Abstract
Neuroimaging studies have revealed associations between intelligence and brain morphology. However, researchers have focused primarily on the anatomical features of the cerebral cortex, whereas subcortical structures, such as the basal ganglia (BG), have often been neglected despite extensive functional evidence on their relation with higher‐order cognition. Here we performed shape analyses to understand how individual differences in BG local morphology account for variability in cognitive performance. Structural MRI was acquired in 104 young adults (45 men, 59 women, mean age = 19.83, SD = 1.64), and the outer surface of striatal structures (caudate, nucleus accumbens, and putamen), globus pallidus, and thalamus was estimated for each subject and hemisphere. Further, nine cognitive tests were used to measure fluid (Gf), crystallized (Gc), and spatial intelligence (Gv). Latent scores for these factors were computed by means of confirmatory factor analysis and regressed vertex‐wise against subcortical shape (local displacements of vertex position), controlling for age, sex, and adjusted for brain size. Significant results (FDR < 5%) were found for Gf and Gv, but not Gc, for the right striatal structures and thalamus. The main results show a relative enlargement of the rostral putamen, which is functionally connected to the right dorsolateral prefrontal cortex and other intelligence‐related prefrontal areas. Hum Brain Mapp 35:1957–1968, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: fluid intelligence, crystallized intelligence, spatial intelligence, basal ganglia, subcortical shape
INTRODUCTION
Neuroimaging evidence has revealed that higher‐order cognitive ability is related to brain morphology [Barbey et al., 2012, 2013; Burgaleta et al., 2012; Colom et al., 2006a,b, 2007, 2009, 2013a,b; Gläscher et al., 2010; Haier et al., 2009; Karama et al., 2009, 2011; Luders et al., 2009]. However, research has mostly focused on the anatomical features of the cerebral cortex, whereas subcortical structures, and more specifically the basal ganglia (BG), have often been neglected. There is, however, an increasing amount of brain activity and lesion studies showing that such structures play a relevant role in cognition. Importantly, many of the cognitive constructs found to be related to BG strongly predict, or are theoretically linked to, human intelligence. For instance, BG are involved in working memory [Cools et al., 2008; Landau et al., 2009; McNab and Klingberg, 2008; Vakil et al., 2004], abstract reasoning [Bellebaum et al., 2008; Monchi et al., 2004], inhibition [Li et al., 2008; Rieger et al., 2003], visuospatial processing [Williams‐Gray et al., 2007], verbal and spatial memory [Abdullaev et al., 1998; Maguire et al., 1998; Moffat et al., 2007], and language processing [Abdullaev et al., 1998], among others.
From a psychometric standpoint, these cognitive constructs fit well within a three‐factor model of intelligence that is widely accepted in current cognitive neuroscience [Colom and Thompson, 2011; Colom et al., 2008, 2009, 2010; Horn, 1985]. This model considers three general factors of cognitive ability: fluid intelligence (Gf), crystallized intelligence (Gc), and visuospatial intelligence (Gv). Gf can be defined as the ability to deal with novel, complex problems, and is an excellent indicator of general intelligence [Colom et al., 2009; McGrew, 2009]. It is strongly correlated with working memory capacity, and is usually measured by means of abstract reasoning tests. Gc is the ability to apply previously acquired knowledge to culture‐loaded problems, and includes language comprehension, lexical knowledge, mathematical skills, and learning of cultural facts. Finally, Gv is defined as the ability to manipulate two‐dimensional and three‐dimensional representations of visual stimuli in working memory [Cattell, 1987; Colom et al., 2008; Gustaffson, 1988, 1984; Horn, 1985; Hunt, 2010]. These three factors are positively correlated, although it has been shown that, to some extent, they are also differentially associated with brain morphology [Colom et al., 2009].
Nevertheless, although basal ganglia functions are likely to play a relevant role in intelligent behavior, evidence on their morphological association with cognitive abilities is sparse. To date, only a few studies have addressed the relation between BG volume and performance in intelligence‐related tasks, with inconsistent results. Jernigan et al. [2001] reported a negative association between caudate nucleus volume and naming latency on a single‐word reading task—a proxy for Gc. Moffat et al. [2007] showed that relative volume of the caudate nucleus (corrected for total intracranial volume) correlated positively with performance in a spatial navigation task, which is related to Gv. Finally, Andreasen et al. [1993] found no significant correlations between total caudate nucleus volume and IQ in young adults, whereas others have reported significant relationships between striatal volume and IQ in childhood [Abernethy et al., 2004; Isaacs et al., 2008]. IQ is a proxy for g, which is highly correlated with Gf [Barbey et al., 2012; Carroll, 1995; Colom et al., 2009; Gläscher et al., 2010; Gustafsson 1984; McGrew, 2009].
The inconsistent relations reported between BG volume and intelligence measures might be due to a number of reasons, such as sample heterogeneity, differences between administered tests, or measured constructs. However, global volumetry is usually not sensitive to regional morphological variability, potentially relevant for cognition. Indeed, basal ganglia segments are known to be functionally specialized [Humphries and Prescott, 2010; MacDonald et al., 2011; Voorn et al., 2004; Wickens et al., 2007], and this specialization is in part related to the function of the cortical region to which specific BG areas are differentially connected [Koziol and Budding, 2009]. Therefore, a regional analysis of BG morphology should be more informative than crude differences in total BG volume.
Here, we investigated the association between intelligence and basal ganglia morphology at the regional level by means of subcortical shape analysis [Patenaude et al., 2011], a methodology that has been proved successful for characterizing anatomical peculiarities of subcortical structures in special populations, such as patients diagnosed with Alzheimer disease, schizophrenia, Parkinson's disease, or obsessive compulsive disorders [Coscia et al., 2009; Harms et al., 2007; Kang et al., 2008; McKeown et al., 2008; Qiu et al., 2008; Xu et al., 2008]. This technique allowed us to observe local morphological variations, independent of global brain size, for the caudate nucleus, the nucleus accumbens, the globus pallidus, and the putamen. In addition, we also included the thalamus in our analyses, given that it is a main component of the cortico‐striato‐thalamo‐cortical loop [Draganski et al., 2008; Johansen‐Berg et al., 2005]. To characterize the intelligence construct, we used a previously validated battery of nine tests assessing fluid, crystallized, and visuospatial cognitive abilities [Bruner et al., 2011; Burgaleta et al., 2012; Colom et al., 2008, 2009].
