Abstract
Spontaneous fluctuations can be measured in the brain that reflect dissociable functional networks oscillating at synchronized frequencies, such as the default mode network (DMN). In contrast to its diametrically opposed task‐positive counterpart, the DMN predominantly signals during a state of rest, and inappropriate regulation of this network has been associated with inattention, a core characteristic of attention‐deficit/hyperactivity disorder (ADHD). To examine whether abnormalities can be identified in the DMN component of patients with ADHD, we applied an independent components analysis to resting state functional magnetic resonance imaging data acquired from 22 male medication‐naïve adults with ADHD and 23 neurotypical individuals. We observed a stronger coherence of the left dorsolateral prefrontal cortex (dlPFC) with the DMN component in patients with ADHD which correlated with measures of selective attention. The increased left dlPFC‐DMN coherence also surfaced in a whole‐brain replication analysis involving an independent sample of 9 medication‐naïve adult patients and 9 controls. In addition, a post hoc seed‐to‐voxel functional connectivity analysis using the dlPFC as a seed region to further examine this region's suggested connectivity differences uncovered a higher temporal coherence with various other neural networks and confirmed a reduced anticorrelation with the DMN. These results point to a more diffuse connectivity between functional networks in patients with ADHD. Moreover, our findings suggest that state‐inappropriate neural activity in ADHD is not confined to DMN intrusion during attention‐demanding contexts, but also surfaces as an insufficient suppression of dlPFC signaling in relation to DMN activity during rest. Together with previous findings, these results point to a general dysfunction in the orthogonality of functional networks. Hum Brain Mapp 35:1261–1272, 2014. © 2013 Wiley Periodicals, Inc.
Keywords: independent components analysis, attention‐deficit/hyperactivity disorder, default mode network, dorsolateral prefrontal cortex, rest, functional magnetic resonance imaging
INTRODUCTION
Besides stimulus‐related activity, the blood oxygen level‐dependent (BOLD) functional magnetic resonance imaging (fMRI) signal captures spontaneous intrinsic activity fluctuations that reflect disparate neural networks oscillating at synchronized frequencies [Fox et al., 1997, 2005, 2006; Tomasi and Volkow, 2011]. These oscillations are coherent within neuroanatomical systems [Greicius et al., 2009], and represent networks that are strongly functionally and anatomically connected, including for instance a somatomotor and a visual network. The default mode network (DMN), one of these intrinsic networks, primarily signals during a state of rest and seems to represent some type of physiological baseline activity of the brain [Fox et al., 1997; Gusnard et al., 2001; Raichle et al., 2001]. DMN signaling is attenuated by the initiation of a cognitive task as a function of the cognitive demand posed by the task [Singh and Fawcett, 2008]. Key nodes of the DMN include the medial prefrontal cortex, the posterior lateral parietal cortex and the precuneus/posterior cingulate cortex [Gusnard et al., 2001; Raichle et al., 2001].
Recent studies have also detected a network diametrically opposed to the DMN termed the task‐positive network (TPN), comprising for instance the dorsolateral prefrontal cortex (dlPFC), the frontal eye fields and the intraparietal sulcus [Fox et al., 2005]. This network functions as a counterpart of the DMN, exhibiting a temporal activity pattern strongly anticorrelated with the DMN with the most prominent synchronized oscillations occurring during the performance of attention‐demanding tasks [Fox et al., 1997, 2005]. The stringent balance between these two networks is critical for attentional processes, and neuroimaging studies in control subjects suggest that an inadequate regulation of the DMN during situations that require cognitive effort render momentary lapses of attention [Eichele et al., 2008; Li et al., 2007; Pessoa et al., 2002; Wagner et al., 1998; Weissman et al., 2006]. More specifically, increases in DMN signaling and deactivation of task‐positive regions were found to predict slower and erroneous performance on tasks assessing various cognitive processes in healthy participants, such as visual memory and selective attention [Eichele et al., 2008; Li et al., 2007; Pessoa et al., 2002; Wagner et al., 1998; Weissman et al., 2006].
Periodic lapses of attention and resulting transient deficits in cognitive performance represent a key characteristic of attention‐deficit/hyperactivity disorder (ADHD). In fact, increased reaction time (RT) variability, an index of attentional lapses, is one of the most consistent manifestations of the disorder [Buzy et al., 2009; Castellanos et al., 2009, 2006] and serves as a reliable predictor of diagnostic severity [Kuntsi et al., 2010]. The default mode interference hypothesis captures a link between ADHD symptoms of inattention and DMN activity, postulating that the transient lapses in attention symptomatic of the disorder reflect DMN signaling persisting into or re‐emerging during attention‐demanding situations [Castellanos et al., 2009; Sonuga‐Barke and Castellanos, 2007]. Indeed, Fassbender et al. 1998 investigated brain activity during a working memory task and observed a lower degree of ventromedial prefrontal cortex deactivation in response to increasing cognitive demands in ADHD children, which was associated with RT variability. In addition, some studies suggest that children with ADHD also exhibit atypical patterns of connectivity and activity in intrinsic functional networks during a state of rest [Fair et al., 2010; Fassbender et al., 2009; Tian et al., 2008; Wang et al., 2009]. During childhood, resting state networks are still undergoing a maturation process [Dosenbach et al., 2010; Fransson et al., 2011; Jolles et al., 2011; Kelly et al., 2009; Uddin et al., 2010], and at an early stage an equivalent of the DMN cannot yet be identified [Fransson et al., 2007]. Subsequently, a developmental trajectory involving weakening of local connectivity and integration across long‐range connections seems to underlie a shift from diffuse local activation patterns to adult‐like focal disparate networks [Dosenbach et al., 2010; Uddin et al., 2010]. Fair et al. 2011 assessed resting state activity in children with ADHD, and demonstrated reductions in the correlated spontaneous activity within the DMN. Interestingly, abnormalities were observed in functional connections that were previously identified as developmentally dynamic, suggesting that the disorder may be characterized by a delay or disruption in the consolidation of the DMN [Fair et al., 2010].
