Abstract
White matter (WM) asymmetries of the human brain have been well documented using diffusion tensor imaging (DTI). However, the relationship between WM asymmetry pattern and cognitive performance is poorly understood. By means of tract‐based spatial statistics (TBSS) and voxel‐based analyses of whole brain, this study examined the WM asymmetries and the correlations between WM integrity/asymmetries and three distinct components of attention, namely alerting, orienting, and executive control (EC), which were assessed by attention network test (ANT). We revealed a number of WM anisotropy asymmetries, including leftward asymmetry of cingulum, corticospinal tract and cerebral peduncle, rightward asymmetry of internal capsule, superior longitudinal fasciculus and posterior corona radiata, as well as heterogeneous asymmetries in anterior corpus callosum and anterior corona radiata (ACR). Moreover, specific correlation was found between asymmetric pattern of inferior frontal ACR and EC performance. Additionally, this study also proposed that there were no significant relationships of WM anisotropy asymmetries to alerting and orienting functions. Further clusters of interest analyses and probabilistic fiber tracking validated our findings. In conclusion, there are a number of differences in WM integrity between human brain hemispheres. Specially, the anisotropy asymmetry in inferior frontal ACR plays a crucial role in EC function. Our finding is supportive of the functional studies of inferior frontal regions and in keeping with the theory of the brain lateralization on human ventral attention system. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: diffusion tensor imaging, asymmetry, attention network test, anterior corona radiata, inferior frontal lobe
INTRODUCTION
Human brain hemispheres differ in their anatomy and function [Toga and Thompson, 2003]. The quantification of brain asymmetries may provide unique information about how homologous regions between cerebral hemispheres function in conjunction as a network or exert irrelevant effects, and therefore extend our understanding of neural structure‐function relationships in health and disease. For example, Fornito et al. [ 2004] found that leftward asymmetry of paracingulate sulcus was associated with better performance on both verbal and spatial tasks. This may be attributed to the underlying hemispheric differences in cortical thickness [Fornito et al., 2008] and functional interactions [Yan et al., 2009] within prefrontal regions. In addition to the normal asymmetries, some neurologic diseases also showed lateralized pathologies between two hemispheres [Koziol et al., 2005; Muhlau et al., 2007]; and disturbances in structural or functional brain asymmetries have been detected in psychotic disorders, such as schizophrenia [Kawasaki et al., 2008; Narr et al., 2001; Takao et al., 2010a; Zhou et al., 2003] and bipolar disorder [Reite et al., 1999].
Voxel‐based morphometry (VBM) method for structural magnetic resonance imaging (MRI) data has been extensively applied to investigate brain asymmetries on grey matter [Luders et al., 2004; Watkins et al., 2001] and white matter (WM) [Good et al., 2001; Zhou et al., 2003]. Compared with structural MRI measures of WM volume or density, diffusion tensor imaging (DTI) provides more information about WM tissue microstructure and organization, because it examines specific fiber tracts and can be used to study connectivity between neural regions of interest (ROI). The most commonly used metric of DTI is fractional anisotropy (FA), which represents the degree of constrained water diffusion along the axons and the myelin [Mori and Zhang, 2006]. Greater FA may reflect greater myelination of WM fibers, greater number of myelinated fibers, or greater longitudinal vs. oblique directional alignment of myelinated fibers in WM tracts.
Nowadays, a rising number of DTI studies concerning WM asymmetries chose the ROI‐based [Huster et al., 2009] or voxel‐based [Ardekani et al., 2007; Jahanshad et al., 2010; Park et al., 2004] analysis of anisotropy maps, and/or tractography‐based analysis [Verhoeven et al., 2010] (for a summary, see Table I). However, there has been much debate about these methods applied for WM asymmetry studies. First, the ROI method is operator dependent and time consuming. The asymmetry results are influenced by the ROI definition and reliability of measurement. Second, in order to keep structural homology between WM structures in the left and right hemispheres, voxel‐based asymmetry analysis usually needs to construct the standard symmetry template for registration. However, the gray/white matter boundary would be ambiguous in this template. Besides, spatially smoothing before voxel‐wise statistics can greatly affect the final results and also increase partial volume effect [Smith et al., 2006; Van Hecke et al., 2010]. Third, the tractography technique also requires the automated or manual segmentation of ROI, and the asymmetry results would be contaminated by many parameters, such as the FA threshold and voxel numbers. In addition, the fiber pathways derived from conventional tractography usually pass through large‐scale WM regions, and hence the resulting asymmetry levels may be vulnerable to the inadequate estimation of fiber orientation in brain areas of fiber crossing. Finally, the registration (linear/nonlinear) methods used in above analyses will alter the relative volumes of different brain regions. As such, differences in hemispheric integrity may reflect not just a signal difference from the same structure in both hemispheres but a lack of homology [Jahanshad et al., 2010].
Table I.
