Abstract
Executive functions that are dependent upon the frontal‐parietal network decline considerably during the course of normal aging. To delineate neuroanatomical correlates of age‐related executive impairment, we investigated the relation between cortical thickness and executive functioning in 73 younger (20–32 years) and 56 older (60–71 years) healthy adults. Executive functioning was assessed using the Wisconsin Card Sorting Test (WCST). Cortical thickness was measured at each location of the cortical mantle using surface‐based segmentation procedures on high‐resolution T1‐weighted magnetic resonance images. For regions involved in WCST performance, such as the lateral prefrontal and parietal cortices, we found that thicker cortex was related to higher accuracy. Follow‐up ROI‐based analyses revealed that these associations were stronger in older than in younger adults. Moreover, among older adults, high and low performers differed in cortical thickness within regions generally linked to WCST performance. Our results indicate that the structural cortical correlates of executive functioning largely overlap with previously identified functional patterns. We conclude that structural preservation of relevant brain regions is associated with higher levels of executive performance in old age, and underscore the need to consider the heterogeneity of brain aging in relation to cognitive functioning. Hum Brain Mapp, 2011. © 2011 Wiley‐Liss, Inc.
Keywords: WCST, right DLPFC, prefrontal cortex, parietal cortex, fronto‐parietal network, structure‐function relationship, performance level, lifespan, healthy aging
INTRODUCTION
Normal aging is accompanied by decline in many cognitive domains, especially those related to fluid abilities [Baltes,1987; Cattell,1971; Horn,1968; Lindenberger,2001], such as reasoning and executive functioning [Bäckman et al.,2000; Li et al.,2004; Rhodes,2004; West,1996]. Executive functions comprise several cognitive abilities, such as working memory, selective attention, inhibition, set shifting, and task switching [Miyake et al.,2000; Teuber1972]. Conjointly, these functions enable flexible and goal‐directed behavior in a changing environment. Age‐related decline in executive functioning may impose difficulties in everyday situations, such as social contacts, street traffic, and diet or drug compliance.
To assess executive functioning, we administered the Wisconsin Card Sorting Test (WCST), a standard neuropsychological test, which requires attention, feedback‐based updating of information in working memory, inhibition of prepotent responses, and shifting of mental set [Grant and Berg1948; Heaton et al.,1993]. In healthy adults, performing the WCST results in increased functional brain activity within a distributed fronto‐parietal network [see Buchsbaum et al.,2005; Nyhus and Barcelo,2009, for reviews]. The right prefrontal (PFC) and parietal cortices are most consistently recruited during the WCST, with less consistent findings regarding activation of the temporal and occipital lobe, as well as subcortical gray matter [Buchsbaum et al.,2005; Nyhus and Barcelo,2009].
Parallel to cognitive decline, advancing age is related to a structural degeneration of the cerebral cortex as indicated by widespread reductions in cortical thickness [Fjell et al.,2009; Hutton et al.,2009; Salat et al.,2004], gray matter density [Sowell et al.,2003], and gray matter volume [Courchesne et al.,2000; Good et al.,2001; Raz et al.,1997,2000,2005; Walhovd et al., 2009]. So far, attempts at linking age‐related structural alterations to executive functioning have yielded mixed results. Fjell et al. [2006], Ziegler et al. [2010], and Van Petten et al. [2004] reported no correlation between cortical thickness and composite scores of various executive measures, whereas Kochunov et al. [2009] found that thicker cortex in many regions was related to better executive performance in adults between 30 and 90 years, but not in adults between 19 and 26 years. These correlations, however, were no longer significant after controlling for age [Kochunov et al.,2009]. In 87 participants aged 20 to 77 years, Raz et al. [2008] observed that, after accounting for the effects of age, sex, and vascular risk factors, the orbitofrontal cortex and the prefrontal white‐matter volumes as well as the 5‐year change in entorhinal cortex volume predicted fluid intelligence level, assessed by Cattell Culture Fair Intelligence and Letter Sets tests. Hartberg et al. [2010] found that thicker cortex in superior temporal, superior frontal, and inferior frontal gyri was related to fewer perseverative errors in the WCST among schizophrenic patients and healthy adults 20–56 years old. Elderkin‐Thompson et al. [2008] found both positive and negative correlations between volume of the frontal lobe gyri and various executive function scores in adults aged 61–88 years. Specifically, greater anterior cingulate volume was associated with less time needed to complete the Stroop Inhibition Task, whereas larger orbitofrontal volumes were associated with poorer verbal fluency. Other studies show that, in older adults, smaller PFC gray‐matter volume [Gunning‐Dixon and Raz,2003] and smaller right frontal‐lobe volume [Hanninen et al.,1997] were related to a higher number of perseverative errors in the WCST, and that larger frontal‐lobe volume was positively linked to the number of completed WCST categories [Schretlen et al.,2000].
At least some of the above findings suggest that structure‐function relationships are more pronounced in late than in early adulthood. A healthy mature brain may function above a critical threshold despite some inter‐individual variability in brain structure. Conversely, in the presence of biological constraints on information processing mechanisms (i.e., when decline in biological resources reaches a certain threshold), such as during aging, the extent of decline in brain structure may be more predictive of cognitive performance [Almeida et al.,2008; Li et al.,2004; Lindenberger et al.,2008; Nagel et al.,2008; Sullivan and Pfefferbaum,2006].
Despite the associations described above, many studies using the WCST have reported negative results. This might reflect relatively small sample sizes, Elderkin‐Thompson et al.,2008, n = 23; Gur et al.,1998, n = 17; Sanfilipo et al.,2002, n = 27, or stem from combining the WCST with other tests, whose neural substrates may not be overlapping [Van Petten et al.,2004]. In addition to issues regarding sample size and use of composite scores of executive functioning, the studies reporting negative results are affected by at least one of the following limitations: (a) use of volumetric measures, which confound cortical thickness, surface area, and folding [Im et al.,2006a,b]; a direct comparison of volumetric measures based on voxel‐based morphometry and cortical thickness measures in 48 healthy adults aged 22–60 years revealed that volumetric measures were less sensitive, had lower signal‐to‐noise ratio, lower T‐scores, and were more confounded by overall brain size than thickness measures [Hutton et al.2009]; (b) gross definition of frontal volume or frontal gyri, pooling together functionally dissociable areas, such as the dorsal and ventrolateral PFC, the orbitofrontal cortex, and premotor areas; and (c) investigation of frontal‐lobe volume only, although the WCST is no longer considered a specific test of prefrontal function [Nyhus and Barcelo,2009].
In this study, we investigated the structural correlates of executive functioning in the adult human brain. Specifically, our goal was twofold: (a) to better characterize the relations between cortical thickness of brain regions associated with the WCST (as defined in previous functional neuroimaging studies) [Buschbaum et al.,2005; Nyhus and Barcelo,2009] and executive functioning and (b) to investigate the effects of age and performance level in modulating this relation.
