Abstract
In recent years, functional imaging studies have revealed a supraspinal network, which is involved in perception and processing of bladder distention. Very little information exists on the cortical representation of C‐fiber transmitted temperature sensation of the human bladder, although C‐fibers seem to be involved in the pathomechanisms of bladder dysfunctions. Our aim was, therefore, to evaluate the outcome of bladder cold stimulation on supraspinal activity using functional magnetic resonance imaging (fMRI). A block design fMRI study was performed in 14 healthy females at the MR‐center of the University of Zurich. After catheterization, all subjects were investigated in a 3.0‐Tesla Scanner. The scanning consisted of 10 repetitive cycles. Each cycle consisted of five conditions: REST, INFUSION, SENSATION, DRAIN 1, and DRAIN 2. Cold saline was passively infused at 4–8°C during scanning. Not more than 100 ml were infused per cycle. Blood‐oxygen‐level‐dependent (BOLD) signal analysis of the different conditions was compared to REST. All activations were evaluated on a random effects level at P = 0.001. Activation of brain regions for bladder cold stimulation (DRAIN 1 period) was found bilaterally in the inferior parietal lobe [Brodmann area (BA) 40], the right insula (BA 13), the right cerebellar posterior lobe, the right middle temporal gyrus (BA 20), and the right postcentral gyrus (BA 3). In conclusion, bladder cooling caused a different supraspinal activation pattern compared to what is known to occur during bladder distention. This supports our hypothesis that cold sensation is processed differently from bladder distension at the supraspinal level. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: bladder cooling, bladder sensations, C‐fibers, fMRI, supraspinal control
INTRODUCTION
There are two main afferent fiber types in the lower urinary tract (LUT), A‐delta (Aδ) and C‐fibers. The myelinated Aδ‐fibers are known to transmit signals of bladder wall distension and therefore, to indicate the degree of bladder filling [Andersson, 2002; Wyndaele and De Wachter, 2008]. The unmyelinated C‐Fibers are thought to transmit signals caused by inflammation, thermal stimuli (e.g., cold), and noxious or chemical stimuli [e.g., vanilloids, resinferatoxin, and changes in urine pH; Andersson, 2002; Jiang et al., 2002; Wyndaele and De Wachter, 2008].
The influence of Aδ‐fiber transmitted information on the activation of supraspinal neuronal structures has been investigated in recent years during different procedures of bladder filling and emptying using positron emission tomography (PET) and functional magnetic resonance imaging [fMRI; Griffiths and Tadic, 2008]. These studies have revealed a complex supraspinal network, involved in the functioning and control of the lower urinary tract (LUT), which includes frontal and orbito‐frontal areas, the anterior cingulate gyrus (ACG), the insula, the thalamus, the cerebellum, the pons, and the periaqueductal gray [PAG; Griffiths and Tadic, 2008].
Still, little is known about the central neural correlates of bladder C‐fiber stimulation. In healthy subjects with a normal LUT function, C‐fibers are regarded as silent [Andersson, 2002; Geirsson et al., 1999; Wyndaele and De Wachter, 2008].
However, there are certain neurological (e.g., spinal cord injury and multiple sclerosis) and other, rather locally confined pathological LUT conditions (e.g., inflammation, overactive bladder, and painful bladder syndrome) that are associated with changes in the sensitization and sprouting of bladder C‐fibers [Andersson, 2002; Fowler, 2002; Mukerji et al., 2006a; Wyndaele and De Wachter, 2008; Yoshimura and Chancellor, 2002]. Thus, changes in C‐fiber activity have been regarded as part of the pathophysiology of LUT dysfunctions, such as increased urination frequency and urgency, as well as suprapubic pain [Mukerji et al., 2006a].
Therefore, investigation of C‐fiber activation and its central nervous system representation are of relevance for understanding LUT function and dysfunctions.
As C‐fibers are sensitive to cold stimulation [Andersson, 2002; Geirsson et al., 1999; Jiang et al., 2002], bladder cooling with rapid cold saline infusion into the bladder would be a safe and feasible way to stimulate C‐fibers during functional brain imaging. Although cooling is not considered a physiological sensation normally arising from the bladder, it has previously been demonstrated to be an useful diagnostic method for investigating functional LUT disturbances [Fall and Geirsson, 1996; Geirsson et al., 1993, 1999; Mukerji et al., 2006a, b] and may also be a good paradigm for studying painful processes in the LUT associated to C‐fiber stimulation [Mukerji et al., 2006a].
Our aim in this study was to investigate the supraspinal neural correlate of bladder cold perception using fMRI in healthy subjects. On the basis of the findings of a previous PET study [Matsuura et al., 2002], we hypothesized that bladder cooling recruits a different cerebral network than bladder filling.
MATERIALS AND METHODS
After approval from the local ethics committee, 14 healthy female subjects gave their written informed consent for participating in the study. Inclusion criteria were as follows: healthy, right‐handed females, aged 18–35 years. Exclusion criteria were: any actual health problems, urinary tract infection (UTI), latex allergy, previous or current pregnancy, symptoms of overactive bladder (OAB) or other LUT symptoms, and ferromagnetic implants.
We included only female subjects for reasons of group homogeneity. Although men and women seem to have a similar overall prevalence for lower urinary tract symptoms like OAB, they show distinct differences in certain symptoms, which might be related to the different anatomy of the male and female LUT [Irwin et al., 2006]. In addition, our experience has shown that catheterization is much less uncomfortable for female subjects than for males.
