Abstract
Methylphenidate (MPH) is an indirect dopaminergic and noradrenergic agonist that is used to treat attention deficit hyperactivity disorder and that has shown therapeutic potential in neuropsychiatric diseases such as depression, dementia, and Parkinson's disease. While effects of MPH on task‐induced brain activation have been investigated, little is known about how MPH influences the resting brain. To investigate the effects of 40 mg of oral MPH on intrinsic functional connectivity, we used resting state fMRI in 54 healthy male subjects in a double‐blind, randomized, placebo‐controlled study. Functional connectivity analysis employing ICA revealed seven resting state networks (RSN) of interest. Connectivity strength between the dorsal attention network and the thalamus was increased after MPH intake. Other RSN located in association cortex areas, such as the left and right frontoparietal networks and the executive control network, showed MPH‐induced connectivity increase to sensory‐motor and visual cortex regions and connectivity decrease to cortical and subcortical components of cortico‐striato‐thalamo‐cortical circuits (CST). RSN located in sensory‐motor cortex areas showed the opposite pattern with MPH‐induced connectivity increase to CST components and connectivity decrease to sensory‐motor and visual cortex regions. Our results provide evidence that MPH does not only alter intrinsic connectivity between brain areas involved in sustained attention, but that it also induces significant changes in the cortico‐cortical and cortico‐subcortical connectivity of many other cognitive and sensory‐motor RSN. Hum Brain Mapp 35:5379–5388, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: intrinsic functional connectivity, resting state fMRI, methylphenidate, ADHD, dopamine agonist
INTRODUCTION
Methylphenidate (MPH) is a widely used treatment for attention deficit hyperactivity disorder (ADHD) that improves symptoms in about 70% of children with ADHD (Arnsten 2006). This improvement has been related to MPH‐induced normalization of altered functional connectivity, e.g., between frontal, striatal, and cerebellar brain regions during sustained attention [Rubia et al., 2009b]. A growing body of evidence has suggested that MPH may positively influence symptoms of other neuropsychiatric diseases like depression [Ng, 2008], Alzheimer's disease [Padala et al., 2010], and Parkinson's disease [Auriel et al., 2009]. Similar to ADHD these disorders are characterized by specific disturbances in intrinsic functional connectivity [Greicius 2008; Mueller et al., 2012a; Mueller et al., 2012b].
The fact that MPH exhibits a broad spectrum of mild to moderate side effects including anorexia, insomnia, and nausea [Yildiz et al., 2011] suggests that MPH does not selectively target circumscribed brain circuits or defined task conditions, but profusely influences the entire central nervous system on a cortical and subcortical level.
MPH is an indirect dopaminergic and noradrenergic agonist with strong dopaminergic effects in the basal ganglia and both dopaminergic and noradrenergic cortical effects [Arnsten, 2006]. The neural sites of performance modulation by MPH can be localized in vivo using functional neuroimaging techniques. Task‐based imaging studies suggest that the magnitude as well as the location of MPH‐mediated DA increase depend on the type and difficulty of the task performed [Cools et al., 2004; Costa et al., 2013; Volkow et al., 2004]. For example, MPH has been shown to modulate neural activity in the striatum during response inhibition [Costa et al., 2013; Vaidya et al., 1998] but in fronto‐parietal regions during working memory [Mehta et al., 2000].
While task‐based imaging studies have demonstrated MPH effects on distinct brain regions under defined task conditions, the impact of MPH on intrinsic brain connectivity on a whole brain level is yet to be elucidated. Gaining greater knowledge about MPH effects on resting state connectivity might have implications in the context of MPH induced changes in task‐related activation since inter‐individual differences in task‐induced blood oxygen level dependent (BOLD) activity can be predicted by resting‐state properties [Mennes et al., 2010, 2011]. More importantly, insight into global MPH effects on the central nervous system might help understand its mechanisms of action in a variety of neuropsychiatric diseases and its spectrum of side effects.
Given that MPH induces wide‐spread activity changes during spatial working memory [Marquand et al., 2011] and based on the described MPH effects and side effects in ADHD [Rubia et al., 2009b; Stiefel and Besag, 2010; Yildiz et al., 2011] and other neuro‐psychiatric diseases [Auriel et al., 2009; Ng, 2008; Padala et al., 2010], we hypothesized that MPH effects are not selective to brain regions involved in sustained attention but involve multiple functional networks on a cortical and subcortical level. To investigate the effects of MPH on intrinsic functional connectivity on a whole brain level we employed functional connectivity MRI (fcMRI), which enables the detection of functional brain networks without task constraints [Fox and Raichle, 2007].
METHODS AND MATERIALS
The effect of MPH on resting state neural connectivity was investigated using resting state fcMRI in a double‐blind, randomized, placebo‐controlled, repeated‐measures study design. This study also comprised the collection of task‐related fMRI data, the results of which have been reported separately [Costa et al., 2013]. The institutional review board of the Faculty of Medicine of the Ludwig‐Maximilians‐University Munich approved the study. All subjects provided written informed consent and received monetary compensation for their participation.
