Abstract
Past neuroimaging studies have focused on identifying specialized functional brain systems for processing different components of reading, such as orthography, phonology, and semantics. More recently, a few experiments have been performed to look into the integration and interaction of distributed neural systems for visual word recognition, suggesting that lexical processing in alphabetic languages involves both ventral and dorsal neural pathways originating from the visual cortex. In the present functional magnetic resonance imaging study, we tested the multiple pathways model with Chinese character stimuli and examined how the neural systems interacted in reading Chinese. Using dynamic causal modeling, we demonstrated that visual word recognition in Chinese engages the ventral pathway from the visual cortex to the left ventral occipitotemporal cortex, but not the dorsal pathway from the visual cortex to the left parietal region. The ventral pathway, however, is linked to the superior parietal lobule and the left middle frontal gyrus (MFG) so that a dynamic neural network is formed, with information flowing from the visual cortex to the left ventral occipitotemporal cortex to the parietal lobule and then to the left MFG. The findings suggest that cortical dynamics is constrained by the differences in how visual orthographic symbols in writing systems are linked to spoken language. Hum Brain Mapp 36:2580–2591, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: connectivity, functional magnetic resonance imaging, reading, Chinese reading, dynamic causal modeling, ventral pathway, dorsal pathway, writing systems
INTRODUCTION
Reading involves the associations of written symbols (i.e., orthography) with sounds (i.e., phonology) and meaningful concepts. Neurobiological models of reading have identified specialized brain regions for the processing of these linguistic components. The left occipitotemporal cortex (vOT) is known to be responsible for extracting visual‐orthographic information of printed words [Ben‐Shachar et al., 2007; Cohen and Dehaene, 2004; Paulesu et al., 2000; Price, 2012; Schlaggar and McCandliss, 2007; Shaywitz et al., 2008; Wandell et al., 2012], while the left temporoparietal regions and the dorsal portion of left inferior frontal gyrus (IFG) are proposed to be involved in letter‐sound transformation and phonological processing [Booth et al., 2002; Cohen et al., 2008; Eden et al., 2004; Gabrieli, 2009; He et al., 2013; Hoeft et al., 2006; Paulesu et al., 2000; Poldrack et al., 1999]. Processing of lexicosemantics encompasses a more widespread set of cortical sites, such as the ventral portion of the left IFG, the left anterior temporal cortex, and the posterior superior temporal region [Bookheimer, 2002; Devlin et al., 2003; Gabrieli et al., 1998; Katzev et al., 2013; Petersen et al., 1988; Poldrack et al., 1999; Richardson et al., 2011].
More recently, great emphasis has been placed on integration and interaction of distributed neural systems for complex brain functions and there has been a rapid growth of studies investigating the neural pathways for visual word recognition [Friston, 2011; Horwitz et al., 1998; Park and Friston, 2013; Price, 2012; Schlaggar and Church, 2009]. It is suggested that visual word processing in alphabetic languages involves both ventral and dorsal neural pathways originating from visual cortex [Booth et al., 2008; Carreiras et al., 2014; Levy et al., 2009; Price, 2012; Richardson et al., 2011; Seghier et al., 2012]. Using dynamic causal modeling (DCM), Richardson et al. [2011] found that during silent reading of English words and sentences, there were multiple routes from the left inferior occipital cortex (iO) to the left anterior superior temporal sulcus (aSTS), one via vOT and the other via the left posterior superior temporal sulcus (pSTS). Hypothetically, the iO→pSTS and vOT→pSTS connections are involved in linking orthography and phonology, the pSTS→aSTS connection is involved in linking phonology and semantics, and the iO→vOT→aSTS connection is involved in linking orthography and semantics. In a functional magnetic resonance imaging (fMRI) study, Levy et al. [2009] used a passive view paradigm with French words and conducted structural equation modeling analyses among four left‐hemisphere regions, including middle occipital gyrus, posterior vOT, posterior parietal cortex, and IFG. They found that reading real words involved both a ventral pathway from the middle occipital gyrus to the posterior vOT and a dorsal pathway to the posterior parietal cortex. Using DCM and an English spelling task, Booth et al. [2008] showed multiple connections from visual cortex to higher‐order language areas, including vOT, superior temporal gyrus, inferior parietal lobule, and IFG in the left hemisphere.
Accumulating evidence has shown that reading of different orthographies places different weights on the ventral and dorsal systems [Duncan et al., 2013; Goswami, 2006; Paulesu et al., 2000; Price, 2012]. For example, in a study using positron emission tomography, Paulesu et al. [2000] compared brain activation in Italian and English readers during explicit (reading aloud words and nonwords) and implicit (feature detection task) reading tasks. They found that reading shallow orthographies such as Italian engaged the dorsal system (i.e., the left temporoparietal regions) to a greater extent, reflecting greater reliance on phonological analysis, while reading deep orthographies such as English recruited the ventral system (i.e., the left vOT) to a greater extent, which might reflect more reliance on retrieving phonology from the orthography of the whole word. In a recent fMRI study, Duncan et al. [2013] compared effective connectivity between lexical decision of Japanese Kanji and syllabographic Hiragana. They found that Kanji, a deeper orthography than Hiragana, increased bidirectional connections between primary visual cortex and left vOT, whereas reading Hiragana increased connection from left IFG to left inferior parietal cortex in the dorsal pathway, which was assumed to reflect a top‐down modulation to guide processes of phonological assembly. Together, these findings suggest that reading deeper orthographies relies more on the ventral pathway, with less involvement of the dorsal pathway.
Written Chinese is among extremely deep orthographies, and thus, it represents an important case for any attempts to test the multiple pathways model for visual word recognition. The Chinese logographic system maps graphic forms (characters) onto morphemes (meanings), rather than phonemes. Chinese characters are defined at the monosyllabic level, with no parts of a character corresponding to phonemes. Hence, there is no such rule as grapheme‐to‐phoneme correspondence rules in Chinese. Visually, Chinese characters are formed with intricate strokes filled in square configurations and the visual complexity increases as the stroke number increases, which is in sharp contrast to the linear structure of alphabetic words [Perfetti et al., 2005; Siok et al., 2004].
