Abstract
Glucose metabolism serves as the central source of energy for the human brain. Little is known about the effects of blood glucose level (BGL) on higher‐order cognitive functions within a physiological range (e.g., after overnight fasting). In this randomized, placebo‐controlled, double blind study, we assessed the impact of overnight fasting (14h) on brain activation during a working memory task. We sought to mimic BGLs that occur naturally in healthy humans after overnight fasting. After standardized periods of food restriction, 40 (20 male) healthy participants were randomly assigned to receive either glucagon to balance the BGL or placebo (NaCl). A parametric fMRI paradigm, including 2‐back and 0‐back tasks, was used. Subclinically low BGL following overnight fasting was found to be linked to reduced involvement of the bilateral dorsal midline thalamus and the bilateral basal ganglia, suggesting high sensitivity of those regions to minimal changes in BGLs. Our results indicate that overnight fasting leads to physiologically low levels of glucose, impacting brain activation during working memory tasks even when there are no differences in cognitive performance. Hum Brain Mapp 36:839–851, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: functional magnetic resonance imaging, working memory, overnight fasting
INTRODUCTION
Glucose is the human brain's most important energy source, as the brain is unable to metabolize nutrients like proteins or fatty acids to the extent necessary to meet its metabolic needs [e.g., Siesjö, 1978; Weiss, 1986]. In vivo studies using labeled 2‐deoxyglucose or 2‐fluorodeoxyglucose have demonstrated a correlation between brain function and brain metabolism [Sokoloff et al., 1977], showing that the proper functioning of all mental processes directly depends on an adequate supply of glucose. The fact that the brain regions that are more active have higher glucose needs, reducing its availability for less active brain regions [McNay et al., 2001], suggests that the amount of available glucose is limited. In the strength model, Gailliot and Baumeister [2007] concluded that self‐control relies on blood glucose as an important but finite source of energy. According to this model, every act of self‐regulation takes up glucose. With increased glucose consumption, self‐regulation becomes more difficult (ego depletion), until it finally fails. Thus, an individual's ability to self‐regulate can diminish with low levels of blood glucose, or, indirectly, through glucose‐consuming processes like demanding cognitive activity [Gailliot and Baumeister, 2007].
Throughout a 24‐h period, blood plasma glucose levels are generally maintained between 72 and 144 mg/dl [Cornblath et al., 2000]. However, each healthy individual has a unique set of responses to main meals, meaning that even stronger fluctuation of blood glucose level (BGL) is physiological. For example, BGLs can vary from about 60–170 mg/dl during the day, in many cases remaining above 120 mg/dl after 2 h following a large meal [Christiansen, 2006]. The level of 60 or 70 mg/dl is commonly cited as the lower limit of normal glucose. At the BGL of 50–55 mg/dl, the first symptoms of hypoglycemia (tremor, palpitations, sweating, hunger, cognitive impairment, behavioral changes, and psychomotor abnormalities) are likely to occur [Cryer, 1997].
Given that neuronal activity of the human brain almost exclusively relies on glucose metabolism [e.g., Siesjö, 1978; Weiss, 1986], an association between BOLD signal and BGL is plausible. Purnell et al. [2011] observed an increase in BOLD signal intensity due to i.v. infusion of glucose in contrast to the infusion of fructose; intensity of BOLD signal was reduced by up to 30% due to hypoglycemia (50 mg/dl) as compared to euglycemia [Anderson et al., 2006; Purnell et al., 2011].
Behavioral and neuroimaging data from studies involving healthy volunteers as well as patients with type 1 and type 2 diabetes show that hyperglycemia and hypoglycemia affect attention control and working memory performance [Bolo et al., 2011; Gailliot and Baumeister, 2007; Sommerfield et al., 2003]. For instance, the study by Sommerfield et al. [Sommerfield et al., 2003] demonstrates that all memory systems (immediate and delayed verbal memory, and immediate and delayed visual memory) are impaired during acute hypoglycemia (∼45 mg/dl), with working memory ‐ a basic cognitive function regulating the ability to remember and manipulate information over a brief period of time [Wager and Smith, 2003]—being particularly susceptible.
Recent research has shown increase of regional blood flow (rCBF) in the thalamus, the medial prefrontal cortex and the globus pallidus in response to hypoglycemia (53 mg/dl) [Arbeláez et al., 2013]. Regional brain activation during hypoglycemia (50 mg/dl) has also been seen in the hypothalamus, the brainstem, the anterior cingulate cortex, the uncus, the putamen [Musen, 2008], the thalamus [Mangia, 2012], and the medial prefrontal cortex [Teves et al., 2004]. In the study done by Rosenthal et al. [2001], slower finger tapping during hypoglycemia (45 mg/dl) was associated with decreased BOLD activation in the right premotor cortex, the supplementary motor area (SMA) and the left hippocampus, and with increased BOLD activation in the left cerebellum and the right frontal pole. In the same study, the behavioral deterioration in four‐choice reaction time (4CRT) was associated with reduction in BOLD activation in the motor and visual systems but with increased BOLD signal in a large area of the left parietal association cortex. Case reports of severe hypoglycemia (20 mg/dl) have revealed regions of restricted diffusion in the temporal and occipital lobes as well as the basal ganglia [Chan et al., 2003]. The findings suggest that some regions respond already to the lower level of hypoglycemia, with other regions being triggered with the increasing severity of the hypoglycemic condition. It can be safely assumed, therefore, that depending on the severity of hypoglycemia and the nature of the applied task, different regions are likely to be involved in response to hypoglycemic conditions. That being said, it is still not easy to define a network involved in hypoglycemia.
There is even less evidence in relation to the effects of more subtle changes in BGL within a physiological range, although this may constitute a major methodological factor affecting results of fMRI studies.
In our prospective, placebo‐controlled, cross‐over fMRI study, we involved a large sample (40 volunteers; 20 male and 20 female) to ensure that the results were adequately representative and that any potential gender effects could be detected. We aimed to examine the effects of physiologically low BGL on working memory performance and associated neural networks, which include larger regions of the prefrontal and posterior parietal cortices, the insula, the ventral visual cortex as well as those of the thalamus and basal ganglia, and the cerebellum [Owen et al., 2005, Rottschy et al., 2012]. The potential effect of physiologically low BGL on the prefrontal cortex, as working memory has mostly been associated with the prefrontal cortex [Gangadhar et al., 2007, Rottschy et al., 2012], which has also been shown to be affected by hypoglycemic conditions [Arbeláez et al., 2013; Musen, 2008; Rosenthal et al., 2001; Teves et al., 2004], was particularly noteworthy. We also aimed to study the effects of physiologically low glucose levels in other brain regions (parietal and visual cortices, basal ganglia and thalamus) also belonging to the “core” working memory network triggered by the n‐back task [Arbeláez et al., 2012, 2013; Mangia, 2012; Musen, 2008; Rosenthal et al., 2001; Rottschy et al., 2012; Teves et al., 2004]. Given that neuroimaging studies related to working memory functions do not necessarily control for BGLs under physiological conditions, our aim was to rule out a possible “metabolic bias” and demonstrate that control of BGL in screening protocols may increase sensitivity of the experimental design.
