Abstract
Odor names refer usually to “source” object categories. For example, the smell of rose is often described with its source category (flower). However, linguistic studies suggest that odors can also be named with labels referring to categories of “practices”. This is the case when rose odor is described with a verbal label referring to its use in fragrance practices (“body lotion,” cosmetic for example). It remains unknown whether naming an odor by its practice category influences olfactory neural responses differently than that observed when named with its source category. The aim of this study was to investigate this question. To this end, functional MRI was used in a within‐subjects design comparing brain responses to four different odors (peach, chocolate, linden blossom, and rose) under two conditions whereby smells were described either (1) with their source category label (food and flower) or (2) with a practice category label (body lotion). Both types of labels induced activations in secondary olfactory areas (orbitofrontal cortex), whereas only the source label condition induced activation in the cingulate cortex and the insula. In summary, our findings offer a new look at olfactory perception by indicating differential brain responses depending on whether odors are named according to their source or practice category. Hum Brain Mapp 35:810–818, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: olfaction, smell, context, verbal, fMRI
INTRODUCTION
The influence of language on perception has been debated for many years. It can be viewed in two ways: (1) Language is a powerful categorization tool and our memory representations largely depend on it: at the beginning of the last century, the thesis of Sapir–Whorf proposed that language determines perception [Marchellesi and Grandin, 1974]; (2) Perception is determined not only by language on a perceptual tabula rasa but also by both the perceptual apparatus itself and discontinuities existing in the perceived world [Berlin and Kay, 1969; Rosch‐Heider, 1971]. These theories, transposed to the world of smells, raise the question of whether lexical knowledge about odors shapes our olfactory percepts.
It has been shown that panelists give higher pleasantness ratings for the odor of products presented with their brand label than for the same odors presented without [Moskowitz, 1979]. Likewise, pleasantness and also intensity and familiarity judgments are enhanced when participants are able to identify the odorant source [Ayabe‐Kanamura et al., 1998] or when the experimenter provides a positive name for the odorant object [Distel and Hudson, 2001]. When verbal information about an odor is available, subjects shift their pleasantness judgment in line with the affective connotation of the label [Herz, 2003]. Such top–down modulation has been found even in children [Bensafi et al., 2007a; Rinck et al., 2011]. Moreover, Dalton [ 1999] showed that health‐related claims also influence valence: the same odorant presented as “harmful,” “healthful,” or “neutral” will evoke more health symptoms when presented as potentially dangerous. Thus labeling odors with positive or negative words (i.e., emotionally intense labels) will influence valence, emotional intensity and pleasantness ratings as compared with neutral, less emotional labels [Djordjevic et al., 2008]. Accordingly, de Araujo et al. [ 2005] showed that the hedonic meaning of the label (edible: e.g., “cheese”; or not: e.g., “body odor”) assigned to an odor differentially affected the activation pattern in cingulate gyrus and orbitofrontal cortex (OFC), two regions implicating in the assignment of value.
In summary, these observations suggest that odor labels are prominent determinants of brain responses to smells. However, other studies suggest that an odor can be named with different verbal labels that may evoke different contexts [Howes, 1986]. Whereas odors are usually named by their contextual source objects, Engen noted that “With respect to subject generated odor names, it has been observed that odors are customarily labeled in terms of personal contextual reference and not by invariant source names” [Dubois and Rouby, 1997]. One may cite for example, “the smell of my grandmother's cakes,” “the medicine for coughs that my mother rubbed on my chest,” “my daddy's aftershave,” etc. Thus, although the process of odor naming produces often categories of “source” objects, categories of “events” or “practices” are also used [Dubois, 2000]. For example, the smell of eugenol is usually qualified as cloves (source category) but can also be reminiscent of a dentist's office (practice category). In the same line, whereas the smells of rose or even peach are often described with their source categories (flower and food), a verbal label referring to their use in fragrance practices (in body lotion for example) can also be used. It remains unknown whether olfactory neural response is dependent on naming an odor by its practice category or its source categories.
To investigate this question, we used functional magnetic resonance imaging (fMRI) in a within‐subjects design comparing brain responses to four different odors (chocolate, peach, linden blossom, and rose) that could be described either by their source category or a verbal label referring to their use in fragrance practices. Particularly, different odor perceptual qualities (food and flower) were used in order to examine whether verbal labeling with practice category or source category affects differently the processing of food odors on the one hand and flower odors on the other hand.
