Abstract
Simultaneous collection of scalp EEG and fMRI has become an important tool for studying the hemodynamic changes associated with interictal epileptiform discharges (IEDs) in persons with epilepsy, and has become a standard presurgical assessment tool in some centres. We previously demonstrated that performing EEG‐fMRI using intracranial electrodes (iEEG‐fMRI) is of low risk to patients in our research centre, and offers unique insight into BOLD signal changes associated with IEDs recorded from very discrete sources. However, it is unknown whether the BOLD response corresponding to IEDs recorded by iEEG‐fMRI follows the canonical hemodynamic response. We therefore scanned 11 presurgical epilepsy patients using iEEG‐fMRI, and assessed the hemodynamic response associated with individual IEDs using two methods: assessment of BOLD signal changes associated with isolated IEDs at the location of the active intracranial electrode, and by estimating subject‐specific impulse response functions to isolated IEDs. We found that the hemodynamic response associated with the intracranially recorded discharges varied by patient and by spike location. The observed shape and timing differences also deviated from the canonical hemodynamic response function traditionally used in many fMRI experiments. It is recommended that future iEEG‐fMRI studies of IEDs use a flexible hemodynamic response model when performing parametric tests to accurately characterize these data. Hum Brain Mapp 36:5252–5264, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: epilepsy, BOLD response, seizure focus, epileptiform discharge
INTRODUCTION
Simultaneous acquisition of scalp EEG and functional magnetic resonance images (fMRI) sensitized to blood oxygenation level‐dependent (BOLD) contrast has been utilized in clinical and research settings to study hemodynamic changes associated with interictal discharges (IEDs). IEDs are an indicator of epileptogenic tissue and provide useful clinical information for seizure localization [Noachtar and Borggraefe, 2009; Ojemann, 1987]. Current literature supports an expanding role for EEG‐fMRI in clinical decision‐making: The BOLD response was found to be concordant with the IED field in 88% of patients studied, and in 64% of these patients, EEG‐fMRI provided information that helped localize the seizure focus [Pittau et al., 2012]. Another study of patients undergoing epilepsy surgery found that surgical resections which completely removed cortical tissue with maximal IED‐correlated BOLD signal lead to seizure freedom in 60% of patients [Thornton et al., 2010]. In patients deemed ineligible for surgery through conventional techniques, EEG‐fMRI improved localization in four of six patients with unclear foci and confirmed multifocality in four of five presumed multifocal patients [Zijlmans et al., 2007].
Despite these findings, not all published literature supports the clinical usefulness of simultaneous scalp EEG‐fMRI. In particular, a recent report assessed the relative value of EEG‐fMRI and EEG source localization as presurgical assessment tools [Elshoff et al., 2012]. Here the localization of the seizure focus as determined by either EEG‐fMRI or EEG source localization was compared with postoperative outcomes in children aged 1 to 17 years. Unlike previous publications showing a strong correlation between seizure freedom and resection of the cortex containing the maximum BOLD cluster determined through EEG‐fMRI in adults [An et al., 2013; Thornton et al., 2010], the authors found that the epileptogenic zones predicted by EEG‐fMRI were within the resection margins of only 33% of the patients that had successful epilepsy surgery (Engel class I–IIb). In contrast, 100% of patients analyzed with EEG source localization had seizure foci within resection margins. The authors suggest that the poor performance of EEG‐fMRI may be related to the low number of discharges that were recorded, or limited spatial coverage of the epileptogenic focus by scalp EEG.
A novel development in the field of simultaneous EEG‐fMRI is the recent utilization of intracranial strip, grid, and depth electrodes instead of scalp electrodes. Simultaneous intracranial EEG‐fMRI (iEEG‐fMRI), after extensive safety testing [Boucousis et al., 2012; Carmichael et al., 2010], has been implemented at 1.5 T [Vulliemoz et al., 2011] and at 3 T by our group [Aghakhani et al., 2015; Cunningham et al., 2012]. The ability to record EEG from a significantly smaller volume of cortical tissue (presumed to be epileptogenic by other modalities) has the potential to improve localization and offer new insights into the BOLD correlates of epileptogenic activity. However, detection of BOLD signal changes is highly dependent on the accuracy of the model describing the expected shape of the BOLD signal change to IEDs. Furthermore, modeling the BOLD response to discharges recorded from intracranial electrodes may advance our understanding of the hemodynamic changes associated with highly localized epileptiform discharges.
Neural activations produce a characteristic change in BOLD signal that is modeled in statistical analyses as a transfer function peaking at 5 to 6 s [Buxton et al., 2004]. Known as the hemodynamic response function (HRF), this model of signal change is ubiquitous in fMRI analyses and typically assumed to be linear, spatially and temporally invariant, and consistent across subjects. However, BOLD signal changes associated with scalp recorded EEG epileptic events have been found to vary in latency, peak amplitude, duration, and shape [Bagshaw et al., 2004; Jacobs et al., 2008]. These variations can have a negative impact on significance testing leading to magnitude misestimates, decreased t values, and increased false negatives [Handwerker et al., 2004]. Additionally, findings from event‐related fMRI experiments point to regional variants of hemodynamic responses as variations in the shape and timing varied between M1 (primary motor cortex) and V1 (primary visual cortex) [Handwerker et al., 2004; Miezin et al., 2000]. By modeling the HRF best corresponding to IEDs obtained from intracranial electrodes, the possibility exists to identify BOLD changes to epileptiform activity corresponding to a discrete region of cerebral cortex.
Therefore, in the present study, we utilized the ability of iEEG‐fMRI to record directly from the presumed epileptic focus to assess BOLD signal changes associated with IEDs arising from different brain regions. Furthermore, we explored the shape and timing of subject‐specific hemodynamic response functions corresponding to IEDs recorded by iEEG. By modeling the HRF best corresponding to discharges recorded by iEEG, we were able to model the BOLD response to highly localized epileptiform activity.
MATERIALS AND METHODS
Intracranial EEG Subjects
Eleven consecutive patients undergoing intracranial video EEG monitoring (VEM) were recruited that met the following inclusion criteria: greater than or equal to 18 years of age, no postimplantation complications (e.g., subdural hematoma), and ability to provide informed consent. This project was approved by the Conjoint Health Research Ethics Board of our institution.