METHODS AND MATERIALS
Participants
Forty healthy (20 male), right‐handed [Oldfield, 1971] participants, all of whom were native speakers of German, took part in the study. Participants were between 20 and 32 years of age (mean = 24.5, SD = 3.4) with body mass index (BMI) ranging from 20 to 24.6 (mean = 22, SD = 1.4). Nine of the female participants reported using hormonal contraceptives. Demographic variables are summarized in Table 1.
Table 1.
Demographic Data
| Test | Female | Male |
|---|---|---|
| n | 20 | 20 |
| Age | 25.2 (3.01) | |
| BMI | 22.0 | 23.2 |
| Education | 12 (10.8%)−13 (89.2%) | |
| MWT‐B (IQ) | 107.3 (14.55) | |
| TMT‐A | 18.9 (5.13) | |
| TMT‐B | 34.3 (11.12) | |
| WMS‐R | 16.5 (2.89) | |
| RWT (K) | 14.8 (3.82) | |
| RWT (GR) | 14.8 (3.03) | |
Age in years; BMI in kg/m2; education in years; MWT‐B in IQ‐points; TMT in s; WMS‐R in point score; RWT in number of mentioned words; The table shows mean values and the standard deviation in brackets. They were no differences between women and men concerning test results exist, except the BMI (P < 0.05).
The exclusion criteria included medical history of psychiatric disorders (including first‐degree relatives), a body mass index (BMI) <20 or >25 kg/m2, left‐handedness, MRI disqualification (metal implants, tattoos, etc.), pregnancy, any type of medication (except hormonal contraceptives), physical impairment or a history of metabolic, cardiovascular, neurological, or psychiatric disorders, abuse of psychoactive substances (alcohol, drugs, smoking, and medication), head trauma or allergies, or pathologically altered lab results for insulin, glucose, adrenaline, and noradrenaline (see below in text).
Participants were recruited via flyers posted on campus and screened on the phone. Suitable participants were invited for further screening tests, which included three major steps. The first step involved evaluation of medical history and physical examination by experienced medical professionals (S.O., N.C., S.V., and A.W.) in the Department of Psychiatry, Psychotherapy and Psychosomatics. In the second phase, neuropsychological tests were carried out by either a psychologist or a specifically trained person (see for results Table 1) to characterize cognitive performance (CPT). The SKIDPIT light was applied [Demal, 1999] to exclude participants with history (or current episode) of psychiatric conditions. In the final step, a blood sample (5 ml) was taken to check for blood levels of insulin, glucose, adrenaline and noradrenaline. The blood tests were intended to rule out pheochromocytoma and insulinoma, as administration of glucagon could lead to a critical increase of arterial blood pressure in case of pheochromocytoma or to a critical decrease in BGL in case of insulinoma.
All participants gave written informed consent prior to participation in the study and were monetarily compensated for their participation. The study protocol was in concordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Medical Faculty, RWTH Aachen University.
Procedure
Before measurements, all subjects had been fasting for at least 14 h (i.e., not consuming food or any beverages that might contain carbohydrates, fat, protein, alcohol, or artificial sweeteners). Participants were not allowed to consume caffeine after 9 a.m. on the day of the scan. The participants were instructed to refrain from physical exercise on the day before the study. All 40 participants were measured twice, with 4 weeks between measurements to account for cyclic hormonal effects. Both fMRI scans were conducted at noon starting between 12 and 1 pm and lasted about 60 min. All measurements were conducted under double blind conditions with one half of the participants randomly assigned to be administered glucagon during the first session and NaCl during the second session and the other half in the opposite order.
Following overnight fasting, the subjects were scanned with their BGL having been elevated through glucagon administration and also in a low normal BGL state induced through sodium chloride. Thus, in one instance it was “overnight fasting condition modulated by glucagon” and, in the other, it was “unmodulated overnight fasting condition”. As the modulated fasting condition was also associated with elevated blood glucose condition, we call this condition “elevated glucose condition” (EGC), and the unmodulated fasting condition is referred to “fasting glucose condition” (FGC).
Glycogen and clamp study are two approaches to contrast glucose levels to the hypoglycemic conditions. The administration of glucagon to control BGL as compared to insulin‐clamp or oral/intravenous administration of glucose was chosen for several reasons. The BGL achieved through glucagon administration reflects naturally occurring postprandial BGL. Insulin clamps conversely create a highly artificial metabolic state, which is stable only in relation to glucose and may strongly interfere with other hormonal systems.
In the elevated BGL Condition (EGC), we applied a 1 mg bolus injection + 0.5 mg/h. of glucagon (GlucaGen® Hypokit, powder, Novo Nordisk, Mainz, in 50 ml Ampuwa®, Fresenius Kabi, Bad Homburg), which resulted in ∼1.5 mg glucagon in EGC. The same total volume of 50ml sodium chloride (NaCl, Ampuwa®, Fresenius Kabi, Bad Homburg) was administered in the physiologically low BGL condition after overnight fasting (FGC).
FMRI measurements started after the bolus injection (of placebo or glucagon) with a 6 min resting state measurement, followed in counterbalanced order by two major tasks: CPT [Rosvold et al., 1956] and a the mood induction paradigm (EmoInd, [Schneider et al., 1994]). The method for mood induction consists of happy or sad facial expressions presented to participants who are asked to simultaneously view the pictures and alter their mood according to the facial expression [Habel et al., 2005; Kohn et al., 2013;Schneider et al., 1994]. Happy or sad pictures were shown in seven sequentially presented blocks of 30 s duration per emotion; each block was followed by three 5‐point Likert‐type scales (shown for 5 s each). Participants were asked to rate how happy, how sad, and how aroused they felt at that time. The three ratings were followed by a fixation cross (low level baseline) lasting 15 s. In total, the mood induction lasted about 14 min (with prescans).
The paradigms were followed by a basal visual stimulation, after which the infusion was terminated. The fMRI session ended with a final resting state measurement, followed by an anatomical scan. Given that this study focuses only on the CPT, the other paradigms are not described further.
Twenty participants were first required to complete a CPT, before starting on the mood induction (EmoInd), the other twenty participants started with mood induction.
On arrival and then subsequently in intervals of 15 min in between fMRI runs, BGLs were measured from capillary blood by finger stick via plasma‐calibrated “Contour” blood glucose meter and test strips (Bayer Vital GmbH, Leverkusen, Germany) according to the manufacturer's recommendations. With 99.3% accuracy, the Contour meter meets International Organization for Standardization accuracy requirements (ISO15197:2003).
Altogether, five samples were taken from all participants for blood glucose tests during the first and the second day of the experiment. The first samples were taken 10 min before fMRI measurements (t 0), the second (t 1) before the first major tasks (the sequence of the two main tasks, EmoInd and CPT, varied), the third (t 2) between the main tasks, the fourth (t 3) after the second main task (EmoInd or CPT), and the last (t 4) at the end of the study schedule (Fig. 1, Table 2).
Figure 1.
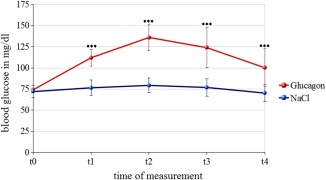
Blood glucose level. BGL and SD under EGC are displayed in red, BGL and SD in prolonged FGC in blue. BGL are sampled approximately every 15 min. Pre (t 0), t 1(∼15min), t 2(∼30min.), t 3(∼45min.) and post (t 4) (∼60min.).