Based on the available evidence regarding BG cortical connectivity patterns, as well as the neuroanatomical and functional substrates of cognitive ability, we expected to find associations between fluid intelligence and morphology of the rostral‐central BG, which are connected to DLPFC and together they comprise an associative network that plays a fundamental role in working memory and higher‐order cognition [Dahlin et al., 2008; Jog et al., 1999; Levy et al., 1997; Martinez et al., 2003; Postuma and Dagher, 2006]. Spatial intelligence is expected to recruit not only prefrontally‐connected areas, but also central‐caudal BG segments linked to right parietal and parieto‐occipital cortices, involved in spatial processing [Jordan et al., 2002; Tang et al., 2010]. Finally, crystallized intelligence should correlate with regions of the basal ganglia that play a role in learning (ventral striatum), language processing [thalamus and putamen; Preston et al., 2010], and arithmetic [left putamen and globus pallidus; Dehaene et al., 1996].
METHOD
Participants
One hundred four young adults (45 males and 59 females; mean age= 19.88, SD = 1.67) took part in this study. Participants were psychology undergraduates and received course credit for their participation. They were screened to exclude anyone with a major medical or psychiatric illness including a history of head injury and substance abuse. Written informed consent was obtained. We have previously reported other brain/intelligence results using part of this sample [Bruner et al., 2011; Burgaleta et al., 2012; Colom et al., 2013a, 2009,b; Martín‐Loeches et al., 2012].
Intelligence Measures
Intelligence was measured by nine standardized tests tapping Horn–Cattell's abstract‐fluid (Gf), verbal‐crystallized (Gc), and spatial intelligence (Gv) [Horn, 1985]. The choice of these specific tests and their fit to the Horn–Cattell model has been found to be successful in previous studies [Colom et al., 2008, 2009]. Examples of items for each task can be found in Figure 1.
Figure 1.
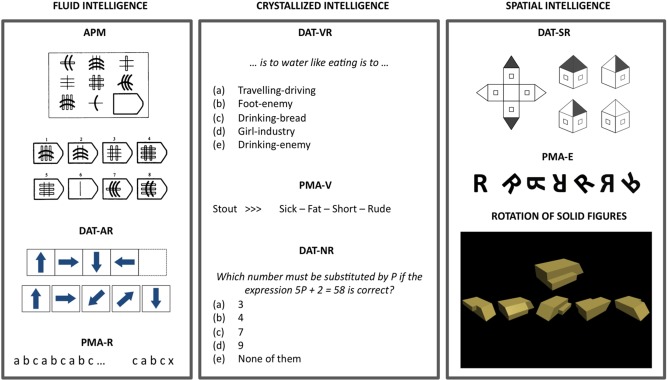
Examples of items for each psychometric test. Test descriptions can be found in the Methods section. APM = Advanced Progressive Matrices Test, PMA‐R = inductive reasoning subtests from the PMA battery, DAT‐AR = abstract reasoning subtest from the DAT battery, PMA‐V = vocabulary subtests from the PMA Battery, DAT‐VR = verbal reasoning subtest from the DAT battery, DAT‐NR = numerical reasoning subtest from the DAT battery, PMA‐S = mental rotation subtest from the PMA battery, DAT‐SR = spatial relations subtest from the DAT battery. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Fluid intelligence (Gf) was measured by the Advanced Progressive Matrices Test (APM, screening version, even numbered items), the inductive reasoning subtest (R) from the Primary Mental Abilities (PMA) Battery, and the abstract reasoning (AR) subtest from the Differential Aptitude Test (DAT‐5) Battery (screening version, even numbered items). The APM comprises a matrix figure with three rows and three columns with the lower right hand entry missing. Participants must choose, among eight alternatives, the one completing the 3 × 3 matrix figure. The score is the total number of correct responses. DAT‐AR is a series test based on abstract figures. Each item includes four figures following a given rule, and the participant must choose one of five possible alternatives. The score is the total number of correct responses. PMA‐R comprises letters' series items. The rule(s) underlying a given sequence of letters must be extracted to select a given letter from a set of six possible alternatives. Only one alternative is correct. The score is the total number of correct responses.
Crystallized intelligence (Gc) was measured by the verbal reasoning (VR) and the numerical reasoning (NR) subtests from the DAT‐5 (screening versions, even numbered items), as well as by the vocabulary (V) subtest from the PMA. DAT‐VR is a verbal reasoning test. A given sentence stated like an analogy must be completed. The first and last words from the sentence are missing, so a pair of words must be selected to complete the sentence from five possible alternative pairs of words. Only one alternative is correct. The score is the total number of correct responses. DAT‐NR consists of quantitative reasoning problems, and the score is the total number of correct responses. PMA‐V is a synonym test. The meaning of four alternative words must be evaluated against a given word that serves as a model. Only one alternative is correct. The score is the total number of correct responses.
Spatial intelligence (Gv) was measured by the Rotation of Solid Figures test, the Mental Rotation (S) subtest from the PMA, and the Spatial Relations (SR) subtest from the DAT‐5 (screening version, even numbered items). Items of the Rotation of Solid Figures test include a model figure and five alternatives that must be evaluated against it. The participant must evaluate which alternative can be rotated within a three‐dimensional space to fit the model figure. Only one alternative is correct. The score is the total number of correct responses. PMA‐S includes, for each item, a model figure and six alternatives that must be evaluated against it. Some alternatives are simply rotated versions of the model figure, whereas the remaining figures are mirror‐imaged. Only the rotated figures must be selected. Several alternatives could be correct for each item. The score is the total number of correct responses (i.e., appropriately selected figures: simply rotated) minus the total number of incorrect responses (i.e., inappropriately selected figures: mirror imaged). DAT‐SR is a mental folding test, and each item is composed by an unfolded figure and four folded alternatives. The unfolded figure is shown at the left, whereas figures at the right depict folded versions. Participants are asked to choose one folded figure matching the unfolded figure at the left. The score is the total number of correct responses.
Measurement Model
We fitted a Confirmatory Factor Analysis (CFA) model to the data using the AMOS program [Arbuckle, 2007]. Three correlated latent factors (Gf, Gc, and Gv) were defined by their respective measures, as described above. It must be noted that, in a previous report using the same sample [Colom et al., 2009], we fitted a similar model but including also a higher‐order g factor. However, Gf was perfectly predicted by g (factor loading = 0.99), so they were not statistically distinguishable at the latent level. Moreover, these two models yield identical goodness‐of‐fit indices. Therefore, here we analyzed the correlated factors model.