Neuroimaging studies have highlighted both similarities and differences in neural systems for reading Chinese and alphabetic languages [Cao et al., 2009; Chen et al., 2008; Ge et al., 2015; Hu et al., 2010; Liu et al., 2015; Perfetti and Tan, 2013; Schlaggar and McCandliss, 2007; Tan et al., 2003, 2005; Wu et al., 2012; Xue et al., 2006; Zhao et al., 2014; Zhang et al., 2014; Zhu et al., 2014]. For example, in a meta‐analysis study, Tan et al. [2005] showed that phonological processing of Chinese and alphabetic languages shared the left ventral prefrontal system and the left vOT, whereas the left temporoparietal cortex uniquely contributed to the grapheme–phoneme conversions in alphabetic languages and the left middle frontal gyrus (MFG) is critically involved in Chinese reading. The left MFG is proposed to serve a crucial role in syllable‐based phonological processing and the coordination of orthographic, phonological, and semantic information during Chinese reading [Kuo et al., 2001, 2003; Li et al., 2010; Perfetti et al., 2006; Siok et al., 2004, 2008; Tan et al., 2003, 2005; Wu et al., 2012; Zhu et al., 2014].
However, it remains largely unknown as to how the neural systems dynamically interact with one another to support Chinese reading, and whether the ventral and dorsal visual pathways are equally involved in printed word recognition in Chinese and alphabetic languages. In this fMRI study, we used DCM and family‐level Bayesian model selection [BMS; Friston et al., 2003; Penny et al., 2010] to examine this question. Bayesian model averaging (BMA) was applied over the wining families of models to examine the effective connectivity among the key regions supporting Chinese reading. We used a phonological task and focused on the process of orthography–phonology mapping by varying the visual‐orthographic complexity of Chinese characters, indexed by the number of strokes. There were behavioral studies showing an effect of stroke number in Chinese character recognition [Coney, 1998; Leong et al., 1987; Hsiao and Cheng, 2013; Yu and Cao, 1992]. We expected that in a phonology‐related task, the “load” of mapping from orthography to phonology becomes heavier as the visual‐orthographic complexity of Chinese characters increases, and this change should be reflected in the change of information flow from visual cortex to other brain regions for higher‐order processing.
MATERIALS AND METHODS
Subjects
Eighteen healthy subjects (9 males) participated in our experiment. All were native Mandarin Chinese speakers and primarily familiar with simplified characters. One subject failed to complete the experiment and the data from this subject were excluded for further analyses. The remaining 17 subjects (9 males), ranging in age from 17 to 23 years (mean age = 20.5 years, SD = 1.28), were strongly right‐handed, as assessed by the handedness inventory [Snyder and Harris, 1993], and had normal or corrected‐to‐normal vision. They were physically healthy and free of neurological disease, head injury and psychiatric disorder. Subjects were paid for their participation and gave informed consent prior to scanning.
Design and Materials
The experimental task in the scanner was a phonology‐based tone judgment task, in which the subjects were asked to decide whether or not a visually exposed character was pronounced as the 4th tone (i.e., the falling tone). We used a tone judgment task because tonal information is part of a phonological code [Spinks et al., 2000], and no component of the characters corresponds to a lexical tone. An arrow direction judgment task was used as a control task, in which subjects judged whether the direction of an arrow was upward or downward.
A blocked design was used, with eight blocks of tone judgment (four blocks with characters of low number of strokes and four blocks with characters of high number of strokes) alternated with eight blocks of arrow judgment. Condition order was counterbalanced. Each experimental block consisted of a 2‐second task instruction and 12 trials, and each arrow‐direction judgment block consisted of a 2‐second task instruction and six trials. On each trial, a black stimulus (character or arrow) was displayed on the center of a white background for 1,000 ms, followed by a 1,000‐ms blank interval. For the tone judgment task, subjects were instructed to press the first button with the right‐hand index finger for positive response and press the second button with the right‐hand thumb for negative response. For the arrow judgment task, they pressed the first button with the index finger for an upward arrow and pressed the second button with the thumb for a downward arrow. All subjects had some practice before scanning. During practice session, the subjects performed the tasks for at least 10 trials to ensure that they understood the task and performed well enough (no less than 90% accuracy). Chinese characters used in the practice session were different from those in the in‐scanner task. The subjects were instructed to perform as quickly and accurately as possible.
Chinese character as a salient visual unit is formed with intricate strokes filled in a square configuration. A stroke, either straight or curved, like “ ” and “
” and “ ,” is the smallest structural unit in the character A total of 96 characters were used in this study, including 48 characters of low number of strokes (8–10 strokes, mean = 9.4, SD = 0.74; e.g.,
,” is the smallest structural unit in the character A total of 96 characters were used in this study, including 48 characters of low number of strokes (8–10 strokes, mean = 9.4, SD = 0.74; e.g., 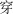 , /chuan1/, meaning to pass through/to wear/to thread;
, /chuan1/, meaning to pass through/to wear/to thread; 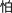 , /pa4/, meaning to fear), and 48 characters of high number of strokes (12–17 strokes, mean = 14.2, SD = 1.27; e.g.,
, /pa4/, meaning to fear), and 48 characters of high number of strokes (12–17 strokes, mean = 14.2, SD = 1.27; e.g.,  , /xu1/, meaning to require/necessity/need;
, /xu1/, meaning to require/necessity/need; 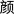 , /yan2/, meaning color/countenance). The characters were either in top‐down or left‐right structure and the structures of characters were matched across conditions. All of the selected characters consisted of two components.
, /yan2/, meaning color/countenance). The characters were either in top‐down or left‐right structure and the structures of characters were matched across conditions. All of the selected characters consisted of two components.
