Abstract
Current knowledge about small‐world networks underlying emotions is sparse, and confined to functional magnetic resonance imaging (fMRI) studies using resting‐state paradigms. This fMRI study applied Eigenvector Centrality Mapping (ECM) and functional connectivity analysis to reveal neural small‐world networks underlying joy and fear. Joy and fear were evoked using music, presented in 4‐min blocks. Results show that the superficial amygdala (SF), laterobasal amygdala (LB), striatum, and hypothalamus function as computational hubs during joy. Out of these computational hubs, the amygdala nuclei showed the highest centrality values. The SF showed functional connectivity during joy with the mediodorsal thalamus (MD) and nucleus accumbens (Nac), suggesting that SF, MD, and Nac modulate approach behavior in response to positive social signals such as joyful music. The striatum was functionally connected during joy with the LB, as well as with premotor cortex, areas 1 and 7a, hippocampus, insula and cingulate cortex, showing that sensorimotor, attentional, and emotional processes converge in the striatum during music perception. The hypothalamus showed functional connectivity during joy with hippocampus and MD, suggesting that hypothalamic endocrine activity is modulated by hippocampal and thalamic activity during sustained periods of music‐evoked emotion. Our study indicates high centrality of the amygdala nuclei groups within a functional network underlying joy, suggesting that these nuclei play a central role for the modulation of emotion‐specific activity within this network. Hum Brain Mapp 35:3485–3498, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: small‐world network, fMRI, Eigenvector Centrality Mapping, music, superficial amygdala, laterobasal amygdala, hypothalamus, striatum, nucleus accumbens
INTRODUCTION
Networks with so‐called “small‐world” properties are characterized by high levels of local clustering among nodes and high global efficiency emerging from short paths that link all nodes of the network [Albert and Barabási, 2002]. Small‐world attributes have been observed in a wide range of complex systems, including communication systems, trade routes, social institutions, computational networks, and neural networks [Bullmore and Sporns, 2009; Sporns and Honey, 2006]. A typical example of a neural network with small‐world properties is a network with provincial hubs (connected to nodes of the same module, or community) and with connector hubs (connected to nodes of different modules) [Bullmore and Sporns, 2009; Pessoa, 2008]. During the last years, small‐world properties of functional brain networks have been investigated with fMRI using partial correlations between pre‐defined cerebral regions [Salvador et al., 2005], wavelet correlations between regional mean time series [Achard et al., 2006; Eryilmaz et al., 2011], hierarchical cluster analysis [Ferrarini et al., 2009; Meunier et al., 2009], and Eigenvector Centrality Mapping (ECM) [Lohmann et al., 2010].
Typically, studies investigating neural networks with small‐world properties have used resting state fMRI to specify network properties of the so‐called default network [Andrews‐Hanna et al., 2010; Bullmore and Sporns, 2009]. Some of these studies examined effects of emotion‐induction on resting‐state networks, such as recall of emotional episodes [Harrison et al., 2008], or presentation of video clips preceding the resting‐state scans [Eryilmaz et al., 2011]. Such experimental paradigms made use of longer time intervals (e.g., 90 s in the study by [Eryilmaz et al., 2011]), thus also shedding light on longer, sustained emotional states (in the range of minutes). This complements the majority of research in affective neuroscience that has focused on relatively brief reactions to short external or internal stimuli (in the range of seconds).
In particular, the use of longer time intervals is required for a more comprehensive understanding of the neural correlates of emotions that usually span over longer time periods (e.g., joy, worry, fear, or sadness), due to three reasons. (1) Findings obtained in functional neuroimaging studies on emotion using short experimental stimuli (in the range of seconds) cannot simply be extrapolated to longer emotional episodes, because activity levels in limbic/paralimbic structures can change significantly over time. For example, combining PET and fMRI data [Salimpoor et al., 2011], it was shown that a music‐evoked frisson (involving exceptionally strong feelings of pleasantness and reward) involves increased dopamine availability in the dorsal striatum during the anticipation of the frisson, whereas during the emotional peak experience of the frisson, dopamine availability increased in the ventral striatum (probably the nucleus accumbens (Nac)). (2) Some emotions (such as tenderness, peacefulness, or joy) might take a few moments to unfold [Koelsch et al., 2006]. (3) While autonomic responses to emotional stimuli are relatively quick (in the range of fractions of a second to seconds), endocrine changes are usually considerably slower (often in the range of minutes, or even longer) [e.g., Gotthardt et al., 1995]. Therefore, neural activity initiating and monitoring such endocrine processes might go unnoticed when investigating only initial reactions to short stimuli (but see [Menon and Levitin, 2005] and [Schwartz et al., 2008], for fMRI studies reporting hypothalamus activation in response to short emotionally salient stimuli).
Previous fMRI experiments using longer stimuli to investigate more sustained emotional states (although not investigating small‐world networks) include music studies that used stimulus durations of 23 s [Ball et al., 2007; Menon and Levitin, 2005] or more [Baumgartner et al., 2006; Mitterschiffthaler et al., 2007; Trost et al., 2012] up to a maximum of 1 min [Koelsch et al., 2006]. Other functional neuroimaging studies using films [Goldin et al., 2005; Hasson et al., 2004; Nummenmaa et al., 2012], music [Lehne et al., in press], or a story [Wallentin et al., 2011] have used even longer stimuli (up to 30 min, [Hasson et al., 2004]), but to achieve a sufficient statistical power these studies used continuous emotion regressors [Goldin et al., 2005; Lehne et al., in press; Wallentin et al., 2011] or continuous inter‐subject correlations [Hasson et al., 2004; Nummenmaa et al., 2012], which inform us about neural correlates of fluctuating emotional experiences, rather than about sustained emotional states.
In this study, we evoked sustained emotional states with music, and applied ECM [Lohmann et al., 2010] in combination with functional connectivity analysis to identify small‐world networks underlying these emotional states. ECM attributes a centrality value to each voxel in the brain such that a voxel receives a large value if it is strongly correlated with many other nodes that are themselves central within the network (Google's Page‐Rank algorithm is a variant of eigenvector centrality). Thus, ECM indicates the “computational hubs” of neural networks with small‐world properties in the human brain [Bullmore and Sporns, 2009; Sporns and Honey, 2006; Tomasi and Volkow, 2011]. Because ECM is based on correlations between time series, it can be applied for time series as long as several minutes (with the upper limit being the maximal length of a scanning session). For example, a previous ECM study with a within‐subjects design compared data of 7.6‐min resting state scans of subjects when they were in states of hunger or satiety (that study reported that eigenvector centrality values were higher during the hungry state in the posterior cingulate cortex and the precuneus) [Lohmann et al., 2010]. Thus, ECM can be applied to resting‐state fMRI scans, but ECM can also be used for the investigation of functional networks beyond those involved in resting‐state activity. Note that, similar to studies using resting‐state fMRI in combination with emotion‐induction methods [Eryilmaz et al., 2011; Harrison et al., 2008], ECM can also be computed for data acquired during different experimental conditions to explore, for example, different small‐world networks underlying different emotions. Another reason for choosing ECM in this study was that ECM is a data‐driven and model‐free approach that is not limited to specific regions of interest, nor to specific frequency bands of interest.