Intracranial VEM data were reviewed by an experienced epileptologist (PF), and used to select electrodes to be recorded from during the iEEG‐fMRI scan. The first seven subjects scanned had two strip or depth electrodes selected for data collection. Electrodes were attached, via commercially available connector blocks (product number L‐SRL‐10DIN; Ad‐Tech, Racine, WI), to a custom‐built, two‐tailed electrode connector capable of recording 19 contacts (Compumedics NeuroScan, Charlotte, NC) and coupled with a commercial scalp EEG‐fMRI system (Maglink RT; Compumedics NeuroScan). The remaining four subjects had up to eight strip or depth electrodes selected for data collection and were attached to a custom built, eight‐tailed electrode connector capable of recording 64 contacts (Compumedics NeuroScan). We have previously established that these connectors are low‐risk in our 3 T environment [Boucousis et al., 2012; Cunningham et al., 2012].
iEEG‐fMRI Acquisition
Subjects underwent 10 to 60 min of fMRI scanning as per the gradual implementation protocol established before the start of the project [Cunningham et al., 2012]. EEG data were continuously collected at 10 kHz using a SynAmps2 amplification/digitization system and Scan 4.4 Software (Compumedics NeuroScan). The first seven patients were scanned using a 3 T GE Signa LX whole body scanner, receive‐only eight‐channel phased‐array head receive/body transmit coil, while the following four were scanned with a 3 T GE Discovery MR750 whole body scanner, receive‐only eight‐channel phased‐array head receive/body transmit coil (GE Healthcare, Waukesha, WI). No adverse events were reported during scanning by any patient. Additionally, subjects were monitored in the seizure unit for a minimum of 24 h after iEEG‐fMRI and no complications were observed.
The MR imaging protocol for the 3 T GE Signa LX scanner included multislice anatomical imaging (spoiled gradient‐recalled echo, TE = min full, TR = 150 ms, flip angle = 18°, 128 × 128 matrix, 24 5 mm thick slices), anatomical three‐dimensional (3D) T1‐weighted imaging (magnetization‐prepared rapid gradient‐echo: TE = min full, TR = 8.9 ms, flip angle = 20°, 384 × 256 × 64 matrix, 2 mm thick slices), a nonlinear shimming sequence to minimize magnetic field inhomogeneities, and fMRI (gradient recalled echo planar imaging sequence, with TE = 30 ms, TR = 1,500 ms, flip angle = 60°, 24‐cm field of view, 64 × 64 matrix, 24 slices 5 mm thick). The MR imaging protocol for the 3 T GE Discovery MR750 included multislice anatomical imaging (spoiled gradient‐recalled echo two‐dimensional multislice sequence, TE = 2.1 ms, TR = 150 ms, flip angle = 18°, 128 × 128 matrix, 24 slices 5 mm thick), anatomical 3D T1‐weighted imaging (TE = 3.8 ms, TR = 9.3 ms, flip angle = 12°, 24‐cm field of view, 320 × 256 × 64 matrix, 2 mm thick slices), a nonlinear shimming sequence to minimize magnetic field inhomogeneities, and fMRI gradient recalled echo planar imaging, TE = 30 ms, TR = 1,500 ms, flip angle = 65°, 24‐cm field of view, 64 × 64 matrix, 24 slices 5 mm thick).
Subjects' heads were immobilized in the head coil using compressible foam cushions. The electrode tails/connector blocks were directed outside of the coil and secured to the scanning table to minimize movement and heating from RF energy deposition. Subjects were not asked to perform a task, and no visual fixation stimulus was presented. Levels of wakefulness were not monitored, but subjects were encouraged to sleep. During the iEEG‐fMRI data collection, the EEG was filtered online for viewing purposes and was monitored in real‐time by an experienced epileptologist (PF). Raw EEG data were stored for offline analyses.
Intracranial EEG Data Processing
All EEG data processing was performed using ScanEdit v4.4 (Compumedics NeuroScan). Gradient switching artifact was removed utilizing average artifact subtraction, which uses the regularity of the gradient switching induced waveform to generate a moving average artifact that can be successfully removed from the baseline EEG [Allen et al., 2000]. There was no cardioballistic artifact seen in the recordings, similar to clinical intracranial recordings, so no removal was necessary.
A low‐pass filter at 30 Hz was applied to the EEG data, and a bipolar montage for each electrode bank was generated. Two experienced electroencephalographers (P.F. and Y.A.) reviewed the EEG data noting the timing, morphology, and location of interictal discharges.
fMRI Data Processing
Functional MRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 6.0, part of FSL (FMRIB's Software Library, http://www.fmrib.ox.ac.uk/fsl). The following pre‐processing was applied: motion correction using MCFLIRT [Jenkinson et al., 2002]; slice‐timing correction using Fourier‐space time‐series phase‐shifting; non‐brain removal using BET [Smith, 2002]; spatial smoothing using a Gaussian kernel with a FWHM of 6.0 mm; high‐pass temporal filtering (Gaussian‐weighted least‐squares straight line fitting, with sigma = 50.0 s). Linear registration of functional data to high‐resolution structural and standard space images was carried out using FLIRT [Jenkinson et al., 2002; Jenkinson and Smith, 2001]. Movement was minimal (<3 mm) in most patients. If a subject had >3 mm translation in any axis, then the fMRI data containing these segments were excluded from analyses. Therefore, motion parameters were not included as regressors.
Region of Interest Selection
A region of interest (ROI) was generated for each subject based on the location of the most active intracranial EEG electrode contact, defined as the maximal amplitude in referential montage and/or phase reversal in bipolar montage. Visual inspection of the isolated IEDs revealed that in the vast majority of events, the spiking activity was maximal in one electrode, with an EEG field in one to three adjacent electrodes.
T1 structural images and 3D rendered CT images with overlaid electrode locations (Atamai Epilepsy Viewer, London, ON, Canada) were used to create an ROI near the active contact. The ROIs were patient‐specific and spherical or hemispherical in shape dependent on whether the electrode was implanted in cortical tissue (depth) or placed on the cortical surface (strip), respectively. The ROIs were created to generously encompass the cortex nearest to the electrode contact with the maximal EEG response. ROIs were then registered to native fMRI data space using FLIRT. The average size of the ROIs was 196 voxels (range: 89 to 548 voxels) as efforts were made to include all cortical tissue near the active electrode that may show an elicited response. The size of the ROIs were then trimmed to only the 50 voxels (∼3.5 cm3) with the most correlated time‐courses using intervoxel cross correlation [Golestani and Goodyear, 2011] to maximize signal‐to‐noise ratio (SNR) and minimize the effect of susceptibility artifact and signal loss immediately adjacent to the electrode contact.
Unlike previous studies examining BOLD signal changes associated with IEDs, the ROI was created without the use of statistical parametric maps of BOLD activation [Aghakhani et al., 2006; Bagshaw et al., 2004; Salek‐Haddadi et al., 2006]. This avoided any bias associated with assumptions of the shape and timing of the hemodynamic response function necessary to model the event‐related fMRI design.
Time‐Course Analyses
To assess the time‐course of BOLD signal changes associated with intracranially recorded IEDs, it was necessary to identify isolated IEDs. Isolated spikes were defined as follows: spikes with maximal response recorded on the same electrode that had no other IEDs occurring at least 3 s (two imaging TRs) before the spike, and 12 s (eight imaging TRs) following it. The average BOLD time‐series of the ROI voxels for each subject was extracted at each occurrence of an isolated IED. In order to average the responses to individual IEDs, t = 0 s was established as the imaging TR <0.75 s from the onset of the spike. The BOLD time‐series from each individual IED was then averaged across runs to generate time‐courses characteristic of the IED for each subject.