MRI Acquisition
Experiment was performed on a 3 T Siemens MRI scanner, using a T2*‐weighted gradient‐echo echo plannar imaging (EPI) sequence (echo time [TE] = 30 ms, repetition time [TR] = 2 s, flip angle = 90°, field of view = 21 cm, slice thickness = 4 mm, and the image matrix = 64 × 64). Thirty axial slices were acquired to cover the whole brain. Visual stimuli were presented through a projector onto a translucent screen and subjects viewed the screen through a mirror attached to the head coil.
fMRI Data Analysis
Statistical Parametric Mapping software package (SPM8; http://www.fil.ion.ucl.ac.uk/spm/) was used for preprocessing and analysis of imaging data. Functional images were first slice timing corrected to correct differences in acquisition time between slices, and then realigned to remove movement artifact. No subject had >2 mm translation or >2° rotation. The images were then spatially normalized to an EPI template based on the the International Consortium for Brain Mapping (ICBM‐152) stereotactic space. Voxels were resampled at a voxel size of 2 × 2 × 2 mm3. An isotropic Gaussican kernel (8 mm full width at half‐maximum) was applied for spatial smoothing. Each time series was high‐pass filtered with a cutoff period set at 128 s to remove low‐frequency drifts. Realignment parameters were included in the first‐level statistical model to regress out movement‐related variance. Contrast images were generated for each subject and were then used to create group contrast images at the second level. Corrections for multiple comparisons across the whole brain were applied (P < 0.05, false discovery rate [FDR] corrected), with an extent threshold of 10 contiguous voxels. Brain regions are estimated from Talairach and Tournoux [1988], after adjustments for differences between MNI and Talariach coordinates with a nonlinear transform.
Effective Connectivity Analyses
Effective connectivity rests on models of the directed causal influence among regions [Friston, 2011]. We used DCM10, as implemented in SPM8, to explore effective connectivity. Different from other approaches, such as Granger causality and structural equation modeling, which operate at the level of the measured signals, DCM combines a neurobiological model of neural dynamics and a biophysical forward model that links neuronal activity to the measured signals [Friston et al., 2003; Stephan and Friston, 2010]. In DCM analyses, BMS tests a set of alternative models (or families of models) and identifies the most likely model given empirical data. The BMS procedure takes into account both model accuracy and model complexity to determine the best model and is not confounded by multiple comparisons [Penny et al., 2004]. Bayesian estimation is used to make inferences about the parameters, which are expressed as the rate or speed of change (in Hz) of activity in one region that is associated with activity in another. Three sets of parameters are estimated, including (1) input parameters that allow influence on specific regions by external stimuli (e.g., sensory stimulation); (2) the intrinsic parameters that describe couplings among regions and can be viewed as the “baseline” connectivity that is present in the system in the absence of external input; and (3) modulatory parameters that capture the changes in the connectivity induced by the experimental manipulations [Friston, 2011; Friston et al., 2003].
Extracting regional time series
Subject‐specific regions of interest (ROIs; 6‐mm sphere) were defined as the local maxima of the contrast of tone judgment (collapsing high‐ and low‐stroke conditions) > arrow judgment, with a liberal threshold of P < 0.05 uncorrected. To ensure that the functional regions were consistent across subjects, ROI selection was guided by group results. We first identified group peak voxel of the four ROIs based on group analyses and then extracted time series from each subject's activation map at the closest maxima within a distance of 8 mm of the group peak voxel [for a similar rationale, see Leff et al., 2008; Seghier et al., 2011]. Because brain activation associated with our task was predominantly left lateralized, only left‐hemisphere regions were included in the effective connectivity analyses. Four left‐hemisphere regions were included, that is, left cuneus (mean coordinates: [−17 −90 5]), left vOT (mean coordinates: [−41 −55 −14]), left superior parietal lobule (SPL, mean coordinates: [−24 −64 44]), and left MFG (mean coordinates: [−47 12 35]). The mean coordinates of the four left‐hemisphere regions are specified as MNI coordinates. The principle eigenvariate was extracted for each ROI and adjusted to the effect of interest.
The DCM model space and family comparisons
The aim of our DCM analyses was to examine whether the ventral (cuneus→vOT), the dorsal pathways (cuneus→SPL), or both pathways were modulated by visual‐orthographic load during orthography–phonology mapping. We performed two‐step analyses. In the first‐step analysis, we constructed models with the four left‐hemispheric regions (i.e., cuneus, vOT, SPL, and MFG) and specified the left cuneus as a region receiving driving input. To keep a reasonable size for the model space and on the basis of previous studies [Duncan et al., 2013], direct connections between cuneus and frontal region were not included. Thus, there were 10 intrinsic connections in the models. The competing models varied as to which connections were modulated by visual‐orthographic load. We compared three families of models, which differed in whether the ventral pathway only, the dorsal pathway only, or both pathways were modulated by experimental manipulation (Fig. 3A). Each family contained 256 models (= 28) and thus resulted in 768 models in total. Family‐level random‐effect BMS was used to compare the three families [Penny et al., 2010]. Exceedance probability (xp), that is, the possibility one family being more likely than the other families to generate the observed data, was used to index the relative superiority of families.
Figure 3.
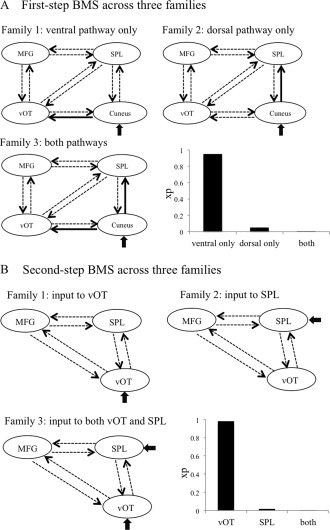
Results from BMS analyses. (A) Family comparison at the first‐step BMS analysis. Solid black arrows indicate the connections used to partition model space into three competitive families that differed in whether the ventral pathway only, the dorsal pathway only, or both pathways were modulated by visual‐orthographic load. The bar graph shows the exceedance probability (xp) for the three families. (B) Family comparison at the second‐step BMS analysis. Three competitive families differed in the location the inputs entered the model, via the vOT only, the SPL only, or both. The bar graph shows the xp for the three families.
In the second‐step connectivity analysis, we included only three left‐hemispheric regions (i.e., vOT, SPL, and MFG) and tested whether the ventral (i.e., vOT) or the dorsal (i.e., SPL) neural system receive driving input. This analysis would help to further elucidate whether the ventral or the dorsal neural system or both of them would be involved in relaying information from early visual area and drive the connectivity within the network. We compared three families of models that differed in where the inputs enter the model, that is, the vOT only, the SPL only, or both vOT and SPL (Fig. 3B). The models contained six intrinsic connections that reciprocally connected the three ROIs. Each model contained one to six modulatory input on the connections, resulting in 63 possible models in each family. Therefore, a total of 189 models were specified for each subject. BMA within the wining family was used to make inferences on the connection parameters within the three‐region models. BMA provides a weighted average of each parameter based on the posterior probability of each model, such that the model with the highest probability makes the greatest contribution to the estimates [Penny et al., 2010]. With the BMA procedure, 10,000 data points are sampled to generate posterior distribution for each parameter. Significance is assessed by the proportion of samples that are different from zero, with threshold set at 0.90 [for a similar procedure, see Lee and Noppeney, 2011; Richardson et al., 2011; Seghier et al., 2011].
