Abstract
Mild traumatic brain injury (mTBI) and post‐traumatic stress disorder (PTSD) are common among recent military veterans and involve substantial symptom overlap, making clinical distinction and effective intervention difficult. Emerging evidence of cerebral white matter abnormalities associated with mTBI may provide a biological measure to inform diagnosis and treatment, but the potentially confounding effects between PTSD and mTBI have largely gone unexamined. We collected diffusion imaging data from 133 recently‐deployed American service members who developed PTSD and/or sustained mTBI, or had neither condition. Effects of PTSD and mTBI on traditional tensor‐based measures of cerebral white matter integrity (fractional anisotropy [FA] and mean diffusivity [MD]) were compared in anatomical regions of interest and individual voxels throughout the brain. Generalized FA (GFA), which allows for multiple fiber orientations per voxel, was also included to improve sensitivity in white matter areas containing crossing or diverging axon bundles. PTSD was consistently associated with high GFA in select brain regions, greater likelihood of regions and voxels with abnormally low MD, and a greater number of voxels with abnormally high FA, while mTBI was associated with fewer high MD regions. Overall, PTSD was associated with more restricted diffusion (low MD) and greater anisotropy (high GFA) in regions of crossing/diverging fibers poorly characterized by a single tensor (FA), suggesting that interstitial fibers may be involved. Contrary to earlier results in a sample without PTSD, mTBI was not associated with anisotropy abnormalities, perhaps indicating the cooccurrence of PTSD and mTBI requires special consideration with regard to structural brain connectivity. Hum Brain Mapp 36:1053–1064, 2015. © 2014 Wiley Periodicals, Inc.
Keywords: mild traumatic brain injury, diffusion magnetic resonance imaging, veterans
INTRODUCTION
One of the most common injuries to military service members in recent conflicts is mild traumatic brain injury (mTBI) from explosive blast [Taber et al., 2006; Warden, 2006]. Approximately 15–25% of American service members deployed to Iraq or Afghanistan reported mTBI (i.e., concussion), and explosive blast was involved in 75% of these incidents [Hoge et al., 2008; Terrio et al., 2009; Wilk et al., 2010]. Furthermore, a study by Schneiderman et al. [2008] found that 35% of veterans who reported deployment experiences consistent with mTBI also reported substantial persistent (i.e., at least 5 months postinjury) postconcussive symptoms (PCS), such as headaches, memory problems, irritability, and sleep problems. Clinically, it can be difficult to determine whether these symptoms are truly due to mTBI rather than post‐traumatic stress disorder (PTSD), which is also common among military veterans [Stein and McAllister, 2009]. The complexity of this determination is increased by evidence that mTBI is more highly associated with PTSD than other types of injuries [Hoge et al., 2008], and PTSD is the strongest predictor of PCS among veterans who experience mTBI during deployment [Schneiderman et al., 2008]. Biological measures that differentiate the long‐term effects of psychological trauma (i.e., PTSD) on the brain from those of direct physiological trauma (i.e., mTBI) would be of high clinical utility in informing prevention, diagnosis, and intervention.
There is emerging evidence from diffusion tensor imaging (DTI) studies that white matter integrity (e.g., axon density and myelination) may be disrupted in both mTBI and PTSD [Niogi and Mukherjee, 2010; Schuff et al., 2011]. DTI is a magnetic resonance imaging (MRI) technique that describes the overall pattern of water diffusion in each spatial unit (i.e., voxel) of the imaged brain as a set of three orthogonal vectors [Basser et al., 1994; Basser and Pierpaoli, 1996]. In white matter, directionally organized barriers (e.g., axon membranes and myelin) cause water to diffuse more easily in the direction of the axon than perpendicular to the axon, making the overall diffusion pattern highly anisotropic [i.e., nonspherical; Beaulieu, 2002]. The degree of anisotropy and total amount of diffusion within each voxel can be quantified by the scalar measures fractional anisotropy (FA) and mean diffusivity (MD), respectively, and are commonly used as measures of overall white matter integrity [Basser and Pierpaoli, 1996; Beaulieu, 2002]. Because healthy white matter (i.e., densely packed, highly myelinated, and well‐organized axons) is associated with directionally restricted diffusion (i.e., high FA and low MD), reduced FA and increased MD are generally considered to be associated with white matter damage or poor integrity [Beaulieu, 2002].
Consistent with the observation of diffuse axonal injury on radiological scans [Mittl et al., 1994], studies of cerebral white matter integrity after mTBI generally report lower FA and higher MD after injury [Aoki et al., 2012; Hulkower et al., 2013; Morey et al., 2013; Niogi and Mukherjee, 2010]. However, the few published studies that have examined veterans have yielded inconsistent findings. Earliest reports [Levin et al., 2010; Sponheim et al., 2011] failed to find effects of military mTBI on white matter integrity for specific regions or voxels, and only one group [MacDonald et al., 2011] reported an effect of mTBI on anisotropy within a priori regions of interest. Subsequent studies involving voxelwise comparisons have reported lower FA associated with mTBI in portions of several major white matter tracts [Morey et al., 2013; Yeh et al., 2014], while others have reported that mTBI was not associated with lower FA at any particular voxels but was associated with a greater number of voxels with abnormally low FA [Davenport et al., 2012; Jorge et al., 2012]. Overall, these findings suggest that military mTBI is likely associated with widespread reductions of FA suggestive of white matter damage that is variable across brain regions or individuals. However, while most DTI studies of military mTBI have collected and reported PTSD symptom data, reflecting the general consensus that post‐traumatic stress is an important consideration in this population, traumatic stress symptom data have generally not been effectively examined in the statistical analyses of these reports. The exceptions are the report by Morey et al. [2013] that entered PTSD severity as one of several regressors and our previous report [Davenport et al., 2012] in which participants who met full diagnostic criteria for PTSD were excluded from analyses, neither of which is adequate to address potentially complex relationships among mTBI, PTSD, and subsequent PCS.
Most studies that have used DTI to study PTSD in military [Schuff et al., 2011] and civilian [Fani et al., 2012; Kim et al., 2005, 2006] populations, have reported lower FA in cerebral white matter, especially in temporal and cingulate regions, though one study [Abe et al., 2006] reported that PTSD was associated with higher FA in the left anterior cingulum. However, these studies explicitly excluded individuals with concussion or blast exposure. An additional study of recent veterans in which mTBI and exposure to blast were not excluded found MD, but not FA, to be a significant predictor of PTSD [Bazarian et al., 2012]. Thus, the complex relationship between PTSD and mTBI may give rise to variable findings of structural connectivity abnormalities depending on how investigators treat these interdependent and mutually confounding variables.