Several fitting measures were considered for testing this reference model. First, the CMIN/DF index because it corrects for the sensitivity of the χ 2 statistic [Jöreskog, 1993]. Values are expected to be around 2.0 for revealing a proper fit. Second, RMSEA is recommended because this index is sensitive to misspecification of the model. Values between 0 and 0.05 indicate good fit, values between 0.05 and 0.08 represent acceptable errors, and values greater than 0.10 are indicative of poor fit [Ackerman et al., 2002; Byrne, 1998; Jöreskog, 1993]. Finally, CFI is also reported; appropriate values for this index must be larger than 0.90 [Marsh and Balla, 1994].
From this model, latent scores were obtained using the imputation function of the AMOS program [Arbuckle, 2007]. These scores capture shared variance among the specific measures and, therefore, reflect fluid, crystallized, and spatial intelligence beyond the specific (and error) variance associated with each measure [Colom et al., 2013a; Miyake et al., 2000]. These latent scores were submitted to imaging analyses.
MRI Acquisition
MRIs were obtained at Ruber International Hospital, Madrid (Spain) with a 3 T scanner (GEHC Waukesha, WI, 3 T Excite HDX), eight‐channels coil. Sequence was FSPGR with IR preparation pulse (TR 5.7 ms, TE 2.4 ms TI 750 ms, flip angle 12°). Sagittal acquisition with 0.8 mm thickness and full brain coverage (220 slices), with a matrix of 266 × 266 and FOV = 24 (isotropic voxels 0.7 cm3).
Segmentation of Subcortical Structures and Statistical Analyses
We applied the Bayesian Appearance Model [Patenaude et al., 2011], as implemented in FIRST which is part of the FSL package (FMRIB, Oxford), to automatically segment the caudate nucleus, the nucleus accumbens, the globus pallidus, the putamen, and the thalamus. FIRST takes into account probabilistic information about shape and intensity of the segmented structures. One advantage of this model over voxel‐based morphometry alternatives is that it does not require tissue classification and arbitrary smoothing; instead, it is directly based on the geometry and location of the structure boundary and thus provides a robust estimation of anatomical boundaries and a higher sensitivity to regional variability. Based on manually segmented training data, FIRST creates a surface mesh for each subcortical structure using a deformable mesh model, the number of vertices for each structure being invariant, thus allowing for vertex‐wise comparisons among subjects.
Quality control was performed by an experienced researcher (M.B.) and 11 subjects were discarded because of poor segmentation of one or more structures. A total sample size of N = 93 was included in further steps.
Vertex coordinates were analyzed in MNI space, where the structural images were affinely registered and where structure models were generated. This allowed to minimize differences in pose and to provide relative deformation indices adjusted for total brain size. F statistics were computed vertex‐wise. Effects of age and gender were partialled out, and resulting F‐maps were corrected for multiple comparisons (FDR < 0.05).
RESULTS
Before our brain imaging analyses, we tested whether the Confirmatory Factor Analysis for the cognitive measures yielded a good fit to the data. The model is shown in Figure 2, along with its respective indices of goodness of fit. Based on these indices, the fit of the model can be considered as excellent.
Figure 2.
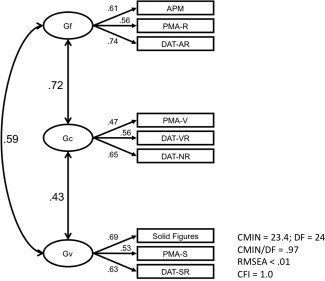
CFA model and model fit for the psychometric measures of intelligence. APM = Advanced Progressive Matrices Test, PMA‐R = inductive reasoning subtests from the PMA battery, DAT‐AR = abstract reasoning subtest from the DAT battery, PMA‐V = vocabulary subtests from the PMA battery, DAT‐VR = verbal reasoning subtest from the DAT Battery, DAT‐NR = numerical reasoning subtest from the DAT battery, PMA‐S = mental rotation subtest from the PMA battery, DAT‐SR = spatial relations subtest from the DAT battery.
Next, we analyzed the correlations between local vertex displacement of each subcortical structure and the latent scores for fluid (Gf), spatial (Gv), and crystallized (Gc) intelligence, controlling for the effects of age and gender. We found significant associations (FDR < 0.05) for Gf and Gv, but not for Gc. Significant correlations were found in the right hemisphere only, for the accumbens, caudate, and putamen. In addition, significant results were also found for Gv in the right thalamus. On the other hand, the shape of the globus pallidus did not correlate with intelligence scores. There were no trends toward significant associations for left‐sided structures, even at a lenient uncorrected threshold of p < 0.05. Furthermore, no significant results were found when the analyses were not adjusted for brain size. Figures 3 and 4 depict a general view of the significant associations between shape of subcortical structures, and Gf and Gv, respectively.
Figure 3.
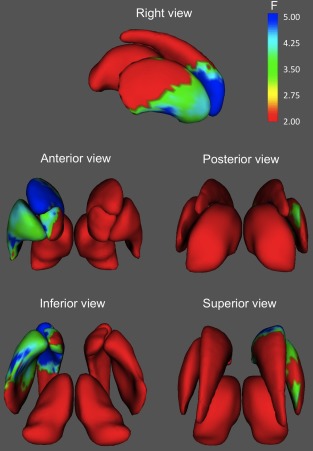
Areas of the basal ganglia and thalamus where shape was significantly associated with fluid intelligence. Only results surviving FDR correction (Q < 0.05) are shown. Significant associations localize primarily in anteroventral areas of the right caudate, accumbens, and putamen. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 4.
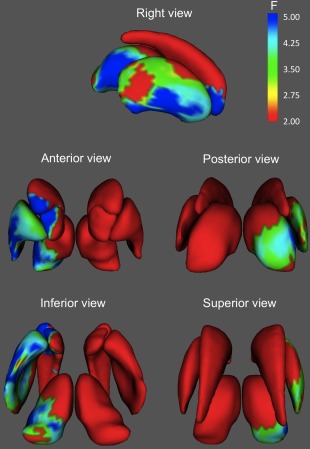
Areas of the basal ganglia and thalamus where shape was significantly associated with spatial intelligence. Only results surviving FDR correction (Q < 0.05) are shown. Significant associations localize primarily in anteroventral areas of the right caudate, accumbens, putamen, and thalamus. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Although the spatial patterns of results were very similar for Gf and Gv, for fluid intelligence the most significant associations were located on the ventral half of the right caudate, whereas for spatial intelligence, regions of higher significance were displaced caudally and ventrally, being located primarily in the ventral pole of the caudate and adjoining regions of accumbens, central lateral, and ventral putamen, and posterior (mainly lateral) thalamus.
