Abstract
Brain stimulation is used to induce transient alterations of neural excitability to probe or modify brain function. For example, single‐pulse transcranial magnetic stimulation (TMS) of the motor cortex can probe corticospinal excitability (CSE). Yet, CSE measurements are confounded by a high level of variability. This variability is due to physical and physiological factors. Navigated TMS (nTMS) systems can record physical parameters of the TMS coil (tilt, location, and orientation) and some also estimate intracortical electric fields (EFs) on a trial‐by‐trial basis. Thus, these parameters can be partitioned with stepwise regression.
Purpose
The primary objective was to dissociate variance due to physical parameters from variance due to physiological factors for CSE estimates. The secondary objective was to establish the predictive validity of EF estimates from spherical head models.
Hypothesis
Variability of physical parameters of TMS predicts CSE variability.
Methods
Event‐related measurements of physical parameters were analyzed in stepwise regression. Partitioned parameter variance and predictive validity were compared for a target‐controlled and a nontarget‐controlled experiment. A control experiment (preinnervation) confirmed the validity of linear data analysis. A bias‐free model quantified the effect of divergence from optimum.
Results
Partitioning physical parameter variance reduces CSE variability. EF estimates from spherical models were valid. Post hoc analyses showed that even small physical fluctuations can confound the statistical comparison of CSE measurements.
Conclusions
It is necessary to partition physical and physiological variance in TMS studies to make confounded data interpretable. The spatial resolution of nTMS is <5 mm and the EF‐estimates are valid. Hum Brain Mapp, 36:40–49, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: transcranial stimulation, human motor cortex, cortical excitability, cortical variability, motor‐evoked potentials, navigated brain stimulation
INTRODUCTION
Motor‐evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) are a well‐established measurement for quantifying changes in corticospinal excitability (CSE) as a surrogate marker of adaptive processes in the human brain [Dimyan and Cohen 2011; Rossini and Rossi 2007]. Whereas MEP onset latencies provide reliable information about neural myelination, the high variability of MEP amplitudes continues to challenge studies with a small number of subjects, for example, in single subjects or small patient populations [Schmidt et al. 2009; Wassermann 2002; Xing et al. 1990]. In addition to physiological variance, CSE variability is also seriously affected by physical variance related to the 3D parameters of the TMS coil in space [Balslev et al. 2007; Brasil‐Neto et al. 1992a, b; De Santo et al. 2007; Mills et al. 1992].
Reducing both the physical and physiological variability of CSE estimates would substantially advance the use of TMS in clinical neuroscience. Additionally, successfully partitioning out physical variance also appears to be critical for TMS studies investigating the dynamics of slow transient brain states. Physiological variability is related to tissue conductivities [Thielscher et al. 2011], brain rhythms [Sauseng et al. 2009; Thut et al. 2012], and a wide range of cognitive states [Bestmann et al. 2008; Claus et al. 1988; Darling et al. 2006; Izumi et al. 1995; Kiers et al. 1993; Mars et al. 2007] as well as peripheral sensory input [Claus et al. 1988], preinnervation [Darling et al. 2006; Hess et al. 1987], and brain dysfunction [Buch et al. 2008; Muller‐Putz and Pfurtscheller 2008; Schmidt et al. 2010]. In contrast, physical variability has been related to TMS 3D parameters in space, that is, coil location, orientation and tilt [Amassian et al. 1989; Balslev et al. 2007; Brasil‐Neto et al. 1992b; De Santo et al. 2007; Ellaway et al. 1998; Mills et al. 1992], and stimulation strength [Brasil‐Neto et al. 1992b; Darling et al. 2006; Kiers et al. 1993; Richter et al. 2013]. Interestingly, anecdotal reports suggest that confounding factors such as background prestimulus muscle activation (preinnervation) related variance can be partitioned out of TMS studies with regression methods [Darling et al. 2006; Haug et al. 1992]. Methodologically this notion is well established, for example, in functional magnetic resonance imaging [Friston 2005]. Partitioning with stepwise regression requires simultaneous event‐related recordings of, for example, physical and physiological parameters. Navigated TMS (nTMS) systems now meet this requirement and can also provide intracortical electric field (EF) estimates of induced EFEF strength. This suggests that stepwise regression algorithms can dissociate physiological (e.g. preinnervation) and physical variance (coil location, ‐tilt, ‐orientation, and EF estimates) as well as test the validity of EF‐derived stimulation strength estimates.
The EF estimates suggest that the TMS system can depict accurately the spatial extent and strength of TMS induced EFs affecting underlying neural structures. For example, an enticing notion is that a dose–response association exists between the EFEF strength estimate and the size of the MEP. Yet, the validity of EF estimates is a matter of controversy and confirmatory experimental data is lacking [Julkunen et al. 2009; Picht et al. 2011; Ruohonen and Ilmoniemi 1999; Thielscher and Wichmann 2009; Wagner et al. 2007]. Thus if software‐derived EF estimates are physiologically relevant, they should to some extent predict the size of an MEP.
In summary, we hypothesized that physical parameters predict MEP size validly, contribute independently to CSE estimate variability and that stepwise regression models can successfully partition each factor contributing to confounding variance. By comparing targeted stimulation (optimal stimulus location, orientation, and tilt parameters) with nontarget‐controlled stimulation (nonoptimal location, orientation, and tilt parameters), the contribution of each specific physical parameter associated with confounding variance can be identified, the predictive validity of EF estimates established and permissible divergence from an optimum quantified.
MATERIALS AND METHODS
Participants
Twenty‐two healthy volunteers (11 females, 11 males; average age 25 years (± 4.31)) were recruited. Prior to the experiments, subjects received a clinical examination screening to exclude any subjects with a predisposition for potential adverse effects of TMS such as neurological disease, medication use, or substance abuse. Twenty volunteers participated in the main experiments. Four subjects (3 females, 1 male; average age 25 years (±5.56), of whom two were new), participated in the control experiment (physiological variance induced by preinnervation). All subjects provided written informed consent before participating in the experiment, which was approved by the Charité – Universtitätsmedizin Berlin Ethics Commission.
