Abstract
Hippocampal atrophy in advanced healthy aging has frequently been reported. However, the vulnerability of different hippocampal subfields to age‐related atrophy is still a source of debate. Moreover, the association of age with the microstructural integrity of subfields is largely unknown. In this study, we investigated the associations between age and volume as well as microstructural integrity of hippocampal subfields using a three‐dimensional (3D) surface mapping approach. Forty‐three healthy older adults spanning the age range from 60 to 85 years underwent T1‐weighted and diffusion‐tensor imaging. Analyses demonstrated an association of age with hippocampal volume predominantly in the most anterior part of the hippocampal head, mainly corresponding to the subiculum. In contrast, the association of age with hippocampal microstructural integrity was mainly confined to regions located in the hippocampal body and tail, corresponding to the subiculum and CA1. Results indicate that age‐related volumetric and microstructural alterations within hippocampal subfields provide complementary information and reflect different age‐related processes. Potential mechanisms underlying the differential associations of age with volume and microstructure of hippocampal subfields are discussed. Hum Brain Mapp 36:3819–3831, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: hippocampal subfields, subiculum, CA1, hippocampal volume, hippocampal microstructure, diffusion‐tensor imaging, healthy aging
INTRODUCTION
The hippocampus has been one of the most studied cerebral structures in neuroimaging research during the last decades. Significant volume reductions have been reported in geriatric depression [Sawyer et al., 2012; Steffens et al., 2011], in Parkinson's disease [Camicioli et al., 2003], and, most consistently, in Alzheimer's disease (AD) [Barnes et al., 2009; Jack et al., 2000]. Hippocampal atrophy is not limited to age‐related psychiatric and neurodegenerative disorders but has also been reported in normal aging. However, the pattern of age‐related atrophy is still a source of debate. The hippocampus is composed of molecularly and functionally distinct subfields, comprising the subiculum, the four cornu ammonis (CA1‐4), and the dentate gyrus (DG) [Duvernoy, 2005]. While some studies reported associations of age with the volume of CA1 and DG and CA3 [Mueller and Weiner, 2009], others found associations of age with the volume of the subiculum and CA1 [Frisoni et al., 2008], the subiculum alone [Chételat et al., 2008], or with all of these subfields [Apostolova et al., 2012]. Moreover, age‐related changes in hippocampal microstructure are barely studied. A more profound understanding of both micro‐ and macrostructural hippocampal changes within normal aging is necessary, not only to gain deeper insights into the healthy aging process but also to more precisely delineate normal and pathological age‐related structural alterations.
Most studies have assessed the hippocampus as a single structure. In the last decade, several sophisticated methods have been developed that enable the investigation of hippocampal subfields based on magnetic resonance imaging (MRI), including surface mapping [Apostolova et al., 2012; Chételat et al., 2008; Frisoni et al., 2008], manual segmentation of ultrahigh resolution images [La Joie et al., 2010; Mueller and Weiner, 2009; Winterburn et al., 2013; Wisse et al., 2012], and automated delineation [Pipitone et al., 2014; Van Leemput et al., 2009; Yushkevich et al., 2010, 2015]. The application of these in vivo methods has led to an increased awareness of different vulnerabilities of hippocampal subfield volumes to age‐related processes. However, the pattern of volumetric alterations of hippocampal subfields is still a source of debate. While several studies reported an association of age with the volume of CA1 [de Flores et al., 2015; Frisoni et al., 2008; Mueller et al., 2007, 2008; Shing et al., 2011; Wisse et al., 2014b] and the subiculum [Chételat et al., 2008; de Flores et al., 2015; Frisoni et al., 2008; La Joie et al., 2010; Thomann et al., 2013; Wang et al., 2003], others found smaller volumes of CA3‐4 and DG with increasing age [Mueller and Weiner, 2009; Pereira et al., 2014; Wisse et al., 2014b]. The discrepancies in the results may be a consequence of the application of different methodological approaches as well as of the investigation of different age‐ranges.
In addition to studies on age‐related hippocampal atrophy the investigation of changes in hippocampal microstructure, as measured by diffusion‐tensor imaging (DTI), is receiving increasing attention. DTI measures the random thermal motion of water molecules in biological tissues, which permits an indirect reconstruction of the structural organization and integrity of brain tissues. The most frequently applied diffusion metrics are mean diffusivity (MD) and fractional anisotropy (FA) [Basser and Pierpaoli, 1996]. MD is thought to be an indicator of microscopic barriers or obstacles in both gray matter (GM) and white matter (WM). In GM regions such as the hippocampus, increasing MD is most likely related to loss of cellular barriers, increases in extracellular fluid caused by shrinkage of neurons, and axonal degeneration [Basser et al., 1994, den Heijer et al., 2012]. FA is thought to quantify the directional dependence of diffusion and reflects the organization of neuronal elements particularly within myelinated fiber bundles [Basser et al., 1994, den Heijer et al., 2012]. Accordingly, FA analyses have primarily been performed in WM regions. In GM regions, the existence of multiple crossing unmyelinated fibers lowers FA, which leads to a low sensitivity of FA to microstructural changes within GM regions.
DTI‐based studies demonstrated a strong positive association between age and hippocampal MD in older adults beyond their 50s. Of note, this association was stronger than the association of age with hippocampal volume [Carlesimo et al., 2010; den Heijer et al., 2012]. However, analyses within these studies were restricted to DTI measures of the entire hippocampus. In spite of the increasing awareness of different vulnerabilities of hippocampal subfield volumes to age‐related processes, to date only one study investigated the relationship between age and the microstructure of specific hippocampal subfields. Using automated subfield segmentation implemented in Free Surfer [Van Leemput et al., 2009], this study demonstrated positive associations between age and MD in CA2‐3. Moreover, a positive association between age and MD in the whole hippocampus and a negative association between age and FA in the subiculum has been found [Pereira et al., 2014]. However, recent studies cast doubts on the accuracy of the automated segmentation procedure applied in this study [de Flores et al., 2015; Pluta et al., 2012; Schoene‐Bake et al., 2014; Wisse et al., 2014a].
The present study was motivated by the need of a more profound understanding of micro‐ and macrostructural changes of hippocampal subfields in normal aging. The study aimed at investigating and comparing the associations of age with volume and microstructural integrity of hippocampal subfields using a semiautomated 3D surface mapping approach. This approach has been validated against manual segmentation using ultrahigh‐resolution MRI [La Joie et al., 2010]. Since several studies demonstrated an acceleration of hippocampal atrophy after the age of 60 years [Pfefferbaum et al., 2013; Raz et al., 2010], this study focused on older adults with an age range of 60–85 years. Other studies that investigated a comparable age range demonstrated strong associations of age with the volume of the subiculum and CA1 [Frisoni et al., 2008; Thomann et al., 2013; Wang et al., 2003]. We expected to find a negative association between age and the volume of the same hippocampal subregions. Moreover, we hypothesized that those hippocampal subfields showing a negative association between age and volume also demonstrate a negative association between age and microstructural integrity. Against the background of the low sensitivity of FA in GM, hippocampal microstructure was quantified exclusively by MD.