A summary of WM asymmetries assessed by DTI
| Studies | Subjects | Methods | Leftward asymmetry | Rightward asymmetry |
|---|---|---|---|---|
| Thiebaut de Schotten et al. [ 2011a] | 40 (20 M) | TBA | CST,B‐W tracts, and frontotemporal segment of AF in males | IFOF and anterior frontal‐parietal connections of AF |
| Thiebaut de Schotten et al. [ 2011b] | 20 (11 M) | TBA | SLF III | |
| Takao et al. [ 2011a] | 224 (161 M) | TBSS | AF, cingulum and CST | Present but not defined |
| Takao et al. [ 2010b] | 109 (51 M) | VBA | Posterior part of IC, AF, cingulum, and CST(no difference in gender) | Frontal WM, UF, PCR, and posterior CC |
| Qiu et al. [ 2011] | 75 (40 M) | TBA | AF | |
| Kang et al. [ 2011] | 56 (28M) | VBA and ROI | Pericortical WM in insula, peri‐Sylvian language regions, and superior parietal and motor regions | Pericortical WM in superior temporal sulcus, superior and paracentral frontal lobe |
| Verhoeven et al. [ 2010] | 78 (3 L; 55 M) | TBA and ROI | FA measure: SLF, temporal part of SLF, CgH, ILF, and SCP; MD measure: temporal part of SLF | FA measure: ATR; MD measure: ATR, CgH, IFOF, UF, and SCP |
| Takao et al. [ 2010a] | 48 (24 M) controls | VBA | AF, cingulum and CST | present but not defined |
| Schulte et al. [ 2010] | 16 (M) controls | TBA | no asymmetry in cingulum (superior, posterior and inferior sectors) | |
| Putnam et al. [ 2010] | 21 (11 M) | TBA | splenium of CC | |
| Propper et al. [ 2010] | 26 (7 L; 9 M) | TBA | AF | |
| O'Donnell et al. [ 2010] | 26 (5 L; 9 M) | histogram | AF, cingulum and occipitofrontal connections | ATR, SLF/anterior segment of AF, UF |
| Lange et al. [ 2010] | 37 (M) controls | ROI | WM FA and MD in superior temporal gyrus | |
| Jahanshad et al. [ 2010] | 374 (154 M) twins | VBA | Temporal lobe | Frontal lobe |
| Trivedi et al. [ 2009] | 51 (21 M) | TBA | Cingulum | |
| Oechslin et al. [ 2009] and Imfeld et al. [ 2009] | 13 (6 M) controls | TBA | SLF, CSF | |
| Lebel and Beaulieu [ 2009] | 183 (97 M) | TBA | AF | |
| Huster et al. [ 2009] | 79 (45 L; 39 M) | ROI | middle cinculum | |
| Hasan et al. [ 2009] | 108 (47 M) | TBA | UF | |
| Dubois et al. [ 2009] | 23 (12 M) infants | VBA and TBA | AF and CST | |
| Upadhyay et al. [ 2008] | 4 M | DTS | AF | |
| Glasser and Rilling [ 2008] | 20 M | TBA | Temporal sectors of AF | |
| Westerhausen et al. [ 2007] | 60 (30 L; 30 M) | ROI | CST at the level of IC | |
| Wakana et al. [ 2007] | 10 (5 M) | TBA | cingulum | CgH |
| Vernooij et al. [ 2007] | 20 (13 L; 9 M) | TBA | AF, no aymmetry in CST | |
| Rodrigo et al. [ 2007a] | 18 (5 M) | TBA | AF and subinsular WM (UF and IFOF) | |
| Rodrigo et al. [ 2007b] | 10 (6 M) controls | TBA | UF | |
| Lutz et al. [ 2007] | 35 (19 M) | ROI | no asymmetry in acoustic pathway | |
| Catani et al. [ 2007] | 50 (30 M) | TBA | B‐W tracts | |
| Bonekamp et al. [ 2007] | 40 (22 M) | ROI | FA measure: SCR, cingulum, and centrum semiovale; MD measure: frontal WM, et al | FA measure: frontal WM; MD measure: body of the CC |
| Barrick et al. [ 2007] | 30 (15 M) | TBA | Pathways connecting temporal lobe to inferior parietal lobule and frontal lobe | Pathway connecting posterior temporal lobe to superior parietal lobule |
| Ardekani et al. [ 2007] | 20 (11 M) | VBA, ROI | anterior limb of external capsule, posterior limb of IC, thalamus, cerebral peduncle and temporal‐parietal regions | Genu, splenium and body of the CC |
| Reich et al. [ 2006] | 20 (5M) | TBA | no asymmetry in CST | |
| Powell et al. [ 2006] | 10 | TBA | B‐W tracts along SLF | |
| Snook et al. [ 2005] | 60 (5 L; 28 M) | ROI | no asymmetry in CC, IC, corana radiata, et al | |
| Parker et al. [ 2005] | 11 (6 M) | TBA | B‐W tracts (AF, external capsule/uncinate fasciculus) | |
| Nucifora et al. [ 2005] | 27 (14 M) | TBA | AF, no aymmetry in CST | |
| Gong et al. [ 2005] | 31 (20 M) | ROI | anterior cingulum | |
| Wang et al. [ 2004] | 20 (M) controls | ROI | anterior cingulum | |
| Park et al. [ 2004] | 32 (M) controls | VBA | Anterior part of CC, cingulum, optic radiation and SCP | Anterior limb of the IC, anterior limb's prefrontal regions, UF and SLF |
| Buchel et al. [ 2004] | 43 (9 L; 25 M) | VBA | AF | |
| Szeszko et al. [ 2003] | 20 (2 L; 9 M) | ROI | frontal lobe FA in women, rather than men | |
| Kubicki et al. [ 2002; 2003] | 18 (M) controls | ROI | UF, cingulum | |
| Cao et al. [ 2003] | 15 (10 M) | ROI | Subinsular WM | |
| Peled et al. [ 1998] | 24 (8 L; 16 M) | ROI | Anterior limb of IC |
The asymmetry results of normal controls in 8 DTI studies of diseases were also included. DTS, diffusion tensor spectroscopy; L, left‐handed; M, males; ROI, region of interest; TBA, tractography‐based analysis; VBA, voxel‐based analysis; AF, arcuate fasciculus; ATR, anterior thalamic radiation; B‐W tracts, tracts connecting Broca's and Wernicke's territories; CC, corpus callosum; CgH, hippocampal part of cingulum; CST, corticospinal tract; IC, internal capsule; IFOF, inferior fronto‐occipital fasciculus; ILF, inferior longitudinal fasciculus; PCR, posterior corona radiata; SCP, superior cerebellar peduncle; SCR, superior corona radiata; SLF, superior longitudinal fasciculus; UF, uncinate fasciculus.
Tract‐based spatial statistics (TBSS), which allows voxel‐wise statistical comparison between individual subjects' DTI data [Smith et al., 2006], has potential advantages for investigation of WM asymmetries. In TBSS, a skeletonized (the centers of WM tracts) FA map is created, and correspondences across subjects are based on distance, rather than by computing a correspondence field for the entire image [Smith et al., 2006]. Moreover, spatial smoothing is no necessary in the image processing, because FA tract skeleton is more Gaussian and lower variable [Takao et al., 2011a]. TBSS has been wildly used to investigate the structure‐behavior correlations [Johansen‐Berg et al., 2007; Kochunov et al., 2010; Rudebeck et al., 2009], and most recently one study has used TBSS to evaluate the effect of scanner on WM asymmetries in elderly people [Takao et al., 2011a]. However, the detailed WM asymmetries assessed by TBSS have not been identified yet.
