fact that unequal sample sizes may have biased comparison results between subjects with and without illusions, more subjects were afterwards tested in the pre‐scanning session until we identified three further subjects insensitive to the illusions in the 100 Hz conditions. Accordingly, nine subjects sensitive to illusions (mean age ± SD: 33.8 ± 4.9 years; three females) and nine subjects insensitive to illusions (mean age ± SD: 31.1 ± 3.5 years; six females) were finally retained for the scanning session, so that the experiment was balanced. Besides, the two subsamples did not differ with respect to age (P = 0.19, two‐tailed Mann–Whitney U test) and gender (P = 0.35, two‐tailed Fisher's exact test), meaning that demographics was not a confounding factor that could have influenced neuroimaging differences between the two subsamples. The vibration devices were triggered via custom software developed using LabVIEW (National Instruments, Austin, TX).
The scanning session was composed of five runs including 12‐s long conditions (epochs) of vibration (R30, L30, R100, L100) and REST (i.e., no vibration). Each vibration condition was repeated three times per run. The order of vibration conditions were randomized within a run and REST epochs were inserted between all vibration conditions to ensure relaxation of muscle spindles. Moreover, REST epochs were followed by a variable delay (mean ISI: 1,000 ms) obtained from exponential distribution [Hagberg et al., 2001], to ensure random subsampling of the brain volume relative to each of the vibration conditions. fMRI time series were acquired over the five runs with a T2*‐weighted gradient echo‐planar imaging sequence (42 interleaved axial slices acquisition; 3 mm thickness; 0.5 mm interslice gap; reco matrix = 64 × 64; field of view = 192 mm × 192 mm; repetition time = 2.8 s; echo time = 30 ms; flip angle = 84°). The scanning planes were parallel to the anterior commissure − posterior commissure and covered the whole brain from the top of the cortex down to the base of the cerebellum. At the end of each run, questions on the illusory movements were asked to the subjects via headphones. For each stimulation condition, we asked whether it induced illusory movements, and if yes, whether all three repetitions (each stimulation condition having been repeated three times within a run) induced illusory movements. Subjects answered the questions using a finger button response system. Answers confirmed the two subsamples of subjects that came out during the prescanning session. Nine subjects reported consistent illusions for the R100 and L100 stimulations in all runs while the other nine subjects did not experience any illusions. We preferred asking the subjects to report the presence of illusions after each run instead of after each stimulation to avoid methodological issues related to movement preparation. Indeed, reporting illusions on an epoch basis would have involved premovement activity of the responding finger in areas that were processing proprioceptive information, including precentral and parietal areas [Mars et al., 2008; Rushworth et al., 2003; Toni et al., 1999]. Structural MRI data was finally acquired using a three‐dimensional T1‐weighted scanning sequence (MPRAGE; repetition time = 9.4 ms; echo time = 4.4 ms; inversion time = 800 ms; field of view = 256 mm × 256 mm × 180 mm, reco matrix = 256 × 256 × 180) to allow anatomical localization of brain activation.
fMRI Time Series Analysis
Analyses of fMRI time series were conducted with SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; available at: http://www.fil.ion.ucl.ac.uk/spm/software/spm8). Each run consisted of 113 scans per subject, including six dummy images of magnetic field saturation that were discarded before analysis. The remaining images were slice‐time corrected using slice #39, which was close to the middle slice in time (42 slices total; interleaved acquisition). After discarding the last two volumes, these images were realigned to the first image of the time series to correct for head movement between scans, and a mean realigned image was created. The realigned images were also “unwarped” to remove residual movement‐related variance [Andersson et al., 2001]. Each structural MRI was then co‐registered to the corresponding mean realigned image. Finally, the functional images were spatially normalized by matching each image to the standard SPM8 EPI template, resampled to 3‐mm isotropic voxel size, and were spatially smoothed using an isotropic three‐dimensional Gaussian kernel (6 mm full‐width at half maximum).
Statistical analysis of the fMRI time series was based on the general linear model (GLM) approach [Friston et al., 1995a,b]. Stimulus‐evoked neural responses were modelled as boxcar regressors time‐locked to the onsets of the vibration (R30, L30, R100, and L100) and REST conditions, and were convolved with the canonical hemodynamic response function of SPM8. Low‐frequency drifts were removed from images using high‐pass filtering (1/128 Hz). First‐level (i.e., subject‐level) linear contrasts including L100 > L30 and R100 > R30 were performed. The resulting statistical parametric maps of the t‐statistic revealed brain activations related to kinaesthetic processing. The eighteen subject‐specific maps were then entered into a second‐level (random effect) full group GLM to derive statistical inferences using one‐sample t‐tests. The threshold for cluster‐wise significance was set at P < 0.05 for the above analyses, corrected using Family Wise Error (FWE) for multiple comparisons, after having applied an auxiliary voxel‐level threshold of P < 0.001 and extent threshold >10 contiguous voxels. These statistical maps were inclusively masked using the maps of the vibration conditions R100 and L100 compared to REST (i.e., L100 > L30 and R100 > R30 were masked with L100 > REST and R100 > REST, respectively), thresholded at P < 0.05 uncorrected for multiple comparisons. Thus, clusters in L100 > L30 and R100 > R30 maps were due to activation induced by L100 and R100 and not deactivation induced by L30 and R30. A conjunction analysis was also conducted [Price and Friston 1997; Worsley and Friston 2000], which consisted in performing L100 > L30 ∩ R100 > R30 to reveal areas that were commonly active during kinaesthetic processing of right and left vibratory stimuli. This analysis was conducted to pinpoint high‐level (non‐limb specific) right‐sided fronto‐parietal regions involved in kinaesthesia [Naito et al., 2007, 2005].