METHODS
Participants
We examined the data of 43 healthy older adults spanning the age range from 60 to 85 years (demographics of the sample are given in Table 1). Participants were recruited via newspaper announcement and advertisements posted in the University Medical Center of the Johannes Gutenberg University Mainz as well as in several public institutions. Prior to study enrolment participants underwent a psychiatric screening interview, including the Diagnostic expert system for psychiatric disorders—Stamm Screening Questionnaire [Wittchen and Pfister, 1997] and the International Diagnostic Checklists for ICD‐10 and DSM‐IV [Hiller et al., 1996]. Participants were excluded if they had a history of psychiatric, neurologic, cerebrovascular, or cognitive illness. To ensure that they were cognitively normal, all participants underwent a broad battery of neuropsychological tests: (a) Verbal learning and memory test [Helmstaedter et al., 2001], which is a German version of the auditory verbal learning test that requires learning of a list of 15 words in five consecutive trials, free recall of these words after each trial, and recognition after 20 minutes; (b) Wechsler memory scale‐revised, digit and block span (forward and backward, respectively) [Wechsler, 1987]; (c) Tower of London, which is a measure of planning and problem solving [Shallice, 1982]; (d) Trail Making Test Parts A and B, which are measures of information processing speed (Part A) and cognitive flexibility (Part B) [Reitan, 1958]. Participants were excluded if the performance in any test was lower than two standard deviations under the group mean. The study was approved by the local Ethics Committee. Written informed consent was provided by all participants according to the Declaration of Helsinki.
Table 1.
Demographic characteristics of the study group
| N | 43 |
| Age (range in years) | 70.02 ± 7.75 (60–85) |
| Education (range in years) | 12.23 ± 3.21 (9–17) |
| Gender | |
| Male (N (%)) | 18 (42%) |
| Female (N (%)) | 25 (58%) |
| Hypertension (N (%)) | 22a (51%) |
Continuous variables are represented as mean ± SD. The range is given in parentheses.
Categorical variables are represented as number. Percentage values are given in parenthesis.
All cases of hypertension were regulated with medication.
MRI Data Acquisition
MRI was conducted on a 3‐T Siemens TrioTim scanner (Siemens, Erlangen, Germany). The identification of currently existing brain pathology (e.g., brain tumors, lacunar, or silent infarcts) was based on PD/T2‐weighted, fluid attenuated inversion recovery‐weighted and time‐of‐flight sequences. To investigate microstructural properties of hippocampal subfields, a diffusion‐weighted, single shot, spin‐echo, echoplanar‐based sequence was performed (30 directions; b = 1,000 s/mm2; matrix dimensions: 128 × 128; slice thickness: 3 mm; spatial resolution: 1.5 × 1.5 × 3 mm; repetition time (TR)/echo time (TE): 7,100 ms/102 ms). To investigate volumetric properties of hippocampal subfields a high‐resolution T1‐weighted magnetization prepared rapid gradient echo sequence was applied (MP‐RAGE; matrix dimensions: 256 × 256 mm; spatial resolution: 0.8 × 0.8 × 0.8 mm; repetition time: 1.770 ms; echo time: 2.38 ms; inversion time: 900 ms; flip angle: 15°; number of slices 224).
Hippocampal Surface Analyses
The relationship between age and volume of hippocampal subfields was investigated using an automated voxel‐based morphometric (VBM) approach combined with projection of the association maps onto a 3D surface view of the hippocampus [Chételat et al., 2008]. This approach was extended by the application of voxelwise analyses on the association between age and DTI measures and their projection onto the same hippocampal 3D surface view. The procedure included the following steps, which are described in detail below: (a) preprocessing and coregistration of the T1‐ and DTI data (see MRI data preprocessing section), (b) application of voxelwise multiple‐linear regression analyses on the relationship between age and GM volume as well as age and DTI measures (see Statistics section), and (c) preparation of a hippocampal region of interest (ROI) and projection of the association maps resulting from the regression analyses on its surface (see 3D hippocampal surface mapping section).
MRI data preprocessing
T1‐weighted images were preprocessed using the VBM protocol implemented in the Statistical Parametric Mapping toolbox (SPM8, Wellcome Trust Centre for Neuroimaging, University College London, England) as described in detail elsewhere [Ashburner and Friston, 2007]. Briefly, images were segmented into probability maps of different tissue classes (GM; WM; cerebrospinal fluid, CSF) using the revised unified segmentation routine of SPM8 [Ashburner and Friston, 2005]. The resulting GM partitions were high‐dimensionally registered to a group template and transformed to MNI space using diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) [Ashburner, 2007]. The GM segments were modulated using the Jacobian determinant to account for effects of local volume changes induced by the spatial normalization (corrected for linear and nonlinear warping) and to preserve the original amount of GM volume. To control the analyses of hippocampal volume for the effect of different brain sizes, the total intracranial volume (TIV) was calculated by summing up the volumes of the GM, WM, and CSF compartments using the VBM8 toolbox.
Diffusion‐weighted imaging data were preprocessed as follows: (a) eddy current and motion correction using the FMRIB Software Library (FSL) (FMRIB Analysis Group, Oxford, UK), (b) adjusting Gradients accordingly by application of the rotational part of the resulting affine transformation, (c) removal of nonbrain tissue using Brain Extraction Tool [Smith, 2002], and (d) calculation of MD images by fitting a single diffusion tensor to the data using CAMINO v.2 [Basser and Pierpaoli, 1996] (Microstructural Imaging Group, University College London, UK, http://cmic.cs.ucl.ac.uk/camino).
Subsequent to the above described preprocessing steps, the DARTEL‐GM segments and MD images of each participant were coregistered using SPM8 by applying the following steps: (a) linear registration of the individual DTI‐b0 images to the T1 images in native space, (b) application of the resulting transformation parameters to the individual MD images, and (c) application of the individual DARTEL transformations (warp fields) resulting from the generation of the modulated GM segments to the coregistered MD images.
Finally, the modulated and spatially normalized GM segments and the spatially normalized MD data were smoothed. A Gaussian kernel of 6 × 6 × 6 mm3 full‐width‐at‐half‐maximum was used for the GM segments. Since the DTI data had a different original spatial resolution, differential smoothing was applied. DTI data were smoothed using a Gaussian kernel of 5.8 × 5.8 × 5.2 mm3 full‐width‐at‐half‐maximum, resulting in an effective smoothness identical to the GM segments smoothed at 6 × 6 × 6 mm3 [Poline et al., 1995; Van Laere and Dierckx, 2001; Villain et al., 2010].