An anatomical study examining the cortical mantle in child and adult patients with ADHD uncovered a relative normalization of laminar thickness deficits in the brains of adults with the disorder in comparison to child patients, among others in the superior parietal cortex and inferior parietal cortex/precuneus [Hoekzema et al., 2012b]. Some functional studies, however, suggest that aberrations in intrinsic network activity within DMN regions can persist into adulthood, and adult patients that do not attain a remission of symptoms through development display abnormal resting state activity patterns [Castellanos et al., 2008; Uddin et al., 2008]. To our knowledge, two magnetic resonance imaging (MRI) studies have investigated DMN activity in adults with ADHD, using measures of functional connectivity or network homogeneity [Castellanos et al., 2008; Uddin et al., 2008]. Castellanos et al. observed a decrease in the negative functional connectivity between the dorsal anterior cingulate cortex and the precuneus/posterior cingulate cortex, a key node of the DMN. Moreover, in line with ADHD child literature, both studies observed reductions in the temporal coherence within the DMN [Castellanos et al., 2008; Uddin et al., 2008]. These results suggest that anomalies in functional network activity can persist into adulthood and comprise a feature of adult ADHD pathophysiology.
To allow an evaluation of the persistent features of resting state network anomalies in ADHD—while also minimizing the within‐group developmental variability associated with child samples—we examined the DMN component in adult patients with ADHD. In contrast to previous studies, we evaluated resting state network activity using independent components analysis (ICA), a data‐driven blind source separation approach that allows a model‐free analysis of whole‐brain fMRI data without the requirement of seed region selection and placement inherent to analyses of functional connectivity. Furthermore, only patients with ADHD who had never received any pharmacological treatment for their condition were recruited for this study, allowing us to account for the potential confounding effects of previous exposure to ADHD medication. The selection of medication‐naïve patients is important in ADHD resting state research, as long‐term and short‐term biochemical, hemodynamic, and metabolic changes have been documented in brain regions associated with the DMN after administration of psychostimulant medication [Andersen et al., 2008; Jezierski et al., 2007; Peterson et al., 2009; Schweitzer et al., 2003]. In fact, methylphenidate was found to suppress default mode activity and enhance task‐positive signaling in healthy subjects [Tomasi et al., 2011; Volkow et al., 2008] and in patients with ADHD [Peterson et al., 2009] during the performance of cognitive tasks. Therefore, in this study, we applied ICA to fMRI resting state data acquired from medication‐naïve adults with ADHD to examine whether this disorder is characterized by abnormalities in the DMN component during rest. We show that anomalies in intrinsic resting state network activity comprise a persistent feature of ADHD pathophysiology, and do not result from previous exposure to stimulant medication. Furthermore, our findings suggest that state‐inappropriate activity in ADHD is not restricted to DMN interference during attention‐demanding tasks, but also surfaces as an inability to regulate signaling of the dlPFC in relation to DMN activity during rest. Taken together with previous results, these findings point to a deficit in the orthogonality of functional networks.
METHODS AND MATERIALS
Participants
Forty‐eight right‐handed male participants (24 patients with ADHD and 24 healthy controls) were recruited for this study over a 3.5‐year period. One ADHD subject had to be removed from the sample due to movement‐related artifacts. In addition, for neurological reasons (cysts), one ADHD and one control subject were excluded from the study.
All patients fulfilled diagnostic criteria for ADHD combined subtype and had never received any pharmacological treatment for their condition. ADHD diagnosis was based on the Diagnostic and Statistical Manual of Mental Diseases, Fourth Edition, Test Revised [DSM‐IV–TR; American Psychiatric Association.Task Force on DSM‐IV, 2000]. The Conners Adult ADHD Diagnostic Interview for DSM‐IV (CAADID) validated in Spanish was applied to all participants [Ramos‐Quiroga et al., 2012]. The purpose of the CAADID is to determine if adult patients meet the first four DSM‐IV‐TR criteria (Criteria A–D). The first section assesses the presence of the DSM‐IV‐TR inattention symptoms during childhood and adulthood (Criterion A), followed by questions about the onset (Criterion B), and pervasiveness of these symptoms (Criterion C). The second section evaluates Criteria A–C for hyperactive–impulsive symptoms. This is followed by a section that assesses all symptoms (Criterion D). The level of impairment was measured with the clinical global impression, included in the CAADID Part II.
The severity of childhood ADHD symptoms was examined with the Wender Utah Rating Scale [WURS; Ward et al., 1993], a self‐administered scale with 61 items that were shown to have good internal consistency and temporal stability [Rossini and O'Connor, 1995; Stein et al., 1995]. The WURS retrospectively evaluates the severity of ADHD symptoms in childhood. It represents a standard instrument in adult ADHD programs for the assessment of childhood symptom severity in adults with ADHD and has been translated and validated in various languages, including Spanish [Allen et al., 2012; Calhoun et al., 2001; Christiansen et al., 2012; Erhardt et al., 2011; Oncu et al., 2005; Retz‐Junginger et al., 2003; Rodriguez‐Jimenez et al., 2001; Yeh et al., 2008]. The severity of ADHD symptoms in the last month before the visit was assessed by the ADHD Rating Scale [ADHD‐RS, DuPaul et al., 1998], which evaluates the ADHD symptoms included in the DSM‐IV‐TR criteria.
Furthermore, the Structured Clinical Interview for DSM Disorders‐I and II (Structured Clinical Interview for Axis I [SCID‐I; First et al., 2002] and Axis II [SCID‐II; First et al., 1997]) were applied to evaluate the presence of comorbid disorders. SCID‐I and SCID‐II allow the assessment of DSM‐IV Criterion E for ADHD. The occurrence of oppositional defiant disorder and conduct disorder during childhood and adolescence was retrospectively examined using the Kiddies Schedule for Affective Disorders and Schizophrenia for School‐age Children [Kaufman et al., 1997]. A history of substance use disorders (SUDs) was considered an exclusion criterion, and no participants with an antecedent of SUD were included in the study. The Vocabulary and Block Design subtests of the Wechsler Adults Intelligence Scale‐III [WAIS; Wechsler, 1997] were applied to obtain an estimate of intelligence quotient (IQ), and only those subjects with an IQ within one standard deviation from the mean were included in the study.
The study was approved by the Hospital Universitari Vall d'Hebron Ethics Committee, and informed consent was obtained from the subjects before their participation in the study. Clinical and demographic data of the sample are depicted in Table 1. Although the differences in age and IQ between the groups were not statistically significant, we included these variables as regressors of no interest in each of the analyses to ensure the variability in these measures did not underlie the observed effects.
Table 1.
