Abstract
Premature birth is associated with an increased risk of cognitive performance deficits that are dependent on working memory (WM) load in childhood. Less clear is whether preterm‐born adults show similar WM impairments, or develop compensatory brain mechanisms that help to overcome prematurity‐related functional deficits, for example, by a workload‐dependent over‐recruitment of WM‐typical areas, and/or engagement of alternative brain networks. In this functional magnetic resonance imaging study, 73 adults born very preterm and/or with very low birth weight (VP/VLBW) and 73 term‐born controls (CON, mean age: 26.5 years) performed a verbal N‐Back paradigm with varying workload (0‐back, 1‐back, 2‐back). Generally, both groups showed similar performance accuracy and task‐typical patterns of brain activations (especially in fronto‐cingulo‐parietal, thalamic, and cerebellar areas) and deactivations (especially in mesial frontal and parietal aspects of the default mode network [DMN]). However, VP/VLBW adults showed significantly stronger deactivations (P < 0.05, cluster‐level corrected) than CON in posterior DMN regions, including right ventral precuneus, and right parahippocampal areas (with adjacent cerebellar areas), which were specific for the most demanding 2‐back condition. Consistent with a workload‐dependent effect, VP/VLBW adults with stronger deactivations (1‐back > 2‐back) in the parahippocampal/cerebellar cluster also presented a greater slowing of response latencies with increasing WM load (2‐back > 1‐back), indicative of higher effort. In conclusion, VP/VLBW adults recruited similar anatomical networks as controls during N‐back performance, but showed an enhanced suppression of posterior DMN regions during higher workload, which may reflect a temporary suppression of stimulus‐independent thoughts that helps to maintain adequate task performance with increasing attentional demands. Hum Brain Mapp 36:1121–1137, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: premature birth; memory, short‐term; executive function; intelligence; functional MRI; parahippocampal gyrus; parietal lobe
Abbreviations
- ACC
Anterior cingulate cortex
- BA
Brodmann area
- BW
Birth weight
- DLPFC
Dorsolateral prefrontal cortex
- DMN
default mode network
- DNTI
Duration of Neonatal Treatment Index
- DSST
Digit Symbol Substitition Test
- EPI
echo‐planar imaging
- EP
extremely preterm
- ELBW
extremely low birth weight
- (f)MRI
(functional) magnetic resonance imaging
- FWE
family‐wise error
- FWHM
Full Width Half Maximum
- GA
Gestational age
- INTI
Intensity of Neonatal Treatment Index
- K‐ABC
Kaufman Assessment Battery for Children
- LNS
Letter Number Sequencing
- MNI
Montreal Neurological Institute
- MPRAGE
Magnetization Prepared Rapid Gradient Echo
- OPTI
Optimality Index (neonatal)
- PCC
Posterior cingulate cortex
- rs‐fMRI
resting‐state fMRI
- RT
Reaction time
- SES
Socioeconomic status
- SENSE
Sensitivity‐Encoded
- STM
Short‐term memory
- VP
very preterm
- VLBW
very low birth weight
- WAIS
Wechsler Adult Intelligence Scales
- WM
Working memory
INTRODUCTION
Being born very or extremely preterm (VP < 32 gestational weeks, EP < 28 weeks gestation) or with a very or extremely low birth weight (VLBW < 1500 g, ELBW < 1000 g) is associated with an elevated risk of perinatal brain injury and abnormal brain development, which can cause long‐term alterations of brain structure and function, and promote cognitive impairments [Baron and Rey‐Casserly, 2010; Hack, 2009; Johnson et al., 2009; Ment et al., 2009; Volpe, 2009; Wolke and Meyer, 1999]. VP/VLBW children [Bhutta et al., 2002], adolescents [Nosarti et al., 2007] and adults [Hack et al., 2002] are more likely to show lower global intelligence, and also present specific deficits in executive functions [e.g., Burnett et al., 2013; Mulder et al., 2009; Nosarti et al., 2007].
Working memory (WM) is a key aspect of executive function [e.g., Miyake et al., 2000], and prerequisite for a broad range of complex cognitive functions that we use to master everyday challenges [e.g., scholar attainments: Griffiths et al., 2013; St Clair‐Thompson and Gathercole, 2006]. There is evidence that prematurity is associated with impaired WM in children [Baron et al., 2012; Mulder et al., 2010], adolescents [Bjuland et al., 2013], and young adults [Hallin et al., 2010]. These WM deficits, combined with slower processing speed, may mediate behavioral problems and lower academic achievement in VP children [Burnett et al., 2013; Mulder et al., 2011a; Rose et al., 2011]. Meanwhile, behavioral findings are less consistent than for other executive functions [Burnett et al., 2013], which could either indicate that WM processes are less vulnerable to prematurity‐related brain alterations, or reflect compensatory mechanisms that help preterm‐born individuals to overcome existing brain dysfunctions.
The engagement of compensatory mechanisms will vary with the cognitive workload of a given task [Hillary, 2008; Just and Varma, 2007]. For example, the 4CAPS model [Just and Varma, 2007] predicts that easy tasks will primarily activate those brain regions which are most specialized and efficient for task demands while increasing task difficulty will initially be compensated by stronger activation of these task‐typical regions. Only tasks that exceed the limited processing resources of the specialized areas will cause a “spillover” to additional brain regions with complementary, but less specialized capacities (e.g., contralateral homologous structures). Although load‐dependent dynamic allocation of limited processing resources is also observed in the normal brain, brain dysfunctions further constrain available processing resources, suggesting that affected individuals will already need to recruit compensatory mechanisms at lower workload levels than healthy individuals. This may also cause an earlier breakdown of behavioral performance with increasing workload, in case that these compensatory mechanisms eventually become overstrained. Recent fMRI studies in non‐WM domains support the idea that prematurity‐related brain alterations promote the compensatory recruitment of alternative brain networks [e.g., Gimenez et al., 2005; Nosarti et al., 2006; Peterson et al., 2002]. Yet, only few studies tested the dynamics of these compensatory responses by explicitly varying the cognitive workload of task conditions: For example, Nosarti et al. [2009] found differential patterns of both enhanced and impaired activations during “easy” and “hard” verbal fluency tasks in young adults born‐preterm [see also: Barde et al., 2012]. Hence, it is highly plausible that similar workload‐dependent variations of compensatory activity exist for WM functions.
A frequent neuroimaging approach to assess WM function that typically incorporates workload manipulations is the N‐back paradigm [Gevins and Cutillo, 1993]. The task instructs participants to attend to a continuous stream of stimuli (letters, pictures, etc.) and indicate whether the current stimulus is identical to that presented n (1, 2, 3…) trials before. With increasing n, WM becomes increasingly taxed by the larger number of stimuli that need to be maintained and updated concurrently. N‐Back paradigms elicit robust brain activation increases in brain regions that are implicated in superordinate cognitive control networks [Niendam et al., 2012], including the dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal regions, the dorsal anterior cingulate (ACC), frontal pole, or medial and lateral posterior parietal areas [Owen et al., 2005; Tomasi et al., 2006]. Concurrently, they induce load‐dependent activation decreases [e.g., Prakash et al., 2012; Tomasi et al., 2006], especially in mesial regions of the “Default Mode Network” (DMN), which includes ventromedial and dorsomedial prefrontal, posterior cingulate (PCC) and ventral precuneus, inferior parietal, and (para)hippocampal and lateral temporal areas [Andrews‐Hanna et al., 2014; Buckner et al., 2008]. Whereas DMN structures frequently show enhanced activation in situations where cognitive processing is focused on self‐generated, internal mental representations [e.g., during mind‐wandering, or episodic memory retrieval: Andrews‐Hanna et al., 2014], DMN deactivations are thought to reflect an adaptive suppression of these stimulus‐independent thoughts when interfering with the cognitively demanding processing of external, task‐related stimuli [Anticevic et al., 2012; Leech and Sharp, 2014].
To date, N‐back imaging data are only available from preterm‐born children: Taylor et al. [2012] report that 7–9‐year old VP children performing a pictorial 1‐back task did not show the DLPFC and ACC activation increases observed in term‐born controls, although direct group contrasts only revealed significant reductions of right parahippocampal and left precuneus activation in the VP group. Griffiths et al. [2013] also observed reduced activation in 11‐year‐old EP/ELBW children during 1‐back and 2‐back tasks with Stroop color‐word interference stimuli, which were mainly located in occipital, supplementary motor, ACC and insular regions, and most prominent for 1‐back and 2‐back conditions where word color had to be attended (i.e., children had to suppress interference by automatically reading the word content). In both studies, lower brain activations were not [Taylor et al., 2012], or only partially [Griffiths et al., 2013] associated with performance deficits, suggesting that recruited brain resources were largely sufficient to cope with task demands.