MATERIAL AND METHODS
Subjects
The subjects were 21 right‐handed female volunteers (mean age: 24.4 ± 2.9 years). They received a moderate financial reward for the time spent in the laboratory. The recording procedure was explained in great detail to the subjects, who provided written consent prior to participation. The study was conducted according to the Declaration of Helsinki and was approved by the Ethics Committee of the Technical University of Dresden Medical Scool (EK number EK43022009). Detailed medical history combined with ENT examination of the nasal cavity and odor perception assessment by the “Sniffin' Sticks” test [Hummel et al., 1997] ascertained that subjects were in good health and had a normal sense of smell.
Stimulus Delivery
Four olfactory stimuli were used (rose, linden blossom, chocolate, and peach odors: Firmenich SA, Switzerland; 1% v/v diluted in propylene glycol), which are fragrances similar to those used in body lotions. The Burghart OM6b olfactometer delivered the olfactory stimuli in a quasi‐rectangular shape, with controlled stimulus onset. Mechanical stimulation was avoided by embedding stimuli in a constant flow of odorless, humidified air of controlled temperature (80% relative humidity; total flow 6 L/min; 36°C) [Kobal, 1981]. A thermally insulated Teflon™ cannula directed the gaseous stimulus from the olfactometer to the subject's nose in the MRI room.
Experimental Procedure and fMRI Experimental Paradigm
The study was performed on a 1.5 T MR‐scanner (Magnetom Sonata; Siemens Medical, Erlangen, Germany). The experiment, which lasted ∼60 min (from arrival to departure of the subject), comprised eight sessions: one for each stimulus condition (chocolate, peach, linden blossom, and rose), presented under either their source label (“food” and “flower”) or a practice label (“body lotion”) (Fig. 1a). To examine how the source and the practice labels used applied and related to the used odors, a control study was run on 10 participants (three males, mean age of 26 ± 3.67 years) who were asked to smell the four odors. Smells were presented without descriptors, but after each odor trial, participants were asked to evaluate how the source labels (food, flower), the practice label (body lotion) and two a priori unrelated labels (spice and medicine) applied to the smell they just perceived. To this end, they were to use a visual rating scale from 0 (not at all applicable) to 10 (extremely applicable). Results revealed that the source label applied the best (mean ± SEM: 6.41 ± 0.28), followed by the practice label (5.72 ± 0.37), the “spice” label (2.67 ± 0.25) and the “medicine” label (2.65 ± 0.38). The statistical analysis revealed an effect of labels (F[3,27] = 31.200, P < 0.0001) reflecting that: (1) the source label was more applicable than both “spice” (t(9) = 9.472, P < 0.0001) and “medicine” (t(9) = 6.397, P < 0.0001) labels, (2) the practice label was more applicable than both “spice” (t(9) = 5.988, P < 0.0002) and “medicine” (t(9) = 5.177, P < 0.0006) labels, (3) the source vs. the practice labels (t(9) = 1.530, P > 0.05) and the “spice” label vs. the “medicine” label (t(9) = 0.056, P > 0.05) did not significantly differ in their applicability. In others words, both the source and the practice labels applied more than the a priori unrelated labels.
Figure 1.
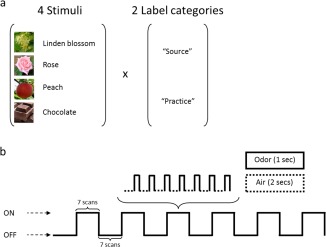
Experimental design and protocol. (a) The experimental design included four olfactory stimuli (linden blossom, rose, peach, and chocolate) presented under two label conditions (source or practice). (b) Schematic representation of the experimental protocol used during the scanning sessions. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For the main fMRI study, each participant went through the eight sessions in random order. Each experimental session in turn comprised six on/off‐block subsessions, with 21‐s blocks presented alternately in On (stimulus‐on) and Off (stimulus‐off) conditions (Fig. 1b).
The fMRI data were collected in 96 volumes per session using a 2D gradient echo–echo planar imaging (GE–EPI) sequence with 36 axial slices (Imaging Matrix: 64 × 64; TR: 3 s; TE: 35 ms; FA: 90°; voxel size: 3 × 3 × 3.75 mm3). In the 12 min immediately following the functional sessions, a high‐resolution T1‐weighted sequence of the brain (3D IR/GR sequence: TR = 2180 ms/TE = 3.93 ms) was acquired for subsequent superimposition of functional data and to exclude any incidental brain pathology.
Before each functional session, participants were verbally given either the source label or the practice label of the odor they were going to smell. After each session, participants were asked to evaluate the stimuli in terms of intensity (on a scale from 0 = “not perceived” to 10 = “extremely intense”) and of pleasantness (on a scale from −5 = “extremely unpleasant” to +5 = “extremely pleasant” with 0 representing “neutral”).
