ABSTRACT
Changing dietary protein and amino acids may impact intestinal barrier function. Experiments were conducted in broiler chickens to evaluate supplementation of L-glutamine, glycine, and L-arginine in a reduced protein (RP) diet. Experiment 1 examined the growth performance of broilers fed 5 dietary treatments: 1) a standard diet; 2) an RP diet (193.9 g/kg CP in grower and 176.9 g/kg CP in finisher); 3) RP diet supplemented with 10 g/kg L-Gln; 4) RP diet supplemented with 10 g/kg Gly; 5) RP diet supplemented with 5 g/kg L-Arg. Each experimental diet was replicated 6 times with 10 birds per replicate. In a subset of 96 birds, experiment 2 tested the 4 RP diets with and without dexamethasone (DEX) to induce leaky gut. Each diet was replicated 24 times. Fluorescein isothiocyanate dextran (FITC-d) was used to test intestinal permeability (IP). Gene expression of selected tight junction proteins in ileal and jejunal tissues was assayed by quantitative PCR. From day 7 to 35, the RP diet increased feed intake (FI) (P < 0.05) and body weight gain (BWG) compared with the standard diet while Gln reduced FI and BWG (P < 0.05) compared with RP. Gly had no effect on BWG or FCR. Supplementation of Arg improved FCR from day 21 to 35 and day 7 to 35. In experiment 2, Arg tended to lower FITC-d (P = 0.086). DEX increased passage of FITC-d into the serum (P < 0.001). The villi surface area was increased in birds fed higher Arg (P < 0.05). DEX and diet interacted (P < 0.01) for jejunal claudin-3 mRNA level where DEX upregulated claudin-3 for all diets except the Arg diet. In summary, with a moderate reduction of protein, satisfactory performance can be obtained. Although Gln and Gly had no demonstrable positive effect on IP and performance of broilers, increasing the dietary Arg by approximately 140% improved FCR and showed indications of improved intestinal barrier function of birds fed an RP diet under a stress model.
Keywords: intestinal barrier, tight junctions, permeability, dexamethasone, reduced protein
INTRODUCTION
Dietary protein content and in particular amino acid composition of the diet are remarkably important to support animal production and health. It is now evident that amino acids are one of the main drivers of feed intake (FI) and growth performance of broilers (Cho, 2012). As a result of extensive breeding programs, modern broiler chickens are now approximately 400% more efficient than breeds grown in 1956 (Zuidhof et al., 2014). This has resulted in increased nutrient specifications which unavoidably increased the total crude protein of the diets in order to provide the desired digestible amino acids in broiler diets. Increased environmental impact, wet litter issues, and ever increasing reliance on quality protein sources are amongst the main drawbacks of a high protein diet. Therefore, in recent years, there has been greater interest in feeding birds with reduced protein (RP) diets supplemented with amino acids. Supplementation of amino acids has been extensively researched in relation to RP diets and the order of limiting amino acids has been identified (Ospina-Rojas et al., 2012). As for the performance of broiler chickens, this practice has often resulted in contradictory results but mostly impaired performance and unbalanced supply of amino acids.
The mucosa of the small intestine has finger-like projections known as villi. A single layer of epithelial cells (enterocytes) covers these villi. The intestinal epithelium is selectively permeable allowing the passage of water, nutrients, and electrolytes, while preventing entry of harmful organisms, toxins, and antigens (Groschwitz and Hogan, 2009). This selective absorption is mediated through complex pathways of paracellular or transcellular permeability. Intestinal permeability (IP) can be regulated or altered by several factors including, but not limited to diet, intestinal microflora, stress, disease, infections, toxins, and drugs (Bischoff et al., 2014). Increased IP is often associated with compromised health, performance, bacterial translocation, immune activation, and lameness (Gilani et al., 2016). In a recent study, Barekatain et al. (2018) found that IP can be maintained in birds fed an RP diet when essential amino acids were supplemented to match a standard protein diet. However, when compared with higher concentrations of amino acids, a higher IP was observed indicating a compromise in intestinal barrier function associated with changes in gene expression of tight junction proteins and some nutrient transporters. Chen et al. (2016) also showed that reducing dietary protein exacerbated the effect of aflatoxin in broiler performance and nutrient utilization along with a numeric tendency to increase IP.
For RP diets, specific amino acids have been extensively studied, with the most promising candidates being glycine (Gly) and serine (Ser) that need to be closely considered when formulating diets (Siegert et al., 2016). Despite abundant data on the performance parameters associated with amino acid supplementation to RP diets, few studies have been conducted to investigate their underlying roles for intestinal barrier function and permeability. A study has shown the effect of Gly on IP in piglets but without consideration of dietary protein (Li et al., 2016). Additionally, some amino acids including glutamine (Gln), arginine (Arg), and threonine (Thr) have been documented to alter intestinal integrity in other species and more recently in poultry (Bortoluzzi et al., 2017).
It is well established that Gln plays a critical role in maintaining gut integrity through provision of energy for rapidly proliferating enterocytes and lymphocytes mediating such effects through tight junctions, mucosal cell proliferation, and differentiation (Soares et al., 2014). Gln is involved in mucin synthesis. N-Acetylglucosamine is a glycoprotein and a component of the mucin that protects mucosal surfaces and its formation is fully dependent on Gln (Coster et al., 2004). Gln may be considered a conditionally essential amino acid during stress, injury, or malnutrition. Under such circumstances the requirement may exceed the endogenous metabolic capacity required to maintain gut integrity and reduce inflammation as immunocytes increase their uptake of Gln (Coster et al., 2004). Previous studies have demonstrated some positive response to Gln in terms of growth performance and gut development in broiler chickens (Bartell and Batal, 2007; Murakami et al., 2007). However, our recent studies have failed to show any positive response to Gln in broiler chickens in terms of gut permeability or performance (Gilani et al., 2018c). Gilani et al. (2018c) found that providing 1% supplemental Gln in drinking water to newly hatched broilers did not reduce IP in delayed fed birds. In another study, Gilani et al. (2018b) supplemented 10 g/kg Gln to a wheat-based diet and found no effect on IP assessed by fluorescein isothiocyanate dextran (FITC-d) uptake or lactulose, mannitol, and rhamnose sugar assays. It should be noted that in both trials (Gilani et al., 2018b, c), the Gln was given alongside a normal commercial diet as opposed to an RP diet. It may be hypothesized that the L-Gln could be more effective when the dietary protein is reduced or the animal is under a serious stressor or disease challenge.
As an essential amino acid for chickens, mounting evidence is emerging on the fundamental roles that Arg plays in various metabolic pathways and regulation of intestinal function and ultimately protein synthesis and performance. The synthesis of nitric oxide (NO) and polyamines is dependent on Arg (Wu and Knabe, 1995). Production of NO along with enterocyte migration is crucial for restoration of epithelial continuity (Jacobi and Odle, 2012). Therefore, Arg can modulate immune responses, inflammation, and the process of recovery from injury or stress. Recent data in poultry suggest potential for increasing feed specification for Arg beyond current industry practices to support optimal growth and intestinal function (Tan et al., 2014a). Supplementation of Arg can therefore improve gut barrier function as shown by reducing ileal permeability measured in Ussing chambers by Zhang et al. (2017). However, the specific role of Arg in RP diets for intestinal barrier function and permeability has not been investigated.
Thus, it was hypothesized that individual amino acids including Gln, Gly, and Arg would positively impact intestinal barrier function in birds fed RP diets. Specifically, the present study investigated the role of increased concentrations of L-Gln, Gly, and L-Arg on growth performance and IP, gene expression of selected tight junction proteins, and intestinal architecture of broiler chickens utilizing a dexamethasone (DEX)-induced model of leaky gut.