It is also possible that the observation of sparse, variable effects of PTSD and mTBI on FA is an indication that the tensor model, which assumes that each voxel contains fibers oriented in a single orientation, is insensitive to the white matter abnormalities associated with these conditions. Specifically, the fibers most prominently affected by mTBI or PTSD may be crossing or diverging fibers (i.e., multiple orientations within a voxel) of cortical white matter rather than major, single‐orientation tracts that are adequately modeled by the diffusion tensor model from which FA and MD are computed. In contrast to the tensor model, the Orientation Distribution Function [ODF; Tuch, 2004] assumes that water molecule diffusion within a macroscopic section of white matter (e.g., MRI voxel) is Gaussian but imposes few limits on the overall shape of the diffusion pattern (i.e., not necessarily elliptical). The ODF can be used to compute generalized fractional anisotropy (GFA), a measure analogous to FA that summarizes integrity across multiple directions within an MRI voxel [Assemlal et al., 2007]. Because computation of the ODF requires the acquisition of more diffusion directions (>30) than the tensor model (>6), it is typically only possible with high angular resolution diffusion imaging (HARDI), which until recently was rare in clinical imaging studies. To date, there are no studies of mTBI or PTSD using ODF‐based measures of white matter integrity that allow assessment of more complex patterns of cerebral white matter bundles where fibers cross or tracts of axons converge or diverge.
To better characterize potential interacting effects of military mTBI and PTSD on neural tissue, while limiting confounding factors of combat exposure and predeployment psychopathology, we extensively assessed PTSD symptoms, experience of mTBI from blast and nonblast sources during deployment, and white matter integrity in a sample of veterans deployed to Iraq or Afghanistan. Effects of PTSD and/or mTBI on white matter integrity were assessed at two spatial levels (i.e., regions of interest and individual voxels) and at two levels of spatial consistency across individuals (i.e., consistent ROI/voxel across individuals, number of abnormal ROIs/voxels). Based on prior investigations, we hypothesized that blast mTBI would be associated with diffuse, heterogeneous white matter abnormalities demonstrated by a greater number of voxels with abnormally low integrity (i.e., low FA, low GFA, and high MD) but no effects in individual regions of interest. In contrast, we hypothesized that PTSD would be associated with more focal disruptions of white matter integrity evident as lower FA, lower GFA, and greater MD in regions previously identified, such as the anterior cingulum and temporal lobe white matter.
METHODS AND MATERIALS
Participants
Participants consisted of 133 veterans of Operations Enduring and Iraqi Freedom recruited from a large sample of National Guard soldiers previously described [Polusny et al., 2011], Minneapolis Veterans Affairs Health Care System patient rosters, veteran community centers, and by word of mouth from other participants or service providers. Fifty‐five of these participants were included in a prior publication that excluded Current PTSD [Davenport et al., 2012]1. Recruitment was designed to target veterans who had previously endorsed experiences consistent with blast‐related mTBI and/or PTSD during their most recent deployment (2–5 years before participation) on a clinical or research instrument, regardless of whether the veteran sought or received treatment. All reports of mTBI and clinical symptoms were independently assessed during study participation. Exclusionary criteria included native language other than English, current or predeployment unstable medical condition that would reasonably be expected to significantly affect brain function (e.g., anoxic episode >10 s, stroke, seizures, multiple sclerosis, etc.), uncorrected visual problems or hearing loss, moderate or severe TBI not due to blast, any predeployment Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision [DSM‐IV‐TR; American Psychiatric Association, 2000] Axis I psychotic or mood disorder, predeployment PTSD according to the Clinician‐Administered PTSD Scale [CAPS; Blake et al., 1995], current or past substance dependence other than nicotine or alcohol, and contraindications to MRI (e.g., metallic implants, shrapnel, and claustrophobia).
Clinical Assessment
All participants completed a clinical interview that included the Structured Clinical Interview for DSM‐IV‐TR [SCID; First et al., 2002], CAPS, and the Minnesota Blast Exposure Screening Tool [MN‐BEST; Nelson et al., 2011]. Participants were also asked whether they had experienced in the past month any of eight features consistent with PCS (memory problems, poor balance, irritability, tinnitus, sensitivity to light/noise, headaches, and insomnia). Symptoms of mTBI were assessed by interview using the MN‐BEST and included altered consciousness (e.g., confusion and disorientation), loss of consciousness (LOC) less than 30 min, post‐traumatic amnesia (PTA) up to 24 h, and neurological symptoms (e.g., headache, tinnitus, nausea, sensitivity to light or noise) immediately after the event. Blast‐related injuries were defined as those in which the individual felt a blast wave and attributed the resultant concussion to its effects. Secondary blast effects, such as being thrown against the ground, were acceptable provided that the blast wave itself was experienced as the source of those effects. Tertiary blast effects, such as being hit by debris, were allowable as part of an overall blast‐related injury but could not be the primary source of injury. The three most significant potential blast‐related and impact‐related TBI events were considered, each of which received a severity score ranging from 0 (no concussion) to a potential maximum of 30 (severe TBI), though only one score was higher than 4 (LOC 5–30 min or PTA <12 h) in the current sample2. All TBI ratings were completed by doctoral‐level neuropsychologists based on descriptions of events secured by trained study interviewers.
DSM‐IV‐TR diagnoses, including PTSD, were finalized by a consensus review process involving advanced doctoral students and doctoral‐level licensed psychologists. Because individuals with predeployment PTSD or Major Depressive Disorder were excluded from participation, these diagnoses by rule had onsets during or after the most recent deployment. Diagnoses were considered “Current” if the individual met full diagnostic criteria at the time of study participation and “Lifetime” if full criteria were met at any point in the individual's lifetime (i.e., past or present). Individuals were separated into 4 groups: mTBI (experience of blast‐related mTBI during most recent deployment; N = 19), PTSD (full criteria for PTSD met at some point as the most recent deployment; N = 31), mTBI+PTSD (meets criteria for both mTBI and PTSD groups; N = 45), and Control (does not meet criteria for any other group; N = 38). Lifetime diagnosis of PTSD was chosen as the grouping variable to increase sensitivity to white matter abnormalities associated with the condition itself rather than symptom expression or treatment response. Table 1 summarizes demographic and clinical characteristics of these groups.
Table 1.