Conversely, it has become a matter of concern to know whether the asymmetries of brain structures modulate cognitive specialization. Up till now, dominance of language function to the left hemisphere and of spatial attention to the right has been commonly considered a distinctive aspect of human brain organization [Iturria‐Medina et al., 2011; Kinsbourne, 1978; Toga and Thompson, 2003]. As evidence of brain asymmetry for attention function accumulated from lesion [Malhotra et al., 2009; Mort et al., 2003], functional [Jansen et al., 2004; Prado et al., 2011] and anatomical [Thiebaut de Schotten et al., 2011b] findings, the concept of attention has become essential for interpreting the ubiquitous patterns of brain asymmetries, such as the right‐ear advantage for verbal material [Hiscock and Kinsbourne, 2011]. Nevertheless, compared with the well‐documented brain left‐hemispheric asymmetry for language [Catani et al., 2007; Gannon et al., 1998; Glasser and Rilling, 2008], there is still no agreement on the neural basis of functional asymmetries for attention, partly due to the inconsistency among the various models of attention systems.
As the central theme in cognitive science, attention refers to both the preparedness for and selection of certain environmental or mental aspects [Raz and Buhle, 2006]. Because of the brain's limited capacity to handle information, the appropriate selection of information for processing becomes especially critical in our daily life. Although many competing theories have proposed a number of potential components of attention, recent brain imaging studies have consistently supported the notion that there are three key distinct subsystems of attention, namely alerting, orienting, and executive control (EC) [Fan et al., 2005; Niogi et al., 2010; Posner and Petersen, 1990; Thiel et al., 2004; Thimm et al., 2010; Westlye et al., 2011]. Briefly, alerting is defined as achieving and maintaining a state of high sensitivity; orienting is the selection of sensory information; and EC is involved with the process of resolving cognitively incongruent stimuli [Posner, 2008].
In order to isolate the functional components of attention and investigate their association, Fan and colleagues [Fan et al., 2005; Fan et al., 2002] invented the attention network test (ANT). Combining the cued response time (RT) and the flanker [Eriksen and Eriksen, 1974] tasks, ANT could provide a means for exploring the behavioral reaction and the brain activity of the alerting, orienting, and EC networks in a single integrated task. Therefore, ANT has been used in numerous researches on normal populations [Fan et al., 2007a, b; Konrad et al., 2005; Niogi et al., 2010; Westlye et al., 2011] as well as patients with neuropsychiatric disorders, such as attention deficit hyperactivity disorder (ADHD) [Adolfsdottir et al., 2008; Konrad et al., 2006], schizophrenia [Gooding et al., 2006; Neuhaus et al., 2007; Opgen‐Rhein et al., 2008; Urbanek et al., 2009], and autism [Keehn et al., 2010].
Although enormous progress has been made to identify neural correlates of attention function, the hemispheric asymmetries of related structure and function are still very controversial. For instance, some studies indicated that RT of visuospatial attention tasks was associated with functional connectivity [Prado et al., 2011] and FA values [Tuch et al., 2005] on the right attention pathways. A pathway of rightward asymmetry connecting the posterior temporal lobe to the superior parietal lobule, which is related to auditory spatial attention, has also been identified [Barrick et al., 2007]. Whereas, a number of previous fMRI studies showed that attention function tended to only activate the left dorsolateral prefrontal cortex [Botvinick et al., 1999; Fan et al., 2007b; MacDonald et al., 2000; Silton et al., 2010], which is a crucial node for EC of attention [Raz et al., 2006]. Furthermore, DTI studies have found that the WM integrity in left anterior corona radiata (ACR) [Niogi et al., 2010], or right anterior thalamic radiation (ATR) [Mamah et al., 2010], was associated with the EC function. The structure–function correlations between alerting and the anterior limb of the internal capsule (left), orienting and the splenium of the corpus callosum [Niogi et al., 2010] as well as left cingulum [Nestor et al., 2007], have also been reported.
The above studies suggest that separately cortical regions and WM tracts may correlate specifically with distinct attention domains. However, these seemingly contradictory results indicate that much remains to be learned about how morphological asymmetries, especially in WM, may underlie specific components of attention function. Therefore, the main purpose of this study was to assess the effects of DTI asymmetries on individual differences in attention performances. We used TBSS and voxel‐based analyses of whole brain to investigate WM integrity asymmetries and for the first time to evaluate their relationships to distinct components of attention. We also validated the estimates through clusters of interest analyses and probabilistic fiber tracking. The results thereby could contribute to our further understanding of the integrated role of brain regions in attention network.
MATERIALS AND METRODS
Subjects
A total of 59 healthy young subjects (31 males) aged 15–19 years were included in the study. All were native speakers of Chinese with normal or corrected‐to‐normal vision. There were three inclusion criteria. (1) They were right‐handed measured with Edinburgh Inventory [Oldfield, 1971]; (2) They had normal neurological exams, no history of psychological illness, and no abnormal findings in conventional brain MR images; (3) The accuracy of ANT performance was not less than 80% and the scores of EC were positive.
The men and women did not differ in mean age (17.3 ± 1.3 vs. 17.5 ± 1.5 years, with the precision of month) and education years (8.2 ± 1.1 vs. 8.4 ± 1.3 years). All subjects were interviewed by Chinese Version of Behavioral Inhibition System and Behavioral Activation System (BIS/BAS) Scale [Carver and White, 1994] and no difference between genders was found. The study was approved by the Human Research Ethics Committees of the Shandong University School of Medicine. Written informed consent was obtained from all participants as well as their parents.
Behavioral Task
A version of the ANT devised by Fan and colleagues [Fan et al., 2005] was adapted as the cognitive task for this study. It could examine the efficiency of the alerting, orienting, and EC networks involved in attention in a single integrated task. Briefly speaking, subjects were instructed to press a button as quickly and accurately as possible to make a left‐right determination of the target, which was a leftward or rightward arrow at the center and flanked on either side by two arrows in the same direction (congruent condition), or in the opposite direction (incongruent condition). The target and flankers were presented until the participant responded or 2000 ms elapsed. A cue (an asterisk) was presented for 200 ms before the appearance of target. There were three cue conditions: no cue (baseline), center cue (at the fixation for alerting), and spatial cue (at the target location for alerting plus orienting).
In each block, the six trial types (three cue conditions by two target conditions) were presented in a predetermined counterbalanced order. Both the durations between cue and target, and between two trials, were varied systematically. Each subject performed a total of six blocks of trials, each block lasting 5 min 42 s and consisting of 36 trials plus 2 buffer trials at the beginning. All the subjects were trained before the formal operation. Stimulus presentation and behavioral response collection were performed using E‐Prime (Psychology Software Tools, Pittsburgh, PA) on the experimental control computer.