Finally, activation differences between subjects who did and did not experience illusory movement of the feet were examined using region of interest (ROI) analysis. Given the low size of the two subsamples of subjects (i.e., nine subjects per subsample), ROI analysis was preferred to whole‐brain analysis that has low power because of the multiple comparisons across voxels [Poldrack, 2007; Saxe et al., 2006]. Specifically, functional ROIs were defined from the group GLM employing a leave one subject out (LOSO) procedure to avoid double dipping [Esterman et al., 2009, 2010; Vul and Kanwisher, 2010].
The subject‐specific maps previously defined were used to perform LOSO GLMs, one with each subject left out. For each of the 18 LOSO GLMs (n = 17 subjects per GLM), significant clusters from L100 > L30, R100 > R30, and L100 > L30 ∩ R100 > R30 maps served to define ROIs, which were applied to the data collected from the subject left out. Maps from the LOSO GLMs were thresholded similarly to those from the full group GLM. Contrast estimates (differences in beta weights) on L100 > L30 and R100 > R30 were then obtained using the left‐out subject's data that was extracted from the ROIs using MarsBaR [Brett et al., 2002; http://marsbar.sourceforge.net/]. ROIs consisted of 6 mm diameter spheres centred on the peaks of activation identified in the LOSO GLMs. Non‐parametric statistics were afterwards run on the contrast estimates to evaluate differences in ROI activations between subjects who did experience illusions and those who did not.
RESULTS
Brain Activation During Muscle Spindles Stimulation
Activations related to right vibratory stimulation, as evaluated using the R100 > R30 contrast, occurred in regions of the right and left frontal and parietal cortices as well as in subcortical structures (Fig. 1A and Table 1). Specifically, the contrast revealed significant activations (P < 0.05, with FWE correction) in three main clusters localized in the left hemisphere (i.e., hemisphere contralateral to the stimulated body side). The first cluster of activation was observed in the left inferior parietal lobule (BA 40). The second cluster included the left inferior frontal gyrus (BA 44), the anterior part of the left insula (BA 13), as well as the claustrum and the putamen on the left side. The third cluster involved areas of the precentral, postcentral, and cingulate gyri, with activation peaks located in the left primary somatosensory cortex (BA 3), the left supplementary motor area (BA 6) and the left anterior cingulate cortex (BA 24). Significant activity in the primary motor cortex (BA 4) was also evident, as shown in Figure 1A. Moreover, it is worth mentioning that although the activation peak was located in the left hemisphere for BA 24, activation also extended to the right hemisphere (Fig. 1A). In addition, the R100>R30 contrast revealed significant clusters of activation (P < 0.05, with FWE correction) in the right hemisphere (i.e., hemisphere ipsilateral to the stimulated body side). These right‐sided clusters included the inferior parietal lobule (BA 40), the inferior frontal gyrus (BA 44), the anterior insula, the claustrum and the putamen. Therefore, stimulation of the right muscle spindles led to bilateral activity in several brain structures. The only exceptions were the sensorimotor areas (i.e., primary somatosensory cortex, primary motor cortex, and supplementary motor area), which showed only contralateral activation to the stimulation site.
Figure 1.
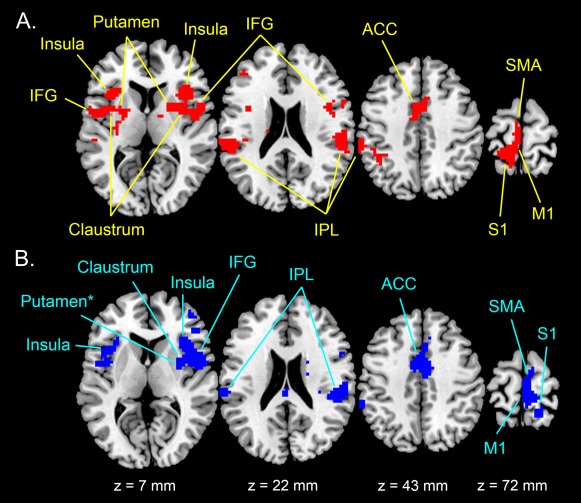
Full‐group activation maps during (A) right (R100 > R30 contrast) and (B) left (L100 > L30 contrast) vibration stimulation rendered on standard T1 template. Maps are thresholded at P < 0.001 uncorrected (t > 3.79) with an extent threshold k = 10 voxels and are inclusively masked using either R100 > REST or L100 > REST (P < 0.05 uncorrected). Clusters of activity (P < 0.05, FWE correction) are indicated with arrows. IFG: inferior frontal gyrus; IPL: inferior parietal lobule; ACC: anterior cingulate cortex; SMA: supplementary motor area; M1: primary motor cortex; S1: primary somatosensory cortex. Note that right putamen during left stimulation did not survive clusterwise FWE correction, as indicated by the asterisk. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 1.