Our experiment used two conditions in which musical stimuli evoked either joy or fear, and a neutral control condition in which neither joy nor fear was evoked. Each condition consisted of one single stimulus block that lasted for 4 min (Fig. 1). That is, there were three blocks per participant, each with a duration of 4 min: one joy, one fear, and one neutral block (ordering of blocks was counterbalanced across subjects). Computational hubs identified in the contrasts between conditions (using ECM) were then used as seed regions for functional connectivity analysis. Functional connectivity was computed separately for each condition, and functional connectivity maps were compared between conditions to identify emotion‐specific functional connections between the identified ECM hubs and other brain structures.
Figure 1.
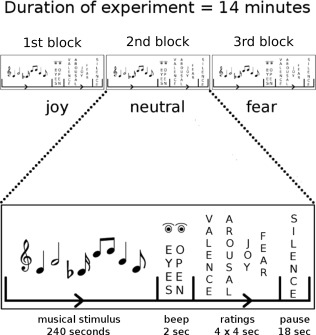
Experimental design. Three blocks of stimuli were presented to each subject. In each block, music of one emotion category (joy, fear, or neutral) was presented for 4 min. Ordering of blocks was counterbalanced across subjects. Participants listened to the music with their eyes closed. The presentation of music was followed by a beep tone signaling to open the eyes and to commence the rating procedure. After each block, four emotion ratings (felt valence, arousal, joy, and fear) were obtained within 16 s.
This approach was used to investigate “small‐world” brain networks underlying joy and fear on the timescale of minutes. Previous studies implicated the amygdala, in particular the lateral and basolateral nuclei, in the evaluation of both positive and negative stimuli [Holland and Gallagher, 2004; LeDoux, 2000; Murray, 2007; Paton et al., 2006], and the central amygdala in initiating behavioral, autonomic, and endocrine responses to such stimuli [LeDoux, 2000]. Moreover, a previous fMRI‐study from our group [Koelsch et al., 2013] using a general linear model analysis and 30‐s‐long musical excerpts evoking joy and fear showed an increase in blood oxygen level dependent (BOLD) signal values in the superficial amygdala (SF) in response to joy (compared with fear) stimuli. In addition, that study [Koelsch et al., 2013] reported increased BOLD signals in response to joy (compared with fear) in the auditory cortex bilaterally, and an increase of BOLD signals during fear (compared with joy) in area 3 of the primary somatosensory cortex. Therefore, we aimed at testing whether the direct contrast of ECMs between the joy and fear condition in the present study (calculated for several minutes of emotional experience) would show differences in these structures. In addition, we expected differences between conditions in neural structures involved in endocrine changes, specifically in the hypothalamus and hippocampus. The hippocampus has dense bidirectional connections with the hypothalamus and is substantially involved in the modulation of hypothalamic endocrine activity [O'Mara, 2005]. Moreover, the hippocampus has previously been implicated in positive music‐evoked emotions such as joy, tenderness, and peacefulness [Koelsch, 2010; Trost et al., 2012].
MATERIALS AND METHODS
Participants
Twenty individuals (aged 21–38 years, mean = 25.55 years, SD = 4.80, 10 females) took part in the experiment. Participants had normal hearing (as assessed with standard pure tone audiometry) and were right‐handed (according to self‐report). None of the participants was a professional musician or a music student. Exclusion criteria were past diagnosis of a neurological or psychiatric disorder, a score on Beck's Depression Inventory [Beck et al., 1993] of ≥13, excessive consumption of alcohol or caffeine during the 24 h before testing, and poor sleep during the previous night. All subjects gave written informed consent; the study was conducted according to the Declaration of Helsinki and approved by the ethics committee of the School of Life Sciences and the Psychology Department of the University of Sussex.
Stimuli
Musical stimuli were selected to evoke (a) joy (CD‐recorded pieces of joyful instrumental music from various epochs and styles), (b) fear (excerpts from soundtracks of suspense movies and video games), or (c) neither joy nor fear (henceforth referred to as neutral stimuli). None of the stimuli contained vocals. Details about the stimuli are provided in the Supporting Information and Supporting Information Table S1. Identical stimuli were also used in a previous study [Koelsch et al., 2013]. There were n = 8 stimuli per condition (joy, fear, and neutral), each stimulus with a duration of 30 s. In contrast to our previous study [Koelsch et al., 2013] in which stimuli were presented in pseudorandomized order, in this study, stimuli of the same emotion category were concatenated into stimulus blocks of 4 min duration per category (see Fig. 1). That is, there was one 4‐min stimulus block per condition (joy, fear, and neutral). No stimulus was repeated, and each stimulus block was presented once to each participant. Importantly, joy, fear, and neutral stimuli were balanced across experimental conditions with regard to tempo (beats per minute), mean F0 pitch, F0 pitch range, F0 pitch variation, pitch centroid values, spectral complexity, and spectral flux. A detailed acoustic analysis of the stimuli is provided in the Supporting Information. In brief, 177 acoustical descriptors were extracted and compared between conditions (joy, neutral, and fear) using one‐way ANOVAs. Significant effects of condition were indicated for 10 acoustical factors (mean and variance of F0 salience, mean and variance of sensory dissonance, mean chord strength, mean key strength, mean and variance of spectral flux, mean spectral crest, and mean spectral complexity). To compensate for these acoustical differences, the values of these psychoacoustic parameters were used in the fMRI data analysis as regressors of no interest (see “Data Analysis” for details).
Procedure
Each participant listened to each of the three different stimulus blocks (each block lasting 4 min, see Fig. 1). Ordering of blocks was counterbalanced across subjects. Participants were asked to listen to the musical stimuli with their eyes closed. Each block of musical stimuli was followed by an interval of 2 s in which a sine‐wave tone of 350 Hz and 1 s duration signaled participants to open their eyes and to commence the rating procedure. During the rating procedure, participants indicated how they felt at the end of each block with regard to valence (pleasantness), arousal, joy, and fear. That is, participants provided ratings about how they actually felt, and not about which emotion they thought each block of stimuli was supposed to express [Gabrielson and Juslin, 2003]. Ratings were obtained with 6‐point Likert scales ranging from 1 (“not at all”) to 6 (“very much”). The time interval for the rating procedure was 16 s. The total length of the fMRI experiment thus amounted to about 14 min. Musical stimuli were presented using Presentation (version 13.0, Neurobehavioral systems, Albany, CA) via MRI compatible headphones (under which participants wore earplugs). Instructions and rating screens were delivered through MRI compatible liquid crystal display goggles (Resonance Technology, Northridge, CA).
MR Scanning
Scanning was performed with a 3 T Siemens Magnetom TrioTim. Before the functional MR measurements, a high‐resolution (1 × 1 × 1 mm) T 1‐weighted anatomical reference image was acquired from each participant using a rapid acquisition gradient echo sequence. Continuous echo planar imaging was used with an echo time of 30 ms and a repetition time (TR) of 2 s. Slice‐acquisition was interleaved within the TR interval. The matrix acquired was 64 × 64 voxels with a field of view of 192 mm, resulting in an in‐plane resolution of 3 mm. Slice thickness was 3 mm with an interslice gap of 0.6 mm (37 slices, whole brain coverage). The acquisition window was tilted at an angle of 30 degrees relative to the AC‐PC line in order to minimize susceptibility artifacts in the orbitofrontal cortex [Deichmann et al., 2002, 2003; Weiskopf et al., 2007]. Given the analyses methods used, a continuous scanning design was preferable for optimum correlation estimations (see “Data Analysis” for details).
Data Analysis
fMRI data were processed using LIPSIA 2.1 [Lohmann et al., 2001]. Data were corrected for slicetime acquisition and normalized into MNI‐space‐registered images with isotropic voxels of 3 mm3. A temporal highpass filter with a cutoff frequency of 1/90 Hz was applied to remove low frequency drifts in the fMRI time series, and a spatial smoothing was performed using a 3D Gaussian kernel and a filter size of 6 mm (full width at half maximum).