The sample comprised right‐handed, nonsmoking, male Caucasian volunteers. To exclude any psychiatric, neurological, and medical disorders, including alcohol and drug abuse, all subjects underwent a baseline screening session, which included an electroencephalogram (EEG), an electrocardiogram (ECG), blood tests, and a detailed interview. Exclusion criteria comprised any psychiatric or neurological condition, smoking, substance abuse, consumption of prescription or over‐the‐counter medication, as well as MR inherent limitations such as metallic implants and claustrophobia. Subjects were asked to abstain from alcohol 24 h prior to each study appointment and from caffeine on the day of the scanning session. Each subject was scanned twice within an interval of ∼1 week. A capsule containing either a dose of 40mg of methylphenidate (MPH) or placebo (P, lactose) was administered orally 60 min prior to each scanning session. The rationale for this latency was based on PET‐data showing that MPH levels in the striatum reach a plateau of highest concentration after about 60 min [Swanson and Volkow, 2003].
Blood samples were taken after each scanning session to determine plasma MPH‐levels.
MR data acquisition was performed at a magnetic field strength of 3.0 Tesla (Magnetom VERIO, Siemens, Erlangen, Germany) using the 12‐channel phased‐array coil supplied by the vendor. Subjects were instructed to rest still, remain awake and keep their eyes closed. Heart rate and respiration were not monitored during the scan.
The resting state functional data was collected using T2*‐weighted echo planar pulse sequence imaging (TE = 30 ms, TR = 3,000 ms, flip angle = 90°, spatial resolution 3*3*4 mm isotropic voxels, transverse orientation, 36 slices fully covering the cerebral cortex and the cerebellum, acquisition time = 6 min). For anatomical reference a high‐resolution isotropic Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence was acquired (TE = 7.6 ms, TR = 14 ms, flip angle 20°, spatial resolution 0.8*0.8*0.8 mm isotropic voxels, FOV 256 × 256, acquisition time = 10 min).
All data analysis steps were performed using FSL software, version 4.16 (http://www.fmrib.ox.ac.uk/fsl/index.html) and Analyses of Functional Images (AFNI) software (http://afni.nimh.nih.gov/afni). Preprocessing using AFNI consisted of (1) slice time correction (first slice as reference, interleaved acquisitions, Fourier interpolation), (2) 3D motion correction, and (3) despiking (removal of extreme time series outliers).
We then used FEAT (FMRI Expert Analysis Tool) version 5.98 [Kosaka et al., 2010] for further fMRI data preprocessing. The first five frames of each functional scan were discarded to allow the MR signal to reach equilibrium. The remaining 115 fMRI volumes for each subject were motion corrected using MCFLIRT (FMRIB's Motion Correction using Linear Image Registration Tool) [Jenkinson et al., 2002], brain extracted using BET (Brain Extraction Tool) [Smith, 2002], normalized to MNI152 template using FLIRT (Functional Linear Image Registration Tool) [Jenkinson et al., 2002], spatially smoothed by a Gaussian kernel with 5 mm full‐width at half‐maximum, and temporally filtered with a high‐pass filter of 0.01 Hz, consistent with previous studies [Biswal et al., 2010; Smith et al., 2009]. Of note, analogous to [Zuo et al., 2010] we did not perform regression of movement parameters, white matter, ventricle, or whole brain signal. We also did not perform movement scrubbing. Preprocessed functional data from the entire data set (MPH and placebo scan) were temporally concatenated across subjects to create a single 4D dataset and decomposed into 20 independent component analysis (ICA) components using the MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) software version 3.10 [Beckmann and Smith, 2004]. For visualization purposes the resulting statistical group maps were thresholded at an average z‐score 2.3 < z < 10 (Fig. 1 and Supporting Information Fig. S1). Seven resting‐state networks of interest were selected visually by comparing with previously defined maps [Smith et al., 2009; Zuo et al., 2010], no network templates were applied.
Figure 1.
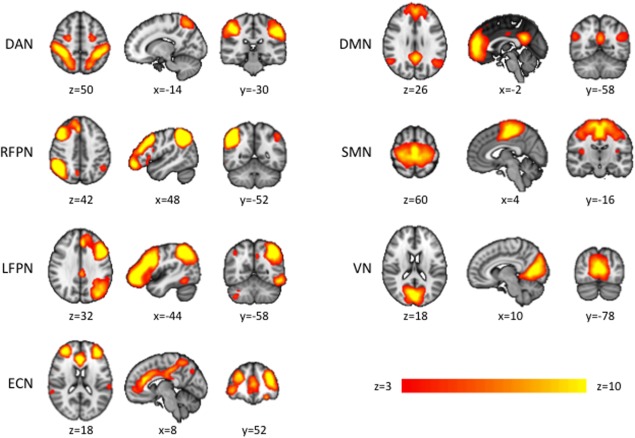
Resting state networks (RSN) of interest identified by group‐ICA of placebo and MPH resting‐state fMRI data. Seven RSN of interest subjected to analysis of MPH‐induced subcortical and cortical functional connectivity changes. Axial and coronal slices are presented in radiological orientation. (DAN dorsal attention network, RFPN and LFPN right‐ and left‐lateralized fronto‐parietal networks, ECN executive control network, DMN default mode network, SMN sensory‐motor network, VN visual network).
