Abstract
In our daily lives, we are confronted with a large amount of information. Because only a small fraction can be encoded in long‐term memory, the brain must rely on powerful mechanisms to filter out irrelevant information. To understand the neuronal mechanisms underlying the gating of information into long‐term memory, we employed a paradigm where the encoding was directed by a “Remember” or a “No‐Remember” cue. We found that posterior alpha activity increased prior to the “No‐Remember” stimuli, whereas it decreased prior to the “Remember” stimuli. The sources were localized in the parietal cortex included in the dorsal attention network. Subjects with a larger cue‐modulation of the alpha activity had better memory for the to‐be‐remembered items. Interestingly, alpha activity reflecting successful inhibition following the “No‐Remember” cue was observed in the frontal midline structures suggesting preparatory inhibition was mediated by anterior parts of the dorsal attention network. During the presentation of the memory items, there was more gamma activity for the “Remember” compared to the “No‐Remember” items in the same regions. Importantly, the anticipatory alpha power during cue predicted the gamma power during item. Our findings suggest that top‐down controlled alpha activity reflects attentional inhibition of sensory processing in the dorsal attention network, which then finally gates information to long‐term memory. This gating is achieved by inhibiting the processing of visual information reflected by neuronal synchronization in the gamma band. In conclusion, the functional architecture revealed by region‐specific changes in the alpha activity reflects attentional modulation which has consequences for long‐term memory encoding. Hum Brain Mapp 35:3972–3987, 2013. © 2013 Wiley Periodicals, Inc.
Keywords: gamma, magnetoencephalography (MEG), electroencephalography (EEG), oscillations, synchronization, episodic memory
INTRODUCTION
To retain the important information we encounter in our daily lives, it is essential to filter relevant from irrelevant information. This implies that efficient memory functioning involves not only successful remembering but also successful forgetting [Johnson, 1994; Van Hooff et al., 2009]. The mechanisms controlling memory encoding have been investigated by directed forgetting, think/no‐think or cued encoding paradigms using various kinds of brain imaging techniques [Anderson and Green, 2001; Anderson et al., 2004; Bauml and Hanslmayr, 2010; Daselaar et al., 2004; Depue et al., 2007; Fawcett and Taylor, 2008; Freunberger et al., 2009; Hanslmayr et al., 2009a, 2010; Norby et al., 2010; Wagner and Davachi, 2001; Wylie et al., 2008]. From these studies, it is clear that the human memory system engages and disengages various brain regions in order to facilitate and prevent memory encoding. To understand the physiological substrates of such mechanisms, oscillatory dynamics might be relevant [Fell and Axmacher, 2011; Siegel et al., 2012; Thut et al., 2012]. For example, neuronal activity in the gamma band has been associated with active processing, such as in memory maintenance and encoding [Gruber et al., 2004; Jensen et al., 2007; Jerbi et al., 2009; Lachaux et al., 2012; Osipova et al., 2006; Palva et al., 2010; Park et al., 2011; Roux et al., 2012]. It has been suggested that alpha band oscillations reflect active inhibition or disengagement of task‐irrelevant brain regions [Foxe and Snyder, 2011; Foxe et al., 1998; Haegens et al., 2011; Hanslmayr et al., 2009a, 2012; Hsieh et al., 2011; Jensen and Mazaheri, 2010; Jokisch and Jensen, 2007; Klimesch et al., 2007; Meeuwissen et al., 2011b; Park et al., 2011; Roberts et al., 2013]. Here, we hypothesized that oscillatory alpha activity can serve to suppress the encoding of irrelevant memories in a setting where new information has to be either remembered or ignored. If the suppression fails, irrelevant memories might be created at the expense of memory performance for the to‐be‐remembered items.
To this end, we have developed a novel long‐term memory paradigm that affords the opportunity to examine brain activity associated with active encoding or ignoring. A cue directed subjects to either encode (remember) or ignore (no‐remember) an upcoming picture presented 2 s later. We applied magnetoencephalography (MEG) to record the ongoing brain activity in order to characterize oscillations from regions associated with intentional memory encoding. We hypothesized that posterior alpha activity following the “No‐Remember” cue will serve to suppress the encoding of irrelevant information. As a consequence, individuals who are better at suppressing the irrelevant information by posterior alpha activity will benefit from an enhanced ability to remember the relevant information. Finally, we predicted that the cue‐directed alpha band suppression would allow for a stronger gamma band response during memory encoding. In short, optimal performance requires the ability to ignore irrelevant information by alpha band suppression in order to remember relevant information.
MATERIALS AND METHODS
Participants
Thirty‐one healthy, young, right‐handed volunteers (15 males, 16 females, 25.0 ± 3.2 years old) participated in this study. However, eight participants who could not perform the task (n = 6; one subject fell asleep, two had a high False Alarm rate comparable to the Hit rate and three misunderstood the task instructions), or had signals with excessive superconducting quantum interference device (SQUID) noise due to a malfunctioning of the MEG system (n = 2) were excluded from the analysis. This left datasets from 23 participants (11 males, 12 females) with a mean age of 24.8 ± 3.1 years to be analyzed. None of the participants had a history of developmental, psychological, or neurological disorders. They all had normal or corrected‐to‐normal vision. The present study was approved by the Institutional Review Board (IRB) at Seoul National University Hospital (IRB No. C‐1007‐156‐325). Written informed consent was obtained from all participants after complete description of the study.
Stimuli
Six hundred and forty real‐life photographs of landscapes and buildings were used. Pictures were obtained from internet websites with resolutions exceeding 480 × 640 pixels (the same stimuli used in Takashima et al. [2006], which excluded well‐known landscapes and buildings). The pictures with a visual angle of 8° horizontally (334 × 250 pixels) were projected to a screen using STIM2TM software (Compumedics Neuroscan, Charlotte, NC). The stimuli were evenly divided into three sets to be used for the conditions: “Remember” (R), “No‐Remember” (NR) and “New.” The “Remember” (R) and “No‐Remember” (NR) conditions were used in both the encoding and recognition sessions and the “New” condition was used only in the recognition session. The stimuli for these three conditions were counterbalanced across subjects. Also, the pictures of landscapes and buildings were equally distributed for the conditions.
Experimental Paradigm and Procedure
The experiment was divided into a training followed by an experimental block. Each block consisted of encoding, interference, and recognition sessions (Fig. 1). In the training block, the stimuli from “No‐Remember” (NR) were not tested in the recognition session. Thus the subjects were naïve about the later test on the “No‐Remember” (NR) items in the recognition session of the experimental block.
Figure 1.
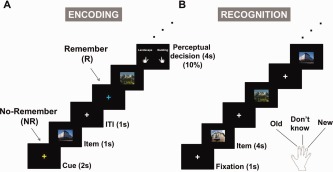
A cued long‐term memory paradigm. (A) In the encoding session, subjects were presented memory items that were pictures of landscapes or buildings. Prior to each item, a “Remember” or “No‐Remember” cue was presented, as indicated by the color of the fixation cross. To ensure that the subjects attended the stimuli, we randomly presented perceptual decision trials. Subjects were told that the “No‐Remember” items would not be tested in the recognition session. (B) In the recognition session, we presented, in random order, all the previous presented items (for both cue types) together with previously unseen new items. The subjects were instructed to indicate if they had seen the items in the encoding session by pressing one of three button corresponding to old, don't know or new. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In the encoding session of the main experiment, 440 pictures (220 pictures for respectively the “Remember” and “No‐Remember” conditions) were presented. First a cue period was shown for 2 s, in which the color of the fixation cross indicated either to remember (e.g., blue, “Remember”) or not to remember (e.g., yellow, “No‐Remember”) the upcoming picture. The color of the cue was counterbalanced across subjects. After the instruction cue, a picture was presented for 1 s, followed by an inter‐trial interval (ITI) of 1 s (Fig. 1A). In 10% of the trials independent of the cue, we asked the subjects to make a perceptual decision to ensure that the subjects perceived the presented stimuli. In these trials, subjects had to decide whether the picture was a landscape or a building as prompted by a question shown after the picture. To prevent motor preparation confounds, they were instructed to press the left or right button (left or right index finger) as instructed by the question screen. This question screen was turned off as soon as a response was given, but was displayed for a maximum of 4 s. The perceptual decision trials were not used in the MEG analysis.