RESULTS
Behavior Results
After excluding trials where participants pressed the wrong key, the average reaction time was 844 ms (SD = 116) for the high‐stroke condition, 825 ms (SD = 120) for the low‐stroke condition, and 476 ms (SD = 52) for the control condition. Paired t‐test analysis showed no reliable difference of reaction time for high‐ versus low‐stroke condition (t (16) = 1.97, P > 0.05). Mean accuracy was 0.88 (SD = 0.08) for the high‐stroke condition, 0.95 (SD = 0.06) for the low‐stroke condition, and 1.00 (SD = 0.006) for arrow judgment. Paired t‐tests indicated higher accuracy for the low‐stroke condition than the high‐stroke condition (t (16) =5.26, P < 0.01).
Imaging Results
Brain activations related to phonological conditions contrasted with control condition overlap to a great extent for high‐stroke and low‐stroke conditions, with peak activation in the left inferior and middle frontal gyri, right superior frontal gyrus, cingulate cortex, left posterior parietal cortex, bilateral cuneus, bilateral lingual gyri, left fusiform gyrus and so on (see Fig. 1 and Table 1). Interestingly, the left temporoparietal regions critical for phonological processing of alphabetic words were not seen in our study, consistent with many of previous studies of Chinese reading [Booth et al., 2006; Dong et al., 2005; Siok et al., 2003, 2004, 2008; Tan et al., 2001].
Figure 1.
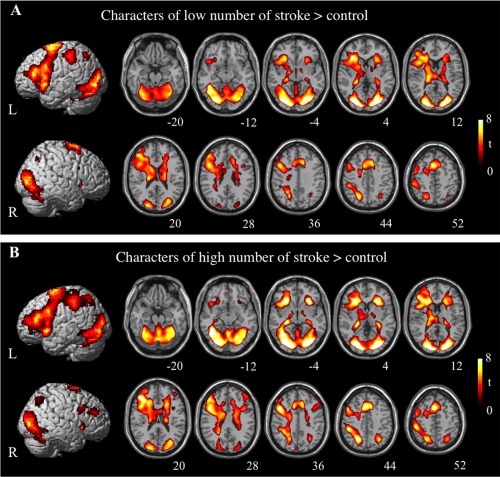
Lateral and axial view of cortical activation associated with tone judgment contrasted with control condition. The significant threshold is P < 0.05 FDR‐corrected. (A) The low‐stroke condition minus the control condition. (B) The high‐stroke condition minus the control condition. L = left hemisphere; R = right hemisphere.
Table 1.
Coordinates of activation peaks
| Coordinates (MNI) | Z | ||||
|---|---|---|---|---|---|
| Regions activated | BA | X | Y | Z | score |
| Characters of low number of stroke > Control | |||||
| Frontal lobe | |||||
| L inferior frontal gyrus | 47 | −32 | 26 | 2 | 5.46 |
| 46 | −46 | 36 | 16 | 4.98 | |
| L middle frontal gyrus | 6/9 | −52 | 6 | 48 | 3.52 |
| L medial frontal gyrus | 6 | −4 | 12 | 52 | 5.35 |
| 6 | −10 | 2 | 66 | 5.13 | |
| L precentral gyrus | 6 | −48 | −4 | 52 | 3.52 |
| L cingulate gyrus | 24 | −20 | −2 | 42 | 3.75 |
| R cingulate gyrus | 32 | 12 | 18 | 40 | 4.79 |
| L insula | −26 | 26 | 16 | 5.28 | |
| −30 | 26 | 14 | 4.92 | ||
| −34 | 26 | 12 | 4.84 | ||
| Parietal lobe | |||||
| L precuneus | 7 | −24 | −62 | 40 | 4.81 |
| 7 | −26 | −54 | 38 | 4.57 | |
| L inferior parietal lobule | 40 | −48 | −38 | 44 | 4.09 |
| 40 | −50 | −38 | 60 | 3.73 | |
| 40 | −56 | −34 | 54 | 4.09 | |
| Occipital lobe | |||||
| L cuneus | 17 | −22 | −86 | 4 | 6.58 |
| L middle occipital gyrus | 18 | −26 | −80 | 0 | 6.01 |
| 18 | −28 | −80 | −12 | 5.86 | |
| R middle occipital gyrus | 18 | 20 | −88 | 8 | 6.25 |
| L lingual gyrus | 17 | −12 | −92 | −4 | 6.04 |
| R lingual gyrus | 18 | 24 | −82 | −2 | 5.82 |
| 18 | 26 | −76 | 0 | 5.72 | |
| 17 | 16 | −86 | −4 | 5.52 | |
| Subcortical regions | |||||
| L thalamus | −20 | −20 | 16 | 5.58 | |
| −26 | −34 | 0 | 4.9 | ||
| −14 | −10 | 0 | 3.38 | ||
| R thalamus | 20 | −12 | 18 | 4.42 | |
| 18 | −20 | 16 | 4.24 | ||
| 24 | −30 | 10 | 3.67 | ||
| 28 | −28 | −2 | 3.96 | ||
| L caudate | −12 | −2 | 22 | 5.06 | |
| R caudate tail | 30 | −38 | 2 | 3.79 | |
| L medial globus pallidus | −18 | −10 | −8 | 4.24 | |
| R medial globus pallidus | 14 | −6 | −2 | 3.75 | |
| R cerebellum | 20 | −66 | −48 | 4.84 | |
| Characters of high number of stroke > Control | |||||
| Frontal lobe | |||||
| L inferior frontal gyrus | 47 | −28 | 24 | −2 | 5.71 |
| 46 | −44 | 36 | 14 | 5.51 | |
| R inferior frontal gyrus | 44/45 | 32 | 8 | 24 | 3.95 |
| L middle frontal gyrus | 46 | −36 | 28 | 20 | 5.41 |
| R middle frontal gyrus | 9/10 | 42 | 46 | 28 | 3.58 |
| 9 | 40 | 12 | 30 | 4.89 | |
| L medial frontal gyrus | 8 | −4 | 16 | 48 | 5.63 |
| 6 | −4 | 12 | 52 | 5.51 | |
| R superior frontal gyrus | 6 | 26 | −4 | 74 | 4.22 |
| R insula | 28 | 22 | −2 | 5.66 | |
| 22 | 26 | 2 | 5.39 | ||
| Parietal lobe | |||||
| L precuneus | 7 | −20 | −64 | 46 | 5.24 |
| R superior parietal lobule | 7 | 30 | −64 | 44 | 4.67 |
| L inferior parietal lobule | 40 | −44 | −38 | 44 | 5.2 |
| Occipital lobe | |||||
| L cuneus | 18 | −18 | −92 | 8 | 6.58 |
| 17 | −20 | −88 | 6 | 6.56 | |
| R cuneus | 17 | 24 | −84 | 6 | 6.73 |
| L lingual gyrus | 17 | −16 | −90 | 0 | 6.13 |
| L middle occiptial gyrus | 18/19 | −28 | −82 | −12 | 6.6 |
| R inferior occipital gyrus | 19 | 36 | −76 | −4 | 6.02 |
| 19 | 34 | −78 | −12 | 5.53 | |
| Temporal lobe | |||||
| L fusiform gyrus | 37 | −40 | −56 | −10 | 6.51 |
| 37 | −40 | −48 | −14 | 6.11 | |
| Subcortical regions | |||||
| L thalamus | −26 | −28 | −4 | 5.79 | |
| R thalamus | 28 | −32 | 2 | 4.82 | |
| R medial globus pallidus | 18 | −12 | 0 | 4.01 | |
| 12 | −6 | 0 | 3.75 | ||
L, left; R, right; BA, Brodmann's area.