The mean signal value per scan was computed and regressed out of each participant's data. Similarly, the movement parameters of each participant were regressed out of the entire fMRI time‐series. In addition, the psychoacoustic parameters that had been identified to differ significantly between experimental conditions (see Supporting Information for details) were regressed out of each respective experimental condition. Thus, variance that could be explained by any of these factors was removed from the fMRI timeseries.
Functional MR data were analyzed using ECM [Lohmann et al., 2010]. On the first‐level of statistical estimations, whole‐brain ECM was computed separately for each participant, and separately for the entire time‐series of each block (i.e., separately for the 4 min block of each condition). No additional filtering was applied, and time‐bins corresponded to the TR (2 s). On the second level of the ECM analysis, ECMs were compared between experimental conditions using voxel‐wise paired sample t‐tests. Results were corrected for multiple comparisons by the use of cluster‐size and cluster‐value thresholds obtained by Monte Carlo simulations with a significance level of P < 0.05 [Lohmann et al., 2008].
The ECM clusters identified by these analyses were then used as seed regions for functional connectivity analyses: Each significant cluster identified by the ECM analysis was used as a functional connectivity seed, by computing the amount of correlation of the average time‐course of activity within each cluster with the activity in all other voxels. Functional connectivity maps were calculated separately for each experimental condition and for each participant, and then normalized across subjects. Subsequently, the normalized maps were compared between experimental conditions using paired t‐tests corrected for multiple comparisons by the use of cluster‐size and cluster‐value thresholds obtained by Monte Carlo simulations with a significance level of P < 0.05 [Lohmann et al., 2008].
RESULTS
Behavioral Results
Behavioral data are shown in Figure 2. Valence (pleasantness) ratings were higher for joy than neutral stimuli (t(19) = 4.30, P < 0.001), and higher for joy than fear stimuli (t(19) = 6.79, P < 0.0001), but did not differ significantly between neutral and fear stimuli (P = 0.59). Arousal ratings did not differ between joy and fear stimuli (P > 0.99), but tended to be higher for joy than neutral stimuli (t(19) = 2.11, P < 0.05), and higher for fear than neutral stimuli (t(19) = 2.45, P < 0.05). Joy ratings were higher for joy than neutral stimuli (t(19) = 4.92, P < 0.0001), and tended to be higher for neutral than fear stimuli (t(19) = 2.55, P < 0.05). Fear ratings were higher for fear than neutral stimuli (t(19) = 3.71, P < 0.01) and higher for neutral than joy stimuli (t(19) = 5.08, P < 0.0001).
Figure 2.
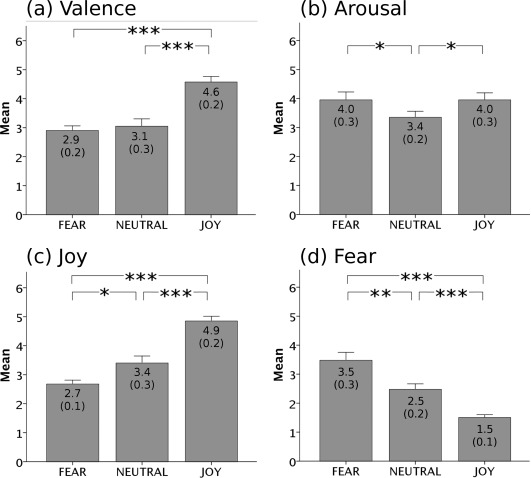
Behavioral ratings. Participants rated their emotional state on four scales: (a) valence, (b) arousal, (c) joy, and (d) fear. Average ratings are depicted separately for each stimulus category (fear, neutral, and joy). Scales ranged from 1 (“not at all”) to 6 (“very much”). Error bars indicate standard error of mean.
Emotion ratings were obtained after each stimulus block (that is, ratings were obtained only once after the joy, once after the fear, and once after the neutral block). To guarantee that joy and fear were not only evoked toward the end of the stimulus blocks, we carried out an additional behavioral experiment with 14 subjects, in which participants performed the emotion ratings after each of the 30‐s‐excerpts (ordering of excerpts was identical to the fMRI experiment, for details see Supporting Information). These ratings showed that, for each of the joy stimuli, joy ratings were higher than ratings for each of the neutral and fear stimuli. Moreover, for each of the fear stimuli, fear ratings were higher than for each of the neutral and joy stimuli (results are shown in Supporting Information Fig. S1). Thus, joy and fear stimuli evoked relatively sustained states of joy or fear, respectively, throughout the stimulus blocks.
fMRI Data
ECMs were computed separately for each emotion condition, and compared between conditions using voxel‐wise t‐tests (corrected for multiple comparisons, P < 0.05, see “Materials and Methods” for details). Results of these tests are listed in Table 1 and shown in Figure 3a. The contrast joy > fear showed significantly higher centrality values in the left SF (70% probability according to [Amunts et al., 2005]) extending into the hippocampal‐amygdaloid transition area, and in the right laterobasal group of the amygdala (LB, 70% probability according to [Amunts et al., 2005]), extending into the superficial group of the amygdala. In the left hemisphere, a cluster of significantly activated voxels protracted from the striatum (putamen and caudate nucleus) into the claustrum and the piriform cortex. Moreover, significant ECM clusters were observed in the hypothalamus bilaterally. In the peak voxels of these clusters, mean centrality values (averaged across subjects) for the joy condition were highest for the left SF (0.43) and right LB (0.42), followed by the left striatum (0.41) and hypothalamus (right: 0.36, left: 0.34). The opposite contrast (fear > joy) did not reveal any cluster with significantly higher centrality values for fear compared with joy. Comparisons with the neutral condition showed that, in the left SF as well as in the hypothalamus, centrality values were significantly higher for joy than for neutral (P < 0.05 for each structure, corrected for multiple comparisons). Note that values of acoustic descriptors that differed between conditions were introduced as regressors of no interest during the pre‐processing (see “Data Analysis” for details). Therefore, it is unlikely that acoustical differences between stimuli contributed to the ECM‐results.
Table 1.
Results of the Eigenvector Centrality Mapping (ECM) contrast joy > fear, corrected for multiple comparisons (P < 0.05)
| MNI coord. | cluster size (mm3) | z‐value: max (mean) | |
|---|---|---|---|
| l Superficial amygdala (70%) | −20 −9 −12 | 432 | 2.83 (2.57) |
| r Laterobasal amygdala (70%) | 28 −2 −18 | 621 | 3.63 (2.88) |
| l Hypothalamus | −4 −2 −13 | 351 | 3.08 (2.66) |
| r Hypothalamusa | 6 0 −11 | – | 3.08 |
| l Striatum | −26 11 −7 | 1,242 | 3.79 (2.71) |
Percentages in brackets indicate anatomical probabilities according to the SPM Anatomy Toolbox [Eickhoff et al., 2005]. The outermost right column indicates the maximal z‐value of voxels within a cluster (with the mean z‐value of all voxels within a cluster in parentheses).
The cluster with the peak voxel in the l hypothalamus had an additional local maximum in the r hypothalamus.
Figure 3.