Navigated Transcranial Magnetic Stimulation
TMS was performed using a focal biphasic figure‐of‐eight coil (70‐mm‐mean wing diameter) connected to a TMS system (eXimia® TMS, Nexstim, Helsinki, Finland). This system uses optical tracking to record the on‐scalp physical parameters (coil location, tilt, and orientation) with a precision of at least 1 mm. The Nexstim eXimia® system provides an aiming tool for repeated stimulation of predefined targets. With this aiming tool stimulations is only possible when the TMS coil location, orientation and tilt are adjusted accurately for a predefined target (Euclidian distance ≤2 mm, tilting and orientation accuracy ≤2°, that is, hereafter referred to as “target‐controlled stimulation”). The calculation of the intracranial EF is based on a spherical individual head model, that is, intracranial EF calculations using over 40,000 spheres that are adjusted locally to the shape and size of the individual head relative also to the physical parameters of the TMS coil in 3D space [Heller and van Hulsteyn 1992; Ravazzani et al. 1996; Ruohonen et al. 1995; Sarvas 1987; and personal communication with Nexstim]. The color‐coded EF estimates can be coregistered and collated with the individual structural magnetic resonance images (GE 3T Signa LX, MPRAGE, 1 mm3 spatial resolution) to enable simultaneous trial‐by‐trial recordings of the on‐scalp parameters as well as the intracortical EF strength estimates (target site and stimulus strength).
Electrophysiological Measures and Experimental Procedure
The stimulation target was the center of gravity (CoG) of the dominant first dorsal interosseus (FDI) muscle [Wassermann et al. 1992]. This reference spot represents the location where minimal stimulation strength elicited the largest motor responses in the contralateral FDI during systematic variation of coil tilt, orientation, and location over well‐established primary motor cortex anatomical landmarks [Rossini et al. 1994; Yousry et al. 1997]. In this study, all subjects were right‐handed as defined by the Edinburgh Handedness Inventory, that is, the left hemisphere was stimulated and electromyography (EMG) was recorded from the right FDI. Five hundred micro volts‐resting motor thresholds (500 µV‐RMT) were defined statistically with 16 MEPs and a 95% confidence interval [efficient maximum‐likelihood threshold detection, see Awiszus and Feistner 2007]. EMG activity was recorded with Neuroline 700 surface electrodes (Ambu®, Ballerup, Denmark) arranged in belly‐tendon montage. The recording device was an integral part of the stimulation system and had a sampling rate of 3,000 Hz per channel and an amplitude resolution of 0.3 µV. Samples were band‐pass filtered (10–500 Hz) by the hardware. MEPs were identified by the eXimia® software with peak‐to‐peak amplitudes. Peak‐to‐peak amplitudes <50 µV were considered to be noise. Preinnervation was defined as the area under the curve of the FDI's EMG signal from 100 to 0 ms prior to stimulation.
In experiment one (E1), 10 subjects received 100 stimuli at 500 µV‐RMT over the primary motor hand representation under “free‐choice” (nontarget‐controlled) conditions with large variations in TMS coil location and orientation. The experienced experimenter was instructed to vary TMS coil location and orientation randomly over the whole primary motor cortex based on anatomical landmarks. The borders of the presumed primary motor hand representation were further defined by a loss of MEPs in the target muscle (i.e., primary motor FDI representation). For all measurements, the test subject was informed to relax and received auditory feedback from the experimenter if the EMG trace from the target muscle exceeded 20 µV. Baseline CSE measurements were obtained with 20 stimuli under target‐controlled conditions.
In experiment two (E2), 10 subjects received 100 stimuli under identical conditions to E1 with the exception that stimulation was target‐controlled (i.e., maximum reduction of coil location, tilt, and orientation variance). The target control was set to allow for a 2 mm maximum distance from the FDI CoG. One subject was excluded due to a technical failure in the target control. In both E1 and E2, preinnervation was typically <5 µV and for all subjects <20 µV as defined by EMG measurements from the target muscle.
In contrast, in a control experiment with the aim of inducing maximal physiological confounding variance (E3), four subjects received target‐controlled stimulation under “baseline” and “preinnervation” conditions. For the preinnervation condition, the test‐subject was informed and trained to perform a pinch movement between the thumb and index finger with maximal force. In the baseline condition, the test‐subject was informed to relax (see E1 and E2). The experimental design was a blocked design with five blocks of 20 s of maximal preinnervation followed by 10 s of minimal preinnervation for the duration of 100 stimuli at 500 µV‐RMT over the FDI CoG. The interstimulus interval was randomized between 3 and 5 s in all experiments.
Signal Processing and Statistical Analyses
Signal processing was performed offline in the Matlab programming environment (MATLAB®, The MathWorks, Gatwick). Navigated parameters were described in terms of Euclidean distance (location on scalp and estimated intracortical sites of maximal EF), degrees (orientation and tilt) and volts per meter (estimated EFEF strength at intracortical target site) relative to the optimal stimulation parameters under the assumption that the curvature of the cortex follows that of the scalp and in this respect plays a secondary role for TMS studies restricted to the primary motor area. The optimal stimulation site was defined relative to the CoG, that is, an amplitude‐weighted average of stimulus locations [Wassermann et al. 1992]. The optimal stimulation orientation and tilt were, in loose analogy, calculated in the same way as the CoG and defined as the “Center of Direction” (CoD) or “Center of Tilt” (CoT). The CSE was defined by the mean value of at least 20 stimuli [Schmidt et al. 2009]. Variability was defined by the coefficient of variation (CV) to account for the interdependency between MEP amplitude and variability [Darling et al. 2006; Kiers et al. 1993]. Multivariate stepwise regression was performed under exclusion of data points larger than five standard deviations from the mean, calculated using centered and scaled data (z‐score). The regression model parameters were initiated with the response variable of MEP amplitudes per TMS event using the following predictors: coil location, tilt, orientation, estimated stimulation strength, and preinnervation per TMS event.