Hemodynamic Response Fitting
To estimate subject‐specific hemodynamic response functions, the averaged time‐courses extracted from the 50‐voxel ROIs were used. This estimation was achieved by modeling the fMRI time‐course as the convolution of a HRF model and the stimulus function. The HRF model implemented was the sum of two gamma functions with six free parameters, similar to previous work estimating the HRF [Handwerker et al., 2004; Shan et al., 2014]:
where are the parameters responsible for the height and direction, shape and scale of each of the two gamma ( functions, respectively. For the stimulus function, delta functions corresponding to the onset times of the isolated discharges were used. The number of isolated spikes identified is shown in Table 1. Fitting of isolated spikes was initialized using parameters as implemented by Shan et al. [2014]. Nonlinear optimization using a modified constrained Nelder‐Mead Simplex algorithm (see [Shan et al., 2014]) was implemented to obtain the six parameters of the subject‐specific HRF that minimized the residual sums of squares (RSS) between the expected and raw time‐courses. We performed careful visual inspection to ensure high fitting accuracy, characterized as low discrepancy between the modeled fMRI time‐course and the actual fMRI time‐course. We also averaged the RSS values across all subjects in order to detect runs of data to be considered as outliers. Runs were considered as outliers if the RSS value was greater than one standard deviation from the group mean. Patients completed two or three runs each. Runs of data were removed from the analysis if the visual inspection revealed a poor fit, and if the run was considered an outlier based on its RSS value. The modeling was performed using in‐house code running on MATLAB [Shan et al., 2014]. For each subject, HRFs were generated for each run of data and averaged to provide a robust subject‐specific response. From each subject‐specific HRF, summary features of the positive response were calculated including the time of onset (onset), time‐to‐peak (TTP), and full width at half maximum (FWHM). For comparison, a canonical HRF was generated using a repetition time equal to the one used in the current study (1,500 ms) and the default parameters implemented previously by others [Shan et al., 2014].
Table 1.
Patient demographic and clinical data
| Subject | Age/sex | Active iEEG electrode | Classification | Scan length | No. isolated IEDs | Total no. IEDs |
|---|---|---|---|---|---|---|
| 1 | 20/♀ | Left posterior temporal | Mesial temp | 10 min | 9 | 216 |
| 2 | 29/♀ | Right mesial temporal | Mesial temp | 20 min | 11 | 184 |
| 2 | 29/♀ | Left mesial temporal | Mesial temp | 20 min | 13 | 277 |
| 3 | 24/♂ | Left mesial temporal | Mesial temp | 60 min | 22 | 2,092 |
| 4 | 29/♀ | Left posterior lateral frontal | Extra temp | 35 min | 8 | 37 |
| 5 | 24/♀ | Middle temporal gyrus | Lateral temp | 11 min | 12 | 75 |
| 6 | 22/♂ | Left anterior parietal | Extra temp | 60 min | 9 | 2,611 |
| 7 | 56/♀ | Left lateral temporal | Lateral temp | 35 min | 14 | 110 |
RESULTS
All 11 subjects underwent simultaneous iEEG‐fMRI without adverse events. Three subjects from the initial group of subjects studied were excluded from analysis because their EEG was uninterpretable due to inadequate grounding of the EEG signal and persistent MR gradient switching EEG artifact. These limitations have since been corrected. One additional dataset was rejected due to excessive patient movement, thus leaving seven patients in the study. One subject (Subject 2) had bilateral, independent, mesial temporal interictal spiking; analyses were performed on each side independently for this subject. Thus a total of eight datasets from seven patients were analyzed.
Clinical and demographic data of subjects whose data were analyzed are summarized in Table 1. The quality of iEEG data recorded during scanning was comparable to iEEG recorded in the seizure‐monitoring unit (Fig. 1). Subject motion during scanning was less than 2 mm from baseline (head position at middle volume) for the majority of the scans. All subjects completed the entire iEEG‐fMRI protocol, except subject 5 who stopped after two fMRI sequences due to a headache that had been experienced since electrode implantation.
Figure 1.
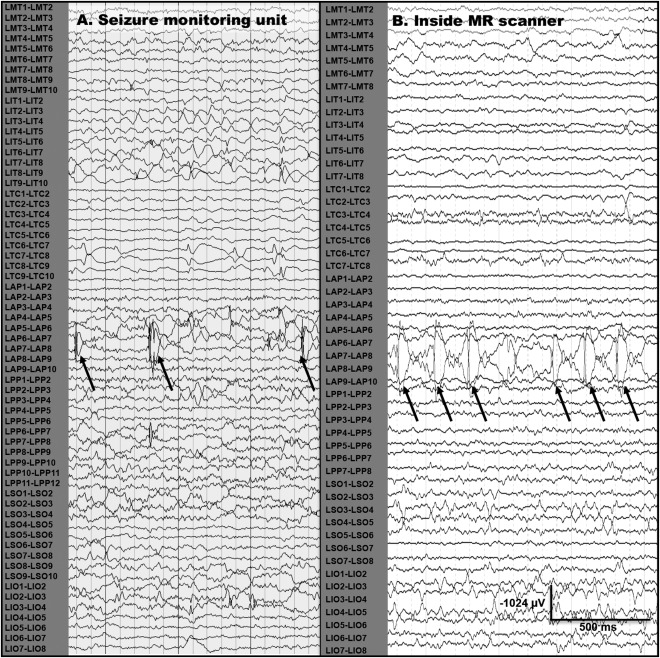
Epileptiform discharges recorded in seizure unit and in MR scanner during iEEG‐fMRI scanning. (A) Epileptiform discharges recorded from a left anterior parietal (LAP) subdural strip electrode during admission to the seizure monitoring unit for subject 6. (B) Epileptiform discharges recorded from the same location in the same patient while undergoing simultaneous iEEG‐fMRI at 3 T.
Interictal Spiking
The total number of interictal spikes recorded for each subject ranged from 37 to 2,611 (Table 1). As mentioned previously, spikes of similar waveform and similar location that had no other IED occurring within 3 s before or 12 s after its occurrence were included in the analysis. While this limited the total number of spikes analyzed, it was essential for determination of the BOLD signal changes associated with single events. Figure 2 illustrates examples of isolated and non‐isolated spikes for a single subject. Using this approach, 9‐22 isolated discharges were identified for each dataset (Table 1). Table 1 also describes the location of each patient's active electrode(s): three subjects had discharges recorded from mesial temporal electrodes, two lateral temporal, and two extratemporal (frontal and parietal).
Figure 2.