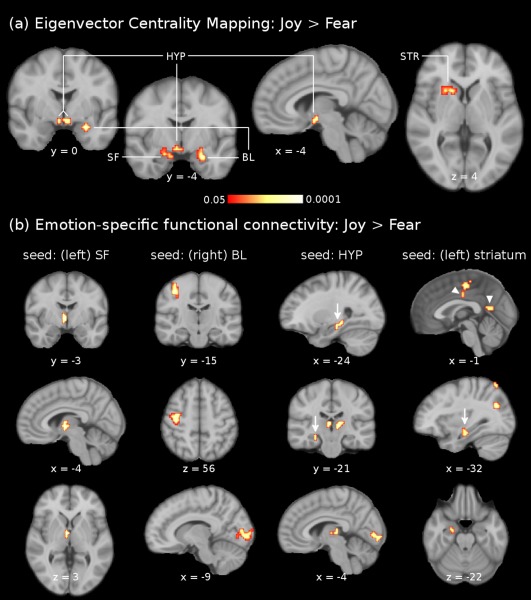
fMRI results. The upper panel (a) shows the comparison of eigenvector centrality maps between joy and fear (joy > fear). Clusters of significantly higher centrality values during joy than fear were indicated in the left superficial amygdala (SF), right laterobasal amygdala (LB), left striatum (STR), and hypothalamus (HYP). These four clusters were used as seed regions for functional connectivity analyses. The results of the comparison of functional connectivity maps between joy and fear (joy > fear) are shown in (b), separately for the four seed regions (left SF: outermost left column, right BL: middle left column, hypothalamus: middle right column, left striatum: outermost right column). Scale for (b) is the same as for (a). The left SF showed emotion‐specific functional connectivity (stronger during joy than fear) with the hypothalamus. The right BL showed stronger functional connectivity during joy than fear with somatosensory cortex (area 3) and primary visual cortex (V1, bottom image of middle left column). The hypothalamus showed emotion‐specific functional connectivity with the hippocampal formation (arrows), thalamus and primary visual cortex (V1, bottom image of middle right column). The left striatum showed stronger functional connectivity during joy than fear with the supplementary motor area, midcingulate cortex and posterior cingulate cortex (arrow‐heads), hippocampal formation (arrow), and laterobasal amygdala (bottom image of outermost right column). Images are shown in neurological convention; all results are corrected for multiple comparisons (P < 0.05). Coordinates refer to MNI space.
Functional connectivity analysis
The ECM clusters were then used as seed regions for functional connectivity analysis, and functional connectivity maps were compared between experimental conditions (that is, for each ECM cluster, the average time‐course of activity was used as seed time‐series in a functional connectivity analysis to identify target regions for which the covariation of activity between seed and target regions was significantly different between experimental conditions, see “Data Analysis” for details). Results (corrected for multiple comparisons, P < 0.05) are listed in Table 2 and summarized in Figures 3b and 4.
Table 2.
Results of the emotion‐specific functional connectivity analyses for the contrast joy > fear, corrected for multiple comparisons (P < 0.05)
| MNI coord. | cluster size (mm3) | z‐value: max (mean) | |
|---|---|---|---|
| (a) l Superficial amygdala | |||
| l Thalamus | −6 −6 4 | 513 | 4.14 (3.38) |
| (b) r Laterobasal amygdala | |||
| l Central sulcus (area 4a, 50%) | −39 −18 52 | 2,403 | 4.95 (3.46) |
| l Central sulcus (area 3a, 60%)a | −35 −26 41 | – | 3.93 |
| l Calcarine sulcus (area 17, 90%) | −6 −95 3 | 3,483 | 3.94 (3.41) |
| (c) l striatum | |||
| Paracentral lobule/SMA (area 6, 70%) | −3 −21 52 | 999 | 4.32 (3.44) |
| Posterior MCCb | 3 −20 41 | – | 3.62 |
| l SPL (area 7A, 80%) | −33 −69 64 | 756 | 4.29 (3.48) |
| l IPS, superior bank (hIP3, 30%) | −39 −39 49 | 1,809 | 3.83 (3.30) |
| l Posterior IPS | −33 −69 31 | 1,593 | 4.89 (3.48) |
| l Precuneus | −3 −57 16 | 405 | 3.55 (3.26) |
| r Postcentral gyrus (area 1, 70%) | 45 −30 58 | 2,106 | 5.88 (3.73) |
| l Anterior perforated substance | −21 −15 −5 | 648 | 3.99 (3.43) |
| l Hippocampus (CA, 70%) | −33 −18 −14 | 459 | 4.34 (3.53) |
| l LB (LB, 100%)c | −23 −6 −24 | – | 3.70 |
| r Hippocampus (CA, 90%) | 29 −16 −15 | 459 | 4.34 (3.53) |
| r Circular insular sulcus | 42 −6 −11 | 1,485 | 4.60 (3.61) |
| (d) Hypothalamus | |||
| l Hippocampus (SUB, 70%) | −26 −23 −14 | 459 | 3.69 (3.30) |
| r Thalamus | 12 −24 7 | 702 | 4.18 (3.40) |
| r Calcarine sulcus (area 17, 100%) | 12 −76 7 | 729 | 3.89 (3.32) |
| l Lingual gyrus (V3v, 60%) | −12 −81 −11 | 5,778 | 3.93 (3.31) |
| Cerebellar vermis (lobule V) | 6 −54 −2 | 648 | 4.57 (3.61) |
Seed‐regions used for the functional connectivity analyses were the clusters observed in the ECM results (ECM contrast joy > fear. For example, the superficial amygdala (which showed higher centrality values during joy compared with fear) showed increased functional connectivity with the thalamus during joy (compared with fear). Percentages in brackets indicate anatomical probabilities according to the SPM Anatomy Toolbox [Eickhoff et al., 2005]. The outermost right column indicates the maximal z‐value of voxels within a cluster (with the mean z‐value of all voxels within a cluster in parentheses). Abbreviations: CA: cornu ammonis of hippocampal formation; IPS: intraparietal sulcus; LB: laterobasal group of amygdala; MCC: midcingulate cortex; SF: superficial group of amygdala; SMA: supplementary motor area; SPL: superior parietal lobule; SUB: subiculum of hippocampal formation; l: left; r: right.
The cluster with the peak voxel in area 4a had an additional local maximum in area 3a.
The cluster with the peak voxel in the paracentral lobule had an additional local maximum in the r posterior MCC.
The cluster with the peak voxel in the l hippocampus had an additional local maximum in the l LB.
Figure 4.
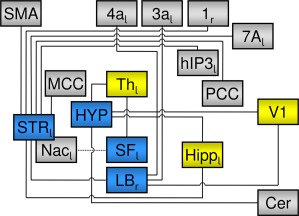
Summary of the observed “small‐world” network. Blue rectangles indicate computational hubs (joy > fear) as indicated by the ECM, lines indicate functional connections to other structures as indicated by the emotion‐specific (joy > fear) functional connectivity analysis. The dotted line connecting SF and Nac indicates that this result was yielded in the uncorrected z‐map (see main text and Supporting Information Fig. S2). Yellow boxes indicate structures that are functionally connected to more than one ECM hub. Subscript letters indicate left (l) or right (r) hemisphere. Abbreviations: Cer: cerebellum: Hipp: hippocampal formation; HYP: hypothalamus; LB: laterobasal group of amygdala; MCC: middle cingulate cortex; Nac: nucleus accumbens; PCC: posterior cingulate cortex; SF: superficial group of amygdala; SMA: supplementary motor area; STR: striatum; Th: Thalamus.