To compare resting state brain networks between conditions (MPH vs. Placebo) we applied dual regression ICA with variance normalization. Dual regression employs the spatial maps derived from the initial group ICA in a linear model fit against each individual fMRI dataset, resulting in matrices describing the temporal dynamics of the corresponding networks for each subject [Beckmann et al., 2005; Filippini et al., 2009]. We used Randomize 2.6 (permutation‐based nonparametric inference, 5000 permutations) to determine voxel‐wise nonparametric statistical contrasts between the two conditions [Nichols and Holmes, 2001] for each of the seven selected networks. Contrasts between the two conditions were considered significant at a FWE‐corrected P‐value (P corr) ≤ 0.005. The voxel threshold for difference clusters was set to a minimum of 50 voxels. Hypotheses tested included two effects: (1) MPH > Placebo and (2) Placebo > MPH.
To test the relation between resting state connectivity and MPH plasma level we additionally performed a GLM analysis. For each of the seven networks of interest voxel‐wise regression coefficients for the MPH‐plasma were estimated by using a mixed‐effects GLM comprising a fixed‐effects first‐level individual analysis and random‐effects second‐level group analysis [Woolrich et al., 2004]. Voxels whose connectivity to the respective network were significantly correlated or anti‐correlated with the MPH plasma level were reported at a FEW‐corrected statistical threshold of P corr < 0.01.
Head motion has recently been shown to differentially impact functional connectivity measures [Van Dijk et al., 2012]. As head motion may be influenced by MPH intake, these motion effects can be mistaken as neuronal MPH effects [Van Dijk et al., 2012]. To control for this potential confounding factor, the mean head motion of each subject, which represents the mean absolute displacement (in mm) of each brain volume relative to the previous volume, was estimated from the translation parameters in x, y, and z direction across all time points. Group comparison was performed using a two‐sample t‐Test. To rule out effects of potentially different signal quality, SNR (signal to noise ratio) was determined for each resting state scan and compared between the MPH and placebo group using a two‐sample t‐Test.
RESULTS
Fifty‐four subjects (age mean ± SD: 23.65 ± 2.97 years) completed the study. However, one subject failed to comply with the exclusion criteria and six subjects performed no resting state sequence during either the placebo or the MPH scan. Three subjects were excluded from further analyses because their MPH plasma level ranged beyond two standard deviations of the group mean (14.39 ± 5.63 ng/mL). Therefore, the final sample for resting state analysis consisted of 44 subjects, all of which had both, a viable placebo and a viable MPH scan with a balanced order of receiving MPH and placebo.
The between condition comparison of head motion, measured by the mean and maximum relative displacement (in mm), revealed no significant difference (mean relative displacement MPH: 0.0416 ± 0.0221, Placebo: 0.0457 ± 0.0347, P = 0.4080, mean maximal displacement: MPH: 0.1224 ± 0.0769, Placebo: 0.1295 ± 0.1648, P = 0.7504). Also, there was no significant between condition difference in signal quality measured by slice SNR (mean slice SNR MPH = 342, mean slice SNR placebo = 293, P > 0.05).
Functional connectivity analysis revealed 20 RSN (Supporting Information Fig. S1), seven of which were manually selected as RSN of interest based on the existing literature [Smith et al., 2009; Zuo et al., 2010, Fig. 1]. The dorsal attention network (DAN), the executive control network (ECN) and the right and left fronto‐parietal networks (RFPN, LFPN) were primarily located in association cortex areas. The sensory‐motor network (SMN) and the visual network (VN) were mainly located in sensory and motor cortex areas. Furthermore, the characteristic pattern of the default mode network (DMN) could be detected. All of these networks were selected as RSNs of interest and underwent further analyses of MPH‐induced connectivity changes.
The DAN showed increased connectivity to the left thalamus and the right insular cortex at P < 0.005 (Table 1) after MPH intake. At a lower significance level (P < 0.05) the DAN displayed MPH‐induced connectivity increase to both thalami. No significant MPH‐induced connectivity decrease could be detected.
Table 1.