While these data suggest that preterm‐born children performing N‐back paradigms activate WM‐related brain networks (especially frontal regions) less effectively, possibly reflecting developmental lag, it remains unknown whether this translates into adulthood, or whether preterm‐born adults develop compensatory mechanisms during later brain maturation. To address this issue, we examined a large cohort of VP/VLBW and term‐born young adults performing a verbal N‐Back fMRI paradigm. The paradigm manipulated cognitive workload by presenting a 0‐back control task, and 1‐back and 2‐back WM tasks. It was hypothesized that if VP/VLBW showed weaker behavioral performance, it would be prominent in, or restricted to, the most demanding 2‐back task: This would converge with childhood data from this cohort [Jaekel et al., 2013] which suggest that the negative impact of low gestational age (GA) is most evident for tasks with higher cognitive workload. Moreover, we expected compensatory activations to preferentially emerge with higher task demands (i.e., stronger for 2‐back than 1‐back). While N‐back studies in clinical populations frequently suggest pronounced activity changes in prototypical task‐relevant regions, consistent with higher “neural effort” [e.g., stronger activation of frontal areas: Callicott et al., 2000; enhanced deactivations in DMN areas: Philip et al., 2013], available fMRI data from other cognitive domains indicate that the altered brain development in individuals born premature may impair activation of canonical networks, and trigger compensatory recruitment of additional brain areas [e.g., Nosarti et al., 2006; Peterson et al., 2002]. As premature populations frequently show lower IQ scores [Bhutta et al., 2002], which may influence N‐back activation patterns [Gray et al., 2003], our group comparisons were controlled for this factor. Finally, to explore whether aberrant VP/VLBW activations were influenced by the degree of immaturity at birth or perinatal risk factors [e.g., Kalpakidou et al., 2012; Narberhaus et al., 2009], we examined whether the alterations in the VP/VLBW group were predicted by GA, birth weight (BW), and the extent of neonatal medical complications.
MATERIAL AND METHODS
This fMRI study is part of the prospective Bavarian Longitudinal Study, a geographically defined whole‐population study of VP/VLBW children and term‐born controls in South Bavaria, Germany [e.g., Riegel et al., 1995; Wolke and Meyer, 1999]. Their developmental status was repeatedly assessed with neurological and psychological test batteries, and parental interviews, at 5 and 20 months (corrected for prematurity), 4;8 years, 6;3 years, 8;5 years, 13 years, and most recently at 25–27 years of age, by specially trained psychologists. After completing the adult assessments, eligible participants were invited to an MRI examination (including the N‐back paradigm) at a separate date. Before entering the study, each participant was carefully screened for MR‐related contraindications (e.g., severe claustrophobia, pregnancy, ferromagnetic implants).
MRI examinations were conducted at two sites: the Department of Neuroradiology of the Klinikum Rechts der Isar, Technische Universität München, and the Department of Radiology of the University Hospital Bonn. The study was approved by local ethics committees. All participants gave written informed consent.
Participants
VP/VLBW group
VP/VLBW infants were recruited from the whole population of infants born alive in Southern Bavaria (70,600) during the period February 1, 1985, to March 31, 1986. Of 682 VP/VLBW children (GA < 32 weeks, and/or BW < 1500g), 172 died during initial hospitalization and 12 died between discharge and 25–27 year assessments. Seven parents did not give consent to participate, and 43 parents and their children were non‐German speakers and excluded as cognitive assessments could not be administered. No contact information was available for 37 VP/VLBW adults. Of the eligible 411 VP/VLBW survivors, 260 participated in adult psychological assessments. This analysis is based on 84 VP/VLBW who performed the N‐back task and completed 25–27 year cognitive assessments. One participant was excluded due to image artifacts, two were excluded due to insufficient task performance. Finally, eight had to be excluded because of excessive scan‐to‐scan movements (>2 mm). In total, the presented analysis included 73 VP/VLBW.
Term‐born controls
A comparison sample of term‐born born infants (GA > 36 weeks) was recruited from normal postnatal wards in the same obstetric hospitals. Of 350 children from the initial cohort, 229 participated in the adult assessments. This analysis is based on 80 term‐born controls with complete N‐Back and cognitive background data. Two participants were excluded because of image artifacts, while for two, response data were missing due to equipment malfunctions. Finally, three had to be excluded because of excessive scan‐to‐scan movements. In total, the presented analysis included 73 controls.
Background Characteristics
Background information for neonatal parameters, including GA, BW, standardized optimality scores for neonatal complications [OPTI: Prechtl, 1967], duration (Duration of Neonatal Treatment Index [DNTI]) and intensity (Intensity of Neonatal Treatment Index [INTI]) of neonatal intensive treatment, duration of ventilation and hospitalization, and socioeconomic status (SES) at birth, were drawn from earlier assessments [further details: Supporting Information; Gutbrod et al., 2000; Riegel et al., 1995]. Developmental cognitive measurements included Griffiths Scales of Baby Abilities [Brandt, 1983; Griffiths, 1976] at 5 and 20 months, and Kaufman‐Assessment Battery for Children [Kaufman and Kaufman, 1983; Melchers and Preuss, 1991] at 6;3 and 8;5 years. To examine dropout‐related selection biases, neonatal and developmental background parameters for the presented VP/VLBW and term‐born subsamples were compared with respective data from those participants in the initial cohort who were not included in the following analyses.
At 25–27 years, Vocabulary, Similarities, Digit Symbol Coding (Digit Symbol Substitition Test [DSST]), Block Design, Matrix Reasoning, and Letter Number Sequencing (LNS) subtests from the German WAIS‐III [von Aster et al., 2006] were administered to derive estimates for Full Scale (FSIQ), Verbal, and Performance IQ. LNS and DSST, which provide independent measures for WM and processing speed, respectively [Lezak et al., 2012], were also examined separately. Additionally, short‐term memory (STM) capacity was measured using the WAIS Digit Span forward.
Experimental Task
The task was presented using Presentation® (Neurobehavioral Systems: Albany, CA). Visual stimuli were projected onto a display in the scanner room, which was viewed through a mirror system mounted on the MR head coil. Responses were recorded with MR‐compatible button boxes.
During each task block, subjects were instructed to attend to a stream of consonants presented one at a time. During 0‐back (which provided an active control condition with minimal WM demand), participants had to respond to each presentation of the letter “X.” During 1‐back, participants had to respond when this letter was identical to the previous letter. During 2‐back, participants had to react when this letter was identical to the penultimate letter. Each task condition was presented four times, in pseudorandomized order. Each task block included 2–3 target stimuli. Task blocks (duration: 35 s) alternated with low‐level baseline blocks (fixation cross, duration: 15 s). Further details are provided in Supporting Information Fig. 1.
For each condition, percentage of correct responses (hit rate), number of false alarms, and median response latency for correct responses were coded, as well as the standard deviation of response times per condition, as an additional indicator for intraindividual response variability [Dykiert et al., 2012].
MRI Data Acquisition
At both sites, MR data were initially acquired on identical Philips Achieva 3T TX systems (Philips, Best, Netherlands), using 8‐channel SENSE head coils. Due to a scanner upgrade, Bonn had to switch to a complementary Philips Ingenia 3T system after n = 17 participants. To account for possible confounds introduced by scanner differences, functional data analyses included scanner identity as covariate.
During the experiment, 240 T2*‐weighted EPI volumes (+ five dummy scans) were acquired (TR = 2595 ms, TE = 35ms, flip angle = 90°, parallel imaging with SENSE = 2; 41 interleaved oblique axial slices, slice thickness = 3.59 mm; field of view= 230 × 230 × 147.2 mm; reconstruction matrix = 64 × 64; reconstructed voxel size = 3.593 mm3). For image registration, high‐resolution T1‐weighted 3D‐MPRAGE were acquired (TI = 1300 ms, TR = 7.7 ms, TE = 3.9 ms, flip angle = 15°; 180 sagittal slices, field of view: 256 × 256 mm, reconstructed voxel size = 13 mm3).
Statistical Analyses for Behavioral Data
Behavioral data were analyzed using SPSS 20 (IBM Corp., Armonk, NY). Frequency distributions for categorical variables were analyzed using χ 2 tests (or Fisher exact tests). Mean differences for continuous variables were analyzed with student's t‐tests for independent samples (with Welch‐Satterthwaite correction for unequal variances). Additionally, N‐back median reaction times were analyzed using mixed repeated measures ANOVA, with group (VP/VLBW vs. control) as between‐, and workload (0‐back < 1‐back < 2‐back) as within‐subject factor. Where Mauchly's test indicated sphericity violation, Greenhouse‐Geisser corrected degrees of freedom were used.
Additionally, associations between N‐Back response parameters and clinical background variables (GA, BW, OPTI) were explored using Pearson correlations.
fMRI Data Analyses
Data were analyzed using SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, UK: http://www.fil.ion.ucl.ac.uk/spm), under MATLAB 7.5 (MathWorks, Natick, MA). Spatial preprocessing of functional data included realignment and unwarping, coregistration of the T1‐weighted image with the mean EPI volume, segmentation of the T1‐weighted image using Unified Segmentation [Ashburner and Friston, 2005], application of segmentation‐derived normalization parameters to the coregistered structural and functional data, and spatial smoothing of the normalized EPI images with a Gaussian kernel (10 mm FWHM).
Functional time series were modeled using General Linear modeling [Friston et al., 1995]. The design matrix included four boxcar regressors (Instruction, 0‐back, 1‐back and 2‐back blocks), treating fixation blocks as implicit baseline. For six participants (3 VP/VLBW, 3 controls), there was a single 1‐back (and in one case, 2‐back) task block where no responses were recorded, calling into question whether they were attending to the task: here, an additional error regressor for these blocks was included as covariate of no interest. In addition, six regressors for individual realignment parameters were included to capture residual movement‐related artifacts [Friston et al., 1996]. Task‐related regressors were convolved with the SPM8 canonical hemodynamic response function. To remove slow frequency signal drifts, high‐pass filtering with 128 s cut‐off was applied. Parameter estimates were generated using Restricted Maximum‐Likelihood estimation, modeling temporal autocorrelation with an AR(1) model.
For each participant, first‐level contrasts were computed, and entered into second‐level random effect analyses [e.g., Penny et al., 2003]. To examine WM‐specific activations, both the 1‐back and 2‐back conditions were contrasted with the 0‐back condition, to control for basic sensory, motor and attentional influences. In general, “activation” will be used if the more demanding condition elicited higher activity levels (e.g., 2‐back > 0‐back: positive contrast estimates) while “deactivation” indicates that the easier condition showed higher activity levels (e.g., 0‐back > 2‐back: negative contrast estimates).