The notion that EF location is largely dependent on the on‐scalp parameters was confirmed by evidence for high correlation between EF location and coil location under nontargeted conditions (adjusted R2 = 82.3636, F = 454, RMSE = 1.19, P < 0.05) over all experiments, significant coil location as well as tilt correlation under targeted conditions (adjusted R2 = 61.17, F = 161, RMSE 0.41. P < 0.05 for location and tilt and R2 = 55.79, F = 251, RMSE = 0.44, P < 0.05 without tilt) and subsequent rank analyses. Rank analyses confirmed that the number of linearly independent signals was smaller than the number of signals analyzed (rank deficient). Full rank data with linearly independent signals is a prerequisite for linear regression analyses. Since EF location is derived from on‐scalp physical parameters (i.e., coil location and tilt), the latter were excluded from EF estimate regression analysis and vice versa. During stepwise regression, any predictors that could explain variance in MEP amplitudes were partitioned out of the dataset. The residuals defined the response variable (i.e., MEP amplitudes) corrected for confounding effects from the predictors (i.e., physical‐, EF parameters or preinnervation). To test the significance of correction for confounding variance, the response variable variance (CV values) were compared with two‐tailed paired t‐tests either (i) before versus after variance partitioning in any given experiment or (ii) after variance partitioning for comparisons between any given two experiments. Figure 1 depicts group‐level comparisons of the beta estimates for each predictor. Table 1 shows the reduction in CV.
Figure 1.
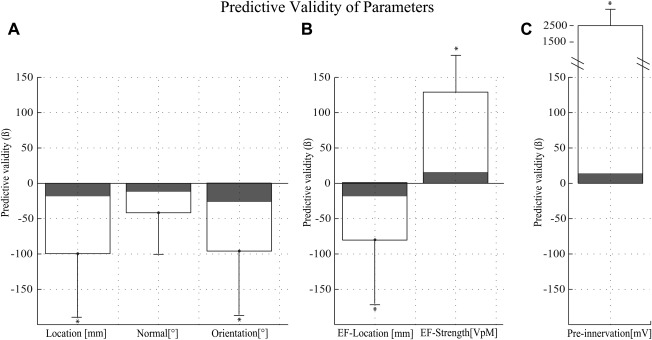
(A) Effect size (beta values) for the individual scalp physical parameters for the E1 “nontargeted” (solid lines) and E2 “targeted” (shaded) experiments. (B) Effect size (beta values) for the estimated intracortical EF strength parameters. (C) Control experiment to validate the regression model. Effect size (beta value) for stimulation without (E2, shaded) and with periods of purposely induced preinnervation (E3 “preinnervation”, solid lines) during stimulation; please note preinnervation is known to modify strongly the size of an MEP (here a more than tenfold effect). Asterisks depict a significant effect (P < 0.05, see Table 1) and evidence for predictive validity. Whiskers depict a 95% confidence interval.
Table 1.
Change in the mean coefficient of variability (CV) due to partitioned variance
| Nontargeted CV | Targeted CV | Preinnervation E3 | |||
|---|---|---|---|---|---|
| Baseline | Partitioned | Baseline | Partitioned | Baseline | Partitioned |
| 0.94 | 0.88* | 0.76 | 0.68 (*) | 0.72 | 0.42* |
| P = 0.002 | P = 0.049 | P = 0.004 | |||
E1 “nontargeted” variable (7% reduction ± 5% SEM), E2 “targeted” (5% reduction, ± 5%) and the control experiment E3 “preinnervation” (43% reduction ± 12%). Asterisks indicate a significant reduction (P < 0.05).
Furthermore, to investigate the susceptibility of CSE estimates to nonoptimal stimulation (e.g., divergence from the CoG), a simple model of targeted versus nontargeted stimulation was established. The reference space of optimal measurements from target‐controlled data ranged from 0° to 2° (CoD and CoT) or 2 mm (CoG). In comparison, nontarget‐controlled data was divided iteratively into data bins of subsequently wider ranges with steps of 1 unit of measurement: for example, from 0 to 3, 0 to 4, 0 to 5, (…) 0 to N mm or angles. Bootstrapping was performed 1,000 times for each data bin. For each bootstrap, 20 MEP amplitudes were chosen at random and the CSE was estimated for the data bin and the reference bin. These CSE estimates were compared under the hypothesis that the reference bin has higher CSE values (one‐tailed homoscedastic t‐test). Figure 2 shows the percentage of significantly different t‐tests per data bin, that is, per parameter deviance from the CoG, CoD, or CoT. The threshold for relevant difference (i.e., random chance) was defined by pairwise comparison with random events both taken only from the reference bin.
Figure 2.
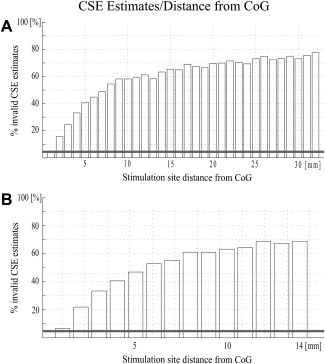
Percentage of CSE estimates that differ significantly per millimeter distance from the CoG were tested with bootstrapping (n = 1,000). Each bootstrap randomly sampled 20 values from two bins to estimate the CSE. These CSE estimates were tested for significant difference (one‐sided t‐test, P < 0.05). Thus, 1,000 CSE estimates from the reference bin (CoG +2 mm) were tested against 1,000 CSE estimates from the reference bin (the horizontal gray line depicts the results as a control for multiple comparisons) or the data bin that encompassed a subsequently larger space (CoG +3 mm, +4 mm …). (A) Results for scalp parameters and (B) intracortical location parameters.
RESULTS
Results from the regression analyses show that during nontargeted stimulation (E1), each individual scalp and intracortical parameter (except tilt, see below for Discussion) was associated significantly with the MEP size (post hoc t‐test, P ≤ 0.05, df: 9). In contrast, during target‐controlled stimulation (E2), there was no evidence for a confounding effect of any individual parameter (post hoc t‐test, P ≥ 0.05, df: 9). Results from the control experiment (E3) with purposely induced preinnervation show, as expected, that preinnervation is a strong predictor of MEP amplitude (P ≤ 0.05, df: 3), thus validating the regression analysis approach. There was no evidence that a model using intracortical parameters (RMSE E1: 285 ± 185, E2: 710 ± 298) was a less accurate predictor than a model using on‐scalp parameters (RMSE E1: 284 ± 184, E2: 703 ± 297). See Figure 1A versus B.