To accomplish these goals, in a sample of 73 younger and 56 older healthy adults, we (a) measured cortical thickness, a clearly defined anatomical property (distance between the gray/white matter boundary and the cortical surface) [Fischl and Dale,2000; MacDonald et al.,2000] that better reflects the surface‐based organization of the cortex than gray‐matter density or gyral/lobar volume [Hutton et al.,2009; Im et al.,2006a,b]; (b) used higher spatial precision of the measurement by calculating cortical thickness at each location of the cortical mantle [Fischl and Dale,2000], using procedures that have been validated against histological [Rosas et al.,2002] and manual [Kuperberg et al.,2003; Salat et al.,2004] measurements; (c) applied a hypothesis‐free search of the entire cerebrum, known to be more sensitive to focal effects than averaging a brain measure over a large region [e.g., Barrick et al.,2010; Voormolen et al.,2010; Ziegler et al.,2010]; and (d) used a single standardized test, WCST, which allows direct comparison to previous functional neuroimaging studies [Buchsbaum et al.,2005; Nyhus and Barcelo,2009]. The study was guided by two main hypotheses. First, we expected that cortical thickness in regions most robustly activated during the WCST, namely (right) lateral prefrontal and parietal cortices, and is positively correlated with WCST performance. Second, we predicted that the association between individual differences in cortical thickness and individual differences in task performance is stronger in older than in younger adults.
MATERIALS AND METHODS
Participants
Seventy‐three younger adults (32 women) between 20 and 32 years of age (M = 25.6 ± 3.1) and 56 older adults (27 women) between 60 and 71 years of age (M = 64.8 ± 2.6) participated. All subjects had received at least eight years of education, were right‐handed, and had no history of psychiatric or neurological disease. Age groups did not differ with respect to schooling (M younger = 16.8 ± 3.3 years; M older = 16.3 ± 3.9 years, P = 0.38). For 50 of the 56 older adults, we have information about vascular risk factors: 19 participants (38%) reported arterial hypertension (defined as blood pressure constantly above 140/90 mm Hg), 7 of the 19 hypertensive participants took anti‐hypertensive medication, 4 (8%) had type 2 diabetes, all of whom took medication but only one was insulin‐dependent, and 5 (10%) reported hypercholesterolemia (two of whom were taking medication).
One of the authors (A.Z.B) evaluated the presence of age‐related WM hyperintensities (WMH) on fluid attenuated inversion recovery (FLAIR) images using the rating scale described by Wahlund et al. [2001]. The presence of moderate WMHs is typical for a normally aged population [Baloh and Vinters,1995]. We estimated WMH volume using a semi‐automated procedure based on FMRIB's Automated Segmentation Tool [FAST 4.1, Zhang et al.,2001], described in more detail in Burzynska et al. [2010]. All younger and 20 older participants had Grade 0 (no lesions), 24 older adults had Grade 1 (a single to several focal WM lesions, M WMH volume = 916.9 ± 700.4 voxels, range 117–2615 voxels), and 12 older participants had Grade 2 (beginning confluence of lesions, M WMH volume = 5767.8 ± 2729.9 voxels, range 3094–13021 voxels). A neuroradiologist examined the structural images of participants with WMH Grade 2 and classified all of them as within the normal range for their age.
Older adults showed deficits in processing speed, as measured by Digit‐Symbol Substitution (M younger = 63.5 ± 10.2, M older = 48.8 ± 12.6, P < 0.001) but higher verbal knowledge, as measured by the Spot‐a‐Word task (M younger = 18.6 ± 5.2, M older = 22.8 ± 5.8, P < 0.001). This pattern of age differences is consistent with the literature on cognitive aging [e.g., Li et al.,2004].
The ethics committee of the Charité University Medicine Berlin and the Max Planck Institute for Human Development approved the study, and written informed consent was obtained from all participants prior to the examination. Participants received financial reimbursement.
Wisconsin Card Sorting Test
We administered a computerized version of the standard 128‐cards WCST [Heaton et al.,1993]. Four reference cards were presented at the top of the screen and a single response card was shown at the bottom centre of the screen. We asked participants to sort the response card to one of the cards presented in the upper half of the screen by pressing one of the four corresponding buttons with the index finger of the right hand. We instructed the participants to perform as quickly and accurately as possible; however, there was no time limit to provide a response. Once a button was pressed, the response could be cancelled within 1550 ms, followed by, again, unlimited time to give an alternative answer. Finally, feedback on the correctness of the answer was communicated visually (“correct” or “false” presented on the screen for 1500 ms) and verbally (spoken via headphones).
This feedback was the only guidance about the sorting rules. In general, the cards could be matched based on three dimensions: color, form, and number. The first sorting principle was color, followed by form and then number. When a person attained 10 consecutive correct responses for a specific category, the sorting principle changed. Because no indication was given about the change, participants were required to make a shift of category to receive positive feedback again. The test either ended after a participant had completed six categories or sorted a set of 128 cards.
We evaluated WCST performance by applying the standard scoring parameters [Heaton et al.,1993], which include the number of categories completed, and the total number of trials needed to complete the first category. Percentage of correct responses and perseverative errors were calculated by dividing absolute values of correct responses and perseverative errors, respectively, by the total number of responses for each participant. Reaction time for correct responses was measured as the time between the presentation of a new key card and the button press of its final (i.e., correct) assignment. Reaction times below 600 ms and above 25 s were considered as error trials and were excluded from the analyses. Statistical analyses were performed using SPSS (v.16, SPSS, Chicago, IL).
MR Acquisition
High‐resolution structural MR scans were acquired using a 3D gradient‐echo T1‐weighted (Fast Low Angle Shot) sequence (TR = 20 ms; TE = 5 ms; flip angle = 30°; matrix = 256 × 256; FOV = 256; 180 slices; resolution = 1 × 1 × 1 mm) on a 1.5 Tesla MR Siemens Magnetom Vision scanner (Siemens, Erlangen, Germany) with a conventional head coil. FLAIR images were acquired on a 1.5 T Siemens Sonata with 40 mT/m gradients and 200 T/m/sec slew rates (Siemens, Erlangen, Germany). All images were obtained parallel to the anterior‐posterior commissure plane with no interslice gap. FLAIR images consisted of 24 five‐mm‐thick slices with an in‐plane resolution of 1.3 × 0.9 mm (240 × 256 matrix, TR/TE/TI = 7500/118/2200 ms). To minimize head movement, we stabilized the head with a vacuum pillow.
Cortical Thickness Analysis
Automated brain tissue segmentation and reconstruction of cortical models was performed on T1‐weighted images using the Freesurfer software, version 4.4 (http://surfer.nmr.mgh.harvard.edu/). In brief, individual T1‐weighted images underwent non‐brain tissue removal, Talairach transformation, creation of representations of the gray/white matter boundaries [Dale et al.,1999; Dale and Sereno,1993], and calculation of the cortical thickness as the distance between the gray/white matter boundary and the pial surface at each point across the cortical mantle [Fischl and Dale,2000]. We screened surface reconstructions to evaluate the success and plausibility of the automatically processed results, as recommended by the software developers. Next, cortical thickness maps were inflated [Fischl et al.,1999a], smoothed using a circularly symmetric Gaussian kernel across the surface with a full width at half maximum of 10 mm, and registered to a spherical atlas to match individual cortical folding patterns across subjects [Fischl et al.,1999b].
Statistical Analysis
Whole‐brain analyses
First, we compared cortical thickness between younger and older adults. Next, we investigated associations between cortical thickness and executive performance in the total sample, controlling for age. Here, we computed a general linear model to assess the relationships among age, cortical thickness at each vertex (measurement point at the cortical mantle), and executive performance. As the index of executive functioning, we selected the percentage of correct responses because its values exhibited the largest variance, the distribution was least skewed in both age groups, and the effect size of the age‐related difference was the largest. All results are shown on inflated standard cortical surface maps with a P‐value ranging from P < 0.001 to P < 0.000001 (see Fig. 1) or P < 0.05 to P < 0.005 (Figs. 2 and 3 ), uncorrected, which is common in surface‐based group analysis of cortical thickness [e.g., Espeseth et al.,2008; Fjell et al.,2006; Walhovd et al.,2006].
