Abstract
Objectives
To ascertain the neural network mediating reading using intraoperative electrostimulation.
Experimental design
A cortical and axonal intraoperative electrical mapping of reading processes was achieved in seven patients who underwent awake surgery for a left occipitotemporal glioma. We performed resection cavity overlapping and superimposition with a diffusion tensor imaging‐based white matter atlas. We assessed the relationship between the location of resection cavities and the occurrence of reading impairments of regular, irregular, and pseudowords.
Principal observations
Intraoperative stimulation of the left posterior inferior temporal cortex (ITCp) elicited reading disturbances. Subcortical stimulation at the anterior portion of the visual word form area (VWFA) induced addressed phonology (irregular words reading) disturbances. Subcortical stimulation of the connection between VWFA and the posterior segment of the arcuate fascicle (AFp) induced both addressed and assembled phonology (irregular and pseudowords reading) disturbances. Postoperative assessment showed that resection of the posterior portion of the inferior longitudinal fascicle (ILFp), connecting the visual cortex to VWFA, induced long‐term and global reading impairment. Resection of the terminations of left AFp in the ITCp‐induced irregular and pseudowords reading disturbances with no impairment of regular words reading. Resection of the anterior portion of ILF did not induce reading impairment.
Conclusions
Our data support an inner posterior‐to‐anterior hierarchical coding of letter strings in the VWFA and a crucial role of the left ILFp to provide visual inputs to the VWFA. Furthermore, we suggest that the AFp is involved in an interactive feedback system between visual and nonvisual information, recruited when reading irregular and pseudowords. Hum Brain Mapp 36:2215–2230, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: superior longitudinal fascicle, inferior longitudinal fascicle, reading, visual word form area, awake surgery, subcortical electrostimulation, connectivity
INTRODUCTION
Although recent studies have emphasized on the neural circuitry implicated in reading [Cummine et al., 2013; Epelbaum et al., 2008; Thiebaut de Schotten et al., 2012; Yeatman et al., 2013], the anatomical connectivity that may be concerned in sustaining reading processes is still poorly documented. Nonetheless, a dual‐route computational model of reading is currently widely accepted in scientific community, mainly based on findings from brain‐damaged patients with acquired dyslexia [Damasio and Damasio, 1983; Greenblatt, 1976; Marshall and Newcombe, 1973; Shallice and Warrington, 1980]. Dual‐route models dissociate the lexical‐semantic pathway devoted to the reading of meaningful words and the phonological pathway devoted to the reading of nonwords and pseudowords [Coltheart et al., 2001; Perry et al., 2007, 2010; Zorzi et al., 1998]. In other words, the lexical‐semantic pathway subserves “addressed phonology” that addresses the pronunciation of all words known to the reader, while the phonological pathway subserves “assembled phonology” that assembles pronunciations based on spelling–sound correspondences [Proverbio et al., 2004; Simos et al., 2002].
Besides, it has also been showed that the first steps before phonological and semantic processing of reading are supported by a visual word form system that connects extrastriate occipital cortex to the left lateral occipitotemporal sulcus cortex, at the anterolateral aspects of the left fusiform gyrus. This region, referred to as the visual word form area (VWFA) [Cohen et al., 2000; Dehaene et al., 2002; Dehaene and Cohen, 2011], receives homolateral visual inputs from the occipital pole via the ventral occipitotemporal (vOT) region [Epelbaum et al., 2008; Vinckier et al., 2007], more precisely via the posterior portion of the inferior longitudinal fascicle (ILFp) [Coello et al., 2013a; Martino et al., 2013a; Yeatman et al., 2013]; and contralateral visual inputs via the corpus callosum (CC) [Molko et al., 2002]. Although the existence of a specific role of the VWFA is still debated [Dehaene and Cohen, 2011; Price, 2013; Price and Devlin, 2003, 2011; Wandell, 2011], its contribution to the reading network is widely accepted [Dehaene et al., 2005; Vinckier et al., 2007; Vogel et al., 2011; Yeatman et al., 2013].
According to current models, visual word recognition is mediated by an interactive system including (i) a feed‐forward orthographic processing of visual inputs that are coded inside the VWFA in an inner posterior‐to‐anterior organization (from letter strings to progressively longer elements that finally form whole words [Dehaene et al., 2005; Vinckier et al., 2007]) and (ii) nonvisual information that are integrated via feedback and lateral connections in the visual system [Dehaene et al., 2005; Twomey et al., 2011]. These bottom‐up and top‐down modulations of orthographic processing of words by the phonological and semantic systems are not entirely understood nor explained by the proposed neurocognitive models of reading [Ans et al., 1998; Coltheart et al., 2001; Marshall and Newcombe, 1973; Perry et al., 2007; Plaut et al., 1996; Seidenberg and McClelland, 1989; Zorzi et al., 1998]. Besides, the anatomical fiber connections of the VWFA, for which knowledge about their functional significance may inform cognitive models of reading, are still a matter of debate. This is mostly due to the numerous fiber crossings encountered in the occipitotemporal area which cannot, at the time, be resolved by diffusion tractography. Indeed, at least six association fiber tracts cross or terminate in this region: the inferior longitudinal fascicle (ILF), the inferior fronto‐occipital fascicle (IFOF), the long segment of the arcuate fascicle (AF) and its posterior vertical segment (AFp), the vertical occipital fascicle (VOF), and the fibers of the tapetum that form the posterior CC.
Indeed, most of the anatomical data concerning neural basis of reading has been provided by functional MRI (fMRI) [Cohen et al., 2000; Dehaene et al., 2010; Dehaene and Cohen, 2011; Henry et al., 2005; Jobard et al., 2003; Mechelli et al., 2003; Mechelli et al., 2005; Simos et al., 2002; Tsapkini et al., 2011; Twomey et al., 2011; Vinckier et al., 2006], event‐related potentials [Proverbio et al., 2004], or magnetoencephalography [Marinkovic et al., 2003]. Hence, the dorsal phonological and ventral lexico‐semantic routes of reading are concepts mainly supported by cortical activations in frontal, parietal, and temporal regions that have been related to different reading tasks involving either phonological or semantic processing of visual information. More specifically, in a review of fMRI studies, cortical activations of the middle temporal gyrus (MTG), basal temporal region, and pars triangularis of the inferior frontal gyrus have been related to lexical processing while cortical activations of the superior temporal gyrus (STG), MTG, supramarginalis gyrus (SMG), and pars opercularis of the inferior frontal gyrus (POp) have been related to phonological processing of reading words [Jobard et al., 2003]. These data provide strong but indirect evidence that the ventral lexico‐semantic pathway of reading might be subserved by the IFOF and the dorsal phonological pathway by the AF. Indeed, the cortical terminations of these two major association bundles correspond to the cortical areas described in fMRI studies [Catani et al., 2005; Duffau et al., 2013; Martino et al., 2013a,b; Sarubbo et al., 2013].
Even if a few recent studies emphasized on fiber connections of the VWFA using diffusion tractography [Cummine et al., 2013; Epelbaum et al., 2008; Yeatman et al., 2013], the only tract that has been demonstrated to be a posterior connection to the VWFA is the ILFp [Epelbaum et al., 2008] whose interruption caused pure alexia (namely, alexia without agraphia or anomia). Other supposed connections to the VWFA are the AFp and the VOF. The AFp has been found to be connected to the VWFA in diffusion tractography studies [Epelbaum et al., 2008; Yeatman et al., 2013] and possibly involved in reading as the temporoparietal connectivity (indirectly measured by the fractional anisotropy in the AFp) is correlated with reading skills [Thiebaut de Schotten et al., 2012]. The VOF, which is not a “classical” association tracts, has been proposed as a possible fiber tract involved in so called “subangular alexia” [Yeatman et al., 2013] described in two reports, one consecutive to a surgical resection of an arteriovenous malformation [Greenblatt, 1976] and the other consecutive to an infarct in the temporoparietal junction [Iragui and Kritchevsky, 1991]. Consequently, neither anterior nor superior disruption of the connectivity of the VWFA that could possibly involve the anterior portion of the ILF (ILFa), the IFOF, or the AF has been reported to cause reading disturbances.
In the present study, we investigated reading abilities of seven patients during awake surgery of diffuse low‐grade gliomas (LGG) involving the occipitotemporal region, and during preoperative and postoperative neuropsychological assessment. For each patient of our series, awake surgery and measure of reading abilities has not been made only for scientific purposes but to obtain a maximal extent of resection of the glioma while preserving neurological functions. This methodology has already been used to investigate language [Khan et al., 2014; Moritz‐Gasser and Duffau, 2013], motor [Almairac et al., 2014; Van Geemen et al., 2014; Kinoshita et al., 2014; Rech et al., 2014; Schucht et al., 2013], or mentalizing processes [Herbet et al., 2013, 2014] but not reading except in one single study [Roux et al., 2004], which only proposed a cortical mapping. Hence, this is the first study to provide in vivo data based on cortical, subcortical, and lesion‐symptom to help understanding brain mechanisms involved during reading.