Brain regions activated during right vibratory stimulus (i.e., R100 > R30 contrast) for the full group
| R100>R30 peak location | Side | x (mm) | y (mm) | z (mm) | t‐Value |
|---|---|---|---|---|---|
| Cluster # 1 (272 voxels) | |||||
| Inf. Par. Lobule (BA 40) | L | −43 | −43 | 40 | 6.81 |
| L | −55 | −31 | 31 | 6.62 | |
| L | −60 | −31 | 43 | 6.58 | |
| Cluster #2 (342 voxels) | |||||
| Inf. Front. Gyrus (BA 44) | L | −48 | 2 | 7 | 7.59 |
| Ant. Insula (BA 13) | L | −36 | 2 | 16 | 6.75 |
| L | −33 | −20 | 10 | 5.81 | |
| Putamen | L | −24 | 2 | 10 | 5.78 |
| Claustrum | L | −30 | 1 | 10 | 5.25 |
| Cluster #3 (225 voxels) | |||||
| Post‐central gyrus (BA 3) | L | −12 | −40 | 70 | 6.33 |
| Sup. Motor Area (BA 6) | L | −3 | −25 | 69 | 5.32 |
| L | −6 | −16 | 67 | 4.94 | |
| L | −3 | 8 | 49 | 4.64 | |
| Ant. Cing. Cortex (BA 24) | L | 0 | 5 | 40 | 5.40 |
| Cluster #4 (410 voxels) | |||||
| Inf. Front. Gyrus (BA 44) | R | 48 | −1 | 7 | 8.51 |
| Claustrum | R | 28 | 8 | 10 | 8.26 |
| Ant. Insula (BA 13) | R | 36 | 2 | 10 | 8.25 |
| Putamen | R | 27 | 8 | 1 | 7.25 |
| Cluster #5 (152 voxels) | |||||
| Inf. Par. Lobule (BA 40) | R | 57 | −34 | 25 | 7.53 |
t‐values refer to significant activation peaks at P < 0.001 (uncorrected for multiple comparisons). In addition, all reported clusters were significantly active at P < 0.05 (FWE correction).
L: left hemisphere; R: Right hemisphere.
On the other hand, the L100 > L30 contrast revealed mainly right‐hemisphere activations (Fig. 1B and Table 2). Large clusters of significant activation (P < 0.05, FWE correction) were observed in the right inferior frontal gyrus (BA 44), including also the right anterior insula (BA 13) and the right claustrum, the right inferior parietal lobule (BA 40), and the right supplementary motor area (BA 6). Activation in BA 6 spread anteriorly into the right anterior cingulate cortex (BA 24) and posteriorly into the primary somatosensory cortex (BA 3) including the primary motor cortex (BA 4). Again, activation of BA 24 also spread to the other (here, left) hemisphere. In addition, the right putamen was also active at a liberal threshold (P < 0.001, uncorrected for multiple comparisons) but did not survive a stringent threshold with FWE correction (Fig. 1B). Only the inferior parietal lobule (BA 40) and the anterior insula (BA 13) were significantly activated (P < 0.05, FWE correction) in the left hemisphere. Interestingly, the right‐hemisphere regions activated during left stimulation (i.e., L100 > L30 contrast) were similar to those activated during right stimulation (i.e., R100 > R30 contrast). In particular, frontal and parietal areas in the right hemisphere seem to be strongly active no matter if the stimulation was on the right or left body side.
Table 2.
Brain regions activated during left vibratory stimulus (i.e., L100 > L30 contrast) for the full group
| L100 > L30 peak location | Side | x (mm) | y (mm) | z (mm) | t‐Value |
|---|---|---|---|---|---|
| Cluster # 1 (182 voxels) | |||||
| Inf. Par. Lobule (BA 40) | R | 60 | −25 | 25 | 10.00 |
| R | 54 | −28 | 19 | 7.13 | |
| Cluster # 2 (398 voxels) | |||||
| Inf. Front. Gyrus (BA 44) | R | 51 | 2 | 7 | 7.71 |
| Ant. Insula (BA 13) | R | 36 | 20 | 7 | 6.84 |
| Claustrum | R | 33 | −1 | 10 | 6.16 |
| Cluster #3 (171 voxels) | |||||
| Post‐central gyrus (BA 3) | R | 15 | −39 | 70 | 7.06 |
| Sup. Motor Area (BA 6) | R | 3 | −19 | 70 | 6.61 |
| R | 3 | −28 | 76 | 6.42 | |
| Ant. Cing. Cortex (BA 24) | R | 9 | −4 | 40 | 5.99 |
| Cluster #4 (137 voxels) | |||||
| Ant. Insula (BA 13) | L | −39 | 5 | 13 | 5.24 |
| Cluster #5 (108 voxels) | |||||
| Inf. Par. Lobule (BA 40) | L | −60 | −40 | 43 | 5.14 |
| L | −66 | −31 | 28 | 5.06 |
t‐values refer to significant activation peaks at P < 0.001 (uncorrected for multiple comparisons). In addition, all reported clusters were significantly active at P < 0.05 (FWE correction).
L: left hemisphere; R: Right hemisphere.
To verify the above observation, the conjunction analysis of L100 > L30 ∩ R100 > R30, which tested for significant clusters active during both right and left vibratory stimuli, was conducted. It revealed two clusters of significantly active voxels (P < 0.05, FWE correction) in the right hemisphere. The first cluster (k = 44 voxels) was located in the right inferior parietal lobule (BA 40), with a peak at x = 57, y = −31, z = 25 mm, and a t‐value of 4.26. The second cluster (k = 88 voxels) belonged to the right inferior frontal gyrus (BA 44), with a peak at x = 51, y = 5, z = 7 mm, and a t‐value of 5.47. This result is again in favor of a dominance of the right hemisphere, especially a right‐sided frontoparietal network, in processing kinaesthetic information.
