Abstract
Processing of reward is the basis of adaptive behavior of the human being. Neural correlates of reward processing seem to be influenced by developmental changes from adolescence to late adulthood. The aim of this study is to uncover these neural correlates during a slot machine gambling task across the lifespan. Therefore, we used functional magnetic resonance imaging to investigate 102 volunteers in three different age groups: 34 adolescents, 34 younger adults, and 34 older adults. We focused on the core reward areas ventral striatum (VS) and ventromedial prefrontal cortex (VMPFC), the valence processing associated areas, anterior cingulate cortex (ACC) and insula, as well as information integration associated areas, dorsolateral prefrontal cortex (DLPFC), and inferior parietal lobule (IPL). Results showed that VS and VMPFC were characterized by a hyperactivation in adolescents compared with younger adults. Furthermore, the ACC and insula were characterized by a U‐shape pattern (hypoactivation in younger adults compared with adolescents and older adults), whereas the DLPFC and IPL were characterized by a J‐shaped form (hyperactivation in older adults compared with younger groups). Furthermore, a functional connectivity analysis revealed an elevated negative functional coupling between the inhibition‐related area rIFG and VS in younger adults compared with adolescents. Results indicate that lifespan‐related changes during reward anticipation are characterized by different trajectories in different reward network modules and support the hypothesis of an imbalance in maturation of striatal and prefrontal cortex in adolescents. Furthermore, these results suggest compensatory age‐specific effects in fronto‐parietal regions. Hum Brain Mapp 35:5153–5165, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: reward anticipation, lifespan, aging, adolescence, fMRI, connectivity
INTRODUCTION
The processing of reward‐related information is fundamental for shaping both simple stimulus‐reward associations as well as complex goal‐directed behavior. Considering human lifespan, motivation, learning, and hedonic behavior are changing. Adolescence is probably the period of life, which is most intensely characterized by hedonic and risky behavior such as drug and alcohol use, careless behavior in traffic and hazardous sexual interactions [Casey et al., 2008; Steinberg, 2008]. While developing into adulthood such risky and hedonic‐oriented behavior normalizes, whereas for the elderly security‐oriented actions become more common. Moreover, age‐related impairments have been reported for reward‐based decision making [Eppinger et al., 2011; Mohr et al., 2010b; Weierich et al., 2011].
The neurobiological basis of reward processing in the human brain has recently been systematically described in a meta‐analysis including 142 brain imaging studies [Liu et al., 2011]. The authors showed that the core areas for reward processing are the ventral striatum (VS, also described as nucleus accumbens) and the orbitofrontal cortex. Additional key nodes of the reward network are prefrontal regions (dorsolateral prefrontal cortex [DLPFC], anterior cingulate cortex [ACC]), parietal regions (inferior parietal lobule [IPL]), and insular cortex. The VS is most active during anticipation of reward [Knutson and Greer, 2008] and also codes the prediction error, the difference between actual and expected outcome [O'Doherty et al., 2003]. The orbitofrontal or ventromedial prefrontal cortex (VMPFC) is usually co‐activated with the VS and is involved in reward value coding [Elliott et al., 2010; Kringelbach, 2005]. The ACC and the insular cortex are closely related to valence processing [Knutson and Greer, 2008; Shackman et al., 2011], whereas the lateral fronto‐parietal network including the DLPFC and the IPL is associated with the integration and representation of reward‐related information [Liu et al., 2011].
Adolescence is a highly vulnerable period of life. Behaviorally, adolescents show highly pronounced risk taking behavior, as measured by self‐reported questionnaires and risk preference tasks [Steinberg, 2008]. These behavioral characteristics have been related neurobiologically to alterations in the reward network in the brain. The cerebral changes in this period of life are characterized by an early maturation of the striatal reward system, while the development of prefrontal top–down control mechanisms is protracted [Casey et al., 2008; Galvan, 2010; Somerville and Casey, 2010]. Neuroimaging studies showed that adolescents show elevated subcortical activity compared with young adults [Galvan et al., 2006, 2007; van Leijenhorst et al., 2010]. Furthermore, in tasks involving inhibition and prefrontal top–down regulation, larger, more diffuse prefrontal activation patterns were observed in adolescents, when compared with adults [Casey et al., 2008]. As maturation proceeds toward adulthood, these diffuse activations turn into more specifically defined neural activation patterns. However, some imaging studies investigating reward anticipation showed no difference between adolescents and younger adults [Cho et al., 2013] or even contradictory results indicating a hypoactivation in adolescents compared with younger adults [Bjork et al., 2004, 2010]. Galvan [2010] concluded in her review that the reward system undergoes dramatic changes during adolescence, but still further studies are needed to explore whether the reward system is hyper‐ or hyporesponsive during adolescence.
With regard to the late adulthood (considering the age above 60 years old) functional imaging studies about reward processing focused mainly on the VS and showed heterogeneous results. Schott et al. [2007] and Dreher et al. [2008] reported decreased striatal activity in older compared with younger participants during reward anticipation. However, Samanez‐Larkin et al. [2007] and Rademacher et al. [2013] found no differences between younger and older adults during reward anticipation. Rademacher et al. [2013] assumed that this discrepancy might be due to different task demands. Although the reward tasks in the studies by Dreher et al. [2008] and Schott et al. [2007] required a decision between two options, the other two studies [Samanez‐Larkin et al., 2007; Rademacher et al., 2013] only required a simple button press and no decisions. Thus, the VS reward anticipation signal may be not affected by age in the context of a simple reward task without a decision component. Considering age‐related alterations in other reward‐related areas during reward anticipation, for instance prefrontal cortex, most studies have not focused on these areas or reported heterogeneous results (e.g., hypoactive DLPFC and hyperactive VMPFC in older adults [Dreher et al., 2008]). However, age‐related alterations are very prominent in non‐reward associated tasks, for example in the fronto‐parietal network. In particular during cognitive demanding tasks, areas involved in information integration and executive functioning (DLPFC and IPL) were reported to be activated less distinctly and more wide‐spread in older compared with younger adults suggesting that they process information less efficiently by requiring more neural resources [Grady, 2012].