A short interference task followed the encoding session in order to reduce recency effects for about 5 min. In this task, the subjects were presented with an arithmetic equation involving addition or subtraction following by a number on a next screen. They were instructed to indicate by button press whether the number was correct or incorrect according to the equation.
In the recognition session, the 440 stimuli, including perceptual decision trials from the encoding session (220 stimuli in each condition), were randomly intermixed with 200 new stimuli (Fig. 1B). Each picture was presented for 4 s and the subjects were instructed to indicate if the picture was presented before (old) or not (new). The fixation screen then followed (1 s). The subjects used the index, middle, and ring fingers of the right hand which was already associated with the appropriate response (i.e., old, don't know, and new) before the experiment. This procedure was also well trained during the training block. The fingers associated with the three responses were counterbalanced across subjects. To reduce guesses, they were instructed to press the don't know button when they were uncertain. Subjects were supposed to respond old even if they remembered that the item was associated with a “No‐Remember” cue, i.e., they were instructed to respond as old or new independently of the cue. The picture disappeared as soon as a response was made, but was displayed for not more than 4 s.
Before the main experiment, the subjects were trained in a block in which 160 pictures, of which 10% were perceptual decision stimuli, were presented during an encoding session. Only the pictures for “Remember” trials were tested for recognition, along with 50 new stimuli as foils. This procedure was the same as in the main experimental phase; however, the “No‐Remember” items were not tested in the recognition session of the training phase. As such the subjects were aware that they later would be tested on the “Remember” stimuli but not the “No‐Remember” stimuli. This was intended to trick subjects into believing that they would not be tested on the “No‐Remember” stimuli. Further, the large number of stimuli used in the main experiment (440 during the encoding) reduced the chances that subjects encoded the “Remember” and “No‐Remember” cues along with a given picture. In short, as confirmed by the behavioral results, our design promoted a strategy in which more “Remember” than “No‐Remember” stimuli were encoded.
The total duration of the experiment was ∼100 min, including the training phase and halfway break (∼20 min) between training and main experiment. After the experiment, all participants were debriefed in a post‐experimental interview, and asked to describe the strategies they used.
Behavioral Analysis
The response trials from the recognition session were characterized as follows. The old and new responses from the “Remember” condition were labeled as R‐hits and R‐misses, respectively, those from the “No‐Remember” condition as NR‐hits and NR‐misses, and those from the “New” condition (the memory foils) as false alarms and correct rejections. The encoding trials were categorized according to subsequent recognition as later R‐hits and later R‐misses for the “Remember” condition, and later NR‐hits and later NR‐misses for the “No‐Remember” condition. Later NR‐hits come from old responses as a result of unsuccessful inhibition of memorization whereas later NR‐misses come from new responses most likely as a result of successful inhibition of memorization as well as fading of the specific memory.
MEG Measurement
Electromagnetic brain activity was recorded using a whole‐head MEG Neuromag (VectorViewTM, Elekta Neuromag Oy, Helsinki, Finland) acquisition system installed at the MEG center of Seoul National University Hospital. It consists of 306 sensors arranged in triplets of two planar gradiometers and one magnetometer. Data were sampled continuously at 1200 Hz (following a 300 Hz low‐pass filter). The vertical and horizontal electrooculogram (EOG) was also measured with EOG electrodes placed near the outer canthus and beneath the left eye for subsequent exclusion of eye movements and blinks artifacts. The electrocardiogram (ECG) was also recorded to remove cardiac artifacts from the data. Before entering the electromagnetically shielded and sound attenuated room, head position indicator (HPI) coils were sparsely attached on the head of each subject, and anatomical landmarks such as nasion and bilateral preauricular points were spatially identified by 3D digitizer (FASTRAKTM, Polhemus, Colchester, VT). Then the subject's head position was registered by localizing HPI coils in the MEG device. This allowed the sources reconstructed from the MEG to be superimposed on structural MR images of each individual with high precision. Before data analysis, a Maxwell filter (Signal Space Separation), which separates brain‐related and external inference signals, was applied to reduce the confounding influence of biological and environmental noises [Taulu and Simola, 2006; Taulu et al., 2005].
Structural MR Image Acquisition
Structural magnetic resonance (MR) images (T1‐weighted gradient‐echo pulse sequence) were acquired at 3.0 T using a Siemens Trio Tim scanner (Siemens, Erlangen, Germany) with the following parameters: 1.0 × 0.98 × 0.98 mm3 voxels; 208 sagittal slices.
Data Analysis
The data analysis was performed in Matlab 2012a (MathWorks, Natick, MA) using the Fieldtrip open source Matlab toolbox developed at the Donders Institute for Brain, Cognition and Behavior, Center for Cognitive Neuroimaging, Nijmegen, The Netherlands [Oostenveld et al., 2011] (http://fieldtrip.fcdonders.nl), and custom scripts. Before the analysis, the data were downsampled at 600 Hz after applying a low‐pass filter at 200 Hz for the computational reason. Trials contaminated by ocular artifacts, SQUID jump and muscle artifacts were manually rejected using visual inspection. Additionally, remaining electrooculographic (EOG) and electrocardiographic (ECG) artifacts were reduced using independent component analysis (ICA). The recorded ECG helped identifying magnetocardiographic (MCG) components. After the artifact rejection the number of trials for later R‐hits, later R‐misses, later NR‐hits, and later NR‐misses were on average 90, 67, 51, and 103 trials, respectively. This also means that the number of trials rejected because of eye movement are not different between “Remember” (157 trials) and “No‐Remember” (154 trials) conditions, i.e., there were not more trials rejected for the “No‐Remember” than the “Remember” trials, as would have been the case if subjects systematically had closed their eyes or made saccades away for the “No‐Remember” cue.
Spectral Analysis
For lower frequencies, time‐frequency representations of power (1–32 Hz) were computed based on a sliding time window (steps of 50 ms) from the data segments covering the whole trial length: cue (2 s), item (1 s), and inter‐trial interval (1 s). The length of the sliding time window was adapted to the frequency, and contained four cycles (i.e., ΔT = 4/f, e.g. 400 ms for 10 Hz). Prior to the Fourier transformation, the data from the sliding time windows were multiplied by a Hanning taper, resulting in adaptive spectral smoothing of Δf ∼1/ΔT. For high frequency ranges (20–200 Hz), a fixed time window of 200 ms was used together with a multitaper approach involving three orthogonal Slepian tapers, which resulted in a spectral smoothing of ∼10 Hz [Percival and Walden, 1993]. The grand‐averaged power values were then calculated for the different conditions and then compared. When conditions are compared, we normalized the power value by the mean of the two conditions.
Source Analysis
To identify the sources of the oscillatory activities we applied a beamforming approach based on an adaptive spatial filter (dynamic imaging of coherent sources, DICS) [Gross et al., 2001]. Cross‐spectral density matrices were calculated from the Fourier transformed data for each condition. We used a Hanning taper for the 10 Hz alpha band, resulting in 3 Hz smoothing for a 500 ms window and three Slepian tapers for the gamma frequency (80 Hz) resulting in a 10 Hz spectral smoothing. Realistically shaped single‐shell descriptions of the brains were constructed from each individual's MRI. The brain volume of each individual subject was divided into a grid with a 0.8 cm resolution and normalized to the template MNI brain (International Consortium for Brain Mapping, Montreal Neurological Institute, Canada) using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The lead field was calculated for each grid point. Then, a spatial filter was constructed for each grid point using the cross‐spectral density matrices for the frequency of interest and the lead fields. The spatial distribution of power of oscillatory activities was estimated in each condition albeit the cross‐spectral densities were calculated for the data combined.
Statistical Analysis
The statistical significance of the spectral power changes was assessed using a cluster‐based nonparametric randomization test [Maris and Oostenveld, 2007] which effectively controls the Type I‐error rate with respect to multiple comparisons over sensors, frequency, and time. This is achieved by clustering neighboring sensors, time, and frequency points. For the first‐level statistics, sensors, frequency and time‐points below a threshold (t test; P < 0.05) were identified from the t statistics and subsequently spatially contiguous points in terms of sensor, frequency and time‐points below this threshold were defined as a cluster. Then, the sum of the t values for a given cluster was used for the cluster‐level statistics. By randomization of the data between conditions across the subjects, the cluster‐level t statistics were created from 5,000 randomization routines. For the second‐level statistics, the P value was estimated according to the proportion of the randomization distribution exceeding the observed maximum cluster‐level statistics.