Image Acquisition
The study was performed in the MR (magnetic resonance)‐Center of the University of Zurich, using a 3.0‐Tesla Scanner (Philips, Achieva) and an eight‐element head coil.
Functional BOLD sensitive images were acquired using a single‐shot gradient echo EPI pulse sequence (TE/TR = 35/3,000 ms, flip angle = 82°, FOV = 220 × 220 mm2, matrix = 128 × 128, slices = 39, slice thickness = 3 mm). Sensitivity encoding (SENSE) with a reduction factor of two was used to minimize the influence of susceptibility artifacts and to maximize the possible number of slices acquired within one TR.
High‐resolution anatomical images were acquired before the functional experiments, using a 3D T1‐weighted gradient echo sequence (TE/TR = 2.3/20 ms, FOV = 220 × 220 mm2, matrix = 256 × 256, slices = 180, slice thickness = 0.75 mm). All images were obtained in an oblique axial orientation covering the entire head.
Experimental Design
Following transurethral catheterization using a nonanesthetic lubricant and a soft 14‐Fr latex catheter, the individual bladder capacity of each subject was determined by manually filling the bladder until strong desire to void (SDV).
Test run
A test run was performed prior to the actual fMRI measurements consisting of 10 repetitive cycles with 5 different conditions per cycle: tREST, tINFUSION, tSENSATION, tDRAIN 1, and tDRAIN 2 (t = test run condition; Table I). During tREST, no specific stimulus was given and the bladder was empty. During tINFUSION, the bladder was passively filled with cold saline (4–8°C) and the time until subjects indicated the beginning of bladder cold sensation was recorded for each cycle. tSENSATION started per definition, when subjects indicated the very first sensation of bladder cooling. During tSENSATION, cold saline was still infused into the bladder. With the start of tDRAIN 1, the infusion stopped and the bladder was drained. During tDRAIN 2, the bladder was empty again. The repetitive, passive infusion and drainage of the cold saline was realized through tubes connected to the transurethral catheter via a simple Y‐connector (see Fig. 1). In‐ and outflow was regulated via two valves, one on the inflow tube (Valve 1) and one on the outflow tube (Valve 2; see Fig. 1), i.e., Valve 1 open and Valve 2 closed = bladder fills with cold saline. Valve 1 closed and Valve 2 open = bladder drains and starts to warm‐up (Table I).
Table I.
Status of the in‐ and outflow valves, the bladder filling, and the subjective sensations during the test run prior to the MR‐scanning (t = test run condition)
| tREST | tINFUSION | tSENSATION | tDRAIN 1 | tDRAIN 2 | |
|---|---|---|---|---|---|
| Duration | 12 s | variable, mean of all subjects: 9.8 ± 1.9 s (range: 6–12 s) | 12 s | 30 s | 30 s |
| Status of the valves at the in‐ and outflow tube | Valve 1 closed, Valve 2 open | Valve 1 open, Valve 2 closed | Valve 1 open, Valve 2 closed | Valve 1 closed, Valve 2 open | Valve 1 closed, Valve 2 open |
| Filling status of the bladder | Bladder is empty | Start of bladder filling with cold saline (4–8°C) | Bladder filling with cold saline (4–8°C) | Bladder is drained | Bladder is empty |
| Subjective sensation | No bladder cold sensation | No bladder cold sensation | First sensation of bladder cooling (=start of SENSATION), which slowly increases with further filling | Further increase in cold sensation up to 12 s, followed by a continuous decline in bladder cold sensation | Slight bladder cold sensation, which further declines until it is completely absent |
Figure 1.
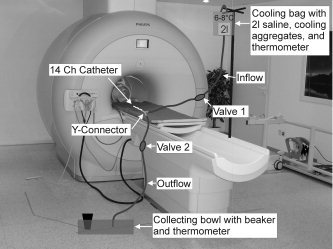
Experimental setup in the magnetic resonance (MR) facility.
The test run was performed to give the subjects an impression of how the cold stimulation feels like, and to determine their subjective sensations and the individual mean time until the cold sensation is perceived after starting INFUSION.
Because of the small catheter diameter, no more than 100 ml could be infused in total, which was controlled with a measuring jug, collecting the drained fluid from the bladder (see Fig. 1).
Regarding the subjective sensations during the test run, we documented the following (Table I): During tREST and tINFUSION, no bladder cooling was indicated. The tINFUSION condition had different durations, as different subjects indicated the start of bladder cold sensation (=start of tSENSATION) at different time points. We used the mean time of the 10 repetitions of tINFUSION in each subject to individually calculate the actual fMRI protocol.
With the beginning of tSENSATION, bladder cold sensation started. All subjects were able to feel cooling within the bladder, which they described as a deep and diffuse coldness that spreads and intensifies with further filling. The sensation of bladder cooling was described as being similar to the sensation felt in the stomach when drinking ice cold water or lemonade except that the location was different, being perceived more inferior in the lower pelvis, just above the pubic bone.
During tDRAIN 1, when the bladder started to drain and no further ice water was infused, the bladder cold sensation still increased during the first 10–12 s of this condition. This increase in cold sensation was followed by an initially slow, and subsequently faster, continuous decline of the sensation. During tDRAIN 2, the bladder was empty and the cold sensation declined further and finally disappeared completely. It took ∼45–50 s after starting to drain the cold saline, until the subjects reported a complete disappearance of any cold sensation.
fMRI measurements
For the fMRI measurements, subjects were placed supine into the scanner and positioned as comfortably as possible. Isolating foam pads on the hip and thigh of the subjects were used to ensure that the in‐ and outflow tubes did not touch the legs or any other body part of the subjects during the functional scans to reduce artifacts and/or somatosensory activations of the touched body region.