Demographic and clinical data of the sample
| ADHD N = 22, mean (SD) | Controls N = 23, mean (SD) | T Value | P Value | |
|---|---|---|---|---|
| Age | 32.82 (10.75) | 29.26 (8.92) | 1.21 | 0.233 |
| IQ index (WAIS vocabulary and block design) | 10.77 (2.54) | 12.07 (2.21) | 1.83 | 0.075 |
| Clinical data | ||||
| WURS | 54.59 (16.57) | 17.40 (14.30) | 8.05 | <0.001 |
| ADHD rating scale | 35.09 (10.01) | 6.87 (6.29) | 11.38 | <0.001 |
| CAARS | ||||
| (A) Inattention/forgetfulness | 23.73 (6.64) | 6.22 (5.05) | 10.08 | <0.001 |
| (B) Hyperactivity/restlessness | 23.64 (7.17) | 8.74 (6.80) | 7.16 | <0.001 |
| (C) Impulsivity/emotional lability | 19.55 (7.13) | 5.35 (4.69) | 7.93 | <0.001 |
| (D) Problems with self‐concept | 9.82 (3.95) | 2.65 (2.37) | 7.35 | <0.001 |
| (E) DSM‐IV inattentive symptoms | 19.09 (4.41) | 4.35 (3.73) | 12.14 | <0.001 |
| (F) DSM‐IV hyperactive‐impulsive symptoms | 18.36 (4.89) | 4.48 (3.52) | 10.90 | <0.001 |
| (G) DSM‐IV total ADHD symptoms | 37.23 (8.12) | 8.83 (6.19) | 13.23 | <0.001 |
| (H) ADHD index | 21.81 (6.50) | 6.26 (4.60) | 9.23 | <0.001 |
CAARS: Conners Adult ADHD Rating Scale‐Self‐Rating Form: Long. The index of IQ was estimated based on the Vocabulary and Block Design tasks of the WAIS ((Vocabulary typical score + Block Design typical score)/2).
fMRI Acquisitions
The MRI images were obtained in a GE 1.5T scanner, equipped with a standard quadrature radiofrequency coil. A vacuum pillow was placed inside the coil to restrict the subjects' head movement. For anatomical reference, a T1‐weighted pulse sequence was used [acquisition parameters: repetition time (TR) = 11.5; echo time (TE) = 4.2; matrix = 256 × 256 × 96; flip angle (FA) = 15°; slice thickness = 1.6]. In addition, functional volumes were acquired using a T2*‐weighted gradient echo sequence [acquisition parameters: TR = 3,000 ms; TE = 60 ms; FA = 90°; field of view (FOV) = 300 mm; gap = 0.5 mm; matrix size = 64 × 64 × 30]. During the fMRI acquisition, the subjects were presented with a fixation cross on a screen during 4 min, and they were asked to relax and fixate on the cross.
Preprocessing
MRI image processing and statistical analyses were conducted with Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm), implemented in Matlab (version 7.8; http://www.mathworks.com). The first three volumes of each subject were discarded to remove non‐steady‐state effects. Spatial interpolation was applied to correct for head motion, using parameters derived from a 6‐parameter rigid body transformation. The individual translation and rotation movement parameters did not exceed a value of 3 mm/° (M ± SD; control group: X: 0.017 ± 0.054; Y: 0.046 ± 0.083; Z: 0.061 ± 0.168; pitch: 0.001 ± 0.004; roll: 0.0002 ± 0.0019; yaw: 0.0008 ± 0.002. ADHD group: X: −0.018 ± 0.108; Y: 0.043 ± 0.149; Z: 0.065 ± 0.192; pitch: 0.001±0.008; roll: 0.0007 ± 0.0027; yaw: 0.0002 ± 0.003). When comparing the head movement parameters between the ADHD and control groups, we observed no significant difference for any of the translation (X: T = 1.38, P = 0.174; Y: T = 0.08, P = 0.933; Z: T = 0.07, P = 948) or rotation parameters (pitch: T = 0.00, P = 1.00; roll: T = 0.79, P = 0.433; yaw: T = 1.20, P = 0.236). Following the realignment, the data were normalized into Montreal Neurological Institute (MNI) space and resampled to a 3‐mm isotropic resolution. Finally, the images of echo planar imaging were smoothed by imposing a 10 mm Full Width at Half Maximum (FWHM) isotropic kernel on the space domain.
ICA
ICA was conducted to analyze resting state data using the Group ICA of fMRI toolbox [GIFT; http://icatb.sourceforge.net/; Calhoun et al., 2001]. ICA is a data‐driven blind source separation strategy that decomposes high‐dimensionality data into maximally independent spatiotemporal components, and is frequently used to extract the DMN component in fMRI data [e.g., Calhoun et al., 2008; Damoiseaux et al., 2006; Fransson et al., 2007; Garrity et al., 2007; Harrison et al., 2008; Uddin et al., 2010]. The used group ICA approach and tests with simulation data are described in detail in publications by the research group of Dr. Calhoun [Allen et al., 2012; Calhoun et al., 2001; Erhardt et al., 2011]. This toolbox implements a group approach comprising an estimation of the independent components (ICs) on concatenated data, which is followed by a computation of the subject‐specific spatial maps and timecourses. Globally, the GIFT approach entails three main steps: (i) compression of the data; (ii) estimation of the ICs in an aggregate dataset; (iii) back reconstruction of the individual ICs.
Regarding the data reduction, the dimensionality of the data was reduced using principal components analysis (PCA), and PCA decomposition was alternated with data concatenation across subjects. The number of independent hemodynamic sources was estimated using the minimal length description criterion, indicating 20 ICs for our functional dataset. Then, using the infomax algorithm, maximally independent components were estimated, and the data were transformed into a linear mixing matrix and 20 ICs. The individual ICs were back reconstructed by multiplying the section of data corresponding to each subject by that subject's mixing matrix. The ICs were then transformed to z score values, which provide an index of the degree of synchronization of the BOLD signal in that voxel with the timecourse of the relevant component.
The DMN component was subsequently selected via an automated process that defines the component that most closely matched the DMN for each individual subject, based on spatial correlation analyses with a DMN template (M ± SD; controls: R = 0.45 ± 0.03; minimum: 0.37; maximum: 0.52; ADHD: R = 0.45 ± 0.04; minimum: 0.40; maximum: 0.51). The main structures constituting the template are the posterior part of the superior parietal cortex, the precuneus, the posterior cingulate cortex, the frontal pole, and the temporoparietal junction. This template is provided as part of the GIFT package and represents regions that have repeatedly been implicated in the DMN. The presence of a frontal component in this default template (the frontal pole) was deemed especially suitable for this study considering the key role of the frontal cortex in the pathophysiology of ADHD [Biederman and Faraone, 2005; Giedd et al., 2001]. The best‐fit components—that is, those that provide the best match with the DMN template—were combined in a second‐level random effects analysis, and two sample t‐tests were applied to compare the DMN component between the groups, including age and IQ as confounding variables. In addition, we performed a correlation analysis with performance on the WAIS symbols task, an index of selective attention, to assess whether activity in brain regions that showed a different pattern of recruitment across the ADHD and control groups was associated with attentional performance. Therefore, a region of interest of the ICA result was applied to this correlation analysis.