Figure 1.
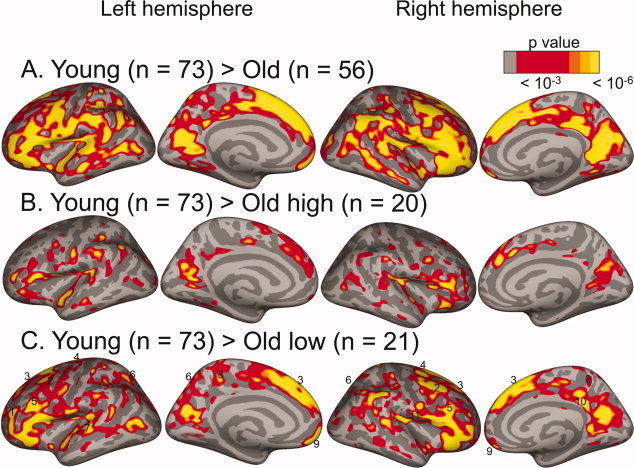
Age‐group comparisons show a strong and spatially distributed negative effect of age on cortical thickness. Red to yellow: regions where cortex was thicker in younger than in (A) older adults, (B) high‐performing older adults, and (C) low‐performing older adults. In (C) numbers indicate, 1: left frontal pole (BA 10), 2: caudal part of the MFG (BA 6/8), 3: lateral superior frontal gyrus (BA 6/8/9), 4: precentral gyrus (BA 4), 5: rostral part of the MFG (BA9/46), 6: posterior parietal cortex (BA 7), 7: left caudal superior temporal gyrus (BA 22), 8: medial postcentral gyrus (BA 5), 9: orbitofrontal cortex (BA 11), and 10: right isthmus cingulate cortex (BA 23).
Figure 2.
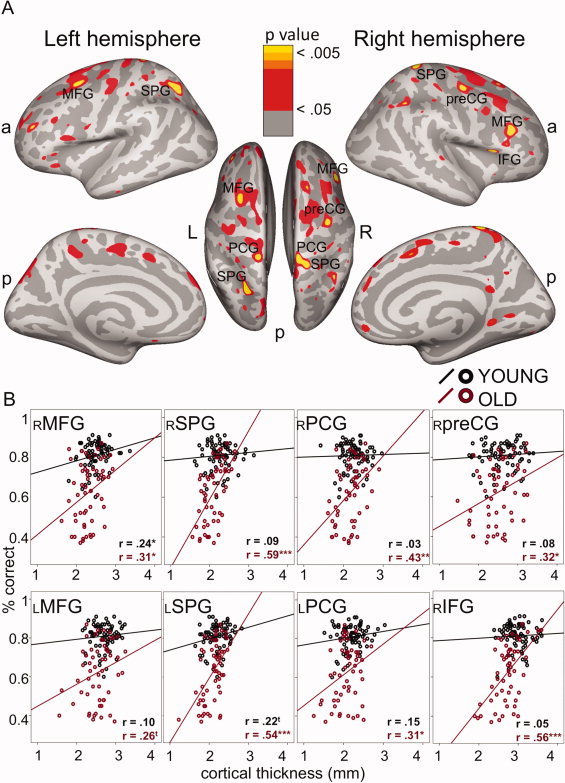
Relationship between cortical thickness and executive performance. A: Thicker cortical mantle was associated with better WCST performance (red‐yellow) in the total sample (corrected for age), and B: Mean cortical thickness from regions at P < 0.005 larger than 30 mm2 in panel A regressed on WCST accuracy. x‐axis: cortical thickness in mm, y‐axis: WCST accuracy (% correct responses); r: Pearson's correlation coefficients, *P < 0.05, **P < 0.01, ***P < 0.001, ttrend (0. 07 > P > 0.05). MFG: middle frontal gyrus, IFG: inferior frontal gyrus, SPG: superior parietal gyrus, preCG: precentral gyrus, PCG: post‐central gyrus. a: anterior, p: posterior, L: left, and R: right. This figure only displays positive correlations. A few areas with significant negative correlations were found in the temporal lobes (see Supporting Information Fig. S2), which are outside the fronto‐parietal WCST network as described in Nyhus and Barcelo, [2009]; Buchsbaum et al., [2005].
Figure 3.
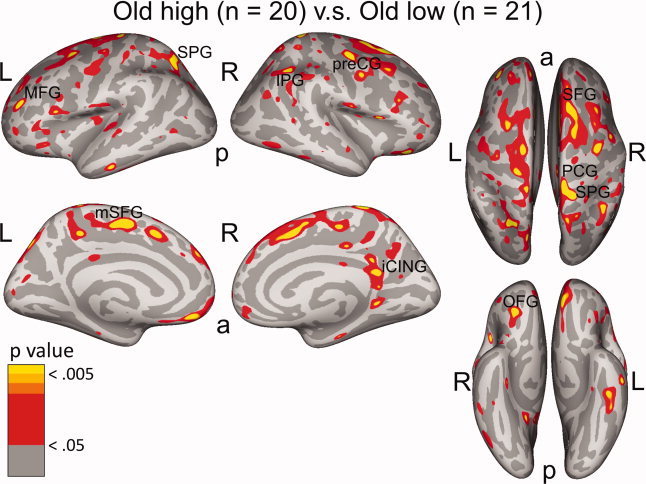
Cortical thickness of high‐ versus low‐performing older adults. In red‐yellow: regions where cortical thickness of high‐performing older adults was thicker than in low‐performing adults. The most highly significant regions (larger than 30 mm2 at P < 0.005 and including vertices at P < 0. 001, see Table III) were: MFG: middle frontal gyrus, SPG: superior parietal gyrus, mSFG: medial superior frontal gyrus, IPG: inferior parietal gyrus, preCG: precentral gyrus, iCING: isthmus cingulate gyrus, OFG: orbitofrontal gyrus. a: anterior, p: posterior, L: left, and R: right.
Post‐hoc region‐of‐interest analyses
Region‐of‐interest (ROI) analyses were used to illustrate characteristics of the positive structure‐performance correlations within the fronto‐parietal network. By using the ROI manual drawing tool in Freesurfer, we manually delineated regions with P < 0.005, which occupied a surface area larger than 30 mm2 in the standard spherical space. Next, the ROIs created in the standard spherical space were mapped onto each participant's surface representations. Finally, mean values of cortical thickness and ROI surface were extracted for each participant in individual space.
First we checked the reliability of variances of cortical thickness and behavioral measures in the ROI analyses in younger and older adults using MPlus software (Muthen & Muthen,Version 5, 2008, Los Angeles, CA). All variances differed significantly from zero (P younger, older < 0.001), indicating that there was enough variance in the data to assess and interpret the correlations.
To test the hypothesis that cortical thickness of the ROIs is related to WCST performance after controlling for age, we performed linear hierarchical regression analyses, where WCST performance was the dependent variable, age was entered as the main independent variable and cortical thickness as the second independent variable. Factorial ANOVAs were calculated per ROI to test for age group × cortical thickness interactions, that is, whether cortical thickness was more predictive of WCST performance in older than in younger adults. To illustrate these findings, we plotted WCST performance against cortical thickness and fitted the regression lines separately for younger and older adults (Fig. 2B).
