Abstract
Heroin addiction is a severe relapsing brain disorder associated with impaired cognitive control, including deficits in attention allocation. The thalamus has a high density of opiate receptors and is critically involved in orchestrating cortical activity during cognitive control. However, there have been no studies on how acute heroin treatment modulates thalamic activity. In a cross‐over, double‐blind, vehicle‐controlled study, 29 heroin‐maintained outpatients were studied after heroin and placebo administration, while 20 healthy controls were included for the placebo condition only. Resting‐state functional magnetic resonance imaging was used to analyze functional integration of the thalamus by three different resting state analysis techniques. Thalamocortical functional connectivity (FC) was analyzed by seed‐based correlation, while intrinsic thalamic oscillation was assessed by analysis of regional homogeneity (ReHo) and the fractional amplitude of low frequency fluctuations (fALFF). Relative to the placebo treatment and healthy controls, acute heroin administration reduced thalamocortical FC to cortical regions, including the frontal cortex, while the reductions in FC to the mediofrontal cortex, orbitofrontal cortex, and frontal pole were positively correlated with the plasma level of morphine, the main psychoactive metabolite of heroin. Furthermore, heroin treatment was associated with increased thalamic ReHo and fALFF values, whereas fALFF following heroin exposure correlated negatively with scores of attentional control. The heroin‐associated increase in fALFF was mainly dominated by slow‐4 (0.027–0.073 Hz) oscillations. Our findings show that there are acute effects of heroin within the thalamocortical system and may shed new light on the role of the thalamus in cognitive control in heroin addiction. Future research is needed to determine the underlying physiological mechanisms and their role in heroin addiction. Hum Brain Mapp 36:5287–5300, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: heroin addiction, heroin effects, thalamus, cognitive control, resting state, functional connectivity
Abbreviations
- BOLD
blood oxygen level‐dependent
- fALFF
fractional amplitude of low frequency fluctuations
- FC
functional connectivity
- fMRI
functional magnetic resonance imaging
- FWE
family‐wise error
- IFG
inferior frontal gyrus
- ReHo
regional homogeneity
INTRODUCTION
Loss of cognitive control, including compromised ability to inhibit impulsive drug‐driven behavior, is a key hallmark of drug addiction [Perry and Carroll, 2008]. For example, it has been shown that heroin addicts have a significantly lower degree of impulse control than normal controls [Lee and Pau, 2002] and have difficulties in shifting attention from one subject to another [Ornstein et al., 2000]. Such deficits in selective attention and impulsivity are common in opiate‐dependent patients [Bracken et al., 2012; King et al., 1999], and even persist in abstinence after stopping a maintenance treatment [Prosser et al., 2009]. On the other hand, regular maintenance treatment may improve cognitive performance in heroin addicts, including attention and executive function [Wang et al., 2014].
Neuroimaging studies have indicated that impaired cognitive control in heroin addicts may arise from abnormal prefrontal brain activity [Pau et al., 2002], in particular from the inferior frontal gyrus (IFG) [Fu et al., 2008; Lee et al., 2005]. Besides prefrontal brain regions, the thalamus is critically implicated in cognitive control and plays a core role in processing and maintaining attention [de Bourbon‐Teles et al., 2014; Lawrence et al., 2003]. There is evidence that the effects of heroin are mostly mediated by µ receptors, which are densest in the cingulate gyrus, ventral tegmental area, cerebellum, thalamus, and hypothalamus [Maurer et al., 1983; Pfeiffer et al., 1982]. Within the thalamus, µ receptors are densest within the dorsomedial, lateroposterior, and laterodorsal thalamic nucleus, as shown by in situ hybridization in post‐mortem studies of humans [Peckys and Landwehrmeyer 1999]. Given the high density of opiate receptors in the thalamus [Apkarian et al., 2005; Lever, 2007], the reduced glucose metabolism in patients in a methadone maintenance program [Galynker et al., 2000; Prosser et al., 2009] and the decreased regional homogeneity in the resting state thalamus (ReHo) in chronic heroin addicts [Qiu et al., 2011], it is conceivable that acute heroin use is associated with abnormal thalamic activity.
However, it is also becoming increasingly apparent that complex psychological processes, such as cognitive control, result from interactions between different brain regions rather than from local activity per se. Within this perspective, the thalamocortical system has an important role in synchronizing the activities of thalamic and cortical neurons and is thus essential in orchestrating and integrating function across different brain regions [Jones 2002; Steriade, 2006]. A critical point is that the thalamus and cortical areas are functionally connected during cognitive control [Aron and Poldrack, 2006]. This evidence suggests that impaired cognitive control in heroin addiction might result not only from abnormal task‐associated activity in the IFG [Fu et al., 2008; Lee et al., 2005], but also from disrupted thalamocortical connectivity.
We have now carried out a cross‐over, double‐blind and vehicle‐controlled study in a clinical sample of heroin‐maintained patients, in which we used resting state functional magnetic resonance imaging (fMRI) to explore how heroin acutely modulates the functional integration of the thalamic system. Resting state fMRI provides a useful tool to study functional integration of brain regions, at the level of large scale neural systems, by analysis of low frequency fluctuations in the blood oxygen level‐dependent (BOLD) signal [Friston, 2009]. We computed thalamocortical functional connectivity (FC) using a seed‐based correlation approach [Biswal et al., 1995], whereas local thalamic low frequency oscillation characteristics were assessed by analysis of ReHo and the fractional amplitude of low frequency fluctuations (fALFF). We expected that acute heroin administration would modulate thalamic functional integration during resting state oscillation.
METHODS
Subjects
This study was carried out with two groups of subjects. Twenty‐nine heroin‐maintained outpatients (19 male) were recruited from the Centre of Substance Use Disorders of the Psychiatric University Hospital of Basel. Inclusion criteria were age older than 18 years, past history of illicit intravenous heroin consumption, and participation in the heroin‐assisted treatment for at least 6 months with an unchanged heroin dose during the previous 3 months. In addition, 20 healthy controls were recruited from the general population in the same geographical area as the patients. Both subject groups were carefully screened, using a semi‐structured clinical interview. The exclusion criteria were a positive alcohol breathalyser test, an additional physical disease, or a comorbid psychiatric disorder. Controls and patients were told to abstain from illicit drug consumption for the duration of the study, as well as to abstain from alcohol intake for 72 hours and from smoking for 2 hours. Nevertheless, 15 patients were tested positive for cocaine and nine patients and five healthy controls for cannabis at one or both points of the measurements. The subjects' characteristics are summarized in Table 1.
Table 1.