Differences in Brain Activation Between Subjects With and Without Kinaesthetic Illusions
Difference in brain activity between subjects who did and did not have kinaesthetic illusions was examined using LOSO GLMs. Since the activation of a high‐level right‐sided network of brain regions distributed from posterior parietal to inferior frontal cortices was expected to be greater in subjects with illusory movement, a first step consisted in determining LOSO ROIs from L100 > L30 ∩ R100 > R30 conjoined activation clusters (P < 0.05, FWE correction). For each of the LOSO GLMs, clusters were significantly activated in the right inferior frontal gyrus (BA 44, Fig. 2A) and the right inferior parietal lobule (BA 40, Fig. 2B). As illustrated in Figure 2, there was a high degree of overlap between the LOSO clusters in both right BA 40 and BA 44, with a large number of voxels surviving in each LOSO fold.
Figure 2.
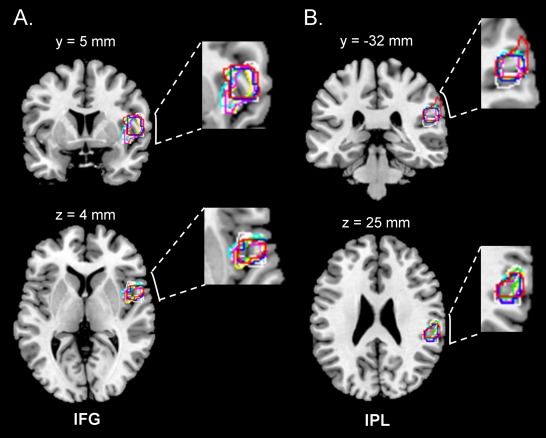
Overlap of leave one subject out (LOSO) clusters in (A) the inferior frontal gyrus (IFG) and (B) the inferior parietal lobule (IPL). The LOSO clusters were obtained from separate LOSO GLMs (here, L100 > L30 ∩ R100 > R30 conjunction), one with each subject left out. Each LOSO cluster was subsequently used to define a region of interest (a 6 mm sphere ROI centred on activation peak), which was applied to the data collected from the subject left out. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Group analysis performed on the above LOSO right‐sided ROIs (BAs 40 and 44) indicated that L100 > L30 contrast estimates were statistically greater than R100>R30 contrast estimates in subjects experiencing illusions (Fig. 3). Statistics of the Wilcoxon signed‐rank tests were, W = 41, P = 0.01 for BA 40, and W = 39, P = 0.02 for BA 44. On the other hand, there was no activation difference between the two contrasts in subjects without illusions. In other words, activation of the right frontoparietal network was amplified by left‐sided stimulation only in subjects who reported kinaesthetic illusions.
Figure 3.
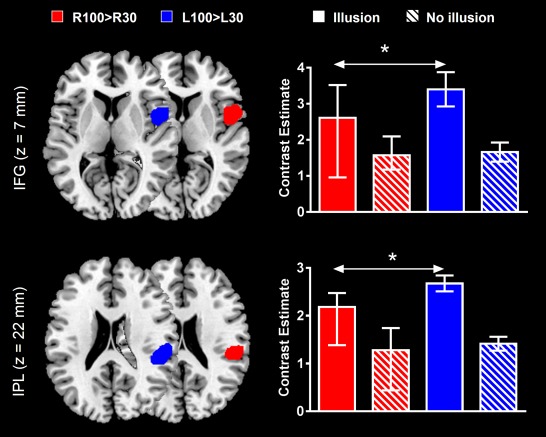
L100 > L30‐ and R100 > R30‐evoked activations in right inferior frontal gyrus (IFG) and right inferior parietal lobule (IPL) regions of interest (ROIs). For each subsample of subjects (i.e., subjects who reported having and not having illusory movements), differences between left‐ and right‐evoked activations are indicated by the arrows (*P <0.05). The clusters reported on the brain slices delineate the overall areas that contained the leave one subject out (LOSO) ROIs that were used to extract contrast estimates. Data in barplots are reported as median ± IQR. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In order to further explore the dominance of this network in kinaesthetic processing and its possible role in the awareness of illusory movement, we also compared activity in right BAs 40 and 44 ROIs (i.e., L100 > L30 contrast) with that in the corresponding ROIs in the left hemisphere (i.e., R100 > R30 contrast). To do so, we defined LOSO ROIs from R100 > R30 activation clusters (P < 0.05, FWE correction) in left BAs 40 and 44 (Fig. 4). L100 > L30 contrast estimates extracted from right BAs 40 and 44 ROIs were statistically larger than R100 > R30 contrast estimates from left hemisphere ROIs in subjects who experienced illusions only (W = 41, P = 0.01 for BA 40, and W = 35, P = 0.04 for BA 44). In addition, Mann‐Whitney U tests indicated that activations (contrast estimates) were significantly larger in subjects who did experience illusions as compared to those who did not in both right BA 40 (U = 4, P = 0.0005) and BA 44 (U = 11, P = 0.008) (Fig. 4). Therefore, while the right frontoparietal network was more active than its left counterpart following contralateral stimulation in subjects who experienced kinaesthetic illusions, this result did not extend to subjects without illusions. In addition, this right‐sided network was more active in the former subjects as compared to the latter.
Figure 4.
