ABSTRACT
Two experiments investigated the effects of lysophospholipid (LPL) supplementation on low-energy and low-nitrogenous diets for broilers. A total of 300 one-day-old male chicks (Ross 308) was allotted to 5 treatments in a completely randomized design. Each group consisted of 6 replicates with 10 birds each. Experimental diet I included positive control (PC) having 3,025 (starter), 3,150 (grower), and 3,200 kcal/kg (finisher) of ME; negative control (NC) was 150 kcal/kg of ME lower than PC, and LPL-05, LPL-10, and LPL-15 treatments were NC + 0.05%, 0.10%, and 0.15% of LPL supplementation, respectively. Experimental diet II included positive control (PC) having a formulated amount of crude protein including Lys and Met + Cys that met the Ross 308 standards; negative control (NC) was 4% lower CP and AA than PC; other treatments were supplemented with LPL at 0.05% (LPL-05), 0.10% (LPL-10), and 0.15% (LPL-15) into the NC, respectively. Experiment I showed that growth performance linearly increased as the LPL inclusion increased (P < 0.001). Broilers fed LPL-10 and LPL-15 increased digestibility of DM (P < 0.05), crude protein (P < 0.01), and total amino acids (P < 0.01) compared to NC. Serum glucose (P < 0.01) and high-density lipoprotein (P < 0.05) concentrations were greater in groups fed LPL-10 than those fed PC. Furthermore, leg muscle increased in birds fed LPL-10 compared with NC (P < 0.05). Experiment II observed a linear response to LPL supplementation in the whole period, in terms of body weight gain (P = 0.015) and feed conversion ratio (P = 0.027). Feeding of 0.15% LPL had promising effects on digestibility of crude protein and ether extract compared with NC (P < 0.01 and P < 0.05, respectively). Overall, LPL could be considered as a feed additive to reduced energy (−150 kcal/kg) or nitrogenous diets (−5%) in order to improve growth performance and nutrient digestibility without adverse effects on lymphoid organs and hepatic enzyme of broilers.
Keywords: lower nutrient diets, lysophospholipid, growth performance, nutrient digestibility, broiler
INTRODUCTION
Feed cost has gradually increased in poultry production worldwide. One approach to minimizing production cost is dietary manipulation of nutrient supplies through improved feed efficiency. However, many reports have shown that undersupply of nutrients could retard growth performance and lead to metabolic disorders (Bregendahl et al., 2002; Kiess et al., 2013). For example, early restriction of nutrients may result in subsequent retarded growth rate of broilers (Butzen et al., 2015). In addition, the birds will over consume to meet their nutrient requirements, leading to increased carcass fat accumulation and feed conversion ratio when limited amounts of nutrients are supplemented (Thomas et al., 1978). In order to prevent detrimental effects of nutrient restrictions and maintain bird performance, interest has grown recently in exogenous feed.
Lysophospholipid (LPL) is a more effective biosurfactant in emulsifying properties than bile salts and soy lecithin because one molecule in the hydrophobic tail is removed (Joshi et al., 2006), indicating more stability in the aqueous environment of the gastrointestinal tract. The beneficial effects of LPL supplementation have been shown to increase the uptake of nutrients, both lipophilic and non-lipophilic substances (Schwarzer and Adams, 1996; Lundbæk et al., 2010). It has been established that LPL is present in cell membranes at higher concentrations to activate the permeability of cell membranes and the formation of ion channels (Maingret et al., 2000; Lundbæk et al., 2010). This mechanism allows the influx of large and small molecules to enter across the cell membrane (Lundbæk and Andersen, 1994). In addition, the lecithin contains smaller micelles than bile salts (Schwarzer and Adams, 1996). Simultaneously delivering free fatty acids into the surface of the absorptive cells leads to increased nutrient absorption. Other functions of modified lecithins, such as modulating T-lymphocytes (Asaoka et al., 1992) and preventing cellular damage, have been demonstrated (Skoura and Hla, 2009). In poultry, Huang et al. (2008) demonstrated that broilers fed 2% soy-lecithin for 42 d lowered concentrations of total cholesterol and low-density lipoprotein cholesterol. Zhang et al. (2011) also observed that inclusion of modified lecithin product at 0.5 g/kg significantly increased fatty acid digestibility and increased metabolizable energy (ME). Additionally, Boontiam et al. (2016) demonstrated that LPL showed significantly reduction in inflammatory responses when broilers were fed diets with lower nutrient density. In laying hens, Han et al. (2010) showed that the inclusion of lysolecithin at 0.10% improved albumen quality. However, there are limited reports investigating the effects of adding LPL biomaterials to low-nutrient diets (Zhao et al., 2015). Consequently, the present study was designed to investigate the effects of dietary LPL supplementation to reduced energy (Experiment I, Exp. I), and CP including essential amino acids (Experiment II, Exp. II) on growth performance, nutrient digestibility, and blood profiles of broiler chickens.
MATERIALS AND METHODS
Bird care and handling were performed in accordance with the Animal Ethics Committee approved by Seoul National University, South Korea.
Birds, Experimental Design, diet, and Management
Two experiments were carried out at a university research farm of Seoul National University (Suwon, South Korea). Three-hundred one-day-old male chicks (Ross 308) were purchased from a commercial hatchery (Busung, South Korea). The chicks were weighed individually before being allotted into 5 treatment groups in a completely randomized design. Each treatment had 6 replicates, consisting 10 birds each, which were similar in both studies.
The 5 dietary treatments for Exp. I consisted of positive control (PC) comprising 3,025 (starter period), 3,150 (grower period), and 3,200 kcal/kg of ME (finisher period); negative control (NC) was 150 kcal/kg of ME lower in each period than PC, NC + 0.05% LPL (LPL-05), NC + 0.10% LPL (LPL-10), and NC + 0.15% LPL (LPL-15), as shown in Table 1.
Table 1.