Statistics
Statistical analyses included the application of voxelwise multiple‐linear regression analyses using SPM8. Analyses were performed using t statistics, resulting in SPM‐t maps. The SPM‐t maps were superimposed onto a hippocampal ROI, as described in the next section. The relationship between age and hippocampal volume was investigated by setting the modulated and smoothed GM segments as dependent variable, age as covariate of interest, and TIV as covariate of noninterest. Further covariates of noninterest were determined by testing the influence of potential confounding variables (i.e., education and gender) on hippocampal volume using simple voxelwise regression analyses. The association between age and hippocampal microstructure was examined by setting the smoothed MD maps as dependent variables and age as covariate of interest. Covariates of noninterest were determined by testing the influence of potential confounding variables (i.e., education and gender) on hippocampal microstructure using simple voxelwise regression analyses.
To ensure that potential associations between hippocampal volume and age are independent of microstructural alterations (and vice versa), additional analyses were performed to evaluate the association of age with hippocampal volume under control of hippocampal microstructure (and vice versa), using Biological Parametric Mapping (BPM). BPM is a statistical toolbox for voxelwise multimodality brain image analyses, which uses the theoretical framework behind the SPM methodology [Casanova et al., 2007]. The independent association of age with hippocampal volume was investigated by setting the modulated and smoothed GM segments as dependent variable, age as covariate of interest, and the smoothed MD data as well as TIV as covariates of noninterest. The independent association of age with hippocampal microstructure was examined by setting the smoothed MD data as dependent variable, age as covariate of interest, and the smoothed GM segments as covariate of noninterest. Further covariates of noninterest were determined as described above. BPM analyses were performed using t statistics, resulting in SPM‐t maps.
3D hippocampal surface mapping
The investigation of hippocampal subfields was based on the superimposition of the SPM‐t maps onto the surface of a 3D view of the left and right hippocampus. 3D representations of the hippocampus were built manually by delineating the left and right hippocampus of a group template (built by averaging the unsegmented T1‐images that have been normalized to MNI space) on coronal slices according to the recently published tracing guidelines of the Harmonized Hippocampal Protocol [Frisoni et al., 2014] (http://www.hippocampal-protocol.net/SOPs/index.php). The resulting binary ROIs were then converted to 3D meshes representing the surface of the hippocampal ROIs. Subsequently, SPM‐t maps were superimposed onto these meshes, using the publicly available software “BrainVISA/Anatomist” (http://www.brainvisa.info). To not restrict the analyses to the outermost layer of the hippocampus and to minimize potential partial volume effects in the DTI analyses, we superimposed the mean of t‐values of voxels located between the outermost layer and a layer 2 mm inside the hippocampus. To complement the projection of SPM‐t maps, we also superimposed corresponding statistical significances onto the 3D mesh. Statistical significance was set at P < 0.001, uncorrected for multiple comparisons. A schematized representation of hippocampal subfields based on the 3D view of the hippocampal ROIs is given in Figure 1‐3A.
Figure 1.
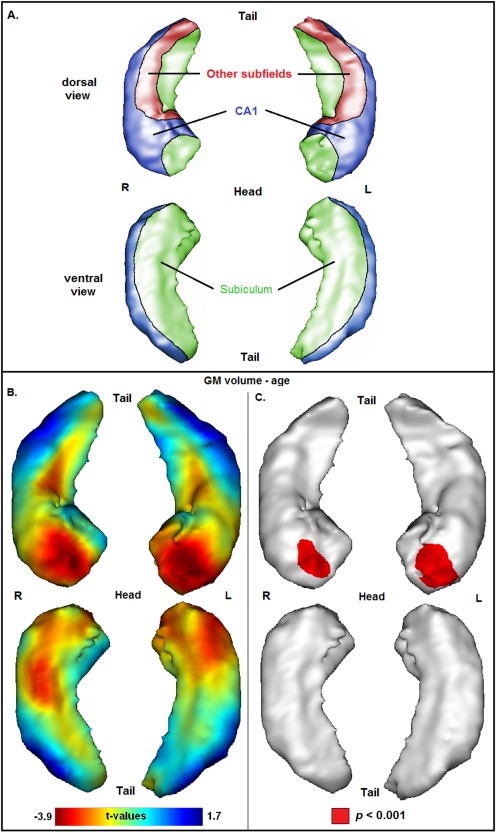
(A) Schematized representation of hippocampal subfields based on a 3D view of the hippocampus (CA1 = blue, subiculum = green, other subfields = red), according to an illustration provided in a previous publication [Chételat et al., 2008]. (B) Projection of the association between age and GM volume onto the 3D hippocampal mesh (SPM t‐map; with total intracranial volume as covariate). (C) Illustration of corresponding significances (in red: P < 0.001, uncorrected for multiple comparisons).
RESULTS
Prior to the application of the main analyses, simple voxelwise regression analyses were performed to test the effect of the potential confounding variables education and gender on hippocampal volume/microstructure. Neither education nor gender was associated with the volume/microstructure of any voxel within the left or right hippocampal ROI. Consequently, gender and education were not included as covariates of noninterest in the main analyses.
Association of Age With Hippocampal Volume
The association between age and hippocampal volume is illustrated in Figure 1. Negative associations between age and volume have been observed bilaterally at the most anterior part of the hippocampal head, mainly corresponding to the subiculum. Furthermore, negative associations have been observed bilaterally at medial‐dorsal parts of the hippocampal body (mainly corresponding to the subiculum), medial‐ventral parts of the hippocampal head (covering the subiculum), and lateral‐ventral parts of the hippocampal head (corresponding to CA1). However, t‐values of these regions were not significant at P < 0.001. Volumes of regions at the level of the hippocampal tail were only weakly related to age. Significant positive associations between age and volume have not been observed.
Association of Age With Hippocampal Microstructure
The association between age and hippocampal microstructure is illustrated in Figure 2. In contrast to the results of the volumetric analyses, the strongest associations between age and MD have been found at the level of the hippocampal body and tail. Positive associations have been observed bilaterally at large medial‐dorsal and medial‐ventral parts of the hippocampal body and tail, mainly corresponding to the subiculum. At the level of the hippocampal body, positive associations have also been observed adjacent to the subiculum in other subfields, mainly corresponding to CA3. Moreover, positive associations have been found at lateral‐ventral parts of the left hippocampal body, corresponding to CA1. At the level of the hippocampal head, positive associations have been observed bilaterally at small medial‐dorsal and lateral‐ventral (left hippocampus) parts, corresponding to CA1. Significant negative associations between age and MD have not been observed.
Figure 2.
