ABSTRACT
Low pathogenicity avian influenza (LPAI) H9N2, highly pathogenic avian influenza (HPAI) H5N1, and H5N8 circulate in Egyptian poultry and cause veterinary and public health burdens. In response, AIV vaccines are commonly used. The main objective of this study was to develop a broad, cross-protective, trivalent vaccine based on circulating AIVs in Egypt. We generated highly replicating avirulent AIVs, H5N1, and H5N8, to be used in combination with H9N2 strain for the generation of an inactivated vaccine. Immunogenicity and protective efficacy of this vaccine were tested. Results showed that a single immunization dose enhanced humoral immune responses giving full protection against challenges with LPAI H9N2, HPAI H5N1, and H5N8 viruses. This efficacious vaccine will reduce the cost of vaccination for poultry growers and is expected to be effective in the field as it is based on contemporary viruses currently in circulation among Egyptian poultry.
Keywords: avian influenza, trivalent, vaccine, Egypt
INTRODUCTION
During the last decade, Egypt suffered from endemic avian influenza viruses (AIV) that cause great economic losses and threaten public health (Aly et al., 2008). Co-circulation of highly pathogenic avian influenza (HPAI) H5N1 and H5N8 as well as low pathogenic avian influenza (LPAI) H9N2 viruses among poultry has been observed. Chances to eradicate these endemic viruses from Egypt are considered to be low as a result of gaps in the application of the recommended AIV control strategies that mainly focused on vaccination (Kayali et al., 2016). At least 24 commercial inactivated AI H5 vaccines were licensed for use against H5N1 in Egyptian poultry (Kayali et al., 2016). Genetic mismatch of vaccine seed strains in commercial products and circulating AIVs has led to vaccination failure and rapid evolution of H5N1 virus as a result of escaping from immune pressure. The current endemic situation of many AIVs subtypes (H5N1, H5N8, and H9N2) in Egyptian poultry, make vaccination using monovalent vaccines costly. Therefore, multi-antigenic vaccine production may provide a solution for such issues. Here we generated highly replicating avirulent H5N1 and H5N8 viruses based on reverse genetics system, to be used in combination with H9N2 strain for the generation of an inactivated trivalent AI vaccine for Egypt. The immunogenicity and protective efficacy of this vaccine against HPAI H5N1, H5N8, and LPAI H9N2 viruses were evaluated.
MATERIALS AND METHODS
Viruses and Vaccine Preparation
The LPAI A/chicken/Egypt/D10802C/2015(H9N2) virus abbreviated as D10802C/H9N2, the HPAI clade 2.3.4.4 A/green-winged teal/Egypt/871/2016(H5N8) (871/H5N8), and HPAI clade 2.2.1.2 A/chicken/Egypt/D10552B/2015(H5N1) (D10552B/H5N1) viruses were used for preparation of experimental vaccines. The multibasic amino acid sequences (PKGEKRRKKR/GLF) and (PLREKRRKR/GLF) at the cleavage site of the H5N1 and H5N8 viruses respectively were transformed into a monobasic form (ETR/GLF) as described previously (Webby et al., 2004).
For H5N1 vaccine strain preparation (rgD10552B/H5N1), a plasmid-based reverse genetics system was applied using 6 internal genes of A/Puerto Rico/8/34(H1N1) and 2 surfaces LP-HA and NA genes of D10552B/H5N1 virus. For H5N8, 2 forms of vaccine seed strains, LP (6+2) 871 and LP full 871 were prepared. LP (6+2) 871 virus was rescued by using 6 internal genes of A/Puerto Rico/8/34(H1N1) virus and 2 surface LP-HA and NA genes of 871/H5N8 virus. LP full 871 included the full genome of 871/H5N8 with altered LP-HA. Rescued reassortant LP H5N8 viruses were individually inoculated into 11-day-old specific pathogen-free embryonated chicken eggs (SPF-ECE) (Koum Oshiem SPF Chicken Farm, Fayoum, Egypt) using equal 104 EID50/0.1 mL titer of each virus. The allantoic fluids were harvested at 24 and 36 h post-infection and titrated using EID50 and HA assays.