Formula and chemical composition of diets in Experiment I (%, as-fed basis)
| Starter period | Grower period | Finisher period | ||||
|---|---|---|---|---|---|---|
| Ingredients | PC | NC | PC | NC | PC | NC |
| Corn small particle-500 | 54.85 | 55.99 | 40.65 | 42.14 | 30.60 | 31.97 |
| Corn coarse particle-325 | 0.00 | 0.00 | 15.00 | 15.00 | 30.00 | 30.00 |
| Soybean meal (45%) | 34.99 | 34.18 | 33.35 | 32.57 | 28.47 | 27.68 |
| Wheat bran | 1.00 | 2.97 | 1.00 | 2.68 | 1.00 | 2.78 |
| Fish meal | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Soybean oil | 3.32 | 1.00 | 5.11 | 2.70 | 5.28 | 2.90 |
| L-lysine sulfate (55%) | 0.42 | 0.43 | 0.15 | 0.17 | 0.12 | 0.14 |
| DL-methionine (98%) | 0.39 | 0.39 | 0.29 | 0.29 | 0.25 | 0.25 |
| Threonine (98%) | 0.12 | 0.12 | 0.04 | 0.04 | 0.03 | 0.03 |
| Choline chloride-50% | 0.06 | 0.06 | 0.05 | 0.05 | 0.05 | 0.05 |
| Dicalcium phosphate | 2.12 | 2.14 | 1.86 | 1.87 | 1.72 | 1.73 |
| Limestone meal | 1.10 | 1.09 | 0.87 | 0.86 | 0.85 | 0.84 |
| Salt | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 |
| NaHCO3 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 |
| Vitamin premix1 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| Mineral premix2 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated compositions3 | ||||||
| ME (kcal/kg) | 3,025 | 2,875 | 3,150 | 3,000 | 3,200 | 3,050 |
| CP (%) | 22.00 | 22.00 | 21.00 | 21.00 | 19.00 | 19.00 |
| Ca (%) | 1.05 | 1.05 | 0.90 | 0.90 | 0.85 | 0.85 |
| Available P (%) | 0.78 | 0.79 | 0.73 | 0.74 | 0.68 | 0.69 |
| Lys (%) | 1.43 | 1.43 | 1.24 | 1.24 | 1.09 | 1.09 |
| Met+cys (%) | 1.07 | 1.07 | 0.95 | 0.95 | 0.86 | 0.86 |
| Thr (%) | 0.94 | 0.94 | 0.83 | 0.83 | 0.74 | 0.74 |
| Trp (%) | 0.26 | 0.26 | 0.25 | 0.25 | 0.22 | 0.22 |
| Analyzed compositions4 | ||||||
| CP (%) | 21.95 | 22.03 | 21.20 | 21.03 | 18.81 | 19.05 |
| Moisture (%) | 10.31 | 10.59 | 10.72 | 11.21 | 10.98 | 10.36 |
| Ash (%) | 5.11 | 5.30 | 5.10 | 4.28 | 5.04 | 5.61 |
| Ether extract (%) | 4.82 | 3.14 | 4.35 | 4.76 | 5.35 | 3.69 |
Provided per kilogram of diet: 8,000 IU of vitamin A (retinyl acetate); 1,600 IU of vitamin D3; 60 mg of vitamin E (dl-α-tocopheryl acetate); 3 mg of vitamin B1; 3 mg of vitamin K; 8 mg of vitamin B2; 4 mg of vitamin B6; 16 μg of vitamin B12; 60 mg of niacin; 2 mg of folic acid; 130 μg of biotin; and 20 mg of calcium pantothenic acid.
Provided per kilogram of diet: 29 mg of CuSO4; 108 mg of ZnSO4; 115 mg of MnSO4.H2O; 0.4 mg of Na2SeO3; and 60 mg of FeSO4.H2O.
Calculated compositions (% as-fed basis).
Analyzed compositions (% as-fed basis).
The 5 dietary treatments for Exp. II were positive control (PC) formulated amounts of CP including Lys, and Met + Cys met the requirement of genetic strain; negative control (NC) was 3 to 4% lower in CP and AA than PC; LPL-05, LPL-10, and LPL-15 treatments were NC + 0.05%, 0.10%, and 0.15% of LPL supplementation, respectively (Table 2). The LPL product (LIPIDOL) was obtained from Easy Bio Inc. (Seoul, South Korea). The diets of 2 experiments were formulated according to the Ross 308 nutrient standard (Aviagen, 2007). Birds were fed experimental diets in 3 feeding programs, starter (one to 7 d of age), grower (8 to 21 d of age), and finisher (22 to 35 d of age) periods.
Table 2.