Table 2.
Blood glucose level
| Time of measurement | NaCl | Glucagon | N | P‐value |
|---|---|---|---|---|
| t 0 | 72.0 (6.98) | 74.2 (9.83) | 37 | 0.251 |
| t 1 | 76.7 (9.29) | 112.1 (15.88) | 34 | <0.001*** |
| t 2 | 79.3 (8.97) | 136.0 (24.24) | 36 | <0.001*** |
| t 3 | 76.9 (10.43) | 126.4 (22.69) | 36 | <0.001*** |
| t 4 | 69.8 (8.70) | 99.7 (22.67) | 35 | <0.001*** |
Difference is significant (P 0.001). Mentioned are the mean values of the BZ at the corresponding time of measurement in mg/dl, the standard deviation is stated in brackets. The number of values is indicated in column “N.”
Infusions were administered by intravenous catheter and perfusion pump (MRI‐Caddy, Medfusion, Duluth, GA) through the left brachial vein. Other blood samples were taken via the i.v. line in the left elbow crease at time points t 0 and t 2 to check for blood levels of insulin, adrenaline and noradrenaline (Table 3). We had lost some blood samples on account of technical difficulties (please refer to the Table 2 and to the Table 3), but none of the participants was excluded from the experiment.
Table 3.
Blood insulin, noradrenalin and adrenalin levels
| Time of measurement of insulin | NaCl | Glucagon | N | P‐value |
|---|---|---|---|---|
| t 0 | 4,67 (3,18) mU/l | 42,06 (21,96) mU/l | 37 | <0.001*** |
| t 2 | 3,25 (1,39) mU/l | 46,45 (30,11) mU/l | 40 | <0.001*** |
| Time of measurement of noradrenalin | NaCl | Glucagon | N | P‐value |
| t 0 | 1,37 (0,70) pmol/l | 1,96 (2,99) pmol/l | 37 | n.s. |
| t 2 | 1,27 (0,57) pmol/l | 2,18 (2,80) pmol/l | 40 | <0.01** |
| Time of measurement of adrenalin | NaCl | Glucagon | N | P‐value |
| t 0 | 194,12(142,40) nmol/l | 194,76 (101,27) nmol/l | 37 | n.s |
| t 2 | 159,85 (89,68) nmol/l | 235,17 (144,36) nmol/l | 40 | <0.001*** |
The standard deviation is stated in brackets. The number of values is indicated in column “N”.
fMRI Paradigm
A parametric fMRI paradigm including 2‐back and 0‐back tasks were used (Fig. 2). As the 0‐back control condition does not require manipulation of information within working memory, it was used as a control task. For working memory and control conditions, single letters were presented one after another for 1,000 ms in the middle of a screen via video goggles (VisuaStim XGA, Resonance Technology, Los Angeles). Participants gave responses via LUMItouch response system (http://ucdirc.ucdavis.edu/techsupport/Lumitouch_brochure.pdf) whenever a prespecified stimulus was presented. In the two back conditions, participants were instructed to press the button when a letter appearing on the screen was identical to the next to last letter. In the 0‐back control condition, participants had to respond to a cue stimulus (every “X” appearing on the screen measures selective attention). The presentation of the letters in control and working memory conditions was randomized with a target probability of .37 (35 targets, 60 distractors). Study volunteers had to perform the paradigm twice, with each run consisting of 9 baseline periods (18 s) and 10 activation periods (30 s). The baseline and activation periods appeared alternately.
Figure 2.
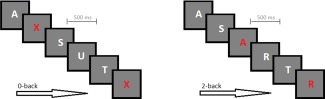
Working memory task. For both CPT conditions, single letters were presented one after another for 1,000 ms. In the 2‐back conditions, participants were instructed to press the button when a letter appearing on the screen was identical to the next to last letter. In the 0‐back control condition, participants had to respond to a cue stimulus (every “X” appearing on the screen).
Analysis of BGL, Demographic, and Behavioral Data
Two‐sample t‐tests were applied to compare BGL between the EGC (glucagon) and FGC (placebo) conditions (t 0–t 5), demographic variables and the neuropsychological tests.
Reaction times (RTs) and the number of correct hits (accuracy calculation) were collected during the fMRI experiment. For accuracy calculations, all types of errors (wrong answers and omissions) were considered.
To test the effect of BGL on CPT, we performed a 2‐way analysis of variance (ANOVA) of the RT and accuracy with the following two factors: CPT (two levels: zero‐ or two‐back) and BGL (two levels: FGC or EGC). To control for order effects, we conducted on further 2 × 2 repeated measures ANOVAs with CPT and FG/EG conditions as within‐subjects variables and paradigm order as between‐subjects factors. To control for order and gender effects, we conducted two further 2 × 2 × 2 repeated measures ANOVAs with CPT and FG/EG conditions as within‐subjects variables, and paradigm order and gender as between‐subjects factors.
All analyses were tested for significance at a threshold of P = 0.05 and SPSS (20) was used for analyses.
ANALYSIS OF FMRI DATA
FMRI data acquisition
Imaging data were acquired in a 3T Trio Tim MRT (Siemens, Erlangen, Germany) using EPI (echo planar imaging), with TR = 2s, TE = 30 ms, 36 slices, ST = 3.3 mm. IG = 0.35 mm, MS = 64 × 64, FOV = 240 × 240 mm, FA = 77. Analyses of functional images were performed with SPM8 (Statistical Parametric Mapping, Wellcome Departement of Cognitive Neurology, London, UK), implemented in Matlab 7.7 (The MathWorks). The images of the time series were realigned with a two‐pass procedure, where the first image (first pass) and the mean image (second pass) were used as reference. Each anatomical scan was used to determine spatial normalization parameters by means of the unified segmentation approach [Ashburner and Friston, 2005]. These normalization parameters were applied to the functional scans, transforming the time series into the standard space defined by the Montreal Neurological Institute (MNI).
During normalization, all images were resampled to a voxel size of 2 × 2 × 2 mm3 and images were later smoothed with an isotropic Gaussian kernel of 8 mm full width at half maximum. Individual time series were analyzed (first level) within the framework of the general linear model (GLM). Box car functions (each of the two CPT conditions under two different BGL conditions) were convolved with the canonical hemodynamic response function (HRF) and then used as predictors in the GLM.
The mean across time in each voxel was modeled by a constant term and low‐frequency drifts were removed using a high‐pass filter with a cutoff period of 128 s. Temporal correlations were modeled by a first‐order regression process in the usual way. The resulting images containing parameter estimates of the both CPT tasks of each subject under glucagon (EGC) and placebo (FGC) were entered into repeated measures ANOVA with mixed effects. The factor “subject” was used for random effects and the two CPT tasks and both EG‐ and FG conditions served as levels of the fixed effects factor.
Main Effects of Working Memory
To demonstrate the effect of working memory, we calculated the t‐contrasts comparing two‐back task against the zero‐back (2‐back>zero‐back and zero‐back>2‐back tasks) for EG and FG conditions, respectively. To demonstrate the regions associated with working memory independently of the BGL, we included the following two t‐contrasts (2‐back > 0‐back under placebo conditions and 2‐back > 0‐back under glucagon administration) into conjunction analysis.