Direct contrast between the high‐stroke and low‐stroke conditions found greater activation for characters with the high number of strokes in regions including bilateral middle frontal gyri (BA 9), left dorsal portion of IFG (BA 44/45), left cingulate gyrus (BA32), bilateral posterior parietal cortex (BA 7/40), bilateral fusiform gyri (BA37/18), and bilateral inferior/middle occipital areas (BA18/19) (Fig. 2 and Table 2). Though many of these regions showed bilateral activation, they were activated more prominently in the left hemisphere. There was no greater activation for the low‐stroke condition than the high‐stroke condition.
Figure 2.
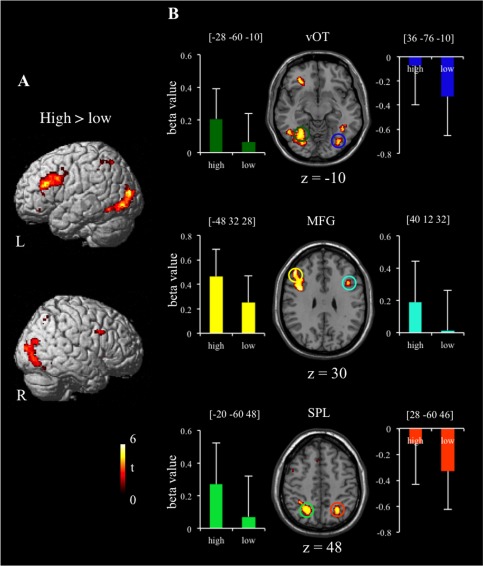
Brain regions significantly activated for high‐stroke condition in comparison with low‐stroke condition, with significant threshold at P < 0.05 FDR‐corrected. (A) Lateral view. (B) Axial sections and parameter estimates (beta values) extracted for high‐stroke and low‐stroke conditions from six ROIs: left vOT (in dark green), right vOT (in blue), left MFG (in yellow), right MFG (in cyan), left SPL (in light green), and right SPL (in orange). Error bars depict standard error of mean (SEM). L= left hemisphere; R = right hemisphere; high = high‐stroke condition; low = low‐stroke condition; vOT = ventral occipitotemporal cortex; MFG = middle frontal gyrus; SPL = superior parietal lobule.
Table 2.
Coordinates of activation peaks: The high‐stroke condition minus the low‐stroke condition
| Coordinates (MNI) | Z | ||||||
|---|---|---|---|---|---|---|---|
| Regions activated | BA | X | Y | Z | score | ||
| Frontal lobe | |||||||
| L middle frontal gyrus | 9 | −48 | 32 | 28 | 4.45 | ||
| R middle frontal gyrus | 9 | 40 | 12 | 32 | 3.63 | ||
| L inferior frontal gyrus | 44 | −40 | 4 | 28 | 4.33 | ||
| 45 | −36 | 22 | 24 | 4.47 | |||
| 47 | −24 | 26 | −2 | 4.28 | |||
| 47 | −32 | 36 | −8 | 3.99 | |||
| R inferior frontal gyrus | 47 | 28 | 22 | −2 | 3.68 | ||
| Cingulate gyrus | 32 | −6 | 22 | 44 | 3.3 | ||
| Parietal lobe | |||||||
| L superior parietal lobule | 7 | −20 | −60 | 48 | 3.77 | ||
| R superior parietal lobule | 7 | 28 | −60 | 46 | 3.84 | ||
| L inferior parietal lobule | 40 | −32 | −48 | 50 | 3.46 | ||
| Occipital lobe | |||||||
| L middle occipital gyrus | 19 | −32 | −86 | 12 | 4.27 | ||
| R middle occipital gyrus | 18 | 26 | −82 | 6 | 4.07 | ||
| L inferior occipital gyrus | 19 | −38 | −80 | −4 | 4.07 | ||
| R inferior/middle occipital gyrus | 18/19 | 28 | −82 | −2 | 3.93 | ||
| Temporal lobe | |||||||
| L fusiform gyrus | 37 | −28 | −60 | −10 | 4.24 | ||
| R fusiform gyrus | 18 | 36 | −76 | −10 | 3.85 | ||
| Subcortical regions | |||||||
| R caudate | 16 | 28 | 6 | 3.39 | |||
| 18 | 34 | 0 | 3.14 | ||||
L, left; R, right; BA, Brodmann's area.
We also conducted ROI analysis on six regions exhibiting significantly greater activation for the characters of the high number of strokes than the characters of the low number of strokes. Six sphere ROIs (8‐mm radius) were defined, including bilateral vOT (coordinate: left [−28 −60 −10]; right [36 −76 −10]), bilateral MFG (left [−48 32 28]; right [40 12 32]) and bilateral SPL (left [−20 −60 48]; right [28 −60 46]). Parameter estimates (beta values) were extracted for each subject from these regions. Figure 2B showed mean beta values for the high‐stroke and the low‐stroke conditions.