In this study, we aimed to investigate developmental and age‐related alterations in the reward‐network during reward anticipation. Striatal reward‐related signals in a simple gambling paradigm might differ between adolescents and both younger and older adults due to imbalanced maturation of the striatum and prefrontal cortex. The same reason of incomplete maturation may lead to age‐related modulation of other key areas of the reward network associated with valence processing (ACC and insula). To further investigate this supposedly reduced top–down regulation in adolescence [Casey et al., 2008; Galvan, 2010; Somerville and Casey, 2010], we investigated functional connectivity of the right inferior frontal gyrus (rIFG), which has been associated with cognitive inhibition processes [Aron et al., 2004; Chambers et al., 2009]. Based on the above mentioned findings, we expected a stronger functional coupling between rIFG and VS for young adults compared with adolescents. According to age‐related changes in fronto‐parietal areas, which are associated with executive functions, we assumed stronger activations in older adults compared with the younger groups probably stemming from inefficient neural information processing.
MATERIALS AND METHODS
Participants
One hundred and fourteen healthy, right‐handed participants were recruited via newspaper and internet advertisements and by contacting senior clubs, bridge clubs, or schools. Twelve participants had to be excluded, because they showed excessive head movements (10; motion cutoff values were 3° for rotation and 3 mm for translation), were non‐compliant or took psychoactive medication (one each). The remaining 102 participants were assigned to three equal sized age groups of 34 participants each: adolescents (mean age = 14.9, SD = 0.52, age range: 13–16, 15 female), younger adults (mean age = 26, SD = 4.3, age range: 19–35, 15 female), and older adults (mean age = 67.5, SD = 5, age range: 61–80, 14 female). For the adolescents we chose 13–16 years old participants, because the imbalance between striatal and prefrontal maturation reaches strongest difference in this mid‐adolescent period [Steinberg, 2008]. The mean age of the recruited older adult group was comparable with other reward studies in aging, but the sample size was larger (mean age of older adults in years: 69 years old (n = 19) in Schott et al. [2007], 66 years old (n = 13) in Dreher et al. [2008], 73 years old (n = 12) in Samanez‐Larkin et al. [2007], and 66 years old (n = 24) in Rademacher et al. [2013]). Age groups did not differ regarding sex distribution (χ 2(2) = 0.1, P = 0.961) and the two adult groups regarding years of education (younger adults: mean = 16.8, SD = 2.8; older adults: mean = 16.3, SD = 3.3; t(65) = 0.78, P = 0.437). The three samples were further characterized in terms of memory span and working memory (WM) capacity (assessed with Digit Span Forward and Backward from the Wechsler Adult Intelligence Scale, Wechsler [1981]). The three groups differed in memory span (F(2,98) = 3.59, P = 0.031) with the two younger groups performing better than the older sample (adolescents > older adults: t(65) = 2.6, P = 0.011; younger adults > older adults: t(65) = 2.2, P = 0.031). Moreover, the groups showed different WM capacity (F(2,98) = 7.18, P = 0.001); the younger adults performed better than the other age groups (younger adults > adolescents: t(65) = 3.2, P = 0.002; younger adults > older adults: t(65) = 3.5, P = 0.001), which is in line with previous research [Grady, 2012; Heinzel et al., 2013; Luciana et al., 2005].
Participants had been screened with standardized questionnaires for the following exclusion criteria: MRI exclusion criteria (e.g., non‐removable ferromagnetic material), left‐handedness, use of psycho‐pharmacological or central nervous system active medication, neurological diseases, psychiatric, and any other clinically relevant disorders. The younger and older adult sample was furthermore screened for Axis I psychiatric disorders according to DSM‐IV (using the structured clinical interview, SCID I [First et al., 2001]). No participant reported an acute Axis I disorder. Adult participants gave written informed consent to the study, and a parent or a person, who has care and custody of the adolescent participant, also gave written informed consent before study participation. The study was approved by the local ethics committee of the Charité—Universitätsmedizin Berlin, Germany, and the Ethics Committee of the German Psychological Society, Münster, Germany.
Slot Machine Paradigm
We chose a slot machine paradigm to provide high accessibility across the life span. The virtual slot machine task was programmed using Presentation software (Version 14.9, Neurobehavioral Systems, Albany, CA) and consisted of three wheels displaying two different types of fruits (alternating cherries (C) and lemons (L)). Above and below the slot machine were two horizontal bars indicating the commands for start and stop of the machine.
The structure of each trial was as follows: At the beginning the wheels stood still and the bars were inactive (gray color). When the bars turned blue (indicating the start of a trial), the participant was able to start the machine by pressing a button with the right hand. After the button press, the bars turned gray again (inactive state) and the three wheels started to rotate vertically with different accelerations for each wheel (exponentially increasing from left to right). When the wheels reached their maximum rotation velocity (1.66 s after button press) the bars turned green. This color change indicated that the participant could stop the machine by pressing the button again. After this second button press, the three wheels successively stopped rotating from left to right. The left wheel stopped after a variable delay of 0.48 and 0.62 s after the button press, while the middle and right wheel were still rotating. The second wheel stopped after an additional variable delay of 0.73 and 1.18 s. The right wheel stopped rotating after the middle wheel with a variable delay of 2.64 and 3.25 s. The stop of the third wheel terminated the trial and a feedback about the current win and the total amount of reward was displayed above the slot machine. The bars changed from gray to blue again and the next trial started after a variable delay ranging between 4.0 and 7.48 s that was characterized by an exponentially decreasing function (see Fig. 1).
Figure 1.
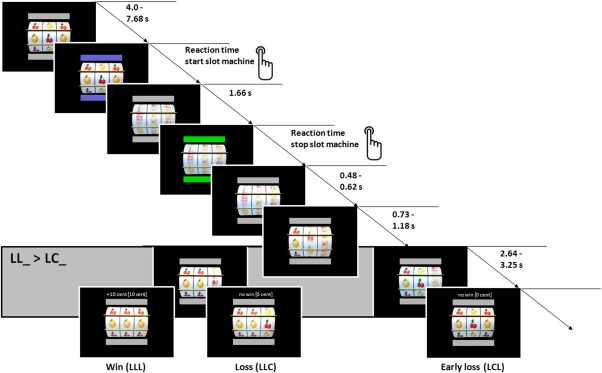
Structure of the slot machine task. FMRI analysis focused on time when the second wheel stopped, when the first two wheels display the same fruit (LL_) or when the first two wheels displayed different fruits (LC_) while the third wheel was still rotating. L = Lemon, C = Cherry. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Participants gained 10 cents per trial, when all fruits in a row were of the same identity (LLL or CCC); if not, participants did not win (LLC, CCL, LCL, CLC, LCC, or CLL). The experiment consisted of 60 trials in total. The slot machine was determined with a fixed number of 20 win trials (CCC or LLL) and 40 pseudo‐randomized loss (LLC or CCL) or early loss (LCL, CLC, LCC or CLL) trials. Participants had no influence on winning or losing and participants won a fixed amount of 200 cents at the end of the task.