Demographic and clinical characteristics of the sample
| Control | mTBI | PTSD | mTBI+PTSD | Test Statistic (χ 2 or F) | P | |
|---|---|---|---|---|---|---|
| N | 38 | 19 | 31 | 45 | ||
| Current PTSD: N (%) | 23 (74) | 37 (82) | 0.71 | n.s. | ||
| Females: N (%) | 6 (16) | 1 (5) | 2 (6) | 0 (0)a | 8.24 | .04 |
| Prior civilian mTBI: N (%) | 19 (50) | 11 (58) | 19 (61) | 23 (51) | 1.17 | n.s. |
| Age, years: Mean (SD) | 33.9 (8.8) | 36.6 (9.4) | 33.3 (9.3) | 30.2 (6.2)b | 3.09 | .03 |
| Number of deployments: N (%) | 6.89 | n.s. | ||||
| 1 | 19 (50) | 6 (32) | 20 (65) | 18 (40) | ||
| 2 | 14 (37) | 10 (53) | 8 (26) | 19 (42) | ||
| 3+ | 5 (13) | 3 (16) | 3 (10) | 8 (18) | ||
| Total duration of deployments, months: Mean (SD)c | 22.1 (7.8) | 28.2 (18.3) | 16.7 (5.7)b | 19.8 (8.6)b | 5.20 | .002 |
| Total current PCSd: Mean (SD) | 1.8 (2.3) | 3.8 (2.2)a | 3.8 (2.0)a | 5.1 (2.0)a | 17.41 | <.001 |
| Lifetime alcohol dependence: N (%) | 7 (18) | 5 (26) | 16 (52)a | 30 (67)a, b | 22.65 | <.001 |
| Lifetime MDD: N (%) | 7 (18) | 7 (37) | 22 (71)a | 31 (69)a | 31.29 | <.001 |
Different than controls.
Different than mTBI.
Total duration of deployments could not be determined for five Controls, 2 PTSD, 3 mTBI, and 1 mTBI+PTSD.
A total of eight postconcussive symptoms were assessed: memory problems, poor balance, irritability, tinnitus, sensitivity to light, sensitivity to noise, headaches, and insomnia.
MDD, major depressive disorder; mTBI, mild traumatic brain injury; PCS, postconcussive symptoms; PTSD, post‐traumatic stress disorder.
In accordance with the Declaration of Helsinki, participants completed an informed consent process that included complete description of the study, and participants were provided monetary compensation for participation after each study procedure. The study protocol was reviewed and approved by the University of Minnesota and Minneapolis Veterans Affairs Medical Center Institutional Review Boards and the U.S. Army Medical Research and Materiel Command.
Image Acquisition and Processing
Images were acquired on a 3 Tesla Siemens Trio (Erlangen, Germany) scanner using a 12‐channel birdcage head coil. Head movements were minimized by placing pads around the participant's head. Localizers were acquired for orientation and prescription of subsequent scans. A high resolution magnetization‐prepared‐rapid acquisition gradient echo structural image (repetition time [TR]=2,530 ms, echo time [TE]=3.65 ms, 240 coronal slices, 256 × 256 matrix, 256 mm field of view [FOV], 1.0 mm thickness) was collected for anatomical alignment and visualization. Two sets of diffusion weighted images aligned to the plane including the anterior and posterior commissures were collected in each of 30 noncollinear directions at b = 800 s/mm2, along with 10 images collected with no diffusion weighting evenly distributed throughout the sequence. Other parameters included TR/TE=9,000/84 ms, 72 oblique axial slices, 128 × 128 matrix, 256 mm FOV, 2.0 mm thickness. A field map of the DTI space was collected immediately following the DTI sequence.
We used the FMRIB software library [FSL; Smith et al., 2004; Woolrich et al., 2009] tools to coregister the diffusion‐weighted images to remove small movements that may have occurred during the sequence, remove eddy currents, and correct field inhomogeneity artifacts using the field map. Three scalar measures (FA, MD, and GFA) of microstructural diffusion properties relevant to white matter integrity were computed at each brain voxel using the FSL Diffusion Toolbox [Behrens et al., 2003] to compute FA and MD and custom Matlab (The Mathworks, Natick, MA) scripts to compute GFA based on the algorithm described by Assemlal et al. [2007]. The FA maps of all participants were nonlinearly aligned to a standard template (International Consortium for Brain Mapping) using FSL's Nonlinear Image Registration Tool and averaged. To further reduce anatomic variability, the aligned FA maps were subsequently nonlinearly registered to the global mean image, and the two resultant warp fields were concatenated and applied to the native FA, MD, and GFA images.
To limit analyses to probable white matter and reduce the influence of gray matter and partial volume voxels, a white matter mask was created as the set of voxels in which FA>0.20 across all individuals and FA>0.25 in at least 90% of individuals (Fig. 3). All individuals were included in the creation of the mask to ensure consistency in its definition across groups.
Figure 3.
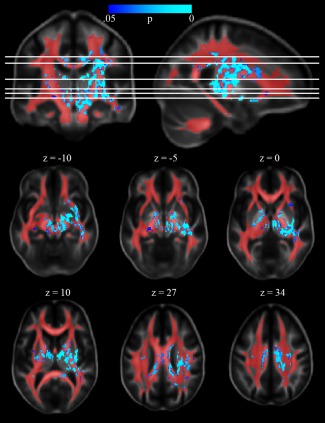
Voxelwise effect of PTSD on GFA. Voxels at which PTSD was associated with higher GFA are shown in blue‐cyan. The white matter mask used to restrict analyses is shown in red. The right side of the image corresponds to the right side of the brain. GFA, generalized fractional anisotropy; PTSD, post‐traumatic stress disorder.
To test hypotheses about integrity within specific tracts, regions of interest were created by thresholding a probabilistic atlas of 20 major white matter tracts in standard space [Mori et al., 2005]. Each ROI was defined as the set of voxels that contained the corresponding tract with at least 25% probability; voxels containing more than one tract were assigned to the tract with the highest probability. The white matter mask was applied to all ROIs to further limit the influence of nonwhite matter voxels. These ROIs were selected because they encompass major subcortical white matter tracts and for comparison to our earlier report [Davenport et al., 2012].
Statistical Analyses
Demographic and clinical characteristics
Gender, history of civilian mTBI, rates of Current PTSD, lifetime diagnoses of Alcohol Dependence and Major Depressive Disorder, and number of deployments were compared across groups using chi‐squared statistics. Age, total current PCS, and total duration of deployments were compared across groups with one‐way ANOVAs. Information about deployments was based on a combination of self‐report and available medical records; deployment durations that could not be determined to within ±3 months (e.g., if only the year of an event was provided) were excluded from the corresponding analyses.