The fMRI was performed following the same paradigm as was used for the test run, with 10 repetitive cycles and 5 different conditions per cycle, namely REST, INFUSION, SENSATION, DRAIN 1, and DRAIN 2 (see Fig. 2). Based on the subjective sensation and timing information from the test run, the fMRI protocol was adapted to the individual mean time of bladder cooling sensation (Table I, Fig. 2). The repetitive bladder cooling was performed in the same way as described for the test run.
Figure 2.
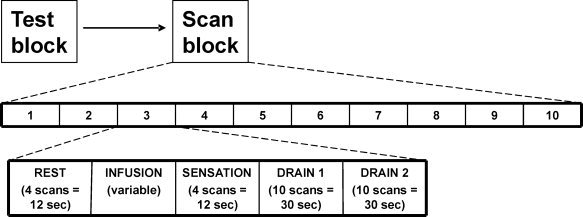
Scanning paradigm of the block design used in this study, indicating the number of repetitions (n = 10) and the sequence of the different scan conditions during each repetition. The repetitions were performed successively without pause.
After completion of all scans, subjects had to indicate on a visual analog scale (VAS) the degree of comfortableness and arousal during the scans. A score of 10 on the VAS indicated “very comfortable/pleasant” or “very calm/low arousal” and a score of −10 indicated “very uncomfortable/painful” or “very excited/high arousal.”
fMRI Data Analysis
The preprocessing of the functional MRI data was performed using BrainVoyager 1.8 (Brain Innovation B.V., Maastricht, The Netherlands) and included motion correction, 4‐mm spatial smoothing, high pass linear trend removal, and temporal filtering (three cycles in time course).
Following motion correction, 2 of the 14 acquired datasets had to be excluded from the final group analysis due to head motion >1.5 mm. Therefore, results (Tables II and III, Figs. 3 and 4) are shown for 12 subjects.
Table II.
Activated (positive t values) and deactivated (negative t values) brain regions during the different scanning conditions
| Condition | Hemisphere | Region | Brodmann area | Talairach coordinates | Cluster size | Peak activation t = 4.4 | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Infusion | Right | Precentral gyrus (frontal lobe) | 4 | 57 | −7 | 26 | 63 | −6.3 |
| Angular gyrus (parietal lobe) | 39 | 41 | −59 | 39 | 110 | −6.4 | ||
| Cerebellum, posterior lobe | 36 | −72 | −41 | 73 | −6 | |||
| Superior frontal gyrus (frontal lobe) | 8 | 23 | 28 | 51 | 200 | −7.1 | ||
| Superior frontal gyrus (frontal lobe) | 10 | 7 | 59 | 24 | 378 | −6.2 | ||
| Superior frontal gyrus (frontal lobe) | 8 | 2 | 45 | 45 | 197 | −5.8 | ||
| Left | Cerebellum, anterior lobe | −10 | −54 | −19 | 266 | −7.2 | ||
| Medial frontal gyrus (frontal lobe) | 10 | −12 | 48 | 12 | 158 | −6.2 | ||
| Sensation | Right | inferior parietal lobe | 40 | 54 | −39 | 49 | 68 | 6.8 |
| middle temporal gyrus | 39 | 41 | −58 | 7 | 154 | −5.7 | ||
| middle temporal gyrus | 39 | 40 | −71 | 14 | 77 | −5.2 | ||
| middle temporal gyrus | 19 | 35 | −62 | 17 | 184 | −6.9 | ||
| superior parietal lobe | 7 | 28 | −48 | 56 | 170 | −5.9 | ||
| frontal lobe, sub‐gyral | 6 | 26 | −1 | 56 | 214 | −7.5 | ||
| Drain 1 | Right | Cerebellum, posterior lobe | 24 | −45 | −37 | 495 | 8.7 | |
| Cerebellum, posterior lobe | 27 | −63 | −36 | 1,021 | 8 | |||
| Cerebellum, posterior lobe | 39 | −50 | −37 | 81 | 5.8 | |||
| Insula | 13 | 36 | −3 | 8 | 77 | 5.4 | ||
| Cerebellum, posterior lobe | 50 | −48 | −20 | 63 | 5.1 | |||
| middle temporal gyrus | 20 | 51 | −33 | −9 | 153 | 5.8 | ||
| postcentral gyrus | 3 | 61 | −17 | 31 | 86 | 5.9 | ||
| inferior parietal lobe | 40 | 61 | −33 | 29 | 555 | 7.4 | ||
| inferior parietal lobe | 40 | 48 | −38 | 43 | 2,310 | 8.4 | ||
| Angular gyrus (parietal lobe) | 39 | 35 | −58 | 37 | 1,973 | 7 | ||
| Left | inferior parietal lobe | 40 | −37 | −55 | 39 | 337 | 6.6 | |
| inferior parietal lobe | 40 | −45 | −49 | 40 | 160 | 5.6 | ||
| inferior parietal lobe | 40 | −50 | −48 | 47 | 51 | 5.7 | ||
| Drain 2 | Right | no activations or deactivations | ||||||
| Left | no activations or deactivations | |||||||
All activations and deactivations are shown as random effects with t = 4.4 (P = 0.001).
Table III.