MRI Data Acquisition
MR imaging was carried out using a 3‐T GE Signa scanner (General Electric Medical Systems, Milwaukee, WI). First, the diffusion MRI was collected using spin‐echo, single shot echo planar imaging sequence (TR/TE = 14,000/75.1 ms, 96 × 96 matrix, FOV = 250 mm, 2.6 mm thick slices, no gap) before the behavioral operation. The DTI scheme included 30 nonlinear diffusion gradients directions with b = 1,000 s/mm2 and 3 nondiffusion‐weighted images (b = 0 s/mm2). Array spatial sensitivity encoding technique (ASSET) was used with an acceleration factor of 2. Acquisition time can be reduced by the ASSET method with less image distortion from susceptibility artifacts [Yu et al., 2008]. From each participant 56 axial slices were acquired and the diffusion sequence was repeated two times to increase signal‐to‐noise ratio.
At the end of the DTI scans, a three‐dimensional volume spoiled gradient‐echo (SPGR) pulse sequence with 174 slices (TR = 6.5 ms, TE = 2.0 ms, matrix of 256 × 256, FOV = 256 mm, FA = 15°, slice thickness = 1.0 mm, no gap) was used to acquire the structural images for anatomical reference.
DATA PROCESSING
Behavioral Data Analysis
Firstly the total accuracy of each subject was calculated and the subjects with poor scores (less than 80%) were excluded in this study. Then the trials with incorrect responses or with RTs longer than 1,500 ms or shorter than 200 ms were also excluded to avoid the influence of the outliers. We also removed responses following erroneous ones to avoid post‐error slowing effect. Since RTs were not normally distributed, we used median RT per condition as raw scores. The accuracy for each of the six trial types was also calculated. Finally, instead of the conventional subtraction measure [Fan et al., 2007b; Fan et al., 2005], we used ratio scores of alerting, orienting, and EC to definite the effects of three attention networks. The formulas were as follows:
DTI Data Analysis
The DTI data was processed using FSL (University of Oxford, UK, http://www.fmrib.ox.ac.uk/fsl). Firstly the DTI volumes were corrected for movement and eddy current distortion using affine registration [Jenkinson and Smith, 2001]. The corrected images were masked to remove skull and nonbrain tissue using the FSL Brain Extraction Tool (BET) [Smith, 2002]. Then the FA images were calculated using the diffusion tensor analysis toolkit (FDT) [Smith et al., 2004].
We then used TBSS to test the asymmetries of fiber tracts. The main procedures were as follows: (1) All subjects' FA data were nonlinearly aligned into MNI152 space (1 × 1 × 1 mm3) and the mean of all aligned FA images was created. (2) The mean FA image was “skeletonized” and only the centers of tracts (maximal FA values) were spared, and voxels with FA values lower than 0.25 were suppressed. (3) The symmetrized mean FA image was generated by flipping and averaging original mean FA image. This was then “skeletonized” to generate the initial symmetric skeleton. (4) The original (asymmetric) skeleton was dilated by one voxel, and then was used to mask the initial symmetric skeleton to ensure that only skeleton structures close to being symmetric in the original data were used in this left‐right analysis. (5) To make sure the final skeleton was exactly symmetric, the masked symmetric skeleton was flipped and remasked with the nonflipped version. Then each subject's aligned FA data was projected onto the final symmetry skeleton, resulting in 4D dataset. (6) To implement the interhemispheric comparison of fibers, the 4D DTI dataset was left–right flipped, and the voxel‐wise difference map between the original and flipped images was created. In this new map, the left side of the image represented the difference of skeleton between the subjects' right and left hemispheres (right–left); voxels on the right had the opposite sign (left–right).
Statistical Analysis
ANOVA of 3 (no, center and spatial cues) × 2 (incongruent and congruent targets) factorial designs was used to test the effects of cues, targets and their interaction on Median RT. Partial correlation analyses between age and the ratio scores of alerting, orienting, and EC were performed, using gender as covariate. We also tested the main effects of sex on ratio scores using independent samples t‐test. To explore the relationships between the distinct ANT components, we correlated each of the RT ratio scores after controlling for sex and age.
The nonparametric 1‐sample t‐test for the difference map was performed by Randomise program (http://www.fmrib.ox.ac.uk/fsl/randomise/index.html), which is based on permutation methods in the FSL, to determine the WM skeleton regions showing significant asymmetries. The output was thresholded at cluster level, which was obtained by first arbitrarily thresholding the raw t‐statistics map on the skeleton, and then corrected for multiple comparisons using the null distribution of the max (across the image) cluster mass. Cluster mass is the sum of all statistic values within the cluster, and has been reported to be more sensitive than cluster size [Bullmore et al., 1999]. After selected a range of thresholds for the raw t‐statistics map and compared the calculation results, we found that a strict threshold was necessary and the cutoff of t ≥ 4 (equal to P ≤ 0.0002) could clearly distinguish the specific skeleton asymmetries in different WM partitions, such as the corpus callosum and superior longitudinal fasciculus (SLF). Thus, we used a strict statistical threshold of t ≥ 4 and only considered clusters of ≥ 50 mm3 (P ≤ 0.005 at cluster level, corrected) that survived thresholding. In order to distinguish the clusters with significant asymmetries between each other, the resulting t‐map was firstly transferred into cluster index map. Then multiple WM atlases implemented in FSL (http://www.fmrib.ox.ac.uk/fsl/data/atlas-descriptions.html) were used for the definition of anatomical regions.
Next, the total determined clusters were binarized to generate a mask (lateralization mask), representing the skeleton regions with significant FA asymmetries. In order to investigate the relationship between fiber asymmetries and performances of attention function, the general linear models (GLMs) were implemented, with FA difference maps of all subjects as dependent variable, ratio scores of three attention networks as fixed factors, age and gender as covariates and the lateralization mask as ROI. We performed separate analyses for alerting, orienting and EC effects to explore the relations to FA asymmetries across attention function. Percent differences of FA values [2(left – right)/(left + right)] were not adopted here in order to avoid over‐emphasizing differences in regions with low anisotropy [Jahanshad et al., 2010].