HA titers of the 3 viruses (rgD10552B/H5N1, LP full 871, and D10802C/H9N2) were adjusted individually using phosphate buffered saline to 7 log2 HA/50μL. Viruses were inactivated by the addition of 0.1% formalin overnight. The complete inactivation process for each antigen used in this study was verified by inoculating the inactivated antigens into 11-day-old SPF-ECE. Inactivated antigens were mixed with adjuvant Montanide ISA 71 VG (Seppic Inc., Puteaux, France) as water in oil (W/O) emulsionin the ratio recommended by the manufacturer's technical manual (30 antigen/70 adjuvant W/W) followed by homogenization for 3 min on ice using a mixer homogenizer. To test the safety of the prepared vaccines, 5 SASSO chickens (3-wk-old) were inoculated intramuscularly by double dose (1 mL) of each form of vaccine then observed for the presence of any clinical signs or local lesions at the site of vaccination for 2 wk (OIE, 2014).
Immunization of SPF Chickens and Seroconversion Determination
A total of 84 2-wk-old SPF chickens were purchased from Koum Oshiem SPF Chicken Farm, Fayoum, Egypt and divided into 5 groups: groups 1 to 3 were composed of 12 chickens each and immunized with H5N1, H9N2, and H5N8 monovalent vaccines, group 4 had 25 chickens that were immunized with the trivalent vaccine, and group 5 of 23 chickens were unimmunized control group. Chickens were vaccinated intramuscularly in the leg with 0.5 mL of the desired vaccine. Serum was collected weekly from all chickens for determination of serological conversion. Specific antibodies were determined using hemagglutination inhibition (HI) and virus microneutralization test (VMN) (WHO, 2002). Homologous AIVs rgD10552B/H5N1, LP (6+2) 871/H5N8, and D10802C/H9N2 were used for titration of specific antibodies in both HI and VMN assays.
Challenge Infection and Viruses Shedding
Virus challenge was conducted at 5 weeks post-vaccination (wpv) at the National Research Centre in Egypt. A total of 7 chickens from groups 1 to 3 (monovalent vaccines) were infected with homologous subtype using HPAIV A/chicken/Egypt/F12505B/2016(H5N1) (F12505B/H5N1), LPAIV A/chicken/Egypt/D7100/2013(H9N2) (D7100/H9N2), and HPAI A/duck/Egypt/F13666A/2017(H5N8) (F13666A/H5N8) virus-es. Chickens from groups 4 and 5 were subjected to challenge infections with the 3 AIVs (7 chickens for each virus). The challenge dose for each virus was 106 EID50/0.5 mL inoculated through intranasal and intratracheal routes. Live virus infection experiments were applied in a negative pressure based biosafety level 3 isolators (PLAS LABS, Lansing, MI). Birds were daily observed for morbidity and mortality for 10 days post-infection (dpi). For determination of viruses shedding, oral and cloacal swabs were obtained from each bird at 3, 5, and 7 dpi, then titrated in egg culture as viruses EID50 per 1 mL was calculated using Reed–Muench method. All animal experiments were approved by the Ethics Committee of the National Research Center, Egypt.
Evaluation of Vaccine at Central Laboratory for Evaluation of Veterinary Biologics (CLEVB)
Another challenge experiment was conducted at CLEVB. One-week-old SPF chickens were immunized as 10 chickens for each monovalent vaccine, 30 for the trivalent vaccine, and 30 chickens as unvaccinated control. By the fourth wpv, chickens were challenged with A/chicken/Egypt/128S/2012(H5N1), A/chicken/Egypt/18FL6/2018(H5N8), and A/chicken/Egypt/114922v/2011(H9N2). Chickens were monitored daily for morbidity and mortality for 10 dpi.