Unless explicitly specified otherwise, all neuroimaging results were corrected for multiple comparisons using a family‐wise error (FWE) correction (p<0.05).
Functional Connectivity Analysis
To examine the specificity of the enhanced connectivity between the left dlPFC and DMN regions suggested by the ICA, we wanted to assess how the subject groups differ in the connectivity of this region—which may play an important role in ADHD pathophysiology—to the rest of the brain. Therefore, to supplement the ICA results, a post hoc seed‐based functional connectivity analysis (FCA) was applied to the resting state fMRI data to examine the temporal coherence of the dlPFC region resulting from the ICA with the rest of the brain, using a seed‐to‐voxel regression strategy with this region as a source ROI. The FCA was performed with the standard approach provided by the Conn toolbox (http://web.mit.edu/swg/software.html). This approach included applying a CompCor strategy for physiological and other noise source reduction and temporally band‐pass filtering the data (0.012 < f < 0.1) to limit the analyses to the band‐pass frequency of interest. The whole‐brain correlation maps were produced by extracting the BOLD timecourse from the seed region and computing the correlation coefficient between that timecourse and the timecourses from all other brain voxels. The group maps were then compared to assess differences in connectivity of the seed region, including age and IQ as nuisance covariates. In addition, to assess the degree of anticorrelation with the DMN, we extracted the beta values for the most negatively correlated DMN region (which was the right precuneus). The beta values were compared across the groups and correlated with performance‐based levels of selective attention, again including age and IQ as covariates of no interest.
RESULTS
ICA
The components that provided the closest match to the DMN were extracted using group ICA, and compared across the groups using a two sample t‐test. The contrast ‘ADHD > control’, comparing the DMN components of the two groups, rendered a focal highly significant cluster in the ventrolateral part of the left dlPFC (−48x26y4z, 324 mm3, T = 6.71, P < 0.001, FWE corrected), indicating a significantly higher connectivity strength of that region with the DMN component in patients with ADHD in comparison to the control group. Although this region was negatively associated with the DMN component in control subjects, patients with ADHD showed a positive beta value for this region (M ± SD; controls: −0.45 ± 0.49 and ADHD: 0.48 ± 0.55). Figure 1 depicts the left dlPFC result obtained by the contrast ‘ADHD > control’. No other regions were observed for this comparison. The contrast ‘control > ADHD’ rendered no results at this threshold (p<0.05, FWE‐corrected). The results of this comparison without including age and IQ as nuisance covariates are reported in Supporting Information.
Figure 1.
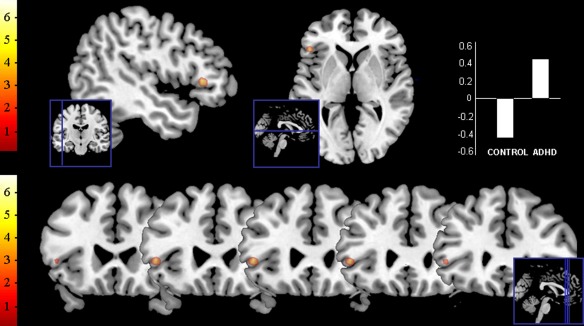
Results of ICA group comparison. The image depicts the result of the two sample t‐test comparing the DMN components of the ADHD and control group (contrast ‘ADHD > control’), at a threshold of P < 0.05 FWE corrected. The beta values for this region are also displayed. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Although ICA is frequently used to investigate functional neural networks and various studies provide support for the group ICA approach implemented in GIFT [Calhoun et al., 2001, 2008; Erhardt et al., 2011], the relative reliability of distinct approaches to apply ICA on group level has not been firmly established [Guo and Pagnoni, 2008; Varoquaux et al., 2010]. Therefore, we have replicated our analysis in an independent sample of nine medication‐naïve adult patients with ADHD and nine controls who were scanned for another ongoing study at our research group (acquired with a Philips 3T scanner, acquisition parameters: TR = 2,000 ms, TE = 25 ms, FA = 90°, FOV = 230 mm, gap = 0.5 mm, and matrix size = 128 × 128 × 35). A whole‐brain analysis of this sample rendered a sole cluster of signal increase in patients with ADHD in comparison to the control group (albeit at a threshold of p<0.001 uncorrected), which was also located in the ventrolateral part of the left dlPFC. ROI analyses confirmed this indeed concerned the same subregion as the cluster resulting from the main analyses (see Supporting Information).
In addition, the DMN components were included in a correlation analysis to assess if the neural activity in this region was associated with attentional performance. The WAIS symbols test, a task measuring selective attention, was used to obtain a performance‐based index of attention. A group comparison including this measure indicated that there was no significant difference in performance of the ADHD and control group (M ± SD; controls: 13.04 ± 2.34 and ADHD: 11.27 ± 3.19; F = 1.61, P = 0.211). This attentional measure was incorporated into a correlation analysis assessing the association between activity in this left dlPFC region and levels of inattention. A negative correlation was observed between left dlPFC activity in the resting state fMRI data and performance on the WAIS symbols test (−48x29y1z, 270 mm3, T = 2.28, P = 0.039, FWE corrected). As a higher score on the WAIS symbols test reflects better performance, this result suggests that higher levels of left dlPFC activity are associated with decreased performance‐based levels of attention. When examining the association with the WAIS symbols for each of the groups separately, we observed a statistical trend for the control group (48x29y4z, T = 2.28, P = 0.058, FWE corrected) and no significant result for the ADHD group (48x26y1z, T = 1.80, P = 0.105, FWE corrected). The correlation results are depicted in Figure 2.
Figure 2.