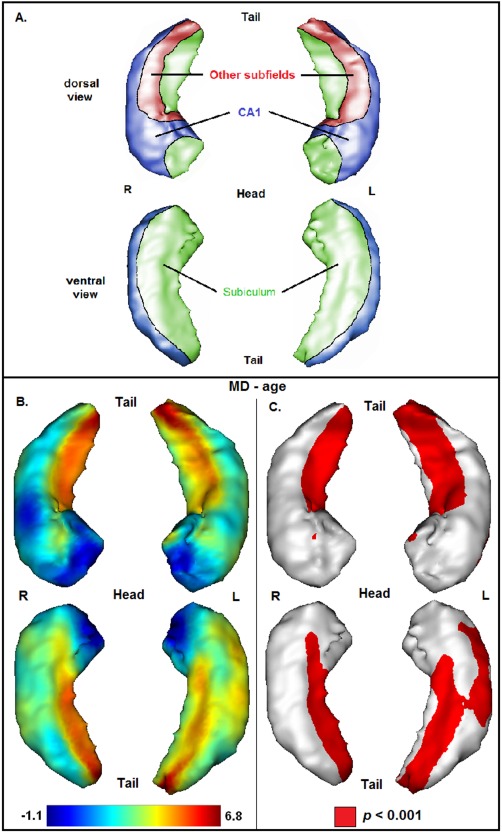
(A) Schematized representation of hippocampal subfields based on a 3D view of the hippocampus (CA1 = blue, subiculum = green, other subfields = red), according to an illustration provided in a previous publication [Chételat et al., 2008]. (B) Projection of the association between age and mean diffusivity (MD) onto the 3D hippocampal mesh (SPM t‐map). (C) Illustration of corresponding significances (in red: P < 0.001, uncorrected for multiple comparisons).
Independent Associations of Age With Hippocampal Volume and Microstructure
The associations between age and hippocampal volume/microstructure under control of the respective imaging covariate of noninterest are illustrated in Figure 3. Analyses on the relationship between age and hippocampal volume controlled for MD demonstrated the same pattern of association that has been found in analyses without controlling for MD as reported in the section “Association of Age with Hippocampal Volume” (Fig. 3A,B). Likewise, analyses on the relationship between age and MD controlled for volume demonstrated the same pattern of association that has been observed without controlling for hippocampal volume as reported in the section “Association of Age with Hippocampal Microstructure” (Fig. 3C,D).
Figure 3.
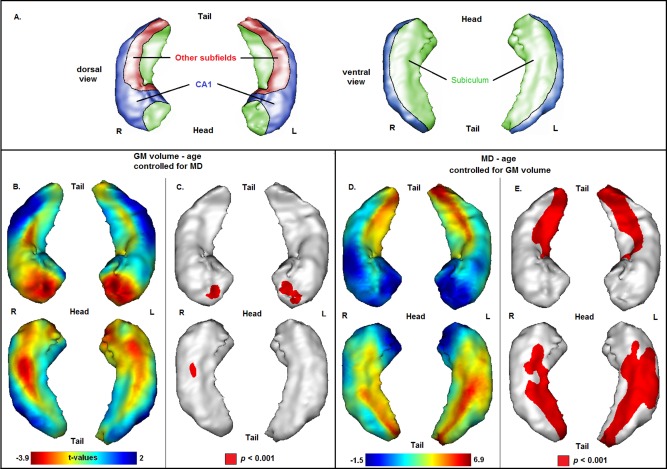
(A) Schematized representation of hippocampal subfields based on a 3D view of the hippocampus (CA1 = blue, subiculum = green, other subfields = red), according to an illustration provided in a previous publication [Chételat et al., 2008]. (B, C): (B) Projection of the association between age and gray matter (GM) volume onto the 3D hippocampal mesh (SPM t‐map; with total intracranial volume and MD as covariates). (C) Illustration of corresponding significances (in red: P < 0.001, uncorrected for multiple comparisons). (D, E): (D). Projection of the association between age and MD onto the 3D hippocampal mesh (SPM t‐map; with GM volume as covariate). (E) Illustration of corresponding significances (in red: P < 0.001, uncorrected for multiple comparisons).
Supplementary Analyses
Additional analyses were performed to investigate potential nonlinear (quadratic) relationships between age and hippocampal volume as well as microstructure using quadratic polynomial regression models. The quadratic models were built by adding an age‐squared term as covariate of interest to the linear models. The variables of the regression analyses were mean‐centered in order to reduce collinearity among predictors (the age‐squared term was calculated from the mean‐centered age). In accordance to the previous analyses, the resulting SPM‐t maps were superimposed onto the hippocampal surface. No quadratic associations between age and hippocampal volume as well as hippocampal MD have been observed (P > 0.001, uncorrected for multiple comparisons; data not shown).
DISCUSSION
This study aimed at investigating and comparing the associations of age with volume and microstructure of hippocampal subfields in a group of cognitively healthy older adults, using a semiautomated 3D surface mapping approach. This approach has been validated against manual segmentation using ultrahigh‐resolution MRI [La Joie et al., 2010].
Association of Age With Hippocampal Volume
In accordance to our hypothesis, linear regression analyses demonstrated a strong negative association between age and the volume of the left and right subiculum. The volume of CA1 showed a tendency for a negative association with age but this relationship did not reach the significance threshold of P < 0.001. Supplementary nonlinear (quadratic) regression analyses did not show any quadratic associations between age and hippocampal volume. The subiculum has been shown to shrink linearly from the age of 20 years while the volume of CA1 is thought to decline nonlinearly across the lifespan with a progressive shrinkage starting at about 50 years [de Flores et al., 2015; Ziegler et al., 2012]. Given the age range of our sample (>60 years), a linear association between age and the volume of CA1 and the subiculum was expected.
An association between age and the volume of the subiculum has been found in several studies [Chételat et al., 2008; Frisoni et al., 2008; La Joie et al., 2010; Wang et al., 2003]. It has been proposed that this association reflects age‐related hippocampal atrophy due to significant neuronal loss accompanying normal aging [Simic et al., 1997; West et al., 1994]. More recent studies using advanced and unbiased methods for cell counting have challenged the belief that age‐related hippocampal atrophy always involves neuronal loss. Instead, hippocampal atrophy could reflect shrinkage of neuronal elements such as dendrites [Miller and O'Callaghan, 2005]. Mechanisms underlying different vulnerabilities of hippocampal subfield volumes to age‐related processes are largely unknown. CA subfields as well as the DG are part of the three‐cell layered archicortex, which is phylogenetically an older part of the brain. The subiculum is part of the phylogenetically younger perarchicortical zone of the mesocortex, which consists of five‐cell layers [Nieuwenhuys et al., 2008]. According to the “last‐in first out theory,” phylogenetically younger brain structures tend to be more susceptible to age‐related neuronal degeneration than phylogenetically older brain structures [Raz, 2000], which might explain the largely selective association of age with the volume of the subiculum.