Statistical Analyses
All statistical analyses were carried out using GraphPad Prism V5 software (GraphPad Inc., San Diego, CA). One-way ANOVA with Tukey post-hoc test was used to compare antibody and virus titers. Differences were considered statistically significant at P-value < 0.05.
RESULTS
Two reassortant LPAI H5N8 viruses [LP (6+2) 871 and LP full 871] viruses were successfully rescued. The rescued viruses were propagated in eggs for 2 passages and did not cause embryo death. Rescued viruses and HP parent virus were grown on eggs and titrated by EID50 and HA assays. We observed that HP A/green-winged teal/Egypt/871/2016 (H5N8) virus showed higher HA and EID50 titers at 24 dpi as compared to LP 871 (6+2) virus (P-value < 0.01) (Figure 1). LP full 871 virus had significantly higher HA and EID50 titers as compared to the LP (6+2) 871 virus (P-value < 0.05) (Figure 1). Thus, the LP full 871 virus was used as H5N8 antigen in vaccine formulation as its titers would be acceptable by vaccine producers.
Figure 1.
Viral growth kinetics of 2 reassortant LPAI H5N8 viruses in ECE by HA and EID50.
Immunogenicity of AIV Inactivated Trivalent Vaccine in SPF Chickens
At the day of vaccination (2 wk of age), sera collected from 10 chicks cross-reacted with H5N1, H5N8, and H9N2 antigens with log2 HI mean titers of 3, 1.9, and 3, respectively. All immunized chickens of trivalent and monovalent groups developed a significant immune response at 3 wpv (P < 0.0001) by both HI (Figure 2) and VMN assays (Figure 3), which increased over time through 4 and 5 wpv. No significant difference of detected antibodies was detected between 3 monovalent and trivalent vaccine either by HI and VMN (P-values < 0.05). Unimmunized chickens had ≤ 3 log2 mean titers against the 3 antigens during the vaccination immunity assessment period which is considered as a non-specific titer (Figures 2 and 3). Neither morbidity nor BW loss were observed in the 4 vaccinated groups compared to the control group during the vaccination follow up period.
Figure 2.
Weekly log2 antibody titers mean of SPF chicken vaccinated with 3 monovalent vaccines, 1 trivalent vaccine, and control chickens against D10552B/H5N1, 871/H5N8, and D10802C/H9N2 AIVs.
Figure 3.
Weekly MN titer mean of SPF chickens immunized with 3 monovalent, 1 trivalent, and chickens against D10552B/H5N1, 871/H5N8, and D10802C/H9N2 AIVs.
Protective Efficacy of Developed AIV Inactivated Trivalent Vaccine in SPF Chickens
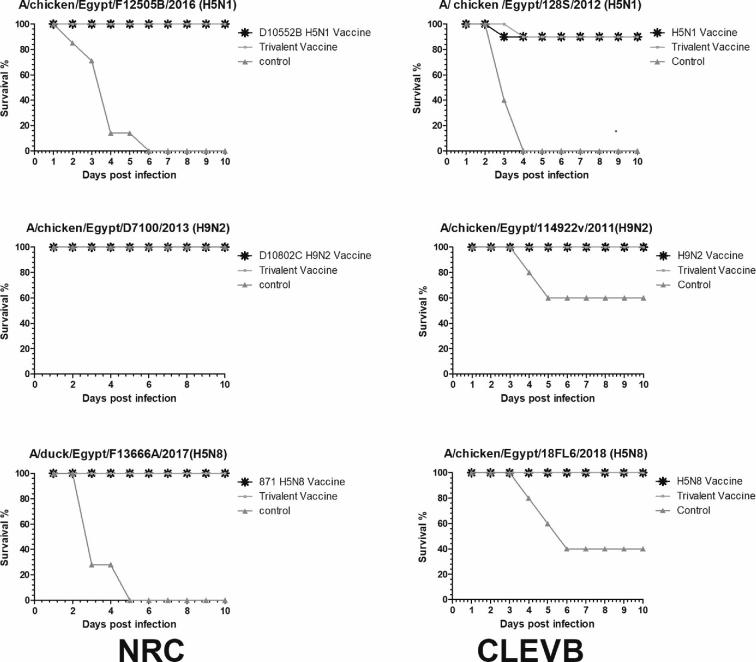
Vaccinated chickens did not show any AI signs and survived post challenge infection with homologous viruses for monovalent and trivalent vaccine groups (Figure 4). All control chickens challenged by HPAIV H5N1 and HPAIV H5N8 died by 5 dpi with typical symptoms of highly pathogenic infection including cyanotic combs and wattles, edema of the head, and shank of leg hemorrhage, while D7100/H9N2 challenged control chicks survived with mild symptoms.