Individual t‐values and Tailarach coordinates for Pons, PAG, and thalamus activations in the 12 subjects
| Subject number | FDR correction 0.05; Cluster threshold 10 voxel | Pons | PAG | Thalamus | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| t value | P value | x | y | z | x | y | z | x | y | z | |
| 1 | 3.17 | 0.0015 | 6 | −26 | −23 | 4 | −11 | −1 | |||
| −9 | −28 | −18 | |||||||||
| −11 | −19 | −29 | |||||||||
| 2 | 2.28 | 0.023 | −6 | −22 | −23 | −14 | −24 | −2 | 14 | −19 | 4 |
| −8 | −25 | −33 | 11 | −25 | −2 | −14 | −19 | 6 | |||
| 9 | −14 | −20 | |||||||||
| 3 | 2.32 | 0.02 | −8 | −23 | −29 | −6 | −19 | −9 | −12 | −22 | 14 |
| 11 | −22 | −30 | −12 | −19 | 9 | ||||||
| −17 | −19 | 1 | |||||||||
| 4 | 2.85 | 0.0043 | −8 | −29 | −27 | −5 | −24 | −9 | −18 | −10 | 7 |
| 6 | −29 | −18 | −1 | −18 | −22 | 17 | −16 | 15 | |||
| 5 | 2.49 | 0.013 | −3 | −17 | −17 | 6 | −25 | −12 | −15 | −20 | 15 |
| −1 | −19 | −9 | −6 | −5 | 0 | ||||||
| −12 | −2 | 10 | |||||||||
| 6 | 2.52 | 0.012 | 8 | −28 | −30 | −6 | −15 | −6 | 5 | −1 | 2 |
| −9 | −19 | −27 | 8 | −15 | −8 | ||||||
| −1 | −19 | −21 | |||||||||
| 7 | 2.47 | 0.014 | −9 | −23 | −24 | 8 | −11 | −6 | 6 | −17 | 7 |
| −2 | −28 | −33 | −5 | −15 | 11 | ||||||
| 10 | −28 | −36 | |||||||||
| 8 | 2.79 | 0.0052 | 3 | −26 | −26 | −13 | −14 | 5 | |||
| −6 | −26 | −20 | 22 | −22 | 0 | ||||||
| 9 | 2.85 | 0.0044 | −4 | −24 | −27 | −4 | −19 | −5 | |||
| 10 | 2.58 | 0.01 | −2 | −27 | −19 | −6 | −16 | −9 | |||
| −2 | −16 | −23 | 9 | −16 | −8 | ||||||
| 11 | 2.57 | 0.01 | −4 | −19 | −15 | 16 | −10 | 16 | |||
| −15 | −24 | 16 | |||||||||
| 12 | 2.9 | 0.0038 | |||||||||
FDR = false discovery rate.
Figure 3.
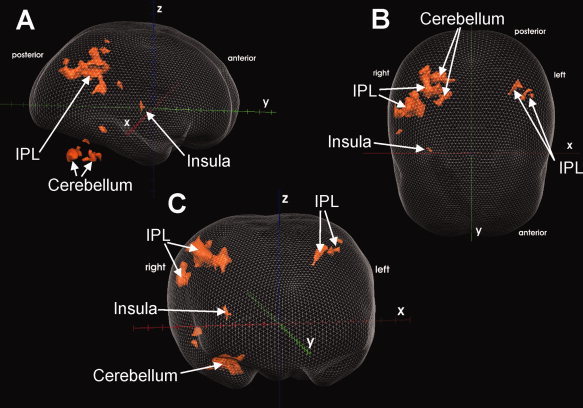
Lateral (A), top (B), and frontal (C) view of a glass brain, showing the supraspinal activations of 12 subjects during the DRAIN 1 condition at random effects level with t = 4.4 (P = 0.001). Only clusters ≥50 contiguous voxel are shown. For detailed description and coordinates of activated regions, see Table II. Talairach axes (x, y, z) are displayed for easier orientation. IPL = inferior parietal lobe.
Figure 4.
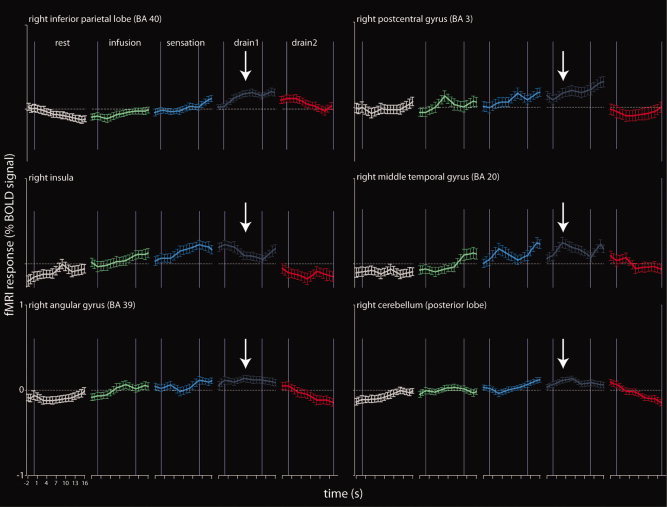
The progression of the BOLD signal during each experimental condition for all activated regions found in the group analysis (Table II). The BOLD signal was extracted from a sphere (10‐mm diameter) around the peak activation in each region for the contrast DRAIN 1–REST.
Next, motion‐corrected data were normalized by interpolation of the 3D anatomical images to iso‐voxel size (1 × 1 × 1 mm3) and subsequent transformation into the Talairach space [Talairach and Tournoux, 1988]. The Talairach coordinates give similar results to the MNI coordinates.
The functional data were then coregistered to the individual anatomical data, using a fully automatic, highly precise, alignment as implemented in the BrainVoyager software package.