For the comparison joy > fear, the left SF showed stronger functional connectivity during joy than during fear with the left mediodorsal thalamus (MD). The right LB showed stronger functional connectivity during joy than during fear stimuli with the left central sulcus (areas 4a and 3a according to [Eickhoff et al., 2005]) and with left primary visual cortex (area 17). The hypothalamus showed stronger functional connectivity during joy than during fear stimuli with the left hippocampal formation (70% probability for subiculum according to [Eickhoff et al., 2005]), bilateral thalamus, V1, V3v bilaterally, and the right cerebellum. Finally, the left striatum exhibited significantly stronger functional connectivity during joy than during fear stimuli with left LB, both left and right hippocampus (70% probability for cornu ammonis according to [Eickhoff et al., 2005]), left anterior perforated substance, right circular insular sulcus, posterior midcingulate cortex (area p24b′ according to [Palomero‐Gallagher et al., 2009]), supplementary motor area (SMA), right postcentral gyrus (area 1), left intraparietal sulcus (IPS, area hIP3 according to [Scheperjans et al., 2008]), left lateral superior parietal lobule (area 7A according to [Scheperjans et al., 2008]), and posterior cingulate cortex (area v23 according to [Palomero‐Gallagher et al., 2009]). None of the comparisons showed stronger functional connectivity during fear (compared with joy).
DISCUSSION
Our data reveal a neural “small‐world” network underlying joy, with the (left) superficial group of the amygdala (SF), the (right) laterobasal group (LB), hypothalamus, and the (left) striatum as computational hubs, as well as a number of functionally connected cortical and subcortical structures (this network is summarized in Fig. 4). These structures include the hippocampus, medial thalamus, cerebellum, and neocortical structures involved in attention, sensorimotor processes, and vision.
Of the observed computational hubs (blue structures in Fig. 4), the left SF (consisting of cortical nuclei, anterior amygdaloid area, amygdalopyriform transition area, and amygdaloid‐hippocampal area) [Amunts et al., 2005] showed the highest centrality values. This indicates that the SF plays a central role for (music‐evoked) joy within the observed small‐world network. This finding is consistent with our previous twin‐study that used the same stimuli with shorter block durations (30 sec) and a general linear model for data analysis [Koelsch et al., 2013]. In that study, BOLD signal intensity increased in the SF during joy, and decreased during fear. The SF receives projections from the (medial) olfactory bulb [Moreno and González, 2007] and has been implicated in intra‐species communication via olfactory stimuli [Moreno and González, 2007]. Moreover, studies with humans showed that SF is also sensitive to non‐olfactory stimuli: an fMRI study by Goossens et al. [2009] observed BOLD signal changes in the SF in response to faces, but not to houses, and a meta‐analysis of functional neuroimaging data showed that trustworthiness as well as attractiveness judgments of faces overlap in the SF [Bzdok et al., 2011]. Based on these findings, it was suggested that the SF is involved in the evaluation of signals with social relevance [Bzdok et al., in press]. The present data, as well as our previous twin‐study [Koelsch et al., 2013], support this notion and indicate that the SF is also sensitive to auditory information. It has previously been argued that individuals perceive music as a stimulus with social significance due to its communicative properties [Cross and Morley, 2008; Koelsch, 2010; Steinbeis and Koelsch, 2008] and its acoustic similarity with affective prosody [Juslin and Laukka, 2003]. The finding that the (left) SF exhibits stronger eigenvector centrality, as well as higher BOLD signal values [Koelsch et al., 2013] in response to joy than fear (and neutral) stimuli suggests that SF is not only sensitive to affective information with social relevance, but that neural activity in the SF differs between social signals that motivate approach and those that do not (nevertheless, note that the SF appears to be also sensitive to social signals that motivate withdrawal [Bzdok et al., 2011], see also below).
The left SF showed functional connectivity during joy (compared with fear) with the mediodorsal thalamus (MD). This replicates results of our previous study [Koelsch et al., 2013], in which the SF also showed emotion‐specific functional connectivity (stronger for joy than for fear) with the MD. Both amygdala and thalamus possess evaluative and mnemonic functions, and efferents to the MD enable the amygdala to influence neural activity in large regions of the (prefrontal) cortex [Aggleton and Mishkin, 1984]. This is consistent with the notion that the SF has a central role in the modulation of activity within networks underlying emotional experiences.
Both amygdala and MD show high density of opiate receptors [Wamsley et al., 1982], and both SF and MD (via the ventral pallidum) project to the Nac [Bzdok et al., in press; Li and Kirouac, 2008]. Such connections have been proposed to modulate approach‐avoidance behavior toward social cues in human interaction [Bzdok et al., 2011], and based on the studies showing projections between SF and Nac [Bzdok et al., in press; Li and Kirouac, 2008] we also investigated possible emotion‐specific functional connectivity between left SF (used as seed region) and Nac in our data, using a lower statistical threshold (uncorrected z‐maps thresholded at P < 0.001 and a voxel‐extent of five voxels). This analysis revealed functional connectivity between SF and the left ventral striatum/Nac that was stronger during joy than during fear (see Supporting Information Fig. S2, and dashed line in Fig. 4). This emotion‐specific functional connection between SF and Nac is consistent with a recent study [Salimpoor et al., 2013] which showed that functional connectivity between these structures correlates with desirability of music (in that study, functional connectivity between the Nac and amygdala predicted whether individuals would decide to buy a song). Taken together, the present results suggest that, in humans, functional connections between SF, MD, and Nac modulate approach‐avoidance behavior in response to socio‐affective signals.
Despite the high centrality value of the left SF (indicating its central functional position within the observed network), only one functional connection (with the left thalamus) was indicated in the contrast of functional connectivity maps when corrected for multiple comparisons. Thus, the high centrality of SF was probably due to a number of functional connections with other structures that did not exceed the level of statistical significance used in our study. Such other connections remain to be specified, for example by using longer block durations (leading to a higher signal‐to‐noise ratio) and perhaps subject‐specific stimuli (that evoke maximal joy responses).
The right LB (consisting of lateral, basolateral, basomedial, and paralaminar nuclei) [Amunts et al., 2005] also showed higher centrality values during joy than fear. The centrality value during joy was lower in LB than in SF, but higher than in the striatum and the hypothalamus. LB is conceived of as the main amygdalar input structure for auditory information (as well as for sensory information from other modalities), and involved in the encoding, evaluation and learning of both positive and negative stimuli [Critchley et al., 2002; Holland and Gallagher, 2004; LeDoux, 2000; Murray, 2007; Paton et al., 2006; Vuilleumier, 2005]. Moreover, LB has been implicated in the generation of expectancies of reinforcers that guide goal‐directed behavior in response to such stimuli [Holland and Gallagher, 2004]. Thus, activity of LB in this study was likely to be due, at least in part, to the coding of the reward value of pleasant music.
The functional connectivity between LB and primary visual cortex is consistent with anatomical projections from the basal nucleus of the amygdala to striate (and prestriate) regions of the occipital cortex [Amaral and Price, 1984]. This functional connection is perhaps related to processes of visual imagery during music listening, which has been proposed as a principle underlying the evocation and amplification of emotion during music listening [Juslin et al., 2010]. The functional connectivity between right LB and left somatosensory cortex (area 3a) parallels direct and indirect anatomical connections between these structures [Murray, 2007; Shi and Cassell, 1999]. These connections might be related to somatosensory aspects, and thus to the subjective feeling component, of emotion [Gray et al., 2007; Harrison et al., 2010; Herwig et al., 2010].