In addition, we investigated relationships between cortical thickness and WCST accuracy separately within the two age groups (see Supporting Information). Finally, we calculated the mean thickness across the cortical gray matter for every participant, separately for each hemisphere.
RESULTS
WCST Performance
Older adults showed lower performance on the WCST than younger adults; they needed more trials to complete the first category (M younger = 14.0 ± 6.4, M older = 33.5 ± 36.5, P < 0.001) and therefore completed less sorting categories altogether (M younger = 6.0 ± 0.1, M older = 4.7 ± 1.7, P < 0.001), made more perseverative errors (M younger = 9.8% ± 3.6, M older = 21.0% ± 10.5, P < 0.001), had a lower percentage of correct answers (M younger = 80.7% ± 6.9, M older = 62.2% ± 15.0 P < 0.001), and had longer response times for correct trials (M younger = 2855 ms ± 539, M older = 4412 ms ± 1322 P < 0.001).
Within each age group, age was normally distributed (Kolmogorov‐Smirnov test, P > 0.05). WCST scores were reliably correlated with age in older adults (r percent correct = −0.28, P = 0.04; r first category = 0.24, P = 0.08; r categories = −0.27, P = 0.04; r perseverative errors = 0.23, P = 0.09; r reaction time = 0.32, P = 0.02), but not in younger adults (r percent correct = 0.01, P = 0.96; r first category = 0.04, P = 0.73; r categories = −0.09, P = 0.43; r perseverative errors = 0.09, P = 0.45; r reaction time = −0.03, P = 0.79).
Associations Between Age Group and Cortical Thickness
The average cortical thickness values were M younger RH = 2.59 ± 0.09, M younger LH = 2.59 ± 0.09, M older RH = 2.44 ± 0.09, and M older LH = 2.44 ± 0.10. As mean cortical thickness was highly correlated between the two hemispheres (r younger RH‐LH = 0.94 P < 0.001, r older RH‐LH = 0.89 P < 0.001), we averaged the thickness of both hemispheres in subsequent analyses (M younger = 2.59 ± 0.09, M older = 2.44 ± 0.09). Older adults had thinner cortex than younger adults (P < 0.001). This observation was confirmed by a vertex‐wise whole‐brain comparison of cortical thickness: There was a strong and spatially distributed negative effect of age group on cortical thickness, regardless of performance level. We observed the strongest effects in the bilateral frontal cortex (superior, middle, inferior, orbital, and ventral precentral gyri), parietal cortex (inferior parietal and supramarginal gyri), posterior cingulate gyrus, and temporal cortex (superior and middle temporal gyri), as well as the medial part of the precuneus (Fig. 1A). None of the examined cortical regions showed significantly thicker cortex in older than in younger adults.
Associations Between Cortical Thickness and WCST Performance
We correlated cortical thickness with percent correct responses on the WCST in all 129 participants, controlling for chronological age. As shown in Figure 2A, a thicker cortical mantle in the fronto‐parietal network, including frontal cortex (bilateral middle frontal gyrus (MFG), as well as right inferior frontal gyrus (IFG), precentral gyrus (preCG), postcentral gyrus (PCG), and superior parietal gyrus (SPG), was related to better WCST performance. The only significant negative correlations (i.e., where thinner cortex was related to better performance on WCST; see Figure S3 in the Supporting Information) were located in the temporal lobes, outside the fronto‐parietal WCST network [Buchsbaum et al.,2005; Nyhus and Barcelo,2009], and therefore were not addressed in the following analyses.
To illustrate the pattern of the positive correlations separately in younger and older adults, we regressed mean cortical thickness on percent correct WCST responses separately for each region larger than 30 mm2 (Fig. 2B). Table I summarizes the anatomical and functional definitions, as well as the coordinates, of the ROIs.
Table I.
Thicker cortex predicts higher WCST accuracy (after controlling for age, n = 129): most significant ROIs from Figure 2 (P < 0.005 across > 30 mm2)
| ROI | Gyrus | BA | Anatomical definition | Talairach coordinates | Peak P‐value | ROI size (mm2) on standard surface | Mean ROI size (mm2) at P < 0.005 |
|---|---|---|---|---|---|---|---|
| Left SPG | Superior parietal | 7 | Somatosensory association cortex | −25, −56, 47 | 0.00008 | 128.4 | 170 ± 59 |
| Right MFG | Rostral middle frontal | 9/46 | Dorsolateral PFC | 43, 27, 22 | 0.001 | 91.6 | 103 ± 29 |
| Right PCG | Dorsal postcentral | 2 | Primary somatosensory cortex | 9, −34, 71 | 0.0002 | 83.6 | 61 ± 13 |
| Right SPG | Superior parietal | 5 | Somatosensory association cortex | 17, −38, 63 | 0.0002 | 81.6 | 98 ± 30 |
| Left MFG | Caudal middle frontal | 8/9 | Dorsolateral PFC | −27, 9, 46 | 0.002 | 71.4 | 83 ± 26 |
| Right pre‐CG | Pre‐central, lateral | 4/6 | Primary motor/premotor cortex | 38, −5, 53 | 0.002 | 43.7 | 45 ± 14 |
| Right IFG | Inferior frontal, pars opercularis | 44 | Ventrolateral PFC | 42, 15, 7 | 0.0002 | 40.6 | 49 ± 12 |
| Left PCG | Dorsal postcentral | 2 | Primary somatosensory cortex | −13, −32, 71 | 0.001 | 36.6 | 33 ± 8 |
We performed multiple hierarchical regression analyses using age and cortical thickness as factors to test whether variance in WCST accuracy is accounted for by cortical thickness in addition to age. The change statistics were significant in all ROIs: right MFG (R 2 change = 0.041, F change (1/126) = 9.82, P < 0.01), left SPG (R 2 change = 0.084, F change (1/126) = 21.80, P < 0.001), right PCG (R 2 change = 0.04, F change (1/126) = 9.51, P < 0.01), right IFG (R 2 change = 0.085, F change (1/126) = 22.03, P < 0.001), right SPG (R 2 change = 0.069, F change (1/126) = 17.40, P < 0.001), left MFG (R 2 change = 0.024, F change (1/126) = 5.52, P < 0.01), left PCG (R 2 change = 0.036, F change (1/126) = 8.43, P < 0.01), and right preCG (R 2 change = 0.028, F change (1/126) = 6.40, P = 0.01). These findings indicate that, beyond age, cortical thickness is an important predictor of WCST performance.
A repeated‐measures ANOVA testing the age group x cortical thickness interaction revealed significant interactions in most of the regions: left SPG [F(1,125) = 14.74; P < 0.001; η2 = 0.076], right IFG [F(1,125) = 18.36; P < 0.001; η2 = 0.095], right PCG [F(1,125) = 11.08; P < 0.01; η2 = 0.065], right preCG [F(1,125) = 4.76; P = 0.03; η2 = 0.031], and right SPG [F(1,125) = 26.83; P < 0.001; η2 = 0.119], reflecting stronger positive associations between cortical thickness and WCST performance in older than in younger adults. In the right MFG the interaction was not reliable [F(1,125) = 2.37; P = 0.13; η2 = 0.017], as thicker cortex was linked to better performance in both younger and older adults. In the left MFG and PCG, the relationships between cortical thickness and performance were significant in older adults and at trend levels in younger adults; hence the interactions were not reliable {left MFG [F(1,125) = 1.90, P = 0.17; η2 = 0.014]; left PCG [(F(1,125) = 3.64, P = 0.06, η2 = 0.025], respectively}. Correlation coefficients for younger and older adults for all ROIs are depicted in Figure 2B. For whole‐brain correlations between cortical thickness and WCST accuracy separately within the younger and older and age groups, see Supporting Information.