To examine load‐dependent group differences for WM‐related activation patterns, 1‐back versus 0‐back and 2‐back versus 0‐back contrast images of both groups were entered into a two‐factorial repeated measures ANOVA, using a Flexible factorial design [(see References section for further details) Gläscher and Gitelman, 2008]. As this did not allow us to control subject‐specific nuisance variables (e.g., IQ), observed interactions were scrutinized by post hoc t‐test comparisons for the 2‐back versus 1‐back contrast (i.e., activation differences between both WM conditions), which included additional covariates for scanner identity, FSIQ and response speed (operationalized by individual median RT for the 0‐back control condition), as the latter variables differed between groups (see: Results), and may influence brain activations [e.g., Gray et al., 2003; Hillary, 2008].
To explore whether activation changes in VP/VLBW adults in areas showing group differences were related to the degree of their prematurity, or severity of neonatal medical complications, follow‐up regressions in this group examined their associations with GA, BW and OPTI.
Contrast maps were thresholded at P < 0.05, family‐wise error (FWE) corrected [based on Gaussian Random field theory: Worsley et al., 1996], with cluster extent k ≥ 10. Anatomical labels for maxima were identified with TalairachClient 2.4.3 (http://www.talairach.org/daemon.html), after converting MNI (Montreal Neurological Institute) coordinates to Talairach space with icbm2tal [Lancaster et al., 2007].
RESULTS
Background Characteristics
The background characteristics of the final sample are presented in Table 1. It included 58 participants from Bonn (n = 33 VP/VLBW, n = 25 controls), and 88 from Munich (n = 40 VP/VLBW, n = 48 controls). VP/VLBW and term‐born controls did not differ in sex, age, maternal age, or SES while VP/VLBW were by definition of lower birth weight, gestation and had longer hospitalization. VP/VLBW had intensive neonatal treatment (special care), on average, for M = 54.3 days (SD 30.7), and were ventilated for M = 11.8 days (SD 16.4). Seven VP/VLBW (no term‐born controls) had cerebral palsy. Dropout analyses indicated that the GA, BW, and duration of hospitalization in both groups were not significantly different from the remaining cohort, while they showed a similar overbalance of males, and higher maternal age. Moreover, the examined VP/VLBW had a higher SES, less frequent history of cerebral palsy, received shorter ventilation, and showed lower optimality scores than the remaining VP/VLBW cohort.
Table 1.
Background characteristics: Comparison of current preterm‐born and control samples, and groupwise dropouts
| VP/VLBW | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Current sample (n = 73) | Not included (n = 338) | Within group: P | Current sample (n = 73) | Not included (n = 235) | Within group: P | Between Current samples: P | ||
| Sex: | Male | n = 44 (60%) | n = 168 (50%) | ns | n = 45 (62%) | n = 109 (46%) | <0.05 | ns |
| Female | n = 29 (40%) | n = 170 (50%) | n = 28 (38%) | n = 126 (54%) | ||||
| Age at examination (years) | 26.5 ± 0.49 | — | — | 26.51 ± 0.53 | — | — | ns | |
| Gestational age (months) | 30.3 ± 2.1 | 30.58 ± 2.34 | ns | 39.85 ± 1 | 39.58 ± 1.23 | ns | <0.001 | |
| Birth weight (grams) | 1331 ± 318 | 1296 ± 305 | ns | 3439 ± 432 | 3366 ± 449 | ns | <0.001 | |
| Maternal age (years) | 29.53 ± 4.42 | 28.3 ± 5.1 (n = 337) | <0.05 | 29.56 ± 5.18 | 28.4 ± 4.7 (n = 234) | ns | ns | |
| Socioeconomic status at birth | Upper | n = 22 (30%) | n = 59 (18%) | <0.05 | n = 25 (34%) | n = 67 (29%) | ns | ns |
| Middle | n = 32 (44%) | n = 141 (42%) | n = 29 (40%) | n = 93 (40%) | ||||
| Lower | n = 19 (26%) | n = 137 (41%) | n = 19 (26%) | n = 75 (32%) | ||||
| Optimality : neonatal | 8.75 ± 2.57 | 9.52 ± 2.71 (n = 336) | <0.05 | 0.33 ± 0.58 | 0.4 ± 0.67 | ns | <0.001 | |
| Duration of hospitalization | 71.52 ± 26.75 | 78.86 ± 38.28 | ns | 6.93 ± 3.23 | 7.34 ± 3.79 (n = 234) | ns | <0.001 | |
| Griffith Scales of Baby Abilities | 5 months | 101.3 ± 17.2 (n = 72) | 94.11 ± 20.95 (n = 312) | <0.01 | 106.2 ± 11 | 106.49 ± 10.79 | ns | <0.05 |
| 20 months | 99.31 ± 10.7 (n = 71) | 90.12 ± 22.53 (n = 299) | <0.001 | 106.4 ± 6.5 | 106.38 ± 6.72 | ns | <0.001 | |
| Kaufman Assessment Battery for Children | 6;3 years | 92.6 ± 11.5 (n = 64) | 83.98 ± 16.88 (n = 267) | <0.001 | 102.4 ± 10.6 | 99.97 ± 11.19 | ns | <0.001 |
| 8;5 years | 96.7 ± 11.2 (n = 69) | 86.01 ± 18.7 (n = 272) | <0.001 | 102.7 ± 9.6 (n = 72) | 100.36 ± 10.2 (n = 233) | ns | 0.001 | |
Within‐group analyses compared present preterm‐born (VP/VLBW) and term‐born (Controls) samples with those preterm‐born and term‐born participants, respectively, not included in this study. Between‐group analyses compared current preterm‐born and term‐born samples. For variables where data were not available for all participants, the actual group size is indicated separately.
The VP/VLBW group showed weaker global cognitive function in childhood, although dropout analyses indicated positive selection of VP/VLBW (but not controls) with better cognitive functioning (Table 1). IQ group differences were still present in adulthood (Table 2). Meanwhile, there were no significant differences for behavioral WM parameters, although VP/VLBW showed a marginally (P = 0.089) lower LNS performance, and there were also no processing speed differences in the DSST (Table 2).
Table 2.
Behavioural performance: Cognitive background measures in adulthood and fMRI task performance
| VP/VLBW (N = 73) | Controls (N = 73) | P‐value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wechsler intelligence scales | M ± SD | M ± SD | ||||||||||||
| Full scale IQ | 96 ± 12.6 | 102 ± 12.4 | <0.01 | |||||||||||
| Verbal IQ | 100.6 ± 13.9 | 105.7 ± 15.3 | <0.05 | |||||||||||
| Performance IQ | 91.4 ± 13.3 | 97.9 ± 10.3 | 0.001 | |||||||||||
| Letter number sequencing | 9.9 ± 3.1 | 10.8 ± 3 | <0.1 | |||||||||||
| Digit‐symbol substitution | 10 ± 3.6 | 10.2 ± 2.8 | ns | |||||||||||
| Digit span forward | 10.5 ± 1.9 | 10.3 ± 1.9 | ns | |||||||||||
| N‐back performance | ||||||||||||||
| Median reaction time (in msec): M (SD) | 0‐back | 498 ± 74 | 474 ± 76 | <0.1 | ||||||||||
| 1‐back | 553 ± 106 | 508 ± 87 | <0.01 | |||||||||||
| 2‐back | 614 ± 130 | 581 ± 121 | ns | |||||||||||
| Standard deviation of reaction times (in msec): M (SD) | 0‐back | 93 ± 72 | 75 ± 43 | <0.1 | ||||||||||
| 1‐back | 130 ± 82 | 113 ± 65 | ns | |||||||||||
| 2‐back | 192 ± 101 | 176 ± 88 | ns | |||||||||||
| Percentage hit rate: N (subjects) | 50% | 60% | 70% | 80% | 90% | 100% | 50% | 60% | 70% | 80% | 90% | 100% | ||
| 0‐back | 0 | 0 | 1 | 0 | 1 | 71 | 0 | 0 | 0 | 0 | 1 | 72 | ns | |
| 1‐back | 1 | 0 | 0 | 1 | 6 | 65 | 0 | 0 | 0 | 0 | 6 | 67 | ns | |
| 2‐back | 0 | 0 | 3 | 5 | 38 | 27 | 0 | 0 | 1 | 5 | 30 | 37 | ns | |
| Number of false alarms: N (subjects) | N = 0 | N = 1 | N = 2 | N = 0 | N = 1 | N = 2 | ||||||||
| 0‐back | 71 | 2 | 0 | 68 | 5 | 0 | ns | |||||||
| 1‐back | 73 | 0 | 0 | 71 | 2 | 0 | ns | |||||||
| 2‐back | 65 | 7 | 1 | 69 | 4 | 0 | ns | |||||||
Abbreviations: N – Number, M – Mean, SD – Standard deviation.
N‐Back Behavioral Performance
No group differences in task accuracy
Both groups showed similar task accuracy (Table 2). For 0‐back and 1‐back, the large majority (≥90%) in both groups showed perfect hit rates. Even collapsing values into dichotomous variables (i.e., hit rate: below 100%/100%) revealed no significant differences (both Ps > 0.7, Fisher exact test). Meanwhile, both groups showed a substantial increase of participants with reduced accuracy in the most difficult 2‐back condition, with half (or more) of the participants showing suboptimal 2‐back target detection, consistent with increased task difficulty. Although visual inspection (Table 2) suggests that there was a higher proportion of VP/VLBW participants in the lower hit rate categories for the 2‐back condition, a Cochrane‐Armitage trend test [see: Agresti, 2002] failed to reach significance (χ 2 (1) = 2.6, P = 0.11). Although post hoc dichotomization of the 2‐back accuracy data indicated that a higher proportion of VP/VLBW (63%) than controls (49%) had suboptimal 2‐back hit rates below 100%, this was not significant (χ 2 (1,146) = 2.8, P = 0.095). False alarms were generally rare, which was similar for both groups (all Fisher exact Ps > 0.2).