Next, inspection of the displacement vectors for each specific analysis allowed to observe in which way shape variability was associated with cognitive performance. For fluid intelligence (Fig. 5) and right putamen, we observed that participants scoring higher presented (1) anteromedial enlargement of the rostral area, (2) anterolateral displacement of the central lateral area, and (3) slight posterior enlargement of the caudal dorsal area. Regarding the right caudate nucleus, we found a significant lateral compression and posterosuperior displacement of the head of the caudate, located right at its boundary with the putamen. Finally, nucleus accumbens showed medial, superior, and anterior displacement of its lateral surface (i.e., at its boundary with the accumbens). Therefore, the general picture suggests that greater Gf scores are found in subjects showing relative anterior expansion of the putamen, that is accompanied by relative lateral "shrinking" of both caudate and accumbens.
Figure 5.
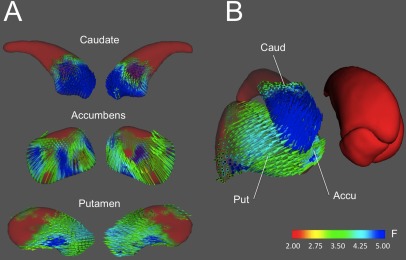
Displacement vectors maps for areas where shape is significantly associated with fluid intelligence. A. Lateral (left) and medial views of the displacement vectors are shown for the right caudate nucleus, nucleus accumbens, and putamen. B. Three‐dimensional joint representation of relevant structures, illustrating the inter‐structure coherence of surface displacements. Note how the anterior enlargement of the right putamen is accompanied by anterior shrinking of the caudate. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Results for spatial intelligence (Fig. 6) clearly resembled those found for Gf, although in this case variations in the shape of the caudate nucleus were associated with Gv at the anterior pole of the caudate head only, as noted above. Accumbens and putamen, on the other hand, presented more significant displacements in their rostral and lateral‐central surfaces, respectively. Again, better performance was related to local expansion of putamen at its boundary with caudate and accumbens, which are also compressed roughly along the same direction as the putamen displacements. Finally, Gv also correlated with the shape of the right thalamus, which showed medial compression of its lateral surface and, to a lesser extent, of its medial surface, as well as caudal expansion of its posterior dorsal surface.
Figure 6.
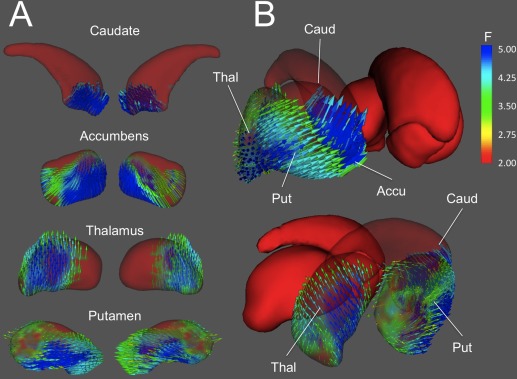
Displacement vectors maps for areas where shape is significantly associated with spatial intelligence. A. Lateral (left) and medial views of the displacement vectors are shown for the right caudate nucleus, nucleus accumbens, thalamus, and putamen. B. Three‐dimensional joint representation of relevant structures, illustrating the inter‐structure coherence of surface displacements. Similarly to the pattern found for fluid intelligence (see Fig. 5), the anterior enlargement of the right putamen is accompanied by anterior shrinking of the caudate. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
Previous studies have shown that basal ganglia are relevant for higher‐order cognition [Abdullaev et al., 1998; Bellebaum et al., 2008; Landau et al., 2009; Maguire et al., 1998; McNab and Klingberg, 2008; Moffat et al., 2007; Monchi et al., 2004; Rieger et al., 2003; Vakil et al., 2004; Williams‐Gray et al., 2007]. Whereas BG volumes have been inconsistently related to intelligence, here we demonstrate, for the first time, that individual differences in BG regional shape correlate with cognitive performance. Relevant associations with fluid (Gf) and spatial (Gv) intelligence were found for all the right striatum structures: caudate nucleus, putamen, and nucleus accumbens. Additionally, shape variability of the right thalamus also correlated significantly with spatial intelligence. On the other hand, highly culture‐loaded tests (Gc) did not yield significant associations with BG shape. Gf comprises mainly abstract reasoning and rule extraction abilities, Gv involves manipulating representations of two‐dimensional and three‐dimensional objects in working memory, and Gc is composed of educational skills such as vocabulary knowledge and arithmetic. Therefore, this pattern of results suggests that striatal and thalamic morphology plays a role in dealing with novel, complex problems that are highly working memory demanding, but not when dealing with problems requiring previously acquired and stored knowledge. This is consistent with previous fMRI studies showing that ventral striatal activity is greatest early in a novel learning task, while it decreases as performance reaches its asymptote [Reiss et al., 2005; Seger et al., 2010].
The functional differentiation of specific striatal areas for cognition has been suggested before, for instance distinguishing between ventral (learning) and dorsal (response execution) regions [Humphries and Prescott, 2010; MacDonald et al., 2011; Schlagenhauf et al., 2013; Voorn et al., 2004; Wickens et al., 2007]. Here we report converging morphological evidence, given that we observed anteroventral, but virtually no caudal and dorsal areas of the basal ganglia, to be significantly relevant for cognitive performance. Results are thus consistent with our initial prediction, and also with previous research highlighting the role of the striatum in an associative working memory corticostriatal network [Dahlin et al., 2008; Martinez et al., 2003; Postuma and Dagher, 2006].
The cellular underpinnings of the observed individual differences in BG shape cannot be determined by the sMRI assessment used here, and might include greater dendritic or axonal arborizations, higher number of neurons, or greater vascularization [Erickson et al., 2010; Schubert et al., 2009]. It has also been suggested that individual differences in striatal volume may reflect, to some extent, variability in dopamine receptor density [Woodward et al., 2009]. Striatal dopamine plays the role of reducing inhibitory pallidal outflow to the thalamus, thus exciting the prefrontal cortex [Landau et al., 2009; Parent and Lavoie, 1993], and a direct correlation has been observed between striatal dopamine and working memory [Cools et al., 2008; Landau et al., 2009]. Therefore, it is possible that morphology of basal ganglia might have an impact on, or is the result of the dopaminergic regulation that the striatum exerts over the prefrontal cortex [Cohen et al., 2002; O'Reilly, 2006]. This possibility should be addressed in future research by concurrently acquiring sMRI and PET data.