MATERIALS AND METHODS
Patients
Between January 2010 and February 2014, seven patients with a LGG (World Health Organization grade II glioma) involving the left lateral and basal temporo‐occipital lobe underwent awake surgery with intraoperative language and reading mapping. Informed consent was obtained from all patients before surgery. Patient characteristics are summarized in Table 1. There were five males and two females ranging in age from 23 to 59 years (mean age 41.9 ± 12.4). Educational level of all patients of the series was >9 years. Seizure was the initial clinical symptom in all patients. The Edinburgh inventory for handedness [Oldfield, 1971] showed that five patients were right‐handed and two patients were ambidextrous. The standard neurological examination before surgery was normal in all cases. The topography of the tumor was accurately analyzed on a preoperative MR images (T1‐weighted and spoiled‐gradient images obtained before and after gadolinium enhancement in the three orthogonal planes, FLAIR axial and T2‐weighted coronal images).
Table 1.
Patients characteristics and surgical findings
| Patients | Age, sex | Handed‐ness | Tumor location | Intraoperative mapping (reading) | Intraoperative cortical mapping (other functions) | Intraoperative subcortical mapping (other functions) |
|---|---|---|---|---|---|---|
| 1 | 59, M | Right handed | Left parahippocampal/fusiform | ILF (basal and medial to the AF: 49): addressed phon disturbances | vPMC (1 and 2): anarthria; mid STG (8): anomia; mid MTG (3): switch French/ English | IFOF (ventricle roof, not represented): sem paraph; AF (50): anomia and phon paraph |
| 2 | 23,M | Right handed | Left post MTG ± post ITG | ILF (50): addressed and assembled phonology disturbance | vPMC and PCG (1 and 2): anarthria; PoCG (3 and 4): articulatory troubles and involuntary tongue movements; posterior STG (5): sem paraph, switch French/English | IFOF (46 and 47): sem paraph and anomia; AF (48 and 49): phon paraph |
| 3 | 40, M | Right handed | Left post MTG and ITG | ILF (48): addressed phon disturbances | vPMC (1 and 2): anarthria; post STG (3): anomia; post MTG (4): phon paraph and pain | IFOF (50): anomia; AF (49): anomia and phon paraph |
| 4 | 30, M | Ambidext | Left parahippocampal/fusiform | Post‐inf MTG (cortex 4): anomia and alexia; ILF (WM 48, not visible, dorsal and lateral to 49): addressed phon disturbances | vPMC (1): anarthria; mid STG (2): anomia; post MTG (3): anomia; post‐inf MTG (4): anomia and alexia | AF (50): phon paraph; OR (49): phosphenes |
| 5 | 42, F | Ambidext, English speaking | Left post MTG | ILF (47): addressed phon disturbances | vPMC (1): anarthria; mid STG (2 and 3): sem paraph and anomia | IFOF (49 and 50): sem paraph and anomia; AF (48): phon paraph and anomia; (46): anomia |
| 6 | 47, F | Right handed | Left fusiform/basal occipital | post ITG (cortex 2): alexia post ILF (WM 40): alexia | post STG (1): anomia; post MTG (3) and occipital lobe: phon paraph and anomia | IFOF (42): anomia without alexia; AF (underneath 3): phon paraph; OR (10 and 11): blurring and phosphenes in the right visual field |
| 7 | 52, M | Right handed | Left fusiform/occipital lobe | post MTG (cortex 6): alexia | Primary motor cortex (1): face; mid‐post STG (2 and 3): anomia | IFOF (46): sem paraph and PPTT disturbance; In the depth of the superior temporal sulcus (47) and of the AG (48): complete loss of consciousness. |
AF: arcuate fascicle; AG: angular gyrus; Ambidext: ambidextrous; F: female; IFOF: inferior frontooccipital fascicle; ILF: inferior longitudinal fascicle; ITG: inferior temporal gyrus; M: male; MTG: middle temporal gyrus; OR: optic radiations; paraph: paraphasia;; PCG: precentral gyrus; phon: phonological; PoCG: postcentral gyrus; post: posterior; sem: semantic; PPTT: pyramidal palm‐tree test; STG: superior temporal gyrus; vPMC: ventral premotor cortex; WM: white matter.
Preoperative and Postoperative Functional Assessment
Language function was assessed by a senior speech therapist pre‐ and postoperatively. In addition, a verbal phonological (saying words beginning by the letter “p” in 2 min) and semantic (saying animals names in 2 min) fluency task [Cardebat et al., 1990], the DO 80 naming task [Metz‐Lutz et al., 1991], and a reading task that consisted in overt reading of regular, irregular, and pseudowords [Nespoulous et al., 1992] were administered to each patient. No formal reliability assessment was conducted on the scores. All the patients were tested in their native language.
Preoperative language and reading examination was performed the day before surgery. Immediate postoperative assessment was achieved 3–5 days after surgery and postoperative assessment was conducted 3 months after surgery. All patients received speech rehabilitation in the postoperative period.
Intraoperative Functional Mapping
All patients underwent awake surgery under local anesthesia to allow functional, especially language, reading, and visual field, cortical and subcortical mapping with direct electrical stimulation. This method has been extensively described in previous studies [Duffau et al., 2002, 2005b, 2008]. After removal of the bone flap and opening of the dura matter, ultrasonography was used on the cortical surface to identify the tumor location. Tumor borders were indicated with sterile letter tags. Prior to glioma resection, the cortex was mapped in all patients. Electrostimulation was delivered to the brain using a 5‐mm spaced tips bipolar stimulator and with a biphasic current intensity between 1.5 and 4 mA (60 Hz, 1 ms single pulse phase, Nimbus, Hemodia). Stimulation of each site lasted maximum 3 s. Each cortical site was stimulated three times. However, no area was stimulated twice in succession to prevent seizures. For language mapping, two tasks were utilized. The first one was counting, where the patients counted from 1 to 10 repeatedly, and the second one was picture naming (DO 80 task), where the patient first read a short sentence “ceci est…” (the French translation of “This is…”) and then named the picture presented on the screen. This allows making a distinction between a speech arrest (no sound) and naming disturbances, that is the patient's inability to name the picture but ability to read the sentence out loud [Duffau et al., 2005b; Duffau, 2013; Vidorreta et al., 2011]. More precisely, an articulatory disturbance (i.e., anarthria) was defined as such when the patient was unable to pronounce correctly the introductive sentence “This is a …” and the name of the picture, because phonetic units were distorted, leading to the production of distorted words with phonemes whose distinctive features were degraded. Phonological paraphasia was defined by the production of words near the target ones but with phonological changes: phoneme elision (e.g., Fag for flag), phoneme addition (e.g., Flagl for flag), change in the distinctive features (e.g., Vlag for flag). For the latter kind of disturbance, some attempts of self‐corrections were sometimes observed. Other disturbance types were anomia and semantic paraphasia, that is the production of a word semantically related to the target word (e.g., Dog for cat).
Both the speech therapist and patient were unaware of when and where stimulations were delivered. If stimulation elicited a reproducible language disturbance that is, at least two of three stimulations induced the same type of deficit (e.g., semantic paraphasia), a sterile number tag was placed on this area to identify the functional epicenter. A photograph of the cortical map was systematically taken before resection. After the completion of cortical mapping, glioma resection was started and subcortical structures were systematically stimulated in order to identify language pathways. During the resection and stimulation of subcortical structures, the patient continued with the naming task and the speech therapist analyzed the language disturbances in real‐time, i.e., articulatory disturbances, anomia, phonemic paraphasia, or semantic paraphasia.