VP/VLBW show a general slowing of response latencies, irrespective of workload
Median reaction times showed significant main effects of workload (F (1.557,224.2) = 111.8, P < 0.001), indicating longer response latencies with increasing task difficulty across groups, and group (F (1,144) = 6.2, P = 0.014), indicating that VP/VLBW were generally responding slower than controls. Importantly, however, there was no group × workload interaction (F (2,146) = 1.15, P = 0.318), indicating that response latencies for VP/VLBW did not increase disproportionally with higher task difficulty. Similarly, the intraindividual standard deviations of response times showed a main effect of workload (F (1.858,267.553) = 73.3, P < 0.001), a marginally significant main effect of group (F (1,144) = 3.9, P = 0.051), but no group × workload interaction (F (1.858,267.553) = 0.01, P = 0.991). Results were hardly altered when additionally controlling for group differences in FSIQ.
No correlation with clinical background data
There were no significant correlations of N‐Back performance scores with clinical background variables (i.e., GA, BW, OPTI) for the VP/VLBW group.
Functional Imaging Data
General overlap of activated and deactivated brain networks
Overall, both groups showed largely similar regional patterns of activations and deactivations (P < 0.05 FWE, voxel‐wise) for the 1‐back and 2‐back WM tasks, as compared to 0‐back control task (Fig. 1). In both groups, WM tasks were associated with activation increases, especially in bilateral DLPFC, SMA, and lateral parietal areas, and (prominently for 2‐back) in the thalamus, dorsal precuneus, anterior insula, and cerebellum. Concurrently, the WM tasks induced similar, mostly bilateral, deactivations in mesial parietooccipital regions (including ventral precuneus and PCC), pregenual ACC and VMPFC, parahippocampal and angular areas, and (prominently for 2‐back) paracentral and posterior insular regions. Consistent with a workload effect, activations and deactivations in both groups were more pronounced for 2‐back, as compared to 1‐back.
Figure 1.
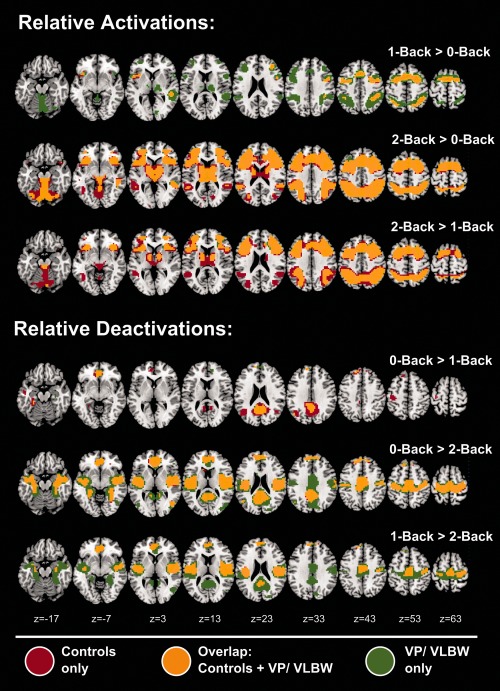
Patterns of relative activation and deactivation during 1‐back and 2‐back working memory tasks in term‐born and preterm‐born adults. The figure shows the anatomical distribution and spatial overlap of the significant activations and deactivations during the 1‐back and 2‐back WM tasks, compared with the 0‐back task (which served as a sensory, motor, and attentional control), and in direct comparison. For comparison, individual contrast maps for both groups were overlaid. Red areas: activation/deactivation for controls only. Green areas: activation/deactivation for VP/VLBW group only. Yellow areas: overlapping activation and deactivation in both groups. All group‐specific maps were threshold at a height threshold of P < 0.05, FWE corrected (voxel‐wise).
VP/VLBW show a load‐dependent enhancement of deactivation in posterior DMN regions
This workload effect was confirmed by a significant ANOVA main effect of task (Supporting Information Fig. 2): Post hoc t‐tests (controlling for additional nuisance variables) confirmed stronger 2‐back activations especially in lateral and medial fronto‐parietal, thalamic and cerebellar networks, and stronger 2‐back deactivations in posteromedial, VMPFC, medial temporal, paracentral and midcingulate, as well as posterior insular areas. While the ANOVA suggested a main effect of group for small clusters in the left insula, and right posterior ACC (Supporting Information Fig. 3), this was not confirmed by post hoc t‐tests.
Critically, there was a significant group × task interaction (P < 0.05 FWE, voxel‐level), indicating load‐dependent group differences in the right ventral precuneus (Table 3, Supporting Information Fig. 4D). Using a slightly less conservative threshold (P < 0.05 FWE, cluster‐level), this region was located in a larger midline cluster that extended into the middle occipital gyrus, and the left cuneus (Supporting Information Fig. 4C). Additionally, significant interactions emerged for a cluster that included right cerebellar vermis, posterior parahippocampal, and fusiform areas (Table 3, Supporting Information Fig. 4B). Post hoc between‐group comparisons for the differential 2‐back versus 1‐back contrast generally confirmed these findings (P < 0.05 FWE, cluster‐level), but indicated that differences concentrated on two more focal regions (Fig. 2, Table 3): The right ventral precuneus, and a cluster at the borderline between the right posterior parahippocampal region and adjacent cerebellar vermis [mainly lobule V: Diedrichsen et al., 2009]. Differential effects in both regions were driven by a stronger deactivation for 2‐back, as compared to 1‐back, in the VP/VLBW group. There were no significant activation differences indicating weaker load‐dependent deactivation for VP/VLBW, and no significant evidence for either weaker or stronger load‐dependent activation increases than controls (Table 3).
Table 3.
Location of significant load‐dependent activation differences between groups
| Cluster statistics | Submaxima (MNI coordinates) | Max. | Anatomical region | |||||
|---|---|---|---|---|---|---|---|---|
| Size (k) | P(FWE) | x | y | z | z | (Talairach daemon) | ||
| F‐test: interaction group × workload | 364 | <0.001 | 16 | −62 | 22 | 4.7 | Precuneus (BA 31, right)a | |
| 37 | −76 | 26 | 4.3 | Middle Occipital Gyrus (BA 19, right) | ||||
| 16 | −72 | 44 | 3.7 | Precuneus (BA 7, right) | ||||
| −6 | −90 | 18 | 3.6 | Cuneus (BA 18, left) | ||||
| −17 | −94 | 11 | 3.5 | Cuneus (BA 17, left) | ||||
| 12 | −58 | 51 | 3.3 | Precuneus (BA 7, right) | ||||
| 192 | 0.001 | 34 | −47 | −25 | 3.9 | Cerebellum: Culmen (right) | ||
| 19 | −44 | −10 | 3.8 | Cerebellum: Culmen (right) | ||||
| 19 | −69 | −21 | 3.8 | Cerebellum: Declive (right) | ||||
| 23 | −29 | −21 | 3.6 | Cerebellum: Culmen (right) | ||||
| 23 | −69 | −43 | 3.3 | Cerebellum: inferior semi‐lunar lobule (right) | ||||
| 16 | −69 | −39 | 3.3 | Cerebellum: inferior semi‐lunar lobule (right) | ||||
| 16 | −62 | −32 | 3.2 | Cerebellum: Nodule | ||||
| Post hoc t‐test with covariates | ||||||||
| Activations (2‐back > 1‐back) | VP/VLBW > Controls | — | — | — | — | — | — | — |
| Controls > VP/VLBW | — | — | — | — | — | — | — | |
| Deactivations (1‐back > 2‐back) | VP/VLBW > Controls | 95 | 0.037 | 16 | −62 | 22 | 4.11 | Precuneus (BA 31, right) |
| 102 | 0.030 | 19 | −44 | −14 | 3.88 | Cerebellum: Culmen (right) | ||
| 23 | −29 | −21 | 3.51 | Cerebellum: Culmen (right) | ||||
| 30 | −47 | −25 | 3.50 | Cerebellum: Culmen (right) | ||||
| Controls > VP/VLBW | — | — | — | — | — | — | — | |
This peak was also significant at P < 0.05 FWE voxel‐level. Abbreviations: VP/VLBW – Very preterm and/ or very low birth weight. FWE – Family‐wise error corrected (cluster‐level). BA – Brodmann Area.
Figure 2.
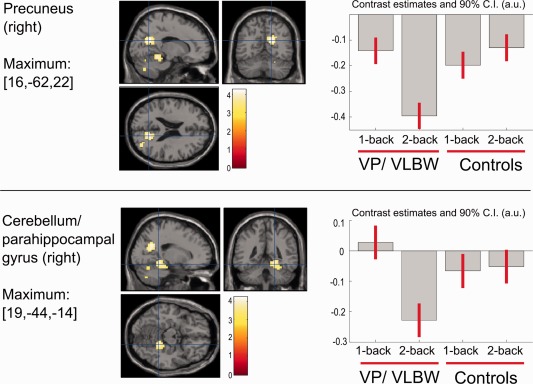
Between‐group differences for the 2‐back versus 1‐back contrast, showing significantly enhanced deactivations for preterm‐born adults during the 2‐back task. Shown are brain regions with significant group differences in the two‐sample t‐test while additionally controlling for nuisance variables (Scanner, Full Scale IQ and response speed). Contrast estimate plots showing relative activation (positive values) or deactivation (negative values) against common 0‐back control task for the local cluster maxima. In both regions, group differences were characterized by stronger deactivations in the VP/ VLBW group during the 2‐back condition. Upper panel: right ventral precuneus cluster, with peak maximum at MNI coordinate [16, −62, 22]. Lower panel: right cerebellar and parahippocampal cluster, peak maximum at MNI coordinate [19, −44, −14]. Statistical maps are presented with a voxel‐wise height threshold of P < 0.001 uncorrected, and cluster size k = 10, for display purposes. Crosshairs indicate the maxima of the two clusters surviving P < 0.05 FWE correction (cluster‐wise). A.u. – Arbitrary units.