DCM Results
In the first‐step connectivity analysis, we used a family‐level random‐effect BMS procedure and compared three families of models, which differed in whether the ventral pathway only, the dorsal pathway only, or both pathways were modulated by visual‐orthographic load during orthography–phonology mapping (Fig. 3A). We found that the family of models with modulation on the ventral pathway only (cuneus→vOT) showed overwhelming evidence (family xp = 0.95) relative to the other two families.
In the second‐step family comparison, we constructed models with three left‐hemispheric regions (i.e., vOT, SPL, and MFG) and compared three families of models that differed in whether inputs entered the model via the vOT only, the SPL only, or both (Fig. 3B). Results showed that driving input most likely entered via left vOT only, with xp being 0.98. Results from BMA analysis of model parameters over the winning family are shown in Table 3 for intrinsic connectivity and in Figure 4 for modulatory connections. There were significant modulatory effects of visual‐orthographic load on the connections from left vOT to left SPL (0.14 Hz), from left SPL to left MFG (0.048 Hz), and from left MFG to left vOT (−0.47 Hz). These results indicated that when the characters had a high number of strokes, information flow from left vOT to left SPL and from left SPL to left MFG increased, whereas information flow from left MFG to left vOT decreased.
Table 3.
Strengths of intrinsic connectivity for the three‐region model (probability values shown in parentheses)
| To | From | ||
|---|---|---|---|
| vOT | SPL | MFG | |
| vOT | / | −0.51 | −0.14 |
| (0.99) | (0.74) | ||
| SPL | 0.87 | / | −0.17 |
| (1.00) | (0.94) | ||
| MFG | 1.22 | −0.48 | / |
| (1.00) | (1.00) | ||
Figure 4.
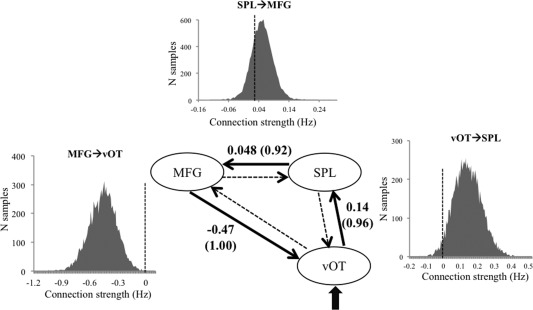
Results from BMA parameter analysis for the three‐region model. The average strength of significant modulatory connection parameters (in Hz) are shown above the thicken lines, with probability values shown in parentheses. The distribution of the 10,000 samples of the posterior densities generated during BMA is presented for the significant modulatory effects.
DISCUSSION
Previous neuroimaging studies have found that visual word recognition in alphabetic languages engages both ventral and dorsal neural pathways from visual cortex to higher‐order language areas [Booth et al., 2008; Levy et al., 2009; Richardson et al., 2011; Seghier et al., 2012], and that reading deep orthographies relies more on the ventral pathway compared to shallow orthographies [Duncan et al., 2013; Paulesu et al., 2000; Price, 2012]. This study directly tested the involvement of the ventral and dorsal pathways during reading of Chinese, an extremely deep orthography. Our results from the two‐step family comparisons converged to suggest that the ventral pathway, but not the dorsal pathway, was critically involved in Chinese visual word processing. First, we found overwhelming evidence that only the ventral pathway (rather than only the dorsal pathway or both ventral and dorsal pathways) was modulated by the visual‐orthographic load. Second, when vOT, SPL, and MFG were included in the models, there was unequivocal evidence suggesting that the driving input entered the models via the ventral system (i.e., vOT) rather than only the dorsal system (i.e., SPL) or both. These findings reveal a significant variation of the neural pathways for reading across writing systems.
Writing systems differ in how the graphic unit maps onto the phonological unit of language. In alphabetic languages, phonology can be represented by different levels of orthographic structures: letters, onset/rimes, syllables, and the whole words [Ziegler and Goswami, 2005]. In deep alphabetic languages like English, inconsistent correspondence between graphemes and phonemes may lead to the development of both dorsal pathway for processing smaller grain size (e.g., phonemes) and ventral pathway for processing larger grain size [e.g., syllables or whole words; Richardson et al., 2011]. In contrast, the logographic Chinese uses characters to represent morphosyllabic but not phonemic information, such that small grain‐size correspondences are not available for Chinese. Such characteristics in written Chinese have led to the suggestion that phonological activation in Chinese characters exhibited threshold‐style activation, in which a full orthographic specification is needed before the activation of word‐level phonology [Perfetti et al., 2005]. Therefore, different relationships between orthography and phonology may explain why reading of logographic Chinese has to go through the left vOT in the ventral pathway, which serves to extract orthographic information of whole words for lexical processing [Dehaene et al., 2005; Shaywitz et al., 2008], whereas alphabetic reading can also be accomplished with the dorsal pathway for serial letter‐sound conversion [Booth et al., 2002; Cohen et al., 2008; Gabrieli, 2009; Paulesu et al., 2000].
In addition, our results highlighted the importance of the connections from vOT to SPL and from SPL to MFG in Chinese reading. As stroke number increased, the left SPL may come to play a more important role in fine‐grained orthographic analysis of the characters. The left MFG was proposed to support coordination of different aspects of linguistic information and the phonological processing at a syllable level during Chinese reading [Kuo et al., 2001, 2003; Liu et al., 2006; Perfetti et al., 2006; Siok et al., 2004, 2008; Song et al., 2013; Tan et al., 2001, 2003, 2005; Wu et al., 2012]. In our study, the left MFG might be more engaged to integrate visual‐orthographic and phonological information when the visual‐orthographic load became heavier. We also noted a strong negative modulation effect on the backward connection from left MFG to left vOT. Such top‐down inhibitory connectivity may enable more efficient information processing in the lower‐level areas that conformed to the processing in higher‐level areas [Cardin et al., 2011; Carreiras et al., 2009].