Because centrality values were highest in SF and LB, our data indicate central functional positions of both the (left) SF and (right) LB within the neural network underlying joy. This observation motivates the hypothesis that the amygdala is centrally involved in the modulation of activity within this network. Such modulation probably includes initiation, maintenance, as well as termination of emotions (note that such modulatory processes can be influenced by voluntary, conscious cognitive activity; [Ochsner and Gross, 2005]). This hypothesis is in accordance with previous studies showing that (in the macaque monkey) the amygdala has connections to most cortical areas, and occupies a central geometric position within a topological map based on structural connectivity patterns [Young et al., 1994]. Moreover, using resting‐state fMRI, functional connectivity was shown between LB and both temporal and frontal regions, and between SF and the entire limbic lobe [Roy et al., 2009]. Thus, the amygdala is a connector hub that is linked with numerous other connector hubs, as well as with numerous provincial hubs [Young et al., 1994], and therefore has a markedly high centrality. This puts the amygdala in a position to integrate cognitive and emotional information [Pessoa, 2008], to modulate activity in emotion‐specific and related cognitive networks, and to switch between different emotional states.
The left striatum was identified as another computational hub (in the comparison joy > fear). Notably, compared with SF, LB, and hypothalamus, the striatum showed by far the largest number of functional connections with other structures, including (left) LB and (left) anterior perforated substance, bilateral hippocampus, posterior middle cingulate cortex, right circular insular sulcus, SMA, area 1, the left IPS (area hIP3), left lateral superior parietal lobule (area 7A), and posterior cingulate cortex (PCC, area v23). The observation of these extensive functional connections of the striatum with other cerebral structures is consistent with anatomical studies showing that the entire neocortex, including sensorimotor and parietal association cortex, sends fibers to both the caudate nucleus and the putamen [Nieuwenhuys et al., 2008]. In addition, the basolateral nucleus of the amygdala [Russchen et al., 1985], the hippocampus [Haber et al., 1990; Parent and Hazrati, 1995] as well as the cingulate cortex [Haber et al., 1990; Parent and Hazrati, 1995] send projections to the ventral striatum including, but not limited to, the NAc. Although sensorimotor, association, and limbic cortical areas project in a segregated manner onto three distinct striatal regions (referred to as associative, sensorimotor, and limbic striatal territories) [Parent and Hazrati, 1995], it is striking that the region identified as the striatal computational hub in our study is located at the borders of all three of these territories. Hence, our data on functional connections of the striatum are in remarkable agreement with anatomical projections to the striatum, and indicate that such projections play a role for emotional processes underlying (music‐evoked) joy. The activation of striatal (caudate nucleus) and sensorimotor areas during joyful music converges with results of a study by Trost et al. [2012] indicating that covert motor activity correlated with joyful music eliciting a tendency to dance. Other studies related sensorimotor activation during music listening also to aesthetic preferences [Kornysheva et al., 2010].
The striatal region observed in our study is different from the ventral part of the striatum (including the NAc) that has been associated with experiences of pleasure and reward [Koelsch, 2010; Salimpoor et al., 2011; Trost et al., 2012], and from the dorsal part of the striatum associated with experiences of reward anticipation during music listening [Salimpoor et al., 2011]. The role of the striatum for emotional processes has first been discussed by MacLean [1972], who proposed that the striatal complex is part of a storage mechanism for learned emotive behaviors, a notion that is corroborated by the functional connections of the striatum with sensorimotor and limbic structures in the present study. MacLean [1972] also proposed that the striatal complex plays a role for behavior involving conspecific recognition and communication in the form of rudimentary, nonverbal signaling. Our results emphasize the significant role of the striatum for emotional processes, and show that the striatum functions as a computational hub in which sensorimotor, attentional, and emotional processes converge during the perception of positive music.
The hypothalamus also showed higher eigenvector centrality during joy (compared with fear). This indicates that the hypothalamus is involved in joy, in particular during longer emotional periods. Activation of the hypothalamus in this study was probably due to endocrine changes. This assumption is supported by the emotion‐specific functional connectivity (stronger during joy than fear) of the hypothalamus with the subiculum of the hippocampal formation. The subiculum has dense bidirectional connections with the hypothalamus [O'Mara, 2005], including projections from the subiculum to the medial preoptic area, the ventromedial and dorsomedial nuclei, and ventral premammillary as well as medial mammillary nuclei [O'Mara, 2005]. The functional significance of these connections is thought to be modulation of hypothalamus‐pituitary‐adrenal (HPA) axis activity (in particular inhibition of HPA‐axis activity mediated by GABAergic neurons) [O'Mara, 2005]. Thus, the subiculum is substantially involved in qualifying (terminating or limiting) HPA axis activity in response to stress. Hence, the present results indicate that the hippocampal formation plays a role for modulating hypothalamic endocrine activity during sustained periods of music‐evoked positive emotion. This finding has important implications for the application of music therapy to reduce stress and stress responses in both clinical and nonclinical settings, although the hormones released during experiences of music‐evoked joy remain to be specified (studies on endocrine effects of music are reviewed in [Koelsch and Stegemann, 2012]).
The fact that hypothalamic activation is rarely observed in fMRI studies on emotion is possibly due to the fact that such changes are relatively slow. Thus, such changes might easily go unnoticed in experiments with shorter stimulus durations. However, two previous fMRI studies with shorter stimuli (positive music and humorous cartoons) reported activation within, or in the close vicinity of the hypothalamus [Menon and Levitin, 2005; Schwartz et al., 2008].
Limitations
Although fear ratings were higher for fear stimuli than for neutral or joy stimuli, we did not observe any effect of fear stimuli in the ECM contrasts. Thus, the fear stimuli were not effective enough to evoke significant centrality changes in computational hubs underlying music‐evoked fear in our study. Future studies might find such changes by using music stimuli in combination with images or movie‐clips (for studies using such combinations and reporting BOLD signal changes in amygdala and hippocampus in response to fear‐evoking music see [Aust et al., in press; Baumgartner et al., 2006; Eldar et al., 2007]). Another limitation is that ECM is not well suited to capture fluctuations in emotional experiences because it yields the strongest results for the analysis of longer time‐series (in this study, each block had a duration of 4 min). Thus, using continuous emotion regressors [Goldin et al., 2005; Lehne et al., in press; Wallentin et al., 2011] or continuous inter‐subject correlations [Hasson et al., 2004; Nummenmaa et al., 2012] is more suitable to investigate this issue. Although the data of our additional behavioral experiment indicate that joy and fear were not evoked to an equal amount by each excerpt in each subject, the use of ECM was justified by the fact that these data also indicate that sustained emotional states of joy and (moderate) fear were evoked (even though the degree of joy or fear varied during the stimulus blocks). A further limitation is that it is likely that other emotions were evoked occasionally, such as surprise in response to deceptive cadences, or emotions such as tenderness, transcendence or power. While we surmise that such factors canceled out (or were lost in noise) across stimuli and participants, future studies might also use, e.g., the Geneva Emotional Music Scale [Zentner et al., 2008] for a more fine‐grained characterization of the emotions evoked by the music stimuli. Finally, centrality values were lateralized in most of the observed computational hubs (left SF, right LB, left striatum). These findings were significant in the corrected centrality maps, but there were also sub‐threshold results in the contralateral homotope structures. Thus, we are hesitant to state that effects are strictly lateralized, and surmise that the results show hemispheric weightings, rather than left/right dichotomies. This is consistent with anatomical studies showing, e.g., that the strongest projections to and from amygdalar nuclei are ipsilateral, but that contralateral projections are typically present as well [e.g., Aggleton and Mishkin, 1984; Kita and Kitai, 1990; McDonald et al., 1996]. The exact nature of possible hemispheric differences observed in this study remains to be specified.