A few studies found an association of age with the volume of CA3‐4 or DG [Mueller and Weiner, 2009; Pereira et al., 2014; Wisse et al., 2014b]. The differences in results between these studies and the present study might be based on differences in the applied methodological approaches. Pereira et al. [2014] used an automated subfield segmentation procedure implemented in FreeSurfer. However, this method is thought to lack reliability when applied to T1‐weighted MRI [de Flores et al., 2015; Pluta et al., 2012; Schoene‐Bake et al., 2014]. Mueller and Weiner [2009] performed manual delineations on 3 consecutive 2‐mm‐thick slices at the level of the hippocampal body using high‐resolution imaging (4‐T, voxel‐size: 0.4 × 0.4 × 2 mm). The hippocampal head and tail were not included in the analyses, which might partly explain the discrepancy with our findings as we found an association between age and volume predominantly in the anterior part (head) of the hippocampus. As Wisse et al. [2014b] applied manual subfield segmentation along the entire hippocampus (with the exception of the most posterior parts of the hippocampus) using ultrahigh‐field MRI (7‐T, voxel‐size: 0.7 × 0.7 × 0.7), the lack of an association between age and the DG in this study may reflect a lack of sensitivity of surface‐based analyses to changes in this structure due to the location of the DG in the middle of the hippocampus. However, the absence of associations between age and volume of CA3‐4 and DG has also been reported using manual delineation [de Flores et al., 2015; La Joie et al., 2010]. The differences in results within studies using manual segmentation might be based on differences in the applied segmentation and normalization procedure as well as differences in the investigated age‐range. Taken together, more studies are needed before a reliable conclusion about the presence or lack of an association between age and volume of individual hippocampal subfields can be drawn.
Importantly, our results demonstrated that negative associations between age and hippocampal volume are most pronounced in regions of the hippocampal head. This result is in line with several publications and suggests an anterior–posterior gradient of age‐related volume decline along the longitudinal axis of the hippocampus [Chen et al., 2010; Hackert et al., 2002; Ta et al., 2012; Wang et al., 2003]. There are, however, some studies that reported a strong vulnerability of medial and posterior parts of the hippocampus to aging [Driscoll et al., 2003; Frisoni et al., 2008; Kalpouzos et al., 2009; Malykhin et al., 2008; Thomann et al., 2013]. The differences in results might be due to differences in the age range of study groups. Of note, our study group was restricted to advanced older adults spanning the age range from 60 to 85 years, including 15 participants aged 75 years and older. Thus, pronounced atrophy of the hippocampal head might characterize advanced aging. In line with this hypothesis, previous studies that demonstrated pronounced atrophy in anterior parts of the hippocampus focused on advanced aging [Chen et al., 2010; Hackert et al., 2002; Wang et al., 2003], whereas studies that found stronger atrophy in posterior parts also included younger adults [Driscoll et al., 2003; Kalpouzos et al., 2009; Malykhin et al., 2008]. Two studies are not in line with this hypothesis, since they found pronounced atrophy in posterior regions although the study groups were restricted to older adults [Frisoni et al., 2008; Thomann et al., 2013]. However, compared to the age distribution of participants of this study, the number of participants aged 75 years and older within these studies was significantly smaller [Thomann et al. 2013: N age > 75 = 0; Frisoni et al. 2008: N age > 75 = 8].
One possible explanation for greater atrophy of the anterior hippocampus in advanced aging may be a dysregulation of the hypothalamic–pituitary–adrenal (HPA)‐axis [Gordon et al., 2013]. The anterior part of the hippocampus is connected with subcortical and hypothalamical nuclei involved in neuroendocrine function. It is a target of stress hormones released by the HPA‐axis (e.g., cortisol) and plays a major role in the regulation of the HPA‐axis [Fanselow and Dong, 2010; Moser and Moser, 1998]. In advanced aging, dysregulations in the activity of the HPA‐axis and concomitant cortisol hypersecretion have frequently been reported [Aguilera, 2011; Lupien et al., 1998; Sapolsky et al., 1986]. These increases in cortisol levels may lead to a predominant atrophy of anterior parts of the hippocampus.
Association of Age With Hippocampal Microstructure
Strong linear associations of age with hippocampal microstructure (as measured by MD) have been observed bilaterally at large parts of the subiculum. Moreover, associations of age with the microstructure of CA1 have been found. These associations were mainly confined to regions of the hippocampal body and tail. At the level of the hippocampal body, associations of age with microstructure have also been observed in regions adjacent to the subiculum (located in other subfields and corresponding to CA3). However, due to the direct proximity to the subiculum, findings within these regions are most likely an artifact of the smoothing of the MD data. Of note, the association of age with hippocampal microstructure was stronger than the association of age with hippocampal volume (age‐microstructure: t‐values up to 6.8, age‐volume: t‐values up to −3.9). Supplementary nonlinear (quadratic) regression analyses did not show any quadratic associations of age with hippocampal microstructure.
MD is thought to be an indicator of microscopic barriers or obstacles in both GM and WM. In GM regions such as the hippocampus, increasing MD is most likely related to loss of cellular barriers, increases in extracellular fluid caused by shrinkage of neurons, and axonal degeneration [Basser et al., 1994; den Heijer et al., 2012]. The subiculum is a pivotal structure that mediates the interaction between the hippocampus and cortical regions. It receives projections from CA1 and several cortical regions and sends projections to the entorhinal cortex and to cortical regions via the fornix [Duvernoy, 2005; Goldman‐Rakic et al., 1984; Mufson and Pandya, 1984]. Moreover, the perforant pathway traverses the subiculum on its way from the entorhinal cortex to DG, CA3 and CA1 [O'Mara et al., 2001]. CA1 receives projections from CA3 via the Schaffer collateral pathway. Moreover, it receives direct projections from the perforant pathway. It sends projections to the subiculum which proceed to the entorhinal cortex and form one of the principle output bundles of the hippocampus [Duvernoy, 2005]. Studies in rats showed that both the subiculum and CA1 are parts of two main neuronal circuits, the perirhinal cortex—lateral entorhinal cortex—hippocampus circuit and the postrhinal cortex—medial entorhinal cortex—hippocampus circuit [Naber et al., 2001]. Given the high proportion of fibers within the subiculum and CA1, age‐related axonal degeneration (possibly accompanied by increases of extracellular fluid) may underlie the observed association between age and MD in both hippocampal subregions. The high proportion of fibers within the subiculum and CA1 compared to other hippocampal subregions may also explain the restriction of the association between age and MD to the subiculum and CA1.