Effects of EGC and FGC
For glucagon (EGC) and placebo (FGC), two directional t‐contrasts comparing two‐back tasks against the baseline were defined. The FGC < EGC and FGC > EGC contrasts were calculated for the zero‐ and n‐back tasks, respectively.
Order and Gender Effects in the Two‐Back Task
To analyze order effects, we entered the images of the two BGL conditions (two levels: FGC or EGC) of each subject into repeated measures ANOVA with mixed effects, and paradigm order (two levels: premood or postmood induction) as an additional between‐subjects factor. Parameter estimates were extracted from entire clusters of activation and were analyzed with SPSS 20. In addition, to test the interaction between paradigm order (premood or postmood induction) and BGL conditions (FGC vs EGC), we performed a two‐way analysis of variance (ANOVA) with the following two factors: order effects (two levels: premood or postmood induction) and BGL (two levels: FGC or EGC conditions) for parameter estimates of each chosen region.
To analyze gender effects, we entered the images of the two BGL conditions (two levels: FGC or EGC) of each subject into repeated measures ANOVA with mixed effects, and gender (two levels: male and female participants) as an additional between‐subjects factor. We contrasted the groups (20 male participants vs. 20 female participants in each condition) under FG and EG conditions in a 2 × 2 repeated measures ANOVA.
Unless otherwise stated, the significance level for all main effects of the imaging data with regard to CPT was set to P < 0.05 after whole‐brain correction (family‐wise error or FWE).
The significance level for each single contrast in the conjunction was also set to P < 0.05 after whole‐brain correction (family‐wise error, or FWE). The clusters of activation in the conjunction were displayed only when they contained >60 contiguous voxels.
For the CPT contrasts in EGC versus FGC, we determined a cluster extent threshold by Monte Carlo simulations (3DClustSim implemented in AFNI). For a threshold at the voxel level of P = 0.001 and spatial properties as presented in this study, 10,000 simulations resulted in an extent threshold of 72 resampled voxels. This procedure guards against a false positive rate above 5% due to multiple testing.
For anatomical localization of functional data, we referred to probabilistic cytoarchitectonic maps [Eickhoff et al., 2005]. The data are reported in MNI (MNI space) coordinates.
RESULTS
Analysis of BGLs
Following the experiment, subjects were asked what they thought was administered at the first and second measurements and answers were below chance level. BGL measured by finger prick from all participants 10 min before fMRI measurements (t 0) did not show significant differences between EGC and FGC. In each participant, the BGL was lower than 80 mg/dl at the beginning of the measurements. In both conditions, the highest level of blood glucose was registered during the time point t 2. In both conditions, the increase of BGL was significant at the time point t 2 as compared to the time point t 0 (NaCl: t(36) = −4.99; P < 0.001; glucagon: t(36) = −15.10; P < 0.001). During the experiment, EGC and FGC differed significantly at all further time points (t 1–t 4) (Fig. 1, Table 2).
In FGC, BGLs remained at a physiologically low level, whereas an increase in blood glucose to physiologically normal or high levels was found after glucagon infusion in EGC (Table 2). At this physiologically low BGL no symptoms of hypoglycemia were expected or detected.
Hormonal Analyses
For adrenalin levels in EGC, FGC and at screening, the repeated measures ANOVA was significant (mean screening = 258.93 pmol/l, SD = 181.07; mean FGC = 178.68 pmol/l, SD = 112.65; mean EGC = 235.30 pmol/l, SD = 120.94; F 2,37 = 9.09, P < 0.001). Noradrenalin levels in FGC, EGC and at screening also differed significantly (mean screening = 2.37 nmol/l, SD = 0.96; mean FGC = 1.32 nmol/l, SD = 0.62; mean EGC = 2.10 nmol/l, SD = 3.0; F 2,37 = 28.31, P < 0.001). In post hoc analysis, we saw that the effects were driven by FGC (NaCl condition) as no significant differences were seen between screening and EGC (glucagon condition) in adrenalin and noradrenalin levels.
Insulin levels also differed significantly between screening, the elevated BGL (EGC) and BGL after overnight fasting (FGC) (screening insulin mean = 12.18 mU/l, SD = 11.43; FGC insulin mean = 3.99 mU/L, SD = 2.0; EGC insulin mean = 45.25 mU/l, SD = 23.8; P < 0.001; F 2.38 = 49.75, P < 0.001). In post hoc analysis, we saw again that the effect was mostly driven by FGC (NaCl condition). There was, however, significant effect (P < 0.001) in the level of insulin between the screening and the elevated glucose condition (EGC). However, the values for insulin in both conditions were physiological after normal meal. Thus, the effect of glucagon on the hormones was comparable with the physiological effects of the meal.
Behavioral Data
The 2‐way CPT × BGL condition (FGC/EGC) analysis of variance (ANOVA) on RT data revealed a significant main effect of CPT (F 1,35 = 165.33, P < 0.001) as RTs in 0‐back as compared to 2‐back task were faster. No other effects were significant.
The 2‐way CPT × condition analysis of variance (ANOVA) on accuracy data revealed a significant main effect of CPT (F 1,35 = 149.02, P < 0.001) as the number of correct hits in 0‐back as compared to 2‐back task was higher. There were no other significant interaction effects.
No significant effect of the factor gender or the factor paradigm order was detected in further analyses.
FMRI RESULTS
Main Effects of Working Memory
To demonstrate the regions associated with working memory independently of the BGL, we included two t‐contrasts (2‐back > 0‐back under placebo conditions and 2‐back > 0‐back under glucagon conditions) into conjunction analysis. The conjunction analysis showed several cortical regions associated with working memory function: (1) bilateral and medial posterior parietal cortex, including the precuneus, the inferior and superior parietal lobules (BA 7, 40); (2) bilateral premotor cortex (BA 6,8); (3) dorsal cingulate/medial premotor cortex, including the SMA (BA 32,6); (4) the bilateral rostral prefrontal cortex or frontal pole (BA10); (5) the bilateral dorsolateral prefrontal cortex (BA 9,46); (6) the bilateral midventrolateral prefrontal cortex (BA 44,45,47), or frontal operculum extending to bilateral insula, and (7) the bilateral occipitotemporal visual cortex, including the bilateral fusiform gyri. Additionally, there was activation in bilateral cerebellum, basal ganglia (right caudate nucleus and left putamen) and bilateral thalamus (Table 4, Fig. 3).
Table 4.