To check the specificity of our findings for WCST, we also examined the relationship of cortical thickness to processing speed (Digit Symbol) and vocabulary (Spot‐a‐Word). Cortical thickness within the eight ROIs was not a significant predictor of processing speed or vocabulary, when entered into the model after age, except for processing speed in the left PCG (R 2 change = 0.029, F change (1/126) = 4.70, P = 0.03). The age × cortical thickness interaction was not reliable for left PCG, suggesting that the age groups did not differ with respect to cortical thickness‐processing speed associations in this region.
Whole‐brain analysis revealed that thickness of regions related to Digit Symbol (bilateral supramarginal gyrus, fusiform gyrus, and left postcentral gyrus, Supporting Information Fig. S4) or Spot‐a‐Word (left pre‐ and post‐central, and medial orbitofrontal gyri, parietal, inferior and medial temporal regions, and right medial paracentral gyrus, Supporting Information Fig. S5) were outside the fronto‐parietal network identified for WCST.
Low‐ vs. High‐performing Older Adults
Behavioral characteristics
As shown in Figure 2B, some older adults performed similarly to younger adults, whereas others showed much lower performance. To investigate potential structural differences between these subgroups, we took the 20 older adults with the lowest (n = 21; the highest accuracy score in this group was shared by two participants) and the 20 older adults with the highest accuracy score on the WCST (n = 20). Older high and low performers did not differ with respect to age (M older high = 64.2 ± 2.1, M older low = 65.6 ± 3.3, P = 0.10), years of education (M older high = 16.4 ± 3.7, M older low = 15.5 ± 4.4, P = 0.45), performance on Digit Symbol (M older high = 51.8 ± 15.4, M older low = 47.5 ± 11.2, P = 0.37), but they differed, of course, significantly with respect to WCST accuracy (M older high = 0.78 ± 0.05, M older low = 0.45 ± 0.06, P < 0.001), number of categories completed (M older high = 6.00 ± 0.00, M older low = 2.86 ± 1.49, P < 0.001), trials to complete first category (M older high = 21.05 ± 13.73, M older low = 55.24 ± 51.05, P = 0.01), percent perseverative errors (M older high = 11.54 ± 3.22, M older low = 31.81 ± 7.76, P < 0.001), and WCST reaction time (M older high = 3895.6 ± 731.2 ms, M older low = 5072.0 ± 1704.1 ms, P = 0.01). Old high and low performers in the WCST also differed in vocabulary (M older high = 25.65 ± 2.81, M older low = 21.19 ± 6.76, P = 0.01). As expected, older low performers, but not older high performers, had lower WCST accuracy than younger adults (P < 0.001 and P = 0.07, respectively). Neither older high nor low performers differed from younger adults with respect to years of education. Both older subgroups were, however, slower on the WCST (P older high, P older low < 0.001), scored significantly lower on Digit Symbol (P older high, P older low < 0.001), and had a higher number of correct responses in Spot‐a‐Word than younger adults (P older high < 0.001, P older low = 0.06).
Taken together, both older adults subgroups showed the expected age‐related decline in processing speed along with superior vocabulary. Critically, older high and older low performers differed specifically with respect to the WCST, but not in processing speed (Digit Symbol).
Structural characteristics
First, older high performers showed thinner global cortical thickness than younger adults (M older high = 2.47 ± 0.07, P < 0.001), and older low performers had thinner cortex than older high performers (M older low = 2.40 ± 0.11, P = 0.03). Next, we performed the same comparisons within the regions involved in WCST (as described in Fig. 2). In all regions, older high performers did not differ in cortical thickness from younger adults, but older low performers had thinner cortex than older high performers (Table II).
Table II.
Differences in cortical thickness among younger, older high‐performing, and older low‐performing adults in WCST‐relevant regions (see Fig. 2 and Table I)
| ROI | Cortical thickness | P‐values | |||
|---|---|---|---|---|---|
| Younger n = 73 | Older high n = 20 | Older low n = 21 | Younger vs. older High | Older low vs. older High | |
| Left SPG | 2.2 ± 0.2 | 2.2 ± 0.2 | 1.9 ± 0.3 | 0.856 | <0.001 |
| Right MFG | 2.5 ± 0.3 | 2.4 ± 0.3 | 2.2 ± 0.3 | 0.195 | 0.027 |
| Right PCG | 2.3 ± 0.3 | 2.3 ± 0.3 | 2.0 ± 0.3 | 0.580 | 0.002 |
| Right SPG | 2.3 ± 0.3 | 2.3 ± 0.2 | 2.0 ± 0.2 | 0.532 | <0.001 |
| Left MFG | 2.7 ± 0.3 | 2.7 ± 0.3 | 2.4 ± 0.4 | 0.661 | 0.020 |
| Right pre‐CG | 2.6 ± 0.4 | 2.7 ± 0.4 | 2.4 ± 0.4 | 0.734 | 0.015 |
| Right IFG | 3.0 ± 0.3 | 2.9 ± 0.3 | 2.5 ± 0.3 | 0.271 | 0.001 |
| Left PCG | 2.1 ± 0.3 | 2.1 ± 0.2 | 1.9 ± 0.3 | 0.307 | 0.028 |
Table III.
Thicker cortex in high ‐ than in low‐performing older adults: most significant ROIs (P < 0.005 across > 30 mm2 and including vertices at P < 0.001, see Fig. 2)
| ROI | Gyrus | BA | Anatomical definition | Talairach coordinates | Peak P‐value | ROI size on average surface (mm2) |
|---|---|---|---|---|---|---|
| Right SFG | Superior frontal | 6/8 | Premotor cortex | 20, 19, 51 | 0.0002 | 196.7 |
| Right SPG | Superior parietal | 5/7 | Somatosensory associatation cortex | 17, −38, 63 | 0.0001 | 138.6 |
| Right PCG | Poscentral | 1/2/3 | Primary somatosensory cortex | 9, −33, 71 | 0.0002 | 136.3 |
| Right preCG | Precentral | 4 | Primary Motor Cortex | 39, −5, 52 | 0.0003 | 99.9 |
| Right IPG | Inferior parietal | 7 | Somatosensory associatation cortex | 42, −49, 41 | 0.0006 | 84.9 |
| Right OBF | Orbitofrontal | 45/47/11 | Orbitofrontal cortex | 24, 30, −10 | 0.0004 | 64.1 |
| Right iCING | Isthmus cingulated | 23/30 | Posterior cingulate cortex | 7, −49, 18 | 0.0009 | 43.1 |
| Left mSFG | Medial superior frontal | 6 | Supplementary motor cortex | −7, −5, 50 | 0.0002 | 184.9 |
| Left SPG | Superior parietal | 7 | Somatosensory associatation cortex | −26, −58, 46 | 0.0002 | 169.0 |
| Left MFG | Middle frontal | 46 | Dorsolateral PFC | −26, 47, 1 | 0.0009 | 77.1 |
We also compared the variances of cortical thickness between younger, older high‐ and older low‐performing persons. The variances did not differ between the groups except for the left MFG, where older low performers displayed larger variance than younger and older high performers (Chi‐square distribution, P < 0.05), and the right SPG, where younger adults had higher variance than both older high and low performers (Chi‐square test, P < 0.01).