Surprisingly, in the target‐controlled stimulation (E2), the reduction of the CV was significant for the reduction of total variance. See Table 1. To confirm this unexpected finding, we performed a retrospective analysis of previous CSE estimates (n = 170) from other targeted experiments. The results verified a significant (P ≤ 0.05) 12.5% reduction in the CV after partitioning out the confounding physical variance. Partitioning results showed that confounding effects were mainly due to fluctuations in coil location (36%) and only to a lesser degree due to fluctuations in coil orientation (<1%), tilt (5%), or stimulus strength (<1%). See Table 2 for further details.
Table 2.
Summary of results from 170 individual CSE estimates from 20 stimuli under target‐controlled conditions in various studies
| CSE (pre) | CSE (post) | STD (pre) | STD (post) | CV (pre) | CV (post) | Confounding Parameter | |||
|---|---|---|---|---|---|---|---|---|---|
| 812.67 | 846.65 | 650.88 | 602.26 | 0.88 | 0.75 | Location | Normal | Orientation | EF strength |
| P = 0.32 | P = 0.15 | P < 0.0001* | 61/170 | 9/170 | 1/170 | 1/170 | |||
Pre‐ and postpartitioning CV, CSE, and STD values are compared. The total number of times that a parameter significantly confounded a CSE estimate is listed under “Confounding Parameter”. Asterisks indicate a significant reduction (P < 0.05) in the comparison pre versus post. Cortical excitability (CSE), Standard deviation (STD), coefficient of variability (CV), electric field (EF).
Additionally, bootstrap analyses were performed on a combined dataset of targeted and nontargeted CSE estimates to quantify the susceptibility of TMS to divergence from the optimal stimulus site, independent of experimental bias. These analyses found that even a surprisingly small divergence led to a statistically significant variance in CSE estimates. To correct for multiple comparisons, values were only considered significant if the effect size was above a threshold defined by 1,000 comparisons of two random CSE estimates both drawn from the reference bin (see horizontal line in Fig. 2). The number of significantly different comparisons within the reference bin was 5%. In contrast, the number of significantly different comparisons between the reference bin and data bins of iteratively larger spread grew as a function of the data spread (i.e., divergence from the FDI CoG). This was the case for both on‐scalp as well as intracortical location estimates. Significant differences corrected for random chance started at 2 mm (∼1%) and reached a maximum at about 10 mm (∼60%, see Fig. 2).
DISCUSSION
The objective of this study was to present new findings concerning the role of physical parameters in TMS studies. The main findings are: (1) CSE measurements are much more susceptible to smaller changes in physical parameters than might be expected from previous studies; (2) there is experimental evidence that the intracortical EFEF estimates derived from spherical models are valid in TMS studies; (3) partitioning confounding variance reduces CSE estimate variability.
MEP Susceptibility to Small Changes in Coil Location, Tilt, and Orientation
In this study, we show that trial‐by‐trial changes of on‐scalp physical parameters are associated closely with the size of a subsequent MEP. This allows us to make inferences about the spatial resolution and orientation selectivity of TMS. Although previous studies showed how strongly coil orientation [Balslev et al. 2007; Brasil‐Neto et al. 1992b; Mills et al. 1992], tilt [Amassian et al. 1989; De Santo et al. 2007], and location [Brasil‐Neto et al. 1992b; Hannula et al. 2005; Hess et al. 1987; Julkunen et al. 2009; Mills et al. 1992] can affect MEP amplitudes, this is the first study to investigate and differentiate these effects on a trial‐by‐trail basis for individual confounding factors. This is possible due to the recent introduction of navigated brain stimulation.
The location of stimulation is arguably the most critical parameter when estimating CSE in TMS studies [Julkunen et al. 2009]. The current understanding is that TMS induces a volley of action potentials, for example, that arises from a primary motor cortex area of <20 mm diameter to produce a finger movement from distributed column‐like output clusters of possibly 0.5–6 mm total diameter [Hess et al. 1987; Kwan et al. 1978; Landgren et al. 1962; Phillips 1967; Roland and Zilles 1996; Schieber 2002; Wise and Jones 1976; Yousry et al. 1997]. The primary motor cortex hand area contains multiple spatially distinct and redundant clusters of neurons [Schieber and Poliakov 1998] with higher functional divergence on the fringe of this representation responsible for variability in the strength of activation of specific lower motor neurons [Asanuma et al. 1976; Kwan et al. 1978; Patton and Amassian 1954; Phillips 1967]. The neural volley arising from TMS activates low‐threshold motor neurons similarly to the mediation of voluntary movement [Hess et al. 1987]. Activation thresholds for motor neurons producing a finger movement were found to be lowest if the induced currents were oriented longitudinally along the principle pyramidal cell axis [Phillips and Porter 1962], that is, tangential to the skull and perpendicular to the central sulcus [Amassian et al. 1992; Marin‐Padilla 1990; Mills et al. 1992; Thielscher et al. 2011]. In line with these notions of fringe variability as well as orientation selectivity, much of the variability found in brain stimulation is understood to be relative to the exact stimulus orientation and location with temporal summation of I‐waves evoked at the representation fringe [Fuhr et al. 1991; Wassermann et al. 1993].
Results from the present study suggest that the spatial resolution of nTMS is possibly as small as 2 mm. This is understood to be the case, as a location change larger than 2 mm resulted in a significant increase in variability (see Fig. 2). Previously, within the framework of fringe variability, the smallest CV on a U‐shaped curve was found at 5 mm divergence from the optimal scalp position and understood to represent the spatial resolution of TMS [Brasil‐Neto et al. 1992b]. However, this finding was limited by methodological restrictions and a floor effect resulting in a lowest‐possible value (highest spatial resolution) of 5 mm. Beyond this limitation in previous findings, the abrupt EF spatial gradient described recently (1 mm 3% reduced strength) [Stokes et al. 2005], findings from intracortical microstimulation in primates [Kwan et al. 1978; Schieber 2002] and the advent of optical navigation with better precision [Gugino et al. 2001; Thielscher et al. 2011] and the event‐related dissociation of confounding physical factors introduced in this study all support the plausibility of the smaller values, that is, higher spatial resolution, found under navigated conditions.