Associations with response measures
To explore whether pronounced deactivations in the 2‐back versus 1‐back contrast were related to VP/VLBW behavioral performance exploratory, post hoc regression analyses examined their associations with 2‐back accuracy and RT measures. While there was no significant association with 2‐back accuracy, and RT variability, brain activity change for 2‐back versus 1‐back showed a moderate negative association with the corresponding percent increase in median RT from 1‐back to 2‐back within the right cerebellar‐parahippocampal cluster (surviving P < 0.05 FWE after small volume correction (SVC) for this region: Table 4; Fig. 3), indicating that those VP/VLBW who showed stronger RT slowing for 2‐back (compared to 1‐back) also presented stronger relative deactivations in this region. Complementary analyses for the controls showed no significant associations (even at a lenient voxel‐wise threshold of P < 0.01 uncorrected).
Table 4.
Brain regions where stronger deactivation (1‐back > 2‐back) was associated with larger median response time increases in the VP/VLBW group
| Cluster | Submaxima (MNI coordinates) | ||||||
|---|---|---|---|---|---|---|---|
| Contrast | Size (k) | P(FWE, SVC) | x | y | z | Maximum z | Anatomical Region (Talairach daemon) |
| 2‐back versus 1‐back contrast: negative correlation with % increase Median response time increase (1‐back to 2‐back) | 4 | 0.03 | 19 | −29 | −18 | 3.1 | Cerebellar culmen |
| 1 | 0.04 | 23 | −36 | −7 | 2.8 | Parahippocampal gyrus (BA 27) | |
Abbreviations: FWE – Family‐wise error corrected. SVC – Small volume corrected. BA – Brodmann Area
Figure 3.
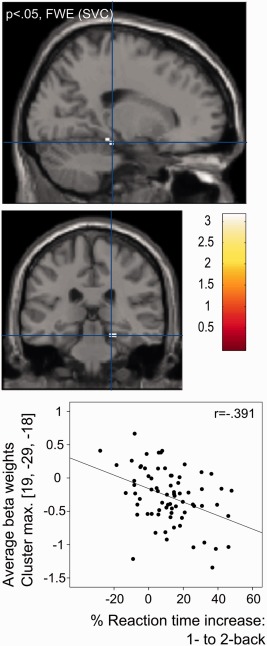
Brain regions showing group differences where stronger deactivations (1‐back > 2‐back) are associated with stronger relative 2‐back response time increases for the preterm‐born group. The upper and middle figure show those aspects of the parahippocampal‐cerebellar cluster in Figure 2 (lower panel) where stronger 2‐back < 1‐back deactivations (indicated by negative beta values) were associated with a higher percentage increase of median response times from 1‐back to 2‐back (P < 0.05 FWE cluster‐level, small volume corrected). The cluster maximum was located in MNI coordinate 19, −29, −18, at the borderline between cerebellum and parahippocampal cortex. The lower diagram shows a scatterplot of the average beta values in this cluster against the percentage increase of median reaction times (1‐back to 2‐back). Complementary analyses in controls showed no significant associations, even at liberal voxel‐wise P < 0.01 uncorrected (corresponding r [19, −29, −18] = 0.026, P < 0.8)
Associations with clinical background parameters
For the regions showing significantly stronger deactivations (i.e., 1‐back > 2‐back) in VP/VLBW, no robust associations with GA, BW, and OPTI of VP/VLBW adults were observed, even with SVC. Exploratory whole brain analyses only found positive associations with GA (P < 0.05 FWE, cluster‐level) in regions where VP/VLBW generally showed activation increases (i.e., 2‐back > 1‐back), including right inferior frontal and precentral gyrus, left and right inferior parietal lobule, and the right superior temporal gyrus, which indicated that VL/VLBW with lower GA showed weaker activation increases in these task‐related areas (Supporting Information Table 1; Supporting Information Fig. 5).
DISCUSSION
In this fMRI study, VP/VLBW and term‐born adults from an epidemiological longitudinal cohort were compared in a verbal N‐back task with varying levels of cognitive workload. Compared to term‐born controls, VP/VLBW showed no significant impairments in behavioral accuracy for the tested workload levels, and similar workload‐dependent patterns of activation increases (especially in frontal, parietal, thalamic, and cerebellar regions) and decreases (especially in DMN areas, such as PCC and ventral precuneus, MPFC, parahippocampal regions), which argue against a large‐scale reorganization of task‐relevant networks (i.e., alternative processing routes) in the N‐Back task. However, within these common networks, VP/VLBW showed enhanced deactivations in posterior DMN areas (including ventral precuneus and parahippocampal areas) that were specific for the more difficult 2‐back condition, suggesting load‐dependent differences which might reflect an adaptive mechanism engaged by VP/VLBW to cope with increasing cognitive workload.
Adults born preterm show a load‐dependent enhancement of posterior DMN deactivation
As a central observation, VP/VLBW adults showed stronger deactivations in posterior DMN regions, primarily the right ventral precuneus and right posterior parahippocampal areas, which were load‐dependent. Within the latter region, deactivation in the VP/VLBW group was also correlated with relatively slower responses during 2‐back.
Although both groups deactivated posteromedial areas during the two WM tasks (Fig. 1), VP/VLBW adults showed pronounced deactivations of the ventral precuneus in the 2‐back, as compared to the 1‐back task (Fig. 2), consistent with a workload‐dependent enhancement of this mechanism in the premature group. While the PCC is a DMN core region, the participation of the precuneus is less clear [Buckner et al., 2008]. Yet, the precise boundaries between these regions is a matter of debate [especially for the BA31 region, which is the relevant area here: Cavanna and Trimble, 2006], and resting‐state connectivity data suggest that at least the ventral precuneus participates in the DMN [Zhang and Li, 2012].
The general finding that DMN deactivation varies with cognitive workload of processed stimuli concurs with earlier observations [e.g., McKiernan et al., 2003]. Recent data suggest that DMN suppression is functionally relevant for certain types of goal‐directed cognitive tasks that direct attention to external stimuli, whereas impairments of this suppression mechanism may relate to cognitive dysfunctions in various brain disorders [Anticevic et al., 2012]. For example, previous studies suggest that stronger suppression of DMN regions (including PCC and precuneus) is associated with more successful performance in experimental tasks that emphasize the goal‐directed processing of external stimuli [e.g., Weissman et al., 2006], including WM‐related tasks [Anticevic et al., 2010]. Possibly, DMN suppression during externally‐guided tasks is necessary to filter out distraction by ongoing, stimulus‐independent thoughts [Anticevic et al., 2012]. This converges with recent theories about attentional functions of the ventral PCC [Leech and Sharp, 2014]. Thus, the observation that VP/VLBW presented enhanced deactivations in the ventral precuneus during the most demanding 2‐back condition might indicate a stronger compensatory filtering of attentional resources that is necessary to focus on the stimulus‐related WM processes, which was probably not necessary for the easier 1‐back condition. This would also concur with a study in individuals with early life stress experiences [Philip et al., 2013], who showed stronger suppression of PCC and additional DMN areas during 2‐back performance.
Complementary deactivations were found in a borderline region between the right cerebellar vermis and adjacent parahippocampal cortex (Fig. 2), which complicates reliable anatomical assignments. While there are cerebellar associations with the DMN, the closest associations exist for more posterior areas [including lobule IX and Crus I/II border area: Buckner et al., 2011]. This cluster overlap with lobule V would be more consistent with a concurrent suppression in somatomotor networks [Krienen and Buckner, 2009; O'Reilly et al., 2010]. Meanwhile, the parahippocampal cortex is implicated in the DMN [Andrews‐Hanna et al., 2014]. Here, deactivations may also reflect a compensatory downregulation, complementary to the ventral precuneus. This would converge with evidence that both regions are participating in a common medial temporal DMN subsystem [Andrews‐Hanna et al., 2010]. Consistent with this notion, deactivations in this region additionally showed a negative association with the corresponding percent increase of median RT for the VP/VLBW only (Table 4; Fig. 3). Considering that participants showed a systematic workload‐dependent RT increase toward the most difficult 2‐back condition, it seems plausible that those VP/VLBW with more pronounced RT prolongation also experienced the highest workload increment during the 2‐back task: Thus, the observation that these participants also showed a stronger downregulation of this region seems compatible with the idea of a compensatory effort. This assertion would have been further strengthened by corresponding associations with RT variability, which was occasionally reported in recent studies [Esterman et al., 2013]. We did not find support for this prediction, although methodological limitations may play a role (discussed below). Therefore, this interpretation remains tentative. Notably, the finding was specific for the VP/VLBW group, as controls did not show complementary associations, even at rather liberal statistical thresholds.