Interestingly, strong differences in patterns of shape variations were observed between caudate, nucleus accumbens, and putamen. More specifically, anterior enlargement of the putamen was accompanied by shrinking of the nucleus accumbens and anteroventral caudate. This could be interpreted in light of the structural and functional connections between these areas and the cortex [Draganski et al., 2008; Koziol and Budding, 2009; Leh et al., 2007; Lehéricy et al., 2004a,b]. Expansion of central‐rostral regions of the right putamen might suggest a higher development of a cortico‐subcortical network involving DLPFC and other prefontal areas that are known to be relevant for fluid intelligence and working memory [Draganski et al., 2008; Lehéricy et al., 2004a]. The slight caudal enlargement of the putamen observed here may be related to areas to which the posterior BG segment is functionally coupled, such as the inferior parietal cortex and the insular cortex [Helmich et al., 2010], regions that are also related to cognitive performance [Jung and Haier, 2007]. Finally, the relative contraction of nucleus accumbens and ventral caudate might involve a reduced number of output connections to the thalamus [Draganski et al., 2008], but also excitatory connections from OFC and prefrontal limbic regions, while leaving intact those caudal‐rostral caudate areas that are connected to DLPFC [Lehéricy et al., 2004a]. Some researchers have reported a negative association between caudate volumes and short‐term memory span [Hokama et al., 1995], which is a key facet of working memory capacity and general intelligence [Colom et al., 2006a,b, 2007; Hornung et al., 2011; Krumm et al., 2009; Martínez et al., 2011]; however, our shape analyses were corrected for total brain volume and thus global linear volumetric effects were not captured. Therefore, our results do not necessarily imply a negative association between BG absolute size and cognitive performance. As an alternative explanation for the observed relative shrinking of the caudate/accumbens, it could be argued that it is a byproduct of the relative enlargement of the putamen and thus it may have no direct functional impact. Because they are neighboring structures, it is to be expected that shape variations in standard space take place simultaneously (and in opposite directions) at their boundary, given that the overall size of the subcortical structures is forced to remain roughly constant across subjects.
We found that thalamic morphology was exclusively related to visuospatial intelligence. Performance in spatial intelligence tasks was associated with superior enlargement of the medial dorsal thalamic surface, as well as with reduction of the posterior ventrolateral region. These results nicely fit with previous studies showing that the dorsal thalamus is a key structure for visuospatial processing [Sommer and Wurtz, 2006] and spatial working memory [Mills et al., 2012]. Johansen‐Berg et al. [2005] summarized results of fMRI studies reporting thalamic activations, and showed that motor paradigms elicited activations in the ventral lateral nucleus, whereas tasks recruiting working memory were associated with patterns of activity localized in the mediodorsal nucleus. Therefore, the relative enlargement of the thalamic mediodorsal nucleus found here might involve an enhanced spatial working memory system, perhaps to the detriment of motor resources. Evidence from thalamic connectivity studies supports the notion that the ventral lateral nucleus is structurally linked to premotor and motor areas [Draganski et al., 2008], whereas the medial dorsal nucleus is likely connected to the parietal and occipital cortices [Johansen‐Berg et al., 2005], that are also relevant for spatial intelligence [Jordan et al., 2002], visual short‐term memory [Todd and Marois, 2004], and spatial working memory [McNab and Klingberg, 2008].
Unexpectedly, shape analysis of the globus pallidus did not yield significant associations with intelligence, even though pallidal activity is related to working memory capacity [McNab and Klingberg, 2008; Mills et al., 2012]. Of course, it is possible that morphology of the globus pallidus is not related to its functional activity. On the other hand, the morphological technique applied here does not differentiate between internal and external globus pallidus, and this could perhaps obscure anatomical variability that is relevant for cognitive abilities. Future research should aim at replicating this finding while increasing the level of anatomical precision.
It is worth noting that we detected significant results for the right BG only, whereas previous fMRI research usually reveals bilateral recruitment of basal ganglia activity [Coull and Nobre, 2008; Ortiz‐Siordia et al., 2008]. However, in PET studies highlighting the role of left striatal dopamine in working memory, it has been argued that the verbal nature of the administered tasks could explain left‐lateralized results [Cools et al., 2008]. In our study, Gf is measured by tests with a high visuospatial load such as Raven's Advanced Progressive Matrices Test [Abad et al., 2004; Ackerman et al., 2002; Colom et al., 2004], whereas Gv evidently pertains to the spatial domain. Therefore, the spatial load of the tests used here could account for our findings, given that visuospatial cognitive abilities are more likely to be associated with the right hemisphere [Jordan et al., 2002; Mottaghy, 2006; Suchan, 2008].
A final comment must be made regarding the limitations of the shape analysis applied in this study. While the Bayesian Appearance Model [Patenaude et al., 2011] allows for fine‐grained regional analysis of subcortical morphology, linking local shape variations with functional segments of the basal ganglia is not straightforward. This is evident when trying to interpret the results in light of the corticostriatal connectivity patterns reported by other authors [e.g., Draganski et al., 2008]. In order to better understand the functional roles of the BG areas found here, future morphometric analyses might benefit from connectivity‐based segmentation of the analyzed structures. This would allow for more precise interpretations of the results, and it would also be possible to make inferences based on the integrity of the structural corticostriatal connections.
CONCLUSION
In this study we provide novel evidence demonstrating that local morphology of the right basal ganglia and right thalamus is associated with fluid and spatial but not with crystallized intelligence. This suggests that striatal and thalamic morphology plays a role in dealing with novel, abstract problems that are highly working memory demanding, but not when dealing with problems requiring manipulation of previously acquired knowledge.