CONCLUSIONS
The present fMRI study used ECM for the investigation of neural networks underlying emotion. Our results reveal a “small‐world” neuroarchitecture of a network underlying joy (evoked and measured over the course of several minutes). The data show that both left superficial (SF) and right laterobasal (LB) nuclear groups of the amygdala play a central role throughout sustained periods of joy. Both SF and LB showed the highest eigenvector centrality values, motivating the hypothesis that the amygdala plays a central role for the modulation (i.e., initiation, maintenance, and termination) of emotions. Results support the recent notion that the SF is sensitive to affective signals with social relevance, in particular to social signals that motivate approach, such as music expressing joy. The functional connectivity between SF and Nac probably modulates approach‐avoidance behavior in response to socio‐affective signals, whereas the functional connectivity between LB and sensory areas appears to be related to somatosensory aspects, and thus to the subjective feeling component, of emotion (in addition to the role of the LB in the evaluation of sensory stimuli). Our data also indicate functional connectivity between hypothalamus and hippocampus, which is likely due to the modulation of HPA axis activity (in particular inhibition of HPA‐axis activity) during sustained joy. Of the four observed computational hubs, the striatal complex had by far the largest number of functional connections to other structures. This highlights the role of the striatal complex in emotion, in particular with regard to emotive sensorimotor functions. The present results are important because they expand the knowledge about neural small‐world networks underlying emotional experience.
Supporting information
Supplementary Information Figure 1.
Supplementary Information Figure 2.
Supplementary Information Table 1.
Supplementary Information Table 2.
Supplementary Information
ACKNOWLEDGMENTS
The authors declare that they have no conflict of interest. The authors thank Vienna Doenni and Yu Fukuda for their assistance during the data collection.
REFERENCES
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E (2006): A resilient, low‐frequency, small‐world human brain functional network with highly connected association cortical hubs. J Neurosci 26:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggleton J, Mishkin M (1984): Projections of the amygdala to the thalamus in the cynomolgus monkey. J Comp Neurol 222:56–68. [DOI] [PubMed] [Google Scholar]
- Albert R, Barabási AL (2002): Statistical mechanics of complex networks. Rev Mod Phys 74:47–101. [Google Scholar]
- Amaral D, Price J (1984): Amygdalo‐cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230:465–496. [DOI] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, Habel U, Schneider F, Zilles K (2005): Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: Intersubject variability and probability maps. Anat Embryol 210:343–352. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust S, Härtwig EA, Koelsch S, Heekeren HR, Heuser I, Bajbouj M: How emotional abilities modulate the influence of early life stress on hippocampal functioning. Soc Cog Affect Neurosci (in press), doi: 10.1093/scan/nst078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze‐Bonhage A, Speck O, Mutschler I (2007): Response properties of human amygdala subregions: Evidence based on functional MRI combined with probabilistic anatomical maps. PLoS One 2:e307, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Lutz K, Schmidt CF, Jäncke L (2006): The emotional power of music: How music enhances the feeling of affective pictures. Brain Res 1075:151–164. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1993): Beck Depression Inventory. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Bullmore E, Sporns O (2009): Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Langner R, Caspers S, Kurth F, Habel U, Zilles K, Laird A, Eickhoff SB (2011): Ale meta‐analysis on facial judgments of trustworthiness and attractiveness. Brain Struct Funct 215:209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB: An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum Brain Mapp (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2002): Fear conditioning in humans: The influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33:653–663. [DOI] [PubMed] [Google Scholar]
- Cross I, Morley I (2008): The evolution of music: Theories, definitions and the nature of the evidence In: Malloch S, Trevarthen C, editors. Communicative Musicality: Exploring the Basis of Human Companionship. Oxford: Oxford University Press; pp 61–82. [Google Scholar]
- Deichmann R, Josephs O, Hutton C, Corfield D, Turner R (2002): Compensation of susceptibility‐induced BOLD sensitivity losses in echo‐planar fMRI imaging. Neuroimage 15:120–135. [DOI] [PubMed] [Google Scholar]
- Deichmann R, Gottfried J, Hutton C, Turner R (2003): Optimized EPI for fMRI studies of the orbitofrontal cortex. Neuroimage 19:430–441. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Eldar E, Ganor O, Admon R, Bleich A, Hendler T (2007): Feeling the real world: Limbic response to music depends on related content. Cereb Cortex 17:2828–2840. [DOI] [PubMed] [Google Scholar]
- Eryilmaz H, Van De Ville D, Schwartz S, Vuilleumier P (2011): Impact of transient emotions on functional connectivity during subsequent resting state: A wavelet correlation approach. Neuroimage 54:2481–2491. [DOI] [PubMed] [Google Scholar]
- Ferrarini L, Veer IM, Baerends E, van Tol MJ, Renken RJ, van der Wee NJ, Veltman D, Aleman A, Zitman FG, Penninx BW, van Buchem MA, Reiber JHC, Rombouts SARB, Milles J (2009): Hierarchical functional modularity in the resting‐state human brain. Hum Brain Mapp 30:2220–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielson A, Juslin PN (2003): Emotional expression in music In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. New York: Oxford University Press; pp 503–534. [Google Scholar]
- Goldin PR, Hutcherson CAC, Ochsner KN, Glover GH, Gabrieli JDE, Gross JJ (2005): The neural bases of amusement and sadness: A comparison of block contrast and subject‐specific emotion intensity regression approaches. Neuroimage 27:26–36. [DOI] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Onur OA, Fink GR, Maier W, Griez E, Schruers K, Hurlemann R (2009): Selective processing of social stimuli in the superficial amygdala. Hum Brain Mapp 30:3332–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthardt U, Schweiger U, Fahrenberg J, Lauer C, Holsboer F, Heuser I (1995): Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. Am J Physiol Regul Integr Comp Physiol 268:R865–R873. [DOI] [PubMed] [Google Scholar]
- Gray MA, Harrison NA, Wiens S, Critchley HD (2007): Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS One 2:e546, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S, Lynd E, Klein C, Groenewegen H (1990): Topographic organization of the ventral striatal efferent projections in the rhesus monkey: An anterograde tracing study. J Comp Neurol 293:282–298. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yücel M (2008): Modulation of brain resting‐state networks by sad mood induction. PLoS One 3:e1794,1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD (2010): The embodiment of emotional feelings in the brain. J Neurosci 30:12878–12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R (2004): Intersubject synchronization of cortical activity during natural vision. Science 303:1634–1640. [DOI] [PubMed] [Google Scholar]
- Herwig U, Kaffenberger T, Jäncke L, Brühl AB (2010): Self‐related awareness and emotion regulation. Neuroimage 50:734–741. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M (2004): Amygdala–frontal interactions and reward expectancy. Curr Opin Neurobiol 14:148–155. [DOI] [PubMed] [Google Scholar]
- Juslin PN, Laukka P (2003): Communication of emotions in vocal expression and music performance: Different channels, same code? Psych Bull 129:770–814. [DOI] [PubMed] [Google Scholar]
- Juslin PN, Liljeström S, Västfjäll D, Lundqvist LO (2010): How does music evoke emotions? Exploring the underlying mechanisms In: Juslin PN, Sloboda JA, editors. Handbook of Music and Emotion: Theory, Research, Applications, 2nd ed. Oxford: Oxford University Press Oxford; pp 605–642. [Google Scholar]