The orientation selectivity of stimulation in non‐nTMS studies was found to be independent of stimulus and preinnervation strength and highest between 0° and 90° [Mills et al. 1992]. Subsequent TMS studies suggested that orientation selectivity can be used to differentiate the cortical representations of individual fingers [Pascual‐Leone et al. 1994] or various neuronal assemblies [Katsuyuki et al. 1997]. In contrast, other studies found an unexpectedly large standard deviation in orientation selectivity [Balslev et al. 2007] or suggest a lack of somatotopy for finger movements in the primary motor hand representation of primates [Schieber and Poliakov 1998]. One suggestion was that this difference in orientation selectivity might be reconciled in future studies using subject‐specific stimulation relative to individual gyrification under navigated conditions [Balslev et al. 2007]. Recent modeling studies provide strong theoretical support for this notion [Thielscher et al. 2011; Thielscher and Wichmann 2009]. In contrast, somewhat surprisingly, recent navigated and non‐nTMS experiments concluded that an orientation more or less perpendicular to the central sulcus seemed to be adequate [Mills et al. 1992; Ruohonen and Karhu 2010] or argued that small fluctuations in TMS coil physical parameters are not critical [Jung et al. 2010; Mutanen et al. 2013; Rosler 2001].
We believe this is not the case. In contrast, the findings of the present study show how finely tuned the location and orientation selectivity of TMS stimulation is. Retrospective analysis of 170 previous CSE estimates confirmed that even smallest fluctuations in TMS coil orientation or location affect CSE estimates even under target‐controlled conditions. Proof‐of‐principle bootstrapping analyses with multiple random comparisons of two independent samples of reliable CSE estimates [Schmidt et al. 2009] rigorously confirmed the statistical significance of confounding physical variance.
The dissociation of confounding physical factors addresses the problem of confounding variance. Yet due to the morphological characteristics of the central sulcus [Thielscher et al. 2011], it seems clear that nonlinear interactions must be expected and that theoretical concerns of possible interaction between coil location, tilt, and orientation in MEP induction exist. Thus, the solution only holds true under the generally used assumption of linear dependency. This study does not address nonlinear interactions, segmentation of the central sulcus, nor the notion of a diffuse distribution of highly circumscribed locations with volleys evoked preferentially from different scalp sites, that is, possibly refuting classic analyses of spatial resolution [Brasil‐Neto et al. 1992b; Wilson et al. 1996]. Despite these constraints, the divergence of physical parameters validly predicted MEP amplitude variability and the control experiment confirmed the efficacy of linear data analyses.
MEP Susceptibility to Small Changes of EF Estimates
Beyond on‐scalp physical parameters, the success of nTMS is critically dependent on the validity of EFEF strength estimates relative to tissue‐specific dielectric constants at a given “peeling” depth below the scalp. One basic notion is that a dose–response association exists between EF‐strength and MEP size. However, there are two fundamental problems with estimating intracortical EFEF strength. First, these dielectric constants are neither likely truly constant nor identical in different subjects [Thielscher et al. 2011; Trillenberg et al. 2012]. Second, it remains unclear whether tissue inconsistencies along the path of stimulation only affect EF estimates insignificantly and intracortical estimates from spherical models are valid in an experimental setting [Ruohonen and Karhu 2010; Thielscher and Wichmann 2009]. A pragmatic prerequisite for use in neuroscience is the experimental proof of physiological relevance, for example, to show that changes in software‐derived EF strength estimates result in associated changes in MEP amplitude.
The findings of this study provide experimental evidence that supports the validity of software‐based sphere model EF estimates. First, we find that EF strength estimates predict MEP amplitudes. Importantly, this is the case for settings with constant maximal stimulator output (MSO) values. These findings confirm and expand on previous studies showing that EF estimates as compared to MSO values provide less variable CSE estimates [Julkunen et al. 2009] and better behavioral modifications [Bashir et al. 2011]. Second, we show that predictive models using EF location estimates instead of on‐scalp coil location and tilt parameters also perform equally well at predicting MEP variability. This confirms and expands on results showing that both coil location and tilt are associated with MEP variability [Brasil‐Neto et al. 1992b; De Santo et al. 2007], that EF estimates are dependent on coil placement, tilt, and individual tissue conductivities [Thielscher et al. 2011] and studies showing that EF‐based cartography is more accurate and reliable than non‐nTMS [Ahdab et al. 2010; Mylius et al. 2013; Picht et al. 2011]. In summary, the results provide novel experimental verification for previous theoretical approaches addressing the importance of orientation selectivity and stimulus site tissue conductivities [Ilmoniemi et al. 1999; Thielscher and Wichmann 2009; Toschi et al. 2009; Wagner et al. 2007].
The results from this study show that sphere models can provide valid EF estimates in the primary motor cortex of healthy test subjects. In contrast, if tissue conductivities are altered, for example, due to a lesion, a more complex model such as a boundary element model or finite element model might be required [Pell et al. 2011; Wagner et al. 2007]. However, the accuracy of sphere models in the presurgical evaluation of patients with pathological tissue alterations has also been established [Picht et al. 2011]. Thus, despite several possibly partially unresolved questions, we believe there is now quite conclusive evidence that supports the full use of nTMS in healthy test subjects.
Partitioning Nonphysiological and Physiological Factors Reduces Variability
Including confounding parameters in stepwise regression models reduces the variability of CSE estimates. This is unsurprising considering the results showing that precise stimulation, that is, least possible deviance from an optimal location, tilt, and orientation, is critical for reliable results [Brasil‐Neto et al. 1992b; Hess et al. 1987; Mills et al. 1992; Pell et al. 2011; Thielscher et al. 2011]. In contrast, however, a recent seminavigated (no EF estimates) study concludes that navigational systems do not improve variability and reproducibility. The authors argue that recording physical parameters and using guidance by magnetic resonance imaging are possibly beneficial but not crucial [Jung et al. 2010]. Similar arguments are found in other studies aiming to reduce EEG artifacts [Mutanen et al. 2013], TMS response variability or to separate cortical from spinal MEP variability [Rosler 2001]. The results from this study show in contrast that even minor physical variability directly influences the validity of statistical inferences and should be accounted for in future TMS studies.