The Talairach transformed contrast images were entered into a group‐level random effect analysis [Friston et al., 1999], i.e., a two‐level procedure of a full mixed‐effects model, to generalize the activation to the population level. This analysis was based on the general linear model (GLM) and used a Gaussian hemodynamic response function (HRF) with commonly used time parameters: 5 s to response peak and 15 s to undershoot peak. The images of all experimental conditions (REST, INFUSION, SENSTION, DRAIN 1, and DRAIN 2) of the first‐level were carried on to the second‐level random‐effects group analysis. Group contrasts were defined by subtraction logic (e.g., [−1 0 0 1 0] to calculate the difference between DRAIN 1 and REST). INFUSION, SENSATION, DRAIN 1, and 2 were separately contrasted to the REST condition.
All (de‐)activations are presented as group results evaluated on random effects level with t = 4.4 (P = 0.001, uncorrected), overlaid on the group averaged anatomical image. In addition, we applied a cluster extent threshold correction, considering only clusters ≥50 contiguous voxel.
A single subject analysis was performed for smaller areas like PAG and pons with P ≤ 0.05 [false discovery rate (FDR) corrected]. For all experimental conditions, we evaluated the BOLD signal changes in the activated regions from the contrast DRAIN 1–REST. The BOLD signal changes were extracted by drawing a sphere (diameter of 10 mm) around the peak activation in a given area. To obtain a higher temporal resolution (i.e., smaller than a TR of 3 s) of the BOLD signal changes, the data between two consecutive data points was interpolated as implemented in the BrainVoyager software package.
RESULTS
All 14 subjects (mean age: 24.8 years, range 21–34 years) included in the study tolerated the catheterization and the fMRI experiment well and indicated no pain during the study. The results present data of 12 subjects, as the datasets of two subjects had to be excluded from the analysis due to overly head motion (>1.5 mm).
Mean comfortableness and arousal scores on the VAS during cold water infusion were −0.8 ± 1.3 and 1.7 ± 3.4, respectively.
The average bladder capacity at SDV was 579.7 ± 179.2 ml. The average time until cooling of the bladder was perceived during the test run was 9.8 ± 1.9 s (range: 6–12 s).
The average saline temperature during INFUSION was (6.1 ± 0.8)°C (range: 4–8°C). The average saline temperature after drainage from the bladder at the beginning of DRAIN 1 condition was (18.3 ± 2.1)°C (range: 11.9–23.7°C).
The INFUSION condition showed only deactivations, mainly in the right frontal lobe [Brodmann area (BA) 8 and 10] and cerebellum (Table II). In the subsequent SENSATION condition, activations were found in the right inferior parietal lobule (IPL, BA 40). Deactivations were observed mainly in the right middle temporal gyrus (BA 19, 39), the right frontal lobe (BA 6), and the right superior parietal lobule (BA 7; Table II).
The DRAIN 1 condition elicited activations in the IPL (BA 40) bilaterally, the right insula (BA 13), the right cerebellar posterior lobe, the right middle temporal gyrus (BA 20), and the right post‐central gyrus (BA 3; Table II, Fig. 3a–c). In the DRAIN 2 condition, neither deactivations nor activations were detected (Table II).
The behavior of the BOLD signal change (%) during each condition confirmed that our scan paradigm was chronologically accurate (see Fig. 4). Figure 4 demonstrates that all activated areas show a remarkably similar BOLD signal change during the different experimental conditions, i.e., a low response during REST and INFUSION, an increasing response during SENSATION, a maximal response during DRAIN 1, and a low response again during DRAIN 2.
Activation of the Pons and PAG were not observed in the group analysis during any condition, even with very low t values. However, in the single subject analysis 10 of 12 subjects and 9 of 12 subjects showed significant activation in the pontine and PAG region, respectively (Table III).
Significant thalamic activation was found in 9 of 12 subjects in the single subject analysis but not in the group analysis (Table III).
Although frontal activation was not observed in the group analysis, each subject showed some scattered frontal activation (mainly in the superior and middle frontal lobes, BA 8, 9, 10, and 46) in the single subject analysis.
ACG activation, which was also not detected in the group analysis, was observed in 6 of 12 subjects. Those ACG activations were rather small clusters, surrounded by several larger clusters with significant deactivation.
DISCUSSION
Nerve Fiber, Cold Receptor, and Clinical Considerations
Cold sensation can be perceived in the urinary bladder as distinct from distention. This is known since bladder cooling experiments have been used in urodynamic investigations [Geirsson et al., 1993; Hellstrom et al., 1991; Mc and Murphy, 1959; Nathan, 1952] and specific cold receptors (TRPM8) have been discovered on bladder afferents in the urothelium and suburothelium [Everaerts et al., 2008; Mukerji et al., 2006b].
Although Geirsson and Fall postulated that the bladder cooling response in humans is mediated by unmyelinated C‐fibers [Geirsson et al., 1999] and some confirmative evidence of this exists from animal studies [cats; Jiang et al., 2002], there is no proof that only C‐fibers can transmit cold sensation in the human LUT. Some authors indicate that cold sensation is also transmitted via Aδ‐fibers [Craig, 2003]. In addition, TRPM8 receptors have been found on both unmyelinated and myelinated bladder afferent fibers [Mukerji et al., 2006b].
Therefore, our fMRI findings may not reflect pure C‐fiber activation, but rather may predominantly indicate TRPM8 activation. As TRPM8 receptors become active at temperatures below 25°C [Mukerji et al., 2006b], it might also explain why cold sensation recruits a different cerebral network as compared to bladder filling with warm (body‐temperature) saline.