Of note, the positive associations between age and MD of the subiculum and CA1 were restricted to the hippocampal body and tail. The density of afferent and efferent projections varies along the longitudinal axis of the hippocampus. In particular, afferent projections from the perirhinal and entorhinal cortices, which provide the main environmental input to the hippocampus, project most densely to medial and posterior parts of the hippocampus [Lavenex and Amaral, 2000; Witter et al., 1989]. This high density of projection fibers to medial and posterior parts might explain the restriction of age‐related alterations in MD to the hippocampal body and tail.
To date, only one study investigated the association of age with the microstructure of hippocampal subfields based on MRI [Pereira et al., 2014]. In line with our results, the authors reported a negative association between age and integrity of the subiculum. However, they also found a negative association between age and integrity of CA2‐3. The discrepancies in study results are most likely based on differences in the methodological assessment of hippocampal subfields. Apart from methodological aspects, differences in the age range between study groups may explain the discrepancies in study results, as participants of the present study were significantly older.
Comparison of the Associations of Age With Hippocampal Volume and Microstructure
Importantly, the association pattern of age with hippocampal volume differed from the association pattern with hippocampal microstructure. Although the strongest associations of age with hippocampal volume and microstructure could be observed in the same hippocampal subfields (subiculum and small parts of CA1), associations between age and hippocampal volume have mainly been found in the hippocampal head, whereas associations between age and hippocampal microstructure have predominantly been observed in the hippocampal body and tail. Moreover, analyses on the relationship between age and hippocampal volume controlled for hippocampal microstructure, and vice versa, demonstrated the same pattern of association that has been found without controlling for hippocampal volume and microstructure, respectively. These results suggest, that alterations in hippocampal volume and microstructure within normal aging reflect largely independent processes. As described above, the observed negative association between age and volume of the hippocampal head in advanced aging may be explained by neuronal shrinkage or loss caused by an age‐related dysregulation of the HPA‐axis and concomitant increases in cortisol secretion. Positive associations between age and MD in hippocampal body and tail in normal aging may reflect age‐related axonal degeneration (possibly accompanied by increases of extracellular fluid) rather than neuronal shrinkage or loss.
Of note, in AD patients alterations in volume and microstructure have been observed in the same hippocampal regions [Fellgiebel and Yakushev, 2011; Yakushev et al., 2010, 2011]. In AD, higher MD may be a consequence of neuronal death rather than a consequence of changes in the integrity of fibers. Thus, in contrast to normal aging, volumetric and diffusivity measures in AD may sensitively detect structural alterations that are based on the same neurobiological mechanisms. Specifically, in AD increased diffusivity and decreased integrity of brain tissue microstructure may be detectable early in the degeneration process when global or regional brain volume reduction is not yet observable. In advanced disease stages, microstructural pathology and atrophy may largely spatially overlap. In other words, in AD atrophy may generally be accompanied by microstructural changes illustrating the preceding damage of brain tissue microstructure, which seems not to be a necessary condition of atrophy in normal aging.
Functional Relevance of Age‐Related Structural Alterations Along the Longitudinal Axis of the Hippocampus
The functional specialization of the hippocampus differs along its longitudinal axis [Poppenk et al., 2013]. The anterior hippocampus is thought to be associated with encoding, vestibular memory and navigation, as well as global spatial representations. Moreover, it is thought to be related to emotion and motivation. The posterior hippocampus has been proposed to be related to retrieval, spatial memory, visual memory and navigation, as well as to local spatial representations [Poppenk et al., 2013]. Against the background of these theoretical proposals for longitudinal‐axis specialization, age‐related alterations in hippocampal volume in anterior regions and age‐related alterations in hippocampal microstructure in medial/posterior regions (as indicated by the present results) are likely to have different functional consequences. However, differences in the functional significance of volumetric and microstructural hippocampal changes in general as well as of changes in specific hippocampal subfields are largely unknown and need to be investigated in future studies.
Limitations
The findings of this study should be considered in the context of some limitations. First, the study is based on a cross‐sectional study design. Potential cohort effects cannot be excluded. Moreover, a cross‐sectional design does not allow for investigations of temporal orders or causalities. Longitudinal studies are necessary to confirm and complement the findings of this study. A further limitation is the rather small sample size of this study. Future studies should confirm the results based on a larger sample size. Another limitation relates to the applied methodological procedure. Although the 3D surface mapping approach underlying this study has been validated against manual segmentation on ultrahigh‐resolution images [La Joie et al., 2010], future studies should confirm the observed results based on manual segmentation procedures. Moreover, surface‐based analyses are rather insensitive to changes in the DG and thus do not allow for a specific evaluation of age‐related structural alterations within this subfield. Finally, the application of ultrahigh‐resolution DTI is necessary. Standard DTI sequences provide only a low resolution that does not allow for the investigation of specific tracts that pass hippocampal subfields. The investigation of such tracts (e.g., perforant pathway) might contribute to a more profound understanding of age differences in the microstructure of hippocampal subfields.
CONCLUSION
This study demonstrated differential associations of age with volume and microstructure of hippocampal subfields in healthy older adults. Although the strongest associations of age with hippocampal volume and microstructure have been observed in the same hippocampal subfields (mainly subiculum and small parts of CA1), associations of age with volume were largely confined to the hippocampal head, whereas associations with microstructural integrity were most pronounced in regions of the hippocampal body and tail. Thus, results indicate that alterations in hippocampal volume and microstructure within advanced healthy aging reflect largely independent processes. Future studies should compare the patterns of volumetric and microstructural alterations within the hippocampus between healthy older adults and AD patients to determine potential differences between normal and pathological aging. Moreover, future studies should investigate differences in the functional significance of volumetric and microstructural hippocampal changes in general as well as of changes in specific subfields.
Disclosure: The authors declare no actual or potential conflicts of interest.