Probabilistic Diffusion Tractography
The clusters identified with above asymmetry analyses and GLMs were then used as seed masks to perform probabilistic diffusion tractography (PDT) [Behrens et al., 2003, 2007]. PDT could estimate a probability distribution function of fiber direction and allow modeling multiple fiber orientations at each voxel. The warpfields of nonlinear registration and the inverse versions were used for the translation between the original space and the MNI152 standard space. Tracts generated by PDT were volumes wherein values at each voxel represented the number of samples (or streamlines) that passed through that voxel. For the elimination of spurious connections, tractography in each subject was thresholded to include only voxels containing at least 100 samples (out of 5,000). These individual tracts were then binarized and summed to produce group probability map. In this map, the value in each voxel represented the number of subjects in whom the pathway passed through that voxel (see colorbars in Figs. 3 and 4).
Figure 3.
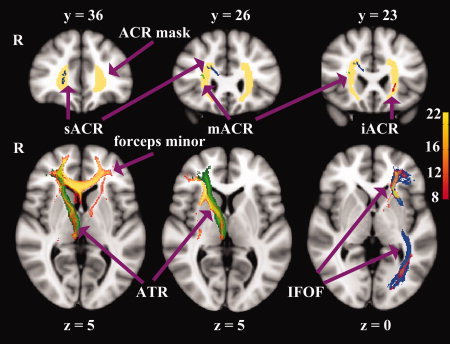
Upper low: Coronal slices (in mm) of the asymmetric regions in ACR overlaid on the standard MNI152 T1 image. The red, green and blue clusters represent the inferior, middle and superior ACR (iACR, mACR, and sACR) regions showing skeleton asymmetries, respectively. The right clusters indicate rightward asymmetry, vice versa. The bilateral ACR templates are displayed in yellow and derived from the ICBM‐DTI‐81 White‐Matter Labels Atlas [Mori et al., 2005]. Lower row: Axial sections of the group probability maps (Red‐Yellow, N = 22) generated from the iACR (right), mACR (middle), and masked sACR (left), respectively. The underlying tracts illustrate the templates of IFOF (inferior fronto‐occipital fasciculus) in blue and ATR (anterior thalamic radiation) in green. R, right hemisphere.
Figure 4.
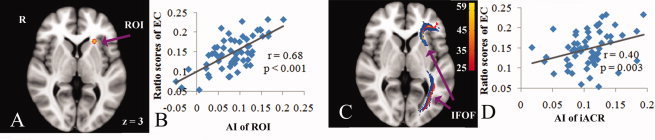
Local correlation between FA asymmetry in inferior part of ACR (iACR) and attention function, and probabilistic tractography from behavior‐related region (N = 59). A: ROI illustrates the correlated region (Red‐Yellow), which is dilated by one voxel for visualization. C: Group probability map (Red‐Yellow, N = 59) generated from PDT of behavior‐related region (14 voxels). The underlying blue tract represents the left thresholded IFOF (inferior fronto‐occipital fasciculus) template. B and D: Scatterplots showing correlations of EC ratio scores with FA asymmetry indexes (AI, Left–Right) derived from ROI (14 voxels) and inferior part of ACR with leftward asymmetry (128 voxels), after controlling for age and gender. r, partial correlation coefficient.
RESULTS
Behavioral Data
On average, the accuracy of ANT performance was very high (94.9%), indicating that the participants understood the instructions and were able to response reliably. There was no significant difference between genders and no correlation with age on overall accuracy. Paired samples t‐tests between the different conditions revealed significant orienting (t = 5.61, P < 0.001) and EC (t = 8.83, P < 0.001) effects on accuracy across subjects, while the alerting effect was absent (t = 1.67, P = 0.10).
As for median RT measures, responses to incongruent stimuli were slower than to congruent, and spatial cues led to the fastest responses while no cue conditions were the slowest of the three cue conditions. Levene's test of equality of error variances was performed and RTs turned out to be equal across the six conditions. ANOVA of 3 × 2 factorial designs showed that the factors of both cue level [F (2, 348) = 50.04; P < 0.001] and target condition [F (1, 348) = 126.29; P < 0.001] were significant. Besides, the interaction between cue and target was also significant [F (2, 348) = 3.21; P = 0.04].
The ratio scores of alerting, orienting and EC effects as well as their correlations were summarized in Table II. One negative value for alerting and one for orienting, as well as one outlier for orienting (0.27), were excluded before the statistical analyses. Only the correlation between alerting and orienting was significant (r = −0.41, P = 0.002), after controlling for age and gender. There were no gender differences in ratio scores (Table II). We did not found age correlations with any of the ratio scores across subjects. The coefficients of determination (R 2) of linear correlation between age and alerting, orienting and EC were 0.0002 (P = 0.97), 0.039 (P = 0.10), and 0.0089 (P = 0.95), respectively (Fig. 1).
Table II.
The ratio scores (Mean ± SD) of attention components and their correlation coefficients (bold)
| Sample size | Alerting | Orienting | EC | |
|---|---|---|---|---|
| Male | 31 | 5.83 ± 3.25 | 10.24 ± 6.02 | 15.60 ± 4.22 |
| Female | 28 | 5.87 ± 3.79 | 10.11 ± 4.19 | 13.85 ± 4.13 |
| t (P) | 0.05 (0.96) | 0.09 (0.92) | 1.60 (0.11) | |
| Total | 59 | 5.85 ± 3.49 | 10.18 ± 5.19 | 14.77 ± 4.24 |
| Alerting | 59 | 1 | ||
| Orienting | 59 | −0.41 (0.002*) | 1 | |
| EC | 59 | −0.03 (0.82) | −0.94 (0.49) | 1 |
The effects of alerting, orienting and EC are expressed in percent relative to the baseline condition. The correlation analyses were adjusted for age and gender. t, the t value of independent samples t‐test. The numbers in parentheses represent P values of statistical analyses. EC, executive control.
Figure 1.
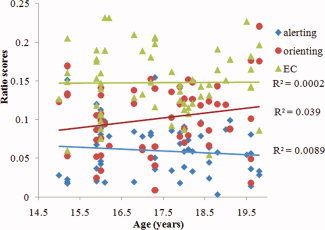
Pearson's correlations between age and ratio scores of alerting, orienting and EC effects. EC, executive control.
Skeleton Asymmetries
The skeleton FA differences between hemispheres were shown in Table III. In general, there was no noteworthy difference in mean FA values of symmetry skeleton between the left and right hemispheres (0.497 ± 0.015 vs. 0.493 ± 0.015) across subjects. However, more voxels in right hemisphere showed prominent asymmetry than in the left (5156 vs. 3240 voxels, the clusters with less than 50 voxels were excluded), after corrected for multiple comparisons of asymmetry analysis.