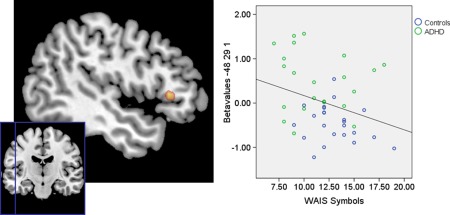
Results of the correlation analyses on the ICA data and measures of attention. Performance on the WAIS symbols test was used as an index of selective attention performance. A higher score on the WAIS symbols test represents increased attentiveness. Results are thresholded at P < 0.05, FWE corrected. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
FCA
In addition, to supplement the ICA results, we also performed a post hoc FCA approach using the left dlPFC region from the ICA as a seed region to further examine the differences between the groups in the functional connectivity of this region with the rest of the brain. However, no differences were observed on a two‐sample t‐test comparing the groups at a threshold of P < 0.05 FWE corrected. When using a more lenient threshold (P < 0.001, uncorrected), we did observe group differences in the functional connectivity of the left dlPFC. While the control group did not show increased temporal coherence of this region with other parts of the brain during rest in comparison to our ADHD sample, the ADHD group showed an increased temporal coherence between the left dlPFC region and a number of regions throughout the brain pertaining to several functional networks, such as the somatomotor network and, as expected, the DMN. The results of the comparison of the whole brain correlation maps at this more lenient threshold (P < 0.001, uncorrected) are indicated in Table 2.
Table 2.
Results of seed‐to‐voxel functional connectivity analysis with the dlPFC as seed region for the contrasts ‘ADHD > control’ and ‘control > ADHD’
| Brain region | MNI | Cluster size (mm3) | T | P | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Control > ADHD | n.s. | ||||||
| ADHD > Control | L Postcentral gyrus | –54 | –4 | 43 | 4617 | 4.86 | <0.001 |
| L Precentral gyrus | –42 | –4 | 40 | — | 4.51 | <0.001 | |
| R Temporal pole | 54 | 8 | –17 | 405 | 4.03 | <0.001 | |
| L Supramarginal gyrus | –63 | –22 | 40 | 54 | 3.70 | <0.001 | |
| L Inferior parietal gyrus | –54 | –28 | 49 | 216 | 3.65 | <0.001 | |
| R Insula | 27 | 20 | –17 | 108 | 3.55 | <0.001 | |
| L Orbitofrontal gyrus | –21 | 17 | –20 | 27 | 3.49 | <0.001 | |
| L Cerebellar tonsil | –12 | –40 | –47 | 54 | 3.39 | <0.001 | |
| R Precentral gyrus | 39 | –25 | 64 | 81 | 3.39 | <0.001 | |
| R Medial prefrontal gyrus | 18 | 17 | –17 | 27 | 3.35 | <0.001 | |
These results are cut off at a threshold of 0.001 uncorrected. No results were obtained when an FWE correction was applied.
In addition, to examine the degree of anticorrelation between this dlPFC region and the DMN, we extracted the beta values for the correlation between the left dlPFC region and the most negatively associated DMN region (the right precuneus). In line with our ICA results, the control subjects indeed exhibited a more negative temporal coherence between the dlPFC and the precuneus (M ± SD; controls: −0.19 ± 0.20, ADHD: −0.09 ± 0.16, F = 5.24, P = 0.027). We also performed a correlation analysis to assess if the degree of anticorrelation between these regions was also associated with attentional performance. Confirming our ICA correlation results, we observed a negative correlation between the FCA beta values and performance on the WAIS symbols test (R = −0.36, P = 0.017).
DISCUSSION
The dlPFC has frequently been associated with ADHD pathophysiology, and converging research findings indicate anatomical alterations and atypical dlPFC signaling—usually hypoactivity—during the performance of various cognitive tasks [Bush et al., 2005; Carmona et al., 2011; Faraone and Biederman, 1998; Hoekzema et al., 2010; Mostofsky et al., 2002; Seidman et al., 2006; Shaw et al., 2011; van 't Ent et al., 2007]. Our findings support an important role of this structure in the disorder. Several cognitive processes rely on dlPFC signaling, including working memory, response inhibition and attention. A number of executive functions, such as semantic working memory and selective attention, have been especially associated with the left hemispheric dlPFC [Hamilton et al., 2010; Vanderhasselt et al., 2010].
In line with its role in attentional processing, the dlPFC represents a key node of the TPN, a network commonly associated with a readiness to respond to environmental cues [Fox et al., 2005]. Neuroimaging studies in healthy subjects suggest that inadequate DMN regulation may underlie lapses of attention during situations that require cognitive effort. For instance, increased activation in DMN nodes such as the precuneus and medial prefrontal cortex preceded errors on a stop‐signal paradigm [Li et al., 2007], while a reduction in task‐positive signaling on memory tasks could predict which words and visual stimuli would later be forgotten [Pessoa et al., 2002; Wagner et al., 1998]. Likewise, a pattern of coincident DMN intrusion and deactivation in regions involved in task performance, such as the anterior cingulate cortex and dlPFC, was found to precede errors on tasks of selective attention [Eichele et al., 2008; Weissman et al., 2006].
Periodic lapses of attention are a key characteristic of ADHD, and these have been postulated to reflect DMN activity persisting into or re‐emerging during attention‐demanding situations [Castellanos et al., 2009; Sonuga‐Barke and Castellanos, 2007]. Indeed, Fassbender et al. 1998 observed a lower degree of ventromedial prefrontal cortex deactivation in response to increasing cognitive demands on a working memory task in ADHD children, which was associated with RT variability. Interestingly, the inability to appropriately regulate DMN activity during the performance of a Stroop task was normalized by treatment with psychostimulant medication [Peterson et al., 2009]. The findings observed in the present study suggest that, besides DMN intrusion during attention‐demanding situations, patients with ADHD exhibit a similar inability to adequately suppress state‐inappropriate TPN signaling from DMN activity during rest.