RESULTS
The MEG data from the encoding session were analyzed for cueing effects, i.e., comparison between “No‐Remember” and “Remember” cues and subsequent memory effects. The encoding trials were categorized according to subsequent recognition as later R‐hits and later R‐misses for the “Remember” condition, and later NR‐hits and later NR‐misses for the “No‐Remember” condition (see “behavioral analysis” in the Material and Methods section).
Behavioral Performance
The behavioral data from the recognition session are reported in Figure 2. The recognition rate for items preceded by the “Remember” cue, i.e., R‐hits (56.7% ± 3.2%) was significantly greater than for items preceded by the “No‐Remember” cue, i.e., NR‐hits (33.3% ± 2.2%) (paired t test; t (22) = 12.3, P < 0.001). Both the R‐hits and NR‐hits were significantly greater than the false alarm rate (24.2% ± 2.0%) (t (22) = 14.5, P < 0.001 and t (22) = 9.0, P < 0.001, respectively). These results demonstrate that the subjects remembered more items following the “Remember” cue compared to the “No‐Remember” cue. Further the low false alarm rate indicates that guessing was relatively low during retrieval. The don't know responses were rated as 5.0% ± 5.3%, 6.4% ± 7.0%, and 6.3% ± 6.7% for “Remember,” “No‐Remember,” and “New” conditions respectively and these trials were not included in the further analyses.
Figure 2.

Behavioral results from the recognition session. The hit‐rate was significantly higher for R‐hits (items preceded by a “Remember” cue) compared to NR‐hits (items preceded by a “No‐Remember” cue). The false alarm rate for completely new items was also relatively low. In short, subjects respectively remembered and ignored the items according to the cue.
For perceptual decision trials, R‐hits (56.5% ± 2.9%) for the “Remember” cued items were significantly higher than for NR‐hits (30.0% ± 2.9%) for the “No‐Remember” cued items (paired t test: t (22) = 9.2, P < 0.001). The mean accuracy for judging the perceptual decision trials was high, i.e., 98.0% ± 0.5% for the “Remember” cue and 87.0% ± 1.6% for the “No‐Remember” cue. Albeit this effect was significantly different, subjects were able to perform the perceptual decisions with high accuracy demonstrating that the subjects were attentive during the course of the experiment.
Alpha Band Activity for “No‐Remember” Versus “Remember” Task Conditions
We first set out to identify electrophysiological modulations according to the “No‐Remember” versus the “Remember” cue. Before comparing the conditions, the absolute power in the alpha band increased for both the “No‐Remember” (Fig. 3A) and “Remember” (Fig. 3B) cues, however, the increase was stronger in the “No‐Remember” cue condition. In particular, the absolute alpha power was strong during the cue presentation interval. During item presentation the alpha power decreased, but more so for the “Remember” compared to the “No‐Remember” condition. We found a highly robust alpha power increase over posterior regions when comparing the trials for the “No‐Remember” to the “Remember” cues. Figure 3C shows a time‐frequency representation of power for 20 posterior sensors over the parieto‐occipital areas. This effect was present during the cue interval and increased further during the item presentation (f = 8–12 Hz; t = 1–2 s and t = 2–3 s; P < 0.05). The modulation in the alpha band is consistent with numerous reports on memory encoding [Bonnefond and Jensen, 2012; Freunberger et al., 2009; Hanslmayr and Staudigl, 2014; Hanslmayr et al., 2009b; Khader et al., 2010; Meeuwissen et al., 2011b; Park et al., 2011; Payne et al., 2013]. We thus focused the subsequent analysis on the 10 Hz band (8–12 Hz when considering spectral smoothing) considering the 1 s time interval just prior to the memory item onset. The topographical distribution of the alpha power difference for the “No‐Remember” and “Remember” cues during the cue interval is shown in Figure 3D. This difference was found to be highly significant (cluster‐level permutation controlling for multiple comparisons over sensors, P < 0.001). Note that the widespread cluster reflects the robustness of the effect in terms of signal to noise ratio, rather than the spatial extent per se of the underlying neuronal source which is shown in Figure 3E. This is an inherent feature of the cluster‐randomization framework in which the size of a given cluster is not directly interpretable. Next, we identified the neuronal sources reflecting the difference in the alpha band during the cue interval (1–2 s) using a beamformer analysis (Fig. 3E). We found that the alpha power increase was dominated by extended sources mainly in posterior parietal regions (cluster‐level permutation, P < 0.05) included in the dorsal attention network [Fox et al., 2006]. When comparing the contrast for the forgotten trials only (NR‐misses vs. R‐misses), we found the same modulation as in Figure 3 (data not shown).
Figure 3.
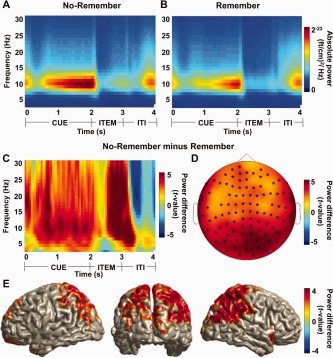
The posterior alpha power (10 Hz) for the “No‐Remember” cue compared to the “Remember” cue. Time‐frequency representations for the “No‐Remember” (A) and the “Remember” (B) cue conditions averaged over 20 posterior sensors. (C) A time‐frequency representation of power calculated for 20 posterior sensors revealed a strong increase in the alpha band when comparing the “No‐Remember” to the “Remember” cue (normalized by the mean of the two conditions). This effect was pronounced in the cue interval (0–2 s), but also during item presentation and beyond (P < 0.05). (D) Topographical distribution of alpha power increases during the cue interval (10 Hz; 1–2 s) was found in a huge cluster including most sensors (cluster‐level permutation, P = 0.0002). (E) Source reconstruction using a beamforming technique of the “No‐Remember” versus “Remember” effect (10 Hz; 1–2 s). The alpha power increase was localized in posterior parietal regions (cluster‐level permutation, P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Correlation Between Alpha Band Difference and Behavioral Performance
Next we set out to investigate the behavioral relevance of the cue‐related alpha power modulation. We correlated the alpha power difference for the cueing effect with the memory performance (assessed by the standard d‐prime measure calculated by R‐hits versus false alarm rates) as well as compliance (assessed by the standard d‐prime measure calculated by R‐hits versus NR‐hits rates). By compliance we here imply encoding intention according to the cue. As a first step, we used the beamformer approach as a spatial filter to extract the alpha band activity from posterior parietal cortex. The spatial filter was centered at the location producing the maximal cue directed difference in the alpha band (as in Fig. 3E; left posterior parietal cortex; MNI coordinates x, y, z = [−8, −58, 32]). The cue‐directed difference in the alpha band was correlated with memory performance over subjects (memory performance: r = 0.52, P = 0.01, Fig. 4A). When we correlated the same cue‐directed alpha power difference with compliance over subjects, we also found a highly significant effect (compliance: r = 0.66, P < 0.001; Fig. 4B). Figure 4B might indicate that the subjects are divided in two groups according to compliance. However, since this was not the case for memory performance (e.g., Fig. 4A) we have not pursued this further.
Figure 4.

The relationship between cue‐related alpha band modulation and behavioral performance. (A) The correlation between alpha power modulation (“No‐Remember” versus “Remember” cue; 10 Hz; 1–2 s) from signals in the posterior parietal cortex (local maxima of the source reconstruction of “No‐Remember” versus “Remember” cue in Fig. 3E; MNI coordinates x, y, z = [−8, −58, 32]) and memory performance (assessed by the standard d‐prime measure calculated by R‐hits versus false alarm rates) over subjects was highly significant (r = 0.52, P = 0.01). (B) The same correlation as in (A) with respect to compliance (assessed by the standard d‐prime measure calculated by R‐hits versus NR‐hits rates) over subjects was also highly significant (r = 0.66, P < 0.001). (C) The regression values between the difference in the alpha band at source level (“No‐Remember” versus “Remember” cue; 10 Hz; 1–2 s) and compliance (cluster‐level permutation, P < 0.05). We found that subjects with a stronger difference in cue‐directed alpha activity in posterior parietal regions were also the subjects who were able to perform the task better. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Next, we performed the analysis using the beamformer approach applied to the full discretized brain volume. We did this by calculating the regression coefficients for the difference in alpha activity (“No‐Remember” versus “Remember” cue) and the measure for compliance at every grid point in the brain volume. These values were subjected to a cluster randomization analysis (testing for nonzero regression coefficients while controlling for multiple comparisons over grid points) that revealed a significant cluster in posterior parietal cortex extending to visual and temporal areas (cluster‐level permutation, P < 0.05; Fig. 4C). These findings demonstrate that subjects who upregulate their alpha band activity in visual processing regions are the ones who successfully prevent the formation of irrelevant memories.