Table III.
FA skeleton regions showing significantly hemispheric asymmetries
| Clusters | Regions | MNI Peak Coordinates | Voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Rightward asymmetry (Right > Left) | |||||
| 1 | Anterior limb of internal capsule extending to posterior limb and external capsule | 16 | 12 | 5 | 1062 |
| 2 | Superior part of ACR, Genu and body of Corpus callosum | 21 | 36 | 1 | 875 |
| 3 | Posterior corona radiata, Splenium of corpus callosum | 33 | −63 | 7 | 829 |
| 4 | Medial lemniscus | 4 | −40 | −40 | 178 |
| 5 | Posterior part of SLF | 35 | −34 | 32 | 176 |
| 6 | WM in superior temporal gyrus | 46 | −24 | 2 | 142 |
| 7 | WM in middle temporal gyrus | 51 | −41 | −5 | 138 |
| 8 | WM in precentral gyrus | 52 | 3 | 20 | 135 |
| 9 | WM in supramarginal gyrus | 47 | −34 | 9 | 133 |
| 10 | Middle part of ACR | 28 | 35 | 5 | 133 |
| 11 | Anterior part of SLF | 40 | −12 | 28 | 110 |
| 12 | External capsule | 31 | 0 | 14 | 109 |
| 13 | Superior corona radiata | 26 | −17 | 28 | 103 |
| 14 | Fornix | 32 | −17 | −10 | 103 |
| Leftward asymmetry (Left > Right) | |||||
| 15 | Medial lemniscus, CST, superior cerebellar peduncle | −2 | −37 | −45 | 548 |
| 16 | Cerebral peduncle | −12 | −26 | −19 | 429 |
| 17 | Genu and body of Corpus callosum | −4 | 27 | 6 | 318 |
| 18 | Middle cerebellar peduncle | −10 | −30 | −41 | 236 |
| 19 | Forceps minor | −17 | 53 | 0 | 159 |
| 20 | Thalamus | −16 | −18 | 11 | 158 |
| 21 | WM in precuneous gyrus | −8 | −59 | 19 | 147 |
| 22 | Inferior part of ACR | −19 | 22 | −6 | 128 |
| 23 | Cingulum (anterior part) | −9 | 21 | 25 | 127 |
ACR, anterior corona radiata; CST, corticospinal tract; SLF, superior longitudinal fasciculus. Minimum cluster size: 100 voxels (100 mm3). P ≤ 0.005 at cluster level, corrected for multiple comparisons. The ACR asymmetries are emphasized in bold font.
As a result, we found most WM structures, such as internal capsule, external capsule, posterior corona radiata, SLF, and temporoparietal WM showed rightward asymmetry. On the contrary, cingulum as well as some fibers in brain stem displayed left‐greater‐than‐right anisotropy asymmetry (Fig. 2, Table III). Besides, both the leftward and rightward asymmetries within some other WM structures, such as corpus callosum (Fig. 2) and ACR (Fig. 3), were revealed in current study. For better visualization of these regions, we discarded the irrelevant clusters in Figures 2 and 3.
Figure 2.
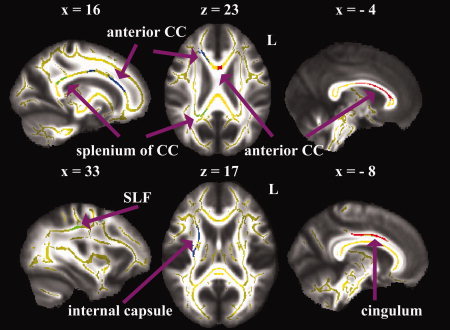
Regions that show skeleton asymmetries. The white matter tracts with leftward asymmetry were shown in red, while those with rightward asymmetry were represented in green and blue. The symmetry skeleton, which is overlaid on the symmetry mean FA image of all subjects, is displayed in yellow. The numbers are the axial and sagittal coordinates in mm. CC, corpus callosum; L, left hemisphere; SLF, superior longitudinal fasciculus.
Relationships between Skeleton Asymmetries and Attention Function
Using asymmetric information in the difference map, statistics of GLMs were performed to correlate the FA differences across the hemispheres with ANT performance. We found that there was a significantly positive correlation of EC ratio scores with the levels of leftward asymmetry in the inferior part of ACR (Fig. 4A,B). The peak coordinate of correlated region is −24, 24, and 3 (x, y, z, in mm) and the voxel number is 14 (P < 0.05, FDR corrected). The statistical results also revealed that there were no significant relationships of alerting and orienting to WM asymmetries.
To verify that it is the asymmetric effect, rather than unilateral influence of WM on the individual variations of attention function, we performed multiple correlation analyses between the ANT ratio scores and bilateral mean FA values as well as their asymmetry indexes in eight clusters of interest. These clusters had been reported to be related with attention function (see Discussion) and showed significant asymmetries during the aforementioned skeleton comparison. The regions included the superior and middle parts of ACR, anterior, and posterior parts of SLF, post limb of internal capsule and splenium of corpus callosum, all of which showed significant rightward asymmetry, and inferior part of ACR as well as cingulum, in which leftward asymmetry was detected (Table IV). To restrict the regions, we respectively isolated post limb of internal capsule, superior part of ACR, and splenium of corpus callosum from cluster 1, 2, and 3 in Table III by means of corresponding structural templates in ICBM‐DTI‐81 White‐Matter Labels Atlas [Mori et al., 2005]. The mean FA values in each of the clusters of interest were extracted from individual symmetric skeleton maps as well as the flipped ones. We then performed correlation analyses between unilateral fiber FA values and behavioral performances using SPSS (IBM Company, US).
Table IV.