A few previous MRI studies have investigated resting state activity in adults with ADHD, using measures of functional connectivity or network homogeneity [Castellanos et al., 2008; Uddin et al., 2008]. Interestingly, these studies consistently observed decreases in the temporal coherence within the DMN in adults with ADHD [Castellanos et al., 2008; Uddin et al., 2008], suggesting a reduction in the within‐network functional connectivity. However, our ICA and FCA findings actually seem to point to an increase in the temporal coherence between distinct functional networks, as underlined by greater dlPFC intrusion into the DMN component during rest, a less stringent anticorrelation between the dlPFC and precuneus, and an enhanced functional connectivity between the dlPFC region and brain structures pertaining to various other neural networks, most significantly the primary motor and somatosensory cortex. In line with a postulated increase in between‐network connectivity, previous studies investigating resting state networks in adults and adolescents with ADHD also support an enhanced coherence between hubs of distinct functional neural networks. Castellanos et al. 2008 performed a functional connectivity study using the dorsal anterior cingulate cortex as a seed region, and observed a reduced anticorrelation—that is, more positive functional connectivity—with the precuneus/posterior cingulate cortex in adults with ADHD. In adolescents with ADHD, the anterior cingulate cortex showed an enhanced temporal coherence with various regions involved in homeostatic autonomic regulation [Tian et al., 2006]. Accordingly, Tian et al. 1995 evaluated the resting state activity index—a measure reflecting both the regional homogeneity and variance of low‐frequency fluctuations—in adolescents with ADHD and observed an increase compared to matched controls in several basic sensory and sensory‐related regions. Hence, whereas the affinity between regions within the same organized neural network may be decreased in comparison to control subjects, these reductions in connectivity do not seem to extend to regions pertaining to distinct networks that typically exhibit low temporal coherence. Together with previous literature, our findings seem to sketch an ADHD brain with more diffusely connected (i.e., enhanced between‐network but reduced within‐network coherence) functional networks in comparison to control subjects. Patients with ADHD may suffer from a general inability to appropriately attenuate low‐frequency oscillations in distinct functional neural networks in response to the cognitive demands posed by the environment, whether suppressing DMN activity during task performance or task‐positive signaling during rest, postulating a dysfunction in the regulation of functional networks.
A state of more diffusely connected networks is reminiscent of the functional architecture associated with a child brain, suggesting that the orthogonality between the functional networks may not have properly consolidated during development in patients with ADHD, a notion in accordance with the developmental delay hypothesis of ADHD [Rubia et al., 2000; Rubia, 2007]. Hence, these findings give rise to the notion that, rather than distinct deficits in various connections in the brain, ADHD may be associated with a more general dysfunction in the regulation of functional neural network activity, potentially related to an incomplete consolidation of these networks during development.
However, rather than reduced state‐dependent regulation, findings of an increased between‐network functional connectivity but reduced within‐network connections, less stringent anticorrelations between functionally opposed networks and intrusion of state‐inappropriate network activity could also be postulated to reflect a set of neural networks operating in the ADHD brain with distinct anatomical architecture. Future studies may clarify this issue by comparing low‐frequency neural network fluctuations across different degrees of cognitive effort to evaluate whether patients with ADHD exhibit a less consistent pattern of activity across repetitions of the same state and more homogeneous patterns of activity across different states. It would also be interesting to examine on a trial‐by‐trial basis whether state‐inappropriate network intrusions underlie the enhanced response variability consistently documented in patients with ADHD.
In the present study, only medication‐naïve patients with ADHD were included, hereby accounting for the contingent confounding effects of previous pharmacological treatment. Controlling for medication use and history is an important factor in ADHD research, as psychostimulant medication has been shown to render long‐term and short‐term biochemical, functional and structural changes in the brain [Andersen et al., 2008; Hoekzema et al., 2012a; Jezierski et al., 2007; Peterson et al., 2009; Schweitzer et al., 2003]. In fact, an fMRI study mapping the effects of psychostimulant treatment on brain activity during a working memory and a visual attention task indicated that, whereas DMN structures such as the posterior cingulate cortex were increasingly suppressed following methylphenidate administration, the BOLD signal was enhanced in several prefrontal and parietal regions in response to an increasing cognitive load [Tomasi et al., 2011]. Enhanced suppression of DMN activity after treatment with methylphenidate was also demonstrated in patients with ADHD [Peterson et al., 2009]. It should be noted, however, that although the strict criteria used for patient recruitment in our study allow us to exclude several important sources of variability, hereby improving the internal validity of the study, the selectivity of the sample also biases its representativeness. For instance, adult patients with ADHD that never received any medication for their condition represent a relatively small part of the ADHD population, hereby reducing the ecological validity of the study. This also applies to the used handedness, gender, subtype and comorbidity criteria.
To summarize, when comparing the ICA‐defined DMN components between the groups, we observed a highly significant cluster in the left dlPFC, indicating a more prominent association of this region with the DMN component of medication‐naïve adults with ADHD in comparison to the control group. Furthermore, activity in this region was related to performance on a selective attention task. Using this region as a seed ROI in a post hoc seed‐to‐voxel FCA approach to further define the differences in connectivity of this region between the groups, we observed increases in functional connectivity with regions pertaining to various other functional neural networks. Confirming the ICA results, we also observed a decreased anticorrelation with the precuneus, a key hub of the DMN, and the degree of anticorrelation was also associated with attentional performance.
These results support the implication of the dlPFC in ADHD pathophysiology, and indicate that aberrations in resting state network activity represent a persistent feature of the disorder, which does not result from previous exposure to ADHD medication. Moreover, our findings suggest that state‐inappropriate activity in ADHD is not restricted to DMN interference during attention‐demanding situations, but also comprises an inability to regulate signaling of the dlPFC—an important task‐positive node involved in various cognitive processes—in relation to DMN activity during rest. Together with results from previous studies, our ICA and FCA results seem to sketch an ADHD brain with more diffusely connected and less selectively regulated functional networks, surfacing as an increased between‐network connectivity, intrusion of state‐inappropriate network activity, and a decreased anticorrelation between functionally opposed networks.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We want to thank TDAH Catalunya for their collaboration and for referring patients for our study.