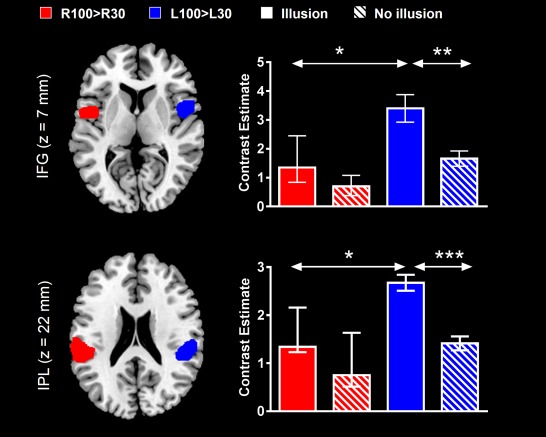
L100 > L30‐ and R100 > R30‐evoked activations in right and left inferior frontal gyri (IFG) and inferior parietal lobules (IPL) regions of interest (ROIs). Differences of activation in ROIs between right and left hemispheres, and between subjects with and without illusions, are indicated by the arrows (*P < 0.05; **P < 0.01; ***P < 0.001). The clusters reported on the brain slices delineate the overall areas that contained the leave one subject out (LOSO) ROIs that were used to extract contrast estimates. Data in barplots are reported as median ± IQR. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Finally, we examined differences in brain activity between the two subsamples of subjects in other areas involved in kinaesthetic processing. The purpose of this analysis was to evaluate whether the greater brain activation in subjects with illusions also generalized to the basic kinaesthetic network. Accordingly, we defined LOSO ROIs in right and left insula, claustrum, anterior cingulate, primary somatosensory cortex and supplementary motor area from L100 > L30 and R100 > R30 activation clusters (P < 0.05, FWE correction), respectively (Fig. 5). The two subsamples of subjects differed only in the left supplementary motor area ROIs, with larger activity in subjects with illusions (U = 8, P = 0.003). Therefore, generalization of increased activation in subjects with illusions to the entire kinaesthetic network appeared rather marginal.
Figure 5.
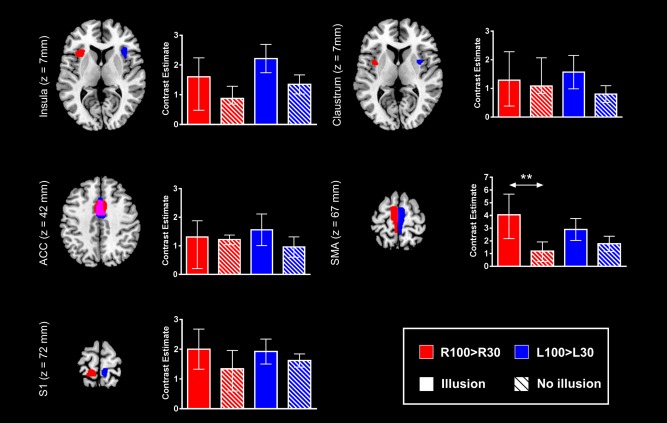
L100 > L30‐ and R100 > R30‐evoked activations in right and left insula, claustrum, anterior cingulated cortex (ACC), supplementary motor area (SMA) and primary somatosensory cortex (S1) regions of interest (ROIs). Activation differences in ROIs between subjects who reported having, and not having, illusory movements is indicated by the arrow (**: P < 0.01). The clusters reported on the brain slices delineate the overall areas that contained the leave one subject out (LOSO) ROIs that were used to extract contrast estimates. Data in barplots are reported as median ± IQR. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In sum, although full‐group GLM results evidenced a right hemisphere dominance in processing kinaesthetic information, with right IPL and IFG consistently activated by right and left vibrations (i.e., a non‐limb‐specific activation) in all subjects, the LOSO ROI analysis showed that these right‐sided brain regions were statistically more active than their left counterparts in response to contralateral stimulation in the subjects with illusions only. Furthermore, the activation of these regions was stronger in subjects with illusions as compared to those without illusions. Therefore, the awareness of illusory movement emerged from boosted activation of the right inferior frontoparietal (IPL‐IFG) network, making stronger the kinaesthetic‐related right hemispheric dominance.
DISCUSSION
The aim of the present study was to identify the neural correlates subserving illusory movement awareness. A first step consisted in characterizing the basic network of kinaesthetic‐related activity, in particular high‐level regions that were active irrespective of whether vibratory stimulation was applied to the right or left ankle. In a second step, we contrasted subjects who experienced illusory movements from those who did not, using region of interest analysis. Separating a posteriori “conscious and non‐conscious subjects” while using exactly the same stimuli and task is considered an elegant approach in the study of consciousness [Dehaene and Naccache, 2001], and has already proven powerful in revealing certain neural correlates of conscious processing [e.g., McIntosh et al., 1999].
A Large‐Scale Asymmetric Network Underlying Kinaesthetic Processing
The many cortical and subcortical regions that were involved in kinaesthetic processing are in good agreement with those reported in previous studies [e.g., Goble et al., 2011, 2012; Kavounoudias et al., 2008; Naito et al., 1999, 2002, 2005, 2007; Romaiguère et al., 2003]. Not surprisingly, activations were found in primary somatosensory (S1/BA 3a) and motor (M1/BA 4) cortices contralateral to the stimulation side. With respect to S1, it is well documented that somatosensory information enters the cerebral cortex through this area, which contains several somatotopic maps of the contralateral half of the body [Dijkerman and de Haan, 2007]. In particular, the region activated in S1 was most likely BA 3a (adjacent to BA 4), where dominant inputs originate from muscle receptors [Phillips et al., 1971; Tanji and Wise, 1981]. The contribution of M1 to kinaesthetic processing has also been widely reported [see Naito, 2004, for a review], and likely relies on primary somatosensory cortex‐to‐primary motor cortex associational connections [Farkas et al., 1999]. Another sensorimotor region that was significantly activated during muscle spindle stimulation was the supplementary motor area (SMA/BA 6). This result adds to evidence that SMA has a clear kinaesthetic function, possibly being interconnected with others brain areas (e.g., parietal cortex) to modulate somatosensory activity [Haggard and Whitford, 2004]. In addition, a midline structure constituent of the kinaesthetic network was the anterior cingulate cortex (BA 24), which has already been reported in previous kinaesthetic mapping studies [Kavounoudias et al., 2008; Romaiguère et al., 2003]. A meta‐analysis based on parcellation showed that this portion of the cingulate cortex is multimodal and multifunctional, being involved in a variety of behavioral domains including the sensorimotor domain [Torta and Cauda, 2011]. The authors further suggested that this portion of the cingulate cortex serves as a “hub,” interconnecting different frontoparietal networks. Thus, this area may have served a higher‐order associative function, supporting large scale functional connections between the frontal and parietal regions involved in kinaesthetic processing.