Data Analysis
fMRI data analysis used SPM8 software (Statistical Parametric Mapping; Wellcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) implemented in Matlab 7 (MathWorks, Natick, MA). After image preprocessing [Ashburner and Friston, 2003], first‐level statistical analysis was implemented. At the individual level, brain activation induced by each of the eight ON odor/label runs was analyzed by comparing the activation pattern for each condition and that obtained with their respective individual OFF nonodorized baseline. The resulting contrasts were then entered into a group analysis using a random effects model [Penny et al., 2003]. Activation coordinates were presented in MNI space. We report results for brain regions known to be involved in processing of food and non food odors and/or modulated by verbal labeling. These regions include the insula, OFC, anterior cingulate cortex, primary olfactory cortex, and amygdala [Bensafi et al., 2012; Croy et al., 2010; de Araujo et al., 2005; Seo et al., 2011; Small et al., 2005]. Loci of activations were thus identified within this brain network of interest delineated using an inclusive mask created with the Pick‐Atlas [Maldjian et al., 2003].
Control for Sniffing
Since sniffing is known to influence neural activity in olfactory areas [Sobel et al., 1998], we examined in a control study whether sniffing could be influenced by odor labeling. Fourteen subjects (five males, mean age of 27 ± 4.27 years) were tested outside the scanner. The exact same experimental design as the one used in the fMRI session was applied with the same four olfactory stimuli (rose, linden blossom, chocolate, and peach odors). As in the fMRI study, each subject went through eight odor sessions in random order (one session for each odor, presented under either their source or practice label). Each session included six 21‐s on/off‐block subsessions. However, in addition to the nasal cannula that directed the gaseous stimulus from the olfactometer to the subject's nose, nasal sniffing was measured simultaneously. To this end, an airflow sensor (AWM720, Honeywell, France) connected to a small nasal cannula positioned in each nostril (Fig. 2a) was used. An example of a sniffing activity during an on‐period is illustrated in Figure 2b. The sniffing signal was amplified and digitally recorded at 100 Hz using LabVIEW software®. Sniffs were preprocessed by removing baseline offsets and aligned in time by setting the point where the sniff entered the inspiratory phase as time 0. Inspired volume and sniff duration were calculated for each condition and each participant.
Figure 2.
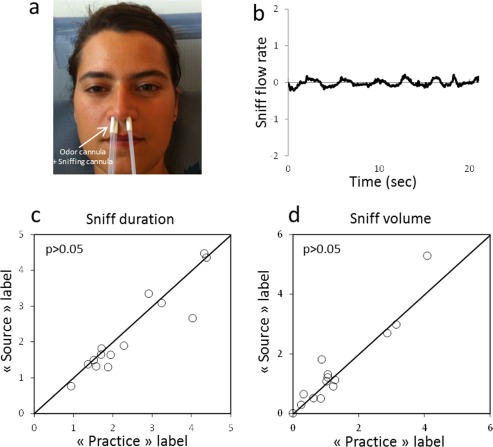
Effect on verbal labeling on sniffing. (a) Odorant tubing and nasal sniffing cannula used to record sniffing. (b) Typical sniffing trace for a subject during an ON period. Sniff duration (c) and sniff volume (d) under the practice label condition and the source label condition. Each circle corresponds to a subject. No significant differences in sniff duration or sniff volume were observed between the two conditions (P > 0.05). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
RESULTS
Psychophysics and Sniffing
To determine whether there were differences in pleasantness and/or intensity between the experimental conditions ANOVAs on pleasantness and intensity ratings with label conditions (source label vs. practice label) as a within‐subjects factor were performed. The verbal conditions did not influence stimulus pleasantness (F[1, 20] = 0.60, P > 0.05) (Fig. 3a) or intensity (F[1, 20] = 0.58, P > 0.05) (Fig. 3b). Moreover, the sniffing control study revealed no significant difference in sniff duration (F[1,14] = 0.489, P > 0.05) (Fig. 2c) and sniff volume (F[1,14] = 0.996, P > 0.05) (Fig. 2d) between the source label condition and the practice label condition, indicating that verbal labeling did not influence sniffing.
Figure 3.