MATERIALS AND METHODS
All experimental procedures were approved by the Animal Ethics Committees of the Primary Industries and Regions South Australia and the University of Adelaide.
Experimental Design and Management
Experiment 1 investigated the growth performance of broiler chickens in which 5 experimental treatments were used in a completely randomized design. Diets were as follows: 1) a standard commercial diet (225.7 g/kg CP and 202.5 g/kg CP for grower and finisher diets, respectively); 2) RP diet (193.9 g/kg CP and 176.9 g/kg CP for grower and finisher diets, respectively); 3) RP diet supplemented with 10 g/kg L-Gln; 4) RP diet supplemented with 10 g/kg Gly; 5) RP diet supplemented with 5 g/kg L-Arg. The L-Gln level was used based on previous literature (Bartell and Batal, 2007). Gly was added in accordance with Waguespack et al. (2009). Additional L-Arg 5 g/kg increased the digestible Arg content of the diet to 17 g/kg within the tested range reported as a positive response in the literature in broilers (Tan et al., 2014a,b). All tested amino acids were added at the expense of wheat. Amino acid specifications were calculated using AMINOChick 2.0 software (Evonik Animal Nutrition) on which basis the diets were formulated at 3,100 and 3,200 kcal/kg AMEn (Table 1).
Table 1.
Ingredient and nutrient composition of basal grower and finisher diets (g/kg unless otherwise noted).
| Ingredients | Grower standard | Grower reduced protein | Finisher standard | Finisher reduced protein |
|---|---|---|---|---|
| Wheat | 374.64 | 477.27 | 425.43 | 519.40 |
| Sorghum | 250.00 | 250.00 | 250.00 | 250.00 |
| Soybean meal 45.7% | 284.40 | 202.40 | 239.30 | 158.40 |
| Meat and bone meal 52.7% | 41.00 | 10.00 | 27.00 | 10.00 |
| Canola oil | 31.01 | 18.64 | 39.84 | 26.66 |
| L-lysine HCL | 2.45 | 5.44 | 2.39 | 5.07 |
| Dl-methionine | 2.79 | 3.54 | 2.43 | 3.11 |
| L-threonine | 1.03 | 2.36 | 0.85 | 2.05 |
| L-valine 98% | 0.23 | 1.89 | 0.11 | 1.58 |
| L-arginine | 0.00 | 2.41 | 0.00 | 2.34 |
| L-isoleucine 98% | 0.00 | 1.53 | 0.00 | 1.40 |
| Sodium chloride | 0.70 | 0.02 | 0.71 | 0.01 |
| Sodium bicarbonate | 2.25 | 3.99 | 2.58 | 4.04 |
| Limestone | 7.16 | 9.21 | 7.11 | 8.38 |
| Dicalcium phosphate | 0.00 | 8.96 | 0.00 | 5.32 |
| Xylanase | 0.05 | 0.05 | 0.05 | 0.05 |
| Phytase | 0.10 | 0.10 | 0.10 | 0.10 |
| Choline chloride 60% | 0.60 | 0.60 | 0.50 | 0.50 |
| Vitamin premix1 | 0.70 | 0.70 | 0.70 | 0.70 |
| Trace mineral premix2 | 0.90 | 0.90 | 0.90 | 0.90 |
| Nutrient composition | ||||
| AMEn (kcal/kg) | 3,100 | 3,100 | 3,200 | 3,200 |
| Protein | 225.7 | 193.9 | 202.5 | 176.9 |
| Fat | 54.85 | 39.71 | 62.15 | 47.62 |
| Fiber | 25.49 | 24.42 | 24.85 | 23.96 |
| Dig3 Lys | 11.50 | 11.50 | 10.19 | 10.20 |
| Dig Met | 5.61 | 5.88 | 5.00 | 5.27 |
| Dig Met+Cys | 8.51 | 8.51 | 7.75 | 7.75 |
| Dig Thr | 7.71 | 7.70 | 6.83 | 6.83 |
| Dig Trp | 2.41 | 2.02 | 2.19 | 1.82 |
| Dig Ile | 8.05 | 8.05 | 7.24 | 7.24 |
| Dig Leu | 15.69 | 13.08 | 14.29 | 11.96 |
| Dig Arg | 12.51 | 11.96 | 10.95 | 10.71 |
| Dig Val | 9.20 | 9.20 | 8.21 | 8.21 |
| Dig His | 4.68 | 3.80 | 4.20 | 3.40 |
| Dig Phe | 9.28 | 7.61 | 8.38 | 6.86 |
| Dig Glyequ | 15.03 | 11.42 | 13.18 | 10.35 |
| Dig Gly | 8.64 | 6.17 | 7.41 | 5.59 |
| Dig Ser | 8.94 | 7.36 | 8.08 | 6.66 |
| Ca | 8.72 | 8.70 | 7.46 | 7.48 |
| Available P | 4.36 | 4.35 | 3.79 | 3.80 |
| Cl | 2.00 | 2.00 | 1.90 | 1.90 |
| Na | 1.60 | 1.60 | 1.60 | 1.60 |
Vitamin concentrate supplied per kilogram of diet: retinol, 12,000 IU; cholecalciferol, 5,000 IU; tocopheryl acetate, 75 mg, menadione, 3 mg; thiamine, 3 mg; riboflavin, 8 mg; niacin, 55 mg; pantothenate, 13 mg; pyridoxine, 5 mg; folate, 2 mg; cyanocobalamin, 16 μg; biotin, 200 μg; cereal-based carrier, 149 mg; mineral oil, 2.5 mg.
Trace mineral concentrate supplied per kilogram of diet: Cu (sulfate), 16 mg; Fe (sulfate), 40 mg; I (iodide), 1.25 mg; Se (selenate), 0.3 mg; Mn (sulfate and oxide), 120 mg; Zn (sulfate and oxide), 100 mg; cereal-based carrier, 128 mg; mineral oil, 3.75 mg.
Digestible.
Male day-old off-sex Ross 308 broiler chickens (n = 396) were purchased from Aviagen Hatchery (Goulburn, NSW) and transferred to the research facility at Roseworthy, SA. All birds were placed in raised-floor pens and fed the same commercial starter diet until day 7 when birds were assigned to the 5 experimental diets. Birds were fed with experimental grower diets from day 7 to 21 and the finisher diets were provided from day 21 to 35 post-hatch. Each diet was replicated 6 times and each replicate/pen housed 10 birds in a total of 30 pens. Each of the 4 RP treatments was given 2 additional pens (8 pens and each pen 12 birds) that were later used for experiment 2. The room temperature of the first day of age was set at 33 to 34°C and then gradually decreased to maintain bird comfort through day 21 when the temperature reached a plateau of 21°C, maintained until the end of the study. Birds were given 23 h of light and 1 h of dark during the first week followed by 16 h light and 8 h dark for the remainder of the study. Feed and fresh water were provided ad libitum throughout the study. Birds were weighed on days 7, 21, and 35, and feed consumption and FCR were determined.
Experiment 2 investigated the effects of L-Gln, Gly, and L-Arg on intestinal barrier function and permeability using a 2 × 4 factorial arrangement of treatments. Four RP treatments were used as the dietary treatment with or without DEX injections used as a leaky gut model. On day 14, a total of 96 birds that had received their respective experimental diets were transferred to individual metabolism cages. Each diet was replicated 24 times by offering each diet to 24 birds. BW and FI were recorded from day 14 to 21 for each bird. On days 14, 16, 18, and 20, half of the birds in each treatment group (n = 12) were injected in breast muscle with DEX (0.5 mg/kg BW) for a total of 4 injections. The DEX solution was prepared according to Wideman and Pevzner (2012). The rest of the birds received sham injections of 0.9% saline solution proportionate to their BW.