Socio‐demographic and diagnostic characteristics of the study sample
| Measurements | Patients (n = 29) | Healthy controls (n = 20) | Between‐group statistics |
|---|---|---|---|
| Age (years), mean (SD) | 41.5 (6.1) | 40.3 (10.9) | T(48) = 0.506 |
| P = 0.615 | |||
| Gender (women/men) | 8/21 | 6/14 | χ 2 = 0.034 |
| P = 0.854 | |||
| Employment (yes/no) | 13/16 | 20/0 | χ 2 = 16.385 |
| P = 0.00005 | |||
| Daily heroin dose (mg/day), mean (SD) | 323.5 (133.3) | – | – |
| Duration of dependence (years), mean (SD) | 20.6 (7.2) | – | – |
| Age at the first‐time heroin use (years), mean (SD) | 18.3 (4.8) | – | – |
| Duration of opioid maintenance (years), mean (SD) | 6.9 (4.5) | – | – |
| Number of cigarettes per day, mean (SD) | 20.76 (9.0) | 11.50 (8.2) | T(48) = 3.664 |
| P = 0.001 | |||
| Cannabis abuse, (yes/no) | 9/20 | 5/15 | χ 2 = 0.211 |
| P = 0.646 | |||
| Cocaine abuse, (yes/no) | 15/14 | 0/20 | χ 2 = 14.909 |
| P = 0.0001 | |||
| BIS total score, mean (SD) | 65.93 (6.9) | 61.15 (5.6) | T(47) = 2.568 |
| P = 0.013 | |||
| BIS first order attention score, mean (SD) | 11.24 (2.0) | 9.10 (1.4) | T(47) = 4.164 |
| P = 0.0001 | |||
| BIS second order attention score, mean (SD) | 16.72 (3.0) | 14.70 (2.1) | T(47) = 2.636 |
| P = 0.011 |
Abbreviations: BIS: Barrett impulsiveness scale; SD: standard deviation.
The study was approved by the local ethics committee and registered with http://clinicaltrials.gov (ID NCT01174927). After receiving a written and oral description of the aim of this study, all participants gave written informed consent statements before inclusion.
Experimental Design
The study was performed using a cross‐over, double‐blind and placebo‐controlled design with two temporally distinct examination sessions; there is a high overlap of the study sample with previously published studies during emotional face processing [Schmidt et al., 2013a], response inhibition [Schmidt et al., 2013b, Schmidt et al., 2014] and reinforcement learning [Walter et al., 2015]. Specifically, resting‐state fMRI data of 29 patients and 20 controls were included in this present thalamo‐cortical analysis, while in a previous analysis of resting‐state limbic network connectivity data of 20 patients and 20 controls from the same sample were included [Schmidt et al., 2015]. All studies derived from the Heroin (Diaphin®) and placebo (saline solution) were administered intravenously through an indwelling intravenous catheter over a period of 30 seconds, as recently described [Walter et al., 2015]. During both sessions (with a time interval of about two weeks), patients received both heroin and placebo. The subjects who received heroin before scanning were administered vehicle after scanning (i.e., 60 min after the first injection), whereas the subjects who received placebo before scanning were administered heroin after scanning. The order was randomized in a balanced manner and did not affect the findings obtained. The administered heroin dose was equivalent to the subjects' individual dose. Healthy controls received only a placebo administration and were examined only once.
Bioanalytical and Behavioral Measurements
Data on plasma levels have recently been published elsewhere [Walter et al., 2015, 2013]. As we used the mean peak levels for later correlation analyses, we provide a brief summary of the analytical method. Plasma levels of diacetylmorphine (heroin) and morphine were quantified in venous ammonium heparinized plasma by high performance liquid chromatography on a 125 × 2 mm i.d. Nucleosil 50 C‐8 ec column with a particle size of 5 µm and a 8 × 3 mm2 i.d. precolumn packed with Nucleosil 120 C‐8 and a particle size of 3 µm, followed by diode array detection. The sample preparation and instrumental conditions were as previously described in detail [Bourquin et al., 1999]. Plasma drug levels were measured three times, at 3, 10, and 60 minutes after drug injection. Individual peak concentrations of diacetylmorphine and morphine (the main psychoactive metabolite of diacetylmorphine) were used for further correlation analysis.
Attention was assessed using the 30‐item self‐report questionnaire of the Barratt Impulsiveness Scale (BIS) [Patton et al., 1995]. Healthy controls and patients completed the questionnaire after receiving the treatment and before the scan. Patients completed the questionnaire in both examination sessions. The BIS questionnaire assesses six first order and three second order factors of impulsivity, including attention, motor, and non‐planning categories, as well as a total impulsivity score. Given the key role of the thalamus in attentional control [de Bourbon‐Teles et al., 2014], only first and second order attention scores were used for subsequent correlation analyses. The values of the first order attention scores range from 5 to 20, where a higher score implies worse attentional control. The second order attention score includes the first order score and scores for cognitive instability, with a range from 8 to 32.
Image Acquisition
Scanning was performed on a 3 Tesla scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany). A 5 minutes resting state condition (20 minutes after substance administration) was examined with a whole brain echo planar imaging (EPI) sequence (TR = 2000 ms, TE = 28 ms, flip angle = 82°, field of view = 228 × 228 mm2, 32 slices, voxel size = 3.6 × 3.6 × 3.8 mm3). In total, 152 EPI volumes were acquired. In addition, images were acquired with a high resolution T1‐weighted magnetization‐prepared rapid acquisition gradient echo (MPRAGE) image (TR = 2000 ms; TE = 3.37 ms; flip angle = 8°; inversion time = 1,000 ms; 176 slices; slice thickness = 1 mm; voxel size = 1 × 1 × 1 mm3).
Image Preprocessing
EPI volumes were preprocessed using statistical parametric mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). EPI volumes of each scanning session were realigned using rigid transformation, normalized by linear and nonlinear transformation into MNI space (Montreal Neurological Institute), by using the ICBM152 template (International Consortium for Brain Mapping), spatially smoothed with a 6 mm full width half maximum (FWHM) Gaussian kernel, and band‐pass filtered for the frequency range of 0.01‐0.08 Hz.
Thalamocortical Connectivity (Seed‐Based Correlation)
Functional data were analyzed using the seed‐driven approach of the CONN toolbox (CONN 13.1; http://web.mit.edu/swg/software.htm) [Whitfield‐Gabrieli and Nieto‐Castanon, 2012]. We defined the bilateral thalamus as seed region of interest for the between‐group (patients and controls) and the between‐treatment (placebo and heroin) analyses. In order to understand between‐treatment differences in more detail, we additionally performed analyses with seeds of the bilateral medial dorsal, lateral posterior and lateral dorsal thalamic nucleus, which are known to show high µ receptor density [Peckys and Landwehrmeyer, 1999]. The masks were generated by WFU PickAtlas [Maldjian et al., 2003]. Preprocessed and band‐pass‐filtered EPI volumes were used for computation of seed‐to‐voxel FC maps. The correlation coefficients between the signal within the thalamic seed and the signal of all other brain voxels were estimated. Confounding signals related to white matter and cerebrospinal fluid were removed through linear regression. Realignment parameters (six dimensions with first order derivative) were defined as first level covariates.