Subsequent Memory Effect in the Alpha Band for Later Forgotten Versus Later Remembered Trials
Next, we investigated the brain activity associated with subsequent memory effect regardless of the task conditions. Thus we sorted the encoding trials according to whether they were later forgotten or later remembered. In the first part of the analysis to be reported below, we combined the “No‐Remember” and “Remember” cues.
The spectral analysis for the subsequent memory effect is shown in Figure 5. A time‐frequency representation of power for 20 posterior sensors over the parieto‐occipital regions when comparing later forgotten to later remembered trials showed a strong alpha power increase during both the cue and item presentation (P < 0.05; Fig. 5A). The posterior alpha power (8–12 Hz) during the cue interval (1–2 s) was highly significant when comparing the later forgotten to the later remembered trials as shown in Figure 5B (cluster‐level permutation controlling for multiple comparisons over sensors, P = 0.008). When localizing the alpha power difference during the cue interval using a beamformer analysis, we identified an extended source in posterior parietal regions (relative power increase with respect to the mean; Fig. 5C). When this difference was statistically tested, it revealed frontal midline structures including the left supplementary motor area (SMA (Brodmann Area 6); MNI coordinates x, y, z = [−32 −8 72]; cluster‐level permutation, P < 0.05; Fig. 5D). The exact location from the randomization analysis should be interpreted with caution since it probably only reveals a peak in an extended network. We concluded that successful memory encoding is associated with a relative decrease in alpha power in an extended area overlapping with the dorsal attention network.
Figure 5.
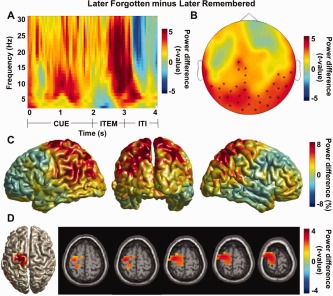
The posterior alpha power (10 Hz) for the later forgotten compared to the later remembered trials. (A) A time‐frequency representation of power calculated for 20 posterior sensors showed a strong increase in the alpha band when comparing the later forgotten to the later remembered trials (normalized by the mean of the two conditions) in the encoding session. The strong alpha power increase was observed in the cue interval as well as during item presentation (P < 0.05). (B) Topographical distribution of alpha power increase during the cue interval (10 Hz; 1–2 s) was found in posterior and central sensors (cluster‐level permutation, P = 0.008) for the later forgotten versus the later remembered trials. (C) Source reconstruction using a beamformer approach to localize the alpha power for later forgotten versus later remembered trials during the cue interval (10 Hz; 1–2 s). The alpha power for the later forgotten trials was relatively increased compared to the later remembered trials (relative power increase with respect to the mean). (D) Statistical testing of the source reconstruction in (C) revealed the involvement of frontal midline structures including the left supplementary motor area (SMA (Brodmann Area 6); MNI coordinates x, y, z = [−32 −8 72]; cluster‐level permutation, P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Subsequent Memory Effect in the Alpha Band for the “No‐Remember” Cue
Given our interests in functional inhibition by alpha band activity in long‐term memory task, we considered brain activity in the cue interval for the “No‐Remember” cue. Specifically we compared later correctly forgotten trials, i.e., later NR‐misses to later unintentionally remembered trials, i.e., Later NR‐hits. When we compared these conditions, we found a significant increase in alpha power predictive of later NR‐misses during the cue interval with respect to 12 central sensors (f = 8–12 Hz; t = 1–2 s; P < 0.05; Fig. 6A). The topographical distribution of alpha power centered at 10 Hz during cue interval (1–2 s) was focused over central regions (permutation, P < 0.05, uncorrected; Fig. 6B). When we localized this effect using a beamformer and found an alpha power increase in frontal midline structures including the left supplementary motor area (SMA (Brodmann Area 6); MNI coordinates x, y, z = [−26, −4, 80]; P < 0.05, uncorrected; Fig. 6C). The left lateralized source in supplementary motor cortex suggests a differential involvement of the extended motor system. However, this is not likely to reflect preparation of button responses in the perceptual decision task since they were independent of the memory cueing. Even though this effect was not significant when controlling for multiple comparisons, it did include sensors from the cluster identified when comparing all the later forgotten versus later remembered trials in Figure 5B. Statistical power is lost when only considering the “No‐Remember” cue trials. We therefore consider the effect reliable and conclude that the modulation in the alpha band reflects the predicted inhibition of irrelevant memory encoding.
Figure 6.
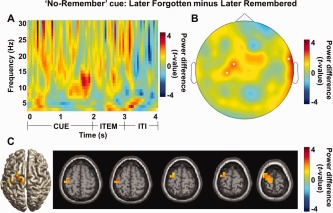
Alpha band activity for subsequent memory effect in the “No‐Remember” condition, i.e., later correctly forgotten trials (later NR‐misses) versus later unintentionally remembered trials (later NR‐hits). (A) A time‐frequency representation of power calculated for 12 central sensors (Neuromag sensor labels: 042, 043, 063, 071, 072, 073, 074, 104, 111, 114, 182, 221) when compared the later NR‐misses to the later NR‐hits (normalized by the mean of the two conditions) revealed an alpha power increase during the cue interval as well as item presentation (P < 0.05). (B) Topographical distribution of the alpha power increase (10 Hz; 1–2 s) for the later NR‐misses versus the later NR‐hits was found over central sensors (P < 0.05, uncorrected). (C) For the same comparison, a beamformer analysis revealed frontal midline structures including the left supplementary motor area (SMA (Brodmann Area 6); MNI coordinates x, y, z = [−26 −4 80]; P < 0.05, uncorrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We next asked if subjects having a larger difference in alpha band power between later NR‐misses and later NR‐hits are also the subjects who are better at ignoring the “No‐Remember” items. To do so, we performed a regression analysis considering the alpha band differences from the beamformer applied to each grid point in the full brain volume (later NR‐misses versus later NR‐hits; f = 8–12 Hz; t = 1–2 s) and the compliance measure (the same analysis as in Fig. 4C). As demonstrated in Figure 7A, this revealed correlations in posterior parietal regions. The effect was consistent with the cueing effect observed in Figure 4C albeit not significant when controlling for multiple comparisons. To further illustrate the results, we computed the correlation between power values from a location in brain volume and compliance over subjects. We here selected a grid point of the local maxima of posterior parietal cortex (precuneus; MNI coordinates x, y, z = [0 −72 64]) from the regression analysis in Figure 7A. This computation revealed a significant positive correlation between power and compliance (r = 0.52, P = 0.01; Fig. 7B). Although this location is included in the sources in Figures 3E and 4C, the correlation should be considered as a trend given it was not robust when considering multiple comparisons.
Figure 7.
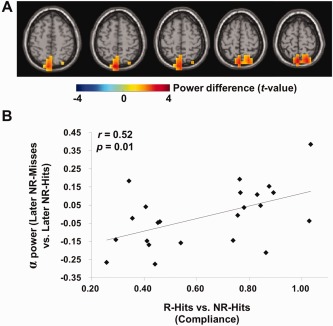
The relationship between cue‐related alpha band modulation for subsequent memory effect in the “No‐Remember” condition, i.e., later NR‐misses versus later NR‐hits and compliance during cue interval (1–2 s). (A) The regression results between source level difference in the alpha band for the subsequent memory effect in the “No‐Remember” condition (later NR‐misses versus later NR‐hits) and compliance. This showed a similar trend as the regression values for the cueing effect (“No‐Remember” cue versus “Remember” cue; Fig. 4C) albeit weaker (permutation, P < 0.05, uncorrected). (B) The correlation between the alpha band effect for subsequent memory effect in the “No‐Remember” condition (later NR‐misses versus later NR‐hits) and compliance over subjects. Here, we selected a grid point of the local maxima of posterior parietal cortex (precuneus; MNI coordinates x, y, z = [0, −72, 64]) from regression analysis in (A). This revealed a significant positive correlation (r = 0.52, P = 0.01). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
We also compared the subsequent memory effect in the alpha band for the “Remember” condition i.e., later R‐hits versus later R‐misses. Even though trends were present consistent with the previous results, no significant effects were identified when taking multiple comparisons into account.