FITC-d Test
The FITC-d test was utilized to assess IP consistent with previous studies (Barekatain et al., 2018; Gilani et al., 2018b). Briefly, at day 21 of age, each bird was orally gavaged with a 1 mL aqueous solution containing 2.2 mg of FITC-d (4,000 kD, Sigma Aldrich, Castle Hill, NSW, Australia). A blood sample was taken from each live bird in a time-dependent manner (150 min after the oral gavage) via the jugular vein. Blood samples were allowed to clot at room temperature for at least 3 h after which all blood tubes were centrifuged at 1,000 g for 15 min and serum samples were separated and kept at –20°C until analysis. Serum samples and standards were analyzed in triplicate for FITC-d concentration using a Synergy MX plate reader (Biotek Instruments, Bedfordshire, UK) with excitation and emission wavelengths set at 485 and 530 nm, respectively.
Sample Collection and Histomorphology
On day 21, after blood collection, birds were euthanized for tissue collection and weight of visceral organs after washing with PBS. The jejunum and ileum of each bird were flushed with PBS solution and then approximately 2 cm of the middle was cut and transferred to a separate vial and snap-frozen in liquid nitrogen before placing the samples in a –80°C freezer prior to molecular analysis. By a similar method, approximately 2 cm of the mid-jejunum was placed in a 10% formalin solution and later used for histological measurements. Hematoxylin and eosin stained sections were used for measurements of villus height (VH) and crypt depth (CD) following the method described by Golder et al. (2011). Villus surface area was directly measured and then computed by Video Pro 32 imaging Software (Leading Edge Pty Ltd; Adelaide, Australia). For each measurement an average of 10 intact villi was used randomly per slide. An Olympus WH B10X\20 microscope (Olympus, Tokyo, Japan) was used for image capture from each slide. All images were processed by Video Pro 32 imaging software.
RNA Extraction and Quantitative PCR
A commercially available kit (RNeasy Plus Universal Tissue Mini kit, Qiagen, Hilden, Germany) was utilized for RNA isolation and quantification as described previously by Gilani et al. (2018a). In summary, PCR assays were designed for claudin-1, claudin-3, zonula occluden 1 (ZO-1), zonula occluden 2 (ZO-2), junctional adhesion molecule 2 (JAM2), and occludin. Concentration and purity of total RNA were confirmed by the agarose gel electrophoresis technique. Housekeeping genes of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and TATA-binding protein (TBP) were selected. However, the GAPDH gene was selected for data normalization as it was the most stable. All the primers related to the studied genes are included in Table 2.
Table 2.
Primers used in real-time quantitative PCR.1
| Target gene | Forward/reverse | Accession number or GenBank ID |
|---|---|---|
| Housekeeping genes | ||
| GAPDH | F: CAACCCCCAATGTCTCTGTT | ENSGALG00000014442 CGNC Symbol; Acc:49077 |
| R: TCAGCAGCAGCCTTCACTAC | ||
| TBP | F: GTCCACGGTGAATCTTGGTT | ENSGALG00000011171 CGNC Symbol; Acc:8484 |
| R: GCGCAGTAGTACGTGGTTCTC | ||
| Tight junction protein genes | ||
| Claudin-1 | F: AAGGTGTACGACTCGCTGCT | ENSGALG00000026862 NP_001013629 |
| R: CAGCAACAAACACACCAACC | ||
| Claudin-3 | F: GCCAAGATCACCATCGTCTC | ENSGALG00000022557 NP_989533 |
| R: CACCAGCGGGTTGTAGAAAT | ||
| ZO-1 | F: CCGCAGTCGTTCACGATCT | ENSGALG00000042552 CGNC Symbol; Acc:415388 |
| R: GGAGAATGTCTGGAATGGTCTGA | ||
| ZO-2 | F: GCCCAGCAGATGGATTACTT | ENSGALG00000015109 CGNC Symbol; Acc:49456 |
| R: TGGCCACTTTTCCACTTTTC | ||
| JAM2 | F: AGACAGGAACAGGCAGTGCT | ENSGALG00000015746 CGNC Symbol; Acc:11746 |
| R: TCCAATCCCATTTGAGGCTA | ||
| Occludin | F: ACGGCAAAGCCAACATCTAC | ENSGALG00000027456 CGNC Not available |
| R: ATCCGCCACGTTCTTCAC | ||
Statistical Analysis
Growth performance data for experiment 1 were analyzed using a 1-way ANOVA of the general linear model procedure of SAS (2012). Data for experiment 2 were analyzed using a 2-way ANOVA to assess the main effect of diets and DEX injection and their interaction. A normal distribution test was performed for all data. For simple performance analysis of the birds reared as a group, 1 pen was considered an experimental unit. For the challenge aspect, each bird, or its respective sample, constituted an experimental unit. All values were present as means with pooled standard error of mean. The least square difference test was used to separate the means if a significant effect was detected between the treatments or for main effects and interaction. The level of significance was considered as P < 0.05, and tendency was considered for 0.05 ≤ P ≤ 0.10.
RESULTS
Growth Performance
The results of the general growth performance of birds are presented in Table 3. Compared to birds fed a standard diet, birds fed RP and Arg-added diets had a higher FI from day 7 to 21 of age (P < 0.05). From day 22 to 35 and from day 7 to 35, birds fed RP diet consumed more feed compared with standard diet and Gln-supplemented diets (P < 0.05). There was no difference between Gln and Arg-supplemented diets in terms of feed consumption at any stage of the growth. Body weight gain (BWG) was not affected from day 7 to 21 of age. During the finisher phase of feeding, birds fed RP and Gly-supplemented diets had a higher BWG compared with standard diet (P < 0.05). When assessed for entire period of study, RP and additional Arg increased BWG in comparison with birds fed standard diet (P < 0.05). Although FCR was not affected from day 7 to 21, supplementation of Arg improved FCR from day 21 to 35 (P < 0.05) and for the entire period of study (day 7 to 35) (P < 0.05).
Table 3.
Growth performance of broilers fed reduced protein (RP) diets supplemented with glutamine, glycine, or arginine.1,2
| Feed consumption (g/bird) | Body weight gain (g/bird) | Feed conversion ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 7–21 | Day 22–35 | Day 7–35 | Day 7–21 | Day 22–35 | Day 7–35 | Day 7–21 | Day 22–35 | Day 7–35 | |
| Treatments | |||||||||
| Standard diet | 1,143b | 2,082b | 3,224b | 828 | 1,294c | 2,122c | 1.381 | 1.608a | 1.527a |
| Control (RP) | 1,216a | 2,284a | 3,500a | 864 | 1,438a | 2,302a | 1.409 | 1.589a | 1.521a |
| RP + Gln | 1,183a,b | 2,079b | 3,262b | 825 | 1,312b,c | 2,137b,c | 1.435 | 1.587a | 1.519a |
| RP + Gly | 1,179a,b | 2,222a,b | 3,402a,b | 849 | 1,405a,b | 2,254a-c | 1.390 | 1.582a,b | 1.510a,b |
| RP + Arg | 1,219a | 2155a,b | 3,375a,b | 876 | 1,398a-c | 2,274a,b | 1.393 | 1.543b | 1.485b |
| SEM3 | 7.7 | 21.4 | 27.2 | 6.4 | 15.1 | 20.1 | 0.0059 | 0.0057 | 0.0039 |
| P value | 0.047 | 0.039 | 0.044 | 0.117 | 0.040 | 0.043 | 0.101 | 0.038 | 0.039 |
Each value represents the mean of 6 replicates.
Means within a column not sharing a superscript differ significantly at the P level shown.
Pooled standard error of mean (n = 30).