Mean FA values and asymmetry indexes (mean ± SD) across subjects in clusters of interest showing significant asymmetries (N = 59)
| Regions | Clusters | Voxels | Mean FA value | Asymmetry index | ||
|---|---|---|---|---|---|---|
| L | R | L − R | 2(L − R)/(L + R) | |||
| Superior part of ACR | 2 | 367 | 0.45 ± 0.04 | 0.54 ± 0.04 | −0.09 ± 0.03 | −0.18 ± 0.06 |
| Middle part of ACR | 10 | 133 | 0.40 ± 0.03 | 0.48 ± 0.04 | −0.08 ± 0.04 | −0.18 ± 0.09 |
| Inferior part of ACR | 22 | 128 | 0.49 ± 0.03 | 0.38 ± 0.04 | 0.10 ± 0.03 | 0.25 ± 0.09 |
| Cingulum | 23 | 127 | 0.60 ± 0.04 | 0.51 ± 0.05 | 0.09 ± 0.03 | 0.17 ± 0.06 |
| Post limb of internal capsule | 1 | 336 | 0.70 ± 0.02 | 0.74 ± 0.02 | −0.04 ± 0.02 | −0.06 ± 0.02 |
| Anterior part of SLF | 11 | 110 | 0.46 ± 0.04 | 0.53 ± 0.05 | −0.06 ± 0.03 | −0.13 ± 0.07 |
| Posterior part of SLF | 5 | 176 | 0.44 ± 0.04 | 0.51 ± 0.04 | −0.06 ± 0.04 | −0.14 ± 0.08 |
| Splenium of corpus callosum | 3 | 333 | 0.68 ± 0.02 | 0.74 ± 0.02 | −0.06 ± 0.02 | −0.09 ± 0.02 |
The cluster numbers correspond with those in Table III. ACR, anterior corona radiata; SLF, superior longitudinal fasciculus; L, left; R, right.
Again, only the leftward asymmetry (left–right) in the inferior part of ACR showed modest but significant correlation (r = 0.40, P = 0.003) with EC effect (Fig. 4D). The correlation still exited (r = 0.38, P = 0.007) even we used another asymmetry index [2(left – right)/(left + right)]. No any other significant correlations (P > 0.05, uncorrected) was detected between WM asymmetries and alerting as well as orienting functions. Note that age and gender were included as covariates in all analyses.
To further explore the presumable relationships between attention function and unilateral fiber integrity across subjects, we employed other GLMs, using original (asymmetric) skeletons of all subjects as dependent variable, and age as well as gender as covariates. However, after FDR corrected of multiple comparisons, no correlations was detected across the entire group.
Probabilistic Tractography
Our main interest here was to assess the fiber pathways arising from behavior‐related region (Fig. 4A) and three asymmetric clusters in ACR (Table IV), so we only tracked the fibers in the prominent side. Differing from the PDT of behavior‐related region, we only chose 22 subjects (11 males, age‐matched between genders) to perform multifiber tracking from the three ACR clusters. As the three clusters showing anisotropy asymmetries were much broader than behavior‐related region, we thresholded the tracking map in each subject using 1,000, rather than 100 samples. Then The JHU White‐Matter Tractography Atlas, which was thresholded to include at least 15% of probability, was chosen as the template to identify the determined tracts.
The behavior‐related region in the left inferior frontal ACR generated sagittal paths connecting inferior frontal region with temporal–occipital cortex (Fig. 4C). Meanwhile, the whole ACR cluster of leftward asymmetry showed the same connecting trajectories (Fig. 3), which exactly overlapped with inferior fronto‐occipital fasciculus (IFOF). In contrast, connections with the two ACR clusters of rightward asymmetry traveled mainly within anterior brain regions (Fig. 3). Here we identified these connections as forceps minor and ATR. Tracking from the middle ACR cluster also generated some fibers that could be considered as IFOF (not shown in the figures).
DISCUSSION
It is often assumed that the attention system comprises three separable functional networks of alerting, orienting, and EC [Fan et al., 2005; Raz and Buhle, 2006]. However, whether such networks evaluated by behavioral performances are independent remains a matter of debate. Our results revealed the interaction between cue and flanker effects, and the negative correlation between alerting and orienting, which could be explained by the incremental effect of spatial as compared with alerting cue [Mahoney et al., 2010]. Such correlation was in line with some previous studies [Callejas et al., 2004; Mahoney et al., 2010; Westlye et al., 2011] but inconsistent with others [Fan et al., 2007a; Fan et al., 2002; Matchock and Toby Mordkoff, 2009; Niogi et al., 2010]. It is speculated that methodological and group differences across studies might account for the controversial results. For instance, one experiment using ANT with various stimulus onset asynchrony (SOA) values has clarified that the cuing effects as well as the interaction between alerting and orienting effect could be influenced by the cue–target time intervals [Fuentes and Campoy, 2008]. Age‐related differences in ANT, especially in alerting effect, have also been reported in previous literatures [Gamboz et al., 2010; Jennings et al., 2007].
Conversely, this study support the independence of EC function [Fan et al., 2002], indicating that ANT could fractionate the distinct components of attention. Robust evidences suggest that selective EC alteration has become an important neuropsychological endophenotype in ADHD [Konrad et al., 2006; Makris et al., 2008] and schizophrenia [Gooding et al., 2006; Neuhaus et al., 2007; Opgen‐Rhein et al., 2008; Urbanek et al., 2009]. We also argued that ratio scores, which had been used to explore the structure–behavior correlations [Westlye et al., 2011], heritability [Fan et al., 2003] and impairment of attention function in diseases [Nestor et al., 2007; Urbanek et al., 2009; Wang et al., 2005], would be more appropriate than RT scores in ANT studies, because the former could isolate the attention system from the overall RT. Recent studies have pointed out that RT in behavioral tasks would be interfered by the effect of speed‐accuracy tradeoff, which modulated the competing demands of response speed and response accuracy [Bogacz et al., 2010].
The voxel‐wise analysis of skeleton difference images revealed a number of regions with a significant FA difference between hemispheres. In contrast to previous tractography‐based asymmetry results [Verhoeven et al., 2010], more observed WM asymmetries showed rightward predominance. Further comparisons showed that most of FA asymmetry indexes (division measurement) in determined clusters obviously exceeded the values in the tractography‐based study [Verhoeven et al., 2010], indicating that VBA and cluster‐mass correction was more appropriate to detect asymmetry effect. Consistent with prior literatures, the current study demonstrated the leftward asymmetry in anterior cingulum [Bonekamp et al., 2007; Gong et al., 2005; Huster et al., 2009; Takao et al., 2011b], cerebral peduncle [Ardekani et al., 2007], and corticospinal tract [Dubois et al., 2009; Takao et al., 2011a; Thiebaut de Schotten et al., 2011a]; and rightward asymmetry in anterior limb of internal capsule [Park et al., 2004], splenium of corpus callosum [Ardekani et al., 2007; Putnam et al., 2010; Takao et al., 2011b], SLF [O'Donnell et al., 2010; Oechslin et al., 2009; Park et al., 2004], and posterior corona radiata [Takao et al., 2011b]. In addition, The leftward FA asymmetry in medial region, and the inverse asymmetry in lateral region of genu and body of corpus callosum revealed in this study might mediated the disputes in previous DTI studies [Ardekani et al., 2007; Bonekamp et al., 2007; Park et al., 2004; Snook et al., 2005], and extend the understanding of rightward thickness asymmetry in anterior body and anterior third of the corpus callosum [Luders et al., 2006].