Formula and chemical composition of diets in Experiment II (%, as fed basis)
| Starter period | Grower period | Finisher period | ||||
|---|---|---|---|---|---|---|
| Ingredients | PC | NC | PC | NC | PC | NC |
| Corn | ||||||
| Fine particle size | 39.22 | 40.91 | 28.83 | 29.90 | 18.77 | 19.33 |
| Coarse particle size | 16.80 | 17.54 | 28.83 | 29.90 | 43.82 | 45.12 |
| Soybean meal (45%) | 30.02 | 27.87 | 24.02 | 22.14 | 19.10 | 17.47 |
| DDGS | 3.00 | 3.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Poultry byproduct | 3.00 | 3.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Soybean oil | 3.20 | 2.95 | 4.61 | 4.39 | 4.83 | 4.63 |
| L-lysine sulfate (55%) | 0.51 | 0.49 | 0.38 | 0.36 | 0.36 | 0.35 |
| DL-methionine (98%) | 0.33 | 0.30 | 0.24 | 0.21 | 0.20 | 0.18 |
| Threonine (98%) | 0.12 | 0.11 | 0.06 | 0.05 | 0.05 | 0.04 |
| Choline chloride (50%) | 0.04 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 |
| Dicalcium phosphate | 1.94 | 1.95 | 1.45 | 1.46 | 1.31 | 1.32 |
| Limestone | 1.24 | 1.25 | 1.03 | 1.04 | 1.01 | 1.01 |
| Salt | 0.33 | 0.33 | 0.30 | 0.30 | 0.30 | 0.30 |
| Vitamin premix1 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| Mineral premix2 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Calculated compositions3 | ||||||
| ME (kcal/kg) | 3,025 | 3,025 | 3,150 | 3,150 | 3,200 | 3,200 |
| CP (%) | 22.00 | 21.14 | 21.00 | 20.24 | 19.00 | 18.34 |
| Calcium (%) | 1.05 | 1.05 | 0.90 | 0.90 | 0.85 | 0.85 |
| Available P (%) | 0.78 | 0.77 | 0.70 | 0.70 | 0.66 | 0.65 |
| Lys (%) | 1.40 | 1.33 | 1.23 | 1.17 | 1.09 | 1.03 |
| Met + cys (%) | 1.04 | 0.99 | 0.95 | 0.91 | 0.86 | 0.82 |
| Analyzed compositions4 | ||||||
| CP (%) | 21.98 | 20.99 | 21.19 | 20.11 | 19.11 | 18.44 |
| Moisture (%) | 10.43 | 10.38 | 10.65 | 10.87 | 10.63 | 10.38 |
| Ash (%) | 4.85 | 4.53 | 5.32 | 5.43 | 5.02 | 5.11 |
| Ether extract (%) | 5.11 | 5.02 | 5.43 | 5.36 | 5.53 | 5.48 |
DDGS = distiller's dried grains with solubles.
Provided per kilogram of diet: 8,000 IU of vitamin A (retinyl acetate); 1,600 IU of vitamin D3; 60 mg of vitamin E (dl-α-tocopheryl acetate); 3 mg of vitamin B1; 3 mg of vitamin K; 8 mg of vitamin B2; 4 mg of vitamin B6; 16 μg of vitamin B12; 60 mg of niacin; 2 mg of folic acid; 130 μg of biotin; and 20 mg of calcium pantothenic acid.
Provided per kilogram of diet: 29 mg of CuSO4; 108 mg of ZnSO4; 115 mg of MnSO4.H2O; 0.4 mg of Na2SeO3; and 60 mg of FeSO4.H2O.
Calculated compositions (% as-fed basis).
Analyzed compositions (% as-fed basis).
During the experimental period, the birds were housed on rice hull on the pen floor (length 158 cm x width 142 cm x height 80 cm) under a controlled temperature. The pens were equipped with pan feeders and bell drinkers. The house temperature was controlled at 35°C (d one to 3), and gradually decreased at -3°C weekly until slaughtering day. The lighting program provided artificial light for 23 h (23L:1D) during one to 29 d and declined to 20L:4D before an 8-day slaughter. Feed and water were supplied ad libitum throughout the experimental period.
Performance Parameters
Bird BW and feed consumption (amounts of added and left feed) were recorded in each pen at the end of hatch, 7, 21, and 35 d of age. Data were used to calculate BW, body weight gain (BWG), feed consumption (FC), and feed conversion ratio (FCR). Mortality rate of birds in each pen was recorded daily to adjust FC. Growth performance parameters were presented in each period and for the overall period.
Metabolic Trial and Digestibility Analyses
From 17 to 23 d of age, 30 chickens were raised in a metabolic cage (one bird/cage) for a 4-day preliminary and a 3-day excreta collection. Fecal samples in each metabolic cage were collected from a plastic tray every 12 h, and then pooled. Contaminants of scales and feather were carefully removed before weighing. The samples of fecal excreta were dried in an oven at 60°C for 72 h, and then ground to pass through a 1.0-mm Wiley mill sieve for subsequent assays. The homogeneous samples of feed and dried excreta were analyzed in triplicate to determine digestibility of DM (procedure 930.15), CP (method 984.13), and ether extract (EE; method 920.39), and total amino acid by oven, the Kjeldahl method, and the Soxhlet analysis, respectively (AOAC International, 2000). Total amino acid was analyzed by high-performance liquid chromatography. Apparent total tract digestibility was calculated as the following equation:
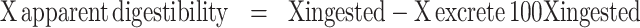 |
where X is illustrated DM, CP, EE, and total amino acids (AA). Digestibility of 17 amino acids (Asp, Thr, Ser, Glu, Gly, Ala, Val, Ile, Leu, Tyr, Phe, Lys, His, Arg, Pro, Met, and Cys) was reported as the total AA digestibility.
Sample Collection and Biochemical Assays (Exp. I)
At slaughtering day (d 35), feed was withdrawn for 2 h before blood collection to avoid interference. A total of 6 birds from each treatment (one bird per replication), with an average BW within 921 ± 3 kg from each group, was randomly selected. Five milliliters of whole blood were collected from a left jugular vein using sterilized needles and syringes. The samples were immediately transferred into non-heparinized tubes and kept at room temperature before being centrifuged at 3,000 x g at 4°C for 10 minutes. Serum was used to analyze triglyceride (TG, #11875540 216, Roche Diagnostic, IN), low-density lipoprotein cholesterol (LDL, #03038807 122, plus 2nd generation Kit, Roche Diagnostic, IN), high-density lipoprotein cholesterol (HDL, #04713311 190, plus 3rd generation Kit, Roche Diagnostic, Indianapolis, IN), glucose (BG, #11876899 216, Glucose Hexokinase Kit, Roche Diagnostic, IN), and aspartate aminotransferase (AST, Bergmeyer et al., 1985) concentrations. The analyses of metabolic profiles were performed according to the manufacturing protocols, and analyzed by a biochemical analyzer (Ciba-Corning model, Express Plus, Ciba Corning, Diagnostics Co., Boston, MA). Each sample was run twice immediately after serum was harvested.