Surprisingly, significant physical variance was identified even with target‐controlled stimulation. Bootstrapping procedures confirmed that this was not due to experimental bias. Thus, as stepwise regression only excludes significant confounders from the residuals, we believe it represents a fairly uncomplicated and worthwhile method that should be added to present preprocessing algorithms for TMS studies even under optimal conditions. Further studies might examine how well regression models can account for preinnervation in clinical studies or examine alternative methods that are possibly less restricted by the assumption of linearity (e.g., Monte Carlo methods or Chaos theory approaches).
Finally, these results suggest that partitioning physical from physiological variance is a prerequisite for studies of the physiological variability of brain states. Brain states are understood to reflect transient dynamic changes of ongoing cortical excitability associated with specific patterns of function and dysfunction [Buch et al. 2008; Thut et al. 2011]. The manipulation of brain states might be a relevant goal of future recovery maximization algorithms [Jackson and Zimmermann 2012; Schmidt et al. 2013; Thut et al. 2011]. Thus, on the one hand, MEP amplitude variability can be a severe limitation in brain stimulation studies [Rosler 2001; Wassermann 2002]. Yet on the other hand, CSE fluctuations are a ubiquitous phenomenon that requires further investigation [Patton and Amassian 1954; Schmidt et al. 2013; Thut et al. 2012]. In TMS studies, dissociating physical from physiological variance is a first step in this direction. This study confirmed that most of the variability in TMS studies is of physiological origin.
The main limitation in partitioning physical from physiological variance is that all physical covariates could have been biased by the experimenters' “random” choice of coil placement. This experimental bias is difficult to account for. The alternative of robotic random stimulation is technically challenging, expensive, and therefore, unlikely to be used in clinical practice. Finally, as bias‐free post hoc analyses confirmed the initial results, we consider this limitation to be minor but open for future debate.
CONCLUSION
TMS studies are more susceptible to physical parameter variance than previously described. Event‐related analyses of this susceptibility helped show that the spatial resolution of TMS is likely higher than previously expected; that EF estimates from sphere models can predict MEP amplitude in healthy test subjects validly; and that fluctuation of coil location can significantly confound CSE estimates even under target‐controlled conditions. Thus, under the assumption that covariates are linearly independent, stepwise regression might be used to partition physical from physiological variance to help make highly variable data more interpretable.
ACKNOWLEDGMENTS
The authors are grateful to K. Manske (Institut für Psychologie und Pädagogik Universität Ulm) for his constructive comments and support in the data analysis and to the German Research Foundation for their financial support.
REFERENCES
- Ahdab R, Ayache SS, Brugieres P, Goujon C, Lefaucheur JP (2010): Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiol Clin 40:27–36. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Cracco RQ, Maccabee PJ (1989): Focal stimulation of human cerebral cortex with the magnetic coil: A comparison with electrical stimulation. Electroencephalogr Clin Neurophysiol 74:401–416. [DOI] [PubMed] [Google Scholar]
- Amassian VE, Eberle L, Maccabee PJ, Cracco RQ (1992): Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain‐shaped volume conductor: The significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol 85:291–301. [DOI] [PubMed] [Google Scholar]
- Asanuma H, Arnold A, Zarzecki P (1976): Further study on the excitation of pyramidal tract cells by intracortical microstimulation. Exp Brain Res 26:443–461. [DOI] [PubMed] [Google Scholar]
- Awiszus F, Feistner H. (2007) Kortikale Reizschwelle In: Siebner H, Ziemann U., editors. Das TMS‐Buch. 1 ed Heidelberg: Springer Medizin; pp 149–158. [Google Scholar]
- Balslev D, Braet W, McAllister C, Miall RC (2007): Inter‐individual variability in optimal current direction for transcranial magnetic stimulation of the motor cortex. J Neurosci Methods 162:309–313. [DOI] [PubMed] [Google Scholar]
- Bashir S, Edwards D, Pascual‐Leone A (2011): Neuronavigation increases the physiologic and behavioral effects of low‐frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr 24:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestmann S, Harrison LM, Blankenburg F, Mars RB, Haggard P, Friston KJ, Rothwell JC (2008): Influence of uncertainty and surprise on human corticospinal excitability during preparation for action. Curr Biol 18:775–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil‐Neto JP, Cohen LG, Panizza M, Nilsson J, Roth BJ, Hallett M (1992a): Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J Clin Neurophysiol 9:132–136. [PubMed] [Google Scholar]
- Brasil‐Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG (1992b): Topographic mapping of the human motor cortex with magnetic stimulation: Factors affecting accuracy and reproducibility. Electroencephalogr Clin Neurophysiol 85:9–16. [DOI] [PubMed] [Google Scholar]
- Buch E, Weber C, Cohen LG, Braun C, Dimyan MA, Ard T, Mellinger J, Caria A, Soekadar S, Fourkas A, Birbaumer N (2008): Think to move: A neuromagnetic brain‐computer interface (BCI) system for chronic stroke. Stroke 39:910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus D, Mills KR, Murray NM (1988): The influence of vibration on the excitability of alpha motoneurones. Electroencephalogr Clin Neurophysiol 69:431–436. [DOI] [PubMed] [Google Scholar]
- Darling WG, Wolf SL, Butler AJ (2006): Variability of motor potentials evoked by transcranial magnetic stimulation depends on muscle activation. Exp Brain Res 174:376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santo G, Tanner P, Witt TN, Gebrecht V, Bötzel K (2007): Investigation of coil tilt by transcranial magnetic stimulation (TMS) of the motor cortex using a navigation system. Clin Neurophysiol 118:e23–e24. [Google Scholar]