Performance data of individually housed birds in experiment 2 are illustrated in Table 4. There was no interaction between DEX and diet for the individual birds. FI and BWG of the birds were reduced (P < 0.001) by DEX injection, whereas diets had no effect. Supplementation of Gly and Arg improved FCR regardless of DEX (P < 0.001). DEX injections increased FCR independently (P < 0.001).
Table 4.
Growth performance of individually housed broilers fed reduced protein (RP) diets supplemented with Gln, Gly, and Arg subjected to dexamethasone (DEX) injections (d 14 to 21).1
| Treatments | Feed intake (g/bird) | Body weight gain (g/bird) | FCR |
|---|---|---|---|
| Main effects | |||
| Diet | |||
| Control (RP) | 619 | 354 | 1.833a |
| RP + Gln | 632 | 349 | 1.871a |
| RP + Gly | 635 | 391 | 1.678b |
| RP + Arg | 636 | 397 | 1.661b |
| DEX | |||
| Sham (n = 48)2 | 666a | 452a | 1.476b |
| Injected (n = 47) | 595b | 294b | 2.046a |
| SEM3 | 4.74 | 3.66 | 0.0146 |
| Source of variation | |||
| Diet | 0.58 | 0.11 | 0.025 |
| DEX | <0.0001 | <0.0001 | <0.0001 |
| Diet × DEX | 0.89 | 0.78 | 0.34 |
Means within a column not sharing a superscript differ significantly at the P level shown for the main effects.
Values in parenthesis represent the number of replicates/birds.
Pooled standard error of mean (n = 95).
Serum FITC-d Concentration
As shown in Figure 1, no significant interaction was found between DEX and diet for FITC-d concentration. Although there was no significant effect of diet, there was a tendency for a lower FITC-d in birds fed additional Arg compared with other treatments (P = 0.086). DEX significantly increased passage of FITC-d into the serum (P < 0.001).
Figure 1.
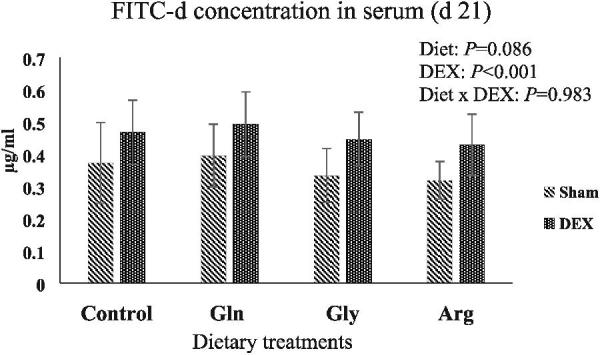
Concentration of FITC-d in serum of broilers on day 21 (error bars represent ± standard deviation).
Jejunal Histomorphological Measurements
Diet and DEX interacted significantly for VH (P < 0.05) and VH:CD (P < 0.05) in sham-injected birds (Table 5). Sham-injected birds fed Arg-added diets had the highest VH, whereas for DEX-injected birds, the VH was the greatest in those fed Gln. In the challenged birds, supplementation of Gln increased (P < 0.05) the VH:CD ratio, whereas there was no difference between the other treatments. The villus surface area was significantly increased in birds fed a higher Arg level compared with other dietary treatments (P < 0.05). DEX decreased the villus surface area in all injected birds (P < 0.001).
Table 5.
Histomorphological measurements of jejunal tissues from broilers fed reduced protein (RP) diets supplemented with Gln, Gly, and Arg subjected to dexamethasone (DEX) injections (day 21).1
| Treatments | Villus height (μm) | Crypt depth (μm) | VH:CD3 | Villus surface area (104 μm2) | |
|---|---|---|---|---|---|
| Main effects | |||||
| Control (RP) | Sham | 1,246b,c | 127 | 10.6b | 2.07 |
| Injected | 1,179c | 119 | 10.5b | 1.56 | |
| RP + Gln | Sham | 1,252b,c | 122 | 10.6b | 1.79 |
| Injected | 1,326a,b | 101 | 13.9a | 1.62 | |
| RP + Gly | Sham | 1,237b,c | 119 | 11.1b | 1.87 |
| Injected | 1,237b,c | 128 | 10.1b | 1.82 | |
| RP + Arg | Sham | 1,399a | 131 | 11.5b | 2.39 |
| Injected | 1,239b,c | 130 | 10.2b | 1.78 | |
| SEM2 | 14.4 | 3.1 | 0.28 | 0.407 | |
| Diet | |||||
| Control (RP) | 1,212 | 123 | 10.5 | 1.82b | |
| RP + Gln | 1,289 | 111 | 12.2 | 1.70b | |
| RP + Gly | 1,237 | 123 | 10.6 | 1.85b | |
| RP + Arg | 1,319 | 130 | 10.8 | 2.08a | |
| DEX | |||||
| Sham | 1,284 | 124 | 10.9 | 2.03a | |
| Injected | 1,245 | 119 | 11.1 | 1.70b | |
| Source of variation | |||||
| Diet | 0.045 | 0.18 | 0.11 | 0.016 | |
| DEX | 0.192 | 0.39 | 0.71 | 0.0002 | |
| Diet × DEX | 0.042 | 0.37 | 0.026 | 0.052 | |
Means within a column not sharing a superscript differ significantly at the P level shown.
Pooled standard error of mean (n = 48).
Villus height to crypt depth ratio.
Relative Weight of Organs
As shown in Table 6, diet and DEX did not interact for relative weight of gizzard, liver, duodenum, jejunum, ileum, spleen, and bursa. Supplementation of Gln increased gizzard weight compared to control and Gly diet (P < 0.05). Both Gly and Gln increased ileal weight compared to control (P < 0.05). The relative weights of duodenum, jejunum, spleen, and bursa were not affected by the dietary treatments. Birds injected with DEX had greater gizzard (P < 0.001), liver (P < 0.001), duodenum (P < 0.05), and ileum (P < 0.01) weights compared to sham-injected birds. Spleen and bursa weights were decreased by DEX injection (P < 0.001).
Table 6.
Relative weight (g/100 g BW) of visceral organs of broilers fed reduced protein (RP) diets supplemented with Gln, Gly, or Arg subjected to dexamethasone (DEX) or sham injections (day 21).1
| Gizzard | Liver | Duodenum | Jejunum | Ileum | Spleen | Bursa | |
|---|---|---|---|---|---|---|---|
| Main effects | |||||||
| Diet | |||||||
| Control (RP) (n = 24)2 | 2.46b | 3.66 | 1.05 | 1.69 | 1.18b | 0.051 | 0.10 |
| RP+ Gln (n = 23) | 2.60a | 3.95 | 1.09 | 1.72 | 1.28a | 0.054 | 0.08 |
| RP+ Gly (n = 24) | 2.36b | 3.75 | 1.09 | 1.73 | 1.29a | 0.052 | 0.09 |
| RP+ Arg (n = 24) | 2.47a,b | 3.45 | 1.10 | 1.75 | 1.24a,b | 0.058 | 0.10 |
| DEX | |||||||
| Sham (n = 48) | 2.38b | 3.18b | 1.06b | 1.72 | 1.21b | 0.064a | 0.14a |
| Injected (n = 47) | 2.57a | 4.23a | 1.11a | 1.72 | 1.29a | 0.044b | 0.05b |
| SEM3 | 0.024 | 0.037 | 0.013 | 0.018 | 0.015 | 0.0013 | 0.003 |
| Source of variation | |||||||
| Diet | 0.016 | 0.065 | 0.384 | 0.767 | 0.038 | 0.304 | 0.185 |
| DEX | 0.0009 | <0.0001 | 0.048 | 0.850 | 0.011 | <0.0001 | <0.0001 |
| Diet × DEX | 0.299 | 0.415 | 0.989 | 0.944 | 0.101 | 0.839 | 0.313 |
Means within a column not sharing a superscript differ significantly at the P level shown for the main effects.