Carcass Measurement (Exp. I)
After blood collection, the same broilers were individually weighed and euthanized by cervical dislocation. Carcass yields of left leg muscle, abdominal fat, liver, bursa of Fabricius, and spleen were removed. Each portion of carcass was weighed immediately using a digital balance (model PB 1501, Mettler, Toledo, OH) and expressed as a relative percentage of live BW.
Statistical Analysis
Data were analyzed using the SAS software package, Version 9.1 (SAS Institute, Cary, NC), following a completely randomized design. The GLM procedure was used to detect significant difference of the treatments for all criteria. A pen was used as an experimental unit for growth performance, whereas an individual bird was used as an experimental unit for nutrient digestibility, blood metabolites, and carcass. Duncan's new multiple range test was to used detect significant differences among treatments. Linear and quadratic effects of dietary LPL supplementation were declared by orthogonal polynomial contrast for all measurements. Probability values of P < 0.05 or P < 0.01 were statistically significant, and P > 0.05 to P < 0.10 was determined as a tendency.
RESULTS
Exp. I
Growth Performance
The effect of LPL supplementation to low-energy diets on growth performance of broiler chickens is summarized in Table 3. Dietary treatments did not affect growth performance in the starter period. Additionally, FC was not affected by dietary treatments in any period. However, birds fed dietary LPL-10 and LPL-15 had greater BW and BWG, and lower FCR than NC (P < 0.01), but there was no difference from PC during the grower, finisher, and overall periods, respectively. A linear reduction in FCR in the starter period was observed as the inclusion level of LPL increased (P = 0.044). Furthermore, the linear and improvements in BW, BWG, and FCR were affected by the inclusion level of LPL during the grower, finisher, and overall periods, respectively (P < 0.001).
Table 3.
| P-value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | PC | NC | LPL-05 | LPL-10 | LPL-15 | SEM | Linear | Quadratic |
| BW (g/bird) | ||||||||
| 1 wk | 155.88 | 154.73 | 157.42 | 157.72 | 158.64 | 1.125 | 0.285 | 0.469 |
| 3 wk | 977.12A | 845.60B | 954.25A | 950.95A | 978.69A | 11.772 | <0.001 | <0.001 |
| 5 wk | 2,078.33A | 1,789.00B | 1,981.67A | 2,024.00A | 2,063.67A | 22.312 | <0.001 | <0.001 |
| BWG (g/bird) | ||||||||
| 0 to 1 wk | 108.88 | 107.73 | 110.42 | 110.72 | 111.64 | 1.126 | 0.285 | 0.469 |
| 2 to 3 wk | 821.23A | 690.87B | 796.83A | 793.23A | 820.05A | 11.591 | <0.001 | <0.001 |
| 4 to 5 wk | 1,101.22A | 943.40B | 1,027.42A,B | 1,073.05A | 1,084.98A | 14.433 | <0.001 | 0.050 |
| Overall | 2,031.33A | 1,742.00B | 1,934.67A | 1,977.00A | 2,016.67A | 22.312 | <0.001 | <0.001 |
| FC (g/bird) | ||||||||
| 0 to 1 wk | 123 | 122 | 126 | 122 | 117 | 1.687 | 0.272 | 0.784 |
| 2 to 3 wk | 1,241 | 1,207 | 1,205 | 1,255 | 1,225 | 9.992 | 0.373 | 0.525 |
| 4 to 5 wk | 1,754 | 1,758 | 1,794 | 1,799 | 1,809 | 12.199 | 0.149 | 0.338 |
| Overall | 3,118 | 3,087 | 3,124 | 3,176 | 3,150 | 16.948 | 0.166 | 0.877 |
| FCR (feed:gain) | ||||||||
| 0 to 1 wk | 1.13 | 1.14 | 1.14 | 1.10 | 1.04 | 0.015 | 0.044 | 0.426 |
| 2 to 3 wk | 1.52B | 1.75A | 1.51B | 1.58B | 1.49B | 0.023 | <0.001 | <0.001 |
| 4 to 5 wk | 1.60C | 1.86A | 1.75B | 1.68B,C | 1.67B,C | 0.021 | <0.001 | 0.051 |
| Overall | 1.54C | 1.77A | 1.62B | 1.61B,C | 1.56B,C | 0.017 | <0.001 | <0.001 |
PC = positive control; NC = negative control; LPL-05 = negative control with 0.05% LPL; LPL-10 = negative control with 0.10% LPL; LPL-15 = negative control with 0.15% LPL.
A total of 300 broilers was raised for 35 d, with an average initial body weight of 47.00 ± 0.00 g and average final weight was 1,987.33 ± 22.31 g.
Least squares means for 6 pens per treatment with 10 broilers per pen (N = 30).
Means in same row with no common superscript differ significantly (P < 0.01).
Nutrient Digestibility
The results of nutrient digestibility in broilers fed low-energy diet supplementation with LPL are presented in Table 4. The LPL-supplemented treatments improved digestibility of DM (P < 0.05), CP (P < 0.01), and total AA (P < 0.01) compared to NC; however, there was no difference from those fed the PC treatment. The CP digestibility also showed the linear (P = 0.001) and quadratic (P = 0.008) improvements as the inclusion level of LPL increased. On the contrary, the digestibility of EE was unaffected by LPL addition among treatments, but a tendency toward improvement was observed (P = 0.065).
Table 4.