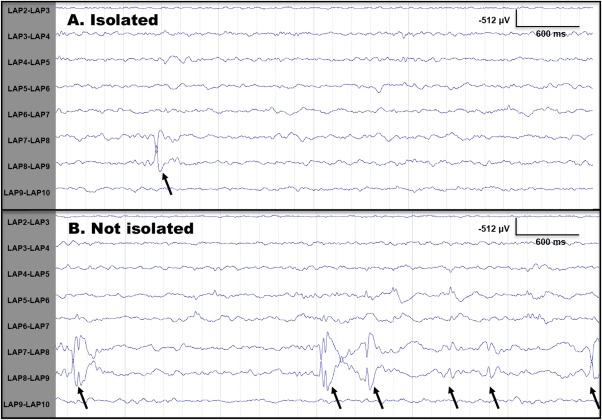
Examples of isolated and non‐isolated epileptiform discharges. (A) Epileptiform discharge recorded from a left anterior parietal (LAP) subdural strip electrode during iEEG‐fMRI scanning for subject 6. This spike would be considered an isolated spike and utilized for analysis as there is at least 3 s prespike, and 10 s postspike with no other epileptiform activity. (B) Epileptiform discharges recorded during the same iEEG‐fMRI study that would not be considered isolated since these did not have an absence of epileptiform activity for 3 s before the spike and 12 s following the spike.
Time‐Course Analyses
Average BOLD time‐series for the IEDs recorded for each subject are shown in Figure 3. The mean peak amplitude was 0.19% (n = 8, range: 0.07 to 0.34%) for BOLD signal increases, and −0.19% (n = 8, range: −0.05 to −0.41%) for BOLD signal decreases. All subjects showed a distinct peak in BOLD signal, but the time‐to‐peak was variable between subjects: for patients with a mesial temporal focus, time‐to‐peak was 8.6 ± 0.7 s (n = 4), whereas it was earlier for the lateral temporal group where both patients (2/2) peaked at 0 s, and later for the extratemporal group where both patients (2/2) had peak signal at 10.5 s. A decrease in BOLD signal immediately following the spike (initial dip) was observed in four datasets [Subject 2 (right), 2 (left), 4, and 6]. A decrease in BOLD signal intensity below baseline after peak intensity (poststimulus undershoot) was observed in 4 datasets [Subject 2 (left), 3, 5, and 7].
Figure 3.
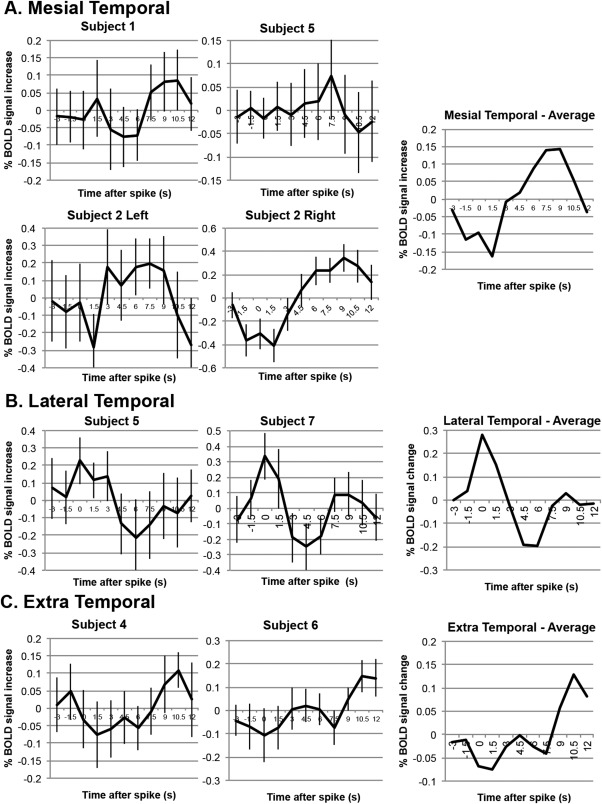
BOLD signal changes associated with epileptiform discharges recorded from intracranial electrodes. (A) BOLD signal changes in four patients associated with epileptiform discharges originating from mesial temporal electrodes. (B) BOLD signal changes in two patients associated with epileptiform discharges originating from lateral temporal electrodes. (C) BOLD signal changes in two patients associated with epileptiform discharges originating from extratemporal electrodes. Standard error reported at each averaged time‐point for every subject.
Hemodynamic Response Fitting
One patient demonstrated poor fits for all runs upon visual inspection. These poor fits were due to noisy data induced by excessive movement—this patient was therefore removed from all analyses for excessive movement, as outlined above. For the remaining seven patients in the analysis, three runs of data from three separate patients [Subjects 2 (right), 5 and 6] were removed from the averaged subject‐specific HRFs for poor fits. The six parameters generating the subject‐specific HRF shapes, and the corresponding summary features of time‐to‐peak, width and onset time of the positive response were obtained. These data are shown in Table 2.
Table 2.
Summary features (onset time of positive response, TTP, FWHM) of the subject‐specific hemodynamic response functions
| Subject | Classification | Onset | TTP | FWHM |
|---|---|---|---|---|
| 1 | MT | 2.9 (+0.7) | 11.9 (+5.9) | 12.5 (+6.8) |
| 2 (left) | MT | 2.0 (−0.2) | 5.5 (−0.5) | 5.2 (−0.5) |
| 2 (right) | MT | 2.5 (+0.3) | 6.5 (+0.5) | 4.7 (−1.0) |
| 3 | MT | 3.4 (+1.2) | 9.7 (+3.7) | 8.8 (+3.1) |
| Average mesial temporal | 2.7 (+0.5) | 8.4 (+2.4) | 7.8 (+2.1) | |
| 5 | LT | 5.0 (+2.8) | 11.7 (+5.7) | 7.5 (+1.8) |
| 7 | LT | 2.8 (+0.6) | 6.7 (+5.7) | 5.1 (−0.6) |
| Average lateral temporal | 3.9 (+1.7) | 9.2 (+3.2) | 6.3 (+0.6) | |
| 4 | ExT | 1.5 (−0.7) | 4.3 (−1.7) | 3.5 (−2.2) |
| 6 | ExT | 2.6 (+0.4) | 6.3 (+0.3) | 5.1 (−0.6) |
| Average extratemporal | 2.1 (−0.2) | 5.3 (−0.7) | 4.3 (−1.4) | |
| Canonical HRF | 2.2 | 6.0 | 5.7 | |
Canonical HRF parameters shown for comparison at bottom and differences between single subject parameters and canonical HRF shown in parenthesis. All values are provided in seconds. MT: mesial temporal, ExT: extratemporal, LT: lateral temporal, TTP: time‐to‐peak of the positive response.