Generally, the observation that the preterm‐born adults show a stronger negative modulation of brain activity in posterior DMN structures argues against the idea that prematurity causes significant long‐term functional impairments in this brain network, as they were recently found in a variety of clinical populations [Anticevic et al., 2012]. This is broadly consistent with the available resting‐state fMRI (rs‐fMRI) literature examining DMN organization in premature populations. While earlier rs‐fMRI studies suggested that preterm‐born infants lack the fragmentary DMN organization patterns observed in term‐born infants at term‐equivalent age [Fransson et al., 2007; Smyser et al., 2010], these initial findings were not confirmed by a later study [Doria et al., 2010]. Available rs‐fMRI data from older cohorts also provide no, or only subtle evidence for long‐term DMN alterations beyond the neonatal phase: for example, Damaraju et al. examined VLBW infants at 18 or 36 months, and found no significant spatial or temporal alterations within the DMN, as compared to term‐born controls [Damaraju et al., 2010]. Recent graph‐theoretical network analyses in preadolescent children with a GA range from 29–42 weeks indicated that lower GA was associated with reduced local network efficiency in posterior medial cortex regions, and a reduced connectivity of “rich club” network structures, including the precuneus [Kim et al., 2014]: as ventral and dorsal parts of the precuneus were not separated in the analysis, it remains uncertain whether this relationship specifically holds for the ventral precuneus region that was relevant in the present study. Another study found no spatial or spectral alterations of DMN function in young VPT adults, although Granger causality analyses suggested reduced interactions with central executive and striatal salience networks [White et al., 2014]. In addition, rs‐fMRI analyses for a large adult cohort that included most participants of this sample [Bäuml et al., in press] showed both an increased functional connectivity in a dorsal part of the right precuneus, and decreased connectivity in bilateral perigenual parts of the PCC, which might indicate some functional reorganization in the posterior DMN during rest, although the relevant areas were not overlapping with the present task‐related findings, but were located in more dorsal aspects of the network.
Although the abovementioned rs‐fMRI data suggest that the spatial and functional organization of the posterior DMN is largely intact in preterm‐born individuals, their recruitment of the network may nevertheless deviate from term‐born controls in a task‐dependent manner, possibly depending on the degree of neonatal injury. For example, a recent task fMRI study [Kalpakidou et al., 2012] examined activity changes in the PCC during the retrieval of word pairs: While term‐born controls showed activation increases during recall [consistent with the general literature suggesting activity increases instead of decreases during the retrieval of episodic memory contents: Huijbers et al., 2012], this activation increase was reduced in VPT adults with no sign of neonatal brain injury, but reverted to a deactivation in VPT adults with neonatal signs of periventricular hemorrhage and ventricular dilation, despite similar task performance in the different groups. This lack of performance differences may result from low task difficulty, but could also reflect the use of a different cognitive strategy in the VPT group with neonatal injury which was associated with a down‐ (instead of up‐) regulation of the posterior DMN. Further research is needed to elucidate posterior DMN functioning in preterm populations.
Relationship to previous WM studies with children born preterm
Deactivation of posterior DMN areas
Load‐dependent deactivations in posterior DMN regions (especially, ventral precuneus) are not consistent across WM studies with preterm‐born children [Griffiths et al., 2013; Mürner‐Lavanchy et al., 2014; Taylor et al., 2012]. Taylor et al. [2012] reported reduced 1‐back activation in parahippocampal and precuneus areas in VP children (although in more rostral and dorsal locations, respectively). Unfortunately, their descriptions allow no directional inferences about whether these group differences were actually driven by weaker activations, or reflect stronger deactivations in the preterm‐born group. The fact that the other studies found no enhanced deactivations in posterior DMN regions might indicate that these children have not yet developed equivalent compensatory mechanisms as our preterm‐born adults. Moreover, their smaller sample size has possibly limited their ability to detect subtle group differences.
Deficient activation of other (task‐positive) WM‐related networks
Taylor et al. [2012] report that VP children did not show similar frontal and ACC activations as control children. While not statistically significant, this converges with Griffiths et al. [2013], who observed that EP/ELBW children showed weaker activation increases in task‐related occipital, supplementary motor, ACC, and insular regions, which might reflect deficient recruitment of these networks (although complementary behavioral deficits were only significant for their most difficult condition). In contrast, activation increases in classical fronto‐cingulo‐parietal networks [Owen et al., 2005] for our adult cohort were not significantly different from controls. Several factors may contribute to these discrepancies: First, the weaker activations in EP/ELBW children could reflect developmental lag that has resolved in adulthood. Second, Griffiths et al. [2013] utilized a complex paradigm that elicited significant behavioral deficits (at least in the most difficult condition). To some extent, the weaker brain activations may be secondary to weaker performance [Price and Friston, 1999]. Meanwhile, our paradigm was not demanding enough to provoke significant performance decrements, but examined a range where compensation was still possible. Third, Griffith et al. selected EP/ELBW children while our cohort mainly examined VP/VLBW adults (n = 16 (22%) EPT/ELBW): due to their stronger prematurity, and its stronger impact on brain development, EP/ELBW children may be less capable to activate the task‐relevant brain networks. Actually, exploratory analyses in our sample indicated that those VL/VLBW with lower GA showed weaker activation increases in right inferior frontal, and left and right inferior parietal regions for the 2‐back > 1‐back comparison, consistent with this argument (Supporting Information Fig. 5). Fourth, our VP/VLBW adults were relatively healthy and showed higher IQ performance compared to all VP/VLBW children [Bhutta et al., 2002], which could further mask prematurity‐related deficits (see below).
Compensatory activation increases
Recently, Mürner‐Lavanchy et al. [2014] examined 7–12 year old VP children with a visuo‐spatial STM retention task. They observed weaker frontal activations than in controls, but also found evidence for enhanced activation, especially in superior frontal areas that were activated by both groups. This might reflect compensatory effort, and would converge with WM studies in other clinical populations [Callicott et al., 2000; but see: Hillary, 2008]. Meanwhile, our results provide no robust evidence for compensatory activation increases. We would speculate that adult VP/VLBW have possibly developed alternative strategies, by regulating their DMN activity.
Interestingly, none of these studies found activation increases in alternative processing pathways, as discussed for other cognitive domains [e.g., Lawrence et al., 2009; Nosarti et al., 2006; Peterson et al., 2002]. WM‐relevant brain networks may either be relatively robust against prematurity‐related brain alterations, or the range for compensatory shifts to functionally equivalent areas [Just and Varma, 2007] is more restricted.
Methodological considerations
Sample size and selection
A major strength of this study is the large group size compared to most fMRI studies with premature populations [e.g., Gimenez et al., 2005; but see: Ment et al., 2006]. Larger sample sizes do not only reduce the probability of missing existing differences due to a lack of power but may also be less prone to reporting bias (i.e., tendencies to report more activation clusters than expected due to sample size) than conventional smaller studies [David et al., 2013]. Moreover, it is a distinctive feature that the VP/VLBW were not drawn from hospital‐based cohorts [e.g., Gimenez et al., 2005; Narberhaus et al., 2009], but came from an epidemiological sample [see also: Griffiths et al., 2013], which should promote the generalizability of findings.
Meanwhile, a methodological drawback is the positive selection of VP/VLBW with relatively high levels of cognitive function, and lower neonatal risks. While the average IQ in the VP/VLBW group was significantly lower, confirming previous studies [Bhutta et al., 2002], drop‐out analyses for childhood data confirmed that the VP/VLBW of the MRI sample had a higher cognitive performance level than the remaining cohort, and were also less impaired in some neonatal parameters. Positive IQ attrition is also observed in other study cohorts that followed premature populations up into adulthood [e.g., Nosarti et al., 2007], and many fMRI studies with preterm‐born participants concentrate on samples with average cognitive abilities [e.g., Lawrence et al., 2009; Narberhaus et al., 2009]. While not invalidating the results, the group differences in this cognitively (and medically) “fitter” subsample may only represent the lower boundary of what could have been observed if more impaired participants were included: possibly, individuals with stronger cognitive impairments and neonatal adversities would have presented worse N‐back performance, and also quantitatively or qualitatively different compensatory activation patterns.
Task design
Behaviorally, performance in both groups proved workload‐sensitive, as indicated by increasing response latencies, and higher proportions of participants with omissions in the most demanding 2‐back condition. Yet, neither general nor workload‐specific performance breakdown was observed in the VP/VLBW group, although they tended to miss 2‐back targets more frequently. This converges with cognitive background data that showed intact STM (Digit Span forward), and only marginally weaker WM performance (Letter‐Number‐Sequencing), and variable WM findings in other premature populations [Bjuland et al., 2013; Burnett et al., 2013; Soria‐Pastor et al., 2009]. Possibly, the positive selection of VP/VLBW with better cognitive (and presumably: WM) function has masked existing deficits.
Moreover, significant behavioral impairments may only appear at higher workload levels [Jaekel et al., 2013] that are not sufficiently taxed by our paradigm, and traditional WM measures. Indeed, Griffith et al. [2013] reported a sharper decline of response accuracy for their 2‐back (versus 1‐back) conditions, but this became significant only for the most difficult color 2‐back condition. Possibly, further workload increases would have unmasked latent VP/VLBW deficits. Adding a 3‐back condition was initially considered, but eventually discarded with the intention to apply a short paradigm that was likely to be adequately performed by most participants [see also: Griffiths et al., 2013], limiting possible performance/activation confounds that frequently complicate interpretations in patient studies [Price and Friston, 1999]. Meanwhile, future studies should consider other WM (e.g., Sternberg) paradigms that provide more flexible approaches for studying parametrical workload manipulations.