Values in parenthesis represent the number of replicates considering one dead bird.
Pooled standard error of mean (n = 97).
Relative mRNA Expression of Genes Related to Tight Junction Proteins
The results of the relative mRNA expression of tight junction proteins in jejunum and ileum of birds are presented in Table 7. For jejunal tissues, compared with control diet, Gln and Gly supplementation reduced the expression of claudin-1 in the absence of any DEX effect. There was an interaction between DEX and diet (P < 0.01) for the expression of jejunal claudin-3 where DEX upregulated claudin-3 for all diets except those supplemented with additional Arg. The relative expression of ZO-1 and ZO-2 was not affected in the jejunum. Diets had no effect on JAM2 and occludin, whereas DEX downregulated (P < 0.01) expression of JAM2 regardless of the diets.
Table 7.
Relative mRNA expression of genes related to tight junction proteins in jejunum and ileum of broilers fed reduced protein diets supplemented with Gln, Gly, or Arg subjected to dexamethasone (DEX) or sham injections (day 21).1,2
| Jejunum | Ileum | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diet | DEX | Claudin-1 | Claudin-3 | ZO-1 | ZO-2 | JAM2 | Occludin | Claudin-1 | Claudin-3 | ZO-1 | ZO-2 | JAM2 | Occludin |
| Control (RP) | Sham | 0.98 | 0.77d,e | 2.41 | 2.09 | 3.10 | 0.95 | 1.11 | 1.69 | 2.47 | 2.02d | 4.11 | 1.16 |
| Injected | 1.00 | 1.49a | 2.19 | 2.47 | 2.99 | 1.12 | 1.49 | 2.76 | 3.28 | 3.64a | 4.33 | 1.77 | |
| RP + Gln | Sham | 0.79 | 0.61e | 2.12 | 1.87 | 3.44 | 0.86 | 0.94 | 1.52 | 2.70 | 2.46c,d | 4.54 | 1.15 |
| Injected | 0.79 | 1.27a,b | 2.01 | 2.16 | 2.37 | 1.01 | 1.19 | 2.39 | 2.88 | 2.82b,c | 3.82 | 1.53 | |
| RP + Gly | Sham | 0.86 | 0.75d,e | 1.98 | 1.74 | 3.68 | 0.91 | 0.87 | 1.75 | 2.62 | 2.35c,d | 4.99 | 1.13 |
| Injected | 0.76 | 1.14b,c | 1.78 | 2.01 | 2.81 | 0.89 | 1.13 | 2.37 | 2.84 | 2.84b,c | 3.04 | 1.33 | |
| RP + Arg | Sham | 0.99 | 0.80d,e | 2.18 | 1.95 | 3.91 | 0.94 | 2.59 | 1.63 | 2.56 | 2.64b,c | 3.94 | 1.20 |
| Injected | 0.87 | 0.89c,d | 1.88 | 1.80 | 2.99 | 0.83 | 1.68 | 2.31 | 2.87 | 3.11a,b | 4.47 | 1.69 | |
| SEM3 | 0.028 | 0.034 | 0.053 | 0.057 | 0.115 | 0.111 | 0.124 | 0.071 | 0.063 | 0.075 | 0.156 | 0.113 | |
| Main effects | |||||||||||||
| Diet | |||||||||||||
| Control (RP) | 0.99a | 1.13 | 2.30 | 2.28 | 3.04 | 1.04 | 1.30b | 2.23 | 2.88 | 2.83 | 4.22 | 1.47 | |
| RP + Gln | 0.79b | 0.94 | 2.06 | 2.01 | 2.90 | 0.93 | 1.07b | 1.95 | 2.79 | 2.64 | 4.18 | 0.93 | |
| RP + Gly | 0.81b | 0.95 | 1.88 | 1.87 | 3.21 | 0.90 | 1.00b | 2.06 | 2.73 | 2.59 | 4.02 | 0.90 | |
| RP + Arg | 0.93a,b | 0.85 | 2.03 | 1.87 | 3.45 | 0.89 | 2.13a | 1.97 | 2.71 | 2.87 | 4.21 | 0.89 | |
| DEX | |||||||||||||
| Sham | 0.91 | 0.73 | 2.17 | 1.91 | 3.52a | 0.91 | 1.38 | 1.65b | 2.59b | 2.37 | 4.39 | 1.16a | |
| Injected | 0.85 | 1.20 | 1.96 | 2.11 | 2.79b | 0.96 | 1.37 | 2.46a | 2.97a | 3.10 | 3.91 | 1.59b | |
| Source of variation | |||||||||||||
| Diet | 0.045 | 0.039 | 0.063 | 0.051 | 0.38 | 0.41 | 0.009 | 0.50 | 0.79 | 0.47 | 0.96 | 0.19 | |
| DEX | 0.36 | <.0001 | 0.058 | 0.09 | 0.003 | 0.45 | 0.98 | <.0001 | 0.004 | <.0001 | 0.13 | <0.0001 | |
| Diet × DEX | 0.76 | 0.007 | 0.93 | 0.37 | 0.46 | 0.37 | 0.23 | 0.67 | 0.27 | 0.015 | 0.063 | 0.39 | |
Means within a column not sharing a superscript differ significantly at the P level shown.
Each value represents the mean of 6 replicates for treatment effects, 12 replicates for the diet effect, and 24 replicates for DEX effect.
Pooled standard error of mean (n = 48).
For ileal tissues, there was no interaction for the measured gene expression of selected tight junction proteins expect for ZO-2. Birds receiving Arg treatment had greater (P < 0.01) expression of claudin-1 compared to the other diets. DEX independently increased the expression of claudin-3 (P < 0.001), ZO-1 (P < 0.01), and occludin (P < 0.001). Through a significant interaction (P < 0.05), DEX upregulated ZO-2 expression only in birds fed control diets.
DISCUSSION
In the present study, grower and finisher diets were slightly reduced in protein in order to keep the diet similar to an industry practicable diet, whereas starter diets were kept the same for all birds. In general, bird performance was above the Ross 308 standards (Aviagen, 2014) across all dietary treatments. Among all dietary treatments, the non-supplemented RP diet had the highest FI when compared with the standard diet suggesting that some nutrients in the standard diet may have been limiting in the RP diet, triggering a compensatory response for birds to adjust their FI accordingly. It is known that birds can adjust their FI in response to various dietary factors in particular amino acids (Dozier et al., 2008). Therefore, the higher BWG of the same birds could be related to higher FI. It is also noteworthy that in the standard protein diet used in this study, the inclusion level of meat and bone meal was unavoidably higher (3.1% higher in grower diets) than the RP diets which may attribute to difference in BWG. Some negative effects of meat and bone meal on broiler performance have been documented (Liu et al., 2016). Nevertheless, similar FCR of the birds fed unsupplemented RP compared with standard protein diet suggests that a moderate reduction in dietary protein is possible without an adverse effect on performance provided that all essential amino acids are met with a relatively similar ratio of amino acids.
Results of the current study suggest that Gly+Ser equivalent (Glyequ on an equimolar basis) of the RP diets (11.4 g/kg in grower and 10.3 g/kg in finisher) did not limit the performance as 10 g/kg additional Gly offered no benefit. These observations are relatively similar to those made by van Harn et al. (2018), who reported that 12.4 g/kg and 11.4 g/kg Glyequ in grower and finisher phase respectively were sufficient to support the growth of birds fed RP diets. Contrarily, the total Gly+Ser level of 21 g/kg recommended by Waguespack et al. (2009) as a minimum requirement is not supported by the current study. The response to Gly is affected by both Gly precursors and metabolites that are dependent on Gly including Thr, total sulfur amino acids, and choline (Kriseldi et al., 2017). The adequate provision of these nutrients in the formulated diets may have limited the response to additional Gly in the diets.