Effects of LPL supplementation to low-energy diets on nutrient digestibility (Exp. I)1
| P-value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | PC | NC | LPL-05 | LPL-10 | LPL-15 | SEM | Linear | Quadratic |
| Nutrient digestibility (%) | ||||||||
| DM | 72.30a | 69.20b | 71.19a | 72.06a | 71.97a | 0.335 | 0.002 | 0.078 |
| CP | 65.88B,C | 64.44C | 67.72A,B | 69.69A | 68.00A,B | 0.536 | 0.001 | 0.008 |
| EE | 88.50 | 81.25 | 86.20 | 85.71 | 83.36 | 0.855 | 0.486 | 0.065 |
| Total AA | 84.59A | 79.25B | 84.83A | 86.98A | 85.66A | 0.717 | <0.001 | 0.062 |
PC = positive control; NC = negative control; LPL-05 = negative control with 0.05% LPL; LPL-10 = negative control with 0.10% LPL; LPL-15 = negative control with 0.15% LPL; DM = dry matter; CP = crude protein; and EE = ether extract.
Means are presented as 6 birds per treatment (N = 30).
Means in same row with no common superscript differ significantly (P < 0.05).
Means in same row with no common superscript differ significantly (P < 0.01).
Blood Profiles
Table 5 shows the effect of LPL in low-energy diets on blood metabolites. Although there were no significant differences among dietary treatments for TG, LDL, and AST concentrations, but HDL (P < 0.05) and glucose (P < 0.01) concentrations of LPL-10 were significantly higher in the low-energy diet group compared with the PC.
Table 5.
Effects of LPL supplementation to low-energy diets on blood profiles (Exp. I)1
| P-value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | PC | NC | LPL-05 | LPL-10 | LPL-15 | SEM | Linear | Quadratic |
| TG (mg/dL) | 29.67 | 28.50 | 28.50 | 29.50 | 31.17 | 0.841 | 0.308 | 0.668 |
| LDL (mg/dL) | 16.00 | 15.67 | 18.00 | 19.50 | 16.33 | 0.864 | 0.700 | 0.837 |
| HDL (mg/dL) | 92.17b | 100.67a,b | 99.33a,b | 102.83a | 100.50a,b | 1.444 | 0.805 | 0.503 |
| BG (mg/dL) | 217.33B | 254.00A | 232.00A,B | 249.67A | 230.17A,B | 4.019 | 0.101 | 0.009 |
| AST (U/L) | 252.17 | 283.17 | 246.17 | 215.83 | 260.00 | 9.648 | 0.309 | 0.868 |
PC = positive control; NC = negative control; LPL-05 = negative control with 0.05% LPL; LPL-10 = negative control with 0.10% LPL; LPL-15 = negative control with 0.15% LPL; TG = triglyceride; LDL = low-density lipoproteins; HDL = high-density lipoproteins; BG = blood glucose; and AST = aspartate aminotransferase.
Means are expressed as 6 birds per treatment (N = 30).
Means in same row with different superscripts significantly differ (P < 0.05).
Means in a same row with different superscripts significantly differ (P < 0.01).
Relative Organ Weights of Carcass
No significant differences were observed for LPL supplementation in relative weights of abdominal fat, liver, bursa of Fabricius, and spleen (Table 6). However, the relative weight of thigh muscle was heavier in the LPL-10 group than that of birds fed NC (P < 0.05), which yielded the poorest weight of thigh muscle (8.03 g/100 g BW).
Table 6.
Effects of LPL supplementation to low-energy diets on relative organ weights of carcass in broiler chickens (Exp. I)1
| P-value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | PC | NC | LPL-05 | LPL-10 | LPL-15 | SEM | Linear | Quadratic |
| Relative organ weights (g/100 g BW) | ||||||||
| Leg muscle | 8.90a,b | 8.03b | 8.93a,b | 9.02a | 8.79a,b | 0.139 | 0.106 | 0.298 |
| Abdominal fat | 2.18 | 1.88 | 2.04 | 1.84 | 1.72 | 0.073 | 0.458 | 0.901 |
| Liver | 2.04 | 2.12 | 1.98 | 2.01 | 1.92 | 0.030 | 0.078 | 0.113 |
| Bursa | 0.25 | 0.30 | 0.28 | 0.24 | 0.27 | 0.009 | 0.228 | 0.916 |
| Spleen | 0.10 | 0.10 | 0.11 | 0.09 | 0.09 | 0.003 | 0.130 | 0.915 |
PC = positive control; NC = negative control; LPL-05 = negative control with 0.05% LPL; LPL-10 = negative control with 0.10% LPL; LPL-15 = negative control with 0.15% LPL.
Means are expressed as 6 birds per treatment (N = 30).
Means in same row with no common superscript differ significantly (P < 0.05).
Exp. II
Growth Performance
Table 7 shows the effect of LPL supplementation on diets with lower CP and AA on broiler performance. The supplementation level of LPL-15 showed improvements in BW (P < 0.05), BWG (P < 0.05), and FC (P < 0.01) in the starter period compared to the PC treatment. Feeding with LPL-15 also showed greater BW in the grower (P < 0.05) and finisher (P < 0.01) periods than for birds fed LPL-05. Furthermore, the improvement in BWG was affected by the LPL-15 inclusion at the grower (P < 0.05), finisher (P < 0.05), and overall (P < 0.01) periods when compared to the LPL-05 treatment. The birds had greater FC in LPL-15 in comparison to LPL-05, which detected significant difference in the finisher and overall periods (P < 0.05). Additionally, the birds fed diets containing LPL-15 observed a reduction in FCR compared to those fed NC diets during the finisher and overall periods (P < 0.05). Linear improvement in BWG was detected as the LPL supplementation increased in the grower (P = 0.031), finisher (P = 0.027), and overall (P = 0.015) periods, as well as a linear reduction in FCR in birds fed LPL treatments in the finisher (P = 0.031) and overall (P = 0.027) periods.
Table 7.