Scanning Procedure
Acquisition of imaging data was conducted at the Berlin Center for Advanced Neuroimaging on the Campus Charité Mitte. A 3 T Siemens TIM Trio Scanner (Erlangen, Germany), equipped with a 12 channel head coil was used. The paradigm was presented via a video projector on a mirror system on top of the head coil. Functional imaging was conducted using axially aligned gradient echo planar imaging (EPI) with the following parameters: 33 slices, descending slice order, time to repeat (TR) = 2 s, time to echo (TE) = 30 ms, field of view (FoV) = 192 × 192, flip angle = 78°, matrix size = 64 × 64, voxel size = 3 × 3 × 3 mm3. For anatomical reference, 3D anatomical images of the whole brain were obtained by T1‐weighted magnetization prepared gradient‐echo sequence (MPRAGE; 192 sagittal slices, TR = 1,900 ms, TE = 2.52 ms, flip angle = 9°, FoV = 256 × 256, matrix size = 256 × 256, voxel size = 1 × 1 × 1 mm3).
Data Analysis
Image processing
MRI data was analyzed using Statistical Parametric Mapping software package (SPM8, Wellcome Department of Imaging Neuroscience, London, UK). EPIs were transformed from Dicom into Nifti file format. We further corrected EPIs for slice timing and head motion and transformed them into the stereotactic normalized standard space of the Montreal Neuroimaging Institute using the unified segmentation algorithm as implemented in SPM8. Finally, EPIs were resampled (voxel size = 3 × 3 × 3 mm3) and spatially smoothed with a 3D Gaussian kernel of 7 mm full width at half maximum.
Statistical analysis with classical event‐related approach
A two‐stage mixed‐effects general linear model (GLM) was applied. On single subject level, event‐related separate regressors were included for gain anticipation (LL_ and CC_) and no gain anticipation (CL_ and LC_). The following separate regressors of no interest were also included: gain (LLL and CCC), loss (CCL and LLC), early loss (CLC, CLL, LCL, and LCC), button presses (after bar changed to blue as well as green), visual flow (rotation of the wheels), and the six rigid body movement parameters. Differential t‐contrasts for gain anticipation (LL_ and CC_) versus no gain anticipation (CL_ and LC_) were calculated and taken to group level analysis. On the second level, these differential t‐contrast images were entered into a full factorial analysis of variance (ANOVA) with the factor group (adolescents, younger adults, older adults).
Whole brain effects were corrected for multiple comparisons using a Monte Carlo simulation based cluster size correction (AlphaSim, Song et al. [2011]). One thousand Monte Carlo simulations revealed a corresponding alpha error probability of P < 0.05, when using a minimum cluster size of 17 adjacent voxels with a statistical threshold of P < 0.001. Activation patterns were first reported for each group separately first and then between group comparisons were reported. According to the meta‐analysis by Liu et al. [2011] about 142 reward studies, activation differences were expected during reward anticipation in the two core areas of reward: VS and VMPFC. Furthermore, we expected effects in the key nodes of the reward network associated with valence processing (ACC and insula) and information integration (DLPFC and IPL). Due to these a priori hypotheses, we further conducted post hoc analyses within the key nodes of this reward network using a region of interest (ROI) analysis. To this end, we used literature‐based ROIs for the VS, VMPFC, ACC, and insula. These ROIs were created by combining previous functional findings regarding reward anticipation (predominantly monetary incentive delay task articles) with anatomical limits to gray brain tissue [Schubert et al., 2008]. Detailed information about the calculation of these ROIs is described in the Supporting Information. For fronto‐parietal brain areas (DLPFC and IPL) standard ROIs as described in the Anatomic Labeling (AAL) brain atlas [Tzourio‐Mazoyer et al., 2002] were used. To test the group effect exclusively within this network, post hoc multivariate analysis of variance (MANOVA) with the factor group (adolescents, younger adults, versus older adults) was calculated using PASW Statistics 18 (SPSS, Chicago, IL). A control ROI analysis with the bilateral Heschl's gyri (primary auditory cortex) was conducted using an anatomical ROI from the AAL brain atlas, because this region should be independent from age effects and experimental paradigm.
Functional connectivity analysis
To test functional coupling within fronto‐striatal reward circuitry, we conducted a functional connectivity analysis between rIFG activity (seed region) and VS. The rIFG plays an important role in the prefrontal cognitive inhibition mechanisms [Aron et al., 2004], particularly the pars triangularis [Chambers et al., 2009]. Specifically, the rIFG is involved not only in motor‐related suppression processes like response inhibition [Aron et al., 2004; Swick et al., 2011], but also in cognitive inhibition processes in the context of semantics [Severens et al., 2011; Xue et al., 2008], beliefs [Goel and Dolan, 2003; Tsujii et al., 2010], and incentive cues [Lorenz et al., 2013]. Therefore, the rIFG is probably a key node for top–down control within the fronto‐striatal reward circuitry.