VP/VLBW showed a general slowing of response times, in line with earlier findings [Strang‐Karlsson et al., 2010]. This might indicate impaired processing speed, which was argued to mediate executive or WM problems in preterm‐born children [Mulder et al., 2011b; Rose et al., 2011]. Yet, the VP/VLBW showed no impaired DSST performance, which also measures processing speed [Lezak et al., 2012; Wechsler, 1997]. Thus, this does most likely not reflect central processing deficits, but relate to specific motor requirements of the task.
Due to the simple response requirements, and the relatively short duration of the paradigm, the behavioral parameters had to be derived from a comparably low number of target stimuli, which means that the reliability of these parameters is necessarily limited. We cannot exclude that this prevented the detection of latent group differences in RT measures. Longer experiments, especially in combination with event‐related designs, also provide better opportunities for more fine‐grained analyses of brain‐behavior relationships: For example, to further explore our observation of tonic deactivations of posterior DMN region during the most difficult 2‐back task blocks, it might be interesting to analyze the relationships between phasic fluctuations of DMN activity and attentional lapses [Weissman et al., 2006] or RT variability [Esterman et al., 2013].
Further statistical considerations
As recent ROI‐based analyses presented by Mürner‐Lavanchy et al. [2014] suggest, data distributions in preterms may show regional deviations from normal distribution, which could reduce the sensitivity of conventional parametric t‐tests. This problem may not be specific for preterm‐born individuals: actually, a methodological investigation in a large‐scale (N = 81) population of normal participants (which provided better power than usual small‐scale studies to check the underlying assumptions) found that deviations from normality were detectable in up to 30% of the examined brain voxels [Thirion et al., 2007]. Therefore, the authors suggest that nonparametric tests may generally provide a preferable analytic strategy. To examine whether this may have influenced our results, we conducted supplementary nonparametric group analyses, using the Statistical Nonparametric Mapping toolbox [SnPM Version 13.1.01: http://warwick.ac.uk/snpm; see also: Nichols and Holmes, 2001]: the analyses provided virtually identical results, suggesting that the findings of our large‐scale study were generally robust.
CONCLUSION
This fMRI study provides novel insights into the functional basis of WM function in VP/VLBW adults. The novel finding of a stronger load‐dependent deactivation in posteromedial DMN areas suggests a stronger modulation of these task‐relevant networks with increasing WM load. This points toward a stronger downregulation of interfering, internally focused thought processes which provides a plausible compensatory mechanism for preserved WM function in relatively high‐functioning preterm‐born adults, although this needs to be corroborated in future research.
Supporting information
Supplementary Information
Supplementary Information Figure 1.
Supplementary Information Figure 2.
Supplementary Information Figure 3.
Supplementary Information Figure 4.
Supplementary Information Figure 5.
Supplementary Information Table 1.
ACKNOWLEDGMENTS
We thank Prof. Hans H. Schild and Prof. Claus Zimmer for enabling this study, staff at the MR facilities in Bonn and Munich for support, and Jason Martin for helpful discussions, for supporting additional data analyses, and proofreading. Moreover, we thank all current and former Bavarian Longitudinal Study Group members who contributed to study organization, recruitment, data collection, management and analyses, including (in alphabetical order): Stephan Czeschka, Claudia Grünzinger, Julia Jäkel, Christian Koch, Diana Kurze, Sonja Perk, Andrea Schreier, Antje Strasser, Julia Trummer, and Eva van Rossum. Most importantly, we thank our study participants for their efforts to take part in this study.
Footnotes
We thank one of the anonymous reviewers for this interesting suggestion.
We thank one of the anonymous reviewers for this interesting suggestion.
REFERENCES
- Agresti A (2002): Categorical data analysis, 2nd ed. New York: Wiley. [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Smallwood J, Spreng RN (2014): The default network and self‐generated thought: Component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, Barch DM (2010): When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage 49:2638–2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X‐J, Krystal JH (2012): The role of default network deactivation in cognition and disease. Trends Cogn Sci 16:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Barde LHF, Yeatman JD, Lee ES, Glover G, Feldman HM (2012): Differences in neural activation between preterm and full term born adolescents on a sentence comprehension task: Implications for educational accommodations. Dev Cogn Neurosci 2:S114–S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron I, Rey‐Casserly C (2010): Extremely preterm birth outcome: A review of four decades of cognitive research. Neuropsychol Rev 20:430–452. [DOI] [PubMed] [Google Scholar]
- Baron IS, Kerns KA, Muller U, Ahronovich MD, Litman FR (2012): Executive functions in extremely low birth weight and late‐preterm preschoolers: Effects on working memory and response inhibition. Child Neuropsychol 18:586–599. [DOI] [PubMed] [Google Scholar]
- Bäuml JG, Daamen M, Meng C, Neitzel J, Scheef L, Jaekel J, Busch B, Baumann N, Bartmann P, Wolke D, Boecker H, Wohlschläger AM, Sorg C: Correspondence between aberrant intrinsic network connectivity and gray‐matter volume in the ventral brain of preterm born adults. Cereb Cortex (in press). doi: 10.1093/cercor/bhu133. [DOI] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ (2002): Cognitive and behavioral outcomes of school‐aged children who were born preterm: A meta‐analysis. JAMA 288:728–737. [DOI] [PubMed] [Google Scholar]
- Bjuland KJ, Løhaugen GCC, Martinussen M, Skranes J (2013): Cortical thickness and cognition in very‐low‐birth‐weight late teenagers. Early Hum Dev 89:371–380. [DOI] [PubMed] [Google Scholar]
- Brandt I (1983): Griffiths Entwicklungsskalen (GES) zur Beurteilung der Entwicklung in den ersten beiden Lebensjahren [Griffiths Developmental Scales for the first two years of life]. Weinheim: Beltz. [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL (2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT (2011): The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AC, Scratch SE, Anderson PJ (2013): Executive function outcome in preterm adolescents. Early Hum Dev 89:215–220. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR (2000): Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 10:1078–1092. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006): The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129:564–583. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Phillips J, Lowe JR, Ohls R, Calhoun VD, Caprihan A (2010): Resting‐state functional connectivity differences in premature children. Frontiers in Systems Neuroscience 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Ware JJ, Chu IM, Loftus PD, Fusar‐Poli P, Radua J, Munafó MR, Ioannidis JPA (2013): Potential reporting bias in fMRI studies of the brain. PLoS ONE 8:e70104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009): A probabilistic MR atlas of the human cerebellum. Neuroimage 46:39–46. [DOI] [PubMed] [Google Scholar]
- Doria V, Beckmann CF, Arichi T, Merchant N, Groppo M, Turkheimer FE, Counsell SJ, Murgasova M, Aljabar P, Nunes RG, Larkman DJ, Rees G, Edwards AD (2010): Emergence of resting state networks in the preterm human brain. Proc Natl Acad Sci USA 107:20015–20020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykiert D, Der G, Starr JM, Deary IJ (2012): Age differences in intra‐individual variability in simple and choice reaction time: Systematic review and meta‐analysis. PLoS ONE 7:e45759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterman M, Noonan SK, Rosenberg M, Degutis J (2013): In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cereb Cortex 23:2712–2723. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U (2007): Resting‐state networks in the infant brain. Proc Natl Acad Sci USA 104:15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2:189–210. [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R (1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35:346–355. [DOI] [PubMed] [Google Scholar]
- Gevins A, Cutillo B (1993): Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr Clin Neurophysiol 87:128–143. [DOI] [PubMed] [Google Scholar]
- Gimenez M, Junque C, Vendrell P, Caldu X, Narberhaus A, Bargallo N, Falcon C, Botet F, Mercader JM (2005): Hippocampal functional magnetic resonance imaging during a face‐name learning task in adolescents with antecedents of prematurity. Neuroimage 25:561–569. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Gitelman D (2008): Contrast weights in flexible factorial design with multiple groups of subjects (Unpublished tutorial). Available at: https://www.jiscmail.ac.uk/cgi-bin/webadmin?A2=ind0803&L=SPM&P=R16629. Accessed on 17 November 2014.