Next, we performed a whole‐brain comparison of the three groups with respect to cortical thickness. Figure 1B shows the comparison between younger and older high‐performing adults, and Figure 1C shows the comparison between younger and older low‐performing adults. Both older groups had a thinner cortical mantle than younger adults in multiple regions. In certain regions, however, cortex in older low performers, but not in older high performers, was thinner than in younger adults. These regions were mainly located in prefrontal and parietal cortex. As portrayed in Figure 1C, they include: left frontal pole (BA 10), left PPG (BA 7), left caudal SPG (BA 22), left medial PCG (BA 5), caudal part of the MFG (BA 6/8), rostral MFG (BA9/46), lateral superior frontal gyrus (SFG, BA 6/8/9), preCG (BA 4), orbitofrontal cortex (OFG, BA 11), and the right isthmus cingulate gyrus (iCING, BA 23). It is also apparent that the difference in cortical thickness in the lateral dorsal and ventral PFC was larger between younger adults and low‐performing older adults than between younger adults and high‐performing older adults. We then compared older low and high performers directly. Here, we used a less stringent P‐threshold in visualizing the data (P < 0.005 instead of P < 0.001), as we expected more subtle differences than in the comparison with younger adults (see Fig. 3 and Table III). Older high performers had thicker cortex than older low performers in left MFG (BA 46/10), left SPG (BA 7), left OFG (BA 11), left medial SFG (BA 6), right SFG (BA 8/9), preCG (BA 4), inferior parietal gyrus (IPG, BA 7), SPG (BA 5/7), PCG (BA 5), and iCING (BA 23/30).
DISCUSSION
The aim of this study was to characterize the link between cortical thickness and executive functioning in younger and older adults. The key results are that: (a) thicker cortex in specific frontal and parietal regions was related to better executive performance, irrespective of age; (b) this association was stronger in later than in earlier adulthood; and (c) cortical thickness in specific regions involved in executive functioning differed between groups that differed with respect to WCST accuracy (low‐performing vs. high‐performing older adults), but did not differ between similarly performing groups (younger adults versus high‐performing older adults).
Cortical Thickness Decreases and Executive Performance Deteriorates With Normal Aging
Younger adults clearly outperformed older adults on the WCST for both accuracy and response speed, and the size of the age‐related differences observed is in agreement with a meta‐analysis of studies on the WCST and aging [Rhodes,2004]. Similarly, we observed age‐related cortical thinning of the entire frontal cortex, the parietal cortex (supramarginal and posterior parietal), the occipital cortex (pericalcarine), and the superior temporal cortex. The largest age difference was seen in the superior and inferior frontal and superior temporal gyri. There was relative sparing of thickness in aging for the interior, medial temporal, and cingulate cortex. These results confirm previous findings regarding patterns of age‐related cortical thinning across different brain regions [Fjell et al.,2009; Hutton et al.,2009; Salat et al.,2004].
Regions Where Thicker Cortex is Related to Better Performance Correspond to Functional Networks Activated During WCST Performance
We showed that thicker cortex in the lateral prefrontal and parietal regions is related to higher WCST performance. These regions resemble closely the “distributed frontoparietal activation pattern” identified in a meta‐analysis of functional imaging studies on the WCST [Buchsbaum et al.,2005]. The observed pattern is also in line with the assertion based on lesion and functional imaging studies that the WCST does not exclusively depend on prefrontal regions. Rather, similar to other executive tasks, WCST performance draws on widespread neuronal networks [Nyhus and Barcelo,2009]. More specifically, regions consistently activated during WCST performance include inferior frontal and middle gyri, parietal cortex, and the postcentral gyrus, but also subcortical gray matter, cerebellum, and the occipital lobe [Buchsbaum et al.,2005]. Two specific cognitive components are thought to be important to successful WCST performance: set shifting and inhibitory control [Buchsbaum et al.,2005]. A conjunction of meta‐analyses of the WCST and two other tasks (task‐switching and go/no‐go) restricted the task‐related activations to the bilateral middle and inferior frontal, bilateral inferior parietal, and right medial frontal gyrus, with the largest overlapping region in the right MFG. Interestingly, these patterns were replicated in a later fMRI study decomposing the cognitive constituents of the WCST regarding their neural underpinnings [Lie et al.,2006]. This study linked activations in certain regions to different cognitive operations: right dorsolateral prefrontal cortex (DLPFC) to executive operations; right ventrolateral PFC/superior parietal cortex to working memory; and anterior cingulate/temporo‐parietal regions to error detection. In addition, we showed that (a) cortical thickness in the aforementioned regions predicted performance only in the WCST, but not on tasks assessing processing speed and vocabulary, and (b) regions where thicker cortex predicted faster processing speed or vocabulary did not overlap with regions associated with WCST performance. These results suggest that the structure‐function relationships we observed are specific to executive functions.
The fact that our results mimic the patterns of task‐related activations described in Buchsbaum et al. [2005] and Lie et al. [2006] has two important implications: First, it suggests that structural characteristics (i.e., cortical thickness) of regions functionally involved in the WCST are predictive of performance. Second, the results confirm the notion that WCST is not a pure prefrontal test, but rather relies on a distributed neuronal network.
The Association Between Cortical Thickness and WCST Performance was Stronger in Older than in Younger Adults
The link between cortical thickness and WCST was stronger in older than in younger adults for all regions examined, and the age group x cortical thickness interaction was significant for bilateral SPG, right IFG, PCG, and preCG. Previous studies also reported stronger structure‐function or structure‐cognition relationships in older than in younger adults. Specifically, thickness in temporoparietal areas correlated with electrophysiological responses in older but not in younger adults, and these relationships mediated executive functions [Fjell et al.,2007]. Also, Kochunov et al. [2009] found that thicker cortex in many brain regions was related to better executive performance in adults between 30 and 90 years, but not in adults between 19 and 26 years. Importantly, we showed that the weak structure‐performance correlations in younger adults were not because of to insufficient variance in cortical thickness, although the variance in WCST accuracy was larger in older than in younger adults. Thus, the restricted range of performance scores may partly explain the lower correlation between cortical thickness and performance observed in younger adults.
Further, the age‐differential relationships are in line with previous findings on age‐related increases in cognitive heterogeneity [Ardila,2007; De Frias et al.,2007]. This increasing heterogeneity is most likely due to age‐related deterioration of biological resources [Lindenberger et al.,2008; Nagel et al.,2008], in the present case age‐related cortical thinning. Rarefaction of dendritic arbors, spines and synapses, cell shrinkage, and cortical myelin loss are thought to underlie nonpathological age‐related alterations in cortical thickness, density, and volume [Burke and Barnes,2006; Morrison and Hof,1997].
The threshold concept invoked in the introduction suggests that between‐person variations are more likely to result in functional impairments if cortical thickness falls below a certain level. In accordance with this notion, we observed that younger adults with thinner cortex performed similarly to those with thicker cortex. By contrast, older adults with thinner cortex performed worse than those with thicker cortex, likely because age‐related thinning had moved the older low‐performing adults below a certain threshold.