Brain regions showing significant MPH‐induced connectivity changes to resting state networks (RSN) of interest
| RSN of interest | P‐value | Cortical/subcortical area showing MPH effect | Number of Voxels | P corrected | X | Y | Z |
|---|---|---|---|---|---|---|---|
| DAN | MPH>P, 0.005 | L T | 47 | 0.002 | −14 | −14 | 0 |
| R insular cortex | 215 | 0.002 | 34 | 16 | 0 | ||
| MPH<P, 0.005 | − | ||||||
| ECN+ | MPH>P, 0.005 | R OL visual cortex (BA 17/18) | 171 | 0.002 | 14 | −92 | 22 |
| Thalamus | MPH<P, 0.005 | BL CB | 344 | 0.001 | −2 | −54 | −26 |
| L CB | 166 | 0.002 | −10 | −74 | −36 | ||
| L T | 84 | 0.002 | −6 | −6 | −4 | ||
| R T | 53 | 0.004 | 16 | −8 | 16 | ||
| LFPN | MPH>P, 0.005 | BL OL visual cortex (BA 17/18) | 4392 | 1 | 14 | −90 | 18 |
| L auditory cortex (BA 41/42) | 176 | 0.001 | −38 | −24 | 10 | ||
| MPH<P, 0.005 | − | ||||||
| RFPN | MPH>P, 0.005 | BL OL visual cortex (BA 17/18) | 7683 | 1 | 22 | −64 | 8 |
| BL PreCG (BA 4/6) and PoCG (BA 1/2/3) | 7663 | 0.001 | 22 | −32 | 56 | ||
| R auditory cortex (BA 41/42) | 740 | 0.001 | 44 | −22 | 12 | ||
| MPH<P, 0.005 | BL T, caudate, putamen | 2785 | 1 | −16 | 6 | −8 | |
| BL CB, BS | 2116 | 0.001 | 8 | −24 | −26 | ||
| DMN | MPH>P, 0.05 | − | |||||
| MPH<P, 0.05 | − | ||||||
| SMN | MPH>P, 0.005 | L T, putamen | 166 | 0.002 | −12 | −4 | 10 |
| R T, putamen | 82 | 0.003 | 22 | 12 | −8 | ||
| MPH<P, 0.005 | BL PreCG (BA 4/6), PoCG (BA 1/2/3) | 6431 | 0.000 | 40 | −22 | 36 | |
| BL OL (BA 17/18) | 3506 | 0.001 | 16 | −84 | 24 | ||
| L secondary somatosensory cortex (BA 43) | 381 | 0.002 | −40 | −18 | 16 | ||
| VN | MPH>P, 0.005 | BL CB and BS | 5916 | 0.000 | −12 | −52 | −50 |
| BL T, caudate, putamen | 4993 | 0.000 | −28 | 10 | −12 | ||
| BL ACC (BA 32) and PCC (BA 23/31) | |||||||
| MPH<P, 0.005 | BL OL visual cortex (BA 17/18) | 4962 | 0.000 | −10 | −74 | −10 | |
| BL PreCG (BA 4/6), PoCG (BA 1/2/3) | 1835 | 0.001 | 8 | −36 | 64 |
DAN, dorsal attention network; ECN, executive control network; DMN, default mode network; LFPN, left fronto‐parietal network; RFPN, right fronto‐parietal network; SMN, somato‐sensory network; VN, visual network; MPH, methylphenidate; P, placebo; T, thalamus; BL, bilateral; L, left; R, right; OL, occipital lobe; PoCG, postcentral gyrus; CB, cerebellum; PreCG, precentral gyrus; BS, brainstem; BA, Brodmann area; paraCG, paracingulate gyrus; ACC, anterior cingulated gyrus; PCC, posterior cingulated gyrus.
While an effect of MPH on cortical and subcortical brain regions involved in attention related processing was to be expected from previous studies [Rubia et al., 2009b; Tomasi et al., 2011; Vaidya et al., 1998], the investigation of MPH effects on other cortical networks was more exploratory. We found that except for the DMN, all of these networks displayed significantly altered connectivity to cortical and subcortical brain regions after MPH intake. Remarkably, all network contrasts (MPH vs. placebo) showed connectivity changes to the same two sets of brain regions.
RSN located in multi‐modal association cortex (LFPN, RFPN, and ECN) showed MPH‐induced connectivity increase to primary, secondary, and tertiary cortical areas such as the sensory‐motor cortex (RFPN derived contrasts), the visual cortex (ECN, LFPN, and RFPN derived contrasts) and the auditory cortex (LFPN and RFPN derived contrast). For detailed results see Figure 2B (brain regions indicated in yellow/orange) and Table 1. Regions that displayed decreased connectivity to these association RSN after MPH intake comprised subcortical structures such as the striatum (RFPN derived contrasts), thalamic nuclei (ECN and RFPN derived contrasts), and cerebellar components (ECN and RFPN derived contrasts). These subcortical structures have previously been described as parts of cortico‐striato‐thalamo‐cortical (CST) circuits (Alexander et al., 1986; Mesulam 2000). For detailed results see Figure 2B (brain regions indicated in blue) and Table 1.
Figure 2.
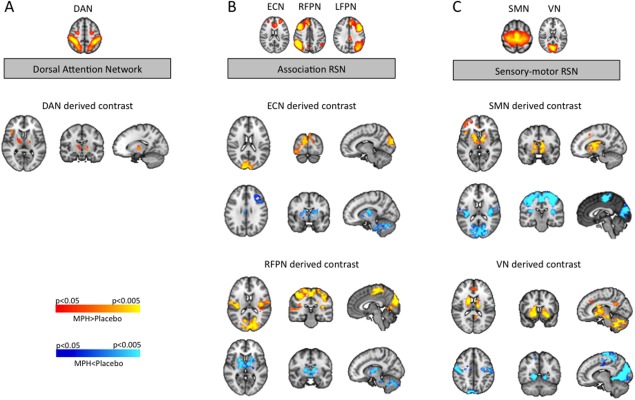
MPH‐induced alterations in neural connectivity derived from resting state network (RSN) comparison (contrast MPH versus placebo). A: The dorsal attention network (DAN) exhibited MPH induced connectivity increase to the bilateral thalamus (P < 0.05), whereat the connectivity increase to the left thalamus reached higher significance levels (P < 0.005). B: Association RSN showed MPH‐induced connectivity increase to sensory‐motor and visual cortex regions and MPH‐induced connectivity decrease to cortical and subcortical components of cortico‐striato‐thalamo‐cortical circuits (CST). C: Sensory‐motor RSN showed MPH‐induced connectivity increase to components of cortico‐striato‐thalamo‐cortical circuits (CST) and MPH‐induced connectivity decrease to sensory‐motor and visual cortex regions. For visualization purposes, contrasts are shown at an FWE‐corrected P‐level of P ≤ 0.05. (ECN executive control network, LFPN left fronto‐parietal network, RFPN right fronto‐parietal network, SMN sensory‐motor network, VN visual network).