Thalamic Regional Homogeneity
For each subject and treatment, ReHo maps were computed using Kendall's coefficient of concordance (KCC), as integrated in the REST toolkit (REST 1.8; http://www.restfmri.net) [Song et al., 2011]. The BOLD time course in preprocessed normalized non‐smoothed EPI volumes was corrected for linear trends and a temporal band‐pass filter of 0.01‐0.08 Hz was applied. KCC was calculated for each voxel time series, in order to measure the similarity of the ranked time series with its 26‐neighbour voxels. The resulting KCC‐ReHo maps were standardized by dividing each voxel by the global mean within a whole brain mask and smoothed with a 6 mm FWHM Gaussian kernel.
Thalamic Fractional Amplitude of Low Frequency Fluctuations
Preprocessed normalized and non‐smoothed EPI volumes were used to compute fALFF maps with the REST toolkit [Song et al., 2011]. The frequency‐filtered time series of each voxel are described by a Fourier series, where the BOLD signal is expressed as a sum of sines and cosines [Davis, 1989]. The ALFF value is the averaged square root of the power spectrum obtained via fast Fourier transformation, whereas fALFF values reflect the ratio of the power spectrum of the signal (0.01‐0.08 Hz) to that of the entire frequency range [Zou et al., 2008]. As categorized in previous research, we also compared thalamic frequency between heroin and placebo treatment by preprocessing and reanalyzing data using slow‐4 (0.027‐0.073 Hz) and slow‐5 (0.01‐0.027 Hz) frequency bands [Buzsaki and Draguhn, 2004; Zhang et al., 2015; Zou et al., 2008]. The computed fALFF maps were standardized by dividing each voxel by the global mean within a whole brain mask and smoothed with a 6 mm FWHM Gaussian kernel.
Statistical Analysis
Between‐group and between‐treatment analyses were performed using a general linear model (GLM) in SPM8. Seed‐based thalamic FC, ReHo and fALFF maps were tested by voxelwise whole brain analysis. Paired t tests were used to test between‐treatment differences in patients and two sample t tests were used to test differences between patients and healthy controls. Treatment difference maps of thalamocortical FC, ReHo, and fALFF were used for whole brain correlation analyses with peak plasma levels of morphine. To investigate only values of positive FC all negative values were excluded within the placebo and heroin treatment. First and second order BIS attention scores were used separately for the correlation analysis with thalamocortical FC, ReHo and fALFF maps after the placebo and heroin administration in heroin‐dependent patients. All analyses were performed using a cluster‐level inference strategy with a cluster‐forming threshold of P < 0.001 and adjusted for family‐wise error (FWE) at P < 0.05 [Hayasaka et al., 2004]. For correlation analyses, we used a binary mask of main treatment differences with a cluster‐forming threshold of P < 0.05 and adjusted for FWE at P < 0.05. Statistical comparisons of demographic and clinical characteristics were performed with SPSS 19 (IBM SPSS Statistics; Armonk, NY: IBM Corp) using independent sample t tests for parametric and χ 2 tests for nonparametric data.
RESULTS
Demographic and Clinical Characteristics
The groups of patients and controls did not differ in age, gender or cannabis use. Patients consumed significantly more cocaine, more cigarettes per day and were less often employed than controls. The demographic and clinical characteristics of the study sample are summarized in Table 1.
Bioanalytical and Behavioral Measurements
The mean plasma peak level of diacetylmorphine was 1042.4 ng/ml and ranged from 40 to 3051 ng/ml. Morphine concentrations showed a mean peak of 976.6 ng/ml and ranged from 39 to 3885 ng/ml. The results on drug plasma levels and their temporal decay have been previously described in more detail [Walter et al., 2015]. Peak plasma levels of diacetylmorphine (P = 0.343, r = 0.203) and morphine (P = 0.863, r = −0.037) did not correlate significantly with daily heroin dose.
No significant differences in BIS total score (P = 0.209, t = 1.286), or first order (t = 0.332, t = 0.987) or second order attention scores (P = 0.124, t = 1.586) were found after heroin and placebo treatment in patients. Relative to healthy controls, patients after heroin treatment showed significantly higher BIS total scores (P = 0.013, t = 2.568), and first order (P = 0.001, t = 4.164), and second order attention scores (P = 0.011, t = 2.636) (Table 1). After placebo treatment, patients showed significantly higher BIS total scores (P = 0‐003, t = 3.115), first order (P < 0.001, t = 4.079), and second order attention scores (P = 0.002, t = 3.246) in comparison to healthy controls.
Thalamocortical Connectivity (Seed‐Based Correlation Analysis)
In comparison to placebo, heroin in patients reduced thalamic FC to the bi‐hemispherical frontal, parietal and temporal regions and increased thalamus FC (Fig. 1, Table 2). Heroin also reduced thalamic FC to parietal, temporal and occipital regions relative to healthy controls, whereas no heroin‐induced increase was found. After placebo administration, patients showed reduced thalamic FC to the temporal gyrus compared with healthy controls.
Figure 1.
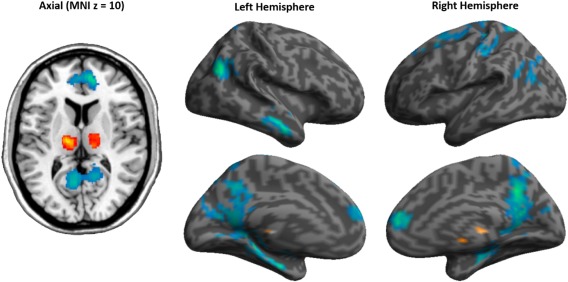
Thalamocortical FC differences between heroin and placebo treatment in heroin‐dependent patients. Red indicates increased FC by heroin and blue indicates reduced FC relative to the placebo treatment. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 2.