Figure 4.
Survival curves of monovalent immunized, trivalent immunized, and control SPF chickens after challenge with different strains of AI H5N1, H9N2, and H5N8 viruses at National Research Centre (NRC), and Central laboratory for evaluation of Veterinary Biologics (CLEVB).
Viral shedding was determined via titration of oropharyngeal and cloacal swabs in eggs by EID50 (Figure 5 and Table S1). All 3 AIVs were detected by 3 dpi in oropharyngeal swabs of the control chickens as the peak of viral shedding was recorded, while only H5N1 and H9N2 (not H5N8) were detected in oropharyngeal swabs of vaccinated groups. Virus shedding in cloacal swabs at 3 dpi varied with virus/vaccine type. Over time, viral shedding declined in vaccinated chickens more than in control groups. Both mono- and tri-valent vaccinated chickens had non-significantly different profile of viral shedding.
Figure 5.
Titration of viral shedding from collected oropharyngeal and cloacal swabs for challenged experiment conducted at NRC after infection of unimmunized and immunized chickens with Egyptian HPAIV A/chicken/Egypt/F12505B/2016(H5N1) (F12505B/H5N1), LPAIV A/chicken/Egypt/D7100/2013(H9N2) (D7100/H9N2), and HPAI A/duck/Egypt/F13666A/2017(H5N8) (F13666A/H5N8) viruses at 3, 5, and 7 dpi. A total of 7 chickens from each group were individually infected. Virus shedding was monitored by titration of Log10 EID50/mL for each collected sample from only live chickens. Each dot represents the viral titer of each chicken. The bar for each vaccine is the mean of viral shedding in the group. The detection limit was <1 Log10 EID50/0.1 mL.
The experimental trivalent and monovalent vaccines were evaluated at CLEVB by challenge infection using different reference Egyptian strains. All vaccinated chicken with H5N8 and trivalent vaccines did not show any AI signs and survived post challenge infection with HP A/chicken/Egypt/18FL6/2018(H5N8) virus (Figure 4). The vaccinated chickens with monovalent H5N1 and trivalent vaccines showed 90% protection against A/chicken/Egypt/128S/2012(H5N1) virus. All control chickens challenged by HP A/chicken/Egypt/128S/2012(H5N1) virus died by 4 dpi with typical symptoms of highly pathogenic infection, while 60% of challenged control chicks with A/chicken/Egypt/18FL6/2018(H5N8) virus died by 6 dpi. All vaccinated chickens with H9N2 and trivalent vaccines did not show any AI signs and survived post challenge infection with LP A/chicken/Egypt/114922v/2011(H9N2) virus while unvaccinated chickens showed 20% mortality rate after 4 dpi that increased to reach 40% by 5 dpi.