In addition to the naming task, a reading test was also performed by the patient [Gil‐Robles et al., 2013; Nespoulous et al., 1992], who had to name real regular and irregular words and pseudowords that were randomly presented on the screen (see Fig. 1). Words used during the reading task derived from the MT86 test [Nespoulous et al., 1992], for which all psycholinguistic criteria (including word frequency and word length) are controlled. As pseudowords reading depends only on assembled phonology without any possible compensation by addressed phonology, an intraoperative inability to read pseudowords while real words could be read was interpreted as an assembled phonology disturbance. By contrast, as irregular words reading depends mostly on addressed phonology, without possible compensation by assembled phonology, an intraoperative inability to read an irregular word was interpreted as an addressed phonology disturbance. Finally, regular words reading can be processed by both addressed and assembled phonology. However, in skill readers, it depends mostly on addressed phonology, which, however, may be compensated by assembled phonology. Thus, during regular words reading, an addressed phonology disturbance may lead to compensatory letter‐by‐letter reading strategy easily identifiable intraoperatively. On the contrary, an assembled phonology disturbance may possibly lead to either no reading difficulty (especially for frequent words) or complete alexia (for infrequent words that would need phonological decoding). If the error was not characterizable (for example when the patient did not produce any sound, or when he/she said “I don't know”) the intraoperative reading task was suspended a few seconds so that the speech therapist could explore in more detail the nature of the error (for instance, by asking the patient to spell the written word). This was possible because when the surgeon interrupts the electrical stimulation (i.e., when the stimulation probe is taken away from the brain), the altered function recovers immediately so that the patient can explain his/her difficulty or feelings.
Figure 1.
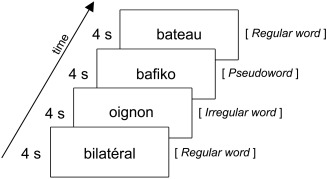
Schema of the reading intraoperative task inspired from the Montreal‐Toulouse MT‐86 test [Nespoulous et al., 1992] and adapted to intraoperative conditions. Single words were consecutively presented on the screen of a laptop computer. The test consisted in a series of 20 words, whose type (regular, irregular, or pseudowords) was presented at random. New words were regularly presented every 4 sec, 0.5 sec after a beep, so that electrical stimulation begun before the word appeared on the screen.
Both intraoperative naming and reading tasks were assessed in the native language of each patient. Bilingual patients were tested in both their first and second languages.
Furthermore, online mapping of the optic radiations was also achieved to prevent hemianopia. To this end, a modified picture‐naming task was used, with presentation of two objects situated diagonally on a screen divided into four quadrants. An image was presented in the quadrant to be saved and another image was presented in the opposite quadrant. Direct subcortical electrostimulation was repeatedly performed without the patient's knowledge, until optic radiations were identified by eliciting transient visual disturbances such as blurred vision, shadow, phosphenes, or even visual illusions [Gras‐Combe et al., 2012]. Finally, a nonverbal semantic association test (the so‐called pyramid and palm trees test) [Howard et al., 1992] was administered. This task involves 52 black‐and‐white drawings presented on a computer screen. For each target picture, two new pictures are proposed, and the patient is asked to match one of these with the target according to a semantic link, by pointing to the matching picture.
To perform the best possible tumor removal and preservation of functional areas, all resections were pursued until eloquent pathways were encountered around the surgical cavity, i.e., resections were achieved according to functional boundaries. Thus, there was no margin left between the surgical cavity and the adjacent functional areas. After tumor removal, a photograph of the cortical and subcortical maps was taken.
Resection Cavity Overlapping
Postoperative high‐resolution three‐dimensional (3D)T1 MRI scans acquired in the third month were normalized to a standardized common space (Montreal Neurological Intitute space) using cost function masking—a method allowing to avoid bias caused by abnormal lesion‐induced radiological signals during the registration process [Andersen et al., 2010; Brett et al., 2001]. Briefly, the lesion is contoured by hand and subsequently transformed into a binarized image. This image is used as a mask during the normalization process, achieved in our study with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) implemented in a MATLAB environment (http://www.mathworks.com). The lesion is then drawn on the normalized scan to yield an individual volume of interest.
Resection cavity reconstructions were overlapped onto the montreal neurological institute (MNI) template. The IFOF, the ILF, and the AF, obtained from a fine‐grained DTI‐based white matter atlas [Thiebaut de Schotten et al., 2011], were also superimposed in this standardized space. The different figures were created using MRIcroGL (http://www.cabiatl.com/mricrogl).
Anatomo‐Functional Correlations
The exact location of the white matter fascicles was determined using both the types of dysfunction during intraoperative electrostimulation mapping and anatomic correlation with postoperative MRI and tractography atlas. Indeed, the control MRI examination carried out 3 months after surgery allowed us to analyze accurately the anatomical location of the eloquent pathways, i.e., by definition, at the periphery of the cavity, where the resection was stopped according to the functional responses elicited by intraoperative stimulation (with no margin). It is worth noting that we have extensively used this reliable and reproducible method in many previous studies [Coello et al., 2013b; Duffau et al., 2002, 2003, 2005a; Duffau, 2008; Van Geemen et al., 2014; Khan et al., 2014; Mandonnet et al., 2007; Schucht et al., 2013].
RESULTS
The results of intraoperative and perioperative reading and language assessments are summarized in Table 1 and Table 2, respectively. Mapping identified eloquent language areas in all patients, indicating crucial participation of the left hemisphere in language, including, importantly, the two ambidextrous patients. In the following, each patient case is described individually.
Table 2.
Perioperative language and reading assessments
| Patients | DO‐80a | Verbal fluency | Reading tasks | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Semantic | Phonological | Regular words (/8) | Pseudowords (/5) | Irregular words (/7) | Global reading score (/20) | ||||||||||||||||||
| pre | post | M3 | pre | post | M3 | Cut‐off | pre | post | M3 | Cut‐off | pre | post | M3 | pre | post | M3 | pre | post | M3 | pre | post | M3 | |
| 1—PD | 80 | 0 | 68 | 45 (+1.9) | 6 (−3.7) | 23 (−1.3) | 25 | 31 (+1.1) | 1 (−3.0) | 24 (+0.2) | 14 | 8 | 8 (S) | 8 | 5 | 0 | 5 | 7 | 0 | 7 | 20 | 8 | 20 |
| 2—JB | 79 | 0 | 77 | 37 (+0.1) | 0 | 35 (−0.1) | 25 | 18 (−0.4) | 0 | 28 (+1.1) | 14 | 8 | 0 | 8 | 5 | 0 | 5 | 7 | 0 | 7 | 20 | 0 | 20 |
| 3—JL | 79 | 0 | 50 | 25 (−1.0) | 0 | 13 (−1.9) | 25 | 33 (+1.8) | 0 | 21 (+0.1) | 14 | 8 | 0 | 8 | 5 | 0 | 5 (S) | 7 | 0 | 6 (S+) | 20 | 0 | 19 |
| 4—EB | 80 | 0 | 79 | 27 (−0.8) | 0 | 39 (+0.3) | 25 | 35 (+2.1) | 0 | 35 (+2.1) | 14 | 8 | 0 | 8 | 5 | 0 | 3 (S) | 7 | 0 | 7 (S) | 20 | 0 | 18 |
| 5—AHb | 80 | 76 | 80 | – | – | – | 19 | – | – | – | 14 | 5/5 | 5/5 | 5/5 | 5 | 1 (S+) | 3 (S) | – | – | – | 10/10 | 6/10 | 8/10 |
| 6—NC | 80 | 80 | 80 | 34 (+0.3) | 31 (+0.1) | – | 19 | 13 (−1.3) | 12 (−1.4) | – | 14 | 8 | 6 (S+) | 8 (S) | 5 | 0 | 4 (S) | 7 | 2 (S+) | 7 (S) | 20 | 8 | 19 |
| 7—JMR | 80 | 70 | 79 | 24 (−1.1) | 12 (−2.9) | 26 (−0.8) | 25 | 22 (−0.1) | 17 (−0.8) | 22 (−0.1) | 15 | 8 | 0 | 8 (S) | 5 (S) | 0 | 4 (S+) | 7 | 0 | 5 (S) | 20 | 0 | 17 |
Z‐scores of verbal fluency tests (whose scores depend on gender and age) are presented in brackets.
pre: preoperative assessment; post: immediate postoperative assessment; M3: third month assessment; S: slow; S+: very slow.
Cut‐off score of the DO‐80 is 73, corresponding to −2 SD.
Foreigner patient (English‐speaking). Verbal fluency task and irregular words reading task were not administrable to this patient. Regular words and pseudowords reading tasks have been adapted from the French versions.
Patient 1
Patient 1 (PD) was a 59‐year‐old right handed bilingual (French/English) male. Preoperative reading, naming, and verbal fluency tasks were normal. Intraoperative reading mapping elicited addressed phonology disturbances when stimulating the white matter anteriorly adjacent to the VWFA, at the postero‐inferior boundary of the resection cavity (Fig. 2, first row, label 49). Resection cavity involved posterior MTG and ITG, anterior fusiform gyrus and parahippocampl gyrus. The anterior portion of ILF was therefore disconnected from the VWFA and partially resected.