REFERENCES
- Abad FJ, Colom R, Rebollo I, Escorial S (2004): Sex differential item functioning in the Raven's Advanced Progressive Matrices: Evidence for bias. Pers Individ Differ 36:1459–1470. [Google Scholar]
- Abdullaev YG, Bechtereva NP, Melnichuk KV (1998): Neuronal activity of human caudate nucleus and prefrontal cortex in cognitive tasks. Behav Brain Res 97:159–177. [DOI] [PubMed] [Google Scholar]
- Abernethy LJ, Cooke RW, Foulder‐Hughes L (2004): Caudate and hippocampal volumes, intelligence, and motor impairment in 7‐year‐old children who were born preterm. Pediatr Res 55:884–893. [DOI] [PubMed] [Google Scholar]
- Ackerman PL, Beier ME, Boyle MO (2002): Individual differences in working memory within a nomological network of cognitive and perceptual speed abilities. J Exp Psychol Gen 131:567–589. [PubMed] [Google Scholar]
- Andreasen NC, Flaum M, Swayze V II, O'Leary DS, Alliger R, Cohen G, Ehrhardt J, Yuh WT. (1993): Intelligence and brain structure in normal individuals. Am J Psychiatry 150:130–134. [DOI] [PubMed] [Google Scholar]
- Arbuckle JL (2007): AMOS, Version 16.0.1. Spring House, PA: AMOS Development Corporation. [Google Scholar]
- Barbey AK, Colom R, Solomon J, Krueger F, Forbes C, Grafman J (2012): An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain 135:1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Paul EJ, Grafman J (2013): Architecture of fluid intelligence and working memory revealed by lesion mapping. Brain Struct Funct [epub ahead of print]. [DOI] [PubMed]
- Bellebaum C, Koch B, Schwarz M, Daum I (2008): Focal basal ganglia lesions are associated with impairments in reward‐based reversal learning. Brain 131:829–841. [DOI] [PubMed] [Google Scholar]
- Bruner E, Martin‐Loeches M, Burgaleta M, Colom R (2011): Midsagittal brain shape correlation with intelligence and cognitive performance. Intelligence 39:141–147. [Google Scholar]
- Burgaleta M, Head K, Álvarez‐Linera J, Martínez K, Escorial S, Haier R, Colom R (2012): Sex differences in brain volume are related to specific skills, not to general intelligence. Intelligence 40:60–68. [Google Scholar]
- Byrne BM (1998): Structural Equation Modelling with LISREL, PRELIS, and SIMPLIS: Basic Concepts, Applications, and Programming. Erlbaum: Mahwah. [Google Scholar]
- Carroll JB (1995): The three‐stratum theory of cognitive abilities In: Flanagan DP, Harrison PL, editors. Contemporary Intellectual Assessment: Theories, Tests, and Issues. New York: Guilford; pp 69–76. [Google Scholar]
- Cattell RB (1987): Intelligence; Their Structure, Growth and Action. Amsterdam: North‐Holland. [Google Scholar]
- Cohen JD, Braver TS, Brown JW (2002): Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol 12:223–229. [DOI] [PubMed] [Google Scholar]
- Colom R, Burgaleta M, Román FJ, Karama S, Álvarez‐Linera J, Abad FJ, Martínez K, Quiroga MA, Haier RJ (2013a): Neuroanatomic overlap between intelligence and cognitive factors: Morphometry methods provide support for the key role of the frontal lobes. Neuroimage 72:143–152. [DOI] [PubMed] [Google Scholar]
- Colom R, Jung RE, Haier RJ (2007): General intelligence and memory span: Evidence for a common neuroanatomic framework. Cogn Neuropsychol 24:867–878. [DOI] [PubMed] [Google Scholar]
- Colom R, Rebollo I, Abad FJ, Shih PC (2006a): Complex span tasks, simple span tasks, and cognitive abilities: A reanalysis of key studies. Mem Cognit 34:158–171. [DOI] [PubMed] [Google Scholar]
- Colom R, Abad FJ, Quiroga MÁ, Shih PC, Flores‐Mendoza C (2008): Working memory and intelligence are highly related constructs, but why? Intelligence 36:584–606. [Google Scholar]
- Colom R, Escorial S, Rebollo I (2004): Sex differences on the progressive matrices are influenced by sex differences on spatial ability. Pers Individ Differ 37:1289–1293. [Google Scholar]
- Colom R, Haier RJ, Head K, Álvarez‐Linera J, Quiroga MÁ, Shih PC, Jung RE (2009): Gray matter correlates of fluid, crystallized, and spatial intelligence: Testing the P‐FIT model. Intelligence 37:124–135. [Google Scholar]
- Colom R, Jung RE, Haier RJ (2006b): Distributed brain sites for the g‐factor of intelligence. Neuroimage 31:1359–1365. [DOI] [PubMed] [Google Scholar]
- Colom R, Stein JL, Rajagopalan P, Martínez K, Hermel D, Wang Y, Álvarez‐Linera J, Burgaleta M, Quiroga MA, Shih PC, Thompson P (2013b): Hippocampal structure and human cognition: Key role of spatial processing and evidence supporting the efficiency hypothesis in females. Intelligence 41:129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom R, Thompson PM (2011): Understanding Human Intelligence by Imaging the Brain In: Chamorro‐Premuzic T, von Stumm S, Furnham A, editors. Handbook of Individual Differences. London: Wiley‐Blackwell. [Google Scholar]
- Colom R, Karama S, Jung RE, Haier RJ (2010): Human intelligence and brain networks. Dialogues Clin Neurosci 12:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M (2008): Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci 28:1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J, Nobre A (2008): Dissociating explicit timing from temporal expectation with fMRI. Curr Opin Neurobiol 18:137–144. [DOI] [PubMed] [Google Scholar]
- Coscia DM, Narr KL, Robinson DG, Hamilton LS, Sevy S, Burdick KE, Gunduz‐Bruce H, McCormack J, Bilder RM, Szeszko PR (2009): Volumetric and shape analysis of the thalamus in first‐episode schizophrenia. Hum Brain Mapp 30:1236–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin E, Neely AS, Larsson A, Bäckman L, Nyberg L (2008): Transfer of learning after updating training mediated by the striatum. Science 320:1510–1512. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Tzourio N, Frak V, Raynaud L, Cohen L, Mehler J, Mazoyer B (1996): Cerebral activations during number multiplication and comparison: A PET study. Neuropsychologia 34:1097–1106. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, Cook PA, Alexander DC, Parker GJM, Deichmann R, Ashburner J, Frackowiak RSJ (2008): Evidence for segregated and integrative connectivity patterns in the human basal ganglia. J Neurosci 28:7143–7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Boot WR, Basak C, Neider MB, Prakash RS, Voss MW, Graybiel AM, Simons DJ, Fabiani M, Gratton G, Kramer AF (2010): Striatal volume predicts level of video game skill acquisition. Cereb Cortex 20:2522–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J, Rudrauf D, Colom R, Paul LK, Tranel D, Damasio H, Adolphs R (2010): Distributed neural system for general intelligence revealed by lesion mapping. Proc Natl Acad Sci USA 10:4705–4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustaffson J (1988): Hierarchical models of individual differences in cognitive abilities In: Sternberg RJ, editor. Advances in the Psychology of Human Intelligence, Vol. 4 New Jersey: LEA. [Google Scholar]