Effects of LPL supplementation to diets with reduced CP including AA on growth performance (Exp. II)1,2
| P-value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | PC | NC | LPL-05 | LPL-10 | LPL-15 | SEM | Linear | Quadratic |
| BW (g/bird) | ||||||||
| 1 wk | 150.77b | 164.92a | 161.78a,b | 160.58a,b | 165.43a | 1.759 | 0.975 | 0.736 |
| 3 wk | 928.67b | 940.00a,b | 933.45b | 938.82a,b | 972.33a | 5.438 | 0.049 | 0.225 |
| 5 wk | 2,203.67A,B | 2,167.22A,B | 2,146.34B | 2,203.54A,B | 2,280.19A | 15.186 | 0.015 | 0.401 |
| BWG (g/bird) | ||||||||
| 0 to 1 wk | 111.77b | 125.92a | 122.78a,b | 121.58a,b | 126.43a | 1.759 | 0.975 | 0.736 |
| 2 to 3 wk | 777.90a,b | 775.08b | 771.66b | 778.23a,b | 806.90a | 4.564 | 0.031 | 0.209 |
| 4 to 5 wk | 1,275.00a,b | 1,227.22a,b | 1,212.90b | 1,264.72a,b | 1,307.85a | 12.511 | 0.027 | 0.599 |
| Overall | 2,164.67A,B | 2,128.22A,B | 2,107.34B | 2,164.54A,B | 2,241.19A | 15.186 | 0.015 | 0.401 |
| FC (g/bird) | ||||||||
| 0 to 1 wk | 117B | 131A | 129A | 127AB | 131A | 1.660 | 0.652 | 0.423 |
| 2 to 3 wk | 1,041 | 1,010 | 1,065 | 1,053 | 1,068 | 10.952 | 0.053 | 0.061 |
| 4 to 5 wk | 2,161a | 2,162a | 2,065b | 2,151a | 2,179a | 14.296 | 0.345 | 0.279 |
| Overall | 3,319a,b | 3,303a,b | 3,259b | 3,331a,b | 3,377a | 16.137 | 0.092 | 0.973 |
| FCR (feed:gain) | ||||||||
| 0 to 1 wk | 1.05 | 1.04 | 1.05 | 1.04 | 1.04 | 0.006 | 0.690 | 0.858 |
| 2 to 3 wk | 1.34a,b | 1.31b | 1.38a | 1.36a,b | 1.32a,b | 0.011 | 0.651 | 0.122 |
| 4 to 5 wk | 1.70a,b | 1.77a | 1.71a,b | 1.70a,b | 1.67b | 0.013 | 0.031 | 0.119 |
| Overall | 1.53a,b | 1.55a | 1.55a,b | 1.54a,b | 1.51b | 0.006 | 0.027 | 0.155 |
PC = positive control; NC = negative control; LPL-05 = negative control with 0.05% LPL; LPL-10 = negative control with 0.10% LPL; LPL-15 = negative control with 0.15% LPL.
A total of 300 chickens with an initial BW of 39 g to an average final BW of 2,200.19 g was fed reduced CP including selected AA.
Least square means for 6 pens per treatment with 10 broilers per pen (N = 30).
Means in a same row with different superscripts significantly differ (P < 0.05).
Means in a same row with different superscripts significantly differ (P < 0.01).
Nutrient Digestibility
The nutrient digestibility of DM, CP, and EE of broiler chickens is shown in Table 8. No significant difference was detected in DM digestibility among treatments. However, significant differences in CP (P < 0.01) and EE (P < 0.05) digestibility were greater in birds that consumed LPL-15 compared to those fed PC and NC. The improvement in CP digestibility also was observed in the linear (P = 0.001) and quadratic (P = 0.005) effects, as well as the linear improvement in EE digestibility found (P = 0.002) as LPL levels increased.
Table 8.
Effects of LPL supplementation to diets with reduced CP including AA on nutrient digestibility (Exp. II)1
| P-value | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | PC | NC | LPL-05 | LPL-10 | LPL-15 | SEM | Linear | Quadratic |
| Nutrient digestibility (%) | ||||||||
| DM | 71.74 | 71.88 | 72.34 | 71.52 | 72.48 | 0.637 | 0.744 | 0.300 |
| CP | 65.34B,C | 64.39C | 67.84A,B | 67.69A,B | 68.55A | 0.457 | 0.001 | 0.005 |
| EE | 83.92b | 84.75b | 86.05a,b | 86.65a,b | 87.91a | 0.460 | 0.002 | 0.056 |
PC = positive control; NC = negative control; LPL-05 = negative control with 0.05% LPL; LPL-10 = negative control with 0.10% LPL; LPL-15 = negative control with 0.15% LPL.
Means are expressed as 6 birds per treatment (N = 30).
Values are expressed as means of 6 birds per treatment (N = 30).
Means in same row with different superscripts significantly differ (P < 0.05).
Means in same row with different superscripts significantly differ (P < 0.01).
DISCUSSION
Growth Performance
The LPL derived from soy-lecithins was shown to be an excellent source of phospholipids (Van Nieuwenhuyzen and Tomás, 2008). Dietary supplementation of LPL had no detrimental effect on performance of broiler chickens, indicating its possible use as a feed additive in lower nutrient diets for broiler chickens. Our study found that feeding LPL at the inclusion levels of 0.10 and 0.15% in reduced energy diets led to a superior increase in BW and BWG of birds, the positive effect being present even at the market age of the finisher phase. Furthermore, dietary inclusion of 0.15% LPL enhanced growth performance in young birds during a starter diet when low-nitrogenous diets were supplemented. The benefits of improved growth performance and reduced FCR of broilers by supplementing modified lecithins was consistent with Zhang et al. (2011), who observed that inclusion of lysophosphatidylcholine at 0.5 g/kg could enhance growth performance in young birds from 1 to 21 d of age via the improvements in weight gain and feed efficiency. Raju et al. (2011) found that broilers fed lysolecithin gain more BW when consuming the lysolecithin at either 25 or 50 g/kg at 21 d of age and 50 g/kg at 35 d of age, whereas feed efficiency improved with 50 g/kg diet. Khonyoung et al. (2015) also found that feeding of 145 mg/kg lysolecithin increased the feed efficiency during the grower period of 7 to 21 d of age. Recently, it was shown that the addition of LPL at 0.5 and 0.10 g/kg in lower metabolizable energy diets of weaning pigs had a compensable effect on energy values, which can further support growth performance of young pigs and those in the latter stages (Zhao et al., 2015). These results are in agreement with the current study, in which the LPL-supplemented birds contributed their nutrient needs for their productivity when LPL was added to reduced nutrient diets.