In conclusion, an analysis of the subsequent memory effect for the “No‐Remember” trials revealed that alpha activity in the cue interval serves to prevent encoding of the irrelevant memory items. This alpha band modulation is predictive of performance over subjects.
Gamma Band Activity for “No‐Remember” Versus “Remember” Task Conditions
Given the well‐known role of gamma band activity in active information processing in cognitive tasks [Fell and Axmacher, 2011; Fries et al., 2007; Jensen et al., 2007; Jutras and Buffalo, 2010; Park et al., 2011; Roberts et al., 2013], we extended the analysis to the higher frequency bands. First we investigated gamma band activity for the “Remember” versus the “No‐Remember” cue. In the cue interval, we did not find any significant effects; however, during the item presentation period, we found a highly robust gamma power increase in posterior regions when comparing the “Remember” to the “No‐Remember” encoding trials (averaged over 8 posterior sensors in Fig. 8B; f = 40–100 Hz; t = 2–3 s; P < 0.05; Fig. 8A). The effect was somewhat broadband (40–100 Hz) and lasted for about 1 s with an onset of about 200 ms after item presentation. The topographical distribution of the gamma power difference for the “Remember” compared to the “No‐Remember” cue at sensor level revealed a highly significant effect over posterior regions (cluster‐level permutation, P = 0.01; Fig. 8B). Source reconstruction of the gamma power increase was localized in posterior parietal cortex, extending into temporal areas bilaterally (maximum MNI coordinates x, y, z = [24 −48 72]; cluster‐level permutation, P < 0.05; Fig. 8C). This source overlaps strongly with the source reflecting the reverse effect in the alpha band during the cue interval (Fig. 3E). These results demonstrated that the encoding of the to‐be‐remembered items is associated with an increase in gamma band activity.
Figure 8.

Modulations of high‐frequency power for the “Remember” to the “No‐Remember” cue. (A) A time‐frequency representation of power when comparing the “Remember” to the “No‐Remember” cue (normalized by the mean of the two conditions) calculated for eight posterior sensors marked in (B). We observed a robust difference in the gamma band during item presentation (P < 0.05). (B) The topography of the gamma band difference at 80 Hz when comparing the “Remember” to the “No‐Remember” condition during item presentation (2–3 s). We found a significant difference over posterior regions (cluster‐level permutation, P = 0.01). (C) Source reconstruction using a beamformer approach to localize the gamma power increase during item presentation (80 Hz; 2–3 s) revealed sources in posterior parietal cortex, extending into temporal cortex bilaterally (maximum MNI coordinates x, y, z = [24 −48 72]; cluster‐level permutation, P < 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Gamma Band Activity for Later Remembered Versus Later Forgotten Trials
Next we examined the subsequent memory effect regardless of the “Remember”/“No‐Remember” cue by comparing the later remembered to the later forgotten trials. We found a robust gamma power increase during item presentation which reflected the subsequent memory effect (averaged over five posterior sensors with power increase in Fig. 9B; f = 40–100 Hz; t = 2–3 s; P < 0.05; Fig. 9A). When we focused on the 80‐Hz band power (70–90 Hz when considering spectral smoothing) during item presentation (2–3 s), the activity was most pronounced in posterior sensors. While consistent with the cueing effect in Figure 8B, it was however not significant when controlling for multiple comparisons over sensors (P < 0.05, uncorrected; Fig. 9B). Next, the source reconstruction of this activity has shown that gamma power for later remembered was increased than the later forgotten trials over posterior parieto‐occipital regions (relative power increase with respect to the mean; Fig. 9C). When this difference was statistically tested, it revealed posterior parietal cortex including precuneus (MNI coordinates x, y, z = [8 −48 56]; P < 0.05, uncorrected; Fig. 9D). Albeit not significant when controlling for multiple comparisons over grid points, the regions of the subsequent memory effect in the gamma band overlapped strongly with the subsequent memory effect in the alpha band (Fig. 5C).
Figure 9.
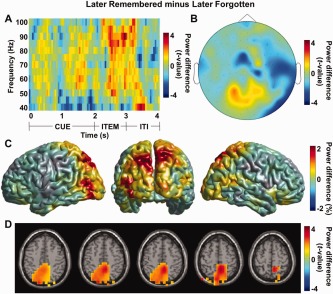
Modulations of high‐frequency power for the later remembered compared to the later forgotten trials. (A) A time‐frequency representation of power when comparing later remembered to the later forgotten trials (normalized by the mean of the two conditions) calculated for five posterior sensors with power increase in (B) (P < 0.05). (B) Topographical distribution of gamma power during item presentation (80 Hz; 2–3 s) increased over posterior sensors. (C) Source reconstruction using the beamformer approach for the later remembered versus the later forgotten trials (80 Hz; 2–3 s). The gamma power for the later remembered trials was relatively increased compared to the later forgotten trials over posterior parieto‐occipital regions (relative power increase with respect to the mean). (D) Statistical testing of the source reconstruction in (C) revealed the involvement of posterior parietal cortex including precuneus (MNI coordinates x, y, z = [8 −48 56]; P < 0.05, uncorrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Correlation Between Alpha Power During Cue Interval and Gamma Power During Item Presentation
As we hypothesized, alpha power increased during the cue interval in response to the “No‐Remember” instruction and gamma power increased during item presentation in response to the “Remember” instruction. Motivated by this fact, we were interested in the relationship between these two oscillatory modulations. To find this relationship, we performed a correlation analysis between alpha power during cue interval (1–2 s; Fig. 3E) and gamma power during item presentation (2–3 s; Fig. 8C) for the “Remember” versus “No‐Remember” cue condition. The source analyses in the previous analyses for both the alpha and gamma band effects produced sources in the precuneus. An analysis involving the overlapping sources of the cueing effect in the alpha band (Fig. 3E) and the gamma band (Fig. 8C) confirmed a common source in the precuneus (data not shown). Thus we investigated the relationship between the power in these two bands using signals from the precuneus (WFU PickAtlas; available from http://fmri.wfubmc.edu/software/PickAtlas). This region includes large portion of posterior parietal cortex and it overlaps with central areas of the dorsal attention network. The alpha power during the cue interval (1–2 s) and gamma power during the item presentation (2–3 s) were extracted from the grid points in left and right precuneus using the beamformer approach and the “Remember” versus “No‐Remember” difference was calculated. Then, these alpha and gamma power values were correlated over subjects. This procedure revealed a significant negative correlation (r = −0.44, P = 0.03; Fig. 10A).
Figure 10.
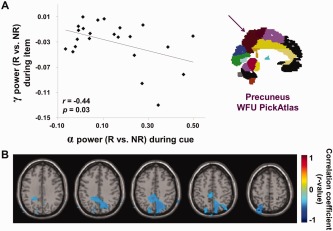
Correlations between alpha power during cue interval (1–2 s) and gamma power during item presentation (2–3 s) for the cueing effect. (A) Correlation between alpha power during cue interval (1–2 s) and gamma power during item presentation (2–3 s) when comparing “Remember” cue to “No‐Remember” cue condition. The power values were derived using a beamformer approach, averaging signals from within the precuneus (ROI analysis; left and right precuneus from the WFU PickAtlas; see insert). We observed a significant negative correlation for these values (r = −0.44, P = 0.03). (B) The correlation analysis in the source domain between alpha power during cue interval (1–2 s) and gamma power during item presentation (2–3 s) when comparing “Remember” cue to “No‐Remember” cue condition. The correlation analysis was performed at every grid point in brain volume and confirmed the negative correlation in posterior parietal cortex (local maxima: precuneus; MNI coordinates x, y, z = [14, −68, 72]; r = −0.54, P < 0.05, uncorrected). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
To identify the brain regions with the strongest correlations, we performed the correlation analysis using the power values from subjects obtained from every grid point in the brain volume using the beamformer approach. We found negative correlations in posterior parietal cortex (local maxima: precuneus; MNI coordinates x, y, z = [14, −68, 72]; r = −0.54, P < 0.05, uncorrected; Fig. 10B). The location is consistent with the regions where the alpha and gamma sources were identified in the previous analysis. We concluded that subjects in which the alpha band power decreases during a “Remember” cue are also subjects in which the gamma band power increases during encoding.