Resting‐state associated thalamocortical functional connectivity (FC)
| Brain region | Cluster size (voxels) | Cluster P value (FWE corrected) | MNI coordinats (x, y, z) | Hemsphere | Z‐value at voxel level |
|---|---|---|---|---|---|
| Heroin > Placebo Treatment | |||||
| Thalamus | 174 | 0.007 | −16, −16, 6 | L | 5.07 |
| −2, −24, 4 | L | 3.53 | |||
| Thalamus | 117 | 0.048 | 6, −14, 4 | R | 3.94 |
| 12, −20, 12 | R | 3.61 | |||
| Heroin < Placebo Treatment | |||||
| Precuneus | 495 | <0.001 | 10, −52, 34 | R | 4.68 |
| 6, −62, 28 | R | 4.05 | |||
| 12, −52, 42 | R | 4.04 | |||
| Lateral Occipital Cortex | 159 | 0.012 | 48, −66, 26 | R | 4.29 |
| Middle Temporal Gyrus | 165 | 0.01 | 58, −6, −26 | R | 4.24 |
| 60, −4, −12 | R | 4.00 | |||
| 58, −12, −18 | R | 3.82 | |||
| Precuneus | 195 | 0.004 | −8, −66, 14 | L | 4.12 |
| −4, −54, 14 | L | 3.42 | |||
| Medial Frontal Gyrus | 118 | 0.046 | 14, 48, 12 | R | 4.11 |
| 6, 54, 8 | R | 3.50 | |||
| −2, 50, 10 | L | 3.44 | |||
| Superior Parietal Lobule | 119 | 0.045 | −22, −54, 66 | L | 4.04 |
| Controls > Heroin Treatment | |||||
| Middle Temporal Gyrus | 507 | <0.001 | 58, −6, −20 | R | 5.00 |
| 50, 2, −32 | R | 4.03 | |||
| 64, −24, −20 | R | 3.91 | |||
| Precentral Gyrus | 162 | 0.025 | 16, −18, 72 | R | 4.68 |
| 14, −24, 66 | R | 3.82 | |||
| 10, −18, 54 | R | 3.68 | |||
| Lateral Occipital Cortex | 349 | <0.001 | 50, −62, 28 | R | 4.60 |
| 56, −54, 22 | R | 4.36 | |||
| Middle Temporal Gyrus | 156 | 0.03 | −64, −8, −26 | L | 4.51 |
| Precuneus | 1,380 | <0.001 | 0, −54, 8 | R | 4.48 |
| 18, −50, 40 | R | 4.35 | |||
| 14, −40, 4 | R | 4.26 | |||
| Controls < Heroin Treatment | |||||
| none | |||||
| Controls > Placebo Treatment | |||||
| Inferior Temporal Gyrus | 237 | 0.004 | −52, −30, −28 | L | 4.09 |
| −62, −26, −30 | L | 3.96 | |||
| −50, −40, −28 | L | 3.80 | |||
| Controls < Placebo Treatment | |||||
| None | |||||
Abbreviations: FWE: family‐wise error; L: left; R: right.
Analyses of FC of specific thalamic nuclei in patients revealed a heroin‐associated reduction in connectivity strength between the precentral gyrus and the lateral dorsal nucleus. The lateral posterior nucleus was instead associated with increased connectivity strength within the thalamus during heroin treatment. The medial dorsal nucleus was associated with the broadest heroin‐associated reduction in connectivity strength, including the temporal, parietal, occipital, and frontal lobe (Table 3).
Table 3.
Resting‐state associated functional connectivity (FC) of specific thalamic nuclei
| Brain region | Cluster size (voxels) | Cluster P‐value (FWE corrected) | MNI coordinats (x, y, z) | Hemisphere | Z‐value at voxel level |
|---|---|---|---|---|---|
| Lateral Dorsal Nucleus: Heroin < Placebo Treatment | |||||
| Precentral Gyrus | 141 | 0.019 | −12, −26, 80 | L | 4.90 |
| Precentral Gyrus | −20, −24, 78 | L | 4.04 | ||
| Precentral Gyrus | −22, −18, 72 | L | 3.27 | ||
| Lateral Dorsal Nucleus: Heroin > Placebo Treatment | |||||
| none | |||||
| Lateral Posterior Nucleus: Heroin < Placebo Treatment | |||||
| none | |||||
| Lateral Posterior Nucleus: Heroin > Placebo Treatment | |||||
| Thalamus | 211 | 0.003 | −10, −16, 6 | L | 4.45 |
| Thalamus | −16, −24, 8 | L | 4.18 | ||
| Thalamus | −6, −24, 8 | L | 372 | ||
| Thalamus | 125 | 0.038 | 12, −10, 8 | R | 4.39 |
| Medial Dorsal Nucleus: Heroin < Placebo Treatment | |||||
| Postcentral Gyrus | 2194 | <0.001 | −32, −30, 52 | L | 4.99 |
| Postcentral Gyrus | −50, −20, 52 | L | 4.77 | ||
| Precentral Gyrus | −12, −16, 80 | L | 4.47 | ||
| Middle Temporal Gyrus | 764 | <0.001 | 58, −6, −24 | R | 4.89 |
| Superior Temporal Gyrus | 58, −4, −12 | R | 4.72 | ||
| Middle Temporal Gyrus | 58, −14, −16 | R | 4.67 | ||
| Precentral Gyrus | 1156 | <0.001 | 10, −20, 80 | R | 4.64 |
| Postcentral Gyrus | 46, −26, 58 | R | 4.60 | ||
| Postcentral Gyrus | 20, −36, 54 | R | 4.40 | ||
| Parahippocampal Gyrus | 254 | 0.001 | 24, −16, −26 | R | 4.60 |
| Parahippocampal Gyrus | 28, −24, −16 | R | 4.44 | ||
| Parahippocampal Gyrus | 26, −34, −16 | R | 4.31 | ||
| Precuneus | 2045 | <0.001 | 10, −60, 26 | R | 4.59 |
| Precuneus | 10, −54, 34 | R | 4.52 | ||
| Cerebellum | −24, −48, −22 | L | 4.51 | ||
| Lateral Occipital Cortex | 242 | 0.001 | 48, −68, 26 | R | 4.38 |
| Angular Gyrus | 54, −58, 34 | R | 3.22 | ||
| Lateral Occipital Cortex | 296 | <0.001 | −46, −70, 12 | L | 4.32 |
| Angular Gyrus | −50, −50, 22 | L | 4.17 | ||
| Cerebellum | −52, −64, 26 | L | 3.58 | ||
| Parahippocampal Gyrus | 149 | 0.017 | −20, −14, −26 | L | 4.29 |
| Parahippocampal Gyrus | −30, −26, −22 | L | 3.82 | ||
| Temporal Fusiform Cortex | −30, −32, −28 | L | 3.64 | ||
| Superior Frontal Gyrus | 151 | 0.016 | 20, 22, 50 | R | 4.24 |
| Middle Frontal Gyrus | 30, 34, 46 | R | 3.20 | ||
| Superior Frontal Gyrus | 175 | 0.008 | −58, −36, −4 | L | 4.18 |
| Middle Frontal Gyrus | −52, −36, −10 | L | 4.11 | ||
| Precentral Gyrus | 183 | 0.006 | −4, −20, 54 | L | 4.07 |
| Precentral Gyrus | 0, −18, 64 | R | 3.74 | ||
| Precentral Gyrus | 12, −16, 58 | R | 3.57 | ||
| Occipital Fusiform Gyrus | 206 | 0.003 | 12, −90, −16 | R | 4.07 |
| Lingual Gyrus | −4, −88, −8 | L | 3.90 | ||
| Occipital Pole | 4, −94, −4 | R | 3.74 | ||
| Medial Dorsal Nucleus: Heroin > Placebo Treatment | |||||
| Thalamus | 216 | 0.002 | −14, −14, 4 | L | 4.73 |
| Thalamus | −10, −18, 10 | L | 4.71 | ||
| Thalamus | −2, −24, 4 | L | 3.71 | ||
| Thalamus | 135 | 0.027 | 12, −18, 12 | R | 4.33 |
| Thalamus | 10, −8, 2 | R | 3.00 | ||
Abbreviations: FWE: family−wise error; L: left; R: right.