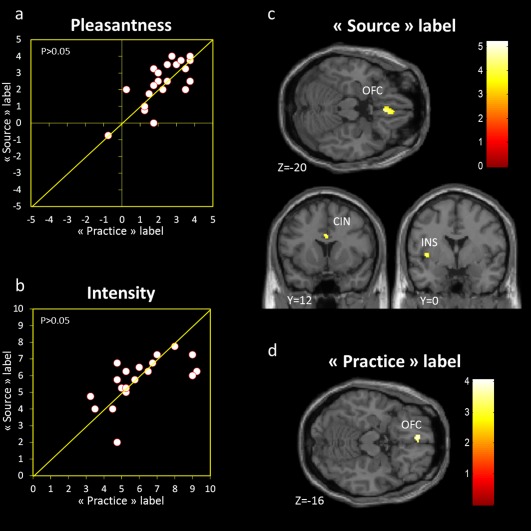
Perceptual ratings and brain activations during the source label condition and the practice label condition. Odor pleasantness (a) and intensity (b) ratings under the practice label condition and the source label condition. Each circle corresponds to a subject. No differences in pleasantness or intensity were observed between the two conditions (P > 0.05). (c) Activation patterns to the source label condition: brain activations were observed in medial orbitofrontal gyrus (OFC), anterior cingulate cortex (CIN), and insula (INS). (d) Activation patterns to the practice label condition: brain activations were observed in medial orbitofrontal gyrus.
fMRI
The main aim of the experiment was to examine the neural correlates of the effect verbal labeling on processing of odors. To test how the type of verbal label (source or practice) would influence neural responses to odors, brain activations were analyzed by contrasting for all odors: (1) the source label condition vs. its odorless baseline condition, and (2) the practice label condition vs. its odorless baseline condition. The areas of significant activation were identified for values exceeding an uncorrected P‐value of 0.001 with a cluster criterion of 20 voxels in the prespecified regions. MNI coordinates (x, y, z) of activated brain areas and t‐map values are presented in parentheses.
Whereas the source label condition induced activation in the insular gyrus bordering the frontal operculum (−36, −16, 20; t = 4.50; −34, −8, 10; t = 4.08; −32, −24, 14; t = 3.49; −40, 0, 2; t = 4.23), in the anterior cingulate cortex (12, 46, 8; t = 4.30; 12, 48, 18; t = 3.24; 0, 12, 28; t = 4.36; 10, 24, 22; t = 4.03; 0, 36, 16; t = 4.30) and in the medial OFC bordering the gyrus rectus (−4, 38, −8; t = 5.22; 6, 34, −8; t = 4.86; −4, 52, −2; t = 4.63; 4, 28, −20; t = 4.63; −6, 36, −16; t = 3.44) (Fig. 3c), the contrast between the practice label condition and its odorless baseline induced activation only in the medial orbitofrontal gyrus bordering the gyrus rectus (−6, 46, −16; t = 4.03; −2, 34, −6; t = 4.07; Fig. 3d). Furthermore, to examine the differential effect of the source label, we contrasted the source label condition with the practice label condition and considered only areas of significant activation at cluster level for values exceeding a P‐value of 0.001 (uncorrected, with a cluster criterion of 15 voxels). In line with the above, induced activation were observed in anterior cingulate cortex (−2, 36, 18; t = 3.89; Fig. 4a–c). In turn, the opposite contrast (practice label vs. source label) did not show any significant activation.
Figure 4.
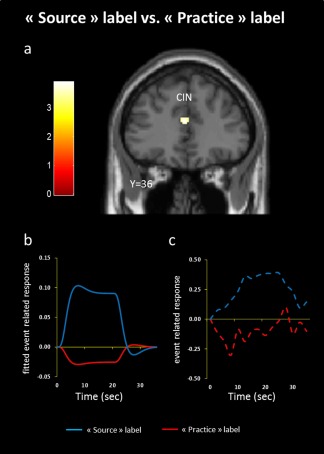
Activation pattern to the source label condition vs. the practice label condition. (a) Brain activations were observed in anterior cingulate cortex (CIN). Fitted (b) and raw (c) event related responses in the functionally activated voxels in anterior cingulate gyrus for the source label condition (blue line) and the practice label condition (red line).
When food odors were considered separately, the source label condition revealed activation in the insular gyrus bordering the frontal operculum (−36, −16, 20; t = 5.55; −38, −22, 14; t = 3.87; −46, −6, 0; t = 3.82; −38, 8, 8; t = 3.41), the anterior cingulate cortex (2, 18, 26; t = 4.72; 4, 38, 22; t = 4.54;−2, 8, 30; t = 3.97; 12, 34, 8; t = 4.71; 16, 42, 6; t = 3.61; 2, 36, 8; t = 3.30) and the medial OFC bordering the gyrus rectus (4, 26, −20; t = 4.05; 6, 34, −6; t = 4.77; −2, 48, −4; t = 4.18; −4, 32, −6; t = 3.99) (Fig. 5a). The condition labeled practice showed activation only in the medial OFC bordering the gyrus rectus (−6, 46, −16; t = 4.19; Fig. 5b). Finally, for flower odors, the source label condition revealed activation in the medial OFC bordering the gyrus rectus (4, 36, −16; t = 4.40; −4, 40, −16; t = 3.81; −6, 48, −16; t = 3.59; −4, 38, −8; t = 4.18) and the anterior cingulate cortex (12, 24, 24; t = 4.37; 10, 26, 16; t = 3.82; 16, 42, 12; t = 4.02; 14, 48, 20; t = 3.41) (Fig. 5c) and the practice label condition did not show any significant activation.