Main effects of working memory task (Conjunction analysis)
| Anatomical region | Side | k | Peak voxel | |||
|---|---|---|---|---|---|---|
| T | x | y | z | |||
| Superior frontal gyrus | R | 29279 | 18.98 | 30 | 4 | 60 |
| Middle frontal gyrus | L | 18.27 | −26 | −2 | 56 | |
| Insula | R | 16.98 | 32 | 24 | −4 | |
| Supplemental motor area | L | 16.87 | −36 | 20 | 44 | |
| Inferior frontal gyrus | L | 16.75 | 46 | 24 | −6 | |
| Middle frontal gyrus | R | 15.88 | 44 | 46 | 32 | |
| Thalamus | L | 13.81 | −10 | −14 | 12 | |
| Precentral gyrus | L | 13.79 | −46 | 4 | 32 | |
| Middle cingulate cortex | R | 13.74 | 8 | 28 | 30 | |
| Inferior parietal lobule | L | 13305 | 21.69 | −36 | −48 | 42 |
| Superior parietal lobule | R | 17.98 | 14 | −72 | 54 | |
| Inferior parietal lobule | R | 17.31 | 42 | −44 | 40 | |
| Superior parietal lobule | L | 16.77 | −26 | −68 | 46 | |
| Angular gyrus | R | 14.21 | 32 | −56 | 42 | |
| Middle occipital gyrus | L | 13.66 | −30 | −74 | 28 | |
| Superior occipital gyrus | R | 13.41 | 28 | −76 | 38 | |
| Cerebellum | R | 6612 | 16.01 | 32 | −62 | −32 |
| Cerebellum | L | 15.58 | −36 | −60 | −34 | |
| Inferior occipital gyrus | L | 8.94 | −54 | −62 | −14 | |
| Inferior temporal gyrus | R | 340 | 6.60 | 64 | −52 | −22 |
| Superior temporal gyrus | L | 284 | 7.08 | −56 | −42 | 12 |
The significance level for each single contrast in the conjunction was also set to P < 0.05 (family‐wise error or FWE).
Figure 3.
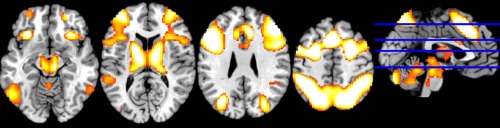
Main effects of working memory task (Conjunction analysis). Regions involved in working memory task independently of the BGL (conjunction analysis). Significance level for each of the two single contrasts in the conjunction was set to an FWE‐corrected threshold of P < 0.05, k = 60.
Comparison between Placebo and Glucagon Conditions in Two‐Back Task
Performing the two‐back task, subjects under physiologically low blood glucose (BG) conditions showed reduced recruitment of the bilateral thalamus, the bilateral basal ganglia, the posterior cingulate cortex and the rostral prefrontal cortex (Table 5, Fig. 4). In both right and left thalami, activations were mainly located in the medial dorsal nucleus. Parameter estimates extracted from entire clusters of activation in the bilateral thalamus, the bilateral basal ganglia and the rostral prefrontal cortex did not reveal any significant correlation with adrenalin or insulin level.
Table 5.
Comparison between placebo and glucagon conditions in two‐back task
| Anatomical region | Side | k | Peak voxel | |||
|---|---|---|---|---|---|---|
| T | x | y | z | |||
| Middle cingulate cortex | L | 179 | 4.07 | −6 | −30 | 34 |
| Paracentral lobule | L | 3.30 | −6 | −32 | 52 | |
| Caudate nucleus | R | 171 | 4.35 | 14 | 16 | 6 |
| Putamen | L | 148 | 4.21 | −18 | 14 | 4 |
| Thalamus | L | 124 | 4.28 | −4 | −16 | 2 |
| Thalamus | R | 3.71 | 10 | −20 | 4 | |
| Thalamus | L | 116 | 4.20 | −16 | −28 | 0 |
| Mid orbital gyurs | L | 95 | 4.50 | −6 | 64 | −8 |
Figure 4.
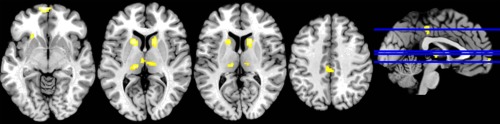
Comparison between placebo and glucagon conditions in two‐back task (glucagon > placebo). Differences of brain activation in the bilateral thalamus, the basal ganglia and the rACC (Monte‐Carlo corrected at P = 0.001, k = 72) between FGC and EGC (FGC > EGC).
Order Effect and the Effect of Gender in the Two‐Back Task
The paradigm order × BGL condition interaction indicated a significant effect in the bilateral thalamus, the right caudate nucleus and the rostral prefrontal cortex (Table 6). Parameter estimates extracted from the bilateral thalamus revealed a significant mean effect of BGL (F 1/18 = 6.603; P = 0.019 and F 1/18 = 5.859; P = 0.026 for the left and the right thalami, respectively), indicating a weaker involvement of the bilateral thalamus after overnight fasting. The significant interaction between the factors paradigm order (premood or postmood induction) and conditions (FGC/EGC) in the right (F 1/18 = 5.281; P = 0.034) and the left (F 1/18 = 4.726; P = 0.043) thalami, respectively demonstrated that the differences between the subjects under EGC (as compared to FGC) were driven mostly by the group of participants who had to perform CPT tasks following mood induction. The post hoc analysis of parameter estimates revealed that a cognitive effort during a previous task (mood induction) induced stronger differences in the right thalamus (t 18; P = 0.005) and the left thalamus (t 18; P = 0.008) between FG and EG conditions in the working memory task (see Fig. 5a,b).
Table 6.
Effect of paradigm order (interaction between BGL and paradigm order)
| Anatomical region | Side | k | Peak voxel | |||
|---|---|---|---|---|---|---|
| F | x | Y | z | |||
| Thalamus | R | 477 | 14.87 | 4 | −8 | 10 |
| Thalamus | L | 11.71 | −16 | −28 | 0 | |
| Mid. orbital gyrus | L | 87 | 13.22 | −8 | 64 | −8 |
| Caudate nucleus | R | 77 | 9.84 | 14 | 16 | 6 |
Figure 5.
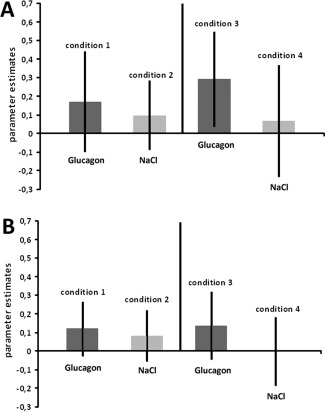
Effect of paradigm order on bilateral thalamus. CPT was administered before mood induction (condition 1 and 2) or CPT was conducted after mood induction (condition 3 and 4). The involvement of bilateral thalamus is affected by paradigm order with the group performing the CPT after mood induction (condition 3 and 4) showing the weakest thalamic involvement in response to overnight fasting (condition 4). Parameter estimates also indicated a weaker involvement of the region after overnight fasting (condition 2 and 4). (a) Parametes estimates in the right thalamus. (b) Parameter estimates in the left thalamus.
The analysis of parameter estimates in the rACC (when we observed task‐induced deactivation) revealed a significant effect of the main factor “condition” (F 1/18 = 5.859; P = 0.026), showing stronger deactivation of the rACC during FGC.
Parameter estimates extracted from the bilateral basal ganglia also revealed a significant mean effect of BGL (F 1/18 = 9,285; P = 0.007 and F 1/18 = 19,254; P < 0,001 for the left and the right caudate nucleus, respectively), indicating a weaker involvement of the region after overnight fasting (Fig. 6a,b). In the right caudate nucleus, they was also a trend toward significant BGL x order effect interaction (F 1/18 = 3,455; P = 0,079). No significant effect of the factor gender was detected in the analyses.
Figure 6.