Thalamic Regional Homogeneity
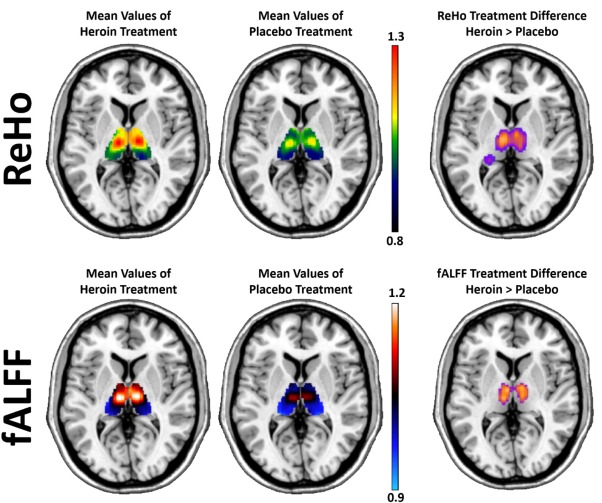
Between‐treatment analysis of ReHo revealed that heroin treatment in patients significantly increased values in the bilateral thalamus, posterior cingulate, postcentral, and precentral gyrus, compared with the placebo treatment (Fig. 2, Table 4). In comparison with heroin treatment, increased ReHo values were found during placebo in the temporal, frontal, and occipital cortex. In comparison with heroin treatment, healthy controls showed increased ReHo values in the frontal cortex.
Figure 2.
Significant positive relation of the heroin‐induced reduction in thalamic FC to the left (128 voxels) and right orbitofrontal cortex (65 voxels) and the left frontal pole (173 voxels) with the peak plasma levels of morphine. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Table 4.
Resting‐state associated regional homogeneity (ReHo)
| Brain region | Cluster size (voxels) | Cluster P‐value (FWE corrected) | MNI coordinats (x, y, z) | Hemisphere | Z‐value at voxel level |
|---|---|---|---|---|---|
| Heroin > Placebo Treatment | |||||
| Posterior Cingulate Gyrus | 639 | <0.001 | 2, −28 30 | R | 5.84 |
| Posterior Cingulate Gyrus | −4, −44 12 | L | 3.30 | ||
| Thalamus | 1,286 | <0.001 | −10, −14 12 | L | 5.38 |
| Thalamus | 6, −8 4 | R | 5.33 | ||
| Thalamus | −18, −34 12 | L | 4.83 | ||
| Postcentral Gyrus | 831 | <0.001 | 2, −38 62 | R | 4.66 |
| Precentral Gyrus | 0, −28 60 | R | 4.63 | ||
| Precentral Gyrus | 2, −20 70 | R | 4.50 | ||
| Heroin < Placebo Treatment | |||||
| Temporal Pole | 414 | 0.006 | −50, 2 −34 | L | 4.76 |
| Inferior Temporal Gyrus | −56, −16 −26 | L | 4.17 | ||
| Middle Temporal Gyrus | −58, −2 −26 | L | 4.01 | ||
| Superior Frontal Gyrus | 381 | 0.009 | 14, 32 62 | R | 4.62 |
| Middle Frontal Gyrus | 38, 22 58 | R | 4.29 | ||
| Middle Frontal Gyrus | 28, 34 52 | R | 4.06 | ||
| Lateral Occipital Cortex | 401 | 0.007 | −36, −90 22 | L | 4.25 |
| Lateral Occipital Cortex | −36, −80 42 | L | 4.22 | ||
| Lateral Occipital Cortex | −48, −78 24 | L | 4.04 | ||
| Controls > Heroin Treatment | |||||
| Frontal Pole | 259 | 0.039 | 20, 56 30 | R | 6.12 |
| Superior Frontal Gyrus | 10, 52 24 | R | 5.41 | ||
| Frontal Pole | 6, 58 28 | R | 4.75 | ||
| Middle Frontal Gyrus | 299 | 0.021 | −36, 22 56 | L | 4.53 |
| Middle Frontal Gyrus | −44, 20 48 | L | 4.29 | ||
| Superior Frontal Gyrus | −18, 30 52 | L | 4.19 | ||
| Controls < Heroin Treatment | |||||
| None | |||||
| Controls > Placebo Treatment | |||||
| None | |||||
| Controls < Placebo Treatment | |||||
| None | |||||
Abbreviations: FWE: family−wise error; L: left; R: right.
Thalamic Fractional Amplitude of Low Frequency Fluctuations
Between‐treatment analysis of fALFF in patients revealed increased values for heroin treatment within the precentral gyrus and the thalamus relative to the placebo treatment (Fig. 2, Table 5). Healthy controls showed increased fALFF values in the precuneus and posterior cingulate gyrus and decreased fALFF values in the cerebellum, midbrain and amygdala in comparison to the heroin treatment. Placebo treatment in patients was associated with increased fALFF values in the cerebellum, inferior temporal, and parahippocampal gyrus.
Table 5.
Resting‐state associated fractional amplitude of low frequency fluctuations (fALFF)
| Brain region | Cluster size (voxels) | Cluster P‐value (FWE corrected) | MNI coordinats (x, y, z) | Hemisphere | Z‐value at voxel level |
|---|---|---|---|---|---|
| Heroin > Placebo Treatment | |||||
| Precentral Gyrus | 540 | 0.001 | 2, −32, 60 | R | 6.06 |
| Precentral Gyrus | 2, −22, 68 | R | 5.62 | ||
| Precentral Gyrus | −4, −26, 54 | L | 3.93 | ||
| Thalamus | 554 | <0.001 | −12, −20, 8 | L | 4.71 |
| Thalamus | −8, −12, 8 | L | 4.67 | ||
| Thalamus | 8, −8, 4 | R | 4.53 | ||
| Heroin < Placebo Treatment | |||||
| none | |||||
| Controls > Heroin Treatment | |||||
| Posterior Cingulate Gyrus | 563 | 0.001 | −4, −50, 32 | L | 5.13 |
| Precuneus | 12, −58, 26 | R | 4.90 | ||
| Precuneus | −10, −60, 22 | L | 4.28 | ||
| Controls < Heroin Treatment | |||||
| Cerebellum | 273 | 0.039 | −30, −58, −26 | L | 4.59 |
| Cerebellum | −38, −58, −32 | L | 3.99 | ||
| Cerebellum | −48, −70, −30 | L | 3.54 | ||
| Midbrain | 550 | 0.001 | −10, −4, −10 | L | 4.37 |
| Amygdala | −26, −2, −18 | L | 4.20 | ||
| Midbrain | −14, −20, −14 | L | 3.81 | ||
| Controls > Placebo Treatment | |||||
| none | |||||
| Controls < Placebo Treatment | |||||
| Cerebellum | 310 | 0.024 | 28, −34, −32 | R | 3.93 |
| Inferior Temporal Gyrus | 46, −38, −24 | R | 3.69 | ||
| Parahippocampal Gyrus | 26, −20, −30 | R | 3.38 | ||
Abbreviations: FWE: family‐wise error; L: left; R: right.