The reason for the existence of cold sensitive receptors in the LUT is still unknown. It has been proposed that they may have the same role in the regulation and maintenance of a stable central core temperature as other thermoreceptors found elsewhere in the body [Geirsson et al., 1999; Lindstrom et al., 2004]. This is supported by the fact that body cooling is usually associated with increased diuresis, and thus, the bladder cooling reflex has presumably evolved to help relieve the thermal ballast in the bladder during cooling stress [Geirsson et al., 1999].
Brain Network of Bladder Thermal Sensation
Although immunohistochemical studies have revealed that cold responsive neurons project to the superficial laminae (I & II) of the dorsal horn in the lumbar and sacral spinal cord of rats [Jiang and Hermanson, 2004], little is known about the supraspinal central representation of bladder cold stimulation. A single PET study exists investigating cerebral response to ice water instillation into the bladder of six subjects, which showed activations of a network including the ACG, inferior and middle frontal gyrus, IPL, hippocampus, and crus cerebri [Matsuura et al., 2002].
In our paradigm, we used repetitive bladder cooling, taking into account the exact time until bladder cold sensation was perceived by the subjects. This is important, as our results indicate that the cold saline (4–8°C) becomes already warmed to 18°C within 18–24 s after infusion. This is not surprising, considering that the bladder lies deep within the inferior abdomen. For this reason, we performed the test run prior to the functional MR‐scanning, to adapt the timing of the scan paradigm accordingly.
The behavior of the BOLD signal during each condition (see Fig. 4) matched the subjective sensations during the test runs (Table I). During SENSATION, the feeling of bladder cooling started and increased, as did the BOLD signal. During DRAIN 1, where the greatest activation was observed, subjects indicated a further increase of bladder cold sensation, followed by a slow and constant decline of the cooling sensation until it disappeared toward the end of DRAIN 2, where no activation was observed. The fact that bladder cold sensation continued to increase during DRAIN 1, although the bladder started to empty, might be due to the fact that the bladder drainage lasted slightly longer than the filling and therefore, some amount of cold saline was still in the bladder during DRAIN 1. Another reason might be an after‐cooling effect with a delayed spreading of cold sensation in the bladder tissue. This can also be observed on the skin, where cold sensation does not disappear immediately following removal of the cooling source.
Assessment of subjective sensations has not been performed during the functional MR‐scans, which might be a limitation of this study, and slight shifts in the timing of sensations in relation to our scan paradigm cannot be entirely excluded. However, assessment of subjective sensations during ongoing scanning would further complicate the experimental procedure and might be a source of additional artifacts.
After functional MR‐scanning, all subjects confirmed a feeling of repeated bladder cooling that matched the timing and sensations felt during the test run.
The SENSATION condition was not prolonged to encompass the entire period of cooling sensation, as this would have caused distention of the bladder. With the 14‐Fr Foley catheter and a filling time of 18–24 s used in the present study, only 80–90 ml saline could flow into the bladder. Thus, with a mean bladder volume of SDV 579.7 ± 179.2 ml, at most only a first sensation of filling was perceived by the subjects, avoiding further distension of the bladder [Wyndaele and De Wachter, 2008].
Similar to the PET study of Matsuura et al., the most prominent and earliest response during bladder cooling was found in the IPL [BA 40; Matsuura et al., 2002]. In contrast to this study, reporting unilateral (left) IPL activation, we observed bilateral activation with a right hemispheric dominance. The IPL is implicated in a series of functions including spatial attention, multimodal sensory integration, oculomotor control, and also in various higher cognitive functions like integrative motor planning and interoception [Abu‐Akel, 2003; Caspers et al., 2006; Clower et al., 2001; Craig, 2002; Fogassi and Luppino, 2005]. Especially the parietal operculum has been implicated in interoception [Craig, 2002]. The plausibility of the specialization of the right IPL in the representation of internally/self‐generated acts finds support in studies reporting a specific involvement of this area in distinguishing self‐produced actions from those generated by others, and studies showing that lesions to this area lead to the loss of corporeal awareness [Abu‐Akel, 2003]. As bladder cooling is a visceral sensation, it is not surprising that especially areas involved in corporeal awareness and self‐perception are activated.
Another important area, involved in viscero‐sensory function, processing of nociceptive inputs, and interoceptive awareness of visceral sensations, is the insula [Kavia et al., 2005]. In contrast to Matsuura et al., who did not find insula activation during bladder cooling, we observed right‐sided insula activation [Matsuura et al., 2002]. In addition, thalamic activation was found in 3/4 of the subjects. The insula, thalamus, and secondary somatosensory cortex (BA 3, Table II) are considered to be the key brain structures for somatic thermal perception [Davis et al., 1998, 1999]. Although the bladder is a visceral organ, similarities between visceral and somatic thermal perception have been previously reported [Strigo et al., 2003]. Moreover, cold water flowing through the urethra might have also contributed to activations in these areas. However, this effect seems to be low, as no activation could be detected during INFUSION.
In a recent fMRI study with urgency incontinent subjects, Tadic et al. demonstrated increased activity in the insula, temporal and parietal lobes, in areas very similar to the ones found during thermal C‐fiber stimulation in our study [Table II; Tadic et al., 2008]. This may further suggest the potential role of C‐fiber function in the etiology and pathogenesis of OAB and urgency incontinence [Andersson, 2002; Fowler, 2002; Mukerji et al., 2006a, b; Wyndaele and De Wachter, 2008; Yoshimura and Chancellor, 2002].