Average white matter integrity within regions of interest
To test whether individual tracts demonstrated group differences in white matter integrity, each integrity measure (i.e., FA, MD, and GFA) was averaged across all voxels included within each ROI. Effects of mTBI, PTSD, and their interaction were tested using 60 (i.e., three measures of integrity in each of 20 regions of interest) two‐way univariate analyses of covariance (ANCOVA), with age entered as the covariate to account for potential age‐related changes [Sullivan and Pfefferbaum, 2003, 2006]. Within each measure of integrity, effects were corrected for multiple comparisons across ROIs using a false discovery rate of q=0.05 [Genovese et al., 2002].
To determine whether mTBI, PTSD, or their interaction had an effect on global integrity, the average of each integrity measure across the entire white matter mask was entered into a two‐way ANCOVA identical to those used on individual ROIs.
Likelihood of regions with abnormal white matter integrity
Given the heterogeneous nature of blast‐related head injury, it is possible that different white matter tracts are affected across individuals, reducing the likelihood of detecting an effect in any single ROI. To determine whether mTBI or PTSD was associated with an increased likelihood of regions with abnormal integrity, without requiring the same regions to be abnormal across individuals, we compared the number of participants with at least one ROI more than two standard deviations from the mean of the Control group. Because the variables of interest were not independent across ROIs, the presence of one region with an abnormally low (or high) value was associated with an increased likelihood that other regions would also have abnormally low (or high) values. Therefore, the dichotomous distinction of having no abnormal regions versus having at least one abnormal region was determined to be more informative than a quantification of the number of abnormal regions.
First, to reduce the likelihood of spurious influences on the occurrence of regions with abnormal integrity, the effect of age was statistically removed from all values using linear regression in each ROI and for each measure of integrity. Next, the mean and standard deviation values across members of the Control group was computed for each integrity measure within each ROI, and these were used to convert all values to z‐scores. The threshold for a region being designated as abnormal was set at two standard deviations (i.e., |z| = 2.0); however, to account for a specific bias recently documented by Watts et al. [2014] that results in underestimating the number of abnormal regions within the group used as the reference (i.e., Controls), a modified threshold of z = ±1.8784 was applied to this group to equalize rates of abnormality due to chance across groups. The rates of having at least one region with an abnormally high value or having at least one region with an abnormally low value were determined for each measure of integrity and compared across the two factors of interest (i.e., PTSD vs. no PTSD and mTBI vs. no mTBI) using chi‐squared tests of independence with one degree of freedom.
Voxelwise comparisons
To determine whether there are groups of white matter voxels, rather than entire tracts, in which integrity is consistently affected by mTBI and/or PTSD across individuals, we ran voxelwise comparisons of each integrity measure using the two‐way ANCOVA described above for ROIs. The FSL program randomise [Anderson and Robinson, 2001] was used to conduct permutation testing at each voxel, and threshold free cluster enhancement [TFCE; Smith and Nichols, 2009] was used to correct results for multiple comparisons.
Our prior investigation of mTBI effects in the absence of PTSD demonstrated that mTBI was associated with a greater number of voxels with abnormally low FA and that the specific voxels affected differed across individuals [Davenport et al., 2012]. To determine whether small regions of voxels were affected by mTBI or PTSD, without requiring spatial consistency across individuals, we used a method based on that first proposed by White et al. [2009] to compare the number of voxels with abnormally high or low FA, GFA, and MD. For each measure of integrity, the effect of age was removed using voxelwise linear regression before creating images of the mean and standard deviation across all individuals in the Control group (Supporting Information Fig. 1). These were used to create z‐score maps for each subject, which were then thresholded at −2.0 and, separately, at +2.0. Again, a threshold of z = ±1.8784 was applied to the Control group to equalize rates due to chance across groups. The number of aberrant (i.e., |z| > 2.0) voxels within the white matter mask was counted for each integrity measure, log‐transformed to normalize the distributions, and effects of PTSD, mTBI, and their interaction were testing using two‐way ANOVAs.
RESULTS
Demographic and Clinical Characteristics
Groups did not differ on history of civilian mTBI and were generally similar in age and gender composition (Table 1). An overall effect of age (F 3,129=3.09, P = 0.03) was due to individuals with only mTBI being somewhat older (mean=36.6 years) than those with mTBI+PTSD (mean=30.2 years), while the PTSD and Control groups were intermediate. Because age was a planned covariate for all analyses, no additional steps were taken to account for potential bias. The Control group included a disproportionately greater number of women relative to the other groups (χ 2=8.24, df=3, P = 0.04); removal of women did not substantially affect the pattern of reported effects.
As expected, total current PCS ratings were lower among Controls than the other groups (F 3,129=17.41, P<0.001), who did not differ from each other. Interestingly, levels were numerically identical in the mTBI and PTSD groups (3.8 out of 8 items) and somewhat higher in the mTBI+PTSD group (5.1 items), supporting the observation that these features are not specific to postconcussive conditions. Both lifetime alcohol dependence and lifetime major depression were more common in the two groups that included individuals with PTSD than in those without PTSD (both χ 2 > 22.6, df=3, P < 0.001). Entering these conditions as covariates in subsequent analyses somewhat reduced significance levels of PTSD‐related effects but did not change the overall pattern, supporting the interpretation that reported effects are due to PTSD rather than comorbid conditions.
The number of deployments was generally low (86% of participants reported less than 3 deployments) and did not differ across groups (χ 2=6.9, df=6, P=0.33), suggesting a similar level of military experience. Among the 122 participants for whom total duration of deployments could be determined within a margin of ±3 months (i.e., excluding 5 Controls, 2 PTSD, 3 mTBI, 1 mTBI+PTSD), the mTBI group had a longer total duration of deployments (28.2 months) than the PTSD and mTBI+PTSD groups (16.7 and 19.8 months; F 3,118=5.20, P=0.002).
Average White Matter Integrity Within Regions of Interest
After correction for multiple comparisons, PTSD was associated with higher GFA in 9 of 20 ROIs (left and right anterior thalamic radiations [L/R ATR], right corticospinal tract [R CST], right inferior fronto‐occipital fasciculus [R IFOF], left and right cingulum, right inferior longitudinal fasciculus (R ILF), right uncinate fasciculus, and right superior longitudinal fasciculus (R SLF); Table 2, Fig. 1), suggesting that PTSD is associated with greater coherence of fibers. PTSD was also associated with lower MD in the R CST (F 1,128=11.97, P=0.001 uncorrected) indicating less overall diffusion and, consistent with effects on GFA, greater white matter integrity. No effects on FA were detected.
Table 2.