The time course of the likely inhibitory related signal in the rIFG was used to conduct a psychophysiological interaction (PPI) analysis. This method allows analyzing the temporal association of a target region (here rIFG) with other brain areas taking a psychological variable into account (here gain anticipation). For each participant, the local maximum of the effect of gain anticipation against no gain anticipation was identified within the rIFG (pars triangularis) ROI (as provided in the AAL atlas). Then the first eigenvariate time series (adjusted for experimental effects of interest) was individually extracted of each local maximum using a sphere of 4 mm. Furthermore, the resulting time course t rIFG was convolved with the experimental event‐related psychological functions of gain anticipation (LL_ and CC_) against no gain anticipation (CL_ and LC_) P gain>nogain to the psycho‐physiological interaction vector X gain>nogain. This vector was reconvolved with the canonical haemodynamic response function and included into a new first level GLM. We further added regressors of no interest to the new GLM: time course t rIFG, the psychological function P gain>nogain, and the six rigid body movement parameters. Linear contrast images of the PPI vector X gain>nogain were calculated for each participant and entered into a second level full factorial ANOVA with the factor group (adolescents, younger adults, older adults). To test functional coupling between rIFG and VS, small volume correction within the literature‐based ROI of the VS (see Supporting Information) was used for between group t‐tests.
RESULTS
Whole Brain Analysis
We calculated an ANOVA with the factor age‐group (adolescents, younger adults, older adults) using the individual differential t‐contrast images for the contrast gain anticipation against no gain anticipation. All results are presented in Figure 2.
Figure 2.

Main effects of brain response during gain anticipation against no gain anticipation for each age group. Results are thresholded with P < 0.05 corrected for multiple comparisons using a Monte Carlo simulation based cluster‐size correction. Coronal (Y = 12, upper column) and axial cuts (Z = −2, middle column) as well as a top view on the brain (lower column) are shown. R = right, L = left, A = anterior, P = posterior. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Group Specific Effects
The three groups showed activation differences in the cortico‐striatal reward network including subcortical (bilateral VS and thalamus), prefrontal (bilateral DLPFC, bilateral, precentral gyrus, ACC, and SMA), anterior insular and parietal areas (bilateral IPL and superior parietal lobule (SPL)). Furthermore occipital areas were activated by gain anticipation. Descriptively, the activation differences in adolescents and older adults were more diffuse, the cluster extents larger and the peak voxel reached higher t‐values. The older adult group also showed a strong recruitment of fronto‐parietal areas (DLPFC, IPL, and SPL). See Figure 2 and Supporting Information Table S1 for details.
Group Comparisons
Younger adults > adolescents and younger adults > older adults. These contrasts revealed no significant effects.
Adolescents > younger adults. A significantly stronger difference was found for the adolescents compared with the younger adult group in the VS (bilateral) and VMPFC (left). Furthermore, activation differences were found in the occipital cortex and middle/posterior cingulate gyrus.
Adolescents > older adults. No significant differences within the reward network were observed.
Older adults > adolescents. The older adult group showed a stronger activation difference compared with the adolescents within a fronto‐parietal network including bilateral IPL, bilateral DLPFC, bilateral inferior frontal gyrus, and superior medial frontal gyrus.
Older adults > younger adults. Similar to the contrast of older adults versus adolescents, a fronto‐parietal network was activated in older adults compared with younger adults. Additionally, activation differences were found in orbital gyrus, bilateral insular cortices and supplementary motor area.
Altogether, the whole brain analysis showed a strong activation of the a priori hypothesized reward network in all groups. However, group comparisons revealed that adolescents activated core reward regions (VS and VMPFC) more strongly than younger adults. Furthermore, older adults showed a stronger recruitment of fronto‐parietal regions compared with both younger groups (see Supporting Information Table S2).
ROI Analysis
Based on the meta‐analysis of Liu et al. [2011] we extracted mean ROI values for each a priori defined brain area within the reward network per participant. A MANOVA with the factor group (adolescents, younger adults, older adults) and the six dependent variables of VS, VMPFC, insula, ACC, DLPFC, and IPL was conducted. The analysis revealed a significant effect of group (F(12, 188) = 5.22, P < 0.001). Follow‐up ANOVAs for each ROI revealed a significant effect within all ROIs. Post hoc least significant difference tests revealed significant differences between adolescents and younger adults in the core reward regions VS and VMPFC as well as in the valence processing associated regions insula and ACC. Furthermore, the difference in VS between adolescents and older adults was significant. Older adults showed a significantly stronger activation difference, when compared with both groups (adolescents and younger adults) in fronto‐parietal regions (DLPFC and IPL) and as compared with only younger adults in ACC and insula. For graphical illustration, see Figure 3. Statistical values of these analyses are listed in the Supporting Information Tables S3 (ANOVAs) and S4 (post hoc tests). A control analysis with the bilateral Heschl's gyri revealed a non‐significant result (F(2) = 0.86, P = 0.425) confirming that the other ROIs were specifically activated by the experimental reward manipulation.
Figure 3.
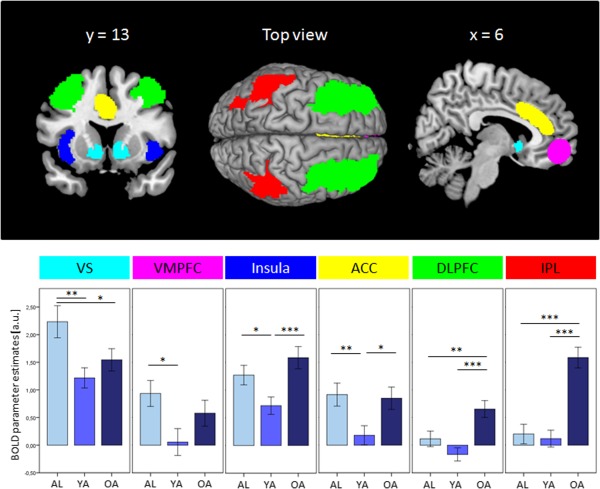
Effects of the ROI analysis during gain anticipation against no gain anticipation for each group. Upper row: Anatomical presentation of ROIs. Bottom row: Bar graphs for each ROI and group (x‐axis). Y‐axis represents the mean BOLD parameter estimates of each ROI in arbitrary units. Error bars represent standard error of means. ROI = region of interest, VS = ventral striatum, VMPFC = ventromedial prefrontal cortex, ACC = anterior cingulate cortex, DLPFC = dorsolateral prefrontal cortex, IPL = inferior parietal lobule, AL = adolescents, YA = younger adults, OA = older adults, a.u. = arbitrary units. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To sum up, the key areas within the reward network show age‐related alterations during reward anticipation. The core areas of reward processing (VS and VMPFC) show significant differences between adolescents and younger adults. Within the valence processing associated regions (insula and ACC) a U‐shape characteristic was found across age. Finally, a J‐shape with a stronger neural recruitment in older adults was observable in fronto‐parietal regions.