RSN located in sensory and motor cortex areas (SMN and VN) showed MPH induced connectivity changes to the same two sets of brain regions (CST components and sensory‐motor cortex). However these connectivity changes exhibited the opposite pattern as compared with the association RSN. Sensory‐motor RSN displayed MPH‐induced connectivity increase to cortical and subcortical components of CST circuits, namely the striatum (SMN and VN derived contrasts), thalamic nuclei (SMN, and VN derived contrasts), and cerebellar components (VN derived contrast). For detailed results see Figure 2C (brain regions indicated in yellow/orange) and Table 1. Regions that displayed decreased connectivity to these sensory‐motor RSN after MPH intake comprised secondary and tertiary cortical areas such as the sensory‐motor cortex (SMN and VN derived contrasts) and the visual cortex (SMN, and VN derived contrasts). For detailed results see Figure 2C (brain regions indicated in blue) and Table 1.
No significant correlation between MPH plasma levels and functional connectivity of any of the seven RSN of interest could be detected.
DISCUSSION
The results of the present study provide evidence that MPH strengthens cortico‐thalamic connectivity of the attention network. Moreover, MPH alters cortico‐cortical and cortico‐subcortical connectivity of various association and sensory‐motor RSN. Across RSN, these shifts affect connectivity to two distinct sets of brain regions, namely components of cortico‐striato‐thalamo‐cortical (CST) circuits and sensory‐motor cortex areas. A general pattern regarding the direction of these shifts could be detected: most RSN located in association cortices showed MPH‐induced connectivity increase to sensory‐motor cortex areas and connectivity decrease to CST components, while RSN located in sensory‐motor cortices displayed the opposite pattern.
MPH Affects Cortico‐Cortical and Cortico‐Subcortical Connectivity
The results of this study indicate that MPH influences cortico‐cortical and cortico‐subcortical intrinsic functional connectivity. MPH‐induced dopaminergic effects have been suggested to originate largely from subcortical regions as MPH binds densely in the striatum [Volkow et al., 1998]. However, effects on both subcortical and cortical levels still seem plausible given the distribution and localization of monoamine neuronal endings that MPH can potentially act on. Dopaminergic terminals are located in many cortical regions including the prefrontal and motor cortex [Lewis et al., 1987] and represent a significant source of afferentiation of the prefrontal cortex [Williams and Goldman‐Rakic 1998]. Dopamine also plays an important role in cortical interneurons [Muly et al., 1998]. The MPH‐induced changes of cortical connectivity observed in this study can therefore result both from indirect subcortical and direct cortical dopaminergic effects.
MPH Affects Connectivity Between RSN and Cortico‐Striato‐Thalamo‐Cortical Circuit Components
In this study MPH was shown to alter connectivity between cortical RSN and cortical and subcortical components of CST circuits. CST circuitry has been described as a set of connections including the striatum that receives glutamatergic input from association and sensory‐motor areas and dopaminergic input from the substantia nigra. Output from the striatum is projected over the globus pallidus and the thalamus back to the frontal cortex as the endpoint of the circuitry [Alexander et al., 1986; Mesulam, 2000]. Increasing dopamine availability by MPH administration can therefore modulate the neural connectivity of components of CST loops, as could be observed in this study.
Alexander and colleagues have described parallel CST circuits that comprise distinct thalamic, striatal and cortical subportions [Alexander et al., 1986]. Our results indicate that MPH affects connectivity in several of these distinct CST circuits. For example, increased connectivity between CST components and the visual network (VN) was most prominent in the ventral striatum, ventral anterior and ventral lateral thalamic nuclei, and in the anterior and posterior cingulate cortex. These components resemble Alexander's anterior cingulate circuit [Alexander et al., 1986]. Decreased connectivity between CST components and the right fronto‐parietal network (RFPN), by contrast, was mainly found in the dorsal striatum and posterior thalamic nuclei. In summary, we found that MPH alters cortico‐subcortical connectivity of different CST circuits including the ventral as well as the dorsal striatum.