Figure 2.
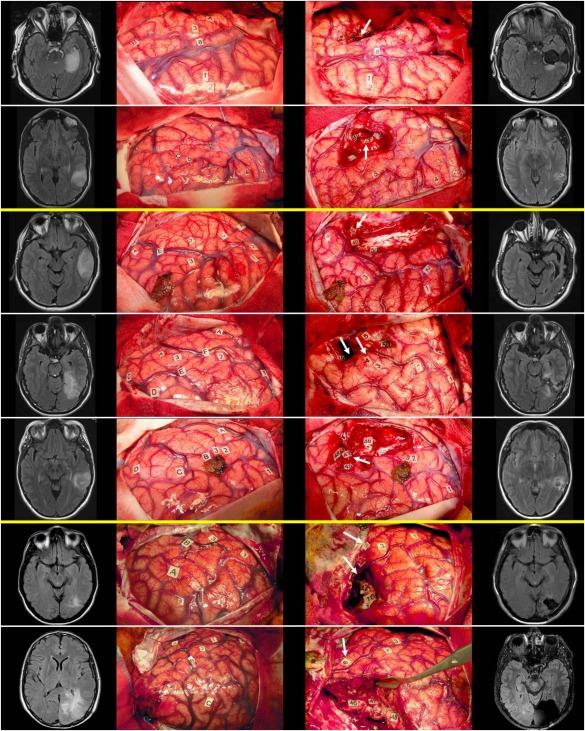
Preoperative FLAIR MRI, preresection cortical mapping photograph, postresection subcortical mapping photograph, and postoperative FLAIR MRI (from left to right) of patients 1–7 (from top to down). Cortical and subcortical stimulation sites eliciting a reading disturbance are tagged by a white arrow on postresection cortical mapping photographs (third column). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Immediate postoperative language and reading assessment revealed a severe phonological disturbance leading to a naming task result at zero and a significative worsening of verbal fluency scores. Regular words reading were possible although difficult, while there was a complete alexia of pseudowords and irregular words. Third month assessment found a complete recovery of reading abilities while naming and verbal fluency had only partially recovered.
Patient 2
Patient 2 (JB) was a 23‐year‐old right‐handed bilingual (French‐English) male. Preoperative language assessment was normal. Intraoperative reading mapping elicited both addressed and assembled phonology disturbances when stimulating the white matter of the basal boundary of the resection cavity (Fig. 2, second row, label 50), at the anterior aspect of the VWFA. Resection cavity involved posterior MTG and ITG, leading to a disconnection of the ILFa
Immediate postoperative assessment elicited a mutism due to severe articulatory and/or phonological impairment while comprehension was preserved. Third month assessment showed a complete recovery of spoken language and reading abilities.
Patient 3
Patient 3 (JL) was a 40‐year‐old right handed monolingual (French) male. Preoperative language and reading assessment was normal. Intraoperative reading mapping elicited addressed phonology disturbance when stimulating the white matter of the ITG, anteriorly adjacent to the VWFA (Fig. 2, third row, label 48). Resection cavity involved the middle and posterior portions of the MTG and ITG, the anterior portion of the STG, and respected the temporal pole and temporo‐mesial structures.
Immediate postoperative assessment revealed a mutism due to severe articulatory and/or phonological impairment with good preservation of comprehension. Third month assessment showed a complete recovery of real regular words reading while reading pseudowords was difficult, and reading irregular words very difficult. Naming and verbal fluency abilities recovered only partially.
Patient 4
Patient 4 (EB) was a 30‐year‐old ambidextrous monolingual (French) male. Preoperative language and reading assessment was normal. Intraoperative reading mapping elicited an alexia when stimulating the posto‐inferior MTG cortex (the stimulation of this site had also induced anomia) and addressed phonology disturbance when stimulating the white matter of the MTG, posteriorly and superiorly adjacent to the VWFA (Fig. 2, fourth row, label 48).
Immediate postoperative assessment showed a mutism and third month assessment a difficulty to read pseudowords and irregular words, while reading regular words, naming, and verbal fluency had completely recovered.
Patient 5
Patient 5 (AH) was a 42‐year‐old ambidextrous monolingual (English) female. Due to her native language, the verbal fluency task could not be administered, as well as the reading task of irregular words. Preoperative assessment was normal. Intraoperative reading mapping elicited addressed phonology disturbance when stimulating the white matter in the depth of the inferior temporal sulcus, at the anterior aspect of the VWFA (Fig. 2, Fifth row, label 47).
Immediate postoperative assessment showed a slight decrease (which did not reach the cut‐off score at the naming task, no deficit at reading regular words but a severe impairment at reading pseudowords. Third month assessment showed a complete recovery except for reading pseudoword, which remained difficult.
Patient 6
Patient 6 (NC) was a 47‐year‐old right‐handed monolingual female. Preoperative language and reading assessment was normal. Intraoperative reading mapping elicited a complete and “pure” alexia, i.e., with no naming impairment when stimulating the posterior ITG cortex (Fig. 2, sixth row, label 2). After resection of ILFp, reading was globally slow. At the end of resection, white matter stimulation in the depth of the posterior ITG, at the posterior aspect of the VWFA (Fig. 2, sixth row, label 40) elicited a complete alexia.
Immediate postoperative assessment showed normal naming and verbal fluency performances, while reading assessment was severely impaired for all types of words. Third month assessment showed an improvement of reading abilities for all types of words, even though reading remained slow.
Patient 7
Patient 7 (JMR) was a 52‐year‐old right‐handed monolingual male. Preoperative assessment showed an impaired reading of pseudowords while reading of real words (regular and irregular) was normal as well as naming and verbal fluency. Intraoperative reading assessment elicited a complete and “pure” alexia when stimulating the posterior MTG cortex (Fig. 2, seventh row, label 6). Note that after resection of ILFp, intraoperative reading was slow but electrical stimulation of the white matter did not worsen reading.
Immediate postoperative assessment showed a significative impairment of naming and semantic verbal fluency, and a complete alexia of all types of words. Third month assessment showed a complete recovery of naming and verbal fluency tasks, and a partial recovery of reading abilities which remained difficult for all kinds of words, especially for pseudowords.
DISCUSSION
We investigated the functional significance of the left lateral and basal occipitotemporal cortex and its anatomical connections in seven patients who underwent awake surgery by means of an intraoperative cortico‐subcortical mapping of reading and language processes (see Fig. 3). In the light of our results, we can outline the reading network and surgical consequences on reading abilities as follows (see Fig. 4).
Figure 3.
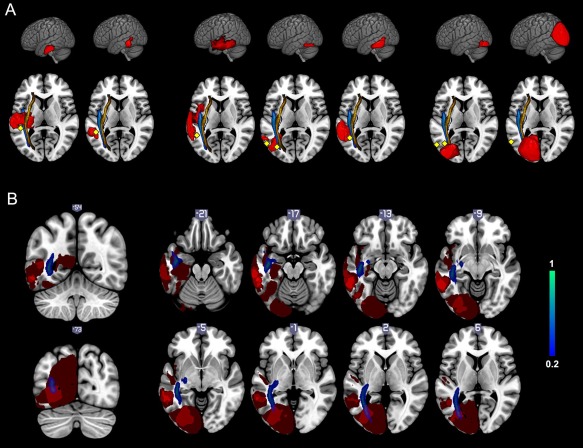
Resection cavity overlapping superimposed on the MNI template. A: Resection cavities of patient 1 to patient 7 (from left to right) on a left lateral 3D view and on a horizontal slice with the ILF in blue and the IFOF in orange. Cortical and subcortical stimulation sites are represented by yellow diamonds. B: Resection cavity overlapping on coronal and axial views showing the ILF in blue and the VWFA (visible from slice −21 to slice 2) respected by the cavities. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Figure 4.