- Gustaffson J (1984): A unifying model for the structure of intellectual abilities. Intelligence 8:179–203. [Google Scholar]
- Haier RJ, Colom R, Schroeder DH, Condon CA, Tang C, Eaves E, Head K (2009): Gray matter and intelligence factors: Is there a neuro‐g? Intelligence 37:136–144. [Google Scholar]
- Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG (2007): Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci 27:13835–13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I (2010): Spatial remapping of cortico‐striatal connectivity in Parkinson's disease. Cereb Cortex 20:1175–1186. [DOI] [PubMed] [Google Scholar]
- Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, Wible CG, O'Donnell BF, Jolesz FA, McCarley RW (1995): Caudate, putamen, and globus pallidus volume in schizophrenia: A quantitative MRI study. Psychiatry Res 61:209–229. [DOI] [PubMed] [Google Scholar]
- Horn J (1985): Remodeling old models of intelligence In: Wolman BB, editor. Handbook of Intelligence. New York: Wiley. [Google Scholar]
- Hornung C, Brunner M, Reuter RAP, Martin R (2011): Children's working memory: Its structure and relationship to fluid intelligence. Intelligence 39:210–221. [Google Scholar]
- Humphries MD, Prescott TJ (2010): The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol 90:385–417. [DOI] [PubMed] [Google Scholar]
- Hunt EB. (2010): Human Intelligence. New York: Cambridge University Press. [Google Scholar]
- Isaacs EB, Gadian DG, Sabatini S, Chong WK, Quinn BT, Fischl BR, Lucas A (2008): The effect of early human diet on caudate volumes and IQ. Pediatr Res 63:308–314. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Ostergaard AL, Fennema‐Notestine C (2001): Mesial temporal, diencephalic, and striatal contributions to deficits in single word reading, word priming, and recognition memory. J Int Neuropsychol Soc 7:63–78. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM (1999): Building neural representations of habits. Science 286:1745–1749. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Behrens TEJ, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, Matthews PM (2005): Functional–anatomical validation and individual variation of diffusion tractography‐based segmentation of the human thalamus. Cereb Cortex 15:31–39. [DOI] [PubMed] [Google Scholar]
- Jordan K, Wüstenberg T, Heinze H, Peters M, Jäncke L (2002): Women and men exhibit different cortical activation patterns during mental rotation tasks. Neuropsychologia 40:2397–2408. [DOI] [PubMed] [Google Scholar]
- Jöreskog K (1993): Testing structural equation models In: Bollen KA, Long JS, editors. Testing Structural Equation Models. Newbury Park: Sage; pp 294–315. [Google Scholar]
- Jung RE, Haier RJ (2007): The Parieto‐Frontal Integration Theory (P‐FIT) of intelligence: Converging neuroimaging evidence. Behav Brain Sci 30:135–154. [DOI] [PubMed] [Google Scholar]
- Kang DH, Kim SH, Kim CW, Choi JS, Jang JH, Jung MH, Lee JM, Kim SI, Kwon JS (2008): Thalamus surface shape deformity in obsessive‐compulsive disorder and schizophrenia. Neuroreport 19:609–613. [DOI] [PubMed] [Google Scholar]
- Karama S, Ad‐Dab'bagh Y, Haier R, Deary I, Lyttelton O, Lepage C, Evans A (2009): Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year‐olds. Neuroimage 47(Suppl 1):S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karama S, Colom R, Johnson W, Deary IJ, Haier R, Waber DP, Lepage C, Ganjavi H, Jung R, Evans AC (2011): Cortical thickness correlates of specific cognitive performance accounted for by the general factor of intelligence in healthy children aged 6 to 18. Neuroimage 55:1443–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol LF, Budding DE (2009): Subcortical Structures and Cognition: Implications for Neuropsychological Assessment. New York: Springer. [Google Scholar]
- Krumm S, Schmidt‐Atzert L, Buehner M, Ziegler M, Michalczyk K, Arrow K (2009): Storage and non‐storage components of working memory predicting reasoning: A simultaneous examination of a wide range of ability factors. Intelligence 37:347–364. [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ (2009): Striatal dopamine and working memory. Cereb Cortex 19:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh SE, Ptito A, Chakravarty MM, Strafella AP (2007): Fronto‐striatal connections in the human brain: A probabilistic diffusion tractography study. Neurosci Lett 419:113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van de Moortele PF, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim DS (2004a): Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann Neurol 55:522–529. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Krainik A, Francois C, Van de Moortele P, Ugurbil K, Kim D (2004b): 3‐D diffusion tensor axonal tracking shows distinct SMA and pre‐SMA projections to the human striatum. Cereb Cortex 14:1302–1309. [DOI] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman‐Rakic PS (1997): Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J Neurosci 17:3870–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Sinha R, & Lee TW (2008): Subcortical processes of motor response inhibition during a stop signal task. Neuroimage 41:1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Toga AW (2009): Neuroanatomical correlates of intelligence. Intelligence 37:156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald PA, MacDonald AA, Seergobin KN, Tamjeedi R, Ganjavi H, Provost JS, Monchi O (2011): The effect of dopamine therapy on ventral and dorsal striatum‐mediated cognition in Parkinson's disease: Support from functional MRI. Brain 134:1447–1463. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ, Frith CD, O'Keefe J (1998): Knowing where and getting there: A human navigation network. Science 280:921–924. [DOI] [PubMed] [Google Scholar]
- Marsh HW, Balla J (1994): Goodness of fit in confirmatory factor analysis: The effects of sample size and model parsimony. Quality Quantity 28:185–217. [Google Scholar]
- Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, Cooper T, Kegeles L, Zarahn E, Abi‐Dargham A, Haber SN, Laruelle M (2003): Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: Amphetamine‐induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab 23:285–300. [DOI] [PubMed] [Google Scholar]
- Martínez K, Burgaleta M, Román FJ, Escorial S, Shih PC, Quiroga MÁ, Colom R (2011): Can fluid intelligence be reduced to ‘simple' short‐term storage? Intelligence 39:473–480. [Google Scholar]