Consequently, it is noteworthy that the cortical thickness‐WCST correlation did not differ reliably between younger and older adults in the right MFG. This finding indicates that the structure of specific cortical regions (i.e., right DLPFC) may influence performance even if biological resources are superior. Toward this end, the importance of the right DLPFC for WCST performance in younger adults was demonstrated in a recent repetitive transcranial stimulation study, showing that stimulation of the right DLPFC during feedback hampers WCST performance in adults aged 19–33 years [Ko et al.,2008].
In sum, the association strength between cortical thickness and executive performance differs by age and brain region. Specifically, in the most task‐relevant cortical regions such as the right DLPFC, individual variations in structure predicted behavior even in young age, whereas in other areas of the network, the cortical mantle may need to undergo structural decline below a certain threshold to influence performance.
Cortical Thickness Differentiates Between Older High and Low Performers on the WCST
Whole‐brain comparison of older high and low performers showed that low‐performing older adults had thinner cortex in prefrontal and parietal regions. Specifically, for regions where thicker cortex predicted higher WCST accuracy in addition to age in the whole sample, thinner cortex distinguished older low‐ from older high‐performing adults, but there was no difference between older high and younger adults. This pattern suggests that structural integrity of regions involved in performing the WCST is important to preservation of high function in late adulthood. In line with these assertions, in recent fMRI research we found that older adults with a more “youth‐like” load‐dependent modulation of the BOLD signal during working memory attained higher levels of performance [Nagel et al.,2009,2010].
Due to its cross‐sectional design, this study did not directly assess the neural mechanisms underlying mean changes in cortical thickness, individual differences in these changes, and their relations to individual differences in cognitive change. However, our finding that the associations between cortical thickness and executive performance increase from early to late adulthood renders it likely that individual differences in rates of cortical change do in fact exist. Common genetic polymorphisms may contribute to this aging‐induced increase in structural heterogeneity [cf. Lindenberger et al.,2008]. For instance, in healthy older adults, individual differences in cortical thickness, in particular in the PFC, are associated with apolipoprotein E [Fan et al.,2010] and catechol‐O‐methyltransferase [Cerasa et al.,2010] genetic polymorphisms. Future longitudinal studies need to determine why certain individuals reach old age with a “youth‐like” cortical mantle, whereas others do not.
CONCLUSIONS
This study revealed that inter‐individual variation in regional cortical thickness in the fronto‐parietal network is related to executive functioning in younger and older adults. Importantly, these effects were specific to WCST, as similar associations were not observed for measures of processing speed and vocabulary. The key brain regions involved in the structure‐performance link resemble closely those identified in functional neuroimaging studies on the WCST. This suggests that microscopic gray‐matter properties, such as dendritic arborization and spine or glial density reflected macroscopically by cortical thickness, may contribute to variation in functional activation patterns. For most of the identified regions, the cortical thickness‐performance association was stronger in older adults, lending support to the threshold concept. In both age groups, however, thicker cortex especially in the right DLPFC was associated with higher WCST accuracy, pointing to the important role of this region in executive functioning. In addition, our data suggest that the degree of structural integrity of regions involved in performing the WCST in old age differentiates high‐ from low‐performing individuals. Future research needs to further delineate and explain the mechanisms contributing to individual differences in brain aging and their relations to cognitive stability and decline.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information.
Acknowledgements
The authors thank Timo von Oertzen for programming the WCST, Hermine Wenzlaff for help with data analysis, and the student assistants for their assistance in data collection.
REFERENCES
- Almeida OP, Schwab SG, Lautenschlager NT, Morar B, Greenop KR, Flicker L, Wildenauer D ( 2008): KIBRA genetic polymorphism influences episodic memory in later life, but does not increase the risk of mild cognitive impairment. J Cell Mol Med 12: 1672–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A ( 2007): Normal aging increases cognitive heterogeneity: Analysis of dispersion in WAIS‐III scores across age. Arch Clin Neuropsych 22: 1003–1011. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Small BJ, Wahlin A, Larsson A ( 2000): Cognitive functioning in very old age In: Craik FI, Salthouse T, editors. Handbook of Aging and Cognition. Mahwah: Erlbaum; p 499–558. [Google Scholar]
- Baloh RW, Vinters HV ( 1995): White matter lesions and disequilibrium in older people. II. Clinicopathologic correlation. Arch Neurol 52: 975–981. [DOI] [PubMed] [Google Scholar]
- Baltes PB ( 1987): Theoretical propositions of lifespan developmental psychology: On the dynamics between growth and decline. Dev Psychol 23: 611–626. [Google Scholar]
- Barrick TR, Charlton RA, Clark, CA , Markus HS ( 2010): White matter structural decline in normal ageing: a prospective longitudinal study using tract‐based spatial statistics. Neuroimage 51: 565–577. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF ( 2005): Meta‐analysis of neuroimaging studies of the Wisconsin card‐sorting task and component processes. Hum Brain Mapp 25: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA ( 2006): Neural plasticity in the ageing brain. Nat Rev Neurosci 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li SC, Lindenberger U, Heekeren HR ( 2010): Age‐related differences in white matter microstructure: Region‐specific patterns of diffusivity. NeuroImage 49: 2104–2112. [DOI] [PubMed] [Google Scholar]
- Cattell RB ( 1971): Abilities: Their structure, growth, and action. Boston: Houghton Mifflin. [Google Scholar]
- Cerasa A, Cherubini A, Quattrone A, Gioia MC, Tarantino P, Annesi G, Assogna F, Caltagirone C, Spalletta G ( 2010): Met158 variant of the catechol‐O‐methyltransferase genotype is associated with thicker cortex in adult brain. Neuroscience 167: 809–814. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA ( 2000): Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology 216: 672–682. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI ( 1993): Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J Cogn Neurosci 5: 162–176. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI ( 1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. Neuroimage 9: 179–194. [DOI] [PubMed] [Google Scholar]
- De Frias CM, Lövden M, Lindenberger U, Nilsson LG ( 2007): Revisiting the dedifferentiation hypothesis with longitudinal multi‐cohort data. Intelligence 35: 381–392. [Google Scholar]
- Elderkin‐Thompson V, Ballmaier M, Hellemann G, Pham D, Kumar A ( 2008): Executive function and MRI prefrontal volumes among healthy older adults. Neuropsychology 22: 626–637. [DOI] [PubMed] [Google Scholar]
- Espeseth T, Westlye LT, Fjell AM, Walhovd KB, Rootwelt H, Reinvang I ( 2008): Accelerated age‐related cortical thinning in healthy carriers of apolipoprotein E epsilon 4. Neurobiol Aging 29: 329–340. [DOI] [PubMed] [Google Scholar]
- Fan M, Liu B, Zhou Y, Zhen X, Xu C, Jiang T ( 2010): Cortical thickness is associated with different apolipoprotein E genotypes in healthy elderly adults. Neurosci Lett 479: 332–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM ( 2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM ( 1999a): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. Neuroimage 9: 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM ( 1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I, Lundervold A, Salat D, Quinn BT, Fischl B, Dale AM ( 2006): Selective increase of cortical thickness in high‐performing elderly‐structural indices of optimal cognitive aging. Neuroimage 29: 984–994. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fischl B, Reinvang I ( 2007): Cognitive function, P3a/P3b brain potentials, and cortical thickness in aging. Hum Brain Mapp 28: 1098–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB ( 2009): High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex 19: 2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS ( 2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14: 21–36. [DOI] [PubMed] [Google Scholar]