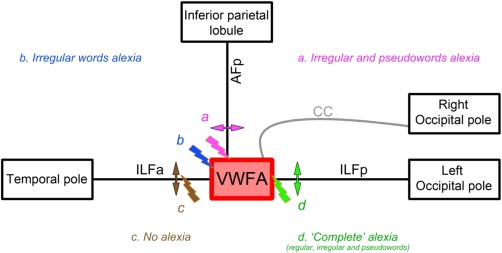
Schema of the circuit including the VWFA, its visual inputs via the ILFp and the CC, and bottom‐up / top‐down modulation via the AFp, as part of the “reading network.” Transient disturbance, i.e., electrical stimulation (thunder symbols) or permanent, i.e., section or resection (arrows) of the represented tracts elicited specific reading impairment. (a) Stimulation and resection of the inferior termination of the AFp induced addressed and assembled phonology disturbance, i.e., irregular and pseudowords reading impairment. (b) Stimulation of the anterior aspect of the VWFA‐induced addressed phonology disturbance, i.e., irregular words reading impairment. (c) Stimulation and resection of the ILFa never induced reading impairment. (d) Stimulation of the posterior aspect of the VWFA, and/or of its CC inputs, and resection of the ILFp‐induced “complete” alexia, i.e., reading impairment of all kinds of regular, irregular and pseudowords. CC, corpus callosum; ILFa, anterior portion of the inferior longitudinal fasciculus; ILFp, posterior portion of the inferior longitudinal fasciculus; AFp, posterior segment of the arcuate fasciculus; VWFA, visual word form area. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Our main intraoperative findings show that (i) intraoperative electrical stimulation of the left posterior inferior temporal cortex‐elicited reading disturbances in three patients; (ii) subcortical stimulation of the anterior portion of the VWFA systematically induced addressed phonology disturbance, i.e., irregular words reading impairment; and (iii) subcortical stimulation of the connection between VWFA and AFp induced both addressed and assembled phonology disturbances, i.e., irregular and pseudowords reading impairment.
On the other hand, our postoperative findings show that (i) resection of the ILFp connecting the visual cortex to the VWFA induced permanent and global reading impairment; (ii) resection of the terminations of the AFp in the left ITGp white matter, located anteriorly and superiorly to the VWFA, induced pseudowords and irregular words reading disturbances in the three patients for whom this region was resected; and (iii), importantly, the resection of the ILFa did not induce reading impairment.
Interpretation of Intraoperative Data
Alexia induced by cortical stimulation
For obvious technical considerations, only the cortical convexity of the occipitotemporal region can be stimulated during awake surgery of diffuse LGG. Thus, the VWFA located in the left lateral occipitotemporal sulcus is not directly exposed during cortical mapping. Nevertheless, for two patients, cortical stimulation of the posterior part of the temporal cortex (precisely the inferior part of the MTG for patient 7 and the ITGp for patient 6) elicited alexia for both words and pseudowords, without anomia. Note that for patient 4, stimulation of the cortical site eliciting alexia also induced anomia. We therefore did not consider this site as being specifically dedicated to reading as it could rather be involved in speech production. Nevertheless, a careful analysis of cortical stimulation sites on Figure 3 shows that the lateral occipitotemporal sulcus, i.e., the VWFA, is situated just a few millimeters in the depth of the labeled sites.
Given that the VWFA was not directly stimulated, our hypothesis to explain alexia during stimulation of the posterior inferior temporal cortex is that a critical cortical relay between the VWFA and the AF may have been disorganized during stimulation of this region. Indeed, it has been proposed that the MTG, STG, SMG, and POp are implicated during the phonological processing, i.e., grapho‐phonological conversion, of visual orthographic information [Jobard et al., 2003]; and it is also known that the AF has cortical terminations in each of these gyri [Catani et al., 2005; Martino et al., 2013b].
If the electrostimulation had disturbed semantic processing, one can suppose that the patients could have used a letter‐by‐letter reading strategy, i.e., they could have used the phonological pathway to compensate the lexical‐semantic pathway. On the contrary, we observed “complete” alexia. In view of dual‐route models of reading [Coltheart et al., 2001; Perry et al., 2010; Zorzi et al., 1998], we can assume that the phonological pathway implicated in grapheme‐phoneme conversion may have been disrupted during stimulation of the posterior temporal cortex.
Addressed phonology disturbance induced by subcortical stimulation
In four of the seven patients of our series, subcortical stimulation of the basal occipitotemporal white matter, anatomically located in the vicinity of the posterior Labbé vein (see Fig. 2), induced transient letter‐by‐letter reading strategy, which demonstrates an “addressed phonology” disturbance [Proverbio et al., 2004; Simos et al., 2002].
Hence, stimulation induced a specific impairment of lexical‐semantic processing of orthographic information, which had to be processed by the sole phonological pathway using “assembled phonology.” In all these four cases, the VWFA and its visual inputs were systematically spared, and the stimulation sites were located in the anterior part of the visual word form system, thus disturbing the visual processing of whole words but not of smaller encoded letter combinations such as bigrams or trigrams, which are processed more posteriorly in the occipitotemporal cortex [Dehaene et al., 2005; Dehaene and Cohen, 2011; Vinckier et al., 2007]. Therefore, we suggest that direct stimulation of the anterior VWFA led to restricted output information from the VWFA. As whole words could not be encoded, small letter combinations needed to be assembled using the phonological route before access to the meaning.
Complete alexia induced by subcortical stimulation
Another interesting finding is the impairment not only of “addressed phonology,” but also of letter‐by‐letter reading during subcortical stimulation in two patients (patients 2 and 6), which signs the impairment of “assembled phonology” [Proverbio et al., 2004; Simos et al., 2002]. The stimulated sites were not involved in aloud speech disturbances.
For patient 2, the subcortical site whose stimulation elicited alexia, was located in the white matter of the posterior ITG (Fig. 2, second row, label 50) between two sites involved in phonological processing (labels 48 and 49). Thus, it is likely that subcortical stimulation of the posterior ITG interrupted the connection between VWFA and AF, inducing the inability for the patient to address the orthographic information toward the phonological dorsal route.
For patient 6, subcortical stimulation alexia site, at the posterior aspect of the VWFA, was not only located in the vicinity of the VWFA (Fig. 2, sixth row, label 40) but was also very close to a site involved in phonological processing (not visible on photography), located just dorsally and laterally to the VWFA. That configuration leading to a transient disruption of the connection between VWFA and AF may explain complete alexia induced by electrical stimulation. Moreover, the ILFp of this patient was disconnected at the time of subcortical stimulation (see Fig. 3). We also know that orthographic visual inputs to the VWFA come not only predominantly from the homolateral primary visual cortex via the inferior and lateral occipital lobe [Marinkovic et al., 2003] but also from the contralateral hemisphere via the corpus callosum [Binder and Mohr, 1992; Carreiras et al., 2009; Cohen et al., 2000; Molko et al., 2002]. Nonetheless, contralateral inputs are much less efficient, i.e., that words presented in the right visual hemifield have a priority access to the VWFA [Finkbeiner et al., 2006], explaining that the section of the left ILFp drastically reduces the visual inputs of the VWFA and, therefore, induces easier disturbance of orthographic processing when stimulating in the vicinity of the VWFA or at the junction between contralateral visual inputs (CC terminations) and VWFA.
It is worth noting that for patient 7, subcortical stimulation did not induce reading disturbance, even though reading had been globally altered after section of the ILFp. Indeed, the lesion involved the occipital lobe so that the resection cavity did not reach the left lateral occipitotemporal sulcus (see Fig. 3, which clearly shows the cortical stimulation site close to the VWFA and the posterior resection cavity distant from the VWFA). In this case, subcortical stimulation site was distant from the VWFA. This observation not only supports the hypothesis that direct stimulation of the VWFA induces pure alexia but also the hypothesis that the VWFA receives visual inputs from the contralateral hemisphere through callosal fibers [Cohen et al., 2000; Molko et al., 2002]—that can partially compensate homolateral visual inputs when interrupted.
Interpretation of Postoperative Assessment
If we consider resection cavity overlapping illustrated in Fig. 3, it is clear that the left lateral occipitotemporal sulcus, where is situated the VWFA, has been left in place in all cases. Furthermore, if we carefully examine subcortical stimulation sites that led to reading disturbance, we can observe that they surround the VWFA, explaining why the resection had to be stopped in all cases before to reach the VWFA in order to preserve reading abilities. This observation strongly supports neuroimaging data that converge on identifying the left lateral occipitotemporal sulcus as a crucial area involved in reading processing [Cohen et al., 2000; Gaillard et al., 2006].
However, even if the cortical site referred to as the VWFA is mandatory for reading, postoperative assessment has identified three different clinical situations considering reading abilities after a surgical lesion in the left occipitotemporal region. These three clinical presentations are discussed further, with emphasis on the possible disconnections created by tumor resection between the VWFA and its visual, phonological, and semantic inputs and/or outputs.
Pure alexia after resection of the posterior portion of the left ILF (disconnection between visual input and VWFA)
In our series, the only two patients who presented long term reading impairment of regular, irregular, and pseudowords were patients 6 and 7. Resection cavity overlapping showed that white matter of the vOT region, namely the ILFp, was resected for those two patients, unlike the other patients of the series (see Fig. 3). Moreover, the resection cavity of patients 6 and 7 did not involve the VWFA itself, and involve neither its anterior, superior, lateral, nor contralateral connectivity. Thus we can assume that reading impairment of these two patients is a consequence of the sole disconnection between VWFA and its homolateral visual inputs.