DISCUSSION
The aim of the current study was to test whether alpha oscillations provide a mechanism for gating long‐term memory encoding. A cue indicated whether pictorial items should be encoded or not. Our first finding was that alpha power in the cue interval already prior to presentation of the memory items was predictive of memory formation. The power increase in the alpha band when comparing the “No‐Remember” to the “Remember” trials was observed both during the cue and item presentation intervals (see Figs. 3C and 5A). Second, we found that alpha activity in posterior parietal regions increased dramatically for the “No‐Remember” compared to the “Remember” cue, prior to the item presentation. Subjects with the strongest cue‐directed alpha power increase performed better on the memory task. Further, during item presentation, we found that there was stronger induced gamma activity for the “Remember” compared to the “No‐Remember” condition. Importantly, the decrease of alpha power before the presentation of the item correlated with the increase in gamma activity during memory encoding when “Remember” was compared to the “No‐Remember” condition.
Several studies have demonstrated strong interactions between attention and long‐term memory encoding (reviewed in Chun and Turk‐Browne [2007]). In this study we demonstrated that attentional gating to the long‐term memory storage is reflected by prestimulus modulations in the alpha band. Importantly, we demonstrated that subjects with a better ability to modulate their posterior alpha activity also were subjects with better memory performance. In future studies it would be of great interest to investigate memory problems in various disorders such as ADHD (attention‐deficit/hyperactivity disorder) and MCI (mild cognitive impairment) as well as in normal aging [Wais et al., 2012] might be explained by an inability to properly modulate the alpha band activity. A second major finding was that the pre‐stimulus alpha modulation was predictive of the post‐stimulus gamma band modulation. Also the degree of modulation was predictive of memory performance. This suggests that the brain state defined by the alpha band activity has a causal influence on the memory encoding reflected by activity in the gamma band.
Alpha Activity in Posterior Parietal Regions Gates Memory Encoding
Our sensory systems are constantly exposed to large amount of information of which only little is of relevance for our behavior. To remember the relevant information, it is essential to have powerful mechanisms that filter out irrelevant information prior to encoding. Our findings suggest that posterior alpha activity is under top‐down control and serves to actively gate information to long‐term memory. This interpretation is consistent with previous findings from attention and working memory studies supporting the idea that posterior alpha activity gates the information flow in an anticipatory manner [Bonnefond and Jensen, 2012; Foxe and Snyder, 2011; Freunberger et al., 2009; Hanslmayr et al., 2009a; Hsieh et al., 2011; Jensen and Mazaheri, 2010; Klimesch et al., 2007; Park et al., 2011; Payne et al., 2013; Roberts et al., 2013]. From a physiological perspective, this gating is achieved through inhibition. For instance, it has been shown that both neuronal firing and the BOLD signal are reduced with a local increase in the alpha activity [Goldman et al., 2002; Haegens et al., 2012; Laufs et al., 2003]. Also, both visual detection and phosphene detection are reduced as posterior alpha activity increases [Hanslmayr et al., 2007; Mathewson et al., 2009; Romei et al., 2008; Stokes et al., 2012; van Dijk et al., 2008]. We extend these principles by demonstrating that alpha power increase during memory cueing reflects the intentional inhibition of visual processing. This inhibition prevents the encoding in long‐term memory. Further we showed that the decrease in alpha power during the cue interval was complemented by an increase in gamma power during item presentation. We therefore propose that alpha power modulation sets the state of the network controlling the subsequent encoding reflected by gamma band synchronization [Gruber et al., 2004; Jutras and Buffalo, 2010; Osipova et al., 2006]. We found the alpha band modulation to be most robust in the dorsal attention network including posterior parietal regions. This is in good agreement with neuroimaging studies demonstrating the involvement of parietal regions in tasks requiring top‐down attentional control [Corbetta and Shulman, 2002; Giesbrecht et al., 2003; Hopfinger et al., 2000; Marzetti et al., 2013; Slagter et al., 2007]. It should be noted that the alpha band modulation is somewhat widespread and does somewhat vary when comparing the different conditions (cf. Figs. 4, 5, and 6); however, the sources do consistently overlap with the dorsal attention network [Fox et al., 2006]. The control of the memory encoding reflected by alpha band modulation should therefore be attributed to the dorsal attention network rather than a single region per se.
It is debated to what extend impaired memory encoding is explained by interference or inhibition [Bauml and Hanslmayr, 2010]. It could be argued that reduced memory encoding after the “No‐Remember” cue is explained by a strategy in which subjects deliberately engage in other mental processes to prevent encoding. For instance it has been demonstrated that tasks not requiring attention to the environment result in increased alpha activity [Ray and Cole, 1985]. As such, the posterior alpha increase might reflect inhibition of visual processing as being a consequence of a strategy where the subjects engage in internal mental processes or even the rehearsal of the previous memory item.
In short, our results provide important new insights into how the encoding of visual information can be prevented. We suggest the alpha band modulation reflects a filter mechanism allowing us to remember only the relevant information when operating in a complex world.
Better Memory Performers had an Improved Ability to Modulate the Posterior Alpha Power
Individuals who remembered more of the relevant information at the expense of the irrelevant information showed better memory performance. Thus individuals who are better at flexible suppressing the irrelevant items will benefit in terms of remembering the relevant items. To understand this mechanism from a physiological perspective, we found that individuals with a stronger difference in the cue‐directed alpha activity in posterior parietal regions were also those with better memory performance.
Gamma Increase in Parietal Regions for the “Remember” Cue During Item Presentation
We observed a gamma band increase when comparing the “Remember” versus “No‐Remember” condition during memory encoding. Several regions including parietal‐occipital‐temporal areas accounted for this gamma band activity. Gamma band activity is typically associated with active neuronal processing in attention and memory tasks [Fell and Axmacher, 2011; Fries et al., 2007; Jensen et al., 2007; Jutras and Buffalo, 2010; Park et al., 2011; Roberts et al., 2013] and it has been shown that spiking phase‐locked to gamma oscillations facilitates synaptic plasticity [Wespatat et al., 2004]. Further, neuronal synchronization in the gamma band in a given region has been proposed to result in a stronger feed‐forward drive [Salinas and Sejnowski, 2001]. In the context of our study, a stronger feed‐forward drive could promote memory encoding. We found a trend toward a subsequent memory effect in the gamma band during item presentation. The reliability of the effect is likely to increase with more trial numbers. Nevertheless, the gamma band subsequent memory effect is consistent with several previous reports [Gruber et al., 2004; Meeuwissen et al., 2011a; Osipova et al., 2006].
Considering broadband nature of gamma band activity in our study, there is a debate to what extend it may represent spectral leakage of multiunit activity rather than genuine oscillatory activity [Buzsaki et al., 2012; Ray and Maunsell, 2011; Scheffer‐Teixeira et al., 2013]. The current data does not allow us to resolve this issue; however, we consider both phenomena interesting. Gamma band oscillations have been implicated in neuronal communications [Fries et al., 2007] whereas high‐frequency multiunit activity has been proposed to directly reflect neuronal processing [Canolty and Knight, 2010].
Cue‐induced Alpha Power Modulation Predict Encoding‐Related Gamma Activity
As we argue above, the alpha power decreases in the cue interval to open the gate to the memory system, whereas the gamma power increases during item presentation to facilitate memory encoding. Our analysis revealed that the encoding‐related alpha decrease observed when comparing the “Remember” to the “No‐Remember” condition predicted the gamma band increase over subjects. This effect was observed in posterior parietal regions included in the dorsal attention network. We suggest that the inhibitory control by the alpha activity sets the state of the network determining the subsequent memory processing reflected in the gamma band.