- Grant DA, Berg EA ( 1948): A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl‐type card‐sorting problem. J Exp Psychol 38: 404–411. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Raz N ( 2003): Neuroanatomical correlates of selected executive functions in middle‐aged and older adults: A prospective MRI study. Neuropsychologia 41: 1929–1941. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, Gur RC ( 1998): A follow‐up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry 55: 145–152. [DOI] [PubMed] [Google Scholar]
- Hanninen T, Hallikainen M, Koivisto K, Partanen K, Laakso MP, Riekkinen PJ Sr., Soininen H ( 1997): Decline of frontal lobe functions in subjects with age‐associated memory impairment. Neurology 48: 148–153. [DOI] [PubMed] [Google Scholar]
- Hartberg CB, Lawyer G, Nyman H, Jönsson EG, Haukvik UK, Saetre P, Bjerkan PS, Andreassen OA, Hall H, Agartz I ( 2010): Investigating relationships between cortical thickness and cognitive performance in patients with schizophrenia and healthy adults. Psychiatry Res 182: 123–133. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. ( 1993). Wisconsin Card Sorting Test Manual revised and expanded. Odessa, Florida: Psychological Assesment Resources; p 230. [Google Scholar]
- Horn JL ( 1968): Organization of abilities and the development of intelligence. Psychol Rev 75: 242–259. [DOI] [PubMed] [Google Scholar]
- Hutton C, Draganski B, Ashburner J, Weiskopf N ( 2009): A comparison between voxel‐based cortical thickness and voxel‐based morphometry in normal aging. Neuroimage 48: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Yoon U, Shin YW, Hong SB, Kim IY, Kwon JS, Kim SI ( 2006a): Fractal dimension in human cortical surface: multiple regression analysis with cortical thickness, sulcal depth, and folding area. Hum Brain Mapp 27: 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, Kim SI ( 2006b): Gender difference analysis of cortical thickness in healthy young adults with surface‐based methods. Neuroimage 15: 31–38. [DOI] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Petrides M, Strafella AP ( 2008): Repetitive Transcranial Magnetic Stimulation of Dorsolateral Prefrontal Cortex Affects Performance of the Wisconsin Card Sorting Task during Provision of Feedback. Int J Biomed Imaging 2008: 143238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Robin DA, Royall DR, Coyle T, Lancaster J, Kochunov V, Schlosser AE, Fox PT ( 2009): Can structural MRI indices of cerebral integrity track cognitive trends in executive control function during normal maturation and adulthood? Hum Brain Mapp 30: 2581–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, Salat DH, Dale AM, Fischl B ( 2003): Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry 60: 878–888. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB ( 2004): Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol Sci 15: 155–163. [DOI] [PubMed] [Google Scholar]
- Lie CH, Specht K, Marshall JC, Fink GR ( 2006): Using fMRI to decompose the neural processes underlying the Wisconsin Card Sorting Test. Neuroimage 30: 1038–1049. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Schere H, Baltes PB ( 2001): The strong connection between sensory and cognitive performance in old age: Not due to sensory acuity reductions operating during cognitive assessment. Psychol Aging 16: 196–205. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Bäckman L ( 2008): Age‐related decline in brain resources modulates genetic effects on cognitive functioning. Front Neurosci 2: 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC ( 2000): Automated 3‐D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage 12: 340–356. [DOI] [PubMed] [Google Scholar]
- MacDonald SWS, Hultsch DF, Dixon RA ( 2003): Performance variability is related to change in cognition: Evidence from the Victoria Longitudinal Study. Psychol Aging 18: 510–523. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD ( 2000): The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cogn Psychol 41: 49–100. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR ( 1997): Life and death of neurons in the aging brain. Science 278: 412–419. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, von Oertzen T, Sander T, Villringer A, Heekeren HR, Bäckman L, Lindenberger U ( 2008): Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci 2: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li S‐C, Nyberg L, Bäckman L, Lindenberger U, Heekeren HR ( 2009): Performance level modulates adult age differences in brain activation during spatial working memory. Proc Natl Acad Sci U S A 106: 22552–22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE, Preuschhof C, Li S‐C, Nyberg L, Bäckman L, Lindenberger U, Heekeren HR: Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J Cogn Neurosci (in press). [DOI] [PubMed] [Google Scholar]
- Nyhus E, Barcelo F ( 2009): The Wisconsin Card Sorting Test and the cognitive assessment of prefrontal executive functions: A critical update. Brain Cogn 71: 437–451. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD ( 1997): Selective aging of the human cerebral cortex observed in vivo: Differential vulnerability of the prefrontal gray matter. Cereb Cortex 7: 268–282. [DOI] [PubMed] [Google Scholar]
- Raz N, Williamson A, Gunning‐Dixon F, Head D, Acker JD ( 2000): Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual‐motor skill. Microsc Res Tech 51: 85–93. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD ( 2005): Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15: 1676–1689. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Ghisletta P, Rodrigue KM, Kennedy KM, Acker JD ( 2008): Neuroanatomical correlates of fluid intelligence in healthy adults and persons with vascular risk factors. Cereb Cortex 18: 718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes MG ( 2004): Age‐related differences in performance on the Wisconsin card sorting test: A meta‐analytic review. Psychol Aging 19: 482–494. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B ( 2002): Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology 58: 695–701. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B ( 2004): Thinning of the cerebral cortex in aging. Cereb Cortex 14: 721–730. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Rotrosen J, Wolkin A ( 2002): Cognitive performance in schizophrenia: relationship to regional brain volumes and psychiatric symptoms. Psychiatry Res 116: 1–23. [DOI] [PubMed] [Google Scholar]
- Schretlen D, Pearlson GD, Anthony JC, Aylward EH, Augustine AM, Davis A, Barta P ( 2000): Elucidating the contributions of processing speed, executive ability, and frontal lobe volume to normal age‐related differences in fluid intelligence. J Int Neuropsychol Soc 6: 52–61. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW ( 2003): Mapping cortical change across the human life span. Nat Neurosci 6: 309–315. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A ( 2006): Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30: 749–61. [DOI] [PubMed] [Google Scholar]
- Teuber HL ( 1972): Unity and diversity of frontal lobe functions. Acta Neurobiol Exp (Wars) 32: 615–656. [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL ( 2004): Memory and executive function in older adults: Relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia 42: 1313–1335. [DOI] [PubMed] [Google Scholar]
- Voormolen EH, Wei C, Chow EW, Bassett AS, Mikulis DJ, Crawley AP ( 2010): Voxel‐based morphometry and automated lobar volumetry: The trade‐off between spatial scale and statistical correction. Neuroimage 49: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P ( 2001): A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke 32: 1318–1322. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Dale AM, Fischl B, Quinn BT, Makris N, Salat D, Reinvang I ( 2006): Regional cortical thickness matters in recall after months more than minutes. Neuroimage 31: 1343–1351. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM: Consistent neuroanatomical age‐related volume differences across multiple samples. Neurobiol Aging (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL ( 1996): An application of prefrontal cortex function theory to cognitive aging. Psychol Bull 120: 272–292. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S ( 2001): Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging 20: 45–57. [DOI] [PubMed] [Google Scholar]
- Ziegler DA, Piguet O, Salat DH, Prince K, Connally E, Corkin S ( 2010): Cognition in healthy aging is related to regional white matter integrity, but not cortical thickness. Neurobiol Aging 3111: 1912–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information.