Nutrient Digestibility
The greater improvement in nutrient digestibility of CP and EE was observed in birds fed diets with reduced energy and nitrogenous nutrients. These findings are established in many reports. For instance, Zhang et al. (2011) observed that a supplementation level of lysophosphatidylcholine at 0.5 g/kg increased the digestion of apparent metabolizable energy of broilers in the grower and finisher phases. An in vivo study also found that lysolecithin increased the digestibility of DM, nitrogen retention, and energy (Jansen et al., 2015). Similar findings were supported in weanling pigs according to Jones et al. (1992), who found that lecithin had beneficial effects on nitrogen, DM, and crude fat digestibility. These results are also consistent with those of Zhao et al. (2015), who observed that supplementation of LPL at 0.05 and 0.10% in reduced energy diets had higher apparent total tract digestibility of DM, nitrogen, and EE than those fed diets with reduced energy and standard diets with no LPL supplementation. Interestingly, the LPL improved the AA digestibility of broiler chickens. Limited data were reported in broiler studies, but a report was presented on laying hens according to Han et al. (2010), who demonstrated that the inclusion of 0.10% lysolecithin could increase digestibility of essential amino acids. The increase in nutrient digestibility with lecithin inclusion may be explained by the modulation effects in fat emulsification (Zhang et al., 2011). In agreement with the present study, the LPL supplementation provided available nutrients under the dietary restrictions through improved EE and CP digestibility.
Blood Profiles
Blood profiles can be used as a marker of health status and malnutrition in poultry. The results showed that birds fed LPL-10 increased concentrations of HDL and BG in broilers. This is consistent with the findings of Huang et al. (2008), who reported that improvement in HDL concentration was found in broilers fed 2% soy-lecithin. It is commonly established that the HDL plays an important function in carrying total cholesterol and free fatty acids from the body's tissue to the hepatocytes for excretion or re-metabolization (Iwata et al., 1992; Spilburgal et al., 2003). The increased level of HDL with LPL supplementation indicated the clearance rate of body cholesterol, which possibly affected hepatic lipogenesis of broilers as reported by Huang et al. (2008). The LPL-10 also showed positively increased BG concentration. The improvement in BG level is usually indicated as an available uptake of glucose, which enhances the metabolic pathway in the hepatocytes (Eiler, 2004). The potential effect of LPL addition on BG concentration detected in this study would alter the metabolism of glucose in order to supply nutrients for their production needs.
Aspartate aminotransferase (AST) is one of the key metabolic enzymes to indicate liver health. This study found that reduced ME diets, either with or without LPL supplementation, showed no alteration in the enzyme activity in broilers, which was similar to that of PC birds. It is indicated that energy supplementation of less 150 kcal/kg is safe for the birds.
Relative Organ Weights of Carcass
Reduced energy diets supplemented with LPL showed no difference in relative weights of abdominal fat, liver, bursa of Fabricius, or spleen. However, dietary LPL-10 addition has a promising effect on the thigh muscles of broiler chickens. This finding was in agreement with a previous report by Huang et al. (2008), who observed that a supplemental level of soy-lecithin at 2% in corn-soybean meal diets increased the relative weight of leg muscles in broilers at 42 d of age. The promoting effects on leg yield from supplementation of lecithin seem to be associated with enhanced lipid and protein facilitations for conversion into muscles (Han et al., 2010; Roy et al., 2010). In general, the muscle contains various mitochondria and is more efficient for generating a large amount of energy; therefore, it is more capable of nutrient accumulation (Oliveira et al., 2013). This results in heavier leg weights when reduced energy diets are fed to the birds. However, no increase of lymphoid organ weight was found, indicating that LPL supplementation was less effective in promoting the immune organs in broiler chickens under the restriction of energy diets.
CONCLUSION
LPL supplementation of at least 0.10% in diets with lower amounts of energy, crude protein, and major amino acids has benefits of increased growth performance, nutrient digestibility, and glucose availability, without toxicity to the hepatic function of broilers.