Dopamine effects on cortico‐striatal circuitry have been described in the context of task dependent fMRI paradigms. In healthy adults, dopamine agonists were shown to modulate task‐related fronto‐striatal connectivity [Honey et al., 2003]. In patients with ADHD, MPH was found to normalize altered task‐induced fronto‐striatal connectivity [Rubia et al., 2009b]. These findings are in line with our result of MPH‐induced alterations of intrinsic connectivity between cortical RSN and striatal components of CST circuits. Modulation of cortico‐striatal connectivity might be relevant in a broad range of neurodevelopmental, neuropsychiatric and movement disorders where impaired cortico‐striatal circuitry is assumed an important aetiological factor [Shepherd, 2013].
This study furthermore detected MPH‐induced connectivity alterations between thalamic nuclei and RSN. Connectivity between the thalamus and the DAN was increased after MPH intake. Reduced integrity of the thalamus is known to result in deficits in spatial attention [Rafal and Posner 1987] and children with ADHD have been reported to display morphological abnormalities of the thalamus [Ivanov et al., 2010]. It is therefore conceivable that MPH reduces attention deficit symptoms in ADHD by strengthening the functional connectivity between the thalamus and cortical regions involved in attention processing, as could be shown in this study.
Our results furthermore indicate MPH‐induced connectivity increase between the thalamus and visual and sensory‐motor RSN. MPH is known to enhance the neural firing rate in primary sensory thalamic areas such as the lateral and medial geniculate nuclei and to attenuate neural activity in polysensory regions of the thalamus such as the pulvinar in rats and cats [Shih et al., 1975]. The results of this study point in a similar direction: thalamic connectivity to primary sensory networks such as the SMN and the VN was increased while thalamic connectivity to polysensory, higher order integration networks such as the ECN and the right fronto‐parietal network (RFPN) was reduced after MPH intake.
This study also provides evidence that MPH alters neural connectivity between the cerebellum and cortical RSN. Cerebellar connectivity to the VN was increased while connectivity to ECN and RFPN was decreased after MPH intake. The cerebellum is a key region for attention and timing functions [Rubia et al., 2009a] and one of the structurally most affected regions in children with ADHD [Rubia et al., 2009a]. MPH has previously been shown to increase task‐related fronto‐cerebellar connectivity in ADHD [Rubia et al., 2009b]. Our results reveal that MPH also modulates baseline connectivity between the cerebellum and the cortex, which may constitute a mechanism of MPH action in ADHD.
MPH Affects Connectivity Between RSN and Sensory‐Motor Cortex Areas
The results of this study indicate that MPH alters connectivity between sensory‐motor cortex areas and RSN. The increased connectivity between sensory‐motor regions and association RSN might contribute to the improvement of inhibitory motor control in patients with ADHD [Buchmann et al., 2006] and healthy adults [Costa et al., 2013] after MPH intake. Patients with ADHD are known to have disinhibited somato‐sensory evoked potentials [Parush et al., 1997]. Increased connectivity between association RSN and somato‐sensory cortex regions might help to reduce and modulate this disinhibition. Our finding of MPH‐induced reduction of connectivity between sensory‐motor areas and sensory‐motor RSN is consistent with reports of MPH‐induced reduction of cerebral blood flow in the primary sensory cortex in ADHD [Lee et al., 2005] and findings of suppressed long latency responses of S1 neurons after administration of low dose MPH [Drouin et al., 2006]. Drouin and colleagues have suggested that MPH may improve sensory attention by increasing extracellular noradrenaline release in S1 cortex and suppressing “noise” [Arnsten and Vijayraghavan, 2006; Drouin et al., 2006]. Our finding might have implications in other neuropsychiatric disorders, such as Parkinson's disease, which is known to display abnormally high resting state connectivity within the primary motor cortex that can be partially normalized by levodopa medication [Wu et al., 2009].
LIMITATIONS
This study investigated MPH‐induced changes of BOLD signal fluctuations in the resting brain. A concern that could be raised is that cardiovascular effects might influence the BOLD signal, especially as MPH is known to enhance blood pressure and heart rate [Volkow et al., 2002]. This issue has been addressed in a study by Rao et al. [2000] who demonstrated that the functional signal in a certain brain area increases with faster finger tapping rates, but not after MPH administration and consecutive modest heart rate increase. Furthermore, studies using direct electrophysiological measurements by EEG and task‐related fMRI studies report concordant results. Since EEG is largely independent of cardiovascular effects, it is unlikely that concordant fMRI results are mainly driven by global hemodynamic effects, e.g., caused by changes in heart rate and blood pressure [Arthurs et al., 2004]. Therefore, a cardiovascular effect of MPH is unlikely to be the sole contributor to MPH‐induced activation increase.
Another question that can be asked is if MPH exhibits direct vasoactive properties, thereby modifying cerebral blood flow. As the BOLD signal is determined by regional cerebral blood flow (rCBF), cerebral metabolic rate of oxygen and cerebral blood volume, a change in BOLD‐derived “resting state connectivity” will never be independent of changes in the underlying hemodynamics. However, it is important to note that these underlying hemodynamic changes are not a global phenomenon but seem to be specific for certain drugs and brain regions. For example, in a MRI study Marquand and colleagues could show that the spatial distribution of drug effects on regional cerebral blood flow accurately discriminates methylphenidate from other drug conditions [Marquand et al., 2012]. MPH‐induced effects on regional cerebral blood flow were found in the caudate nucleus, the midbrain, the thalamus, and the cerebellum. Interestingly, these regions are very similar to the ones we found to exhibit MPH‐induced connectivity changes. Further studies will be needed to determine if these blood flow changes are solely caused by MPH‐induced changes in neuronal transmission and activity or if part of these changes are related to direct vascular MPH‐effects.