Indeed, current neural models of reading usually admit that the first step of visual words recognition is a feed‐forward processing of the visual input from the occipital pole through the vOT cortex to the VWFA. This VWFA is, therefore, a crucial integration center for visual information before its distribution to both the lexical‐semantic and the phonological networks, which are engaged in the meaning and the spelling of words. Moreover, as aforementioned, even if the CC provides connections between the VWFA and contralateral visual inputs [Binder and Mohr, 1992; Cohen et al., 2000; Molko et al., 2002], it has been shown that words presented in the right hemifield have priority access to the VWFA [Finkbeiner et al., 2006], explaining why a lesion of the left ILFp results in severe slowing down of reading abilities. Our observation is then comparable to others already discussed in literature [Dejerine, 1892; Epelbaum et al., 2008; Greenblatt, 1976], which describe “pure alexia” as a disconnection syndrome.
Pseudowords and irregular words alexia after disconnection between VWFA and AF
Three patients of our series (patients 3, 4, and 5) presented long‐lasting pseudowords and irregular words‐reading impairment while regular words reading was preserved. Observation of the resection cavities (see Fig. 5) demonstrates that the left ITGp was the only structure resected in all these three cases and was spared in other patients of the series. As cortical stimulation of the resected regions did not elicited reading disturbance, we can suppose that the region involved is the white matter of the left ITGp. Moreover, the ILFa was resected in patients 3 and 5 but not in patient 4, allowing the conclusion that the ILFa is neither crucial for reading, nor responsible for the impairment of pseudowords and irregular words reading. Therefore, as resection cavities in the ITGp remained lateral to the ILF and did not involve the ILFp nor the CC, it is highly possible that the inferior termination of the AFp was the interrupted structure. In other words, pseudowords and irregular words reading is impaired when the VWFA is disconnected from the AFp, even when visual inputs of the VWFA are preserved.
Figure 5.
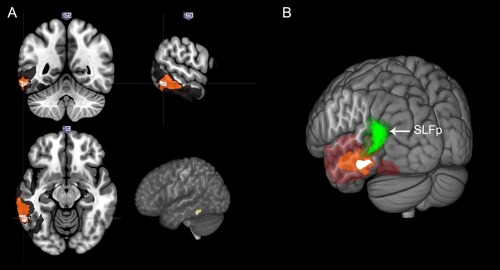
A: Resection cavity overlapping of patients 3, 4, and 5, superimposed on the MNI template. The three cavities overlap on the left posterior inferior temporal gyrus and its underlying white matter. B: Posterolateral 3D view of resection cavity overlapping on the MNI template, with left AFp in green. Inferior termination of the AFp corresponds with the region of maximum overlap. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Reading aloud irregular words, which do not contain common print‐to‐sound correspondence, is likely to be highly automatized, thus mainly requiring the “addressed phonology” and depending little on “assembled phonology.” On the other hand, the pronunciation of pseudowords, which by definition do not possess entry in the “mental lexicon,” is dependent upon phonological decoding, i.e., “assembled phonology.” In other words, irregular words and pseudowords reading should depend on different pathways involved in the reading network. Thus, to explain that reading out loud irregular and pseudowords can be impaired by an unique lesion, we propose that reading these words do not rely on a simple serial, feed‐forward neural system, but rather on feedback connections linking visual to nonvisual information to create an interactive system for visual words recognition [Twomey et al., 2011]. According to our observations, these feedback connections might therefore be involved in both the semantic and the phonological pathways. More particularly, this interactive system should be mostly recruited when spelling‐sound incoherence (the case for irregular words) or when absence of meaning (the case of pseudowords) is detected; it should also rely on bottom‐up / top‐down regulation subserved by the AF (and/or AFp), after visual information have been feed‐forward processed via the ILFp to the VWFA. The hypothesis that the AFp is crucial for reading and involved in both semantic and phonological pathways, is reinforced by our intraoperative observations of addressed and assembled phonology difficulties when stimulating the white matter of the superior aspect of the VWFA in the vicinity of sites involved in phonological processing (see Fig. 2 and Table 1).
Interestingly, even if the IFOF has been systematically identified during surgery by the occurrence of semantic paraphasia, it was in all cases located dorsally and medially to intraoperative reading sites and to postoperative resection cavities (see Fig. 3). Hence, we can assume that the IFOF is not involved in reading difficulties observed in patients 3, 4, and 5. On the other hand, these patients had a preserved reading of regular words, which might, therefore, be processed via the lexical‐semantic pathway, namely the IFOF, without the need for any feedback interaction of the AF.
Complete recovery of reading performances
Finally, two patients of our series (patients 1 and 2) showed a complete recovery of reading abilities 3 months after surgery. Consequently, for these two patients, we can postulate that either the entire reading network has been preserved, or it has been fully compensated.
Analysis of the resection cavities (Fig. 3) shows that they are the most anterior of our series, sparing the ITGp and its underlying white matter which were resected in the abovementioned group with impaired irregular and pseudowords reading. Besides, the ventral segment of the IFOF and the ILFa are the only association tracts that may have potentially been damaged by the resection. Thus, neuroanatomical networks of reading, including major input and output connectivity of the VWFA, must have been spared. Consequently, we can correctly suppose that neither the ventral segment of the IFOF, nor the ILFa (and, therefore, nor the uncinate fascicle [UF]), although usually described as components of the ventral semantic stream [Duffau et al., 2013], are crucial for reading. Interestingly, this could be considered in disagreement with a recent study which showed a relationship between orthographic lexical semantic processing in the ventral stream and fractional anisotropy in the UF [Cummine et al., 2013]. However, we would rather consider that the UF and, therefore, the indirect ventral stream is indeed recruited during semantic processing of reading but, as for speech, this indirect stream might not be essential (i.e., probably compensable by the IFOF) [Duffau et al., 2013; Mandonnet et al., 2007].
Limitations of the Study
Maximizing tumor resection while avoiding permanent neurologic deficits was the principal goal of surgery in these seven patients. Electrical stimulation was performed to detect functional boundaries and was therefore restricted to certain areas. As a result, further stimulation sites leading to various reading responses might have been missed. Moreover, intraoperative time constraints limited subtle explorations. For example, we did not explore the possible effect of word frequency on the depth of reading impairment. Likewise, we did not perform any repetition task which would have been able to help differentiating a real assembled phonology difficulty from a grapheme–phoneme conversion problem (i.e., we cannot state if a difficulty in reading pseudowords is associated with a difficulty in repeating pseudowords).
On the other hand, it is worth noting that no previous study investigated reading during surgery. To our knowledge, only two studies explored reading with intraoperative cortical stimulation. Mani et al. [2008] demonstrated the existence of a cortical center for visual words recognition, by electrical cortical stimulation using subdural electrodes placed on the basal occipitotemporal cortex for presurgical planning of epilepsy. This area was located in the left lateral fusiform gyrus and the left lateral occipitotemporal sulcus. Another intraoperative study of reading [Roux et al., 2004] found cortical reading‐specific sites preferentially located in the left inferior parietal or posterior temporal cortex. Our study is, therefore, the first to provide preoperative, intraoperative, and postoperative clinical and anatomical data on reading abilities. However, from a clinical perspective, our patient sample was too small to draw definitive conclusion about the importance of spared or sacrificed fibers for functional reading outcome. This is also true for cortical stimulations, which elicited a reading‐specific site for only two patients. Further studies are required to investigate the influence of subcortical reading mapping on the occurrence of postoperative reading disturbances.
We also have to acknowledge that we did not perform DTI or fMRI before surgery. Nonetheless, even if the VWFA position might vary significantly between subjects [Yeatman et al., 2013], it is interesting to note that the use of intraoperative electrical mapping allowed the identification of eloquent cortical and subcortical structures in all cases, with very good neurological outcome. Furthermore, the method of using postoperative MRI with superimposed white matter tracts obtained from an atlas of healthy subjects may have limitations in brain tumors due to possible geometric distortion and displacement. However, we would like to insist on the fact that distortion is very rare in low‐grade gliomas, conversely to high‐grade gliomas. In addition, the possible displacement induced by the tumor has disappeared on postoperative MRI, because in essence the glioma was removed. This is supported by the fact that, on the resection cavity overlapping maps, the subcortical structures identified during electrical mapping (i.e., at the periphery of the cavity, where the resection was stopped according to the functional responses elicited by intraoperative stimulation) perfectly matched with the white matter tracts obtained from atlas of healthy subjects.