The kinaesthetic network also included the anterior insular cortex (AIC), regions medial to this cortex (i.e., claustrum, putamen), and regions of the (pre)frontal (IFG/BA 44) and posterior parietal (IPL/BA 40) cortices. Overall, these regions had bilateral activity in response to right stimulation while their activity was restricted to the right hemisphere for left stimulation. Such greater involvement of right‐sided regions in processing kinaesthetic information was further supported by conjoined right IFG and IPL activations for both right and left stimulations. Again, these findings confirm previous observations, both as regards the regions that processed kinaesthetic information and the dominance of the right hemisphere in processing this information [e.g., Goble et al., 2012; Naito et al., 2007, 2005]. Although right hemisphere dominance in human kinaesthesia remains to be understood, an explanation would be that such asymmetry is functional, emerging as the brain develops to avoid interference between language and somatic functions [Naito et al., 2005]. The activation of the putamen also deserves to be highlighted, as only very few studies reported activation of this structure in human kinesthesia [Goble et al., 2011, 2012]. Accordingly, our study provides further evidence that the putamen plays a significant role in human kinaesthetic processing, possibly directing inputs from pre‐ and post‐central areas [e.g., BA 4, S1; Kunzle, 1975, 1977] to frontal lobe areas, including SMA and prefrontal areas [Alexander et al., 1986; Middleton and Strick, 2002]. On the other hand, our results question a common suggestion made about the neural basis of illusory movements. Indeed, the AIC along with the claustrum and the IFG are considered neural correlates of consciousness [e.g., Craig, 2009; Crick and Koch, 2005; Dehaene and Changeux, 2011]. Accordingly, it has been proposed that activation of these regions in association with activation of the IPL, which underpins elements of one's own movement and body [Daprati et al., 2010; Blakemore and Frith, 2003], are responsible for experiences of illusory limb movements [Goble et al., 2012; Naito et al., 2007]. However, half of our population did not experience illusions despite this typical activation pattern. Therefore, we should perhaps reconsider the idea that right frontal and parietal coactivations per se would trigger illusory movements (i.e., an on/off mechanism with the “switch on” bringing illusions).
Neural Correlates of Illusory Movement Awareness
The main result from the present study was that subjects who experience illusory movements differed from those who did not have illusions through larger activation of the right frontoparietal network, including right IFG and IPL. These data clearly suggest that activation of this network alone is insufficient to drive subjective experience of movement but needs to reach a certain threshold. This result is in line with previous studies on perceptual awareness, which revealed stronger activation of a distributed network, including inferior frontal and parietal areas, only when visual, auditory or tactile stimuli were consciously accessible [Boly et al., 2007; Dehaene et al., 2006, 2001; Sadaghiani et al., 2009]. In the same vein, a study by Hasson et al. [2007], using the MacGurk illusion, dissociated brain areas that code for audiovisual stimuli from those that code for speech percept (i.e., what the subject consciously hears). Whereas the auditory cortex coded for the sensory properties of the input, the left posterior IFG (pars opercularis) and the anterior IPL coded for the speech percept. Interestingly, the authors demonstrated that activations of these areas were particularly strong in subjects highly sensitive to speech percept. Therefore, on condition that IFG and IPL activations become strong, these two regions code for the perceived conscious content related to sensory stimuli, with left‐ and right‐sided regions showing specialization in coding for what is consciously heard (speech illusion) and what is consciously felt (kinaesthetic illusion), respectively. Though our data are in favor of a threshold‐based mechanism subserving the emergence of illusory movements, we did not examine the possibility that the characteristics of the illusions, such as the vividness, the duration or the strength [Ehrsson et al., 2004; Hagura et al., 2007; Naito et al., 1999], may be reflected in an activation gradient once the threshold is reached. Clearly, examining whether activity of the right frontoparietal network is associated with psychological ratings when a percept of limb movement is formed should be on the research agenda.
Turning to the action domain, several studies revealed a central role for the right parietal cortex in the awareness of one's action [Desmurget and Sirigu, 2009; Desmurget et al., 2009]. Using direct cortical stimulation, the authors reported that strong stimulation of the posterior parietal cortex (BAs 39 and 40) triggered illusory movements. They proposed that high intensities of stimulation in these areas primed motor representations to consciousness, likely recruiting circuitries usually responsible for movement monitoring through forward computations (i.e., predictions made about the movement before its onset). In the present study, illusory movements may also have resulted from activation of such executive network. If true, our results further suggest that this network is implemented not only in the right posterior parietal cortex but also extends to the opercular part of the right IFG. Interestingly, this is in keeping with a study by Berti et al. [2005] in the domain of self‐monitoring of action, which showed that the network of conscious monitoring of motor acts is implemented in areas also related to the programming of motor acts, particularly right BA 44. In addition, our results are in agreement with the proposal that experiencing one's own body in motion mainly involves the right IPL [Daprati et al., 2010], although we may further speculate that right frontoparietal brain regions are required for conscious perception of our own body in motion.