Figure 5.
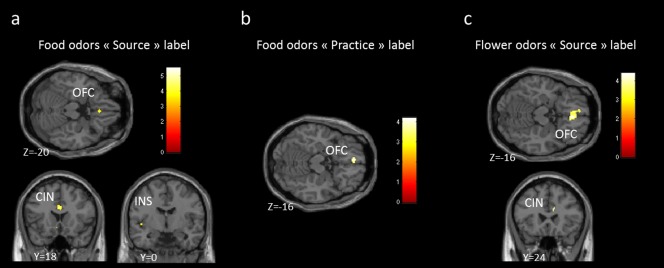
Brain activations during the source label condition and the practice label condition for both food odors and flower odors. (a) Activation patterns to the source label condition for the food odors: brain responses were seen in medial orbitofrontal gyrus (OFC), anterior cingulate cortex (CIN) and insula (INS). (b) medial orbitofrontal activation (OFC) in response to the practice label for the food odors. (c) medial orbitofrontal (OFC) and cingulate cortex (CIN) activations in response to the source label for the flower odors.
DISCUSSION
The main aim of the study was to explore the effect of verbal odor labeling on fMRI substrates of odor perception. Interestingly, common neural activity patterns were observed when odors were labeled with a source label or with a practice label notably in the OFC (including the gyrus rectus and the medial part of the orbitofrontal gyrus). The involvement of the OFC in olfactory processing has been shown by a large number of neuroimaging studies. Orbitofrontal activity in response to odors is modulated by cognitive experimental tasks [Zatorre et al., 2000], reflects assignment of hedonic value [Anderson et al., 2003; Bensafi et al., 2007b; Gottfried et al., 2002; Rolls et al., 2003] and its modulation by verbal labeling has been shown in odor processing [de Araujo et al., 2005]. Our results confirm the involvement of this brain area in olfactory perception, and they also emphasize the fact that whereas the activity within this region is modulated by the hedonic value of verbal labels used to described smells [de Araujo et al., 2005], it is not sensitive to the quality or meaning of the label (source vs. practice) used to described olfactory stimuli.
Another aspect of this study is that neural activity in the cingulate cortex and the insular gyrus were greater under the source label condition compared to the practice label condition, and such modulation was not sustained by changes in odor intensity, or odor pleasantness. Sniffing measurements collected in the control study outside of the scanner did not reveal any significant differences between the two label conditions, rendering unlikely that difference in nasal breathing accounted for the neural activation patterns observed here. Regarding the cingulate cortex, it is usually activated in response to chemosensory stimuli [Cerf‐Ducastel and Murphy, 2006; Croy et al., 2010; Reske et al., 2010; Sabri et al., 2005; Small et al., 2005]. An investigation in humans proposed the cingulate cortex as a multi‐integrative structure in processing chemosensory stimuli: for example, Small et al. showed increased activity in this brain region when a tastant and an odorant were concurrently perceived [Small et al., 2004]. In line with our findings, de Araujo et al. showed that neural activation to an odorant stimulus in cingulate cortex was modulated by the concurrent presentation of a positive compared to a negative verbal label [de Araujo et al., 2005]. Moreover, prior studies of odor processing in humans showed that reading olfaction‐related words induced activation in the anterior cingulate cortex [Gonzalez et al., 2006]. Combined with the above, our findings highlight a role of this area in binding olfactory representations of source objects and their lexical representations.