- Martín‐Loeches M, Bruner E, la Cuetara JM, Colom R (2012): Correlation between corpus callosum shape and cognitive performance in healthy young adults. Brain Struct Funct [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- McGrew KS (2009): CHC theory and the human cognitive abilities project: Standing on the shoulders of the giants of psychometric intelligence research. Intelligence 37:1–10. [Google Scholar]
- McKeown M, Uthama A, Abugharbieh R, Palmer S, Lewis M, Huang X (2008): Shape (but not volume) changes in the thalami in Parkinson disease. BMC Neurol 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNab F, Klingberg T (2008): Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci 11:103–107. [DOI] [PubMed] [Google Scholar]
- Mills KL, Bathula D, Dias TG, Iyer SP, Fenesy MC, Musser ED, Stevens CA, Thurlow BL, Carpenter SD, Nagel BJ, Nigg JT, Fair DA (2012): Altered cortico‐striatal‐thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Front Psychiatry 3:2. [DOI] [PMC free article] [PubMed]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000): The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognit Psychol 41:49–100. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Kennedy KM, Rodrigue KM, Raz N (2007): Extrahippocampal contributions to age differences in human spatial navigation. Cereb Cortex 17:1274–1282. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A (2004): Neural bases of set‐shifting deficits in Parkinson's disease. J Neurosci 24:702–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottaghy FM (2006): Interfering with working memory in humans. Neuroscience 139:85–90. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC (2006): Biologically based computational models of high‐level cognition. Science 314:91–94. [DOI] [PubMed] [Google Scholar]
- Ortiz‐Siordia LE, Alvarez‐Amador L, Gonzalez‐Pina R (2008): Anatomic and topographic models of the cerebral areas that activates during the linguistic functions. Rev Neurol 47:653–658. [PubMed] [Google Scholar]
- Parent A, Lavoie B (1993): The heterogeneity of the mesostriatal dopaminergic system as revealed in normal and parkinsonian monkeys. Adv Neurol 60:25–33. [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011): A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56:907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Dagher A (2006): Basal ganglia functional connectivity based on a meta‐analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex 16:1508–1521. [DOI] [PubMed] [Google Scholar]
- Preston JL, Frost SJ, Mencl WE, Fulbright RK, Landi N, Grigorenko E, Jacobsen L, Pugh KR (2010): Early and late talkers: School‐age language, literacy and neurolinguistic differences. Brain 133:2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A, Younes L, Miller MI, Csernansky JG (2008): Parallel transport in diffeomorphisms distinguishes the time‐dependent pattern of hippocampal surface deformation due to healthy aging and the dementia of the Alzheimer's type. Neuroimage 40:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss JP, Campbell DW, Leslie WD, Paulus MP, Stroman PW, Polimeni JO, Malcolmson KA, Sareen J (2005): The role of the striatum in implicit learning: A functional magnetic resonance imaging study. Neuroreport 16:1291–1295. [DOI] [PubMed] [Google Scholar]
- Rieger M, Gauggel S, Burmeister K. (2003): Inhibition of ongoing responses following frontal, nonfrontal, and basal ganglia lesions. Neuropsychology 17:272–282. [DOI] [PubMed] [Google Scholar]
- Schlagenhauf F, Rapp MA, Huys QJ, Beck A, Wustenberg T, Deserno L, Buchholz HG, Kalbitzer J, Buchert R, Bauer M, Kienast T, Cumming P, Plotkin M, Kumakura Y, Grace AA, Dolan RJ, Heinz A (2013): Ventral striatal prediction error signaling is associated with dopamine synthesis capacity and fluid intelligence. Hum Brain Mapp 34:1490--1499. [DOI] [PMC free article] [PubMed]
- Schubert MI, Porkess MV, Dashdorj N, Fone KCF, Auer DP (2009): Effects of social isolation rearing on the limbic brain: A combined behavioral and magnetic resonance imaging volumetry study in rats. Neuroscience 159:21–30. [DOI] [PubMed] [Google Scholar]
- Seger CA, Peterson EJ, Cincotta CM, Lopez‐Paniagua D, Anderson CW (2010): Dissociating the contributions of independent corticostriatal systems to visual categorization learning through the use of reinforcement learning modeling and Granger causality modeling. Neuroimage 50:644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH (2006): Influence of the thalamus on spatial visual processing in frontal cortex. Nature 444:374–377. [DOI] [PubMed] [Google Scholar]
- Suchan B (2008): Neuroanatomical correlates of processing in visual and visuospatial working memory. Cogn Process 9:45–51. [DOI] [PubMed] [Google Scholar]
- Tang CY, Eaves EL, Ng JC, Carpenter DM, Mai X, Schroeder DH, Condon CA, Colom R, Haier RJ (2010): Brain networks for working memory and factors of intelligence assessed in males and females with fMRI and DTI. Intelligence 38:293–303. [Google Scholar]
- Todd JJ, Marois R (2004): Capacity limit of visual short‐term memory in human posterior parietal cortex. Nature 428:751–754. [DOI] [PubMed] [Google Scholar]
- Vakil E, Blachstein H, Soroker N (2004): Differential effect of right and left basal ganglionic infarctions on procedural learning. Cogn Behav Neurol 17:62–73. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM (2004): Putting a spin on the dorsal‐ventral divide of the striatum. Trends Neurosci 27:468–474. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Budd CS, Hyland BI, Arbuthnott GW (2007): Striatal contributions to reward and decision making: Making sense of regional variations in a reiterated processing matrix. Ann NY Acad Sci 1104:192–212. [DOI] [PubMed] [Google Scholar]
- Williams‐Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA (2007): Catechol O‐methyltransferase val158met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci 27:4832–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Zald DH, Ding Z, Riccardi P, Ansari MS, Baldwin RM, Cowan RL, Li R, Kessler RM (2009). Cerebral morphology and dopamine D2/D3 receptor distribution in humans: A combined [18F]fallypride and voxel‐based morphometry study. Neuroimage 46:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Valentino DJ, Scher AI, Dinov I, White LR, Thompson PM, Launer LJ, Toga AW (2008): Age effects on hippocampal structural changes in old men: The HAAS. Neuroimage 40:1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]