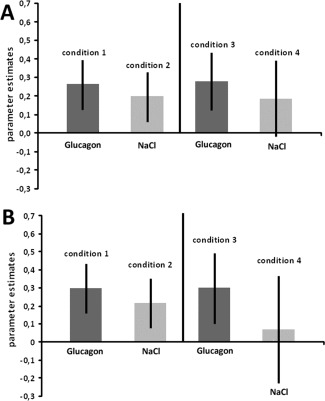
CPT was administered before mood induction (condition 1 and 2) or CPT was conducted after mood induction (condition 3 and 4). Parameter estimates extracted from the bilateral caudate nucleous indicated a weaker involvement of the region after overnight fasting (condition 2 and 4). (a) Parametes estimates in the right caudate nucleus. (b) Parameter estimates in left caudate nucleus.
DISCUSSION
In healthy volunteers, we investigated the effects of physiologically low (after overnight fasting) compared to elevated (after the administration of glucagon) BGLs on a neural network associated to working memory processes. In both conditions, the working memory task strongly activated the bilateral frontal and parietal cortices, the insula, the thalamus, the basal ganglia and the cerebellum. Cognitive performance was not affected by modulation of the BGL, while, neuronally, the fasting condition was associated with weaker involvement of the dorsal midline thalamus, a region with the highest glucose uptake among the thalamic nuclei [Cho et al., 2011], and the bilateral basal ganglia. As far as we know, this is the first evidence that in healthy humans the bilateral dorsal midline thalamus and basal ganglia are highly sensitive to increase of BGL within a physiological range (e.g., as a response to overnight fasting) during working memory processes, even when there are no differences in behavioral performance.
Glucose‐Mediated Differences in the Bilateral Dorsal Midline Thalamus in Working Memory
There is some evidence of the involvement of the dorsal midline thalamus in the regulation of BGL and hypoglycemia [Arbeláez et al., 2008, 2012; Teves et al., 2004], and also the effects of hypoglycemia on the thalamus during working memory tasks [Bolo et al., 2011]. The analysis of resting regional cerebral blood flow (CBF) differences identified neuronal activation during hypoglycemia (<55 mg/dl) in bilateral medial prefrontal cortex (MPFC) [Teves et al., 2004] and bilateral thalamus [Arbeláez et al., 2008; Teves et al., 2004]. Slightly subphysiological plasma glucose concentrations of 65mg/dl, compared to euglycemic conditions (90 mg/dl), increased activity only in the dorsal midline thalamus [Arbeláez et al., 2012]. In response to working memory tasks during acute hypoglycemia (50mg/dl), activation in the bilateral frontal and parietal cortices, the insula, the thalamus, and the cerebellum decreased [Bolo et al., 2011]. In line with the observation by Bolo et al [Bolo et al., 2011], the behavioral performance in our study did not differ between the glycemic conditions. However, unlike us, Bolo et al., observed neuronal differences between conditions in the bilateral insula, and the bilateral frontal and parietal cortices. Collectively, these studies indicate a hierarchy among brain responses to falling plasma glucose concentrations, with the dorsal midline thalamus being particularly susceptible to changes in plasma glucose concentrations, and the initial changes (as seen in our study) being registered soon after overnight fasting. Previous research and also results of our study suggest a decrease of thalamic involvement [a part of the default‐mode functional network; Greicius et al., 2007] in cognitive tasks during hypoglycemia and overnight fasting and an increase in resting condition.
In both BGL conditions, we also observed task‐induced deactivation in rACC, a region with high metabolic activity during rest, which is suppressed during cognitively demanding tasks [Greicius et al., 2007]. However, after overnight fasting, lesser rACC deactivation was seen, suggesting that fluctuations of the resting state network might be affected by BGL.
The effects of overnight fasting were also evident in the left putamen and the right caudate nucleus, in addition to the thalamus and the rACC. The putamen, the thalamus and the caudate belong to subcortical regions that show significantly greater activation in the hungry state compared to the satiated state [Tataranni et al., 1999], constituting the key brain regions involved in craving [Hommer, 1999] and in the pathophysiology of eating disorder [Delvenne et al., 1999; Van der Stelt and Di Marzo, 2003). Conversely, a distinct bilateral rCBF increase (without any significant effect on the BGL) after central insulin administration has been seen in the putamen and the caudate nucleus [Schilling et al., 2013], suggesting that these regions are targets for insulin signaling in the CNS. We did not find any significant association between the insulin or adrenalin levels and brain activation; however, as these effects are strongly interconnected, they cannot be completely ruled out or separated from the effects of BGL [for review see Filippi et al., 2013].
In the overnight fasting condition, thalamic involvement was affected by the paradigm order, as the previous task (the emotion induction task) induced stronger differences in the thalamus. The group performing the working memory task after mood induction showed the weakest thalamic involvement in response to unmodulated fasting state glucose levels. In response to an activated condition (through administration of glucagon), thalamic involvement increased with the working memory task performance following mood induction. Laboratory studies have found that after participants complete an initial self‐control task, they perform worse on a second self‐control task compared to participants whose initial task did not require self‐control [for review see Gailliot and Baumeister, 2007]. The model of Gailliot and Baumeister [2007] suggests that the first act of self‐control consumes or depletes some resource, thereby impairing self‐regulation on the subsequent task. Based on this model, we propose that the duration of exposure to physiologically low glucose levels and the previous effort (in the form of mood induction task) may compromise resources [Gailliot and Baumeister, 2007], leading to further decline in thalamic involvement.
Replication of a Robust Network Underlying Working Memory Performance
In response to N‐back, we saw a widely distributed network underlying the active maintenance of perceptual representation. In particular, we observed simultaneous involvement of larger regions of the prefrontal and posterior parietal cortices, insula, ventral visual cortex as well as those of the thalamus and basal ganglia, and the cerebellum. Involvement of those regions in working memory processes is confirmed by quantitative meta‐analysis of functional neuroimaging data [Owen et al., 2005, Rottschy et al., 2012].
Although working memory has mostly been associated with the prefrontal cortex [Gangadhar et al., 2007], recent findings increasingly suggest that subcortical structures (basal ganglia, thalamus) also play a crucial role in working memory processes. A study by McNab and Klingberg [2008] identified the basal ganglia (as part of the basal ganglia–thalamo‐cortical circuit [Alexander et al., 1986] as the regulator of information entering into working memory. The microstructure of the medial dorsal nucleus, a region strongly linked to the orbito‐frontal cortex [Bruyn, 1989], was shown to be a predictor of working memory performance quality [Piras et al., 2010]. Clinical trials demonstrated the impairments in working memory [Dagenbach and Kubat‐Silman, 2001; Kubat‐Silman et al., 2002] and executive functions (Van der Werf et al., 2003) following lesions in the medial dorsal nucleus.
LIMITATIONS AND SUGGESTIONS FOR FURTHER RESEARCH
We did not directly measure BGL in the brain, as would have been possible with positron emission tomography, or look at CBF. Thus, the possibility that modulation of BGL may differentially interfere with the HRF in our conditions cannot be ruled out. That being said, we would argue that such modulation is unlikely as blood glucose in the brain is highly regulated and differences in peripheral blood glucose may not have such a strong impact on the brain. Additionally, such modulation would most likely lead to a systematic effect, which would produce strong one‐sided differences, which is not the case in our sample.