The shape and timing of each subject's HRF relative to the canonical HRF is shown in Figure 4. Similar to the time‐course analyses, results of the HRF fitting varied between patients and groups: the onset time of the positive response varied between 2.0 s (−0.2 s compared with canonical) and 5.0 s (+2.8 s vs. canonical). Both the mesial and lateral temporal groups had average onset times greater than the canonical HRF (mesial temporal average 2.7 ± 0.3 s, +0.5 s vs. canonical, lateral temporal average 3.9 ± 1.1 s, +1.7 s vs. canonical), while the extratemporal group had an onset time very similar to the canonical function (extratemporal average 2.1 ± 0.4 s, −0.1 s vs. canonical). The time‐to‐peak of the BOLD response varied similarly: the shortest TTP was 4.3 s (−1.7 s vs. canonical) while the longest was 11.9 s (+5.9 s vs. canonical). Again, both the mesial and lateral temporal groups had average TTP greater than the canonical HRF (mesial temporal average 8.4 ± 1.5 s, +2.4 s vs. canonical, lateral temporal average 9.2 ± 2.5 s, +3.2 s vs. canonical), while the extratemporal group had an onset time more similar to the canonical function (extratemporal average 5.3 ± 1.0 s, −0.7 s vs. canonical). The FWHM values ranged from 3.5 s (−2.2 s vs. canonical) to 12.5 s (+6.8 s vs. canonical). Group‐wise, the mesial temporal average was 7.8 ± 1.8 s (+2.1 s vs. canonical), lateral temporal average was 6.3 ± 1.2 s (+0.6 s vs. canonical), and the extratemporal average was 4.3 ± 0.6 s (−1.4 s vs. canonical).
Figure 4.
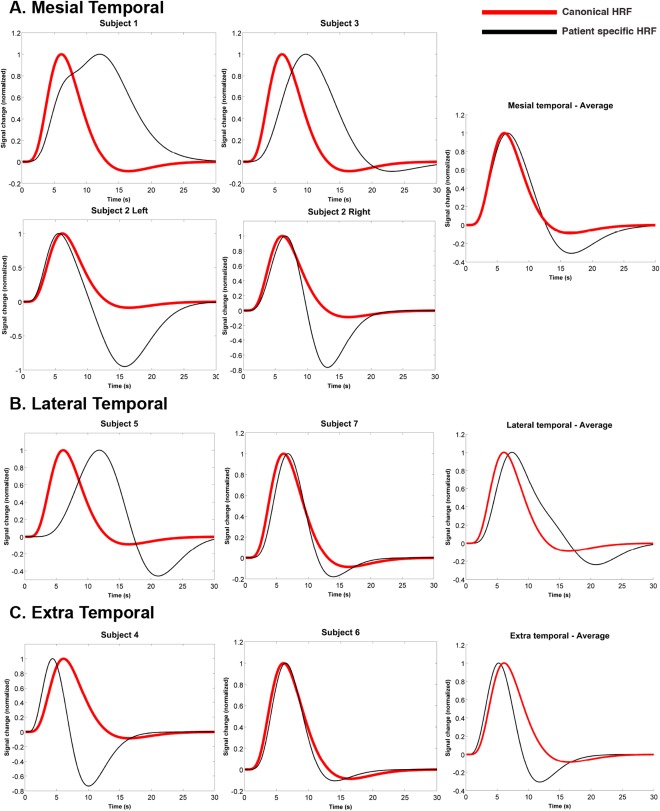
Estimated subject‐specific hemodynamic response functions (black line) with the canonical HRF (red line) overlaid for comparison. The shape and timing of each subject's HRF relative to the canonical HRF as determined by fitting analysis for (A) patients with epileptiform discharges originating from mesial temporal electrodes, (B) patients with epileptiform discharges originating from lateral temporal electrodes, and (C) patients with epileptiform discharges originating from extratemporal electrode. The average function for all patients in each group is compared with the canonical HRF in the rightmost graph. The six parameters generating the subject‐specific HRF functions and the corresponding summary features were time‐to‐peak, width, and onset time of positive response.
DISCUSSION
The successful integration of simultaneous intracranial EEG and fMRI has provided unique insight into BOLD signal changes associated with epileptiform activity. While previous work examining the hemodynamic response to interictal discharges utilized scalp EEG [Bagshaw et al., 2004; Salek‐Haddadi et al., 2006], recording IEDs with intracranial electrodes allows for the examination of BOLD signal changes from a much more discrete source. We sought to assess the hemodynamic changes associated with IEDs using two well‐published methods for characterizing the hemodynamic response to neuronal events: BOLD time‐course analysis and HRF fitting. Our goal was to assess the feasibility of utilizing the canonical HRF (Glover model) in intracranial EEG‐fMRI analysis as the scalp EEG‐fMRI literature is divided: many laboratories convolve IED timings with a canonical HRF, while many others use flexible convolution models.
We found that hemodynamic responses to IEDs recorded by iEEG varied by spike location and subject, and rarely matched the shape and timing of the canonical HRF. Indeed, the averaged time‐courses from mesial temporal IEDs (three subjects with four separate datasets) showed a time‐to‐peak of 8.6 s, a difference of >3.5 s from the canonical HRF peaks of 5 to 6 s (Fig. 3). Extratemporal IEDs (n = 2, 1 frontal and 1 parietal) had an even more delayed time‐to‐peak of 10.5 s. In contrast, patients with lateral temporal discharges (n = 2) had an early time‐to‐peak of 0.0 s (Fig. 3). These findings suggest that BOLD responses associated with IEDs recorded from a discrete tissue source via iEEG varies by location of epileptogenic tissue, and is dissimilar to the widely used canonical HRF in scalp EEG‐fMRI.
Our finding that lateral temporal discharges were associated with BOLD signal changes peaking at 0 seconds is consistent with previous scalp EEG‐fMRI studies showing BOLD perturbations before IED onset [Grouiller et al., 2010; Hawco et al., 2007; Jacobs et al., 2009]. Maximum activation was found for most patients using the HRF model that peaked at 1 s post‐IED, suggesting that neural changes undetectable by scalp EEG recordings occur before IEDs [Hawco et al., 2007]. Another study of four patients examined intracranial EEG recordings from the location corresponding to an early BOLD response seen by scalp EEG‐fMRI [Pittau et al., 2011]. Only one of those subjects showed an IED recorded by iEEG, suggesting that the early BOLD response most likely reflects metabolic or physiological processes not associated with synchronized neural activity.
Time‐Course Analysis Versus HRF Fitting
We observed differences between the time‐course and HRF fitting data for TTP and other measures. Specifically, the TTPs were not appreciably different for the mesial temporal group (8.6 ± 0.7 vs. 8.4 ± 1.5 s for time‐course vs. fitting analysis, respectively). However, differences between the analyses were seen for the lateral temporal group (0.0 ± 0.0 s vs. 9.2 ± 2.5 s) and extratemporal group (10.5 ± 0.0 vs. 5.3 ± 1.0 s). In addition, the pre‐spike BOLD changes identified in the current study, highlighted in Figure 3, were also not captured in the HRF fitting analyses as demonstrated in Figure 4.
The differences between the two analyses are likely related to separate methodologies and in particular, the data used for each of the respective analyses. The time‐series analysis examined isolated epochs of data seeking to visualize the raw BOLD signal changes associated with a single IED. Using this analysis, we looked for differences in the shape and timing of the BOLD signal time‐course between patients, between discharge locations, and compared these time‐courses to the canonical HRF. We also included both prespike signal changes in the time‐course analysis because pre‐spike perturbations may help further elucidate the neurophysiologic changes associated with interictal spiking.