Finally, we also have to acknowledge that, even though postoperative speech rehabilitation might have an impact on third month assessment, this impact is unfortunately not quantifiable as speech rehabilitation has not been controlled. We can only presume that the type of speech therapy (i.e., therapy only focused on reading, vs. global therapy on lexicon, cognitive, auditory, and visual processes) might influence postoperative scores, but this statement should be the target of future works.
CONCLUSION
This is the first study combining intraoperative direct stimulation mapping of reading abilities and postoperative reading assessment, correlated with resection cavity overlapping and MR diffusion tractography atlas. Taken as a whole, our data demonstrate that (i) resection of the left ILFa does not induce alexia; that (ii) resection of the left ILFp leads to “pure alexia,” namely alexia without aphasia nor agraphia, by disconnecting the VWFA from its main visual inputs; that (iii) the most anterior part of the VWFA is required to allow “addressed phonology” through the lexical‐semantic pathway, i.e., to read irregular words; and that (iv) inferior termination of the AFp in the ITGp is a crucial part of the network involved in assembled phonology and in reading aloud pseudowords and irregular words (Fig. 4).
Hence, we can propose that (i) further surgical procedures involving the ITGp and its subcortical white matter should emphasize on a reading task dedicated to pseudowords and irregular words and (ii) that further studies should consider this region as a potential important anatomical pathway between orthographic processing of visual words in the VWFA and both phonological and semantic modulation.
REFERENCES
- Almairac F, Herbet G, Moritz‐Gasser S, Duffau H (2014): Parietal network underlying movement control: Disturbances during subcortical electrostimulation. Neurosurg Rev 37:513–517. [DOI] [PubMed] [Google Scholar]
- Andersen SM, Rapcsak SZ, Beeson PM (2010): Cost function masking during normalization of brains with focal lesions: Still a necessity? NeuroImage 53:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ans B, Carbonnel S, Valdois S (1998): A connectionist multiple‐trace memory model for polysyllabic word reading. Psychol Rev 105:678. [DOI] [PubMed] [Google Scholar]
- Binder JR, Mohr JP (1992): The topography of callosal reading pathways. A case‐control analysis. Brain J Neurol 115 (Pt 6):1807–1826. [DOI] [PubMed] [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J (2001): Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage 14:486–500. [DOI] [PubMed] [Google Scholar]
- Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y (1990): Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level. Acta Neurol Belg 90:207–217. [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estévez A, Lozano A, Devlin JT, Price CJ (2009): An anatomical signature for literacy. Nature 461:983–986. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, ffytche DH (2005): Perisylvian language networks of the human brain. Ann Neurol 57:8–16. [DOI] [PubMed] [Google Scholar]
- Coello AF, Duvaux S, De Benedictis A, Matsuda R, Duffau H (2013a): Involvement of the right inferior longitudinal fascicle in visual hemiagnosia: A brain stimulation mapping study: Case report. J Neurosurg 118:202–205. [DOI] [PubMed] [Google Scholar]
- Coello AF, Moritz‐Gasser S, Martino J, Martinoni M, Matsuda R, Duffau H (2013b): Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks: A review. J Neurosurg 119:1380–1394. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene‐Lambertz G, Hénaff MA, Michel F (2000): The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split‐brain patients. Brain J Neurol 123 (Pt 2):291–307. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J (2001): DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychol Rev 108:204–256. [DOI] [PubMed] [Google Scholar]
- Cummine J, Dai W, Borowsky R, Gould L, Rollans C, Boliek C (2013): Investigating the ventral‐lexical, dorsal‐sublexical model of basic reading processes using diffusion tensor imaging. Brain Struct Funct 220:445–455. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H (1983): The anatomic basis of pure alexia. Neurology 33:1573–1583. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L (2011): The unique role of the visual word form area in reading. Trends Cogn Sci 15:254–262. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec HG, Poline JB, Le Bihan D, Cohen L (2002): The visual word form area: A prelexical representation of visual words in the fusiform gyrus. Neuroreport 13:321–325. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F (2005): The neural code for written words: A proposal. Trends Cogn Sci 9:335–341. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, Dehaene‐Lambertz G, Kolinsky R, Morais J, Cohen L (2010): How learning to read changes the cortical networks for vision and language. Science 330:1359–1364. [DOI] [PubMed] [Google Scholar]
- Dejerine JJ (1892): Contribution à l'étude anatomo‐pathologique et clinique des différentes variétés de cécité verbale.
- Duffau H (2008): The anatomo‐functional connectivity of language revisited. New insights provided by electrostimulation and tractography. Neuropsychologia 46:927–934. [DOI] [PubMed] [Google Scholar]
- Duffau H (2013): The reliability of asleep‐awake‐asleep protocol for intraoperative functional mapping and cognitive monitoring in glioma surgery. Acta Neurochir Wien 155:1803–1804. [DOI] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Sichez N, Denvil D, Lopes M, Sichez JP, Bitar A, Fohanno D (2002): Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo‐functional study. Brain 125:199–214. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Denvil D, Lopes M, Capelle L (2003): The articulatory loop: Study of the subcortical connectivity by electrostimulation. Neuroreport 14:2005–2008. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Mandonnet E, Peruzzi P, Tzourio‐Mazoyer N, Capelle L (2005a): New insights into the anatomo‐functional connectivity of the semantic system: A study using cortico‐subcortical electrostimulations. Brain 128:797–810. [DOI] [PubMed] [Google Scholar]
- Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, Van Effenterre R, Capelle L (2005b): Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: A comparative study between two series without (1985‐96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 76:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Peggy Gatignol ST, Mandonnet E, Capelle L, Taillandier L (2008): Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with Grade II glioma in the left dominant hemisphere. J Neurosurg 109:461–471. [DOI] [PubMed] [Google Scholar]
- Duffau H, Herbet G, Moritz‐Gasser S (2013): Toward a pluri‐component, multimodal, and dynamic organization of the ventral semantic stream in humans: Lessons from stimulation mapping in awake patients. Front Syst Neurosci 7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum S, Pinel P, Gaillard R, Delmaire C, Perrin M, Dupont S, Dehaene S, Cohen L (2008): Pure alexia as a disconnection syndrome: New diffusion imaging evidence for an old concept. Cortex 44:962–974. [DOI] [PubMed] [Google Scholar]
- Finkbeiner M, Almeida J, Caramazza A (2006): Letter identification processes in reading: Distractor interference reveals an automatically engaged, domain‐specific mechanism. Cogn Neuropsychol 23:1083–1103. [DOI] [PubMed] [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clémenceau S, Volle E, Hasboun D, Dupont S, Baulac M, Dehaene S, Adam C (2006): Direct intracranial, fMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron 50:191–204. [DOI] [PubMed] [Google Scholar]
- Van Geemen K, Herbet G, Moritz‐Gasser S, Duffau H (2014): Limited plastic potential of the left ventral premotor cortex in speech articulation: Evidence From intraoperative awake mapping in glioma patients: Ventral premotor cortex and speech. Hum Brain Mapp 35:1587–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil‐Robles S, Carvallo A, Jimenez Mdel M, Gomez Caicoya A, Martinez R, Ruiz‐Ocana C, Duffau H (2013): Double dissociation between visual recognition and picture naming: A study of the visual language connectivity using tractography and brain stimulation. Neurosurgery 72:678–686. [DOI] [PubMed] [Google Scholar]
- Gras‐Combe G, Moritz‐Gasser S, Herbet G, Duffau H (2012): Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways: Clinical article. J Neurosurg 117:466–473. [DOI] [PubMed] [Google Scholar]
- Greenblatt SH (1976): Subangular alexia without agraphia or hemianopsia. Brain Lang 3:229–245. [DOI] [PubMed] [Google Scholar]
- Henry C, Gaillard R, Volle E, Chiras J, Ferrieux S, Dehaene S, Cohen L (2005): Brain activations during letter‐by‐letter reading: A follow‐up study. Neuropsychologia 43:1983–1989. [DOI] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Bonnetblanc F, Moritz‐Gasser S, Duffau H (2013): Is the right frontal cortex really crucial in the mentalizing network? A longitudinal study in patients with a slow‐growing lesion. Cortex 49:2711–2727. [DOI] [PubMed] [Google Scholar]