The absence of a positive response to 10 g/kg L-Gln suggested that the RP wheat-based diet did not require additional L-Gln, at least at the tested level. A recent trial found no effect of L-Gln on performance of Ross 308 broilers in wheat and sorghum-based diets with recommended amino acids and protein content (Barekatain and Toghyani, 2019). Additionally, most of the published effect of L-Gln is based on a corn/soybean meal-based diet which contains less Gln than a wheat-based diet (Zulkifli et al., 2016; Kriseldi et al., 2017). Although the exact mechanism by which L-Gln reduced the FI of RP diet cannot be immediately explained by the data of the current experiment, it may be possible that additional L-Gln along with sufficient glutamic acid present in the wheat-based diet could increase satiety through regulating gastric distension or eating behavior (Jordi et al., 2013). Conversely, liver size in birds fed Gln was tended to be higher than any other treatment in this study. The change in liver weight could be a function of FI indicating changes in energy expenditure (Zaefarian et al., 2019). Further research is required to elucidate FI regulation of Gln in avian species.
Additional supplementation of Arg (5 g/kg) raised the Arg content of the diets to approximately 140% of the Ross 308 recommendation resulting in a significant improvement in feed efficiency both in individual and group-housed birds. This was accompanied by an increase in VH and absorptive surface area observed in this study that are consistent with data reported by Tan et al. (2014a). The positive growth promoting effect of Arg is mainly attributed to the actions of its metabolites such as NO or polyamines and increase in production of IgA (Zhang et al., 2017). The increased surface area as observed in the current study also provides an opportunity for increased absorption and digestion of nutrients which ultimately can support the growth of the birds.
Similar to the birds raised as a group, Arg improved the FCR. However, additional Gly resulted in an improved FCR of the individual birds during 1-wk measurement. As there was no effect of Gly in group-raised bird chickens, this observation may have been attributed to a high rate of growth in a short, 1 wk, assessment from day 14 to 21 as well as the change in housing conditions (cage vs floor).
For the challenge component of the current study, the severe reduction of FI and BWG as a result of DEX injections was expected and consistent with previous studies (Wideman Jr and Pevzner, 2012; Barekatain et al., 2018). DEX as an exogenous glucocorticoid (GC) has a profound effect on energy homeostasis and glucose metabolism. Such alterations occur in various tissues ultimately impacting performance of the animal (Pasieka and Rafacho, 2016). Besides, preferential binding of DEX to the type II GC receptor induces physiological stress effects including immunosuppression (Turner et al., 2012). The profound effect of DEX on immune organs was evidenced by bursa and spleen atrophy and an enlarged liver. The proportionate increase in relative weight of the gizzard, duodenum, and ileum may have also been a reflection of a possible muscle atrophy caused by DEX-induced stress (Song et al., 2011).
Intestinal architecture is another factor by which intestinal absorptive ability is evaluated. With no significant changes in CD, the reduced villus surface area in DEX-injected bird may have been driven by changes in villus width. However, such a reduction in villus surface area could indicate a potential adverse effect on nutrient absorption, primarily glucose (Li et al., 2009). A depressed feed consumption in DEX treatment could also be another explanation for changes in intestinal morphology.
Tight junction proteins are adhesive junctional molecules that link epithelial cells together. These proteins are fundamentally important in controlling IP thereby facilitating the passage of ions and solutes intracellularly while blocking the entry of unwanted microorganisms, antigens, and related toxins (Groschwitz and Hogan, 2009). In the current study, the FITC-d test was used as a reliable indicator of IP, mainly through the paracellular pathway documented across many species. In a compromised intestinal barrier, the passage of FITC-d from mucosal side to the serosal side will increase, leading to an elevation of FITC-d in blood. In the current study, repeated DEX injections significantly increased FITC-d in the blood confirming the successful inducement of the leaky gut. This is consistent with a previous study (Barekatain et al., 2018), where administration of DEX increased IP. Different mechanisms have been proposed for the effect of exogenous GC on IP, for which bacterial–mucosal cell interactions is of great importance (Spitz et al., 1994). Besides, corticotrophin-releasing hormone, mast cells, cholinergic and adrenergic nerves have been suggested to mediate the stress-induced permeability changes (Santos et al., 1999). The increased IP caused by DEX was accompanied with differential gene expression of tight junctions in both jejunal and ileal tissues. Most of the tight junction genes, with the exception of jejunal JAM2, were overly expressed in most likely a compensatory response to DEX to restore IP. This differential gene expression may have been associated with the highly dynamic nature of the tight junctions where these proteins can be remodeled and redistributed in response to various stimuli including nutrient transporters present in different regions of the small intestine (Barekatain et al., 2018). In a compromised barrier function status, the expression of barrier forming tight junctions including claudin 1 and 3 would be expected to decline. Therefore, it is possible that the effect of DEX includes factors beyond expression and distribution of tight junctions.
In broilers, recent evidence suggests that dietary protein, balance, and concentration of amino acids can influence IP (Soomro et al., 2017; Barekatain et al., 2018). Although the dietary effect on IP was not statistically significant, there was a tendency for a lower IP as shown by blood concentration of FITC-d in birds fed 0.5 g/kg additional Arg. Although the protective effects of Arg during coccidial challenge (Tan et al., 2014a) or endotoxins (Tan et al., 2014b) reduced IP from Eimeria/C. perfringens challenge in Ussing chambers in birds (Zhang et al., 2017) and pigs (Corl et al., 2008) have been shown, data are scarce on the effect of Arg on in vivo IP in poultry. In the current experiment, an Arg independent effect was observed for the ileal mRNA expression of claudin-1 being upregulated. This increase in claudin-1 expression, as a barrier forming tight junction protein, alongside a numeral decrease in serum FITC-d of the same birds suggests a protective role of Arg for the intestinal barrier in birds fed RP diets. Although a molecular mechanism of intestinal barrier regulation by Arg is not fully understood, it is thought that Arg effects are mediated through the actions of polyamines and NO and changes in the microbiota composition (Sellmann et al., 2017) that were not directly studied here. By an interaction, the increase of claudin-3 observed in the birds injected with DEX was only limited in the Arg-fed group. Zhang et al. (2017) reported that feeding broilers with a diet containing 1.87% Arg prevented the changes in claudin-1 mRNA expression caused by an Eimeria/C. perfringens challenge. This notable interaction highlights the significance of Arg in stress-associated responses and hypercatabolic states, suggesting that Arg may be an important amino acid under stress conditions when the gut barrier is compromised. Considered together, the positive effect of additional Arg tends to support that the bird requirement for Arg for optimal barrier function and growth may be higher than the current industry recommendations.