Alpha Activity in Frontal Midline Structures Might Prevent Encoding of Irrelevant Information
When we compared the brain activity for later correctly forgotten to later remembered trials regardless of task conditions, we found significant alpha power increase in frontal structures including the supplementary motor area (SMA) which also partly overlap with the dorsal attention network. The same regions were found in the subsequent memory effect for the “No‐Remember” cue condition (later NR‐misses > later NR‐hits). This finding is consistent with previous studies suggesting that the SMA plays an inhibitory role [Aron, 2011; Boy et al., 2010; Chen et al., 2009, 2010; Ikeda et al., 2000; Konishi et al., 2011; Mostofsky et al., 2003; Shadmehr and Holcomb, 1999; Sumner et al., 2007]. It is well known that the SMA being part of the dorsal attention network is not only involved in motor‐related functions, but is also involved in attention and memory [Chein and Fiez, 2001; Cole and Schneider, 2007; Hopfinger et al., 2000; Ortuno et al., 2005; Simon et al., 2002]. A meta‐analysis by Kim [2011] on subsequent memory effects in fMRI studies revealed that the SMA was consistently found to be involved in successful memory formation. This modulation in the BOLD signal might be related to the prestimulus alpha power decrease for the later remembered items regardless of task conditions and the NR‐hits in our study. The cue‐related activity in our paradigm could be translated into internally generated actions for the preparation of memory items to be remembered or not. This resembles cue‐driven cognitive operations in other types of paradigms [Aron, 2011; Jaffard et al., 2007; Mostofsky et al., 2003]. Future work is required to better understand how the alpha activity produced in frontal regions and posterior parietal cortex are associated. Even though this effect was not significant when controlling for multiple comparisons, it did include sources from the cluster identified when comparing all the later forgotten versus later remembered trials in Figure 5. Statistical power is lost when only considering the “No‐Remember” cue trials. We therefore consider the effect reliable and conclude that the modulation in the alpha band reflects the predicted inhibition of irrelevant memory encoding.
Oscillatory Brain Activity Predicting Memory Encoding
Prestimulus oscillatory activity has been found to predict memory encoding in other studies as well [Addante et al., 2011; Fell et al., 2011; Gruber et al., 2013; Guderian et al., 2009]. For instance, Gruber et al. [2013] recently reported that encoding‐related theta activity reflected memory encoding in an EEG study using a monetary reward expectancy paradigm. Guderian et al. [2009] also found theta band activity to predict episodic encoding in an MEG study. In addition to theta power enhancement, Fell et al. [2011] observed alpha power enhancement for successful memory encoding before stimulus presentation in an intracranial EEG study. They suggested that theta and alpha band activity reflects top‐down control in preparation for memory processing. Consistently, we suggest that alpha band activity in the dorsal attention network is predictive of memory encoding.
CONCLUSION
In conclusion, posterior alpha activity gates memory encoding. Memory is improved if one manages to block out irrelevant information by an increase in alpha activity. Finally, alpha and gamma activities interact such that a decrease in the alpha band activity allows for stimulus‐induced memory encoding reflected in the gamma band. As such, posterior alpha activity might play an important role in real‐life situations where we are confronted with massive amounts of information. Under such circumstances, it is essential to have powerful mechanisms that suppress the irrelevant input so that the relevant information can be remembered. Future work employing measures of cross‐frequency coupling between brain regions may shed further light on the network properties and interactions between oscillations in different frequency bands.
ACKNOWLEDGMENTS
The authors thank Dr. Atsuko Takashima for providing the stimuli and helpful comments on the analysis. They also thank Professor Cheongtag Kim, Professor Inah Lee, and Professor Myung‐Sun Kim for comments on the manuscript.
REFERENCES
- Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C (2011): Prestimulus theta activity predicts correct source memory retrieval. Proc Natl Acad Sci USA 108:10702–10707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MC, Green C (2001): Suppressing unwanted memories by executive control. Nature 410:366–369. [DOI] [PubMed] [Google Scholar]
- Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JD (2004): Neural systems underlying the suppression of unwanted memories. Science 303:232–235. [DOI] [PubMed] [Google Scholar]
- Aron AR (2011): From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol Psychiatry 69:e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauml KH, Hanslmayr S (2010): Forgetting in the no‐think paradigm: Interference or inhibition? Proc Natl Acad Sci USA 107:E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O (2012): Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr Biol 22:1969–1974. [DOI] [PubMed] [Google Scholar]
- Boy F, Husain M, Singh KD, Sumner P (2010): Supplementary motor area activations in unconscious inhibition of voluntary action. Exp Brain Res 206:441–448. [DOI] [PubMed] [Google Scholar]
- Canolty RT, Knight RT (2010): The functional role of cross‐frequency coupling. Trends Cogn Sci 14:506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein JM, Fiez JA (2001): Dissociation of verbal working memory system components using a delayed serial recall task. Cereb Cortex 11:1003–1014. [DOI] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH (2009): Control of prepotent responses by the superior medial frontal cortex. Neuroimage 44:537–545. [DOI] [PubMed] [Google Scholar]
- Chen X, Scangos KW, Stuphorn V (2010): Supplementary motor area exerts proactive and reactive control of arm movements. J Neurosci 30:14657–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Turk‐Browne NB (2007): Interactions between attention and memory. Curr Opin Neurobiol 17:177–184. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W (2007): The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage 37:343–360. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R (2004): When less means more: Deactivations during encoding that predict subsequent memory. Neuroimage 23:921–927. [DOI] [PubMed] [Google Scholar]
- Depue BE, Curran T, Banich MT (2007): Prefrontal regions orchestrate suppression of emotional memories via a two‐phase process. Science 317:215–219. [DOI] [PubMed] [Google Scholar]
- Fawcett JM, Taylor TL (2008): Forgetting is effortful: Evidence from reaction time probes in an item‐method directed forgetting task. Mem Cognit 36:1168–1181. [DOI] [PubMed] [Google Scholar]
- Fell J, Axmacher N (2011): The role of phase synchronization in memory processes. Nat Rev Neurosci 12:105–118. [DOI] [PubMed] [Google Scholar]
- Fell J, Ludowig E, Staresina BP, Wagner T, Kranz T, Elger CE, Axmacher N (2011): Medial temporal theta/alpha power enhancement precedes successful memory encoding: Evidence based on intracranial EEG. J Neurosci 31:5392–5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006): Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA 103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC (2011): The role of alpha‐band brain oscillations as a sensory suppression mechanism during selective attention. Front Psychol 2:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP (1998): Parieto‐occipital approximately 10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport 9:3929–3933. [DOI] [PubMed] [Google Scholar]
- Freunberger R, Fellinger R, Sauseng P, Gruber W, Klimesch W (2009): Dissociation between phase‐locked and nonphase‐locked alpha oscillations in a working memory task. Hum Brain Mapp 30:3417–3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P, Nikolic D, Singer W (2007): The gamma cycle. Trends Neurosci 30:309–316. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, Mangun GR (2003): Neural mechanisms of top‐down control during spatial and feature attention. Neuroimage 19:496–512. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J Jr, Cohen MS (2002): Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport 13:2487–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R (2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber MJ, Watrous AJ, Ekstrom AD, Ranganath C, Otten LJ (2013): Expected reward modulates encoding‐related theta activity before an event. Neuroimage 64:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber T, Tsivilis D, Montaldi D, Muller MM (2004): Induced gamma band responses: An early marker of memory encoding and retrieval. Neuroreport 15:1837–1841. [DOI] [PubMed] [Google Scholar]
- Guderian S, Schott BH, Richardson‐Klavehn A, Duzel E (2009): Medial temporal theta state before an event predicts episodic encoding success in humans. Proc Natl Acad Sci USA 106:5365–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Nacher V, Luna R, Romo R, Jensen O (2011): Alpha‐oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc Natl Acad Sci USA 108:19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegens S, Luther L, Jensen O (2012): Somatosensory anticipatory alpha activity increases to suppress distracting input. J Cogn Neurosci 24:677–685. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T (2014): How brain oscillations form memories—A processing based perspective on oscillatory subsequent memory effects. Neuroimage 85 Pt 2:648–655. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Aslan A, Staudigl T, Klimesch W, Herrmann CS, Bauml KH (2007): Prestimulus oscillations predict visual perception performance between and within subjects. Neuroimage 37:1465–1473. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Leipold P, Pastotter B, Bauml KH (2009a): Anticipatory signatures of voluntary memory suppression. J Neurosci 29:2742–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanslmayr S, Spitzer B, Bauml KH (2009b): Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cereb Cortex 19:1631–1640. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Leipold P, Bauml KH (2010): Anticipation boosts forgetting of voluntarily suppressed memories. Memory 18:252–257. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Fellner MC (2012): Oscillatory power decreases and long‐term memory: The information via desynchronization hypothesis. Front Hum Neurosci 6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR (2000): The neural mechanisms of top‐down attentional control. Nat Neurosci 3:284–291. [DOI] [PubMed] [Google Scholar]