To conclude, this study provides important evidence suggesting that visual word recognition of Chinese characters engages the ventral pathway from visual cortex to vOT, but not the dorsal pathway from visual cortex to the left superior temporal or parietal regions. The ventral pathway, however, is linked to the SPL and the left MFG, so that a dynamic neural network is built, with information flowing from the visual cortex (cuneus) to vOT to SPL and to MFG. This dynamic system reinforces whole‐character‐based phonology, which is related to visual‐orthographic complexity. It represents sharp contrast to alphabetic languages in which both ventral and dorsal pathways are critically involved during word recognition. Cortical dynamics is constrained by the differences in how graphic symbols are linked to spoken language in writing systems.
REFERENCES
- Ben‐Shachar M, Dougherty RF, Deutsch GK, Wandell BA (2007): Differential sensitivity to words and shapes in ventral occipitotemporal cortex. Cereb Cortex 17:1604–1611. [DOI] [PubMed] [Google Scholar]
- Bookheimer S (2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25:151–188. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM (2002): Functional anatomy of intra‐ and cross‐modal lexical tasks. Neuroimage 16:7–22. [DOI] [PubMed] [Google Scholar]
- Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, Zhang L, Ding GS, Deng Y, Liu L (2006): Specialization of phonological and semantic processing in Chinese word reading. Brain Res 1071:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Mehdiratta N, Burman DD, Bitan T (2008): Developmental increases in effective connectivity to brain regions involved in phonological processing during tasks with orthographic demands. Brain Res 1189:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Peng D, Liu L, Jin Z, Fan N, Deng Y, Booth JR (2009): Developmental differences of neurocognitive networks for phonological and semantic processing in Chinese word reading. Hum Brain Mapp 30:797–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin V, Friston KJ, Zeki S (2011): Top‐down modulations in the visual form pathway revealed with dynamic causal modeling. Cereb Cortex 21:550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estévez A, Lozano A, Devlin JT, Price CJ (2009): An anatomical signature for literacy. Nature 461:983–986. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Armstrong BC, Perea M, Frost R (2014): The what, when, where, and how of visual word recognition. Trends Cogn Sci 18:90–98. [DOI] [PubMed] [Google Scholar]
- Chen HC, Vaid J, Bortfeld H, Boas DA (2008): Optical imaging of phonological processing in two distinct orthographies. Exp Brain Res 184:427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S (2004): Specialization within the ventral stream: The case for the visual word form area. Neuroimage 22:466–476. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Vinckier F, Jobert A, Montavont A (2008): Reading normal and degraded words: Contribution of the dorsal and ventral visual pathways. Neuroimage 40:353–366. [DOI] [PubMed] [Google Scholar]
- Coney J (1998): The effect of complexity upon hemispheric specialization for reading Chinese characters. Neuropsychologia 36:149–153. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F (2005): The neural code for written words: A proposal. Trends Cogn Sci 9:335–341. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS (2003): Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cognit Neurosci 15:71–84. [DOI] [PubMed] [Google Scholar]
- Dong Y, Nakamura K, Okada T, Hanakawa T, Fukuyama H, Mazziotta JC, Shibasaki H (2005): Neural mechanisms underlying the processing of Chinese words: An fMRI study. Neurosci Res 52:139–145. [DOI] [PubMed] [Google Scholar]
- Duncan KJK, Twomey T, Jones ŌP, Seghier ML, Haji T, Sakai K, Price CJ, Devlin JT (2013): Inter‐ and intrahemispheric connectivity differences when reading Japanese Kanji and Hiragana. Cereb Cortex 24:1601–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, Dietz NAE, Agnew JA, Flowers DL (2004): Neural changes following remediation in adult developmental dyslexia. Neuron 44:411–422. [DOI] [PubMed] [Google Scholar]
- Friston KJ (2011): Functional and effective connectivity: A review. Brain Connect 1:13–36. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny WD (2003): Dynamic causal modelling. Neuroimage 19:1273–1302. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE (2009): Dyslexia: A new synergy between education and cognitive neuroscience. Science 325:280–283. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE (1998): The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA 95:906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Peng G, Lyu B, Wang Y, Zhuo Y, Niu Z, Tan LH, Leff AP, Gao JH (2015): Cross‐language differences in the brain network subserving intelligible speech. Proc Natl Acad Sci USA 112:2972–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U (2006): Neuroscience and education: From research to practice? Nat Rev Neurosci 7:406–413. [DOI] [PubMed] [Google Scholar]
- He Q, Xue G, Chen C, Chen C, Lu ZL, Dong Q (2013): Decoding the neuroanatomical basis of reading ability: a multivoxel morphometric study. J Neurosci 33:12835–12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor‐Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA (2006): Neural basis of dyslexia: A comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci 26:10700–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC (1998): Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA 95:8939–8944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao JH, Cheng L (2013): The modulation of stimulus structure on visual field asymmetry effects: The case of Chinese character recognition. Q J Exp Psychol 66:1739–1755. [DOI] [PubMed] [Google Scholar]
- Hu W, Lee HL, Zhang Q, Liu T, Geng LB, Seghier ML, Shakeshaft C, Twomey T, Green DW, Yang YM, Price CJ (2010): Developmental dyslexia in Chinese and English populations: Dissociating the effect of dyslexia from language differences. Brain 133:1684–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzev M, Tüscher O, Hennig J, Weiller C, Kaller CP (2013): Revisiting the functional specialization of left inferior frontal gyrus in phonological and semantic fluency: The crucial role of task demands and individual ability. J Neurosci 33:7837–7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Duann JR, Wu YT, Ho LT, Hung D, Tzeng OJL, Hsieh JC (2001): A left‐lateralized network for reading Chinese words: A 3 T fMRI study. Neuroreport 12:3997. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Yeh TC, Lee CY, Wu Y, Chou CC, Ho LT, Hung DL, Tzeng OJL, Hsieh JC (2003): Frequency effects of Chinese character processing in the brain: An event‐related fMRI study. Neuroimage 18:720–730. [DOI] [PubMed] [Google Scholar]