At the neurocomputational level, the larger activation of the right frontoparietal network in our subjects aware of the illusions fits well with the Global Neuronal Workspace (GNW) model of conscious processing [Dehaene and Changeux, 2005, 2011; Dehaene et al. 2006]. This model was derived from experiments contrasting perceived and unperceived stimuli and proposes that conscious processing occurs when information is made available to different brain association areas (e.g., frontal and parietal cortices) through long‐distance connections, as those of the superior longitudinal fascicle connecting frontal and parietal opercula [Makris et al., 2005]. In this framework, conscious processing result from a two‐stage process: (1) at an early stage, sensory signals progress through sensory areas to high‐level association areas (i.e., bottom‐up connections), and (2) at a later stage, if the spread of activation to high‐level areas is sufficiently intense, these areas exchange information through self‐sustaining reverberant activation that favors convergence toward a single percept, also projecting information back to sensory areas (i.e., top‐down connections). Assuming that consciousness is a unifying property that emerges from a broad frontoparietal network regardless of the physiological context (motor, perceptual), the second stage of the process may have been incomplete in subjects who did not experience illusory movements (i.e., an incomplete reverberating loop), hindering emergence of conscious movement representation (i.e., illusory ankle plantar‐flexion movements) from kinaesthetic signals. A relevant way to test this assumption would be to examine time sequences related to the (un)awareness of illusory movement using electroencephalography. Indeed, conscious processing, according to the GNW model, should come with large and late (>300 ms) increases in power and synchrony in beta and gamma bands in frontoparietal networks [Dehaene and Changeux, 2011]. Accordingly, one may expect particular features with respect to these hallmarks in subjects without illusions. Moreover, examining whether individual differences in the neural correlates of the awareness of illusory movement are genuine or evolve throughout life is another important issue. This should help to further understand the mechanisms that lead to motor awareness and, more largely, add to current theories of conscious processing.
ACKNOWLEDGMENTS
The authors thank Dr. Jennifer T. Coull for useful discussions and comments on the draft.
REFERENCES
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Hutton C, Ashburner J, Turner R, Friston K (2001): Modeling geometric deformations in EPI time series. Neuroimage 13:903–919. [DOI] [PubMed] [Google Scholar]
- Berti A, Bottini G, Gandola M, Pia L, Smania N, Stracciari A, Castiglioni I, Vallar G, Paulesu E (2005): Shared cortical anatomy for motor awareness and motor control. Science 309:488–491. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Frith C (2003): Self‐awareness and action. Curr Opin Neurobiol 13:219–224. [DOI] [PubMed] [Google Scholar]
- Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, Phillips C, Peigneux P, Maquet P, Laureys S (2007): Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA 104:12187–12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB (2002): Region of interest analysis using an SPM toolbox. In: 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, June 2–6. Neuroimage 16: abstract 497. [Google Scholar]
- Craig AD (2009): How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Crick FC, Koch C (2005): What is the function of the claustrum? Philos Trans R Soc B 360:1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daprati E, Sirigu A, Nico D (2010): Body and movement: Consciousness in the parietal lobes. Neuropsychologia 48:756–762. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP (2005): Ongoing spontaneous activity controls access to consciousness: A neuronal model for inattentional blindness. PLoS Biol 3:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP (2011): Experimental and theoretical approaches to conscious processing. Neuron 70:200–227. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C (2006): Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci 10:204–211. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L (2001): Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition 79:1–37. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Rivière D (2001): Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci 4:752–758. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Reilly KT, Richard N, Szathmari A, Mottolese C, Sirigu A (2009): Movement intention after parietal cortex stimulation in humans. Science 324:811–813. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Sirigu A (2009): A parietal‐premotor network for movement intention and motor awareness. Trends Cogn Sci 13:411–419. [DOI] [PubMed] [Google Scholar]
- Dijkerman HC, de Haan EH (2007): Somatosensory processes subserving perception and action. Behav Brain Sci 30:189–201; discussion 201–239. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE (2004): That's my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 305:875–877. [DOI] [PubMed] [Google Scholar]
- Esterman M, Chiu YC, Tamber‐Rosenau BJ, Yantis S (2009): Decoding cognitive control in human parietal cortex. Proc Natl Acad Sci USA 106:17974–17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Tamber‐Rosenau BJ, Chiu YC, Yantis S (2010): Avoiding non‐independence in fMRI data analysis: leave one subject out. Neuroimage 50:572–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas T, Kis Z, Toldi J, Wolff JR (1999): Activation of the primary motor cortex by somatosensory stimulation in adult rats is mediated mainly by associational connections from the somatosensory cortex. Neuroscience 90:353–361. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, Turner R (1995a): Analysis of fMRI time‐series revisited. Neuroimage 2:45–53. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, and Frackowiak RSJ (1995b): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Doumas M, Wenderoth N, Swinnen SP (2011): Brain activity during ankle proprioceptive stimulation predicts balance performance in young and older adults. J Neurosci 31:16344–16352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Van Impe A, Geurts M, Van Hecke W, Sunaert S, Wenderoth N, Swinnen SP (2012): The neural basis of central proprioceptive processing in older versus younger adults: An important sensory role for right putamen. Hum Brain Mapp 33:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg GE, Zito G, Patria F, Sanes JN (2001): Improved detection of event‐related functional MRI signals using probability functions. Neuroimage 14:1193–1205. [DOI] [PubMed] [Google Scholar]