CONCLUSIONS
In conclusion, the results of this study demonstrate that MPH does not selectively target brain regions involved in sustained attention or other executive functions but that it profusely affects intrinsic connectivity on a whole brain level, including brain stem and midbrain regions that can be linked to typical MPH side effects such as insomnia, loss of appetite, and nausea. Our results furthermore suggest that across resting state networks MPH induces shifts in intrinsic functional connectivity to two distinct sets of brain regions, namely components of CST circuits and sensory‐motor cortex areas, indicating that these brain regions might be especially prone to MPH modulation. These findings have implications in a broad range of neuro‐psychiatric diseases that are characterized by cortico‐cortical and cortico‐subcortical dysconnectivity and that might therefore benefit from MPH treatment.
Supporting information
Supporting Information Figure S1.
REFERENCES
- Alexander GE, DeLong MR, Strick PL (1986): Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381. [DOI] [PubMed] [Google Scholar]
- Arnsten AF (2006): Stimulants: Therapeutic actions in ADHD. Neuropsychopharmacology 31:2376–2383. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Vijayraghavan S (2006): Staying in touch with methylphenidate: AHDH and sensory processing. Focus on "methylphenidate enhances noradrenergic transmission and suppresses mid‐ and long‐latency sensory responses in the primary somatosensory cortex of awake rats". J Neurophysiol 96:524–525. [DOI] [PubMed] [Google Scholar]
- Arthurs OJ, Stephenson CM, Rice K, Lupson VC, Spiegelhalter DJ, Boniface SJ, Bullmore ET (2004): Dopaminergic effects on electrophysiological and functional MRI measures of human cortical stimulus‐response power laws. Neuroimage 21:540–546. [DOI] [PubMed] [Google Scholar]
- Auriel E, Hausdorff JM, Giladi N (2009): Methylphenidate for the treatment of Parkinson disease and other neurological disorders. Clin Neuropharmacol 32:75–81. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM (2004): Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23:137–152. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM (2005): Investigations into resting‐state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360:1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP (2010): Toward discovery science of human brain function. Proc Natl Acad Sci USA 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann J, Gierow W, Weber S, Hoeppner J, Klauer T, Wittstock M, Benecke R, Haessler F, Wolters A (2006): Modulation of transcallosally mediated motor inhibition in children with attention deficit hyperactivity disorder (ADHD) by medication with methylphenidate (MPH). Neurosci Lett 405(1‐2):14–18. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Katner JS, Nelson DL, Hemrick‐Luecke SK, Threlkeld PG, Heiligenstein JH, Morin SM, Gehlert DR, Perry KW (2002): Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 27:699–711. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW (2004): Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci 24:1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Riedel M, Pogarell O, Menzel‐Zelnitschek F, Schwarz M, Reiser M, Moller HJ, Rubia K, Meindl T, Ettinger U (2013): Methylphenidate effects on neural activity during response inhibition in healthy humans. Cereb Cortex 23:1179–1189. [DOI] [PubMed] [Google Scholar]
- Drouin C, Page M, Waterhouse B (2006): Methylphenidate enhances noradrenergic transmission and suppresses mid‐ and long‐latency sensory responses in the primary somatosensory cortex of awake rats. J Neurophysiol 96:622–632. [DOI] [PubMed] [Google Scholar]
- Engert V, Pruessner JC (2008): Dopaminergic and noradrenergic contributions to functionality in ADHD: The role of methylphenidate. Curr Neuropharmacol 6:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE (2009): Distinct patterns of brain activity in young carriers of the APOE‐epsilon4 allele. Proc Natl Acad Sci USA 106:7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Greicius M (2008): Resting‐state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol 21:424–430. [DOI] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, Ng V, Fletcher PC, Williams SC, Brown J, Bullmore ET (2003): Dopaminergic drug effects on physiological connectivity in a human cortico‐striato‐thalamic system. Brain 126(Part 8):1767–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Bansal R, Hao X, Zhu H, Kellendonk C, Miller L, Sanchez‐Pena J, Miller AM, Chakravarty MM, Klahr K, Durkin K, Greenhill LL, Peterson BS (2010): Morphological abnormalities of the thalamus in youths with attention deficit hyperactivity disorder. Am J Psychiatry 167:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kosaka H, Omori M, Munesue T, Ishitobi M, Matsumura Y, Takahashi T, Narita K, Murata T, Saito DN, Uchiyama H, Morita T, Kikuchi M, Mizukami K, Okazawa H, Sadato N, Wada Y (2010): Smaller insula and inferior frontal volumes in young adults with pervasive developmental disorders. Neuroimage 50:1357–1363. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kim BN, Kang E, Lee DS, Kim YK, Chung JK, Lee MC, Cho SC (2005): Regional cerebral blood flow in children with attention deficit hyperactivity disorder: Comparison before and after methylphenidate treatment. Hum Brain Mapp 24:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH (1987): The distribution of tyrosine hydroxylase‐immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci 7:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquand AF, De Simoni S, O'Daly OG, Williams SC, Mourao‐Miranda J, Mehta MA (2011): Pattern classification of working memory networks reveals differential effects of methylphenidate, atomoxetine, and placebo in healthy volunteers. Neuropsychopharmacology 36:1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquand AF, O'Daly OG, De Simoni S, Alsop DC, Maguire RP, Williams SCR, Zelaya FO, Mehta MA (2012): Dissociable effects of methylphenidate, atomoxetine and placebo on regional cerebral blood flow in healthy volunteers at rest: A multi‐class pattern recognition approach. Neuroimage 60:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW (2000): Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci 20:RC65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, Milham MP (2010): Inter‐individual differences in resting‐state functional connectivity predict task‐induced BOLD activity. Neuroimage 50:1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Castellanos FX, Milham MP (2011): Linking inter‐individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage 54:2950–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M (2000): Principles of Cognitive and Behavioural Neurology. New York: Oxford University Press.