With the HRF fitting analysis, pre‐spike perturbations were not modeled. Instead, this analysis aimed to provide insights that could facilitate future statistical methods for iEEG‐fMRI analyses. To achieve this aim, the HRF fitting analysis examined the entire time‐series to determine the best‐fitting parameters of the HRF model that best characterized BOLD changes associated with all isolated IEDs for each patient. The HRF fitting required consistency with fMRI statistical analyses using the general linear model (GLM), which builds GLM regressors by modeling the entire time‐series. We therefore only modeled post‐IED BOLD signal changes in this analysis. This method, when used with t tests within the GLM, is commonly utilized to identify BOLD signal changes corresponding to IEDs [Lu et al., 2006; Salek‐Haddadi et al., 2003].
The discrepancies between the HRF fitting and time‐course analysis highlights caveats associated with this common practice. The time‐courses, as shown in Figure 3, clearly demonstrate pre‐IED BOLD signal changes that are not captured by the HRF fitting. This exemplifies how the BOLD response to IEDs recorded by intracranial EEG deviates from the canonical HRF, and warrants the need for more flexible modeling. We recommend that these models be flexible in response shape, timing, and onset time around the IED in order to capture pre‐IED BOLD changes.
In addition, some of the apparent discrepancy between techniques may be related to differences in time‐scale between Figures 3 and 4. The raw BOLD time‐course analysis examined a total of 15 s (3 s pre‐IED and 12 s post‐IED) while the fitting analysis required 30 s post‐IED, as this is approximately the length of the HRF. With this in mind, similarities in the results obtained with the two analyses can be seen: for example, Subject 2 left and right, the HRF fitting analyses revealed a prominent undershoot (Fig. 4A). This is not immediately visible on the corresponding time‐course analysis in Figure 3A; however, the decreasing signal amplitude at the termination of the time‐course at time +12 s indicates that, should the time‐course have been temporally extended, an undershoot may have been visible. Thus, there is probable agreement between the two analyses in this regard.
Location‐Dependant HRF Variability
The variability in time‐to‐peak BOLD intensity by anatomical location presented in our study has been seen in a previous scalp EEG‐fMRI study [Kang et al., 2003]. The authors examined multiple areas of activation in the frontal, parietal, and temporal lobes from a single IED type in each of eight patients and found peak intensity times varied between 3.5 and 6.2 s, varying even within the same subject. Both of our analyses showed variation between locations, being most apparent in the time‐course analysis: mesial and extratemporal discharges had time‐to‐peak BOLD intensity values of 8.6 and 10.5 s respectively, while the peak intensity was found at the time of the spiking event (0.0 s) for lateral temporal discharges. This finding may reflect metabolic changes occurring before the IED in the lateral temporal cortex. A more recent study comparing the HRF between two patient groups (hippocampal sclerosis vs. focal cortical dysplasia) found that only the peak HRF amplitude varied between groups, while other HRF parameters remained similar [Watanabe et al., 2014]. Since the locations of lesions were variable by patient, this work may imply the hemodynamic response to IEDs recorded via scalp EEG‐fMRI do not vary by location as seen in the intracranial EEG‐fMRI data presented here. Further exploration is warranted.
Intersubject variability that is not location‐dependant may also contribute to some of the differences observed in the present study. Intersubject HRF variability has been demonstrated in a number of previous studies in a variety of brain locations using different paradigms [Aguirre et al., 1998; Handwerker et al., 2004; Lindquist et al., 2009; Lindquist and Wager, 2007; Shan et al., 2014]. The contribution of intersubject variability to the present study is not known since each patient was unique, having different IEDs arising from different brain regions.
Recently published animal work may provide some insight into the location‐dependant variability seen in our study [Goense et al., 2012]. Applying high‐resolution fMRI at 4.7 T with a visual paradigm in macaque monkeys, differences in cerebral blood flow (CBF) and cerebral blood volume (CBV) responses were observed between the six cortical layers. This layer‐specific variability could also manifest as BOLD signal variability that is dependent on the cortical layer generating the epileptiform activity or the type of cortex generating the IEDs (e.g., six‐layered temporal neocortex vs. three‐layered hippocampal allocortex). It is therefore tempting to speculate that different IED‐generating cortical layers might explain some of the observed time‐to‐peak differences between mesial and lateral temporal BOLD responses to IEDs in the present study. However, further validation is required as evaluating BOLD responses to IEDs generated from different cortical layers is beyond the scope of this study.
HRF Variability in EEG‐fMRI
The earliest work characterizing the HRF in response to IEDs was a study of four patients with focal epilepsy [Benar et al., 2002]. The timing of IEDs were convolved with the canonical Glover HRF and the BOLD signal time‐course was extracted from voxels deemed active through this analysis, resulting in the “true” hemodynamic response for each IED. They found all HRFs to have a shape resembling the Glover model, although the positive deflection was wider, the time‐to‐peak was delayed (6–7 s), and three out of four patients had a negative undershoot after the peak. These results differ from our data that demonstrate hemodynamic responses distinct from the Glover model.
There are two potential explanations. First, the previous study used scalp EEG‐fMRI whereas we employed iEEG‐fMRI. The nature of BOLD signal changes associated with very focal IEDs recorded intracranially may be distinct from those recorded by scalp EEG, as discussed in the following section. Secondly, only voxels found to be active through a standard convolution were examined in the previous study [Benar et al., 2002]. Indeed, the authors state that BOLD responses too different from the Glover model may have been missed. We avoided this limitation by selecting ROIs solely based on the location of the active iEEG electrode contact, since iEEG allowed greater precision in localizing the region generating IEDs.
Another, larger study of 34 datasets of 10‐channel scalp EEG‐fMRI concluded that IEDs primarily elicit physiological hemodynamic responses that are well captured in analyses using the canonical HRF [Salek‐Haddadi et al., 2006]. In contrast, two more recent publications suggest that hemodynamic changes associated with IEDs may not match the canonical HRF, in agreement with our study [Pittau et al., 2011; Proulx et al., 2014]. The observed differences between these scalp EEG‐fMRI studies and the earlier ones could be related to increased SNR and BOLD sensitivity through technological advances: the literature implying a canonical BOLD response to IEDs was collected at 1.5 T before 2006, whereas the studies that observed variable hemodynamic responses were performed at 3 T and published later (2011 and 2014).
The importance of considering non‐canonical HRFs with IEDs was highlighted in a 2006 study [Lu et al., 2006]. Here, different HRF models were used to identify which model was most sensitive to BOLD signal change associated with scalp‐recorded IEDs. Comparing parametric and nonparametric HRF models, the flexible deconvolution model was deemed most sensitive, resulting in larger volumes of activation and a greater sensitivity to activation undetected by the other HRFs. The flexible deconvolution model does not impose an a priori shape or timing restrictions as the optimal HRF is estimated directly from the data, which are divided into estimation and detection sets [Lu et al., 2007]. These findings highlight the advantages of using patient‐specific HRFs for detecting BOLD signal changes to IEDs, as deviation from the canonical HRF reduces sensitivity.