Regions of interest in which PTSD was associated with higher GFA
| Region | F | P (uncorrected) |
|---|---|---|
| R ATR | 13.39 | <0.001 |
| R CST | 11.83 | 0.001 |
| L ATR | 8.02 | 0.005 |
| R IFOF | 7.74 | 0.006 |
| R ILF | 6.90 | 0.010 |
| R cingulum | 6.84 | 0.010 |
| R uncinate | 6.40 | 0.013 |
| L cingulum | 6.16 | 0.014 |
| R SLF | 5.94 | 0.016 |
| L CST | 4.74 | 0.031 |
All regions in which univariate ANOVA resulted in P < 0.05 are shown.
Bold face represents effects significant after correction for multiple comparisons.
R, right; L, left; ATR, anterior thalamic radiations; CST, corticospinal tract; IFOF, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; PTSD, post‐traumatic stress disorder; GFA, generalized fractional anisotropy.
Figure 1.

Regions of interest with elevated GFA associated with PTSD. Regions in which PTSD was associated with higher GFA included bilateral anterior thalamic radiations (ATR; red), bilateral cingulum (blue), right corticospinal tract (CST; green), right inferior fronto‐occipital fasciculus (IFOF; yellow), right inferior longitudinal fasciculus (ILF; cyan), right superior longitudinal fasciculus (SLF; light green), and right uncinate fasciculus (magenta).
PTSD was also associated with higher global GFA (F 1,128=8.35, P=0.005), indicating there are possibly additional voxels outside the twenty ROIs investigated (e.g., cortical white matter) in which PTSD is associated with higher GFA. Neither the main effect of mTBI on GFA nor the interaction between PTSD and mTBI was significant, and no differences in global FA or MD were detected in relation to PTSD or mTBI.
Number of Regions With Abnormal White Matter Integrity
As seen in Figure 2 (top), the two groups that included individuals with PTSD (i.e., PTSD and mTBI+PTSD groups) contained disproportionately more individuals with at least one region of abnormally high GFA (χ 2=4.47, P=0.034), consistent with the association between PTSD and higher GFA observed in individual ROIs. Contrary to hypotheses of spatially heterogeneous reductions in white matter integrity, mTBI tended to be associated with a lower likelihood of regions with abnormally low GFA (χ 2=3.12, P=0.078). Mirroring the effects on GFA, PTSD tended to be associated with higher likelihood of regions with abnormally low MD (χ 2=3.19, P=0.074; Fig. 2, middle), and mTBI was associated with lower likelihood of regions with high MD (χ 2=4.21, P=0.040). Given that measures of overall diffusivity (e.g., MD) are typically inversely related to measures of anisotropy (e.g., GFA), the effects seen for these measures are completely complementary. However, it is notable that the PTSD group had the highest proportion of individuals with a region of abnormally high MD and the highest proportion of individuals with a region of low MD, whereas the mTBI group had the lowest proportions of both types. Therefore, while both reported effects appear to be in the same direction (i.e., toward low MD and away from high MD), their manifestations at the level of individual groups appear to be in opposition.
Figure 2.

Number of individuals with at least one region of abnormal integrity. The percentage of individuals in each group observed to have at least one ROI of abnormally high (dark bar) or low (light bar) value of (top) GFA, (middle) MD, or (bottom) FA, are depicted. FA, fractional anisotropy; GFA, generalized fractional anisotropy; MD, mean diffusivity; mTBI, mild traumatic brain injury; PTSD, post‐traumatic stress disorder; ROI, region of interest.
There were no effects of PTSD or mTBI on the percentage of individuals with abnormally high or low FA (all P>0.13; Fig. 2, bottom).
Voxelwise Comparisons
PTSD was associated with higher GFA in one large (31,610 mm3) cluster of voxels encompassing portions of the corpus callosum, cingulum, SLF, CST, internal capsule, ILF, and IFOF, as well as 8 additional clusters (total size 343 mm3) encompassing bilateral external capsule, bilateral middle cerebellar peduncle, right anterior thalamic radiation, and left superior cerebellar peduncle (Fig. 3). Incidentally, effects were observed to be lateralized to the right hemisphere, which contained 77% of significant voxels. Neither the effect of mTBI nor the interaction between mTBI and PTSD was significant. Effects of mTBI and PTSD on FA or MD were not significant.
Consistent with the results of the voxelwise comparison, PTSD was associated with a greater number of voxels with abnormally high GFA (F 1,129=10.35, P=0.002) and tended to be associated with a greater number of voxels with abnormally high FA (F 1,129=3.128, P=0.079). No effects of mTBI were detected.
Potential Effects of Deployment Duration or Postconcussive Symptoms
Because the total duration of deployments and number of postconcussive symptoms differed across groups, it is possible that these factors contribute to the differences in white matter integrity observed. To explore this possibility, we correlated each of these variables with the average FA, GFA, and MD in all ROIs, including the global mask, and with the number of voxels with abnormally high or abnormally low FA, GFA, or MD. Each correlation was conducted across all groups at alpha of 0.05 and within each group at alpha of 0.0125 (i.e., 0.05 divided by 4 groups), though these alpha levels may still be somewhat liberal.
Total duration of deployments did not correlate with any measure of white matter integrity, indicating that exposure to military situations alone does not have a direct impact on brain connectivity. Across all groups, number of PCS negatively correlated with FA (i.e., higher PCS associated with lower FA) in the forceps major (r=−0.17, P=0.045), left IFOF (r=−0.18, P=0.043), and left ILF (r=−0.20, P=0.02). This negative association between PCS and FA was strongest in the PTSD group, in which the left ILF demonstrated a moderately strong correlation (r=−0.43, P=0.02) and 7 additional ROIs (including the global mask) demonstrated effects with P<0.05 (r<−0.36); however, these effects did not survive correction for multiple comparisons using alpha of 0.0125. Finally, PCS was negatively correlated with MD in the right CST (r=−0.17, P=0.049) when looking across the entire sample. The addition of PCS as a covariate to analyses generally strengthened existing effects but did not alter the overall pattern of results.
DISCUSSION
We used conventional and novel diffusion imaging measures to assess long‐term effects of blast‐related mTBI and postdeployment PTSD on white matter integrity in a sample of recent American military service members. At each level of analysis, PTSD was associated with high GFA, putatively representing high integrity of white matter fibers. The association between PTSD and high white matter integrity was also demonstrated by observations of low MD and high FA in a subset of analyses, though the tensor‐based measures (MD and FA) were substantially less sensitive to effects of PTSD than GFA. The overall pattern of effects suggests that PTSD is associated with high white matter integrity in a consistent set of regions across individuals, and that this effect is most apparent when multiple fiber orientations are considered through the use of measures based on higher order models (i.e., GFA). The only significant effect of mTBI was a lower likelihood of regions with high MD, which was mirrored by the trend toward a lower likelihood of regions with low GFA and is contrary to hypotheses of diffuse disruptions to white matter integrity associated with mTBI.