PPI Analysis
To test functional coupling within fronto‐striatal reward circuitry during reward anticipation, a functional connectivity analysis was conducted with the seed in right inferior frontal gyrus (rIFG) pars triangularis that is associated with cognitive inhibition processes [Aron et al., 2004; Chambers et al., 2009]. To justify this analysis, we first checked, whether the effect of gain anticipation revealed a significant effect within the rIFG. Small volume correction of a contrast over all groups revealed a significant effect within this ROI (MNI coordinates [x y z] = 42, 26, 1; t(99) = 7.5, P < 0.001).
We conducted a small volume analysis within the VS for the comparisons, younger adults versus adolescents, younger adults versus older adults, and adolescents versus older adults. Only one significant effect was found for the comparison younger adults versus adolescents in the right VS (MNI coordinates [x y z] = 12, 11, 4; t(99) = 3.1, P = 0.039) indicating a stronger negative coupling between the rIFG and the right VS for younger adults compared with adolescents. In other words, the temporal negative correlation during gain anticipation versus no gain anticipation was stronger in younger adults than adolescents (see Fig. 4).
Figure 4.
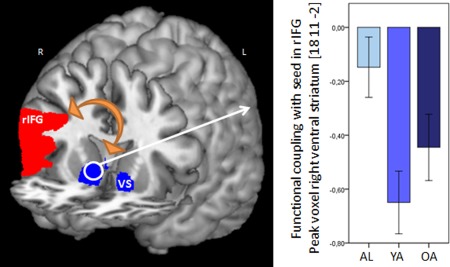
PPI analysis. Left panel: Schematic illustration of PPI analysis. A temporal correlation with the seed in the rIFG pars triangularis (red area) was calculated. Results are presented for the target region VS (blue area). Right panel: Younger adults showed a stronger negative functional coupling between rIFG and right VS compared with adolescents. Error bars represent standard error of means. rIFG = right inferior frontal gyrus, VS = ventral striatum, AL = adolescents, YA = younger adults, OA = older adults. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Exploratory Analysis: Relationship Between Imaging Data and WM Performance
We found a stronger recruitment of fronto‐parietal areas in older adults compared with the two younger groups. These areas are associated with executive functions such as WM performance [Grady, 2012]. In WM tasks with low task demand, the WM network is recruited more strongly in older adults compared with younger adults at the same performance level (see discussion for more details). To test, whether the activation difference in fronto‐parietal regions during reward anticipation (low cognitive demand) is associated with executive functions, we correlated WM performance assessed by a Digit Span Backwards with the brain activity during reward anticipation in the above reported ROIs.
A significant negative correlation (Spearman's rho) was observed between performance in Digit Span Backward and brain activity in insula (r(101) = −0.265, P = 0.007), DLPFC (r(101) = −0.265, P = 0.007), and IPL (r(101) = −0.342, P = 0.001). We further tested this relationship within each group separately and found significant correlations only within the older adults in insula (r(34) = −0.453, P = 0.007) and IPL (r(34) = −0.394, P = 0.021). That means, the lower the WM performance, the higher the neural recruitment of insula and IPL in older adults.
DISCUSSION
The aim of this study was to investigate developmental and age‐related alterations in the reward network during reward anticipation. Changes in functional activity across the lifespan were characterized in different network components in different trajectories: The core areas of reward processing (VS and VMPFC) were characterized by a higher activation in the adolescents compared with both adult groups, the valence processing associated areas (ACC and insula) were characterized by a U‐shaped relationship (lower activation in younger adults compared with both adolescents and older adults), and executive function associated areas (DLPFC and IPL) were characterized by a J‐shape (highest activation in older adults) relationship of age and brain activation. We further showed a more pronounced negative functional coupling between the inhibition‐related area rIFG and the reward core area VS in the younger adults relative to adolescents.
Core Areas of Reward Processing
Consistent with previous research investigating reward anticipation, adolescents showed stronger recruitment of reward core areas VS and VMPFC compared with younger adults. This finding fits to previous research exploring this hyperresponsive striatum by a maturation imbalance between the early matured striatum and a protracted development of the prefrontal cortex [Galvan et al., 2007; Galvan, 2010; Somerville and Casey, 2010]. Furthermore, this result contradicts the findings by Bjork et al. [2004, 2010] showing a hyporesponsive striatal response to reward cues. The reason for this discrepancy might be related to the age‐range of the participants. The Bjork studies had a wider age range (12–17) than the other studies (Galvan et al., 2006, 2007: 13–17; van Leijenhorst et al., 2010: 14–15) which might be crucial due to the assumption that the striatal‐prefrontal maturation imbalance is strongest during mid‐adolescence between 13 and 16 years [Steinberg, 2008]. Therefore, this study only included adolescent participants within the specific age range between 13 and 16. Furthermore, different task designs might also have an influence on results. Whereas the Bjork studies used monetary incentive delay tasks with abstract cues, developed for adults [Knutson et al., 2001], the other studies used cartoons [Galvan et al., 2006, 2007] or a slot machine task [van Leijenhorst et al., 2010]. The latter stimuli may probably be more appealing for adolescents than abstract cues [Richards et al., 2013]. Together, our results support the hypothesis of a hyperresponsive striatum to reward cues during adolescence.
According to the results observed in the adult groups, we did not find a difference between younger and older adults during reward anticipation, which coincides with findings by Samanez‐Larkin et al. [2007] and Rademacher et al. [2013]. Our data support the assumption that no striatal age‐related differences are observable within an adult age range, when only a simple reaction task was used without a decision component [Rademacher et al., 2013]. In contrast, when the investigated tasks included a decision between at least two options, two studies found differences between younger and older adults [Dreher et al., 2008; Schott et al., 2007]. Future studies should investigate this issue systematically by varying response complexity (e.g., reaction task versus decision between two options).