REFERENCES
- Allen EA, Erhardt EB, Wei Y, Eichele T, Calhoun VD (2012): Capturing inter‐subject variability with group independent component analysis of fMRI data: A simulation study. Neuroimage 59:4141–4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Task Force on DSM‐IV (2000):Diagnostic and Statistical Manual of Mental Disorders: DSM IV‐TR. Washington, DC:American Psychiatric Association. [Google Scholar]
- Andersen SL, Napierata L, Brenhouse HC, Sonntag KC (2008): Juvenile methylphenidate modulates reward‐related behaviors and cerebral blood flow by decreasing cortical D3 receptors. Eur J Neurosci 27:2962–2972. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV (2005): Attention‐deficit hyperactivity disorder. Lancet 366:237–248. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ (2005): Functional neuroimaging of attention‐deficit/hyperactivity disorder: A review and suggested future directions. Biol Psychiatry 57:1273–1284. [DOI] [PubMed] [Google Scholar]
- Buzy WM, Medoff DR, Schweitzer JB (2009): Intra‐individual variability among children with ADHD on a working memory task: An ex‐Gaussian approach. Child Neuropsychol 15:441–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD (2008): Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp 29:828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona S, Hoekzema E, Ramos‐Quiroga JA, Richarte V, Canals C, Bosch R, Rovira M, Carlos SJ, Bulbena A, Tobena A, Casas M, Vilarroya O (2011): Response inhibition and reward anticipation in medication‐naive adults with attention‐deficit/hyperactivity disorder: A within‐subject case–control neuroimaging study. Hum Brain Mapp 33:2350–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Kelly C, Milham MP (2009): The restless brain: Attention‐deficit hyperactivity disorder, resting‐state functional connectivity, and intrasubject variability. Can J Psychiatry 54:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di MA, Biswal B, Sonuga‐Barke EJ, Rotrosen J, Adler LA, Milham MP (2008): Cingulate–precuneus interactions: A new locus of dysfunction in adult attention‐deficit/hyperactivity disorder. Biol Psychiatry 63:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Sonuga‐Barke EJ, Milham MP, Tannock R (2006): Characterizing cognition in ADHD: Beyond executive dysfunction. Trends Cogn Sci 10:117–123. [DOI] [PubMed] [Google Scholar]
- Christiansen H, Kis B, Hirsch O, Matthies S, Hebebrand J, Uekermann J, bdel‐Hamid M, Kraemer M, Wiltfang J, Graf E, Colla M, Sobanski E, Alm B, Rosler M, Jacob C, Jans T, Huss M, Schimmelmann BG, Philipsen A (2012): German validation of the conners adult ADHD rating scales (CAARS) II: reliability, validity, diagnostic sensitivity and specificity. Eur Psychiatry 27:321–328. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF (2006): Consistent resting‐state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov‐Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR Jr, Barch DM, Petersen SE, Schlaggar BL (2010): Prediction of individual brain maturity using fMRI. Science 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R (1998):The ADHD rating scale‐IV: Checklist, norms, and clinical interpretation. New York:Guilford Press. [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, von Cramon DY, Ullsperger M (2008): Prediction of human errors by maladaptive changes in event‐related brain networks. Proc Natl Acad Sci USA 105:6173–6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt EB, Rachakonda S, Bedrick EJ, Allen EA, Adali T, Calhoun VD (2011): Comparison of multi‐subject ICA methods for analysis of fMRI data. Hum Brain Mapp 32:2075–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL, Blythe MS, Giwa A, Schmitt CF, Nigg JT (2010): Atypical default network connectivity in youth with attention‐deficit/hyperactivity disorder. Biol Psychiatry 68:1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Biederman J (1998): Neurobiology of attention‐deficit hyperactivity disorder. Biol Psychiatry 44:951–958. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB (2009): A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res 1273:114–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, William BW, Benjamin LS (1997):Interview for DSM‐IV Axis II Personality Disorders SCID‐II. Washington, DC:American Psychiatric Press. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams JBW (2002):Structured Clinical Interview for DSM‐IV‐TR Axis I Disorders, Research Version, Patient Edition. New York:Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fox MD, Raichle ME (2007): Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8:700–711. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005): The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME (2006): Coherent spontaneous activity accounts for trial‐to‐trial variability in human evoked brain responses. Nat Neurosci 9:23–25. [DOI] [PubMed] [Google Scholar]
- Fransson P, Aden U, Blennow M, Lagercrantz H (2011): The functional architecture of the infant brain as revealed by resting‐state FMRI. Cereb Cortex 21:145–154. [DOI] [PubMed] [Google Scholar]
- Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U (2007): Resting‐state networks in the infant brain. Proc Natl Acad Sci USA 104:15531–15536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD (2007): Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 164:450–457. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Molloy E, Castellanos FX (2001): Brain imaging of attention deficit/hyperactivity disorder. Ann N Y Acad Sci 931:33–49. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF (2009): Resting‐state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex 19:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Pagnoni G (2008): A unified framework for group independent component analysis for multi‐subject fMRI data. Neuroimage 42:1078–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME (2001): Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2:685–694. [DOI] [PubMed] [Google Scholar]
- Hamilton AC, Martin RC, Burton PC (2010): Converging functional magnetic resonance imaging evidence for a role of the left inferior frontal lobe in semantic retention during language comprehension. Cogn Neuropsychol 26:685–704. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Ortiz H, Fornito A, Pantelis C, Yucel M (2008): Modulation of brain resting‐state networks by sad mood induction. PLoS One 3:e1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos‐Quiroga JA, Canals C, Moreno A, Richarte Fernández V, Picado M, Bosch R, Duno L, Soliva JC, Rovira M, Bulbena A, Tobena A, Casas M, Vilarroya O (2012a): Stimulant drugs trigger transient volumetric changes in the human ventral striatum. Brain Struct Funct. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Ramos‐Quiroga JA, Richarte Fernández V, Picado M, Bosch R, Soliva JC, Rovira M, Vives Y, Bulbena A, Tobena A, Casas M, Vilarroya O (2012b): Laminar thickness alterations in the fronto‐parietal cortical mantle of patients with attention‐deficit/hyperactivity disorder. PLoS One 7:e48286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Carmona S, Tremols V, Gispert JD, Guitart M, Fauquet J, Rovira M, Bielsa A, Soliva JC, Tomas X, Bulbena A, Ramos‐Quiroga A, Casas M, Tobena A, Vilarroya O (2010): Enhanced neural activity in frontal and cerebellar circuits after cognitive training in children with attention‐deficit/hyperactivity disorder. Hum Brain Mapp 31:1942–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezierski G, Zehle S, Bock J, Braun K, Gruss M (2007): Early stress and chronic methylphenidate cross‐sensitize dopaminergic responses in the adolescent medial prefrontal cortex and nucleus accumbens. J Neurochem 103:2234–2244. [DOI] [PubMed] [Google Scholar]