Implications
Our study suggests that analyses of iEEG‐fMRI data using only the canonical HRF for convolution may not accurately model BOLD signal changes associated with IEDs. Thus, future studies should use more flexible models for IED event convolution. For example, multiple‐hemodynamic‐function methods have been applied to scalp EEG‐fMRI data, whereby IED events are convolved with four hemodynamic response functions peaking at 3, 5, 7, and 9 s and included as regressors in the same general linear model (e.g. [Aghakhani et al., 2006; Bagshaw et al., 2004; Benar et al., 2006; Moeller et al., 2009; Pittau et al., 2012]). Another method that may be useful for iEEG‐fMRI datasets is event‐related Independent Components Analysis (eICA) [Masterton et al., 2013]. The developers assert that eICA provides a less constrained analysis, compared with canonical convolution, that is ideal for detecting non‐canonical BOLD responses.
It is possible that differences in hemodynamic responses to IEDs are greater between subjects than between regions in the same subject. While the shape of the HRFs reported here deviated across subjects, the within‐subject HRFs for subject 2 who had bilateral independent mesial temporal IEDs were similar to one another. Though a firm conclusion cannot be made based on results from this one subject, the data are consistent with a previous study that showed greater between‐subject HRF variability than within [Proulx et al., 2014]. The authors also used subject‐specific HRFs generated from an auditory‐motor task to analyze scalp EEG‐fMRI obtained from the same subject. This resulted in a 12% increase in peak t‐value and a doubling of cluster‐sizes of significant voxels compared with using the canonical HRF alone [Proulx et al., 2014]. However, it is unclear if this approach can be applied to iEEG‐fMRI data since cortical sensory or motor activations may not accurately predict the BOLD response from very localized sources of epileptiform discharges. Furthermore, interevent variability in the BOLD response warrants further investigation. Slight variability in the IED electrographic features such as morphology, amplitude, and timing may result in BOLD response variability as demonstrated previously where applying ICA to EEG‐fMRI data revealed correlations between the peak amplitudes of IED and HRFs [LeVan et al., 2010]. In the current study, we did not characterize inter‐event variability; rather we averaged all BOLD signal changes to IEDs to create robust patient‐specific responses. However intracranial EEG‐fMRI, due to its sensitivity to electrographic features, offers the ideal platform to investigate this in the future.
Limitations
We examined relatively few of the total number of discharges recorded in each subject. For example, 2,092 IEDs were recorded for subject 3, however, only 22 (1.1%) discharges met our criteria for being isolated. While a large number of IEDs were excluded, we felt it is was essential to limit our analyses to isolated discharges in order to examine the BOLD response to single, uncontaminated IEDs as recorded by intracranial EEG. Notably, the number of discharges included in our analyses is similar to a previous study that examined 6 to 27 IEDs in four patients that found similar, but not as significant, variability in the shape and timing of the hemodynamic response [Benar et al., 2002].
As we have previously demonstrated, intracranial electrodes cause signal loss and susceptibility artifact within 20 mm of the electrode [Boucousis et al., 2012]. We addressed this issue by generating regions of interest larger than the immediate area surrounding the active electrode contact, then trimming the size of the ROI using intervoxel cross correlation [Golestani and Goodyear, 2011]. This allowed us to only analyze voxels outside the region of susceptibility artifact, but still close to the most active contact.
CONCLUSION
Interictal epileptiform discharges recorded by intracranial EEG during simultaneous fMRI are associated with BOLD signal time‐courses that are distinct from the widely used canonical hemodynamic response function and vary by patient and IED location. Also, patient‐specific response functions determined through fitting analysis are appreciably different than the canonical HRF. Our data are unique as we recorded IEDs using intracranial electrodes, implying that the generators of the recorded spiking activity originated from a much smaller volume of tissue than previously studied with scalp EEG‐fMRI. The differences between the canonical HRF and the hemodynamic changes presented here suggest that flexible models of analysis should be used to analyze iEEG‐fMRI data. This will more accurately capture the breadth of BOLD signal changes associated with focal epileptiform discharges and may in turn, lead to a better understanding of epileptogenic networks.
Supporting information
Supporting Information
REFERENCES
- Aghakhani Y, Beers CA, Pittman DJ, Gaxiola‐Valdez I, Goodyear BG, Federico P (2015): Co‐localization between the BOLD response and epileptiform discharges recorded by simultaneous intracranial EEG‐fMRI at 3T. Neuroimage 7:755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghakhani Y, Kobayashi E, Bagshaw AP, Hawco C, Benar CG, Dubeau F, Gotman J (2006): Cortical and thalamic fMRI responses in partial epilepsy with focal and bilateral synchronous spikes. Clin Neurophysiol 117:177–191. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, Zarahn E, D'Esposito M (1998): The variability of human, BOLD hemodynamic responses. Neuroimage 8:360–369. [DOI] [PubMed] [Google Scholar]
- Allen PJ, Josephs O, Turner R (2000): A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage 12:230–239. [DOI] [PubMed] [Google Scholar]
- An D, Fahoum F, Hall J, Olivier A, Gotman J, Dubeau F (2013): Electroencephalography/functional magnetic resonance imaging responses help predict surgical outcome in focal epilepsy. Epilepsia 54:2184–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw AP, Aghakhani Y, Benar CG, Kobayashi E, Hawco C, Dubeau F, Pike GB, Gotman J (2004): EEG‐fMRI of focal epileptic spikes: Analysis with multiple haemodynamic functions and comparison with gadolinium‐enhanced MR angiograms. Hum Brain Mapp 22:179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benar CG, Gross DW, Wang Y, Petre V, Pike B, Dubeau F, Gotman J (2002): The BOLD response to interictal epileptiform discharges. Neuroimage 17:1182–1192. [DOI] [PubMed] [Google Scholar]
- Benar CG, Grova C, Kobayashi E, Bagshaw AP, Aghakhani Y, Dubeau F, Gotman J (2006): EEG‐fMRI of epileptic spikes: Concordance with EEG source localization and intracranial EEG. Neuroimage 30:1161–1170. [DOI] [PubMed] [Google Scholar]
- Boucousis SM, Beers CA, Cunningham CJ, Gaxiola‐Valdez I, Pittman DJ, Goodyear BG, Federico P (2012): Feasibility of an intracranial EEG‐fMRI protocol at 3T: risk assessment and image quality. Neuroimage 63:1237–1248. [DOI] [PubMed] [Google Scholar]