- Dimyan MA, Cohen LG (2011): Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol 7:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway PH, Davey NJ, Maskill DW, Rawlinson SR, Lewis HS, Anissimova NP (1998): Variability in the amplitude of skeletal muscle responses to magnetic stimulation of the motor cortex in man. Electroencephalogr Clin Neurophysiol 109:104–113. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2005): Models of brain function in neuroimaging. Annu Rev Psychol 56:57–87. [DOI] [PubMed] [Google Scholar]
- Fuhr P, Cohen LG, Roth BJ, Hallett M (1991): Latency of motor evoked potentials to focal transcranial stimulation varies as a function of scalp positions stimulated. Electroencephalogr Clin Neurophysiol 81:81–89. [DOI] [PubMed] [Google Scholar]
- Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual‐Leone A, Grimson E, Weisenfeld N, Kikinis R, Shenton ME (2001): Transcranial magnetic stimulation coregistered with MRI: A comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clin Neurophysiol 112:1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula H, Ylioja S, Pertovaara A, Korvenoja A, Ruohonen J, Ilmoniemi RJ, Carlson S (2005): Somatotopic blocking of sensation with navigated transcranial magnetic stimulation of the primary somatosensory cortex. Hum Brain Mapp 26:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug BA, Schonle PW, Knobloch C, Kohne M (1992): Silent period measurement revives as a valuable diagnostic tool with transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 85:158–160. [DOI] [PubMed] [Google Scholar]
- Heller L, van Hulsteyn DB (1992): Brain stimulation using electromagnetic sources: Theoretical aspects. Biophys J 63:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NM (1987): Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol 388:397–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Ruohonen J, Karhu J (1999): Transcranial magnetic stimulation—A new tool for functional imaging of the brain. Crit Rev Biomed Eng 27:241–284. [PubMed] [Google Scholar]
- Izumi S, Findley TW, Ikai T, Andrews J, Daum M, Chino N (1995): Facilitatory effect of thinking about movement on motor‐evoked potentials to transcranial magnetic stimulation of the brain. Am J Phys Med Rehabil 74:207–213. [DOI] [PubMed] [Google Scholar]
- Jackson A, Zimmermann JB (2012): Neural interfaces for the brain and spinal cord—Restoring motor function. Nat Rev Neurol 8:690–699. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Saisanen L, Danner N, Niskanen E, Hukkanen T, Mervaala E, Kononen M (2009): Comparison of navigated and non‐navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. Neuroimage 44:790–795. [DOI] [PubMed] [Google Scholar]
- Jung NH, Delvendahl I, Kuhnke NG, Hauschke D, Stolle S, Mall V (2010): Navigated transcranial magnetic stimulation does not decrease the variability of motor‐evoked potentials. Brain Stimul 3:87–94. [DOI] [PubMed] [Google Scholar]
- Katsuyuki S, Ugawa Y, Yasuo T, Ritsuko H, Toshiaki F, Ichiro K (1997): Preferential activation of different I waves by transcranial magnetic stimulation with a figure‐of‐eight‐shaped coil. Exp Brain Res 113:24–32. [DOI] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, Fang J (1993): Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 89:415–423. [DOI] [PubMed] [Google Scholar]
- Kwan HC, MacKay WA, Murphy JT, Wong YC (1978): Spatial organization of precentral cortex in awake primates. II. Motor outputs. J Neurophysiol 41:1120–1131. [DOI] [PubMed] [Google Scholar]
- Landgren S, Phillips CG, Porter R (1962): Cortical fields of origin of the monosynaptic pyramidal pathways to some alpha motoneurones of the baboon's hand and forearm. J Physiol 161:112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin‐Padilla M (1990): Three‐dimensional structural organization of layer I of the human cerebral cortex: A Golgi study. J Comp Neurol 299:89–105. [DOI] [PubMed] [Google Scholar]
- Mars RB, Bestmann S, Rothwell JC, Haggard P (2007): Effects of motor preparation and spatial attention on corticospinal excitability in a delayed‐response paradigm. Exp Brain Res 182:125–129. [DOI] [PubMed] [Google Scholar]
- Mills KR, Boniface SJ, Schubert M (1992): Magnetic brain stimulation with a double coil: The importance of coil orientation. Electroencephalogr Clin Neurophysiol 85:17–21. [DOI] [PubMed] [Google Scholar]
- Muller‐Putz GR, Pfurtscheller G (2008): Control of an electrical prosthesis with an SSVEP‐based BCI. IEEE Trans Biomed Eng 55:361–364. [DOI] [PubMed] [Google Scholar]
- Mutanen T, Maki H, Ilmoniemi RJ (2013): The effect of stimulus parameters on TMS‐EEG muscle artifacts. Brain Stimul 6:371–376. [DOI] [PubMed] [Google Scholar]
- Mylius V, Ayache SS, Ahdab R, Farhat WH, Zouari HG, Belke M, Brugieres P, Wehrmann E, Krakow K, Timmesfeld N, Schmidt S, Oertel WH, Knake S, Lefaucheur JP (2013): Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: Inter‐rater reliability, accuracy, and influence of gender and age. Neuroimage 78:224–232. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Cohen LG, Brasil‐Neto JP, Hallett M (1994): Non‐invasive differentiation of motor cortical representation of hand muscles by mapping of optimal current directions. Electroencephalogr Clin Neurophysiol 93:42–48. [DOI] [PubMed] [Google Scholar]
- Patton HD, Amassian VE (1954): Single and multiple‐unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol 17:345–363. [DOI] [PubMed] [Google Scholar]
- Pell GS, Roth Y, Zangen A (2011): Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol 93:59–98. [DOI] [PubMed] [Google Scholar]
- Phillips CG (1967): Corticomotoneuronal organization. Projection from the arm area of the baboon's motor cortex. Arch Neurol 17:188–195. [DOI] [PubMed] [Google Scholar]
- Phillips CG, Porter R (1962): Unifocal and bifocal stimulation of the motor cortex. J Physiol 162:532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picht T, Schmidt S, Brandt S, Frey D, Hannula H, Neuvonen T, Karhu J, Vajkoczy P, Suess O (2011): Preoperative functional mapping for rolandic brain tumor surgery: Comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery 69:581–588; discussion 588. [DOI] [PubMed] [Google Scholar]