REFERENCES
- Aguilera G (2011): HPA axis responsiveness to stress: Implications for healthy aging. Exp Gerontol 46:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova LG, Green AE, Babakchanian S, Hwang KS, Chou Y‐Y, Toga AW, Thompson PM (2012): Hippocampal atrophy and ventricular enlargement in normal aging, mild cognitive impairment and Alzheimer's disease. Alzheimer Dis Assoc Disord 26:17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner I, Friston K. 2007. Voxel‐based morphometry In: Friston K, Ashburner S, Kiebel S, Nichols T, Penny W, editors. Statistical Parametric Mapping: The Analysis of Functional Brain Images. London: Academic Press; pp. 92–100. [Google Scholar]
- Ashburner J (2007): A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ (2005): Unified segmentation. Neuroimage 26:839–851. [DOI] [PubMed] [Google Scholar]
- Barnes J, Bartlett JW, van de Pol LA, Loy CT, Scahill RI, Frost C, Thompson P, Fox NC (2009): A meta‐analysis of hippocampal atrophy rates in Alzheimer's disease. Neurobiol Aging 30:1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C (1996): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson Ser B 111:209–219. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA (2003): Parkinson's disease is associated with hippocampal atrophy. Mov Disord 18:784–790. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Cherubini A, Caltagirone C, Spalletta G (2010): Hippocampal mean diffusivity and memory in healthy elderly individuals A cross‐sectional study. Neurology 74:194–200. [DOI] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, Laurienti PJ, Burdette JH, Hayasaka S, Flowers L, Wood F, Maldjian JA (2007): Biological parametric mapping: A statistical toolbox for multimodality brain image analysis. Neuroimage 34:137–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Chuah LY, Sim SK, Chee MW (2010): Hippocampal region‐specific contributions to memory performance in normal elderly. Brain Cogn 72:400–407. [DOI] [PubMed] [Google Scholar]
- Chételat G, Fouquet M, Kalpouzos G, Denghien I, De La Sayette V, Viader F, Mézenge F, Landeau B, Baron J‐C, Eustache F (2008): Three‐dimensional surface mapping of hippocampal atrophy progression from MCI to AD and over normal aging as assessed using voxel‐based morphometry. Neuropsychologia 46:1721–1731. [DOI] [PubMed] [Google Scholar]
- de Flores R, La Joie R, Landeau B, Perrotin A, Mezenge F, de La Sayette V, Eustache F, Desgranges B, Chetelat G (2015): Effects of age and Alzheimer's disease on hippocampal subfields: Comparison between manual and FreeSurfer volumetry. Hum Brain Mapp 36:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, der Lijn Fv Vernooij MW, de Groot M, Koudstaal P, der Lugt Av Krestin GP, Hofman A, Niessen WJ Breteler MM (2012): Structural and diffusion MRI measures of the hippocampus and memory performance. Neuroimage 63:1782–1789. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ (2003): The aging hippocampus: Cognitive, biochemical and structural findings. Cereb Cortex 13:1344–1351. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM (2005): The Human Hippocampus: Functional Anatomy, Vascularization and Serial Sections with MRI. Berlin: Springer. [Google Scholar]
- Fanselow MS, Dong H‐W (2010): Are the dorsal and ventral hippocampus functionally distinct structures?. Neuron 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellgiebel A, Yakushev I (2011): Diffusion tensor imaging of the hippocampus in MCI and early Alzheimer's disease. J Alzheimer's Dis 26:257–262. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Ganzola R, Canu E, Rüb U, Pizzini FB, Alessandrini F, Zoccatelli G, Beltramello A, Caltagirone C, Thompson PM (2008): Mapping local hippocampal changes in Alzheimer's disease and normal ageing with MRI at 3 Tesla. Brain 131:3266–3276. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Jack CR, Bocchetta M, Bauer C, Frederiksen KS Liu Y, Preboske G, Swihart T, Blair M Cavedo E (2014): The EADC‐ADNI harmonized protocol for manual hippocampal segmentation on magnetic resonance: Evidence of validity. Alzheimer's Dement 11:111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman‐Rakic P, Selemon L, Schwartz M (1984): Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience 12:719–743. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Blazey T, Benzinger TL, Head D (2013): Effects of aging and Alzheimer's disease along the longitudinal axis of the hippocampus. J Alzheimer's Dis 37:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackert V, Den Heijer T, Oudkerk M, Koudstaal P, Hofman A, Breteler M (2002): Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage 17:1365–1372. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Lendt M, Lux S. 2001. VLMT: Verbaler Lern‐und Merkfähigkeitstest. Göttingen: Beltz Test. [Google Scholar]
- Hiller W, Zaudig M, Mombour W. 1996. IDCL: International Diagnostic Checklists for ICD‐10 and DSM‐IV. Seattle: Hogrefe & Huber Publishers. [Google Scholar]
- Jack C, Petersen R, Xu Y, O'brien P, Smith G, Ivnik R, Boeve B, Tangalos E, Kokmen E (2000): Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 55:484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron J‐C, Landeau B, Mevel K, Godeau C, Barré L, Constans J‐M, Viader F, Eustache F (2009): Voxel‐based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging 30:112–124. [DOI] [PubMed] [Google Scholar]
- La Joie R, Fouquet M, Mézenge F, Landeau B, Villain N, Mevel K, Pélerin A, Eustache F, Desgranges B, Chételat G (2010): Differential effect of age on hippocampal subfields assessed using a new high‐resolution 3T MR sequence. Neuroimage 53:506–514. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG (2000): Hippocampal‐neocortical interaction: A hierarchy of associativity. Hippocampus 10:420–430. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, De Santi S, Convit A, Tarshish C, Nair NPV, Thakur M, McEwen BS, Hauger RL, Meaney MJ (1998): Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1(1:69–73.): [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Bouchard TP, Camicioli R, Coupland NJ (2008): Aging hippocampus and amygdala. Neuroreport 19:543–547. [DOI] [PubMed] [Google Scholar]
- Miller D, O'Callaghan J (2005): Aging, stress and the hippocampus. Ageing Res Rev 4:123–140. [DOI] [PubMed] [Google Scholar]
- Moser M‐B, Moser EI (1998): Functional differentiation in the hippocampus. Hippocampus 8:608−619. [DOI] [PubMed] [Google Scholar]
- Mueller SG, Weiner MW (2009): Selective effect of age, Apo e4, and Alzheimer's disease on hippocampal subfields. Hippocampus 19:558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Stables L, Du A, Schuff N, Truran D, Cashdollar N, Weiner M (2007): Measurement of hippocampal subfields and age‐related changes with high resolution MRI at 4T. Neurobiol Aging 28:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Schuff N, Raptentsetsang S, Elman J, Weiner MW (2008): Selective effect of Apo e4 on CA3 and dentate in normal aging and Alzheimer's disease using high resolution MRI at 4 T. Neuroimage 42:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Pandya DN (1984): Some observations on the course and composition of the cingulum bundle in the rhesus monkey. J Comp Neurol 225:31–43. [DOI] [PubMed] [Google Scholar]
- Naber PA, Lopes da Silva FH, Witter MP (2001): Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus 11:99–104. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, Van Huijzen C. 2008. The Human Central Nervous System: A Synopsis and Atlas. Berlin: Springer. [Google Scholar]