- Haggard P, Whitford B (2004): Supplementary motor area provides an efferent signal for sensory suppression. Brain Res Cogn Brain Res 1:52–58. [DOI] [PubMed] [Google Scholar]
- Hagura N, Takei T, Hirose S, Aramaki Y, Matsumura M, Sadato N, Naito E (2007): Activity in the posterior parietal cortex mediates visual dominance over kinesthesia. J Neurosci 27:7047–7053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Skipper JI, Nusbaum HC, Small SL (2007): Abstract coding of audiovisual speech: Beyond sensory representation. Neuron 56:1116–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavounoudias A, Roll JP, Anton JL, Nazarian B, Roth M, Roll R (2008): Proprio‐tactile integration for kinesthetic perception: an fMRI study. Neuropsychologia 46:567–575. [DOI] [PubMed] [Google Scholar]
- Kunzle H (1975): Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res 88:195–209. [DOI] [PubMed] [Google Scholar]
- Kunzle H (1977): Projections from the primary somatosensory cortex to basal ganglia and thalamus in the monkey. Exp Brain Res 30:481–492. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN 2005: Segmentation of subcomponents within the superior longitudinal fascicle in humans: A quantitative, in vivo, DT‐MRI study. Cereb Cortex 15:854–869. [DOI] [PubMed] [Google Scholar]
- Mars RB, Coles MG, Hulstijn W, Toni I (2008): Delay‐related cerebral activity and motor preparation. Cortex 44:507–520. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Rajah MN, Lobaugh NJ (1999): Interactions of prefrontal cortex in relation to awareness in sensory learning. Science 284:1531–1533. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2002): Basal‐ganglia 'projections' to the prefrontal cortex of the primate. Cereb Cortex 12:926–935. [DOI] [PubMed] [Google Scholar]
- Naito E (2004): Sensing limb movements in the motor cortex: How humans sense limb movement. Neuroscientist 10:73–82. [DOI] [PubMed] [Google Scholar]
- Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE (1999): Illusory arm movements activate cortical motor areas: A positron emission tomography study. J Neurosci 19:6134–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito E, Nakashima T, Kito T, Aramaki Y, Okada T, Sadato N (2007): Human limb‐specific and non‐limb‐specific brain representations during kinesthetic illusory movements of the upper and lower extremities. Eur J Neurosci 25:3476–3487. [DOI] [PubMed] [Google Scholar]
- Naito E, Roland PE, Ehrsson HH (2002): I feel my hand moving: A new role of the primary motor cortex in somatic perception of limb movement. Neuron 36:979–988. [DOI] [PubMed] [Google Scholar]
- Naito E, Roland PE, Grefkes C, Choi HJ, Eickhoff S, Geyer S, Zilles K, Ehrsson HH (2005): Dominance of the right hemisphere and role of area 2 in human kinesthesia. J Neurophysiol 93:1020–1034. [DOI] [PubMed] [Google Scholar]
- Phillips CG, Powell TP, Wiesendanger M (1971): Projection from low‐threshold muscle afferents of hand and forearm to area 3a of baboon's cortex. J Physiol 217:419–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA (2007): Region of interest analysis for fMRI. Soc Cogn Affect Neurosci 2:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ (1997): Cognitive conjunction: A new approach to brain activation experiments. Neuroimage 5:261–270. [DOI] [PubMed] [Google Scholar]
- Radovanovic S, Korotkov A, Ljubisavljevic M, Lyskov E, Thunberg J, Kataeva G, Danko S, Roudas M, Pakhomov S, Medvedev S, Johansson H (2002): Comparison of brain activity during different types of proprioceptive inputs: A positron emission tomography study. Exp Brain Res 143:276–285. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP (1982): Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47:177–190. [DOI] [PubMed] [Google Scholar]
- Roll JP, Vedel JP, Ribot E (1989): Alteration of proprioceptive messages induced by tendon vibration in man: A microneurographic study. Exp Brain Res 76:213–222. [DOI] [PubMed] [Google Scholar]
- Romaiguère P, Anton JL, Roth M, Casini L, Roll JP (2003): Motor and parietal cortical areas both underlie kinaesthesia. Brain Res Cogn Brain Res 16:74–82. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Johansen‐Berg H, Göbel SM, Devlin JT (2003): The left parietal and premotor cortices: Motor attention and selection. Neuroimage 1:S89–S100. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A (2009): Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci 29:13410–13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N (2006): Divide and conquer: a defense of functional localizers. Neuroimage 30:1088–1096; discussion 1097–1099. [DOI] [PubMed] [Google Scholar]
- Tanji J, Wise SP (1981): Submodality distribution in sensorimotor cortex of the unanesthetized monkey. J Neurophysiol 45:467–481. [DOI] [PubMed] [Google Scholar]
- Toni I, Schluter ND, Josephs O, Friston K, Passingham RE (1999): Signal‐, set‐ and movement‐related activity in the human brain: An event related fMRI study. Cereb Cortex 9:35–49. [DOI] [PubMed] [Google Scholar]
- Torta DM, Cauda F (2011): Different functions in the cingulate cortex, a meta‐analytic connectivity modeling study. Neuroimage 56:2157–2172. [DOI] [PubMed] [Google Scholar]
- Vul E, Kanwisher N (2010): Begging the question: The non‐independence error in fMRI data analysis In: Hanson S, Bunzl M, editors. Foundational Issues in Human Brain Mapping. Cambridge, MA: MIT Press. [Google Scholar]
- Worsley KJ, Friston KJ (2000): A test for a conjunction. Stat Prob Lett 47:135–140. [Google Scholar]