REFERENCES
- AOAC International. 2000. Official Methods of Analysis of AOAC International, 17th ed.Gaithersburg, MD, USA. [Google Scholar]
- Asaoka Y., Oka M., Yoshida K., Sasaki Y., Nishizuka Y.. 1992. Role of lysophosphatidylcholine in T-lymphocyte activation: Involvement of phospholipase A2 in signal transduction through protein kinase C. Proc. Natl. Acad. Sci. USA. 89:6447–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviagen 2007. Ross 308 broiler: Nutrition specifications. Ross Breeders Limited, Newbridge, Midlothian, Scotland, UK. [Google Scholar]
- Bergmeyer H. U., Horder M., Rej R.. 1985. Approved recommendations of IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase. J. Clin. Chem. Biochem. 24:467–510. [PubMed] [Google Scholar]
- Boontiam W., Jung B., Kim Y. Y.. 2016. Effects of lysophospholipids supplementation to lower nutrient diets on growth performance, intestinal morphology, and blood metabolites in broiler chickens. Poult. Sci. Doi:10.3382/ps/pew269. [DOI] [PubMed] [Google Scholar]
- Bregendahl K., Sell J. L., Zimmerman D. R.. 2002. Effect of low-protein diets on growth performance and body composition of broiler chicks. Poult. Sci. 81:1156–1167. [DOI] [PubMed] [Google Scholar]
- Butzen F. M., Vieira M. M., Kessler A. M., Aristimunha P. C., Marx F. R., Bockor L., Ribeiro A. M. L.. 2015. Early feed restriction in broilers. II: Body composition and nutrient gain. J. Appl. Poult. Res. 24:198–205. [Google Scholar]
- Eiler H. 2004. Endrocrine glands. Pages621–669 In Dukes Physiology of Domestic Animals, 12th ed.Reese W.O., ed Ithaca, N.Y. Comstock. [Google Scholar]
- Han Y. K., Jin Y. H., Kim J. H., Thacker P. A.. 2010. Influence of enzyme and/or lysolecithin supplementation on performance, nutrient digestibility and egg quality for laying hens. Trends Anim. Vet. Sci. J. 1:28–35. [Google Scholar]
- Huang J., Yang D., Gao S., Wang T.. 2008. Effects of soy-lecithin on lipid metabolism and hepatic expression of lipogenic genes in broiler chickens. Livest. Sci. 118:53–60. [Google Scholar]
- Iwata T., Hoshi S., Takehisa F., Tsutsumi K., Furukawa Y., Kimura S.. 1992. The effect of dietary safflower phospholipid and soybean phospholipid on plasma and liver lipids in rats fed a hypercholesterolemic diet. J. Nutr. Sci. Vitaminol. 38:471–479. [DOI] [PubMed] [Google Scholar]
- Jansen M., Nuyens F., Buyse J., Leleu S., Van Campenhout L.. 2015. Interaction between fat type and lysolecithin supplementation in broiler feeds. Poult. Sci. 94:2506–2515. [DOI] [PubMed] [Google Scholar]
- Jones D. B., Hancock J. D., Harmon D. L., Walker C. E.. 1992. Effects of exogenous emulsifiers and fat sources on nutrient digestibility, serum lipids, and growth performance in weanling pigs. J. Anim. Sci. 70:3473–3482. [DOI] [PubMed] [Google Scholar]
- Joshi A., Paratkar S. G., Thorat B. N.. 2006. Modification of lecithin by physical, chemical and enzymatic methods. Eur. J. Lipid Sci. Technol. 108:363–373. [Google Scholar]
- Khonyoung D., Yamauchi K., Suzuki K.. 2015. Influence of dietary fat sources and lysolecithin on growth performance, visceral organ size, and histological alteration in broiler chickens. Livest. Sci. 176:111–120. [Google Scholar]
- Kiess A. S., Manangi M. K., Cleveland B. M., Wilson M. E., Blemings K. P.. 2013. Effect of dietary lysine on hepatic lysine catabolism in broilers. Poult. Sci. 92:2705–2712. [DOI] [PubMed] [Google Scholar]
- Lundbæk J. A., Andersen O. S.. 1994. Lysophospholipids modulate channel function by altering the mechanical properties of lipid bilayers. J. Gen. Physiol. 104:645–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundbæk J. A., Collingwood S. A., Ingólfsson H. I., Kapoor R., Andersen O. S.. 2010. Lipid bilayer regulation of membrane protein function: Gramicidin channels as molecular force probes. J. R. Soc. Interface. 7:373–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel A. J., Lesage F., Lazdunski M., Honoré E.. 2000. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J. Biol. Chem. 275:10128–10133. [DOI] [PubMed] [Google Scholar]
- Oliveira J. E., Druyan S., Uni Z., Ashwell C. M., Ferket P. R.. 2013. Metabolic profiling of late-term turkey embryos by microarrays. Poult. Sci. 92:1011–1028. [DOI] [PubMed] [Google Scholar]
- Raju M. V. L. N., Rao S. V. R., Chakrabarti P. P., Rao B. V. S. K., Panda A. K., Devi B. L. A. P., Sujatha V., Reddy J. R. C., Sunder G. S., Prasad R. B. N.. 2011. Rice bran lysolecithin as a source of energy in broiler chicken diet. Br. Poul. Sci. 52:769–774. [DOI] [PubMed] [Google Scholar]
- Roy A., Haldar S., Mondal S., Ghosh T. K.. 2010. Effects of supplemental exogenous emulsifier on performance, nutrient metabolism, and serum lipid profile in broiler chickens. Vet. Med. Int. 10:1–9. DOI: 10.4061/2010/262604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer K., Adams C. A.. 1996. The influence of specific phospholipids as absorption enhancer in animal nutrition. Eur. J. Lipid Sci. Technol. 98:304–308. [Google Scholar]
- Skoura A., Hla T.. 2009. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc. Res. 82:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilburg C. A., Goldberg A. C., McGrill J. B., Stenson W. F., Racette S. B., Bateman J., McPherson T. B., Ostlund R. E.. 2003. Fat-free foods supplemented with soy stanol-lecithin power reduce cholesterol absorption and LDL cholesterol. J. Am. Diet Assoc. 103:577–581. [DOI] [PubMed] [Google Scholar]
- Thomas O. P., Twinning P. V. Jr., Bossard E. H.. 1978. The lysine and sulfur amino acid requirements for broilers. Pages27– 35 in Proceeding of the Georgia Nutrition Conference, Atlanta, GA. [Google Scholar]
- Van Nieuwenhuyzen W., Tomás M. C.. 2008. Update on vegetable lecithin and phospholipid technologies. Eur. J. Lipid. Sci. Technol. 110:472–486. [Google Scholar]
- Zhang B., Haitao L., Zhao D., Guo Y., Barri A.. 2011. Effect of fat type and lysophosphatidylcholine addition to broiler diets on performance, apparent digestibility of fatty acids, and apparent metabolizable energy content. Anim. Feed. Sci, Technol. 163:177–184. [Google Scholar]
- Zhao P. Y., Li H. L., Hossain M. M., Kim I. H.. 2015. Effect of emulsifier (lysophospholipids) on growth performance, nutrient digestibility and blood profile in weanling pigs. Anim. Feed. Sci. Technol. 207:190–195. [Google Scholar]