- Mueller S, Keeser D, Reiser MF, Teipel S, Meindl T (2012a): Functional and structural mr imaging in neuropsychiatric disorders, Part 1: Imaging techniques and their application in mild cognitive impairment and Alzheimer disease. AJNR Am J Neuroradiol 33:1845–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S, Keeser D, Reiser MF, Teipel S, Meindl T (2012b): Functional and structural MR imaging in neuropsychiatric disorders, Part 2: Application in Schizophrenia and Autism. AJNR Am J Neuroradiol 33:2033–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muly EC III, Szigeti K, Goldman‐Rakic PS (1998): D1 receptor in interneurons of macaque prefrontal cortex: Distribution and subcellular localization. J Neurosci 18:10553–10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng B (2008): Methylphenidate and depression. J Clin Psychopharmacol 28:116–117; author reply 117–118. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP (2001): Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum Brain Mapp 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padala PR, Burke WJ, Shostrom VK, Bhatia SC, Wengel SP, Potter JF, Petty F (2010): Methylphenidate for apathy and functional status in dementia of the Alzheimer type. Am J Geriatr Psychiatry 18:371–374. [DOI] [PubMed] [Google Scholar]
- Parush S, Sohmer H, Steinberg A, Kaitz M (1997): Somatosensory functioning in children with attention deficit hyperactivity disorder. Dev Med Child Neurol 39:464–468. [DOI] [PubMed] [Google Scholar]
- Rafal RD, Posner MI (1987): Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci USA 84:7349–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SM, Salmeron BJ, Durgerian S, Janowiak JA, Fischer M, Risinger RC, Conant LL, Stein EA (2000): Effects of methylphenidate on functional MRI blood‐oxygen‐level‐dependent contrast. Am J Psychiatry 157:1697–1699. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Christakou A, Taylor E (2009a): Impulsiveness as a timing disturbance: Neurocognitive abnormalities in attention‐deficit hyperactivity disorder during temporal processes and normalization with methylphenidate. Philos Trans R Soc Lond B Biol Sci 364:1919–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E (2009b): Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication‐naive children with ADHD during a rewarded continuous performance task. Neuropharmacology 57(7‐8):640–652. [DOI] [PubMed] [Google Scholar]
- Shepherd GM (2013): Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 14:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM, Khachaturian ZS, Barry H III, Reisler KL (1975): Differential effects of methylphenidate on reticular formation and thalamic neuronal activity. Psychopharmacologia 44:11–15. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009): Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel G, Besag FM (2010): Cardiovascular effects of methylphenidate, amphetamines and atomoxetine in the treatment of attention‐deficit hyperactivity disorder. Drug Saf 33:821–842. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND (2003): Serum and brain concentrations of methylphenidate: Implications for use and abuse. Neurosci Biobehav Rev 27:615–621. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS (2011): Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage 54:3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD (1998): Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proc Natl Acad Sci USA 95:14494–14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL (2012): The influence of head motion on intrinsic functional connectivity MRI. NeuroImage 59:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ (2002): Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: Results from imaging studies. Eur Neuropsychopharmacol 12:557–566. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N (1998): Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry 155:1325–1331. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM (2004): Dopamine in drug abuse and addiction: Results from imaging studies and treatment implications. Mol Psychiatry 9:557–569. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman‐Rakic PS (1998): Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex 8:321–345. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM (2004): Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage 21:1732–1747. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang L, Chen Y, Zhao C, Li K, Chan P (2009): Changes of functional connectivity of the motor network in the resting state in Parkinson's disease. Neurosci Lett 460:6–10. [DOI] [PubMed] [Google Scholar]
- Yildiz O, Sismanlar SG, Memik NC, Karakaya I, Agaoglu B (2011): Atomoxetine and methylphenidate treatment in children with ADHD: The efficacy, tolerability and effects on executive functions. Child Psychiatry Hum Dev 42:257–269. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP (2010): Reliable intrinsic connectivity networks: Test‐retest evaluation using ICA and dual regression approach. Neuroimage 49:2163–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure S1.