With regard to the insula, the statistical analysis revealed that this activation was more prominent for the food odors. The insula is a central area in processing food sensory stimuli including odors. Indeed, whereas the insula is activated by gustatory stimuli, most of the neuroimaging studies in humans also report activations in this brain region in response to olfactory stimulation [Cerf‐Ducastel and Murphy, 2001; de Araujo et al., 2003; Rolls et al., 2003]. In addition, there is evidence that patients with insular lesions exhibit also smell deficits [Stevenson et al., 2008]. Another important function of the insula is its involvement in multisensory integration and learning: one functional study showed supra‐additive response in the insula to congruent (sweet vanilla) but not incongruent (salty vanilla) odor‐taste mixtures, suggesting that taste‐odor integration occurring in this brain area depends upon learning and experience [Small et al., 2004]. Thus, food odors have the ability to activate the insula and it is through odor‐taste learning mechanisms that the strength between insular neurons that fire together is enhanced to the extent that upon future sensation of the odor, the taste cells that had been activated by the flavor stimulus may come to be sensitive to the smell alone [Small, 2008]. It is however important to note that an odor is never perceived in isolation, but always in a particular context whereby lexical cues play a prominent role [Bensafi et al., 2007a; Distel and Hudson, 2001; Herz, 2003; Moskowitz, 1979]. When available, words may also be determinant in odor‐taste integration and our findings highlight an increased insular response that may be related to such previous association between an odor, a taste and verbal cues referring to the name of the source food. It is nevertheless noteworthy that a complementary statistical analysis showed that whereas food odors and flower odors did not differ in hedonic valence (P > 0.05), they differed in perceived intensity (P < 0.05). Thus, we cannot discard the possibility that the insula activation reflects intensity differences. However, since food odors were perceived as less intense than flower odors, one may expect that they would induce less activity, which was not the case in this study.
In summary, the important new point being made in our study is that the quality of the verbal inputs (source or practice) is very important in influencing the activity of secondary or tertiary brain areas activated by odors; some of the findings, however, deserve discussion. First, contrasting different source labels with a single practice label may render the comparison not fully balanced. The possibility that this experimental choice may explain part of the present observations cannot be discarded at this stage and further work using equivalent number of labels in each condition (source and practice) are needed to test this alternative. Second, we see a difference in brain areas when the same odor is presented with one of two labels but no control of this is provided. One may therefore assume that the effect is reflected by the label alone and not by the odor. To address this concern, an analysis was run comparing all the OFF periods (that do not contain any olfactory stimulus) from the source condition on the one hand and the practice condition on the other hand. Whatever the contrast (source vs. practice or practice vs. source), no significant activation was seen suggesting that the effect of label is observed only in the presence of olfactory stimuli. Thirdly, it is not clear why no activation in primary olfactory areas such as the piriform cortex (PC) were observed. Actually, a notable feature of past and more recent neuroimaging studies in odor perception is the inconsistent activation of the PC in response to odors. This recurrent lack of PC activation may be due to several factors such as the sequences used in past fMRI studies that were often associated with susceptibility artifacts (signal loss) at air–tissue interfaces, olfactory habituation, respiration and the stimulation route (orthonasal vs. retronasal) (see Bensafi [ 2012], for a review). Although these experimental factors should be considered, we do not think that they are involved in the current observation. Indeed, PC activations were observed in a recent study that used a very similar protocol to ours [Burke et al., 2012]. A more prominent factor that may explain the lack of primary olfactory cortex activity is the putative top–down modulation of the label that likely occurred when the participant smelled the odor. Such top–down modulation of PC activity was already observed in past neuroimaging studies. For example, Savic et al. [ 2000] showed that, whereas a passive odor sensation task was able to activate the PC, when participants were engaged in a task requiring them to discriminate the quality of the odor percepts, no PC activation was seen. In another study, Kareken et al. [ 2003] observed PC activation during a detection task, but not during an identification task. In the same vein, Qureshy et al. [ 2000] found PC activation during a passive smelling task but not during an odor naming task.
In conclusion, by manipulating the verbal context, we showed that recovery of neural olfactory representation depends on the available lexical information. These findings are in line with a large set of data suggesting that contextual and verbal information present during odor encoding is a prominent factor in the emergence of olfactory representations [Ayabe‐Kanamura et al., 1998; Bensafi et al., 2007a; Bulsing et al., 2007, 2010; Dalton, 1999; de Araujo et al., 2005; Distel and Hudson, 2001; Djordjevic et al., 2008; Moskowitz, 1979; Poncelet et al., 2010a, 2010b]. Odors are generally emitted by a food, flower, place, animal, perfume, cosmetic, or person, and it is of this context that we are aware concurrently with the smell. Coding is known to be holistic in the case of olfaction, closely dependent on the exposure context, which conditions access to memory traces and the ability to name and describe [Schab, 1991]. “Context,” as far as olfaction is concerned, comprises the stimulation situation, including the task or instructions given to the subject [Bensafi et al., 2002, 2003; Rouby et al., 2009; Zatorre et al., 2000], and this study shows that verbal label categories (source or practice) are powerful shapers of the neural response to odors.