In the context of current neurobiological models of PTSD emphasizing frontotemporal connectivity, especially among the hippocampus, prefrontal cortex, anterior cingulate, and amygdala [Simmons and Matthews, 2012], it is not surprising that effects of PTSD on measures of white matter integrity would include frontal white matter (i.e., bilateral anterior thalamic radiations and cingulum) or frontotemporal tracts (i.e., R IFOF, ILF, and uncinate fasciculus). Effects of PTSD on GFA and MD in the R CST, and on GFA in the right SLF, would not be expected given the simple frontotemporal model but were among the areas in which Schuff et al. [2011] reported effects of PTSD on FA in veterans. Moreover, the observation that both the ROI and voxelwise effects of PTSD on GFA were predominantly lateralized to the right hemisphere is consistent with a report by Engdahl et al. [2010] in which right‐sided MEG synchrony abnormalities associated with PTSD were proposed to reflect a propensity for re‐experiencing past experiences. This hypothesis, based on evidence from electrical stimulation and temporal lobe epilepsy studies [e.g., Fried, 1997], may have broad implications for the development and maintenance of PTSD symptoms and warrants direct testing in the future.
While the locations of effects are consistent with existing knowledge, the observation of PTSD associated with greater directional restriction of diffusion (i.e., higher GFA and lower MD) is inconsistent with the majority of DTI studies of PTSD reporting lower FA [Fani et al., 2012; Kim et al., 2005, 2006]; however, higher FA associated with PTSD has been reported [Abe et al., 2006], and OCD has also been found to be associated with higher FA in the internal capsule and lower MD in the cingulum [Lochner et al., 2012]. Furthermore, two recent reports of a longitudinal study by Sekiguchi and colleagues [Sekiguchi, Kotozaki, et al., 2014; Sekiguchi, Sugiura, et al., 2014] found that higher state anxiety or CAPS scores several months following a substantial earthquake were correlated with greater increases in FA, relative to levels prior to the earthquake, in cingulum and uncinate regions. The authors propose that residual anxiety following the stressful event may cause overuse of the frontotemporal pathways underlying anxiety regulation, which results in increased integrity of those fibers. Alternatively, in light of recent reports of positive correlations between trait anxiety and FA in the uncinate fasciculus [Clewett et al., 2014; Modi et al., 2013; Montag et al., 2012] and several additional areas when looking exclusively at men [Montag et al., 2012], it is also possible that these regions of abnormally high GFA represent vulnerability factors present before the trauma or development of symptoms. Additional longitudinal research and consideration of comparison groups may help to further clarify the significance of elevated diffusion MRI measures of white matter integrity.
The observation of strong, consistent effects of PTSD on GFA but very limited effects on FA suggests that diffusion abnormalities occur in regions of complex white matter configurations (e.g., crossing/diverging fibers) that cannot be modeled by a single tensor. Unlike the tensor model on which FA is based, the ODF model is better able to model complex architecture and, consequently, differences in architecture across groups [Fritzsche et al., 2010]. Given that GFA and FA were highly correlated (median r=0.86 across the 20 ROIs), the additional information accounted for by GFA relative to FA (i.e., additional orientations) is apparently critical to its sensitivity to effects of PTSD. Consistent with this interpretation, Figure 3 demonstrates that most voxels in which PTSD was associated with greater GFA are located outside of major single‐orientation tracts. However, comparison of Figures 1 and 3 demonstrates that many significant voxels are located within the 9 regions of interest in which PTSD was associated with higher GFA. While this correspondence between analyses at the regional and voxel levels is expected, it raises the question of why these ROIs, which presumably reflect coherent tracts, show effects of GFA but not FA. One likely explanation is that while these ROIs correspond well to known single‐orientation tracts, they also capture voxels that include the interstitial fibers that diverge from the main tracts and, in some cases, voxels that include fibers from multiple tracts (e.g., voxels at the borders of adjacent tracts in Fig. 1). Thus, the increased sensitivity of GFA relative to FA may indicate that PTSD is associated with abnormalities in axon fibers diverging from the core of the tract more so than the integrity of those core fibers.
Effects of mTBI on measures of white matter integrity were limited to a lower likelihood of regions with high MD and a trend toward a lower likelihood of regions with low GFA, both of which had small effect sizes (ϕ=0.178 and 0.155, respectively). These results are directly contrary to the hypotheses regarding mTBI in two ways. First, the detection of effects at the ROI level but not the voxel level contradicts the hypothesis that the effects of mTBI are too diffuse to alter the overall integrity of a tract. Indeed, only one study [MacDonald et al., 2011] has detected differences in DTI‐based measures of white matter integrity between mTBI participants and controls at the level of a priori regions of interest. However, given the rarity with which MD and GFA have been used in previous studies of mTBI, it is possible that the effects observed here would have been present in previous studies. Second, the observation of mTBI being associated with a lower likelihood of regions with abnormally high MD or low GFA is contrary to the hypothesis that mTBI is associated with diffuse damage to axons and the surrounding myelin, which would increase overall diffusivity (MD) and lower the anisotropy (GFA). While it is possible that the inclusion of all participants in the definition of the white matter mask eliminated some voxels in which mTBI was associated with low FA (i.e., below the masking threshold), it also ensured that voxels contaminated by other tissues (e.g., gray matter, corticospinal fluid) were excluded consistently across groups. Additional analyses demonstrated that the use of a mask based only on Controls did not alter any of the significant results reported, suggesting that any bias introduced by inclusion of all individuals was minimal. An alternative interpretation is that physiological processes, such as inflammation due to psychosocial stress [Christensen et al., 2004], limit the diffusivity, though given that effects were small and limited to a single level of analysis, some caution in interpretation is warranted.
Strengths and Limitations
There are several unique strengths of this study. The control group consisted of deployed military service members and thus deployment stress and characteristics of military samples are matched across groups. Furthermore, because the majority of participants were not seeking treatment, the results are likely generalizable to broad military populations rather than only those seen clinically. Participants were generally free of predeployment Axis I psychopathology, so psychopathology evident in groups more likely developed in association with deployment. Nonmilitary TBI was carefully documented and rated for participants, so the possible effects of previous mTBI from contact sports or other civilian activities could be assessed. Finally, all military mTBI involved an explosive blast so the contrasts test whether the presence of blast mTBI is particularly damaging to cerebral white matter.