DISCUSSION
The complicated situation of AIVs in Egypt added additional economic risk on poultry industry in Egypt as a result of combined enzootic state of 3 antigenically different subtypes AIVs, clade 2.2.1.2 H5N1 (since 2006), GI like H9N2 (since 2011), and clade 2.3.4.4 H5N8 (since 2016) (Aly et al., 2008; Kandeil et al., 2017; Kayali et al., 2014, 2016; Yehia et al., 2018). Vaccination in Egypt is the primary tool for controlling AIVs in poultry. Vaccine seed strains of licensed commercial vaccines were based either on classical LPAI H5NX viruses or on Rg H5N1 having HA and NA genes of H5N1 on a backbone of A/Puerto Rico/8/1934(H1N1) strain. The majority of commercial influenza vaccines had limited protection effects against circulating viruses due to diversity in genetic and antigenic patterns between circulating viruses and antigens contained in those vaccines (Kandeil et al., 2018; Kayali et al., 2013). Although some commercial inactivated H5 vaccines showed 100% protection against H5N8 under laboratory conditions, they did not prevent virus shedding against circulating H5N8 viruses in Egypt (Kandeil et al., 2018). The genetic dissimilarity and poor reactivity between H5 commercial vaccines used in Egypt and currently circulating H5N8 viruses proves that the vaccines might not be effective in the field or introduce only partial protection and thus might lead to vaccine-induced escape mutant strains.
In this study, we constructed reverse genetics H5N8 virus (LP (6+2) 871) containing HA and NA gene segments from H5N8 virus on a PR8 backbone. Growth yield of the reassortant LP (6+2) 871 virus was limited. Previous studies showed that some LP H5N1 vaccine seed strains produced by reverse genetics in a backbone A/Puerto Rico/8/34(H1N1, PR8) virus as a donor strain for 6 internal segments grow suboptimally in ECE (Harvey et al., 2008; Horimoto et al., 2007). Therefore, a seed virus with more efficient growth in ECE is needed to ensure an adequate supply of H5N8 antigen content. Thus, the LP full 871 virus was used as H5N8 antigen in vaccine formulation.
A single immunization with the current trivalent vaccine enhanced humoral immune response and provided full protection based on the 2 independent experiments conducted at NRC and CLEVB against challenges with different strains of LPAI H9N2, HPAI H5N1, and HPAI H5N8 viruses. The use of this vaccine might reduce the cost of vaccination for poultry growers leading to better vaccination coverage. If vaccination continues to be the primary method of control of AIVs in Egypt, this trivalent vaccine is recommended.
The difference in mortality rates among the challenged unvaccinated chickens of the 2 experiments conducted at NRC and CLEVB might be attributed to the difference in genetic constellation of 2 challenge strains used. A previous study showed multiple introductions of HP influenza A(H5N8) viruses into poultry in Egypt(Salaheldin et al., 2018).
The findings of this study may have been affected by the following limitations. The developed inactivated trivalent vaccines conferred protection for chickens against different subtypes of AIVs circulating in Egypt under laboratory conditions, thus the efficacy of this vaccine for different species of poultry (ducks, turkey, geese, etc.) under field conditions needs further studies. The vaccination schedule used in this study is suitable for broiler chickens that have a short life-span and further studies are needed to investigate the long term immunity and recommended doses for layer and breeder chickens. Biosecurity and safety factors associated with preparation of inactivated vaccines based on full LP H5N8 will need to be assessed further in case this strain is used in vaccine manufacturing.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services (under contract HHSN272201400006C), the Deanship of Scientific Research, King Abdulaziz University, Jeddah, Saudi Arabia, contract number I-005-436, and the Egyptian Science and Technology Development Fund (under grant number 5175).
SUPPLEMENTARY DATA
Table S1. AIVs shedding titration of oropharyngeal and cloacal swabs at 3, 5, and 7 days post challenge.