- Herbet G, Lafargue G, Bonnetblanc F, Moritz‐Gasser S, Menjot de Champfleur N, Duffau H (2014): Inferring a dual‐stream model of mentalizing from associative white matter fibres disconnection. Brain 137:944–959. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson KE (1992): The pyramids and palm trees test. Thames Valley Test Co. Thames Valley Test Company, Bury St Edmunds. [Google Scholar]
- Iragui VJ, Kritchevsky M (1991): Alexia without agraphia or hemianopia in parietal infarction. J Neurol Neurosurg Psychiatry 54:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio‐Mazoyer N (2003): Evaluation of the dual route theory of reading: A metanalysis of 35 neuroimaging studies. Neuroimage 20:693–712. [DOI] [PubMed] [Google Scholar]
- Khan OH, Herbet G, Moritz‐Gasser S, Duffau H (2014): The role of left inferior fronto‐occipital fascicle in verbal perseveration: A brain electrostimulation mapping study. Brain Topogr 27:403–411. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, de Champfleur NM, Deverdun J, Moritz‐Gasser S, Herbet G, Duffau H (2014): Role of fronto‐striatal tract and frontal aslant tract in movement and speech: An axonal mapping study. Brain Struct Funct 2014 Aug 3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H (2007): Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain 130:623–629. [DOI] [PubMed] [Google Scholar]
- Mani J, Diehl B, Piao Z, Schuele SS, Lapresto E, Liu P, Nair DR, Dinner DS, Luders HO (2008): Evidence for a basal temporal visual language center: Cortical stimulation producing pure alexia. Neurology 71:1621–627. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E (2003): Spatiotemporal dynamics of modality‐specific and supramodal word processing. Neuron 38:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JC, Newcombe F (1973): Patterns of paralexia: A psycholinguistic approach. J Psycholinguist Res 2:175–199. [DOI] [PubMed] [Google Scholar]
- Martino J, da Silva‐Freitas R, Caballero H, Marco de Lucas E, Garcia‐Porrero JA, Vazquez‐Barquero A (2013a): Fiber dissection and diffusion tensor imaging tractography study of the temporoparietal fiber intersection area. Neurosurgery 72:87–97; discussion 97–98. [DOI] [PubMed] [Google Scholar]
- Martino J, De Witt Hamer PC, Berger MS, Lawton MT, Arnold CM, de Lucas EM, Duffau H (2013b): Analysis of the subcomponents and cortical terminations of the perisylvian superior longitudinal fasciculus: A fiber dissection and DTI tractography study. Brain Struct Funct 218:105–121. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno‐Tempini M, Price C (2003): Neuroimaging studies of word and pseudoword reading: Consistencies, inconsistencies, and limitations. Cogn Neurosci J Of 15:260–271. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Ralph MAL, Patterson K, Mcclelland JL, Price CJ (2005): Dissociating reading processes on the basis of neuronal interactions. J Cogn Neurosci 17:1753–1765. [DOI] [PubMed] [Google Scholar]
- Metz‐Lutz M, Kremin H, Deloche G, Hannequin D, Ferrand I, Perrier D, Quint S, Dordain M, Bunel G, Cardebat D (1991): Standardisation d'un test de dénomination orale: Contrôle des effets de l'âge, du sexe et du niveau de scolarité chez les sujets adultes normaux. Rev Neuropsychol 1:73–95. [Google Scholar]
- Molko N, Cohen L, Mangin JF, Chochon F, Lehéricy S, Le Bihan D, Dehaene S (2002): Visualizing the neural bases of a disconnection syndrome with diffusion tensor imaging. J Cogn Neurosci 14:629–636. [DOI] [PubMed] [Google Scholar]
- Moritz‐Gasser S, Duffau H (2013): The anatomo‐functional connectivity of word repetition: Insights provided by awake brain tumor surgery. Front Hum Neurosci 7:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nespoulous J, Joanette Y, Lecours A (1992): Protocole Montréal‐Toulouse d'examen linguistique de l'aphasie. MT‐86. Module initial M1B. Lab Théophile Alajouanine Montr.
- Oldfield RC (1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Perry C, Ziegler JC, Zorzi M (2007): Nested incremental modeling in the development of computational theories: The CDP+ model of reading aloud. Psychol Rev 114:273. [DOI] [PubMed] [Google Scholar]
- Perry C, Ziegler JC, Zorzi M (2010): Beyond single syllables: Large‐scale modeling of reading aloud with the Connectionist Dual Process (CDP++) model. Cognit Psychol 61:106–151. [DOI] [PubMed] [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K (1996): Understanding normal and impaired word reading: Computational principles in quasi‐regular domains. Psychol Rev 103:56. [DOI] [PubMed] [Google Scholar]
- Price CJ (2013): Current themes in neuroimaging studies of reading. Brain Lang 125:131–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT (2003): The myth of the visual word form area. Neuroimage 19:473–481. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT (2011): The interactive account of ventral occipitotemporal contributions to reading. Trends Cogn Sci 15:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proverbio AM, Vecchi L, Zani A (2004): From orthography to phonetics: ERP measures of grapheme‐to‐phoneme conversion mechanisms in reading. J Cogn Neurosci 16:301–317. [DOI] [PubMed] [Google Scholar]
- Rech F, Herbet G, Moritz‐Gasser S, Duffau H (2014): Disruption of bimanual movement by unilateral subcortical electrostimulation: Network Subserving Bimanual Movement. Hum Brain Mapp 35:3439–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F‐E, Lubrano V, Lauwers‐Cances V, Trémoulet M, Mascott CR, Démonet J‐F(2004): Intra‐operative mapping of cortical areas involved in reading in mono‐ and bilingual patients. Brain J Neurol 127:1796–1810. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, Benedictis A, Maldonado IL, Basso G, Duffau H (2013): Frontal terminations for the inferior fronto‐occipital fascicle: Anatomical dissection, DTI study and functional considerations on a multi‐component bundle. Brain Struct Funct 218:21–37. [DOI] [PubMed] [Google Scholar]
- Schucht P, Moritz‐Gasser S, Herbet G, Raabe A, Duffau H (2013): Subcortical electrostimulation to identify network subserving motor control: Subcortical Stimulation and Motor Control. Hum Brain Mapp 34:3023–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL (1989): A distributed, developmental model of word recognition and naming. Psychol Rev 96:523. [DOI] [PubMed] [Google Scholar]
- Shallice T, Warrington E (1980): Single and multiple component central dyslexic syndromes In: Coltheart M, Patterson K, Marshall JC, editors. Deep Dyslexia. London: Routledge and Kegan Paul; pp 119–145. [Google Scholar]
- Simos PG, Breier JI, Fletcher JM, Foorman BR, Castillo EM, Papanicolaou AC (2002): Brain mechanisms for reading words and pseudowords: An integrated approach. Cereb Cortex N Y N 1991 12:297–305. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell'Acqua F, Allin M, Walshe M, Murray R, Williams SC, Murphy DGM, Catani M (2011): Atlasing location, asymmetry and inter‐subject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage 54:49–59. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Cohen L, Amemiya E, Braga LW, Dehaene S (2012): Learning to read improves the structure of the arcuate fasciculus. Cereb Cortex 24:989–995. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Vindiola M, Rapp B (2011): Patterns of brain reorganization subsequent to left fusiform damage: fMRI evidence from visual processing of words and pseudowords, faces and objects. Neuroimage 55:1357–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey T, Kawabata Duncan KJ, Price CJ, Devlin JT (2011): Top‐down modulation of ventral occipito‐temporal responses during visual word recognition. Neuroimage 55:1242–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidorreta JG, Garcia R, Moritz‐Gasser S, Duffau H (2011): Double dissociation between syntactic gender and picture naming processing: a brain stimulation mapping study. Hum Brain Mapp 32:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinckier F, Naccache L, Papeix C, Forget J, Hahn‐Barma V, Dehaene S, Cohen L (2006): “What” and “where” in word reading: ventral coding of written words revealed by parietal atrophy. J Cogn Neurosci 18:1998–2012. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L (2007): Hierarchical coding of letter strings in the ventral stream: Dissecting the inner organization of the visual word‐form system. Neuron 55:143–56. [DOI] [PubMed] [Google Scholar]
- Vogel AC, Miezin FM, Petersen SE, Schlaggar BL (2011): The putative visual word form area is functionally connected to the dorsal attention network. Cereb Cortex 22:537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA (2011): The neurobiological basis of seeing words. Ann N Y Acad Sci 1224:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Rauschecker AM, Wandell BA (2013): Anatomy of the visual word form area: Adjacent cortical circuits and long‐range white matter connections. Brain Lang 125:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi M, Houghton G, Butterworth B (1998): Two routes or one in reading aloud? A connectionist dual‐process model. J Exp Psychol Hum Percept Perform 24:1131. [Google Scholar]