In the present study, supplementation of RP diet with 10 g/kg Gln had no effect on gut permeability assessed by FITC-d. Our results are in agreement with recent observations by Gilani et al. (2018c), who found no effect of 1% Gln in young broilers assessed by FITC-d and older age broilers (day 38) studied by both FITC-d and dual-sugars tests (Gilani et al., 2018b). The positive role of Gln in reducing permeability of the Caco-2 intestinal epithelial cell line has been documented whereby Gln deprivation increased permeability and decreased tight junction expression of claudin-1, occludin, and ZO-1 (DeMarco et al., 2003; Li et al., 2004). Several potential mechanisms are reported in the literature, one of which is through modulation of tight junctions (Kim and Kim, 2017). Glutamine possesses a strong potential to affect IP through modulation of tight junctions via the MAKP-dependent signal transduction pathway (Ulluwishewa et al., 2011). The gene expression assays in the present study revealed no effect of Gln on any of the selected tight junctions except a reduction in mRNA level of jejunal claudin-1. The largely unaffected tight junction expression is consistent with the lack of change in IP of the same birds fed Gln-supplemented diets. However, the only positive effects associated with Gln were related to VH and VH:CD in DEX-injected birds which may indicate that Gln can be beneficial for intestinal development under stress conditions. Future studies on Gln should focus on a lower inclusion level or under pathological conditions to elucidate the mechanistic response of the intestinal barrier of broilers to Gln. Nevertheless, it is prudent to assume that the provision of Gln as a synthesizable amino acid in chickens and its presence in wheat-based diets offered to the birds have been sufficient to support a normal IP and the barrier function of these animals. Therefore, beyond a certain level of Gln in the diet there is no apparent effect at least in a normal and healthy flock. In the current study, it was also hypothesized that a reduction in dietary protein would possibly provide an opportunity for Gln to influence IP and a possible reduction of the IP induced by GC. This lack of Gln effect further highlights that the reduction of protein did not present a deficiency of amino acids to the extent that IP could be significantly influenced.
To the authors’ knowledge, in this study, for the first time the effects of Gly on IP and tight junction expression have been reported for poultry. Although the mRNA expression of jejunal claudin-1 and ZO-2 was downregulated in response to Gly supplementation, the changes in these tight junctions were not associated with any significant change in IP assessed by FITC-d concentration in the serum. Gly has been shown to be effective in maintaining the barrier function of intestinal enterocytes isolated from the jejunum of piglets evidenced by decreasing the transepithelial electrical resistance (TEER) and expression of tight junction proteins (Li et al., 2016). The in vitro-based TEER method on cell isolates is a more sensitive test compared to in vivo techniques including FITC-d used in this study that may explain the discrepancy of the observations. Further studies at the molecular level may provide a possible explanation on anti-inflammatory or specific pathways that Gly could affect the regulation of tight junctions. Reducing the dietary protein further to what was adapted in the current study could also present a Gly deficiency under which changes in IP could possibly be observed.
In conclusion, the results of the current study suggest that increasing Arg levels of a RP diet can be beneficial in terms of performance, intestinal absorptive area, and strengthening intestinal barrier function through an effect on tight junction gene expression and permeability under stress induced by DEX. Gln at 10 g/kg had no demonstrable effect on IP and had some negative effects on tight junction expression. The additional Gly offered to birds fed RP diets did not exhibit consistent benefit in terms of IP or growth performance when assessed for grower and finisher diets. Further molecular assays in terms of intestinal inflammatory response and metabolic pathways are required to investigate the potential for individual amino acids to protect against intestinal disorders caused by stress.
ACKNOWLEDGMENTS
This project was supported by the Chicken Meat Program of AgriFutures Australia. Authors acknowledge technical assistance from SARDI technical staff and scientists, Derek Schultz, Kylee Swanson, Soressa Kitessa, and Carolyn de Koning.
REFERENCES
- Aviagen. 2014. Ross 308 Broiler: Performance Objectives. Ross Breeders Limited, Newbridge, Midlothian, Scotland, UK. [Google Scholar]
- Barekatain R., Nattrass G., Tilbrook A., Chousalkar K., Gilani S.. 2018. Reduced protein diet and amino acid concentration alter intestinal barrier function and performance of broiler chickens with or without synthetic glucocorticoid. Poult. Sci. 10.3382/ps/pey563. [DOI] [PubMed] [Google Scholar]
- Barekatain R., Toghyani M.. 2019. High dietary zinc and glutamine do not improve the performance or reduce excreta moisture of broiler chickens fed diets with and without magnesium supplementation. Poult. Sci. 10.3382/ps/pez098. [DOI] [PubMed] [Google Scholar]
- Bartell S. M., Batal A. B.. 2007. The effect of supplemental glutamine on growth performance, development of the gastrointestinal tract, and humoral immune response of broilers. Poult. Sci. 86:1940–1947. [DOI] [PubMed] [Google Scholar]
- Bischoff S. C., Barbara G., Buurman W., Ockhuizen T., Schulzke J.-D., Serino M., Tilg H., Watson A., Wells J. M.. 2014. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoluzzi C., Rochell S., Applegate T.. 2018. Threonine, arginine, and glutamine: influences on intestinal physiology, immunology, and microbiology in broilers. Poult. Sci. 97:937–945. [DOI] [PubMed] [Google Scholar]
- Chen X., Naehrer K., Applegate T.. 2016. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 95:1312–1325. [DOI] [PubMed] [Google Scholar]
- Chen J., Tellez G., Richards J. D., Escobar J.. 2015. Identification of potential biomarkers for gut barrier failure in broiler chickens. Front. Vet. Sci. 2 10.3389/fvets.2015.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M. 2012. The impact of diet energy and amino acid content on the feed intake and performance of broiler chickens. Master Thesis, University of Saskatchewan; SK. Canada. [Google Scholar]
- Corl B. A., Odle J., Niu X., Moeser A. J., Gatlin L. A., Phillips O. T., Blikslager A. T., Rhoads J. M.. 2008. Arginine activates intestinal p70S6k and protein synthesis in piglet rotavirus enteritis. J. Nutr. 138:24–29. [DOI] [PubMed] [Google Scholar]
- Coster J., McCauley R., Hall J.. 2004. Glutamine: metabolism and application in nutrition support. Asia Pac. J. Clin. Nutr. 13:25–31. [PubMed] [Google Scholar]
- DeMarco V. G., Li N., Thomas J., West C. M., Neu J.. 2003. Research communication: glutamine and barrier function in cultured Caco-2 epithelial cell monolayers. J. Nutr. 133:2176–2179. [DOI] [PubMed] [Google Scholar]
- Dozier W. III, Kidd M., Corzo A.. 2008. Dietary amino acid responses of broiler chickens. J. Appl. Poult. Res. 17:157–167. [Google Scholar]
- Gilani S., Howarth G., Kitessa S., Forder R., Tran C., Hughes B.. 2016. New biomarkers for intestinal permeability induced by lipopolysaccharide in chickens. Anim. Prod. Sci. 56:1984–1997. [Google Scholar]
- Gilani S., Howarth G., Nattrass G., Kitessa S., Barekatain R., Forder R., Tran C., Hughes R.. 2018a. Gene expression and morphological changes in the intestinal mucosa associated with increased permeability induced by short-term fasting in chickens. J. Anim. Physiol. Anim. Nutr. 102:e653–e661. [DOI] [PubMed] [Google Scholar]