Unlike Matsuura, we did not observe significant activation in the ACG or frontal regions in our group results [Matsuura et al., 2002]. However, the single subject analysis revealed ACG activations in half of the subjects and some frontal activation in each of the subjects. Nevertheless, these activations were too scattered to result in any significant activation in the group analysis. In previous studies, the ACG was implicated in the sensation of bladder fullness (i.e., strong desire to void, urgency) and micturition control including pelvic floor contractions [Blok et al., 1997; Di Gangi Herms et al., 2006; Griffiths et al., 2007; Mehnert et al., 2008; Seseke et al., 2006]. The frontal cortices are thought to be responsible for planning complex social behaviors, and activation in the right inferior frontal gyrus has been implicated in decision making and motivational control, such as whether or not micturition should take place [Kavia et al., 2005]. However, our study did not involve any specific task like pelvic floor muscle contraction, micturition, or withholding or attempted micturition, which might explain the low or scattered activation of ACG and frontal areas.
In our study, we did not detect pontine activation in the group analysis, similar to the findings of Matsuura et al. [ 2002]. However, most subjects showed activations in the pontine region in the single subject analysis. The pontine micturition center (or M‐region) and the pontine continence center [or L‐region; Kavia et al., 2005] show activation during micturition and storage‐related tasks, respectively [Blok et al., 1997; Nour et al., 2000; Seseke et al., 2006]. However, filling the bladder with only small amounts (≤100 ml) of cold water without voiding or withholding of urine, as in our experimental condition, was not expected to elicit pontine activation in these specific areas.
Activation of the PAG area was observed in 9 of the 12 subjects in the single subject analysis, but not in the overall group analysis. The PAG is an important relay station for sensations arising from visceral organs [Craig, 2002; Holstege, 2005] and therefore, a more robust activation of this region was expected. Failure to detect activation in the group analysis may be due to methodological limitations related to inadequate spatial and temporal resolution of both fMRI and PET, and also increased susceptibility to movement‐related artifacts in the brainstem region.
In general, activations and deactivations appeared to be predominantly located in the right hemisphere, which indicates that processing LUT sensations may show a hemispheric dominance, as has been previously reported [Blok et al., 1997; Mehnert et al., 2008]. Hemispheric asymmetry is not uncommon in cerebral control and coordination of human body functions and has been observed for other functions like tactile discrimination [Harada et al., 2004] and human respiratory control [Corfield et al., 1995].
CONCLUSION
In conclusion, our findings indicate that bladder cooling involves a different supraspinal network than the one reported to be involved during bladder distention in previous PET and fMRI studies. The main difference is a pronounced parietal activity during bladder cooling, with only scarce frontal and brain stem activity. This suggests that bladder cooling is processed in the brain differently than bladder distention. These findings might provide new insights for further research in patients with OAB and painful bladder syndromes, as bladder cold receptor expression and bladder cold perception seem to be altered in those patients. Whether these differences in brain activation are related to the conduction of signals via different afferent fiber types (Aδ‐ vs. C‐fibers) and their supposed specificity toward certain sensations (e.g., distention vs. cold temperature) remains unclear, as cold receptors have been found on both fiber types. More basic research will be necessary to further elucidate the exact type of afferent fibers responsible for transmitting various bladder sensations in humans.
Acknowledgements
The authors thank Ms. Aline Bonvin for her valuable assistance and support during the MR‐measurements.
REFERENCES
- Abu‐Akel A ( 2003): A neurobiological mapping of theory of mind. Brain Res Brain Res Rev 43: 29–40. [DOI] [PubMed] [Google Scholar]
- Andersson KE ( 2002): Bladder activation: Afferent mechanisms. Urology 59( 5, Suppl 1): 43–50. [DOI] [PubMed] [Google Scholar]
- Blok BF, Willemsen AT, Holstege G ( 1997): A PET study on brain control of micturition in humans. Brain 120 ( Part 1): 111–121. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K ( 2006): The human inferior parietal cortex: Cytoarchitectonic parcellation and interindividual variability. Neuroimage 33: 430–448. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL ( 2001): The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci 21: 6283–6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield DR, Fink GR, Ramsay SC, Murphy K, Harty HR, Watson JD, Adams L, Frackowiak RS, Guz A ( 1995): Evidence for limbic system activation during CO2‐stimulated breathing in man. J Physiol 488 ( Part 1): 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD ( 2003): Interoception: The sense of the physiological condition of the body. Curr Opin Neurobiol 13: 500–505. [DOI] [PubMed] [Google Scholar]
- Davis KD, Kwan CL, Crawley AP, Mikulis DJ ( 1998): Functional MRI study of thalamic and cortical activations evoked by cutaneous heat, cold, and tactile stimuli. J Neurophysiol 80: 1533–1546. [DOI] [PubMed] [Google Scholar]
- Davis KD, Lozano RM, Manduch M, Tasker RR, Kiss ZH, Dostrovsky JO ( 1999): Thalamic relay site for cold perception in humans. J Neurophysiol 81: 1970–1973. [DOI] [PubMed] [Google Scholar]