Valence Processing Associated Areas: U‐Shape
In a recent meta‐analysis about reward processing, Liu et al. [2011] discussed neural processing in the insula and ACC in the context of valence processing. More specifically, in the context of reward, there is evidence that the insula is associated with two different functions. On the one hand, the insula is likely to be involved in the subjective integration of affective information [Singer et al., 2009]. Furthermore, the anticipation of reward is a salient event and might lead to a subjective state of arousal and awareness [Craig, 2009]. The anticipation of gain during slot machine gambling in this study was a highly salient and arousing event that may thus have driven the insula activation. On the other hand, activity in the insula may be also associated with uncertainty [Huettel et al., 2005; Paulus et al., 2003; Preuschoff et al., 2008]. In this study, high uncertainty is given during gain anticipation (LL_ or CC_) compared with no gain anticipation (LC_ or CL_), when a certain loss feedback was followed. We believe that the insula activation observed here may reflect both arousal and uncertainty.
Still, the question arises why insula activation follows a U‐shaped pattern across age groups. Considering the insula function of arousal coding, van Leijenhorst et al. [2010] found elevated insular activation in adolescents compared with younger adults during gain anticipation. Due to the early maturation of the striatal reward areas during adolescence, reward cues might be very salient and may lead to an enhanced arousal in adolescence compared with young adults probably resulting in the insular hyperactivity in adolescents. In another aging study, decreased activation in older adults compared with younger adults during loss anticipation (against a neutral condition) was observed in the insula [Samanez‐Larkin et al., 2007]. The authors argued that older adults experience negative emotions (e.g., disappointment after a loss) as less arousing compared with younger adults [Carstensen and Mikels, 2005]. In this study, we contrasted reward anticipation trials against trials when a gain was already impossible (LC_) and a certain loss was followed. Thus, the stronger activation difference in older adults might be driven by a weaker neural processing in the loss trials as shown by Samanez‐Larkin et al. [2007].
Another explanation of the U‐shaped pattern across age groups might relate to the neural processing of uncertainty, which is also related to the insular cortex. Adolescents and older adults may not be as efficient as the younger adults in estimating uncertainty or outcome probabilities [Eppinger et al., 2011; van Leijenhorst et al., 2010; Mohr et al., 2010b; Weierich et al., 2011]. Speculatively, a neural correlate of this impairment may be an insular hyperactivation, which might be interpreted as inefficient neural processing and an attempt to compensate that is accompanied by a stronger neural recruitment [Grady, 2012]. To sum up, the U‐shape developmental trajectory across the age groups might reflect an ineffective ability to estimate reward probabilities and different subjective experienced arousal across the age groups.
Regarding the ACC, a recent review by Shackman et al. [2011] described the ACC as a hub, which links reward‐ to motor‐related information, to shape emotional‐driven behavior. In the context of positive effect, the authors underlined the importance of the ACC for the coding of uncertainty and for the anticipation of reward. The expectation of the anticipated reward [Shenhav et al., 2013] might reflect the activation difference in the ACC across age groups in this study and could be interpreted similar to the changes in the insula: The estimation of expected reward probability might be inefficient in adolescents and older adults compared with younger adults, leading to increased compensation efforts.
Executive Function Related Areas: J‐Shape
In this study, we found increased DLPFC and IPL activity in older adults compared with adolescents and younger adults. In general, these areas are involved in executive functions [Nee et al., 2013] and attentional control processes [Corbetta and Shulman, 2002]. Furthermore, DLPFC activity was associated to learning of conditioned relationships between cue and consequence [Fletcher et al., 2001] and IPL activity was related to representation of numerical stimuli and mathematical operations [Kadosh et al., 2005] as well as to integration of reward‐related information [Liu et al., 2011; Mohr et al., 2010a].
In a recent review about age‐related neural changes, Grady [2012] showed that many working memory (WM) studies reported a hyperactivation in the WM network in older adults compared with younger adults during low WM loads. Conducting a WM task demands strongly executive functions and attentional processes and leads neurally to a recruitment of the brain areas IPL and DLPFC amongst others [Nee et al., 2013; Owen et al., 2005]. To achieve the same performance, older adults recruit the WM network more intensely than younger adults under low difficulty conditions. Transferred to this study, we assume that gain anticipation leads to an increased attentional focus to the third still rotating wheel of the slot machine, which may require a low cognitive demand. Possibly this demand is reflected by the neural recruitment of brain areas associated with executive functions. We tested this hypothesis with an exploratory correlational analysis between WM performance and brain activity during reward anticipation. Negative relationships were observed only for the older adults in the insula and IPL, not in younger adults or adolescents. The lower the WM performance, the stronger the activation differences in these areas. We assume that for many older adults (especially these with low WM performance) the cognitive task demands are relatively high, which may be neurally reflected by a stronger recruitment of areas associated with executive functions. This hyperactivation in older adults might be an age‐specific compensatory mechanism for inefficient neural processing [Grady, 2012]. Moreover, the present fronto‐parietal hyperactivation pattern in older adults spatially overlaps with the activity pattern across a variety of WM tasks as found in a recent meta‐analysis [Nee et al., 2013] and also the pattern of the neural correlate of Digit Span Backward in older adults [Sun et al., 2005].
Fronto‐Striatal Functional Connectivity Changes
The striatal hyperactivation during reward anticipation in adolescence has previously been explained by an immaturity of the prefrontal cognitive control network [Casey et al., 2008; Galvan, 2010; Somerville and Casey, 2010]. This hypothesis is not only reflected by activation differences in frontal and striatal reward‐related areas, but also in the connectivity within this circuitry. Maturation processes are structurally associated with strengthenings of relevant connections and consequently with an improvement of cognitive capabilities like for instance top–down inhibition [Casey et al., 2008; Luna et al., 2010; Steinberg, 2008].
Consistent with this connectivity assumption, we found a stronger negative functional coupling between the inhibition associated area rIFG and the VS in younger adults compared with adolescents. This finding suggests that inhibition related activation in rIFG probably coincides with a temporal deactivation in the VS in younger adults, which was not seen in adolescents. This finding is also in line with a structural developmental study showing a relationship between diffusion of white matter tracts, increasing age and cognitive control [Liston et al., 2006] and a developmental functional connectivity study demonstrating that a stronger fronto‐striatal coupling was accompanied by increasing age and impulsive choice behavior in a delay discounting task [Christakou et al., 2011].
Furthermore, in this study, older adults also showed a negative coupling between rIFG and VS, but the coupling strength did not differ between older adults and adolescents or younger adults suggesting an intermediate coupling strength in older adults.