- O'Mara SM, Commins S, Anderson M, Gigg J (2001): The subiculum: A review of form, physiology and function. Prog Neurobiol 64:129–155. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Valls‐Pedret C, Ros E, Palacios E, Falcón C, Bargalló N, Bartrés‐Faz D, Wahlund LO, Westman E Junque C (2014): Regional vulnerability of hippocampal subfields to aging measured by structural and diffusion MRI. Hippocampus 24:403–414. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, Sullivan EV (2013): Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85years) measured with atlas‐based parcellation of MRI. Neuroimage 65:176–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipitone J, Park MTM, Winterburn J, Lett TA, Lerch JP, Pruessner JC, Lepage M, Voineskos AN, Chakravarty MM (2014): Multi‐atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. Neuroimage 101:494–512. [DOI] [PubMed] [Google Scholar]
- Pluta J, Yushkevich P, Das S, Wolk D (2012): In vivo analysis of hippocampal subfield atrophy in mild cognitive impairment via semi‐automatic segmentation of T2‐weighted MRI. J Alzheimer's Dis 31:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poline J‐B, Worsley KJ, Holmes AP, Frackowiak R, Friston KJ (1995): Estimating smoothness in statistical parametric maps: Variability of p values. J Comput Assist Tomogr 19:788–796. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L (2013): Long‐axis specialization of the human hippocampus. Trends Cogn Sci 17:230–240. [DOI] [PubMed] [Google Scholar]
- Raz N. 2000. Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings In: Craik FIM, Salthouse TA, editors. The Handbook of Aging and Cognition. Mahwah, NJ: Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U (2010): Trajectories of brain aging in middle‐aged and older adults: Regional and individual differences. Neuroimage 51:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. 1958. Trail Making Test: Manual for Administration, Scoring and Interpretation. Indianapolis: Indiana University Medical Center. [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS (1986): The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr Rev 7:284–301. [DOI] [PubMed] [Google Scholar]
- Sawyer K, Corsentino E, Sachs‐Ericsson N, Steffens DC (2012): Depression, hippocampal volume changes, and cognitive decline in a clinical sample of older depressed outpatients and non‐depressed controls. Aging Ment Health 16:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoene‐Bake JC, Keller SS, Niehusmann P, Volmering E, Elger C, Deppe M Weber B (2014): In vivo mapping of hippocampal subfields in mesial temporal lobe epilepsy: Relation to histopathology. Hum Brain Mapp 35:4718–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T (1982): Specific impairments of planning. Philos Trans R Soc Lond B, Biol Sci 298:199–209. [DOI] [PubMed] [Google Scholar]
- Shing YL, Rodrigue KM, Kennedy KM, Fandakova Y, Bodammer N, Werkle‐Bergner M, Lindenberger U, Raz N (2011): Hippocampal subfield volumes: Age, vascular risk, and correlation with associative memory. Front Aging Neurosci 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic G, Kostovic I, Winblad B, Bogdanovic N (1997): Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. J Comp Neurol 379:482–494. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, McQuoid DR, Payne ME, Potter GG (2011): Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry 19:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta AT, Huang SE, Chiu MJ, Hua MS, Tseng WYI, Chen SHA Qiu A (2012): Age‐related vulnerabilities along the hippocampal longitudinal axis. Hum Brain Mapp 33:2415–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann PA, Wüstenberg T, Nolte HM, Menzel PB, Wolf RC, Essig M, Schröder J (2013): Hippocampal and entorhinal cortex volume decline in cognitively intact elderly. Psychiatry Res: Neuroimaging 211:31–36. [DOI] [PubMed] [Google Scholar]
- Van Laere KJ, Dierckx RA (2001): Brain perfusion SPECT: Age‐ and sex‐related effects correlated with voxel‐based morphometric findings in healthy adults 1. Radiology 221:810–817. [DOI] [PubMed] [Google Scholar]
- Van Leemput K, Bakkour A, Benner T, Wiggins G, Wald LL, Augustinack J, Dickerson BC, Golland P, Fischl B (2009): Automated segmentation of hippocampal subfields from ultra‐high resolution in vivo MRI. Hippocampus 19:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villain N, Fouquet M, Baron J‐C, Mézenge F, Landeau B, de La Sayette V, Viader F, Eustache F, Desgranges B, Chételat G (2010): Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer's disease. Brain 133:3301–3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Swank JS, Glick IE, Gado MH, Miller MI, Morris JC, Csernansky JG (2003): Changes in hippocampal volume and shape across time distinguish dementia of the Alzheimer type from healthy aging. Neuroimage 20:667–682. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1987. WMS‐R, Wechsler Memory Scale‐Revised Manual. Harcourt Javanovich, New York: Psychological Corporation. [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC (1994): Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet 344:769–772. [DOI] [PubMed] [Google Scholar]
- Winterburn JL, Pruessner JC, Chavez S, Schira MM, Lobaugh NJ, Voineskos AN, Chakravarty MM (2013): A novel in vivo atlas of human hippocampal subfields using high‐resolution 3T magnetic resonance imaging. Neuroimage 74:254–265. [DOI] [PubMed] [Google Scholar]
- Wisse L, Gerritsen L, Zwanenburg JJ, Kuijf HJ, Luijten PR, Biessels GJ, Geerlings MI (2012): Subfields of the hippocampal formation at 7T MRI: In vivo volumetric assessment. Neuroimage 61:1043–1049. [DOI] [PubMed] [Google Scholar]
- Wisse LE, Biessels GJ, Geerlings MI (2014a): A critical appraisal of the hippocampal subfield segmentation package in freesurfer. Front Aging Neurosci 6:261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LEM, Biessels GJ, Heringa SM, Kuijf HJ, Koek DL, Luijten PR, Geerlings MI, Impairment UVC (2014b): Hippocampal subfield volumes at 7T in early Alzheimer's disease and normal aging. Neurobiol Aging 35:2039–2045. [DOI] [PubMed] [Google Scholar]
- Wittchen H, Pfister H. 1997. DIA‐X Interview. Frankfurt: Swets Test Services. [Google Scholar]
- Witter M, Van Hoesen G, Amaral D (1989): Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci 9:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakushev I, Müller MJ, Lorscheider M, Schermuly I, Weibrich C, Dellani PR, Hammers A, Stoeter P, Fellgiebel A (2010): Increased hippocampal head diffusivity predicts impaired episodic memory performance in early Alzheimer's disease. Neuropsychologia 48:1447–1453. [DOI] [PubMed] [Google Scholar]
- Yakushev I, Gerhard A, Müller MJ, Lorscheider M, Buchholz H‐G, Schermuly I, Weibrich C, Hammers A, Stoeter P, Schreckenberger M (2011): Relationships between hippocampal microstructure, metabolism, and function in early Alzheimer's disease. Brain Struct Funct 216:219–226. [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Wang H, Pluta J, Das SR, Craige C, Avants BB, Weiner MW, Mueller S (2010): Nearly automatic segmentation of hippocampal subfields in in vivo focal T2‐weighted MRI. Neuroimage 53:1208–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding SL, Gertje EC, Mancuso L, Kliot D, Das SR, Wolk DA (2015): Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Hum Brain Mapp 36:258–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G, Dahnke R, Jäncke L, Yotter RA, May A, Gaser C (2012): Brain structural trajectories over the adult lifespan. Hum Brain Mapp 33:2377–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]