- Hsieh LT, Ekstrom AD, Ranganath C (2011): Neural oscillations associated with item and temporal order maintenance in working memory. J Neurosci 31:10803–10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Ohara S, Matsumoto R, Kunieda T, Nagamine T, Miyamoto S, Kohara N, Taki W, Hashimoto N, Shibasaki H (2000): Role of primary sensorimotor cortices in generating inhibitory motor response in humans. Brain 123 (Part 8):1710–1721. [DOI] [PubMed] [Google Scholar]
- Jaffard M, Benraiss A, Longcamp M, Velay JL, Boulinguez P (2007): Cueing method biases in visual detection studies. Brain Res 1179:106–118. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A (2010): Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front Hum Neurosci 4:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Kaiser J, Lachaux JP (2007): Human gamma‐frequency oscillations associated with attention and memory. Trends Neurosci 30:317–324. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Ossandon T, Hamame CM, Senova S, Dalal SS, Jung J, Minotti L, Bertrand O, Berthoz A, Kahane P, Lachaux JP (2009): Task‐related gamma‐band dynamics from an intracerebral perspective: Review and implications for surface EEG and MEG. Hum Brain Mapp 30:1758–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson HM (1994): Processes of successful intentional forgetting. Psychol Bull 116:274–292. [Google Scholar]
- Jokisch D, Jensen O (2007): Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27:3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutras MJ, Buffalo EA (2010): Synchronous neural activity and memory formation. Curr Opin Neurobiol 20:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader PH, Jost K, Ranganath C, Rosler F (2010): Theta and alpha oscillations during working‐memory maintenance predict successful long‐term memory encoding. Neurosci Lett 468:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H (2011): Neural activity that predicts subsequent memory and forgetting: A meta‐analysis of 74 fMRI studies. Neuroimage 54:2446–2461. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S (2007): EEG alpha oscillations: The inhibition‐timing hypothesis. Brain Res Rev 53:63–88. [DOI] [PubMed] [Google Scholar]
- Konishi S, Watanabe T, Jimura K, Chikazoe J, Hirose S, Kimura HM, Miyashita Y (2011): Role for presupplementary motor area in inhibition of cognitive set interference. J Cogn Neurosci 23:737–745. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Axmacher N, Mormann F, Halgren E, Crone NE (2012): High‐frequency neural activity and human cognition: Past, present and possible future of intracranial EEG research. Prog Neurobiol 98:279–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek‐Haddadi A, Preibisch C, Krakow K (2003): EEG‐correlated fMRI of human alpha activity. Neuroimage 19:1463–1476. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R (2007): Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 164:177–190. [DOI] [PubMed] [Google Scholar]
- Marzetti L, Della Penna S, Snyder AZ, Pizzella V, Nolte G, de Pasquale F, Romani GL, Corbetta M (2013): Frequency specific interactions of MEG resting state activity within and across brain networks as revealed by the multivariate interaction measure. Neuroimage 79:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Gratton G, Fabiani M, Beck DM, Ro T (2009): To see or not to see: Prestimulus alpha phase predicts visual awareness. J Neurosci 29:2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuwissen EB, Takashima A, Fernandez G, Jensen O (2011a): Evidence for human fronto‐central gamma activity during long‐term memory encoding of word sequences. PLoS One 6:e21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeuwissen EB, Takashima A, Fernandez G, Jensen O (2011b): Increase in posterior alpha activity during rehearsal predicts successful long‐term memory formation of word sequences. Hum Brain Mapp 32:2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Schafer JG, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, Pekar JJ (2003): fMRI evidence that the neural basis of response inhibition is task‐dependent. Brain Res Cogn Brain Res 17:419–430. [DOI] [PubMed] [Google Scholar]
- Norby S, Lange M, Larsen A (2010): Forgetting to forget: On the duration of voluntary suppression of neutral and emotional memories. Acta Psychol (Amst) 133:73–80. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM (2011): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortuno FM, Lopez P, Ojeda N, Cervera S (2005): Dysfunctional supplementary motor area implication during attention and time estimation tasks in schizophrenia: A PET‐O15 water study. Neuroimage 24:575–579. [DOI] [PubMed] [Google Scholar]
- Osipova D, Takashima A, Oostenveld R, Fernandez G, Maris E, Jensen O (2006): Theta and gamma oscillations predict encoding and retrieval of declarative memory. J Neurosci 26:7523–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Monto S, Palva JM (2010): Graph properties of synchronized cortical networks during visual working memory maintenance. Neuroimage 49:3257–3268. [DOI] [PubMed] [Google Scholar]
- Park H, Kang E, Kang H, Kim JS, Jensen O, Chung CK, Lee DS (2011): Cross‐frequency power correlations reveal the right superior temporal gyrus as a hub region during working memory maintenance. Brain Connect 1:460–472. [DOI] [PubMed] [Google Scholar]
- Payne L, Guillory S, Sekuler R (2013): Attention‐modulated alpha‐band oscillations protect against intrusion of irrelevant information. J Cogn Neurosci 25:1463–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival DB, Walden AT (1993): Spectral Conventional Univariate Techniques. New York: Cambridge University Press. [Google Scholar]
- Ray WJ, Cole HW (1985): EEG alpha activity reflects attentional demands, and beta activity reflects emotional and cognitive processes. Science 228:750–752. [DOI] [PubMed] [Google Scholar]
- Roberts BM, Hsieh LT, Ranganath C (2013): Oscillatory activity during maintenance of spatial and temporal information in working memory. Neuropsychologia 51:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romei V, Brodbeck V, Michel C, Amedi A, Pascual‐Leone A, Thut G (2008): Spontaneous fluctuations in posterior alpha‐band EEG activity reflect variability in excitability of human visual areas. Cereb Cortex 18:2010–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ (2012): Gamma‐band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci 32:12411–12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ (2001): Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb HH (1999): Inhibitory control of competing motor memories. Exp Brain Res 126:235–251. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK (2012): Spectral fingerprints of large‐scale neuronal interactions. Nat Rev Neurosci 13:121–134. [DOI] [PubMed] [Google Scholar]
- Simon SR, Meunier M, Piettre L, Berardi AM, Segebarth CM, Boussaoud D (2002): Spatial attention and memory versus motor preparation: Premotor cortex involvement as revealed by fMRI. J Neurophysiol 88:2047–2057. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Giesbrecht B, Kok A, Weissman DH, Kenemans JL, Woldorff MG, Mangun GR (2007): fMRI evidence for both generalized and specialized components of attentional control. Brain Res 1177:90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG, Atherton K, Patai EZ, Nobre AC (2012): Long‐term memory prepares neural activity for perception. Proc Natl Acad Sci USA 109:E360–E367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Nachev P, Morris P, Peters AM, Jackson SR, Kennard C, Husain M (2007): Human medial frontal cortex mediates unconscious inhibition of voluntary action. Neuron 54:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Jensen O, Oostenveld R, Maris E, van de Coevering M, Fernandez G (2006): Successful declarative memory formation is associated with ongoing activity during encoding in a distributed neocortical network related to working memory: A magnetoencephalography study. Neuroscience 139:291–297. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J (2006): Spatiotemporal signal space separation method for rejecting nearby interference in MEG measurements. Phys Med Biol 51:1759–1768. [DOI] [PubMed] [Google Scholar]
- Taulu S, Simola J, Kajola M (2005): Applications of the signal space separation method. IEEE Trans Sign Proc 53:3359–3372. [Google Scholar]
- Thut G, Miniussi C, Gross J (2012): The functional importance of rhythmic activity in the brain. Curr Biol 22:R658–R663. [DOI] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O (2008): Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooff JC, Whitaker TA, Ford RM (2009): Directed forgetting in direct and indirect tests of memory: Seeking evidence of retrieval inhibition using electrophysiological measures. Brain Cogn 71:153–164. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Davachi L (2001): Cognitive neuroscience: Forgetting of things past. Curr Biol 11:R964–R967. [DOI] [PubMed] [Google Scholar]
- Wais PE, Martin GM, Gazzaley A (2012): The impact of visual distraction on episodic retrieval in older adults. Brain Res 1430:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wespatat V, Tennigkeit F, Singer W (2004): Phase sensitivity of synaptic modifications in oscillating cells of rat visual cortex. J Neurosci 24:9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie GR, Foxe JJ, Taylor TL (2008): Forgetting as an active process: An FMRI investigation of item‐method‐directed forgetting. Cereb Cortex 18:670–682. [DOI] [PubMed] [Google Scholar]