Reward Network Alteration Across the Lifespan and Their Relationship to Structural Brain Changes
Considering the developmental and age‐related changes in the three discussed reward modules, three distinct developmental trajectories have been found in this study. The reasons for these alterations are complex and manifold. Brain development across the lifespan is characterized by changes in the neurotransmitter systems, in gray and white matter structures as well as by psychological and behavioral changes [Casey et al., 2008; Galvan, 2010; Goh, 2011; Good et al., 2001; Luna et al., 2010; Raz et al., 2005; Sowell et al., 2003]. In closing, we want to briefly outline these ontogenetic changes in relationship to these findings in the three discussed reward modules.
The hyperactivation of the core areas of reward processing (VS and VMPFC) during adolescence may be associated with the higher levels of dopamine during adolescence [Galvan, 2010; Wahlstrom et al., 2010] and the lower inhibitory top–down control might be caused by a not fully matured prefrontal cortex [Casey et al., 2008; Galvan, 2010; Somerville and Casey, 2010]. During late adulthood, striatal reward signal seems to be preserved in simple tasks. In more complex reward‐based tasks requiring strategic decision making for optimizing reward, older adults showed behavioral impairments and alterations in neural activity [Eppinger et al., 2011; Marschner et al., 2005; Mell et al., 2009; Mohr et al., 2010b]. Speculatively, older adults failed to integrate striatal reward signals for decision making. An impairment in the gating function of the striatal signal to the prefrontal cortex may be the reason for this finding, which further could be related to age‐related structural changes (dopaminergic decline and demyelination of white matter tracks).
Furthermore, in this study, a U‐shape activation pattern was observed in brain areas related to valence processing. We suggest that this age‐related association emerges from psychological and behavioral changes, in particular emotional valence attribution. During adolescence, rewarding stimuli are highly salient and may lead to impulsive and risk‐taking behavior [Casey et al., 2008; Galvan, 2010]. Elderly participants also show an “positivity effect” and focus more strongly to positive than negatively valenced information [Carstensen and Mikels, 2005] probably due to the limited life time perspective [Mather and Carstensen, 2005]. Thus, two different mechanisms—an impaired prefrontal cognitive control (adolescents) and a strong emotion regulation to positive stimuli (older adults)—may result in stronger neural recruitment in adolescents and older adults compared with young adults.
Finally, in this study, the age‐related hyperactivation in brain areas associated with executive functions seems to be unique for the older adult group. This finding might indicate an age‐related inefficient neural information processing and might therefore be a compensatory mechanism for age‐related gray matter loss [Grady, 2012].
CONCLUSION
Taken together, in a large sample of 102 participants we were able to demonstrate that functional lifespan‐related changes are present in different reward processing modules with different trajectories. These findings are fundamental to human neurobiology and behavior, as reward processing forms the basis of any adaptive behavior of the human being and apparently underlies lifespan‐related changes. We believe that this work increases the understanding of the particular key nodes within the frontal, parietal, and subcortical reward network and the lifespan‐related changes.
ACKNOWLEDGMENTS
The authors thank J. Frenzel, L. Adam, S. Luo, S. Golde, and E. Flemming for assistance during data collection.
Supporting information
Supporting Information
REFERENCES
- Aron AR, Robbins TW, Poldrack RA (2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8:170–177. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW (2004): Incentive‐elicited brain activation in adolescents: Similarities and differences from young adults. J Neurosci 24:1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Chen G, Hommer DW (2010): Adolescents, adults and rewards: Comparing motivational neurocircuitry recruitment using fMRI. PLoS ONE 5:e11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA (2005): At the intersection of emotion and cognition aging and the positivity effect. Curr Dir Psychol Sci 14:117–121. [Google Scholar]
- Casey BJ, Jones RM, Hare TA (2008): The adolescent brain. Ann N Y Acad Sci 1124:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA (2009): Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev 33:631–646. [DOI] [PubMed] [Google Scholar]
- Cho YT, Fromm S, Guyer AE, Detloff A, Pine DS, Fudge JL, Ernst M (2013): Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. NeuroImage 66:508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K (2011): Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. NeuroImage 54:1344–1354. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Craig AD (Bud) (2009): How do you feel—Now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. [DOI] [PubMed] [Google Scholar]
- Dreher J‐C, Meyer‐Lindenberg A, Kohn P, Berman KF (2008): Age‐related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci USA 105:15106–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Agnew Z, Deakin JFW (2010): Hedonic and Informational functions of the human orbitofrontal cortex. Cereb Cortex 20:198–204. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Hämmerer D, Li S‐C (2011): Neuromodulation of reward‐based learning and decision making in human aging. Ann N Y Acad Sci 1235:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J (2001): Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID‐I/P W/ PSY SCREEN). New York: New York State Psychiatric Institute. [Google Scholar]
- Fletcher PC, Anderson JM, Shanks DR, Honey R, Carpenter TA, Donovan T, Papadakis N, Bullmore ET (2001): Responses of human frontal cortex to surprising events are predicted by formal associative learning theory. Nat Neurosci 4:1043–1048. [DOI] [PubMed] [Google Scholar]
- Galvan A (2010): Adolescent development of the reward system. Front Hum Neurosci 4. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2826184/. [DOI] [PMC free article] [PubMed]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ (2006): Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk‐taking behavior in adolescents. J Neurosci 26:6885–6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare T, Voss H, Glover G, Casey BJ (2007): Risk‐taking and the adolescent brain: Who is at risk? Dev Sci 10:F8–F14. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ (2003): Explaining modulation of reasoning by belief. Cognition 87:B11–B22. [DOI] [PubMed] [Google Scholar]
- Goh JOS (2011): Functional dedifferentiation and altered connectivity in older adults: Neural accounts of cognitive aging. Aging Dis 2:30–48. [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Fristen KJ, Frackowiak RSJ (2001): A Voxel‐Based Morphometric Study of Ageing in 465 Normal Adult Human Brains. NeuroImage. 14:21–36. [DOI] [PubMed] [Google Scholar]