- Kita H, Kitai S (1990): Amygdaloid projections to the frontal cortex and the striatum in the rat. J Comp Neurol 298:40–49. [DOI] [PubMed] [Google Scholar]
- Koelsch S (2010): Towards a neural basis of music‐evoked emotions. Trends Cogn Sci 14:131–137. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Fritz T, Cramon DY, Müller K, Friederici AD (2006): Investigating emotion with music: An fMRI study. Hum Brain Mapp 27:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelsch S, Skouras S, Fritz T, Herrera P, Bonhage C, Küssner MB, Jacobs AM (2013): Neural correlates of music‐evoked fear and joy: The roles of auditory cortex and superficial amygdala. Neuroimage 81:49–60. [DOI] [PubMed] [Google Scholar]
- Koelsch S, Stegemann T (2012): The brain and positive biological effects in healthy and clinical populations In: MacDonald R, Kreutz D, Mitchell L, editors. Music, Health and Well‐Being. Oxford: Oxford University Press; pp 436–456. [Google Scholar]
- Kornysheva K, von Cramon DY, Jacobsen T, Schubotz RI (2010): Tuning‐in to the beat: Aesthetic appreciation of musical rhythms correlates with a premotor activity boost. Hum Brain Mapp 31:48–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE (2000): Emotion circuits in the brain. Ann Rev Neurosci 23:155–184. [DOI] [PubMed] [Google Scholar]
- Lehne M, Rohrmeier M, Koelsch S: Functional neuroimaging of tension: An fMRI study with music. Soc Cogn Affect Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Kirouac GJ (2008): Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 506:263–287. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Müller K, Bosch V, Mentzel H, Hessler S, Chen L, von Cramon DY (2001): Lipsia—A new software system for the evaluation of functional magnet resonance images of the human brain. Comp Med Imaging Graph 25:449–457. Available at: http://www.cns.mpg.de/lipsia. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Neumann J, Müller T, Lepsien J, Turner R (2008): The multiple comparison problem in fMRI—A new method based on anatomical priors In: Ghassan H, Rafeef A, editors. Workshop on Analysis of Functional Medical Images. New York University, New York, NY, USA: pp 179–187. [Google Scholar]
- Lohmann G, Margulies DS, Horstmann A, Pleger B, Lepsien J, Goldhahn D, Schloegl H, Stumvoll M, Villringer A, Turner R (2010): Eigenvector Centrality Mapping for analyzing connectivity patterns in fMRI data of the human brain. PLoS One 5:e10232, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean PD (1972): Cerebral evolution and emotional processes: New findings on the striatal complex. Ann NY Acad Sci 193:137–149. [DOI] [PubMed] [Google Scholar]
- McDonald A, Mascagni F, Guo L (1996): Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 71:55–75. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ (2005): The rewards of music listening: Response and physiological connectivity of the mesolimbic system. Neuroimage 28:175–184. [DOI] [PubMed] [Google Scholar]
- Meunier D, Achard S, Morcom A, Bullmore E (2009): Age‐related changes in modular organization of human brain functional networks. Neuroimage 44:715–723. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Fu CH, Dalton JA, Andrew CM, Williams SC (2007): A functional MRI study of happy and sad affective states evoked by classical music. Hum Brain Mapp 28:1150–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno N, González A (2007): Evolution of the amygdaloid complex in vertebrates, with special reference to the anamnio‐amniotic transition. J Anat 211:151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA (2007): The amygdala, reward and emotion. Trends Cogn Sci 11:489–497. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, Huijzen CV (2008): The Human Central Nervous System. Berlin: Springer. [Google Scholar]
- Nummenmaa L, Glerean E, Viinikainen M, Jääskeläinen IP, Hari R, Sams M (2012): Emotions promote social interaction by synchronizing brain activity across individuals. Proc Natl Acad Sci USA 109:9599–9604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ (2005): The cognitive control of emotion. Trends Cogn Sci 9:242–249. [DOI] [PubMed] [Google Scholar]
- O'Mara S (2005): The subiculum: What it does, what it might do, and what neuroanatomy has yet to tell us. J Anat 207:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomero‐Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K (2009): Receptor architecture of human cingulate cortex: Evaluation of the four‐region neurobiological model. Hum Brain Mapp 30:2336–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Hazrati LN (1995): Functional anatomy of the basal ganglia. I. The cortico‐basal ganglia‐thalamo‐cortical loop. Brain Res Rev 20:91–127. [DOI] [PubMed] [Google Scholar]
- Paton JJ, Belova MA, Morrison SE, Salzman CD (2006): The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2008): On the relationship between emotion and cognition. Nat Rev Neurosci 9:148–158. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP (2009): Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen F, Bakst I, Amaral D, Price J (1985): The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res 329:241–257. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, Benovoy M, Larcher K, Dagher A, Zatorre RJ (2011): Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat Neurosci 14:257–262. [DOI] [PubMed] [Google Scholar]
- Salimpoor VN, van den Bosch I, Kovacevic N, McIntosh AR, Dagher A, Zatorre RJ (2013): Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science 340:216–219. [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E (2005): Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex 15:1332–1342. [DOI] [PubMed] [Google Scholar]
- Scheperjans F, Eickhoff SB, Hömke L, Mohlberg H, Hermann K, Amunts K, Zilles K (2008): Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18:2141–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Ponz A, Poryazova R, Werth E, Boesiger P, Khatami R, Bassetti CL (2008): Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain 131:514–522. [DOI] [PubMed] [Google Scholar]
- Shi CJ, Cassell M (1999): Cascade projections from somatosensory cortex to the rat basolateral amygdala via the parietal insular cortex. J Comp Neurol 399:469–491. [DOI] [PubMed] [Google Scholar]
- Sporns O, Honey CJ (2006): Small worlds inside big brains. Proc Natl Acad Sci USA 103:19219–19220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeis N, Koelsch S (2008): Understanding the intentions behind man‐made products elicits neural activity in areas dedicated to mental state attribution. Cereb Cortex 19:619–623. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2011): Association between functional connectivity hubs and brain networks. Cereb Cortex 21:2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost W, Ethofer T, Zentner M, Vuilleumier P (2012): Mapping aesthetic musical emotions in the brain. Cereb Cortex 22:2769–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P (2005): How brains beware: Neural mechanisms of emotional attention. Trends Cogn Sci 9:585–594. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Nielsen AH, Vuust P, Dohn A, Roepstorff A, Lund TE (2011): Amygdala and heart rate variability responses from listening to emotionally intense parts of a story. Neuroimage 58:963–973. [DOI] [PubMed] [Google Scholar]
- Wamsley J, Zarbin M, Young W, Kuhar M (1982): Distribution of opiate receptors in the monkey brain: An autoradiographic study. Neuroscience 7:595–613. [DOI] [PubMed] [Google Scholar]
- Weiskopf N, Hutton C, Josephs O, Turner R, Deichmann R (2007): Optimized epi for fMRI studies of the orbitofrontal cortex: Compensation of susceptibility‐induced gradients in the readout direction. Magn Reson Mater Phys Biol Med 20:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MP, Scanneil JW, Burns GA, Blakemore C (1994): Analysis of connectivity: Neural systems in the cerebral cortex. Rev Neurosci 5:227–250. [DOI] [PubMed] [Google Scholar]
- Zentner M, Grandjean D, Scherer KR (2008): Emotions evoked by the sound of music: Characterization, classification, and measurement. Emotion 8:494–521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information Figure 1.
Supplementary Information Figure 2.
Supplementary Information Table 1.
Supplementary Information Table 2.
Supplementary Information