REFERENCES
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N (2003): Dissociated neural representations of intensity and valence in human olfaction. Nature Neurosci 6:196–202. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K (2003): Spatial normalization using basis function In: Frackowiak RSJ, editor.Human Brain Function, 2nd ed. Amsterdam:Academic Press. [Google Scholar]
- Ayabe‐Kanamura S, Schicker I, Laska M, Hudson R, Distel H, Kobayakawa T, Saito S (1998): Differences in perception of everyday odors: A Japanese‐German cross‐cultural study. Chem Senses 23:31–38. [DOI] [PubMed] [Google Scholar]
- Bensafi M (2012): The role of the piriform cortex in human olfactory perception: Insights from functional neuroimaging studies. Chemosens Percept 5:4–10. [Google Scholar]
- Bensafi M, Rouby C, Farget V, Vigouroux M, Holley A (2002): Asymmetry of pleasant vs. unpleasant odor processing during affective judgment in humans. Neurosci Lett 328:309–313. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Rouby C, Farget V, Bertrand B, Vigouroux M, Holley A (2003): Perceptual, affective, and cognitive judgments of odors: Pleasantness and handedness effects. Brain Cogn 51:270–275. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Rinck F, Schaal B, Rouby C (2007a): Verbal cues modulate hedonic perception of odors in 5‐year‐old children as well as in adults. Chem Senses 32:855–862. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Sobel N, Khan RM (2007b): Hedonic‐specific activity in piriform cortex during odor imagery mimics that during odor perception. J Neurophysiol 98:3254–3262. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Iannill iE, Poncelet J, Seo HS, Gerber J, Rouby C, Hummel T (2012): Dissociated representations of pleasant and unpleasant olfacto‐trigeminal mixtures: An fMRI study. PLoS One 7:e38358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin B, Kay P (1969): Basic color terms. Their universality and evolution. LA:University of California Press. [Google Scholar]
- Bulsing PJ, Smeets MA, Hummel T, van den Hout MA (2007): Influence of chemosensory pain‐expectancy on olfactory event‐related potentials. Neuroimage 38:164–170. [DOI] [PubMed] [Google Scholar]
- Bulsing PJ, Smeets MA, Gemeinhardt C, Laverman M, Schuster B, van den Hout MA, Hummel T (2010): Irritancy expectancy alters odor perception: Evidence from olfactory event‐related potential research. J Neurophysiol 104:2749–2756. [DOI] [PubMed] [Google Scholar]
- Burke SM, Veltman DJ, Gerber J, Hummel T, Bakker J (2012): Heterosexual men and women both show a hypothalamic response to the chemo‐signal androstadienone. PLoS One 7:e40993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Murphy C (2001): fMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses 26:625–637. [DOI] [PubMed] [Google Scholar]
- Cerf‐Ducastel B, Murphy C (2006): Neural substrates of cross‐modal olfactory recognition memory: An fMRI study. Neuroimage 31:386–396. [DOI] [PubMed] [Google Scholar]
- Croy I, Schellong J, Gerber J, Joraschky P, Iannilli E, Hummel T (2010): Women with a history of childhood maltreatment exhibit more activation in association areas following non‐traumatic olfactory stimuli: A fMRI study. PLoS One 5:e9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P (1999): Cognitive influences on health symptoms from acute chemical exposure. Health Psychol 18:579–590. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Kringelbach ML, McGlone F, Phillips N (2003): Taste‐olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur J Neurosci 18:2059–2068. [DOI] [PubMed] [Google Scholar]
- de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I (2005): Cognitive modulation of olfactory processing. Neuron 46:671–679. [DOI] [PubMed] [Google Scholar]
- Distel H, Hudson R (2001): Judgement of odor intensity is influenced by subjects' knowledge of the odor source. Chem Senses 26:247–251. [DOI] [PubMed] [Google Scholar]
- Djordjevic J, Lundstrom JN, Clement F, Boyle JA, Pouliot S, Jones‐Gotman M (2008): A rose by any other name: Would it smell as sweet? J Neurophysiol 99:386–393. [DOI] [PubMed] [Google Scholar]
- Dubois D (2000): Categories as acts of meaning: The case of categories in olfaction and audition. Cogn Sci Q 1:35–68. [Google Scholar]
- Dubois D, Rouby C (1997): Une approche de l'olfaction: Du linguistique au neuronal. Intellectica 24:9–20. [Google Scholar]
- Gonzalez J, Barros‐Loscertales A, Pulvermuller F, Meseguer V, Sanjuan A, Belloch V, Avila C (2006): Reading cinnamon activates olfactory brain regions. Neuroimage 32:906–912. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ (2002): Functional heterogeneity in human olfactory cortex: An event‐related functional magnetic resonance imaging study. J Neurosci 22:10819–10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz RS (2003): The effect of verbal context on olfactory perception. J Exp Psychol Gen 132:595–606. [DOI] [PubMed] [Google Scholar]