A notable methodological limitation is the relatively low strength of the diffusion gradients (b=800 mm2/s). While this value is similar to those commonly used in clinical DTI studies, it is not ideal for ODF modeling. Consequently, the ability to resolve multiple fiber orientations may have been limited, and GFA may have been underestimated. Such a limitation would be expected to be consistent across individuals and, if anything, bias against detecting group differences, so it is remarkable that this measure was the one most significantly associated with PTSD in this sample. Future studies would benefit from using a higher b‐value [e.g., 1,500–3,000 mm2/s; Jones et al., 2013] to better model the ODF and, thus, more accurately determine GFA. Better estimation of the ODF may also allow more detailed investigation of the type of alteration to diffusion properties involved in PTSD.
Finally, a limitation inherent in the majority of MRI studies of mTBI and PTSD in veterans to date is the lack of predeployment imaging data. Without longitudinal imaging data, it is impossible to determine whether reported effects represent vulnerability factors (i.e., were present prior to combat exposure), sequelae of psychological or physiological trauma (e.g., PTSD, mTBI), or are related to other deployment factors (e.g., chronic stress, poor nutrition, and dehydration).
CONCLUSION
In summary, we have investigated the possibility that PTSD and mTBI exert independent effects on white matter integrity in a large sample of military veterans. We have demonstrated that PTSD was associated with higher GFA in a variety of tracts, especially on the right side, and that mTBI had little or no effect on white matter integrity. Because these two conditions often cooccur in military veterans, this report addresses a critical limitation of studies that investigate PTSD and mTBI in isolation. Moreover, this is the first report to apply GFA to the investigation of these conditions.
Supporting information
Supplementary Information
ACKNOWLEDGMENTS
The authors would like to thank Nikki Fraser, Mallory Skorheim, and Joel Nelson for assistance in MRI data acquisition; Ryan Muetzel and Bryon Mueller for support in neuroimaging data processing and analysis; Christophe Lenglet and Iman Aganj for providing guidance regarding ODF modeling; Michael Armstrong, Amanda Ferrier‐Auerbach, Molly Charlesworth, and Emily Johnson for clinical assessment of participants; Kathleen McGuire, Nathaniel Nelson, Gregory Lamberty, Bridget Doan, and Daniel Goldman for consensus review of TBI and clinical diagnoses.
Conflict of interest: Nothing to report
Footnotes
All 19 mTBI participants and 28 of 38 Control participants (see group definitions below), as well as 5 of 31 PTSD participants and 3 of 45 mTBI+PTSD participants, were included in a prior publication.
One 27 year old male participant who met criteria for both mTBI and PTSD reported a TBI event involving LOC between 30 minutes and 2 hours. Removal of this participant did not change any reported effect.
REFERENCES
- Abe O, Yamasue H, Kasai K, Yamada H, Aoki S, Iwanami A, Ohtani T, Masutani Y, Kato N, Ohtomo K (2006): Voxel‐based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res 146:231–242. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson M, Robinson J (2001): Permutation tests for linear models. Aust N Z J Stat 43:75–88. [Google Scholar]
- Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H (2012): Diffusion tensor imaging studies of mild traumatic brain injury: A meta‐analysis. J Neurol Neurosurg Psychiatry 83:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assemlal H‐E, Tschumperle D, Brun L (2007): Fiber tracking on HARDI data using robust ODF fields, Vol. 3. In: 2007 IEEE International Conference on Image Processing, San Antonio, TX. IEEE. pp III–133–III–136).
- Basser PJ, Pierpaoli C (1996): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 111:209–219. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8661285. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D (1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103:247–254. Available at: http://www.ncbi.nlm.nih.gov/pubmed/8019776. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Donnelly K, Peterson DR, Warner GC, Zhu T, Zhong J (2012): The relation between posttraumatic stress disorder and mild traumatic brain injury acquired during Operations Enduring Freedom and Iraqi Freedom. J Head Trauma Rehabil 28:1–12. doi: 10.1097/HTR.0b013e318256d3d3. [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002): The basis of anisotropic water diffusion in the nervous system—A technical review. NMR Biomed 15:435–455. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen‐berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM (2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50:1077–1088. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM (1995): The development of a Clinician‐Administered PTSD Scale. J Trauma Stress 8:75–90. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7712061. [DOI] [PubMed] [Google Scholar]
- Christensen J, Holcomb J, Garver DL (2004): State‐related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry Res 130:71–78. [DOI] [PubMed] [Google Scholar]
- Clewett D, Bachman S, Mather M (2014): Age‐related reduced prefrontal‐amygdala structural connectivity is associated with lower trait anxiety. Neuropsychology 28:631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport ND, Lim KO, Armstrong MT, Sponheim SR (2012): Diffuse and spatially variable white matter disruptions are associated with blast‐related mild traumatic brain injury. NeuroImage 59:2017–2024. [DOI] [PubMed] [Google Scholar]
- Engdahl B, Leuthold AC, Tan H‐RM, Lewis SM, Winskowski AM, Dikel TN, Georgopoulos AP (2010): Post‐traumatic stress disorder: a right temporal lobe syndrome? J Neural Eng 7:066005. [DOI] [PubMed] [Google Scholar]
- Fani N, King TZ, Jovanovic T, Glover EM, Bradley B, Choi K, Ely T, Gutman DA, Ressler KJ (2012): White matter integrity in highly traumatized adults with and without post‐traumatic stress disorder. Neuropsychopharmacology 37:2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (2002): Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Patient Edition. (SCID‐I/P). New York: Biometrics Research, New York State Psychiatric Institute.