- Di Gangi Herms AM, Veit R, Reisenauer C, Herms A, Grodd W, Enck P, Stenzl A, Birbaumer N ( 2006): Functional imaging of stress urinary incontinence. Neuroimage 29: 267–275. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Gevaert T, Nilius B, De Ridder D ( 2008): On the origin of bladder sensing: Tr(i)ps in urology. Neurourol Urodyn 27: 264–273. [DOI] [PubMed] [Google Scholar]
- Fall M, Geirsson G ( 1996): Positive ice‐water test: A predictor of neurological disease? World J Urol 14 ( Suppl 1): S51–S54. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Luppino G ( 2005): Motor functions of the parietal lobe. Curr Opin Neurobiol 15: 626–631. [DOI] [PubMed] [Google Scholar]
- Fowler CJ ( 2002): Bladder afferents and their role in the overactive bladder. Urology 59 ( 5, Suppl 1): 37–42. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ ( 1999): How many subjects constitute a study? Neuroimage 10: 1–5. [DOI] [PubMed] [Google Scholar]
- Geirsson G, Fall M, Lindstrom S ( 1993): The ice‐water test—A simple and valuable supplement to routine cystometry. Br J Urol 71: 681–685. [DOI] [PubMed] [Google Scholar]
- Geirsson G, Lindstrom S, Fall M ( 1999): The bladder cooling reflex and the use of cooling as stimulus to the lower urinary tract. J Urol 162: 1890–1896. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD ( 2008): Bladder control, urgency, and urge incontinence: Evidence from functional brain imaging. Neurourol Urodyn 27: 466–474. [DOI] [PubMed] [Google Scholar]
- Griffiths D, Tadic SD, Schaefer W, Resnick NM ( 2007): Cerebral control of the bladder in normal and urge‐incontinent women. Neuroimage 37: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, Saito DN, Kashikura K, Sato T, Yonekura Y, Honda M, Sadato N ( 2004): Asymmetrical neural substrates of tactile discrimination in humans: A functional magnetic resonance imaging study. J Neurosci 24: 7524–7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellstrom PA, Tammela TL, Kontturi MJ, Lukkarinen OA ( 1991): The bladder cooling test for urodynamic assessment: Analysis of 400 examinations. Br J Urol 67: 275–279. [DOI] [PubMed] [Google Scholar]
- Holstege G ( 2005): Micturition and the soul. J Comp Neurol 493: 15–20. [DOI] [PubMed] [Google Scholar]
- Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, Coyne K, Kelleher C, Hampel C, Artibani W, Abrams P. ( 2006): Population‐based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur Urol 50: 1306–1314; discussion 1314–1315. [DOI] [PubMed] [Google Scholar]
- Jiang CH, Hermanson O ( 2004): Cooling of the urinary bladder activates neurons in the dorsal horn of the spinal cord. Neuroreport 15: 351–355. [DOI] [PubMed] [Google Scholar]
- Jiang CH, Mazieres L, Lindstrom S ( 2002): Cold‐ and menthol‐sensitive C afferents of cat urinary bladder. J Physiol 543 ( Part 1): 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavia RB, Dasgupta R, Fowler CJ ( 2005): Functional imaging and the central control of the bladder. J Comp Neurol 493: 27–32. [DOI] [PubMed] [Google Scholar]
- Lindstrom S, Mazieres L, Jiang CH ( 2004): Inhibition of the bladder cooling reflex in the awake state: An experimental study in the cat. J Urol 172 ( 5, Part 1): 2051–2053. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Kakizaki H, Mitsui T, Shiga T, Tamaki N, Koyanagi T ( 2002): Human brain region response to distention or cold stimulation of the bladder: A positron emission tomography study. J Urol 168: 2035–2039. [DOI] [PubMed] [Google Scholar]
- McDonald DF, Murphy GP ( 1959): Quantitative studies of perception of thermal stimuli in the normal and neurogenic urinary bladder. J Appl Physiol 14: 204–206. [DOI] [PubMed] [Google Scholar]
- Mehnert U, Boy S, Svensson J, Michels L, Reitz A, Candia V, Kleiser R, Kollias S, Schurch B ( 2008): Brain activation in response to bladder filling and simultaneous stimulation of the dorsal clitoral nerve—An fMRI study in healthy women. Neuroimage 41: 682–689. [DOI] [PubMed] [Google Scholar]
- Mukerji G, Waters J, Chessell IP, Bountra C, Agarwal SK, Anand P ( 2006a): Pain during ice water test distinguishes clinical bladder hypersensitivity from overactivity disorders. BMC Urol 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji G, Yiangou Y, Corcoran SL, Selmer IS, Smith GD, Benham CD, Bountra C, Agarwal SK, Anand P ( 2006b): Cool and menthol receptor TRPM8 in human urinary bladder disorders and clinical correlations. BMC Urol 6: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan PW ( 1952): Thermal sensation in the bladder. J Neurol Neurosurg Psychiatry 15: 150–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour S, Svarer C, Kristensen JK, Paulson OB, Law I ( 2000): Cerebral activation during micturition in normal men. Brain 123 ( Part 4): 781–789. [DOI] [PubMed] [Google Scholar]
- Seseke S, Baudewig J, Kallenberg K, Ringert RH, Seseke F, Dechent P ( 2006): Voluntary pelvic floor muscle control—An fMRI study. Neuroimage 31: 1399–1407. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Duncan GH, Boivin M, Bushnell MC ( 2003): Differentiation of visceral and cutaneous pain in the human brain. J Neurophysiol 89: 3294–3303. [DOI] [PubMed] [Google Scholar]
- Tadic SD, Griffiths D, Schaefer W, Resnick NM ( 2008): Abnormal connections in the supraspinal bladder control network in women with urge urinary incontinence. Neuroimage 39: 1647–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Coplanar Stereotactic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Wyndaele JJ, De Wachter S ( 2008): The sensory bladder (1): An update on the different sensations described in the lower urinary tract and the physiological mechanisms behind them. Neurourol Urodyn 27: 274–278. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Chancellor MB ( 2002): Current and future pharmacological treatment for overactive bladder. J Urol 168: 1897–1913. [DOI] [PubMed] [Google Scholar]