In depth analysis of slow‐4 and slow‐5 frequency bands within patients revealed increased slow‐5 fALFF values in the precentral gyrus during heroin treatment. Slow‐4 fALFF was associated with increased values in the bilateral thalamus during heroin treatment (Table 6).
Table 6.
Differences in slow‐5 and slow‐4 amplitude of low frequency fluctuations (fALFF) bands in heroin and placebo treatment
| Brain region | Cluster size (voxels) | Cluster P‐value (FWE corrected) | MNI coordinats (x, y, z) | Hemisphere | Z‐value at voxel level |
|---|---|---|---|---|---|
| fALFF slow‐5: Heroin > Placebo Treatment | |||||
| Precentral Gyrus | 1305 | <0.001 | 2, −20, 70 | R | 4.29 |
| Precentral Gyrus | 20, −28, 66 | R | 4.22 | ||
| Precentral Gyrus | 6, −28, 70 | R | 4.13 | ||
| fALFF slow‐5: Heroin < Placebo Treatment | |||||
| None | |||||
| fALFF slow‐4: Heroin > Placebo Treatment | |||||
| Thalamus | 814 | <0.001 | 8, −8, 4 | R | 4.96 |
| Thalamus | −8, −12, 8 | L | 4.97 | ||
| Thalamus | 8, −24 4 | R | 3.90 | ||
| fALFF slow‐4: Heroin < Placebo Treatment | |||||
| None | |||||
fALFF slow‐4: 0.027–0.073 Hz; fALFF slow‐5: 0.01–0.027 Hz; FWE: family‐wise error; L: left; R: right.
Correlation Analyses
Correlation analyses showed that peak plasma levels of morphine correlated positively with heroin‐induced reduction in thalamic FC to the frontal orbital cortex, mediofrontal cortex and frontal pole (Table 7). By analyzing heroin and placebo treatment separately, we found a negative correlation between first‐order attention score of BIS (heroin treatment) and fALFF values (heroin treatment), within a cluster including the thalamus, midbrain and hypothalamus (Table 8, Fig. 3). No correlation was found between first and second order attention scores of BIS and treatment‐dependent thalamocortical FC and ReHo.
Table 7.
Positive correlations between morphine metabolites and regional treatment difference values in thalamocortical functional connectivity (FC), regional homogeneity (ReHo), and fractional amplitude of low frequency fluctuations (fALFF)
| Brain region | Cluster size (voxels) | Cluster P value (FEW corrected) | MNI coordinates (x, y, z) | Hemisphere | Z value at voxel level |
|---|---|---|---|---|---|
| Thalamocortical FC and morphine plasma levels | |||||
| Frontal Orbital Cortex | 52 | 0.046 | −24, 12, −20 | L | 5.41 |
| ReHo and morphine plasma levels | |||||
| None | |||||
| fALFF and morphine plasma levels | |||||
| None | |||||
Abbreviations: fALFF: fractional amplitude of low frequency fluctuations; FC: functional connectivity; FWE: family‐wise error; L: left; R: right; ReHo: regional homogeneity.
Table 8.
Negative correlation of first‐order BIS attention score with fractional amplitude of low frequency fluctuations (fALFF)
| Brain region | Cluster size (voxels) | Cluster P‐value (FWE corrected) | MNI coordinates (x, y, z) | Hemisphere | Z‐value at voxel level |
|---|---|---|---|---|---|
| fALFF (Heroin treatment) and first‐order BIS | |||||
| Midbrain | 238 | 0.049 | 4, −10, −14 | R | 4.20 |
| Thalamus | 10, −16, −6 | R | 4.17 | ||
| Hypothalamus | 8, −6, −8 | R | 3.34 | ||
Abbreviations: BIS: Barrett impulsiveness scale; fALFF: fractional amplitude of low frequency fluctuations; FWE: family‐wise error; L: left; R: right; ReHo: regional homogeneity.
Figure 3.
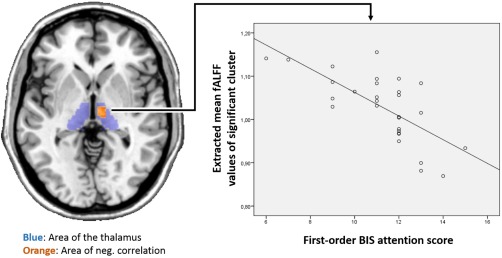
Negative correlation of first‐order BIS attention score with heroin treatment related fALFF. Spectral colours indicate mean thalamic fALFF values of heroin and placebo treatment. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
In this study, we investigated whether acute heroin treatment leads to alterations in spontaneous neural activity within the thalamocortical system. We performed three different resting state analyses to detect modulations of functional integration and segregation of thalamic low frequency oscillation and examined whether these effects were related to clinical characteristics and behavioral indices of attention. We could show that, in comparison to placebo treatment and healthy controls, heroin acutely reduced thalamocortical FC to multiple cortical regions, including parts of the frontal cortex. The heroin‐induced reductions in FC from the thalamus to the mediofrontal cortex, orbitofrontal cortex and frontal pole were positively correlated with plasma levels of morphine. Furthermore, and in contrast to reductions in thalamic FC, we found increased thalamic ReHo and fALFF values during heroin treatment. The heroin‐associated increase in fALFF values were driven by alterations in the slow‐4 oscillation band. Plasma levels of heroin were positively correlated with treatment differences in fALFF in the bilateral lateral occipital cortex, while fALFF within the right thalamus correlated negatively with attention scores measured by BIS.
Impaired cognitive control plays an important role in the compulsive and drug‐seeking behavior of drug‐dependent subjects [Perry and Carroll, 2008]. It has repeatedly been shown that this deficit in cognitive functioning in heroin addicted individuals is accompanied by reduced activity in the right IFG and ACC [Fu et al., 2008; Lee et al., 2005]. Our group has recently extended these findings by showing that acute heroin administration not only impairs cognitive control by reducing IFG and ACC activity in heroin‐dependent patients, but also effective connectivity from the ACC to the IFG during a cued Go/No‐Go task [Schmidt et al., 2014; Schmidt et al., 2013b]. However, besides the crucial interplay between the dorsal ACC and IFG, the IFG is also functionally connected with the thalamus during cognitive functioning (i.e. response inhibition) [Aron and Poldrack, 2006]. In the present study, we found that acute heroin treatment reduced FC between the thalamus and frontal brain regions, which suggests that these reductions may modulate cognitive functioning in heroin‐dependent subjects. This finding adds to our previous results that acute heroin administration not only reduces ACC activity and its connectivity to the IFG [Schmidt et al., 2014; Schmidt et al., 2013a], but also the connectivity from the thalamus to the frontal cortex. The reduced thalamocortical FC after heroin treatment may be regarded as desynchronized activity between the thalamus and the cortex and may contribute to impairment in the alerting attention network. Heroin‐associated reduction in frontal perfusion and gray matter volume, as shown in our previous studies [Denier et al., 2013a, 2013b], may be associated with reduction in thalamofrontal FC.