- Fried I (1997): Auras and experiential responses arising in the temporal lobe. J Neuropsychiatry Clin Neurosci 9:420–428. Available at: http://journals.psychiatryonline.org/data/Journals/NP/3847/420.pdf. [DOI] [PubMed] [Google Scholar]
- Fritzsche KH, Laun FB, Meinzer H‐P, Stieltjes B (2010): Opportunities and pitfalls in the quantification of fiber integrity: What can we gain from Q‐ball imaging? NeuroImage 51:242–251. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA (2008): Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med 358:453–463. [DOI] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML (2013): A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol 34:2064–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R (2013): White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. NeuroImage 73:239–254. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Acion L, White T, Tordesillas‐Gutierrez D, Pierson R, Crespo‐Facorro B, Magnotta VA (2012): White matter abnormalities in veterans with mild traumatic brain injury. Am J Psychiatry 169:1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Lyoo IK, Kim SJ, Sim M, Kim N, Choi N, Jeong D‐U, Covell J, Renshaw PF (2005): Disrupted white matter tract integrity of anterior cingulate in trauma survivors. NeuroReport 16:1049–1053. Available at: http://journals.lww.com/neuroreport/Abstract/2005/07130/Disrupted_white_matter_tract_integrity_of_anterior.4.aspx. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Jeong D‐U, Sim ME, Bae SC, Chung A, Kim MJ, Chang KH, Ryu J, Renshaw PF, Lyoo IK (2006): Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology 54:120–125. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde E, Troyanskaya M, Petersen NJ, Scheibel R, Newsome M, Radaideh M, Wu T, Yallampalli R, Chu Z, Li X (2010): Diffusion tensor imaging of mild to moderate blast‐related traumatic brain injury and its sequelae. J Neurotrauma 27:683–694. [DOI] [PubMed] [Google Scholar]
- Lochner C, Fouché J‐P, du Plessis S, Spottiswoode B, Seedat S, Fineberg N, Chamberlain SR, Stein DJ (2012): Evidence for fractional anisotropy and mean diffusivity white matter abnormalities in the internal capsule and cingulum in patients with obsessive‐compulsive disorder. J Psychiatry Neurosci 37:193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL (2011): Detection of blast‐related traumatic brain injury in U.S. military personnel. N Engl J Med 364:2091–2100. Available at: http://www.nejm.org/doi/full/10.1056/nejmoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Kauder DR, Gennarelli TA, Alburger GW (1994): Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. Am J Neuroradiol 15:1583–1589. [PMC free article] [PubMed] [Google Scholar]
- Modi S, Trivedi R, Singh K, Kumar P, Rathore RKS, Tripathi RP, Khushu S (2013): Individual differences in trait anxiety are associated with white matter tract integrity in fornix and uncinate fasciculus: Preliminary evidence from a DTI based tractography study. Behav Brain Res 238:188–192. [DOI] [PubMed] [Google Scholar]
- Montag C, Reuter M, Weber B, Markett S, Schoene‐Bake J‐C (2012): Individual differences in trait anxiety are associated with white matter tract integrity in the left temporal lobe in healthy males but not females. Neuroscience 217:77–83. [DOI] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Selgrade ES, Massoglia D, Liu C, Weiner J, Marx CE, Cernak I, McCarthy G (2013): Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum Brain Mapp 34:2986–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Zijl PCMV, Nagae‐Poetscher LM (2005): MRI Atlas of Human White Matter. Amsterdam, The Netherlands: Elsevier Science. [Google Scholar]
- Nelson NW, Hoelzle JB, McGuire KA, Ferrier‐Auerbach AG, Charlesworth MJ, Sponheim SR (2011): Neuropsychological evaluation of blast‐related concussion: Illustrating the challenges and complexities through OEF/OIF case studies. Brain Injury 25:511–525. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P (2010): Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil 25:241–255. [DOI] [PubMed] [Google Scholar]
- Polusny MA, Erbes CR, Murdoch M, Arbisi PA, Thuras P, Rath MB (2011): Prospective risk factors for new‐onset post‐traumatic stress disorder in National Guard soldiers deployed to Iraq. Psychol Med 41:687–698. [DOI] [PubMed] [Google Scholar]
- Schneiderman AI, Braver ER, Kang HK (2008): Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in Iraq and Afghanistan: persistent postconcussive symptoms and posttraumatic stress disorder. Am J Epidemiol 167:1446–1452. [DOI] [PubMed] [Google Scholar]
- Schuff N, Zhang Y, Zhan W, Lenoci M, Ching C, Boreta L, Mueller SG, Wang Z, Marmar CR, Weiner MW, Neylan TC (2011): Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: An MRI study. NeuroImage 54:S62–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi A, Kotozaki Y, Sugiura M, Nouchi R, Takeuchi H, Hanawa S, Nakagawa S, Miyauchi CM, Araki T, Sakuma A, Taki Y, Kawashima R (2014): Long‐term effects of postearthquake distress on brain microstructural changes. BioMed Res Int 2014:180468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi A, Sugiura M, Taki Y, Kotozaki Y, Nouchi R, Takeuchi H, Araki T, Hanawa S, Nakagawa S, Miyauchi CM, Sakuma A, Kawashima R (2014): White matter microstructural changes as vulnerability factors and acquired signs of post‐earthquake distress. PLoS One 9:e83967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Matthews SC (2012): Neural circuitry of PTSD with or without mild traumatic brain injury: A meta‐analysis. Neuropharmacology 62:598–606. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE (2009): Threshold‐free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44:83–98. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Mcguire KA, Suk S, Davenport ND, Aviyente S, Bernat EM, Lim KO (2011): Evidence of disrupted functional connectivity in the brain after combat‐related blast injury. Neuroimage 54:S21–S29. [DOI] [PubMed] [Google Scholar]
- Stein M, McAllister T (2009): Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am J Psychiatry 166:768–776. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A (2003): Diffusion tensor imaging in normal aging and neuropsychiatric disorders. Eur J Radiol 45:244–255. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12595109. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A (2006): Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30:749–761. [DOI] [PubMed] [Google Scholar]
- Taber KH, Warden DL, Hurley RA (2006): Blast‐related traumatic brain injury: What is known? J Neuropsychiatry Clin Neurosci 18:141–145. [DOI] [PubMed] [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Seally K, Bretthauer R, Warden D (2009): Traumatic brain injury screening: Preliminary findings in a US army Brigade Combat Team. J Head Trauma Rehabil 24:14–23. [DOI] [PubMed] [Google Scholar]
- Tuch DS (2004): Q‐ball imaging. Magn Reson Med 52:1358–1372. [DOI] [PubMed] [Google Scholar]
- Warden D (2006): Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil 21:398–402. [DOI] [PubMed] [Google Scholar]
- Watts R, Thomas A, Filippi CG, Nickerson JP, Freeman K (2014): Potholes and Molehills: Bias in the diagnostic performance of diffusion‐tensor imaging in concussion. Radiology 272:217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T, Schmidt M, Karatekin C (2009): White matter “potholes” in early‐onset schizophrenia: A new approach to evaluate white matter microstructure using diffusion tensor imaging. Psychiatry Res: Neuroimaging 174:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk JE, Thomas JL, McGurk DM, Riviere LA, Castro CA, Hoge CW (2010): Mild traumatic brain injury (concussion) during combat: lack of association of blast mechanism with persistent postconcussive symptoms. J Head Trauma Rehabil 25:9–14. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM (2009): Bayesian analysis of neuroimaging data in FSL. Neuroimage 45(Suppl 1):S173–S186. [DOI] [PubMed] [Google Scholar]
- Yeh P‐H, Wang B, Oakes TR, French LM, Pan H, Graner J, Liu W, Riedy G (2014): Postconcussional disorder and PTSD symptoms of military‐related traumatic brain injury associated with compromised neurocircuitry. Hum Brain Mapp 35:2652–2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