- Ravazzani P, Ruohonen J, Grandori F, Tognola G (1996): Magnetic stimulation of the nervous system: Induced electric field in unbounded, semi‐infinite, spherical, and cylindrical media. Ann Biomed Eng 24:606–616. [DOI] [PubMed] [Google Scholar]
- Richter L, Trillenberg P, Schweikard A, Schlaefer A (2013): Stimulus intensity for hand held and robotic transcranial magnetic stimulation. Brain Stimul 6:315–321. [DOI] [PubMed] [Google Scholar]
- Roland PE, Zilles K (1996): Functions and structures of the motor cortices in humans. Curr Opin Neurobiol 6:773–781. [DOI] [PubMed] [Google Scholar]
- Rosler KM (2001): Transcranial magnetic brain stimulation: A tool to investigate central motor pathways. News Physiol Sci 16:297–302. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S (2007): Transcranial magnetic stimulation: Diagnostic, therapeutic, and research potential. Neurology 68:484–488. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C (1994): Non‐invasive electrical and magnetic stimulation of the brain, spinal cord and roots: Basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91:79–92. [DOI] [PubMed] [Google Scholar]
- Ruohonen J, Ilmoniemi RJ (1999): Modeling of the stimulating field generation in TMS. Electroencephalogr Clin Neurophysiol Suppl 51:30–40. [PubMed] [Google Scholar]
- Ruohonen J, Karhu J (2010): Navigated transcranial magnetic stimulation. Neurophysiol Clin 40:7–17. [DOI] [PubMed] [Google Scholar]
- Ruohonen J, Ravazzani P, Grandori F (1995): An analytical model to predict the electric field and excitation zones due to magnetic stimulation of peripheral nerves. IEEE Trans Biomed Eng 42:158–161. [DOI] [PubMed] [Google Scholar]
- Sarvas J (1987): Basic mathematical and electromagnetic concepts of the biomagnetic inverse problem. Phys Med Biol 32:11–22. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gerloff C, Hummel FC (2009): Spontaneous locally restricted EEG alpha activity determines cortical excitability in the motor cortex. Neuropsychologia 47:284–288. [DOI] [PubMed] [Google Scholar]
- Schieber MH (2002): Motor cortex and the distributed anatomy of finger movements. Adv Exp Med Biol 508:411–416. [DOI] [PubMed] [Google Scholar]
- Schieber MH, Poliakov AV (1998): Partial inactivation of the primary motor cortex hand area: Effects on individuated finger movements. J Neurosci 18:9038–9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S, Cichy RM, Kraft A, Brocke J, Irlbacher K, Brandt SA (2009): An initial transient‐state and reliable measures of corticospinal excitability in TMS studies. Clin Neurophysiol 120:987–993. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Holst E, Irlbacher K, Oltmanns F, Merschhemke M, Brandt SA (2010): A case of pathological excitability located with navigated‐TMS: Presurgical evaluation of focal neocortical epilepsy. Restor Neurol Neurosci 28:379–385. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Scholz M, Obermayer K, Brandt SA (2013): Patterned Brain Stimulation, What a Framework with Rhythmic and Noisy Components Might Tell Us about Recovery Maximization. Front Hum Neurosci 7:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, Mattingley JB (2005): Simple metric for scaling motor threshold based on scalp‐cortex distance: Application to studies using transcranial magnetic stimulation. J Neurophysiol 94:4520–4527. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Wichmann FA (2009): Determining the cortical target of transcranial magnetic stimulation. Neuroimage 47:1319–1330. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Opitz A, Windhoff M (2011): Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage 54:234–243. [DOI] [PubMed] [Google Scholar]
- Thut G, Miniussi C, Gross J (2012): The functional importance of rhythmic activity in the brain. Curr Biol 22:R658–R663. [DOI] [PubMed] [Google Scholar]
- Thut G, Veniero D, Romei V, Miniussi C, Schyns P, Gross J (2011): Rhythmic TMS causes local entrainment of natural oscillatory signatures. Curr Biol 21:1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi N, Welt T, Guerrisi M, Keck ME (2009): Transcranial magnetic stimulation in heterogeneous brain tissue: Clinical impact on focality, reproducibility and true sham stimulation. J Psychiatr Res 43:255–264. [DOI] [PubMed] [Google Scholar]
- Trillenberg P, Bremer S, Oung S, Erdmann C, Schweikard A, Richter L (2012): Variation of stimulation intensity in transcranial magnetic stimulation with depth. J Neurosci Methods 211:185–190. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero‐Cabre A, Pascual‐Leone A (2007): Noninvasive human brain stimulation. Annu Rev Biomed Eng 9:527–565. [DOI] [PubMed] [Google Scholar]
- Wassermann EM (2002): Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol 113:1165–1171. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG (1992): Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol 85:1–8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Pascual‐Leone A, Valls‐Sole J, Toro C, Cohen LG, Hallett M (1993): Topography of the inhibitory and excitatory responses to transcranial magnetic stimulation in a hand muscle. Electroencephalogr Clin Neurophysiol 89:424–433. [DOI] [PubMed] [Google Scholar]
- Wilson SA, Day BL, Thickbroom GW, Mastaglia FL (1996): Spatial differences in the sites of direct and indirect activation of corticospinal neurones by magnetic stimulation. Electroencephalogr Clin Neurophysiol 101:255–261. [DOI] [PubMed] [Google Scholar]
- Wise SP, Jones EG (1976): The organization and postnatal development of the commissural projection of the rat somatic sensory cortex. J Comp Neurol 168:313–343. [DOI] [PubMed] [Google Scholar]
- Xing J, Katayama Y, Yamamoto T, Hirayama T, Tsubokawa T (1990): Quantitative evaluation of hemiparesis with corticomyographic motor evoked potential recorded by transcranial magnetic stimulation. J Neurotrauma 7:57–64. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P (1997): Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120 (Pt 1):141–157. [DOI] [PubMed] [Google Scholar]