- Howes (1986): Le sens sans parole: Vers une anthropologie de l'odorat. Anthropologie et Sociétés 10:29–45. [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G (1997): 'Sniffin' sticks': Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD (2003): Functional anatomy of human odor sensation, discrimination, and identification in health and aging. Neuropsychology 17:482–495. [DOI] [PubMed] [Google Scholar]
- Kobal G (1981): Elektrophysiologische untersuchungen des menschlichen geruchssinns. Thieme Verlag, Stuttgart. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003): An automated method for neuroanatomic and cytoarchitectonic atlas‐based interrogation of fMRI data sets. Neuroimage 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- Marchellesi J, Grandin B (1974): Introduction à la sociolinguistique. Paris:Larousse Université. [Google Scholar]
- Moskowitz H (1979): Mind, body and pleasure: An analysis of factors which influence sensory hedonics In: Kroeze JHA, editor.Preference Behaviour and Chemoreception. London:Information Retrieval; pp 131–144. [Google Scholar]
- Penny WD, Holmes AP, Friston KJ (2003): Random effects analysis In: Frackowiak RSJ, Friston KJ, Frith C, Dolan R, Price CJ, Zeki S, Ashburner J, Penny WD, editors.Human Brain Function. New York:Academic Press. [Google Scholar]
- Poncelet J, Rinck F, Bourgeat F, Schaal B, Rouby C, Bensafi M, Hummel T (2010a): The effect of early experience on odor perception in humans: Psychological and physiological correlates. Behav Brain Res 208:458–465. [DOI] [PubMed] [Google Scholar]
- Poncelet J, Rinck F, Ziessel A, Joussain P, Thevenet M, Rouby C, Bensafi M (2010b): Semantic knowledge influences prewired hedonic responses to odors. PLoS One 5:e13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshy A, Kawashima R, Imran MB, Sugiura M, Goto R, Okada K, Inoue K, Itoh M, Schormann T, Zilles K and others (2000): Functional mapping of human brain in olfactory processing: A PET study. J Neurophysiol 84:1656–1666. [DOI] [PubMed] [Google Scholar]
- Reske M, Kellermann T, Shah NJ, Schneider F, Habel U (2010): Impact of valence and age on olfactory induced brain activation in healthy women. Behav Neurosci 124:414–422. [DOI] [PubMed] [Google Scholar]
- Rinck F, Barkat‐Defradas M, Chakirian A, Joussain P, Bourgeat F, Thevenet M, Rouby C, Bensafi M (2011): Ontogeny of odor liking during childhood and its relation to language development. Chem Senses 36:83–91. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kringelbach ML, de Araujo IE (2003): Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci 18:695–703. [DOI] [PubMed] [Google Scholar]
- Rosch‐Heider E (1971): “Focal” color areas and the development of color names. Dev Psychol 4:447–455. [Google Scholar]
- Rouby C, Pouliot S, Bensafi M (2009): Odor hedonics and their modulators. Food Qual Prefer 8:545–549. [Google Scholar]
- Sabri M, Radnovich AJ, Li TQ, Kareken DA (2005): Neural correlates of olfactory change detection. Neuroimage 25:969–974. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P (2000): Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26:735–745. [DOI] [PubMed] [Google Scholar]
- Schab FR (1991): Odor memory: Taking stock. Psychol Bull 109:242–251. [DOI] [PubMed] [Google Scholar]
- Seo HS, Iannilli E, Hummel C, Okazaki Y, Buschhuter D, Gerber J, Krammer GE, van Lengerich B, Hummel T (2011): A salty‐congruent odor enhances saltiness: Functional magnetic resonance imaging study. Hum Brain Mapp [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM (2008): Flavor and the formation of category‐specific processing in olfaction. Chemosens Percept 1:136–146. [Google Scholar]
- Small DM, Voss J, Mak YE, Simmons KB, Parrish T, Gitelman D (2004): Experience‐dependent neural integration of taste and smell in the human brain. J Neurophysiol 92:1892–1903. [DOI] [PubMed] [Google Scholar]
- Small DM, Gerber JC, Mak YE, Hummel T (2005): Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 47:593–605. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD (1998): Sniffing and smelling: Separate subsystems in the human olfactory cortex. Nature 392:282–286. [DOI] [PubMed] [Google Scholar]
- Stevenson RJ, Miller LA, Thayer ZC (2008): Impairments in the perception of odor‐induced tastes and their relationship to impairments in taste perception. J Exp Psychol Hum Percept Perform 34:1183–1197. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Jones‐Gotman M, Rouby C (2000): Neural mechanisms involved in odor pleasantness and intensity judgments. Neuroreport 11:2711–2716. [DOI] [PubMed] [Google Scholar]