ETHICAL APPROVAL
Ethical guidelines for the care and use of animals were reviewed and approved from animal ethical committees at NRC and CLEVB. This article does not contain any studies with human participants performed by any of the authors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Aly M. M., Arafa A., Hassan M. K.. 2008. Epidemiological findings of outbreaks of disease caused by highly pathogenic H5N1 avian influenza virus in poultry in Egypt during 2006. Avian Dis. 52:269–277. [DOI] [PubMed] [Google Scholar]
- Harvey R., Wheeler J. X., Wallis C. L., Robertson J. S., Engelhardt O. G.. 2008. Quantitation of haemagglutinin in H5N1 influenza viruses reveals low haemagglutinin content of vaccine virus NIBRG-14 (H5N1). Vaccine 26:6550–6554. S0264-410X(08)01314-5 [pii]. [DOI] [PubMed] [Google Scholar]
- Horimoto T., Murakami S., Muramoto Y., Yamada S., Fujii K., Kiso M., Iwatsuki-Horimoto K., Kino Y., Kawaoka Y.. 2007. Enhanced growth of seed viruses for H5N1 influenza vaccines. Virology 366:23–27. doi:10.1016/j.virol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeil A., El-Shesheny R., Maatouq A., Moatasim Y., Cai Z., McKenzie P., Webby R., Kayali G., Ali M. A.. 2017. Novel reassortant H9N2 viruses in pigeons and evidence for antigenic diversity of H9N2 viruses isolated from quails in Egypt. J. Gen. Virol. 98:548–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeil A., Sabir J. S. M., Abdelaal A., Mattar E. H., El-Taweel A. N., Sabir M. J., Khalil A. A., Webby R., Kayali G., Ali M. A.. 2018. Efficacy of commercial vaccines against newly emerging avian influenza H5N8 virus in Egypt. Sci. Rep. 8:9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayali G., Kandeil A., El-Shesheny R., Kayed A. S., Gomaa M. M., Maatouq A. M., Shehata M. M., Moatasim Y., Bagato O., Cai Z., Rubrum A., Kutkat M. A., McKenzie P. P., Webster R. G., Webby R. J., Ali M. A.. 2014. Active Surveillance for Avian Influenza Virus, Egypt, 2010−2012. Emerg. Infect. Dis. 20:542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayali G., Kandeil A., El-Shesheny R., Kayed A. S., Gomaa M. R., Kutkat M. A., Debeauchamp J., McKenzie P. P., Webster R. G., Webby R. J., Ali M. A.. 2013. Do commercial avian influenza H5 vaccines induce cross-reactive antibodies against contemporary H5N1 viruses in Egypt? Poult. Sci. 92:114–118. [DOI] [PubMed] [Google Scholar]
- Kayali G., Kandeil A., El-Shesheny R., Kayed A. S., Maatouq A. M., Cai Z., McKenzie P. P., Webby R. J., El Refaey S., Kandeel A., Ali M. A.. 2016. Avian Influenza A(H5N1) Virus in Egypt. Emerg. Infect. Dis. 22:379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIE 2014. Avian influenza Manual of Diagnostic Tests, OIE, Paris, France. [Google Scholar]
- Salaheldin A. H., El-Hamid H. S., Elbestawy A. R., Veits J., Hafez H. M., Mettenleiter T. C., Abdelwhab E. M.. 2018. Multiple introductions of influenza A(H5N8) virus into poultry, egypt, 2017. Emerg. Infect. Dis. 24 doi:10.3201/eid2405.171935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webby R. J., Perez D. R., Coleman J. S., Guan Y., Knight J. H., Govorkova E. A., McClain-Moss L. R., Peiris J. S., Rehg J. E., Tuomanen E. I., Webster R. G.. 2004. Responsiveness to a pandemic alert: use of reverse genetics for rapid development of influenza vaccines. Lancet North Am. Ed. 363:1099–1103. S0140673604158923 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2002. WHO Manual on Animal Influenza Diagnosis and Surveillance, WHO, Geneva, Switzerland [Google Scholar]
- Yehia N., Naguib M. M., Li R., Hagag N., El-Husseiny M., Mosaad Z., Nour A., Rabea N., Hasan W. M., Hassan M. K., Harder T., Arafa A. A.. 2018. Multiple introductions of reassorted highly pathogenic avian influenza viruses (H5N8) clade 2.3.4.4b causing outbreaks in wild birds and poultry in Egypt. Infect. Genet. Evol. 58:56–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.