In contrast to the heroin‐induced reduction in thalamocortical FC, we found enhancement of local thalamic parameters, including fALFF and ReHo values. While resting state FC can be taken as a measure of coupling in oscillation between distant regions, ALFF/fALFF measures reflect the extent of BOLD‐associated spontaneous oscillation and so indirectly its neural activity [Duff et al., 2008; Fransson, 2006]. ReHo is a good index of local integrity as assessed by oscillation synchronisation [Zang et al., 2004]. However, the direct relationships of the three parameters, FC, fALFF, and ReHo, are not yet fully understood. There are only two studies examining long term ALFF changes in heroin addicts, but no ALFF changes were found in the thalamus. Compared to healthy controls, heroin addicts showed decreased ALFF values in the temporal and frontal lobe, including the orbitofrontal cortex and the ACC. Moreover, heroin addicts showed decreased ALFF in the right caudate that correlated with the duration of heroin use [Wang et al., 2013]. A previous study in heroin‐dependent individuals showed decreased ReHo values in the thalamus relative to healthy controls [Qiu et al., 2011]. This discrepancy may result from differences in the included patient samples, such as differences in the durations of heroin use, duration of maintenance treatment or dose of maintenance treatment. Furthermore, the placebo treatment in our study may induced a state of heroin withdrawal [Schmidt et al., 2013a], resulting in a different psychological state from that of the patients in Qiu et al. [2011]. However, despite this discrepancy, the authors concluded that this decrease in the ReHo values in the bilateral medial dorsal nucleus might mediate the attention deficits in chronic heroin users. In accordance with such an interpretation, we could show that fALFF values in the right thalamus after heroin treatment were negatively correlated with subjective scores on attentional control. This corresponds with a previous study showing a negative correlation between attentional BIS scores in codeine‐dependent subjects and ReHo values in the thalamus [Qiu et al., 2013] as ReHo and fALFF values are known to be correlated. More work is needed to understand the relation between thalamic ReHo and ALFF alteration and attentional control.
Opioid‐associated alterations in thalamic functionality are well documented in animals. In particular, Brunton and Charpak showed that a systemically administered µ‐opioid agonist inhibited the entire thalamus by inducing hyperpolarization and shifting thalamic cell firing from the tonic to the bursting mode [Brunton and Charpak, 1998]. The burst firing mode is characteristic of thalamic relay neurons [Jahnsen and Llinas, 1984] and plays an important role in slow wave sleep and drowsiness [Livingstone and Hubel, 1981; Steriade et al., 1993]. We may speculate that our findings of heroin‐induced reduction in thalamocortical FC and enhancement of fALFF and ReHo reflect a shift towards the thalamic burst firing mode on a higher functional level.
Some limitations need to be considered. Our patients were recruited from a population which mainly consisted of individuals with long standing polysubstance use, including cocaine consumption. Although this problem is virtually inevitable when chronic heroin‐dependent individuals are examined, cocaine and other drug use may have confounded our findings. However, the greatest differences were found between heroin and placebo treatment within patients.
Another limitation is that BIS is not a direct psychometric measurement of attention and impulsivity. Therefore the BIS scores are stable within patients, did not differ between treatments, but may be substantially influenced by recurring heroin effects. A more direct cognitive assessment of attention and impulsivity would help to clarify the acute modulation of heroin on attentional control functions and whether this effect is related to the reported alterations in thalamic activities.
In conclusion, our results showed that acute heroin administration leads to abnormalities in functional integration and segregation of thalamic resting‐state oscillation. Abnormalities were partially related to clinical characteristics and plasma levels of morphine. Reduced thalamocortical FC and altered intrinsic thalamic oscillation characteristics may explain some deficits in cognitive functioning in heroin‐dependent patients. Further research is needed to elucidate the relationship between thalamic function and cognitive control in heroin addiction.
ACKNOWLEDGMENTS
We would like to thank the staff of the heroin‐assisted treatment in Basel (Janus) who helped us to conduct this study. In particular, we thank Jeannette Kaiser, Sandra Gropp, Eva Müller and Rainer Kreider. All authors declare that they have no conflicts of interest.
REFERENCES
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005): Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA (2006): Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. J Neurosci 26:2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS (1995): Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 34:537–541. [DOI] [PubMed] [Google Scholar]
- Bourquin D, Bundeli P, Lehmann T, Brenneisen R (1999): Diacetylmorphine and its metabolites in plasma by HPLC with diode‐array and atmospheric pressure ionization mass spectrometric detection. J Liq Chrom Rel Technol 22:2663–2674. [Google Scholar]
- Bracken BK, Trksak GH, Penetar DM, Tartarini WL, Maywalt MA, Dorsey CM, Lukas SE (2012): Response inhibition and psychomotor speed during methadone maintenance: impact of treatment duration, dose, and sleep deprivation. Drug Alcohol Depend 125:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J, Charpak S (1998): mu‐Opioid peptides inhibit thalamic neurons. J Neurosci 18:1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Draguhn A (2004): Neuronal oscillations in cortical networks. Science 304:1926–1929. [DOI] [PubMed] [Google Scholar]
- Davis, F.H. (1989) Fourier Series and Orthogonal Functions. New York: Dover Publications. [Google Scholar]
- de Bourbon‐Teles J, Bentley P, Koshino S, Shah K, Dutta A, Malhotra P, Egner T, Husain M, Soto D (2014): Thalamic control of human attention driven by memory and learning. Curr Biol 24:993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denier, N , Gerber, H , Vogel, M , Klarhofer, M , Riecher‐Rossler, A , Wiesbeck, GA , Lang, UE , Borgwardt, S , Walter, M (2013a): Reduction in cerebral perfusion after heroin administration: a resting state arterial spin labeling study. PloS One 8:e71461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denier, N , Schmidt, A , Gerber, H , Schmid, O , Riecher‐Rossler, A , Wiesbeck, GA , Huber, CG , Lang, UE , Radue, EW , Walter, M , Borgwardt, S (2013b): Association of frontal gray matter volume and cerebral perfusion in heroin addiction: a multimodal neuroimaging study. Front Psychiatry 4:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff EP, Johnston LA, Xiong J, Fox PT, Mareels I, Egan GF (2008): The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Hum Brain Mapp 29:778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P (2006): How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44:2836–2845. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2009): Modalities, modes, and models in functional neuroimaging. Science 326:399–403. [DOI] [PubMed] [Google Scholar]
- Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L, Ming‐Fan Yang Z (2008): Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett 438:322–326. [DOI] [PubMed] [Google Scholar]
- Galynker II, Watras‐Ganz S, Miner C, Rosenthal RN, Des Jarlais DC, Richman BL, London E (2000): Cerebral metabolism in opiate‐dependent subjects: effects of methadone maintenance. Mt Sinai J Med 67:381–387. [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE (2004): Nonstationary cluster‐size inference with random field and permutation methods. NeuroImage 22:676–687. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinas R (1984): Electrophysiological properties of guinea‐pig thalamic neurones: an in vitro study. J Physiol 349:205–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG (2002): Thalamic circuitry and thalamocortical synchrony. Philos Trans R Soc Lond B Biol Sci 357:1659–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VL, Brooner RK, Kidorf MS, Stoller KB, Mirsky AF (1999): Attention deficit hyperactivity disorder and treatment outcome in opioid abusers entering treatment. J Nerv Ment Dis 187:487–495. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA (2003): Multiple neuronal networks mediate sustained attention. J Cogn Neurosci 15:1028–1038. [DOI] [PubMed] [Google Scholar]
- Lee TM, Pau CW (2002): Impulse control differences between abstinent heroin users and matched controls. Brain Inj 16:885–889. [DOI] [PubMed] [Google Scholar]
- Lee TM, Zhou WH, Luo XJ, Yuen KS, Ruan XZ, Weng XC (2005): Neural activity associated with cognitive regulation in heroin users: a fMRI study. Neurosci Lett 382:211–216. [DOI] [PubMed] [Google Scholar]
- Lever JR (2007): PET and SPECT imaging of the opioid system: receptors, radioligands and avenues for drug discovery and development. Curr Pharm Des 13:33–49. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH (1981): Effects of sleep and arousal on the processing of visual information in the cat. Nature 291:554–561. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. NeuroImage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Maurer R, Cortes R, Probst A, Palacios JM (1983): Multiple opiate receptor in human brain: an autoradiographic investigation. Life Sci 33:231–234. [DOI] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW (2000): Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology 23:113–126. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES (1995): Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774. [DOI] [PubMed] [Google Scholar]
- Pau CW, Lee TM, Chan SF (2002): The impact of heroin on frontal executive functions. Arch Clin Neuropsychol 17:663–670. [PubMed] [Google Scholar]
- Peckys D, Landwehrmeyer GB (1999): Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience 88:1093–1135. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME (2008): The role of impulsive behavior in drug abuse. Psychopharmacology 200:1–26. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Pasi A, Mehraein P, Herz A (1982): Opiate receptor binding sites in human brain. Brain Res 248:87–96. [DOI] [PubMed] [Google Scholar]
- Prosser J, London ED, Galynker II (2009): Sustained attention in patients receiving and abstinent following methadone maintenance treatment for opiate dependence: performance and neuroimaging results. Drug Alcohol Depend 104:228–240. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Lv X, Su H, Jiang G, Tian J, Zhuo F, Han L, Zhang X (2013): Reduced regional homogeneity in bilateral frontostriatal system relates to higher impulsivity behavior in codeine‐containing cough syrups dependent individuals. PloS One 8:e78738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YW, Han LJ, Lv XF, Jiang GH, Tian JZ, Zhuo FZ, Su HH, Lin CL, Zhang XL (2011): Regional homogeneity changes in heroin‐dependent individuals: resting‐state functional MR imaging study. Radiology 261:551–559. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Borgwardt S, Gerber H, Schmid O, Wiesbeck GA, Riecher‐Rossler A, Bendfeldt K, Smieskova R, Lang UE, Rubia K, Walter M (2014): Altered prefrontal connectivity after acute heroin administration during cognitive control. Int J Neuropsychopharmacol 1–11. [DOI] [PubMed] [Google Scholar]
- Schmidt, A. , Borgwardt, S. , Gerber, H. , Wiesbeck, G.A. , Schmid, O. , Riecher‐Rössler, A. , Smieskova, R. , Lang, U.E. , Walter, M. (2013a) Acute effects of heroin on negative emotional processing: relation of amygdala activity and stress‐related responses. Biol Psychiatry 76:289–296. [DOI] [PubMed] [Google Scholar]
- Schmidt, A. , Denier, N. , Magon, S. , Radue, E.W. , Huber, C.G. , Riecher‐Rossler, A. , Wiesbeck, G.A. , Lang, U.E. , Borgwardt, S. , Walter, M. (2015) Increased functional connectivity in the resting‐state basal ganglia network after acute heroin substitution. Transl Psychiatry. 2015 Mar 24;5:e533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Walter M, Gerber H, Schmid O, Smieskova R, Bendfeldt K, Wiesbeck GA, Riecher‐Rössler A, Lang UE, Rubia K, McGuire P, Borgwardt S (2013b): Inferior frontal cortex modulation with an acute dose of heroin during cognitive control. Neuropsychopharmacology 38:2231–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011): REST: a toolkit for resting‐state functional magnetic resonance imaging data processing. PloS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M (2006): Grouping of brain rhythms in corticothalamic systems. Neuroscience 137:1087–1106. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ (1993): Thalamocortical oscillations in the sleeping and aroused brain. Science 262:679–685. [DOI] [PubMed] [Google Scholar]
- Walter M, Denier N, Gerber H, Schmid O, Lanz C, Brenneisen R, Riecher‐Rossler A, Wiesbeck GA, Scheffler K, Seifritz E, McGuire P, Fusar‐Poli P, Borgwardt S (2015): Orbitofrontal response to drug‐related stimuli after heroin administration. Addict Biol 20:570–579. [DOI] [PubMed] [Google Scholar]
- Walter M, Gerber H, Kuhl HC, Schmid O, Joechle W, Lanz C, Brenneisen R, Schächinger H, Riecher‐Rössler A, Wiesbeck GA, Borgwardt SJ (2013): Acute effects of intravenous heroin on the hypothalamic‐pituitary‐adrenal axis response: a controlled trial. J Clin Psychopharmacol 33:193–198. [DOI] [PubMed] [Google Scholar]
- Wang GY, Wouldes TA, Kydd R, Jensen M, Russell BR (2014): Neuropsychological performance of methadone‐maintained opiate users. J Psychopharmacol 28:789–799. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhu J, Li Q, Li W, Wu N, Zheng Y, Chang H, Chen J, Wang W (2013): Altered fronto‐striatal and fronto‐cerebellar circuits in heroin‐dependent individuals: a resting‐state FMRI study. PloS One 8:e58098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Nieto‐Castanon A (2012): Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L (2004): Regional homogeneity approach to fMRI data analysis. NeuroImage 22:394–400. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhu C, Chen H, Duan X, Lu F, Li M, Liu F, Ma X, Wang Y, Zeng L, Zhang W (2015): Frequency‐dependent alterations in the amplitude of low‐frequency fluctuations in social anxiety disorder. J Affect Disord 174:329–335. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF (2008): An improved approach to detection of amplitude of low‐frequency fluctuation (ALFF) for resting‐state fMRI: fractional ALFF. J Neurosci Meth 172:137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]