- Gilani S., Howarth G., Tran C., Barekatain R., Kitessa S., Forder R., Hughes R.. 2018b. Reduced fasting periods increase intestinal permeability in chickens. J. Anim. Physiol. Anim. Nutr. 102:e486–e492. [DOI] [PubMed] [Google Scholar]
- Gilani S., Howarth G. S., Tran C. D., Kitessa S. M., Forder R. E., Barekatain R., Hughes R. J.. 2018c. Effects of delayed feeding, sodium butyrate and glutamine on intestinal permeability in newly-hatched broiler chickens. J. Appl. Anim. Res. 46:973–976. [Google Scholar]
- Golder H., Geier M., Forder R., Hynd P., Hughes R.. 2011. Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. Br. Poult. Sci. 52:500–506. [DOI] [PubMed] [Google Scholar]
- Groschwitz K. R., Hogan S. P.. 2009. Intestinal barrier function: molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 124:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi S. K., Odle J.. 2012. Nutritional factors influencing intestinal health of the neonate. Adv. Nutr. 3:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordi J., Herzog B., Camargo S. M., Boyle C. N., Lutz T. A., Verrey F.. 2013. Specific amino acids inhibit food intake via the area postrema or vagal afferents. J. Physiol. 591:5611–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.-H., Kim H.. 2017. The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. 18:E1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriseldi R., Tillman P., Jiang Z., Dozier W. III. 2017. Effects of glycine and glutamine supplementation to reduced crude protein diets on growth performance and carcass characteristics of male broilers during a 41-day production period. J. Appl. Poult. Res. 26:558–572. [Google Scholar]
- Li Y., Cai H., Liu G., Dong X., Chang W., Zhang S., Zheng A., Chen G.. 2009. Effects of stress simulated by dexamethasone on jejunal glucose transport in broilers. Poult. Sci. 88:330–337. [DOI] [PubMed] [Google Scholar]
- Li N., Lewis P., Samuelson D., Liboni K., Neu J.. 2004. Glutamine regulates Caco-2 cell tight junction proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 287:G726–G733. [DOI] [PubMed] [Google Scholar]
- Li W., Sun K., Ji Y., Wu Z., Wang W., Dai Z., Wu G.. 2016. Glycine regulates expression and distribution of Claudin-7 and ZO-3 proteins in intestinal porcine epithelial cells. J. Nutr. 146:964–969. [DOI] [PubMed] [Google Scholar]
- Liu S. Y., Cowieson A. J., Selle P. H.. 2016. The influence of meat-and-bone meal and exogenous phytase on growth performance, bone mineralisation and digestibility coefficients of protein (N), amino acids and starch in broiler chickens. Anim. Nutr. 2:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A., Sakamoto M., Natali M., Souza L., Franco J.. 2007. Supplementation of glutamine and vitamin E on the morphometry of the intestinal mucosa in broiler chickens. Poult. Sci. 86:488–495. [DOI] [PubMed] [Google Scholar]
- Ospina-Rojas I., Murakami A., Eyng C., Nunes R., Duarte C., Vargas M.. 2012. Commercially available amino acid supplementation of low-protein diets for broiler chickens with different ratios of digestible glycine + serine: Lysine. Poult. Sci. 91:3148–3155. [DOI] [PubMed] [Google Scholar]
- Pasieka A., Rafacho A.. 2016. Impact of glucocorticoid excess on glucose tolerance: clinical and preclinical evidence. Metabolites 6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J., Saunders P. R., Hanssen N. P., Yang P.-C., Yates D., Groot J. A., Perdue M. H.. 1999. Corticotropin-releasing hormone mimics stress-induced colonic epithelial pathophysiology in the rat. Am. J. Physiol. Gastrointest. Liver Physiol. 277:G391–G399. [DOI] [PubMed] [Google Scholar]
- Sellmann C., Degen C., Jin C. J., Nier A., Engstler A. J., Alkhatib D. H., De Bandt J.-P., Bergheim I.. 2017. Oral arginine supplementation protects female mice from the onset of non-alcoholic steatohepatitis. Amino Acids 49:1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. 2012. SAS® Qualification Tools User’s Guide. Version 9.4. SAS Institute Inc., Cary, NC. [Google Scholar]
- Siegert W., Wild K., Schollenberger M., Helmbrecht A., Rodehutscord M.. 2016. Effect of glycine supplementation in low protein diets with amino acids from soy protein isolate or free amino acids on broiler growth and nitrogen utilisation. Br. Poult. Sci. 57:424–434. [DOI] [PubMed] [Google Scholar]
- Soares A. D., Costa K. A., Wanner S. P., Santos R. G., Fernandes S. O., Martins F. S., Nicoli J. R., Coimbra C. C., Cardoso V. N.. 2014. Dietary glutamine prevents the loss of intestinal barrier function and attenuates the increase in core body temperature induced by acute heat exposure. Br. J. Nutr. 112:1601–1610. [DOI] [PubMed] [Google Scholar]
- Song Z., Zhang X., Zhu L., Jiao H., Lin H.. 2011. Dexamethasone alters the expression of genes related to the growth of skeletal muscle in chickens (Gallus gallus domesticus). J. Mol. Endocrinol. 46:217–225 [DOI] [PubMed] [Google Scholar]
- Soomro R. N., Hu R., Qiao Y., El-Hack M. E. A., Abbasi I. H. R., Mohamed M. A. E., Bodinga B. M., Alagawany M., Yang X., Yao J.. 2017. Effect of dietary protein sources and amino acid balances on performance, intestinal permeability and morphology in broiler chickens. Int. J. Pharmacol. 13:378–387. [Google Scholar]
- Spitz J., Hecht G., Taveras M., Aoys E., Alverdy J.. 1994. The effect of dexamethasone administration on rat intestinal permeability: The role of bacterial adherence. Gastroenterology 106:35–41. [DOI] [PubMed] [Google Scholar]
- Tan J., Applegate T. J., Liu S., Guo Y., Eicher S. D.. 2014a. Supplemental dietary L-arginine attenuates intestinal mucosal disruption during a coccidial vaccine challenge in broiler chickens. Br. J. Nutr. 112:1098–1109. [DOI] [PubMed] [Google Scholar]
- Tan J., Liu S., Guo Y., Applegate T. J., Eicher S. D.. 2014b. Dietary L-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 111:1394–1404. [DOI] [PubMed] [Google Scholar]
- Turner A. I., Keating C. L., Tilbrook A. J.. 2012. Sex differences and the role of sex steroids in sympatho-adrenal medullary system and hypothalamo-pituitary adrenal axis responses to stress. Pages 115–136 in Sex Steroids Kahn S. M., ed. InTech, Croatia. [Google Scholar]
- Ulluwishewa D., Anderson R. C., McNabb W. C., Moughan P. J., Wells J. M., Roy N. C.. 2011. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 141:769–776. [DOI] [PubMed] [Google Scholar]
- van Harn J., Dijkslag M., van Krimpen M.. 2018. Glycine plus serine requirement of broilers fed low-protein diets: a dose response study. Wageningen: Wageningen Livestock Research; https://library.wur.nl/WebQuery/wurpubs/539325. [Google Scholar]
- Waguespack A. M., Powell S., Bidner T. D., Southern L. L.. 2009. The glycine plus serine requirement of broiler chicks fed low-crude protein, corn-soybean meal diets. J. Appl. Poult. Res. 18:761–765. [Google Scholar]
- Wideman R. Jr, Pevzner I.. 2012. Dexamethasone triggers lameness associated with necrosis of the proximal tibial head and proximal femoral head in broilers. Poult. Sci. 91:2464–2474. [DOI] [PubMed] [Google Scholar]
- Wu G., Knabe D. A.. 1995. Arginine synthesis in enterocytes of neonatal pigs. Am. J. Physiol. Regul. Integr. Comp. Physiol. 269:R621–R629. [DOI] [PubMed] [Google Scholar]
- Zaefarian F., Abdollahi M. R., Cowieson A., Ravindran V.. 2019. Avian liver: the forgotten organ. Animals 9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Lv Z., Li H., Guo S., Liu D., Guo Y.. 2017. Dietary L-arginine inhibits intestinal Clostridium perfringens colonisation and attenuates intestinal mucosal injury in broiler chickens. Br. J. Nutr. 118:321–332. [DOI] [PubMed] [Google Scholar]
- Zuidhof M., Schneider B., Carney V., Korver D., Robinson F.. 2014. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 93:2970–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulkifli I., Shakeri M., Soleimani A.. 2016. Dietary supplementation of L-glutamine and L-glutamate in broiler chicks subjected to delayed placement. Poult. Sci. 95:2757–2763. [DOI] [PubMed] [Google Scholar]