- Buxton RB, Uludag K, Dubowitz DJ, Liu TT (2004): Modeling the hemodynamic response to brain activation. Neuroimage 23:S220–S233. [DOI] [PubMed] [Google Scholar]
- Carmichael DW, Thornton JS, Rodionov R, Thornton R, McEvoy AW, Ordidge RJ, Allen PJ, Lemieux L (2010): Feasibility of simultaneous intracranial EEG‐fMRI in humans: A safety study. Neuroimage 49:379–390. [DOI] [PubMed] [Google Scholar]
- Cunningham CB, Goodyear BG, Badawy R, Zaamout F, Pittman DJ, Beers CA, Federico P (2012): Intracranial EEG‐fMRI analysis of focal epileptiform discharges in humans. Epilepsia 53:1636–1648. [DOI] [PubMed] [Google Scholar]
- Elshoff L, Groening K, Grouiller F, Wiegand G, Wolff S, Michel C, Stephani U, Siniatchkin M (2012): The value of EEG‐fMRI and EEG source analysis in the presurgical setup of children with refractory focal epilepsy. Epilepsia 53:1597–1606. [DOI] [PubMed] [Google Scholar]
- Goense J, Merkle H, Logothetis NK (2012): High‐resolution fMRI reveals laminar differences in neurovascular coupling between positive and negative BOLD responses. Neuron 76:629–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani AM, Goodyear BG (2011): Regions of interest for resting‐state fMRI analysis determined by inter‐voxel cross‐correlation. Neuroimage 56:246–251. [DOI] [PubMed] [Google Scholar]
- Grouiller F, Vercueil L, Krainik A, Segebarth C, Kahane P, David O (2010): Characterization of the hemodynamic modes associated with interictal epileptic activity using a deformable model‐based analysis of combined EEG and functional MRI recordings. Hum Brain Mapp 31:1157–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Ollinger JM, D'Esposito M (2004): Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. Neuroimage 21:1639–1651. [DOI] [PubMed] [Google Scholar]
- Hawco CS, Bagshaw AP, Lu Y, Dubeau F, Gotman J (2007): BOLD changes occur before epileptic spikes seen on scalp EEG. Neuroimage 35:1450–1458. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Hawco C, Kobayashi E, Boor R, LeVan P, Stephani U, Siniatchkin M, Gotman J (2008): Variability of the hemodynamic response as a function of age and frequency of epileptic discharge in children with epilepsy. Neuroimage 40:601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Moeller F, Boor R, Stephani U, Gotman J, Siniatchkin M (2009): Hemodynamic changes preceding the interictal EEG spike in patients with focal epilepsy investigated using simultaneous EEG‐fMRI. Neuroimage 45:1220–1231. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S (2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S (2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Kang JK, Benar C, Al‐Asmi A, Khani YA, Pike GB, Dubeau F, Gotman J (2003): Using patient‐specific hemodynamic response functions in combined EEG‐fMRI studies in epilepsy. Neuroimage 20:1162–1170. [DOI] [PubMed] [Google Scholar]
- LeVan P, Tyvaert L, Gotman J (2010): Modulation by EEG features of BOLD responses to interictal epileptiform discharges. Neuroimage 50:15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Meng Loh J, Atlas LY, Wager TD (2009): Modeling the hemodynamic response function in fMRI: Efficiency, bias and mis‐modeling. Neuroimage 45:S187–S198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Wager TD (2007): Validity and power in hemodynamic response modeling: A comparison study and a new approach. Hum Brain Mapp 28:764–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Bagshaw AP, Grova C, Kobayashi E, Dubeau F, Gotman J (2006): Using voxel‐specific hemodynamic response function in EEG‐fMRI data analysis. Neuroimage 32:238–247. [DOI] [PubMed] [Google Scholar]
- Lu Y, Grova C, Kobayashi E, Dubeau F, Gotman J (2007): Using voxel‐specific hemodynamic response function in EEG‐fMRI data analysis: An estimation and detection model. Neuroimage 34:195–203. [DOI] [PubMed] [Google Scholar]
- Masterton RA, Jackson GD, Abbott DF (2013): Mapping brain activity using event‐related independent components analysis (eICA): Specific advantages for EEG‐fMRI. Neuroimage 70:164–174. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL (2000): Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage 11:735–759. [DOI] [PubMed] [Google Scholar]
- Moeller F, Tyvaert L, Nguyen DK, LeVan P, Bouthillier A, Kobayashi E, Tampieri D, Dubeau F, Gotman J (2009): EEG‐fMRI: Adding to standard evaluations of patients with nonlesional frontal lobe epilepsy. Neurology 73:2023–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noachtar S, Borggraefe I (2009): Epilepsy surgery: A critical review. Epilepsy Behav 15:66–72. [DOI] [PubMed] [Google Scholar]
- Ojemann GA (1987): Surgical therapy for medically intractable epilepsy. J Neurosurg 66:489–499. [DOI] [PubMed] [Google Scholar]
- Pittau F, Dubeau F, Gotman J (2012): Contribution of EEG/fMRI to the definition of the epileptic focus. Neurology 78:1479–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittau F, Levan P, Moeller F, Gholipour T, Haegelen C, Zelmann R, Dubeau F, Gotman J (2011): Changes preceding interictal epileptic EEG abnormalities: Comparison between EEG/fMRI and intracerebral EEG. Epilepsia 52:1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx S, Safi‐Harb M,L, Van P, An D, Watanabe S, Gotman J (2014): Increased sensitivity of fast BOLD fMRI with a subject‐specific hemodynamic response function and application to epilepsy. Neuroimage 93:59–73. [DOI] [PubMed] [Google Scholar]
- Salek‐Haddadi A, Diehl B, Hamandi K, Merschhemke M, Liston A, Friston K, Duncan JS, Fish DR, Lemieux L (2006): Hemodynamic correlates of epileptiform discharges: An EEG‐fMRI study of 63 patients with focal epilepsy. Brain Res 1088:148–166. [DOI] [PubMed] [Google Scholar]
- Salek‐Haddadi A, Lemieux L, Merschhemke M, Friston KJ, Duncan JS, Fish DR (2003): Functional magnetic resonance imaging of human absence seizures. Ann Neurol 53:663–667. [DOI] [PubMed] [Google Scholar]
- Shan ZY, Wright MJ, Thompson PM, McMahon KL, Blokland GG, de Zubicaray GI, Martin NG, Vinkhuyzen AA, Reutens DC (2014): Modeling of the hemodynamic responses in block design fMRI studies. J Cereb Blood Flow Metab 34:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton R, Laufs H, Rodionov R, Cannadathu S, Carmichael DW, Vulliemoz S, Salek‐Haddadi A, McEvoy AW, Smith SM, Lhatoo S, Elwes RD, Guye M, Walker MC, Lemieux L, Duncan JS (2010): EEG correlated functional MRI and postoperative outcome in focal epilepsy. J Neurol Neurosurg Psychiatry 81:922–927. [DOI] [PubMed] [Google Scholar]
- Vulliemoz S, Carmichael DW, Rosenkranz K, Diehl B, Rodionov R, Walker MC, McEvoy AW, Lemieux L (2011): Simultaneous intracranial EEG and fMRI of interictal epileptic discharges in humans. Neuroimage 54:182–190. [DOI] [PubMed] [Google Scholar]
- Watanabe S, An D, Safi‐Harb M, Dubeau F, Gotman J (2014): Hemodynamic response function (HRF) in epilepsy patients with hippocampal sclerosis and focal cortical dysplasia. Brain Topogr 27:613–619. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS (2007): EEG‐fMRI in the preoperative work‐up for epilepsy surgery. Brain 130:2343–2353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