- Jolles DD, van Buchem MA, Crone EA, Rombouts SA (2011): A comprehensive study of whole‐brain functional connectivity in children and young adults. Cereb Cortex 21:385–391. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997): Schedule for Affective Disorders and Schizophrenia for School‐Age Children‐Present and Lifetime Version (K‐SADS‐PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Di MA, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP (2009): Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex 19:640–657. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Wood AC, Rijsdijk F, Johnson KA, Andreou P, Albrecht B, rias‐Vasquez A, Buitelaar JK, McLoughlin G, Rommelse NN, Sergeant JA, Sonuga‐Barke EJ, Uebel H, van der Meere JJ, Banaschewski T, Gill M, Manor I, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Steinhausen HC, Faraone SV, Asherson P (2010): Separation of cognitive impairments in attention‐deficit/hyperactivity disorder into 2 familial factors. Arch Gen Psychiatry 67:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Yan P, Bergquist KL, Sinha R (2007): Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage 38:640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Cooper KL, Kates WR, Denckla MB, Kaufmann WE (2002): Smaller prefrontal and premotor volumes in boys with attention‐deficit/hyperactivity disorder. Biol Psychiatry 52:785–794. [DOI] [PubMed] [Google Scholar]
- Oncu B, Olmez S, Senturk V (2005): Validity and reliability of the Turkish version of the Wender Utah Rating Scale for attention‐deficit/hyperactivity disorder in adults. Turk Psikiyatri Derg 16:252–259. [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L (2002): Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron 35:975–987. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S (2009): An FMRI study of the effects of psychostimulants on default‐mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry 166:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001): A default mode of brain function. Proc Natl Acad Sci USA 98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos‐Quiroga JA, Bosch R, Richarte Fernández V, Valero S, Gómez‐Barros N, Nogueira M, Palomar G, Corrales M, Sáez‐Francàs N, Corominas M, Real A, Vidal R, Chalita PJ, Casas M (2012): Validez de criterio y concurrente de la versión española de la Conners Adult ADHD Diagnostic Interview for DSM‐IV. Rev Psiquiatr Salud Ment (Barc) 5:229–235. [DOI] [PubMed] [Google Scholar]
- Retz‐Junginger P, Retz W, Blocher D, Stieglitz RD, Georg T, Supprian T, Wender PH, Rosler M (2003): Reliability and validity of the Wender‐Utah‐Rating‐Scale short form. Retrospective assessment of symptoms for attention deficit/hyperactivity disorder. Nervenarzt 74:987–993. [DOI] [PubMed] [Google Scholar]
- Rodriguez‐Jimenez R, Ponce G, Monasor R, Jimenez‐Gimenez M, Perez‐Rojo JA, Rubio G, Jimenez A, Palomo T (2001): Validation in the adult Spanish population of the Wender Utah Rating Scale for the retrospective evaluation in adults of attention deficit/hyperactivity disorder in childhood. Rev Neurol 33:138–144. [PubMed] [Google Scholar]
- Rossini ED, O'Connor MA (1995): Retrospective self‐reported symptoms of attention‐deficit hyperactivity disorder: Reliability of the Wender Utah Rating Scale. Psychol Rep 77:751–754. [DOI] [PubMed] [Google Scholar]
- Rubia K (2007): Neuro‐anatomic evidence for the maturational delay hypothesis of ADHD. Proc Natl Acad Sci USA 104:19663–19664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, Andrew C, Bullmore ET (2000): Functional frontalisation with age: Mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev 24:13–19. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Lee DO, Hanford RB, Tagamets MA, Hoffman JM, Grafton ST, Kilts CD (2003): A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology 28:967–973. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J (2006): Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention‐deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry 60:1071–1080. [DOI] [PubMed] [Google Scholar]
- Shaw P, Gilliam M, Liverpool M, Weddle C, Malek M, Sharp W, Greenstein D, Evans A, Rapoport J, Giedd J (2011): Cortical development in typically developing children with symptoms of hyperactivity and impulsivity: support for a dimensional view of attention deficit hyperactivity disorder. Am J Psychiatry 168:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KD, Fawcett IP (2008): Transient and linearly graded deactivation of the human default‐mode network by a visual detection task. Neuroimage 41:100–112. [DOI] [PubMed] [Google Scholar]
- Sonuga‐Barke EJ, Castellanos FX (2007): Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev 31:977–986. [DOI] [PubMed] [Google Scholar]
- Stein MA, Sandoval R, Szumowski E, Roizen N, Reinecke MA, Blondis TA, Klein Z (1995): Psychometric characteristics of the Wender Utah Rating Scale (WURS): reliability and factor structure for men and women. Psychopharmacol Bull 31:425–433. [PubMed] [Google Scholar]
- Tian L, Jiang T, Liang M, Zang Y, He Y, Sui M, Wang Y (2008): Enhanced resting‐state brain activities in ADHD patients: A fMRI study. Brain Dev 30:342–348. [DOI] [PubMed] [Google Scholar]
- Tian L, Jiang T, Wang Y, Zang Y, He Y, Liang M, Sui M, Cao Q, Hu S, Peng M, Zhuo Y (2006): Altered resting‐state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neurosci Lett 400:39–43. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND (2011): Association between functional connectivity hubs and brain networks. Cereb Cortex 21:2003–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND, Wang GJ, Wang R, Telang F, Caparelli EC, Wong C, Jayne M, Fowler JS (2011): Methylphenidate enhances brain activation and deactivation responses to visual attention and working memory tasks in healthy controls. Neuroimage 54:3101–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, Milham MP (2008): Network homogeneity reveals decreased integrity of default‐mode network in ADHD. J Neurosci Methods 169:249–254. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Menon V (2010): Typical and atypical development of functional human brain networks: Insights from resting‐state FMRI. Front Syst Neurosci 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van 't Ent D, Lehn H, Derks EM, Hudziak JJ, Van Strien NM, Veltman DJ, De Geus EJ, Todd RD, Boomsma DI (2007): A structural MRI study in monozygotic twins concordant or discordant for attention/hyperactivity problems: Evidence for genetic and environmental heterogeneity in the developing brain. Neuroimage 35:1004–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhasselt MA, De RR, Leyman L, Baeken C (2010): Role of the left DLPFC in endogenous task preparation: Experimental repetitive transcranial magnetic stimulation study. Neuropsychobiology 61:162–168. [DOI] [PubMed] [Google Scholar]
- Varoquaux G, Sadaghiani S, Pinel P, Kleinschmidt A, Poline JB, Thirion B (2010): A group model for stable multi‐subject ICA on fMRI datasets. Neuroimage 51:288–299. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM (2008): Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One 3:e2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL (1998): Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science 281:1188–1191. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, Zhong Q, Wang Y (2009): Altered small‐world brain functional networks in children with attention‐deficit/hyperactivity disorder. Hum Brain Mapp 30:638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW (1993): The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry 150:885–890. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1997):Wechsler Adult Intelligence Scale‐III. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG (2006): The neural bases of momentary lapses in attention. Nat Neurosci 9:971–978. [DOI] [PubMed] [Google Scholar]
- Yeh CB, Gau SS, Kessler RC, Wu YY (2008): Psychometric properties of the Chinese version of the adult ADHD Self‐report Scale. Int J Methods Psychiatr Res 17:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