We did not compare different experimental settings for the administration of glucose to rule out possible side effects of glucagon administration. Further experimental conditions would have led to a more complex design and logistical difficulties. Furthermore, we tried to control metabolic side effects of glucagon by measuring a large array of hormones associated with glucagon functioning, and, also, performing correlations between the adrenalin and insulin and brain activation. However, the previously demonstrated effects (even in the absence of a significant effect on the BGL) on brain activation following central insulin administration [Schilling et al., 2013] suggest that the hormonal effect (both of glucagon and insulin) on our results cannot be completely ruled out. Further research will be required to dissect insulin and glucagon signaling in the CNS. Another limitation of our study is the lack of a non‐food‐deprived control group, owing to which the effects of fasting and those of plasma glucose concentration cannot be completely separated. It was, however, beyond the scope of our current study given its within‐subject double‐blind design. The inclusion of a non‐food‐deprived control group might have interesting implications for future research.
We also believe that the level of difficulties might have played a role in the lack of effects seen in behavioral performance during the working memory task. The study included only young, healthy and cognitively fit participants, all students from the university in Aachen. For these participants, the 2n‐back task might not have been difficult enough. Alternatively, increasing the task difficulty level (e.g., performing of a 3n‐back) could demonstrate differences in working memory. Thus, task difficulty should be taken into account when studying the effects of low BGL or hypoglycemia on working memory.
Another pertinent question is, why cognitively demanding tasks are associated with relatively weak involvement of the regions forming working memory network while at rest those regions are more strongly involved? And whether resting state network is affected by changes in BGL? Nevertheless, as this study is the first to use glucagon in this context, it may pave the way for more elaborate use of glucagon in the modulation of glucose metabolism.
CONCLUSION
The results of our study, replicate a robust network underlying working memory functions, emphasizing a powerful contribution of the thalamus and basal ganglia to working memory processes within this network. Physiologically low BGL after overnight fasting was linked to weaker involvement of the bilateral dorsal midline thalamus and bilateral basal ganglia, indicating the high sensitivity of these regions to changes in BGLs. These findings are in line with previous research suggesting the contribution of basal ganglia and thalamus to the regulation of hypoglycemic states [Arbeláez et al., 2012] and hunger [Tataranni et al., 1999]. No differences were found with respect to the involvement of the prefrontal and parietal regions, nor did behavioral performance differ between the conditions. Collectively, the findings suggest that the dorsal midline thalamus is one of the first regions to respond to increase of BGL even within a physiological range. This assumption is strongly supported by the fact that initial synaptic activation was seen only in the dorsal midline thalamus as plasma glucose concentrations dropped just below physiological levels (65 mg/dl; Arbeláez et al., 2012]. The differences in the prefrontal and parietal regions, as observed in the studies of Bolo et al., [2011] and Teves et al., [2004], are likely to be seen first during hypoglycemic conditions, when the first differences in CPT are also expected to appear [Sommerfield et al., 2003].
Our results suggest that the effects of physiologically low levels of blood glucose (e.g., after overnight fasting) on brain activation have to be taken into account along with paradigm order as a possible confounder even when behavioral outcomes are similar between physiologically low and normal glucose levels.
Basal ganglia and thalamus are involved in a wide range of pathological conditions, for example, eating disorder, obsessive‐compulsive disorder, addiction or schizophrenia [Delvenne et al., 1999; Klingner et al., 2014; Tataranni et al., 1999; Van der Stelt and Di Marzo, 2003; Welter et al., 2011]. The thalamus is recognized as an essential node in a range of higher‐order cognitive circuits despite the common perception of it being a mere sensory information relay center [Alcauter et al., 2014]. Perception and experience of emotions are affected by disorders of the basal ganglia [Paradiso et al., 2013; Pell and Leonard, 2003]. Given the importance of these regions, we believe that the effects of physiologically low levels of blood glucose (e.g., after overnight fasting) on brain activation ought to be taken into account as a possible confounder even when behavioral outcomes are similar between low and normal glucose levels. Based on our results, we propose that a concrete cut off of 80 mg/dl BGL needs to be suggested to exclude the passable effects.
The effect of gender, conversely, did not seem to play a significant role, as we did not see any differences between female and male participants either on the level of brain activation or behavioral performance.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Supplementary Information
ACKNOWLEDGMENT
This work is subject to the Doctoral Thesis of L.K.
REFERENCES
- Alcauter S, Lin W, Smith JK, Short SJ, Goldman BD, Reznick JS, Gilmore JH, Gao W (2014): Development of thalamocortical connectivity during infancy and its cognitive correlations. J Neurosci 34:9067–9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Anderson AW, Heptulla RA, Driesen N, Flanagan D, Goldberg PA, Jones TW, Rife F, Sarofin H, Tamborlane W, Sherwin R, Gore JC (2006): Effects of hypoglycemia on human brain activation measured with fMRI. Mag Resn Imaging 24:693–697. [DOI] [PubMed] [Google Scholar]
- Arbeláez AM, Powers WJ, Videen TO, Price JL, Cryer PE (2008): Attenuation of counterregulatory responses to recurrent hypoglycemia by active thalamic inhibition: A mechanism for hypoglycemia‐associated autonomic failure. Diabetes 57:470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeláez AM, Rutlin JR, Hershey T, Powers WJ, Videen TO, Cryer PE (2012): Thalamic activation during slightly subphysiological glycemia in humans. Diabetes Care 35:2570–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeláez AM, Su Y, Thomas JB, Hauch AC, Hershey T, Ances BM . (2013): Comparison of regional cerebral blood flow responses to hypoglycemia using pulsed arterial spin labeling and positron emission tomography. Plos One 8:e60085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Bolo NR, Musen G, Jacobson AM, Weinger K, McCartney RL, Flores V, Renshaw PF, Simonson DC (2011): Brain activation during working memory is altered in patients with type 1 diabetes during hypoglycemia. Diabetes 60:3256–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruyn RPM (1989): Thalamic aphasia. J Neurol 236:21–25. [DOI] [PubMed] [Google Scholar]
- Chan R, Erbay S, Oljeski S, Thaler D, Bhadelia R (2003): Case report: Hypoglycemia and diffusion‐weighted imaging. J Comput Assist Tomogr 27:420–423. [DOI] [PubMed] [Google Scholar]
- Cho ZH, Son YD, Kim HK, Kim NB, Choi EJ, Lee SY, Chi JG, Park CW, Kim YB, Ogawa S (2011): Observation of glucose metabolism in the thalamic nuclei by fusion PET/MRI. J Nucl Med 52:401–404. [DOI] [PubMed] [Google Scholar]
- Christiansen JS (2006): What is normal glucose? – Continuous Glucose Monitoring Data from Healthy Subjects, On the occasion of the Annual Meeting of the EASD, Copenhagen. Available at: http://www.diabetes-symposium.org/index.php?menu=view&id=322, accessed date August 23, 2014.