- Gray JR, Chabris CF, Braver TS (2003): Neural mechanisms of general fluid intelligence. Nat Neurosci 6:316–322. [DOI] [PubMed] [Google Scholar]
- Griffiths R (1976): The abilities of babies: A study in mental measurement. Amersham: Association of Research in Infant and Child Development. [Google Scholar]
- Griffiths ST, Gundersen H, Neto E, Elgen I, Markestad T, Aukland SM, Hugdahl K (2013): fMRI: Blood oxygen level‐dependent activation during a working memory‐selective attention task in children born extremely preterm. Pediatr Res 74:196–205. [DOI] [PubMed] [Google Scholar]
- Gutbrod T, Wolke D, Soehne B, Ohrt B, Riegel K (2000): Effects of gestation and birth weight on the growth and development of very low birthweight small for gestational age infants: A matched group comparison. Arch Dis Child Fet Neonat Ed 82:F208–F214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack M (2009): Adult outcomes of preterm children. J Dev Behav Pediatr 30:460–470. [DOI] [PubMed] [Google Scholar]
- Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N (2002): Outcomes in young adulthood for very‐low‐birth‐weight infants. N Engl J Med 346:149–157. [DOI] [PubMed] [Google Scholar]
- Hallin AL, Hellstrom‐Westas L, Stjernqvist K (2010): Follow‐up of adolescents born extremely preterm: Cognitive function and health at 18 years of age. Acta Paediatr 99:1401–1406. [DOI] [PubMed] [Google Scholar]
- Hillary FG (2008): Neuroimaging of working memory dysfunction and the dilemma with brain reorganization hypotheses. J Int Neuropsychol Soc 14:526–534. [DOI] [PubMed] [Google Scholar]
- Huijbers W, Vannini P, Sperling RA, C M P, Cabeza R, Daselaar SM (2012): Explaining the encoding/retrieval flip: Memory‐related deactivations and activations in the posteromedial cortex. Neuropsychologia 50:3764–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaekel J, Baumann N, Wolke D (2013): Effects of gestational age at birth on cognitive performance: A function of cognitive workload demands. PLoS ONE 8:e65219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Fawke J, Hennessy E, Rowell V, Thomas S, Wolke D, Marlow N (2009): Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics 124:e249–e257. [DOI] [PubMed] [Google Scholar]
- Just MA, Varma S (2007): The organization of thinking: What functional brain imaging reveals about the neuroarchitecture of complex cognition. Cogn Affect Behav Neurosci 7:153–191. [DOI] [PubMed] [Google Scholar]
- Kalpakidou AK, Allin MP, Walshe M, Giampietro V, Nam KW, McGuire P, Rifkin L, Murray RM, Nosarti C (2012): Neonatal brain injury and neuroanatomy of memory processing following very preterm birth in adulthood: An fMRI study. PLoS ONE 7:e34858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N (1983): Kaufman Assessment Battery for Children (K‐ABC). Circle Pines, MN: American Guidance Service. [Google Scholar]
- Kim D‐J, Davis EP, Sandman CA, Sporns O, O'Donnell BF, Buss C, Hetrick WP (2014): Longer gestation is associated with more efficient brain networks in preadolescent children. Neuroimage 100:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL (2009): Segregated fronto‐cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence EJ, Rubia K, Murray RM, McGuire PK, Walshe M, Allin M, Giampietro V, Rifkin L, Williams SC, Nosarti C (2009): The neural basis of response inhibition and attention allocation as mediated by gestational age. Hum Brain Mapp 30:1038–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ (2014): The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D (2012): Neuropsychological Assessment, 5th ed. New York: Oxford University Press. [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera‐Thompson J, Binder JR (2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15:394–408. [DOI] [PubMed] [Google Scholar]
- Melchers P, Preuss U (1991): K‐ABC: Kaufman Assessment Battery for Children—Deutschsprachige Fassung. Frankfurt am Main: Swets & Zeitlinger. [Google Scholar]
- Ment LR, Peterson BS, Vohr B, Allan W, Schneider KC, Lacadie C, Katz KH, Maller‐Kesselman J, Pugh K, Duncan CC, Makuch RW, Constable RT (2006): Cortical recruitment patterns in children born prematurely compared with control subjects during a passive listening functional magnetic resonance imaging task. J Pediatr 149:490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment LR, Hirtz D, Hüppi PS (2009): Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol 8:1042–1055. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD (2000): The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognit Psychol 41:49–100. [DOI] [PubMed] [Google Scholar]
- Mulder H, Pitchford NJ, Hagger MS, Marlow N (2009): Development of executive function and attention in preterm children: A systematic review. Dev Neuropsychol 34:393–421. [DOI] [PubMed] [Google Scholar]
- Mulder H, Pitchford NJ, Marlow N (2010): Processing speed and working memory underlie academic attainment in very preterm children. Arch Dis Child Fet Neonat Ed 95:F267–F272. [DOI] [PubMed] [Google Scholar]
- Mulder H, Pitchford NJ, Marlow N (2011a): Inattentive behaviour is associated with poor working memory and slow processing speed in very preterm children in middle childhood. Br J Educ Psychol 81:147–160. [DOI] [PubMed] [Google Scholar]
- Mulder H, Pitchford NJ, Marlow N (2011b): Processing speed mediates executive function difficulties in very preterm children in middle childhood. J Int Neuropsychol Soc 17:445–454. [DOI] [PubMed] [Google Scholar]
- Mürner‐Lavanchy I, Ritter BC, Spencer‐Smith MM, Perrig WJ, Schroth G, Steinlin M, Everts R (2014): Visuospatial working memory in very preterm and term born children—Impact of age and performance. Dev Cogn Neurosci 9:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus A, Lawrence E, Allin MP, Walshe M, McGuire P, Rifkin L, Murray R, Nosarti C (2009): Neural substrates of visual paired associates in young adults with a history of very preterm birth: Alterations in fronto‐parieto‐occipital networks and caudate nucleus. Neuroimage 47:1884–1893. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2001): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niendam T, Laird A, Ray K, Dean Y, Glahn D, Carter C (2012): Meta‐analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Rubia K, Smith AB, Frearson S, Williams SC, Rifkin L, Murray RM (2006): Altered functional neuroanatomy of response inhibition in adolescent males who were born very preterm. Dev Med Child Neurol 48:265–271. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Giouroukou E, Micali N, Rifkin L, Morris RG, Murray RM (2007): Impaired executive functioning in young adults born very preterm. J Int Neuropsychol Soc 13:571–581. [DOI] [PubMed] [Google Scholar]
- Nosarti C, Shergill SS, Allin MP, Walshe M, Rifkin L, Murray RM, McGuire PK (2009): Neural substrates of letter fluency processing in young adults who were born very preterm: Alterations in frontal and striatal regions. Neuroimage 47:1904–1913. [DOI] [PubMed] [Google Scholar]
- O'Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen‐Berg H (2010): Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 20:953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E (2005): N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Hum Brain Mapp 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Holmes AP, Friston KJ (2003): Random effects analysis In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Friston KJ, Price CJ, Zeki S, Ashburner J, Penny WD, editors. Human Brain Function, 2nd ed. San Diego, CA: Academic Press; pp 843–850. [Google Scholar]
- Peterson BS, Vohr B, Kane MJ, Whalen DH, Schneider KC, Katz KH, Zhang H, Duncan CC, Makuch R, Gore JC, Ment LR (2002): A functional magnetic resonance imaging study of language processing and its cognitive correlates in prematurely born children. Pediatrics 110:1153–1162. [DOI] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Price LH, Carpenter LL, Kuras Y, Clark US, Niaura RS (2013): Early life stress is associated with greater default network deactivation during working memory in healthy controls: A preliminary report. Brain Imaging Behav 7:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Heo S, Voss MW, Patterson B, Kramer AF (2012): Age‐related differences in cortical recruitment and suppression: Implications for cognitive performance. Behav Brain Res 230:192–200. [DOI] [PubMed] [Google Scholar]
- Prechtl HF (1967): Neurological sequelae of prenatal and perinatal complications. Br Med J 4:763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ (1999): Scanning patients with tasks they can perform. Hum Brain Mapp 8:102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel K, Ohrt B, Wolke D, Österlund K (1995): Die Entwicklung gefährdet geborener Kinder bis zum fünften Lebensjahr [The Development of Children Born at Risk until Their Fifth Year of Life]. Stuttgart, Germany: Ferdinand Enke Verlag. [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ (2011): Modeling a cascade of effects: The role of speed and executive functioning in preterm/full‐term differences in academic achievement. Dev Sci 14:1161–1175. [DOI] [PubMed] [Google Scholar]
- Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, Neil JJ (2010): Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 20:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria‐Pastor S, Padilla N, Zubiaurre‐Elorza L, Ibarretxe‐Bilbao N, Botet F, Costas‐Moragas C, Falcon C, Bargallo N, Mercader JM, Junque C (2009): Decreased regional brain volume and cognitive impairment in preterm children at low risk. Pediatrics 124:e1161–e1170. [DOI] [PubMed] [Google Scholar]
- St Clair‐Thompson HL, Gathercole SE (2006): Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Q J Exp Psychol 59:745–759. [DOI] [PubMed] [Google Scholar]
- Strang‐Karlsson S, Andersson S, Paile‐Hyvarinen M, Darby D, Hovi P, Raikkonen K, Pesonen AK, Heinonen K, Jarvenpaa AL, Eriksson JG, Kajantie E (2010): Slower reaction times and impaired learning in young adults with birth weight <1500 g. Pediatrics 125:e74–e82. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Donner EJ, Pang EW (2012): fMRI and MEG in the study of typical and atypical cognitive development. Neurophysiol Clin 42:19–25. [DOI] [PubMed] [Google Scholar]
- Thirion B, Pinel P, Mériaux S, Roche A, Dehaene S, Poline J‐B (2007): Analysis of a large fMRI cohort: Statistical and methodological issues for group analyses. Neuroimage 35:105–120. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L (2006): Common deactivation patterns during working memory and visual attention tasks: An intra‐subject fMRI study at 4 Tesla. Hum Brain Mapp 27:694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ (2009): Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol 8:110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Aster M, Neubauer A, Horn R (2006): Wechsler Intelligenztest für Erwachsene WIE. Deutschsprachige Bearbeitung und Adaptation des WAIS‐III von David Wechsler (2., korrigierte Auflage). Frankfurt: Pearson Assessment. [Google Scholar]
- Wechsler D (1997): WAIS‐III Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG (2006): The neural bases of momentary lapses in attention. Nat Neurosci 9:971–978. [DOI] [PubMed] [Google Scholar]
- White TP, Symington I, Castellanos NP, Brittain P, Walsh SF, Nam K‐W, Sato JR, Allin MPG, Shergill SS, Murray RM, Williams SCR, Nosarti C (2014): Dysconnectivity of neurocognitive networks at rest in very‐preterm born adults. NeuroImage Clin 4:352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolke D, Meyer R (1999): Cognitive status, language attainment, and prereading skills of 6‐year‐old very preterm children and their peers: The Bavarian Longitudinal Study. Dev Med Child Neurol 41:94–109. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4:58–73. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li C‐SR (2012): Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage 59:3548–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information Figure 1.
Supplementary Information Figure 2.
Supplementary Information Figure 3.
Supplementary Information Figure 4.
Supplementary Information Figure 5.
Supplementary Information Table 1.