- Grady C (2012): The cognitive neuroscience of ageing. Nat Rev Neurosci 13:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S, Schulte S, Onken J, Duong Q‐L, Riemer TG, Heinz A, Kathmann N, Rapp MA (2013): Working memory training improvements and gains in non‐trained cognitive tasks in young and older adults. Aging Neuropsychol Cogn 0:1–28. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G (2005): Decisions under uncertainty: Probabilistic context influences activation of prefrontal and parietal cortices. J Neurosci 25:3304–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh RC, Henik A, Rubinsten O, Mohr H, Dori H, van de Ven V, Zorzi M, Hendler T, Goebel R, Linden DEJ (2005): Are numbers special?: The comparison systems of the human brain investigated by fMRI. Neuropsychologia 43:1238–1248. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D (2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21:RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Greer SM (2008): Anticipatory affect: Neural correlates and consequences for choice. Philos Trans R Soc B Biol Sci 363:3771–3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML (2005): The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci 6:691–702. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA (2010): What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex 20:61–69. [DOI] [PubMed] [Google Scholar]
- Liston C, Watts R, Tottenham N, Davidson MC, Niogi S, Ulug AM, Casey BJ (2006): Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb Cortex 16:553–560. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J (2011): Common and distinct networks underlying reward valence and processing stages: A meta‐analysis of functional neuroimaging studies. Neurosci Biobehav Rev 35:1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz RC, Krüger J‐K, Neumann B, Schott BH, Kaufmann C, Heinz A, Wüstenberg T (2013): Cue reactivity and its inhibition in pathological computer game players. Addict Biol 18:134–146. [DOI] [PubMed] [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS (2005): The development of nonverbal working memory and executive control processes in adolescents. Child Dev 76:697–712. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, O'Hearn K (2010): What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn 72:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, Heekeren HR (2005): Reward‐based decision‐making and aging. Brain Res Bull 67:382–390. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL (2005): Aging and motivated cognition: The positivity effect in attention and memory. Trends Cogn Sci 9:496–502. [DOI] [PubMed] [Google Scholar]
- Mell T, Wartenburger I, Marschner A, Villringer A, Reischies FM, Heekeren HR (2009): Altered function of ventral striatum during reward‐based decision making in old age. Front Hum Neurosci 3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PNC, Biele G, Heekeren HR (2010a): Neural processing of risk. J Neurosci 30:6613–6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr PNC, Li S‐C, Heekeren HR (2010b): Neuroeconomics and aging: Neuromodulation of economic decision making in old age. Neurosci Biobehav Rev 34:678–688. [DOI] [PubMed] [Google Scholar]
- Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, Jonides J (2013): A meta‐analysis of executive components of working memory. Cereb Cortex 23:264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ (2003): Temporal difference models and reward‐related learning in the human brain. Neuron 38:329–337. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E (2005): N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB (2003): Increased activation in the right insula during risk‐taking decision making is related to harm avoidance and neuroticism. NeuroImage 19:1439–1448. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P (2008): Human insula activation reflects risk prediction errors as well as risk. J Neurosci 28:2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Salama A, Gründer G, Spreckelmeyer KN (2013): Differential patterns of nucleus accumbens activation during anticipation of monetary and social reward in young and older adults. Soc Cogn Affect Neurosci. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD (2005): Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex 15:1676–1689. [DOI] [PubMed] [Google Scholar]
- Richards JM, Plate RC, Ernst M (2013): A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev 37:976–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez‐Larkin GR, Gibbs SEB, Khanna K, Nielsen L, Carstensen LL, Knutson B (2007): Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci 10:787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Niehaus L, Wittmann BC, Schütze H, Seidenbecher CI, Heinze H‐J, Düzel E (2007): Ageing and early‐stage Parkinson's disease affect separable neural mechanisms of mesolimbic reward processing. Brain J Neurol 130:2412–2424. [DOI] [PubMed] [Google Scholar]
- Schubert R, Ritter P, Wustenberg T, Preuschhof C, Curio G, Sommer W, Villringer A (2008): Spatial attention related SEP amplitude modulations covary with BOLD signal in S1‐A simultaneous EEG‐fMRI study. Cereb Cortex 18:2686–2700. [DOI] [PubMed] [Google Scholar]
- Severens E, Kühn S, Hartsuiker RJ, Brass M (2011): Functional mechanisms involved in the internal inhibition of taboo words. Soc Cogn Affect Neurosci. Available at: http://scan.oxfordjournals.org/content/early/2011/05/23/scan.nsr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011): The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12:154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD (2013): The expected value of control: An integrative theory of anterior cingulate cortex function. Neuron 79:217–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K (2009): A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci 13:334–340. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey B (2010): Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol 20:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X‐W, Dong Z‐Y, Long X‐Y, Li S‐F, Zuo X‐N, Zhu C‐Z, He Y, Yan C‐G, Zang Y‐F (2011): REST: A toolkit for resting‐state functional magnetic resonance imaging data processing. PLoS ONE 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nat Neurosci 6:309–315. [DOI] [PubMed] [Google Scholar]
- Steinberg L (2008): A social neuroscience perspective on adolescent risk‐taking. Dev Rev 28:78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhang X, Chen X, Zhang P, Bao M, Zhang D, Chen J, He S, Hu X (2005): Age‐dependent brain activation during forward and backward digit recall revealed by fMRI. NeuroImage 26:36–47. [DOI] [PubMed] [Google Scholar]
- Swick D, Ashley V, Turken U (2011): Are the neural correlates of stopping and not going identical? Quantitative meta‐analysis of two response inhibition tasks. NeuroImage 56:1655–1665. [DOI] [PubMed] [Google Scholar]
- Tsujii T, Masuda S, Akiyama T, Watanabe S (2010): The role of inferior frontal cortex in belief‐bias reasoning: An rTMS study. Neuropsychologia 48:2005–2008. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002): Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M (2010): Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev 34:631–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1981): Wechsler adult intelligence scale‐revised manual. N Y Psychol Corp 84–85. [Google Scholar]
- Weierich MR, Kensinger EA, Munnell AH, Sass SA, Dickerson BC, Wright CI, Barrett LF (2011): Older and wiser? An affective science perspective on age‐related challenges in financial decision making. Soc Cogn Affect Neurosci 6:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G, Aron AR, Poldrack RA (2008): Common neural substrates for inhibition of spoken and manual responses. Cereb Cortex 18:1923–1932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