- Cornblath M, Hawdon JM, Williams AF, Aynsley‐Green A, Ward‐Platt MP, Schwartz R, Kalhan SC (2000): Controversies regarding definition of neonatal hypoglycemia: Suggested operational thresholds. Pediatrics 105:1141–1145. [DOI] [PubMed] [Google Scholar]
- Cryer PE (1997): Hypoglycemia: Pathophysiology, Diagnosis, and Treatment. New York: Oxford University Press. [Google Scholar]
- Dagenbach D, Kubat‐Silman AK (2001): Human verbal working memory impairments associated with thalamic damage. Int J Neurosci 111:67–87. [DOI] [PubMed] [Google Scholar]
- Delvenne V, Goldman S, De Maertelaer V, Lotstra F (1999): Brain glucose metabolism in eating disorders assessed by positron emission tomography. Int J Eat Disorder 25:29–37. [DOI] [PubMed] [Google Scholar]
- Demal U (1999): SKIDPIT‐light Screeningbogen. Universitaet Wien.
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Filippi B, Abraham M, Yue JY, Lam TT (2013): Insulin and glucagon signaling in the central nervous system. Rev Endocr Metab Disord 14:365–375. [DOI] [PubMed] [Google Scholar]
- Gailliot MT, Baumeister RF (2007): The physiology of willpower: Linking blood glucose to self‐control. Pers Soc Psychol Rev 11:303–327. [DOI] [PubMed] [Google Scholar]
- Gangadhar G, Joseph D, Chakravarthy VS (2007): An oscillatory neuromotor model of handwriting generation. Int J Digit Acc Res 10:69–84. [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF (2007): Resting‐state functional connectivity in major depression: Abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 62:429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel U, Klein M, Kellermann T, Shah NJ, Schneider F (2005): Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage 26:206–214. [DOI] [PubMed] [Google Scholar]
- Hommer D (1999): Functional imaging of craving. Alcohol Res Health 23:187–196. [PMC free article] [PubMed] [Google Scholar]
- Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, Sauer H, Nenadic I (2014): Thalamocortical connectivity during resting state in schizophrenia. Eur Arch Psychiatry Clin Neurosci 264:111–119. [DOI] [PubMed] [Google Scholar]
- Kohn N, Falkenberg I, Kellermann T, Eickhoff SB, Gur RC, Habel U (2013): Neural correlates of effective and ineffective mood induction. Soc Cogn Affect Neurosci 9:864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubat‐Silman AK, Dagenbach D, Absher JR (2002): Patterns of impaired verbal, spatial, and object working memory after thalamic lesions. Brain Cognition 50:178–193. [DOI] [PubMed] [Google Scholar]
- S Mangia, N Tesfaye, F De Martino, AF Kumar, P Kollasch, AA Moheet, LE Eberly, ER Seaquist (2012): Hypoglycemia‐induced increases in thalamic cerebral blood flow are blunted in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab 32:2084–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Klingberg T (2008): Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci 11:103–107. [DOI] [PubMed] [Google Scholar]
- McNay EC, McCarty RC, Gold PE (2001): Fluctuations in brain glucose concentration during behavioral testing: Dissociations between brain areas and between brain and blood. Neurobiol Learn Mem 75:325–337. [DOI] [PubMed] [Google Scholar]
- G Musen, DC Simonson, NR Bolo, A Driscoll, K Weinger, A Raji, J Théberge, PF Renshaw, AM Jacobson (2008): Regional brain activation during hypoglycemia in type 1 diabetes. J Clin Endocrinol Metab 93:1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird R, Bullmore E (2005): N‐back working memory paradigm: A meta‐analysis of normative functional neurimaging studies. Hum Brain Mapp 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso S, Ostedgaard K, Vaidya J, Ponto LB, Robinson R (2013): Emotional blunting following left basal ganglia stroke: The role of depression and fronto‐limbic functional alterations. Psychiatry Res 211:148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pell MD, Leonard CL (2003): Processing emotional tone from speech in Parkinson's disease: A role for the basal ganglia. Cogn Affect Behav Neurosci 3:275–288. [DOI] [PubMed] [Google Scholar]
- Piras F, Caltagirone C, Spalletta G (2010): Working memory performance and thalamus microstructure in healthy subjects. Neuroscience 171:496–505. [DOI] [PubMed] [Google Scholar]
- Purnell JQ, Klopfenstein BA, Stevens AA, Havel PJ, Adams SH, Dunn TN, Krisky C, Rooney WD (2011): Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabet Obes Metab 13:229–234. [DOI] [PubMed] [Google Scholar]
- Rosenthal JM, Amiel SA, Yágüez L, Bullmore E, Hopkins D, Evans M, Pernet A, Reid H, Giampietro V, Andrew CM, Suckling J, Simmons A, Williams SCR (2001): The effect of acute hypoglycemia on brain function and activation: A functional magnetic resonance imaging study. Diabetes 50:1618–1626. [DOI] [PubMed] [Google Scholar]
- Rosvold H, Mirsky A, Sarason I, Bransome E, Beck L (1956): A continuous performance test of brain damage. J Consult Psych 20:343–350. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Fox PT, Eickhoff SB (2012): Modelling neural correlates of working memory: A coordinate‐based meta‐analysis. Neuroimage 60:830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling TM, Ferreira de Sá DS, Westerhausen R, Strelzyk F, Larra MF, Hallschmid M, Savaskan E, Oitzl MS, Busch HP, Naumann E, Schächinger H (2013): Intranasal insulin increases regional cerebral blood flow in the insular cortex in men independently of cortisol manipulation. Hum Brain Mapp 35:1944–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Gur RE, Muenz LR (1994): Standardized mood induction with happy and sad facial expressions. Psychiat Res 51:19–31. [DOI] [PubMed] [Google Scholar]
- Siesjö BK (1978): Brain energy metabolism and catecholaminergic activity in hypoxia, hypercapnia and ischemia. J Neural Transm Suppl 17–22. [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Rosiers MHD, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M (1977): The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916. [DOI] [PubMed] [Google Scholar]
- Sommerfield AJ, Deary IJ, Frier BM (2003): Moderate hypoglycemia impairs multiple memory functions in healthy adults. Neuropsychology 17:125–132. [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E (1999): Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci USA 96:4569–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves D, Videen TO, Cryer PE, Powers WJ (2004): Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci USA 101:6217–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Stelt M, Di Marzo V (2003): The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: Implications for neurological and psychiatric disorders. Eur J Pharmacol 480:133–150. [DOI] [PubMed] [Google Scholar]
- Van der Werf YD, Scheltens P, Lindeboom J, Witter MP, Uylings HBM, Jolles J (2003): Deficits of memory, executive functioning and attention following infarction in the thalamus; a study of 22 cases with localised lesions. Neuropsychologia 41:1330–1344. [DOI] [PubMed] [Google Scholar]
- Wager, T ., Smith, E . (2003) Neuroimaging studies of working memory. Cogn Affect Behav Neurosci 3:255–274. [DOI] [PubMed] [Google Scholar]
- Weiss V (1986): From memory span and mental speed toward the quantum mechanics of intelligence. Pers Individ Dif 7:737–749. [Google Scholar]
- Welter ML, Burbaud P, Fernandez‐Vidal S, Bardinet E, Coste J, Piallat B, Borg M, Besnard S, Sauleau P, Devaux B, Pidoux B, Chaynes P, Tézenas du Montcel S, Bastian A, Langbour N, Teillant A, Haynes W, Yelnik J, Karachi1 C and Mallet L (2011): Basal ganglia dysfunction in OCD: Subthalamic neuronal activity correlates with symptoms severity and predicts high‐frequency stimulation efficacy. Transl Psychiatry 1:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Supplementary Information