- Lee HL, Noppeney U (2011): Long‐term music training tunes how the brain temporally binds signals from multiple senses. Proc Natl Acad Sci USA 108:E1441–E1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff AP, Schofield TM, Stephan KE, Crinion JT, Friston KJ, Price CJ (2008): The cortical dynamics of intelligible speech. J Neurosci 28:13209–13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong CK, Cheng PW, Mulcahy R (1987): Automatic processing of morphemic orthography by mature readers. Lang Speech 30:181–197. [DOI] [PubMed] [Google Scholar]
- Levy J, Pernet C, Treserras S, Boulanouar K, Aubry F, Démonet J, Celsis P (2009): Testing for the dual‐route cascade reading model in the brain: An fMRI effective connectivity account of an efficient reading style. PLoS One 4:e6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QL, Bi HY, Zhang JX (2010): Neural correlates of the orthographic neighborhood size effect in Chinese. Eur J Neurosci 32:866–872. [DOI] [PubMed] [Google Scholar]
- Liu CL, Hue CW, Chen CC, Chuang KH, Liang KC, Wang YH, Wu CW, Chen JH (2006): Dissociated roles of the middle frontal gyri in the processing of Chinese characters. Neuroreport 17:1397–1401. [DOI] [PubMed] [Google Scholar]
- Liu K, Shi L, Chen F, Waye MMY, Lim CKP, Cheng PW, Luk SHH, Mok WCT, Chua WCW, Wang D (2015): Altered topological organization of brain structural network in Chinese children with developmental dyslexia. Neuroscience Letters 589:169–175. [DOI] [PubMed] [Google Scholar]
- Park HJ, Friston K (2013): Structural and functional brain networks: From connections to cognition. Science 342:1238411. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M (2000): A cultural effect on brain function. Nat Neurosci 3:91–96. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Mechelli A, Friston KJ (2004): Comparing dynamic causal models. Neuroimage 22:1157–1172. [DOI] [PubMed] [Google Scholar]
- Penny WD, Stephan KE, Daunizeau J, Rosa MJ, Friston KJ, Schofield TM, Leff AP (2010): Comparing families of dynamic causal models. PLoS Comput Biol 6:e1000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti CA, Tan LH (2013): Write to read: The brain's universal reading and writing network. Trends Cogn Sci 17:56–57. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Liu Y, Tan LH (2005): The lexical constituency model: Some implications of research on Chinese for general theories of reading. Psychol Rev 112:43–59. [DOI] [PubMed] [Google Scholar]
- Perfetti CA, Tan LH, Siok WT (2006): Brain‐behavior relations in reading and dyslexia: Implications of Chinese results. Brain Lang 98:344–346. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331:585–589. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10:15–35. [DOI] [PubMed] [Google Scholar]
- Price CJ (2012): A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage 62:816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson FM, Seghier ML, Leff AP, Thomas MSC, Price CJ (2011): Multiple routes from occipital to temporal cortices during reading. J Neurosci 31:8239–8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD (2007): Development of neural systems for reading. Annu Rev Neurosci 30:475–503. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Church JA (2009): Functional neuroimaging insights into the development of skilled reading. Curr Dir Psychol Sci 18:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Josse G, Leff AP, Price CJ (2011): Lateralization is predicted by reduced coupling from the left to right prefrontal cortex during semantic decisions on written words. Cereb Cortex 21:1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Neufeld NH, Zeidman P, Leff AP, Mechelli A, Nagendran A, Riddoch JM, Humphreys GW, Price CJ (2012): Reading without the left ventral occipito‐temporal cortex. Neuropsychologia 50:3621–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Morris R, Shaywitz BA (2008): The education of dyslexic children from childhood to young adulthood. Annu Rev Psychol 59:451–475. [DOI] [PubMed] [Google Scholar]
- Siok WT, Jin Z, Fletcher P, Tan LH (2003): Distinct brain regions associated with syllable and phoneme. Hum Brain Mapp 18:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH (2004): Biological abnormality of impaired reading is constrained by culture. Nature 431:71–76. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH (2008): A structural–functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci USA 105:5561–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder PJ, Harris LJ (1993): Handedness, sex, and familial sinistrality effects on spatial tasks. Cortex 29:115. [DOI] [PubMed] [Google Scholar]
- Song R, Zhang J, Wang B, Zhang H, Wu H (2013): A near‐infrared brain function study of Chinese dyslexic children. Neurocase 19:382–389. [DOI] [PubMed] [Google Scholar]
- Spinks JA, Liu Y, Perfetti CA, Tan LH (2000): Reading Chinese characters for meaning: The role of phonological information. Cognition 76:B1–B11. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ (2010): Analyzing effective connectivity with functional magnetic resonance imaging. Wiley Interdiscip Rev Cogn Sci 1:446–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers. [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH (2001): The neural system underlying Chinese logograph reading. Neuroimage 13:836–846. [DOI] [PubMed] [Google Scholar]
- Tan LH, Spinks JA, Feng CM, Siok WT, Perfetti CA, Xiong J, Fox PT, Gao JH (2003): Neural systems of second language reading are shaped by native language. Hum Brain Mapp 18:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT (2005): Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: A meta analysis. Hum Brain Mapp 25:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell BA, Rauschecker AM, Yeatman JD (2012): Learning to see words. Annu Rev Psychol 63:31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CY, Ho MHR, Chen SHA (2012): A meta‐analysis of fMRI studies on Chinese orthographic, phonological, and semantic processing. Neuroimage 63:381–391. [DOI] [PubMed] [Google Scholar]
- Xue G, Chen C, Zhen J, Dong Q (2006): Language experience shapes fusiform activation in processing a logographic artificial language: an fMRI Training Study. Neuroimage 31:1315–1326. [DOI] [PubMed] [Google Scholar]
- Yu B, Cao H (1992): A new exploration on the effect of stroke number in the identification of Chinese characters. Acta Psychol Sin 24:120–126. [Google Scholar]
- Zhang M, Chen C, Xue G, Lu ZL, Mei LL, Xue H, Wei M, He Q, Li J, Dong Q (2014): Language‐general and ‐specific white matter microstructural bases for reading. Neuroimage 98:435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wang X, Frost SJ, Sun W, Fang SY, Mencl WE, Pugh KR, Shu H, Rueckl JG (2014): Neural division of labor in reading is constrained by culture: A training study of reading Chinese characters. Cortex 53:90–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Nie YX, Chang CQ, Gao JH, Niu ZD (2014): Different patterns and development characteristics of processing written logographic characters and alphabetic words: An ALE meta‐analysis. Hum Brain Mapp 35:2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U (2005): Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychol Bull 131:3–29. [DOI] [PubMed] [Google Scholar]