Interestingly, we also found the heterogeneous asymmetries in ACR, which continues caudally as the internal capsule and consists of a mixture of projection, association, and callosal fibers [Mori et al., 2005; Wakana et al., 2004]. Probabilistic fiber tracking demonstrated that the clusters with rightward asymmetry were occupied by ATR, forceps minor, and IFOF, while the cluster showing leftward predominance contained fibers connecting inferior frontal cortex to lower edge of insular lobe and temporal‐occipital regions. Here we also defined the left bundle as IFOF. However, it needs to be declared that IFOF and inferior longitudinal fasciculus share most of the projections at the posterior temporal and occipital lobes, while the uncinate fasciculus and IFOF share the projections at the frontal lobe [Wakana et al., 2004]. Asymmetries in ATR and IFOF are highly genetically influenced [Jahanshad et al., 2010]. ATR carried nerve fibers between thalamus and prefrontal cortex. The rightward asymmetry of ATR integrity was in congruence with previous studies [O'Donnell et al., 2010; Verhoeven et al., 2010]. The incongruent asymmetries in local regions of IFOF also support the dorsal‐ventral anatomic segmentation of IFOF [Martino et al., 2010], although further studies are needed to clarify the exact segmentation in DT images. Finally, since leftward asymmetry in forceps minor is also existent, we propose that bidirectional connectivity between hemispheres [Bitan et al., 2010] coincides with current findings.
In addition to the examination of asymmetries in WM integrity, we also investigated the relationship between WM asymmetries and distinct components of attention—alerting, orienting, and EC. With the combination of voxel‐based analyses and ROIs approach, we found that left‐greater‐than‐right FA asymmetry in inferior frontal ACR, was positively correlated with EC ratio scores. Because larger EC scores were indicative of worse performance as a result of longer RTs required for conflict resolution [Fan et al., 2003], our finding implicated that greater fiber integrity difference across bilateral inferior frontal ACR provided an disadvantageous influence on EC performance. Further tractography from correlated regions generated sagittal fiber paths connecting inferior frontal lobe to temporal–occipital regions. Group probalility map verified that these tracts corresponded to IFOF.
The finding of inferior frontal–temporal–occipital tracts associated with EC function is strongly supported by previous studies. EC of attention refers to the higher order processes involved in the self‐regulation of behavior [Garavan et al., 2002], cognition [Marklund et al., 2007; Smith and Jonides, 1999; Tomita et al., 1999], and emotion [Pessoa, 2009]. It is construed as the monitoring and resolution of conflict between computations (such as decision making, error detection or regulation of thoughts and feelings) in different neural areas [Raz and Buhle, 2006]. Accordingly, temporoparietal cortex and inferior frontal cortex together constitute the ventral attention system, which is specialized for the detection of behaviorally relevant stimuli, particularly when they are salient or unexpected [Corbetta and Shulman, 2002]. Meanwhile, IFOF provided the main anatomical connections for ventral attention system [Umarova et al., 2010], and reductions of WM integrity in IFOF are associated with deficits of executive function in patients with first‐episode psychosis [Perez‐Iglesias et al., 2010] or chronic trauma [Kraus et al., 2007].
Specially, the significant correlation between ACR integrity asymmetry and EC function indicated that EC of attention was underpinned by both hemispheres. As in the classic Stroop [MacLeod, 1991] task and Simon task [Simon and Berbaum, 1990], EC in ANT is assessed by subtracting RTs to congruent or neutral stimuli from those to incongruent ones. Previous functional imaging studies showed that EC function as measured by the ANT was associated with the blood‐oxygenation‐level dependent (BOLD) [Fan et al., 2005] as well as oscillatory [Fan et al., 2007a] activity in bilateral inferior frontal cortex among healthy controls. Using Stroop task, one study also found the activation in bilateral inferior frontal cortex, which showed functional connectivity with superior frontal area and anterior cingulate gyrus [Kemmotsu et al., 2005]. Additionally, attention function was also linked to WM anisotropy in bilateral pericallosal frontal regions [Madden et al., 2007]. Niogi et al [ 2008; 2010] found that correlation between EC scores of ANT and FA values within a ROI in the ACR was significant in the left hemisphere, and appeared a nonsignificant trend in the right. It was speculated that both the left and right ACR regions played a role in EC and the right contributions might be apparent in studies with great power [Niogi et al., 2010]. Furthermore, damage in either side of ACR due to mild [Niogi et al., 2008] or severe [Kraus et al., 2007] trauma could induce deficit in the attention domain.
To our knowledge, such a significant relationship between brain WM asymmetry and EC performance has never previously been described. The negative effect of leftward asymmetry on EC function also suggests that fibers connecting inferior frontal and posterior temporal–occipital regions in the right hemisphere confer an advantage for EC function. This finding is supported by theory of rightward asymmetry for human sustained attention [Pardo et al., 1991]. Moreover, many of the paradigms involving response inhibition have focused on ventrolateral regions (primarily within BA 47 and 45) within the right inferior frontal gyrus [Aron et al., 2004]. It is thought that inhibitory control processes during EC or response selection could enhance the cognitive efficiency by inhibiting the irrelevant stimulus dimension and emphasizing the required response. Consistently, behavioral performance of Simon task showed strong correlation with both fMRI and DTI characteristics of the right inferior frontal region [Forstmann et al., 2008]. In addition, attention tasks that activate the brain central–executive network (including the dorsolateral prefrontal cortex and posterior parietal cortex) have been consistently shown to evoke decreased activation in the default‐mode network (including the ventromedial prefrontal cortex and posterior cingulated cortex), and the right ventrolateral prefrontal cortex and anterior insula (IFOF travels in the WM between insular cortex and putamen) play a major role in switching between these two networks [Sridharan et al., 2008].
For deeper explanation, asymmetrical brains, for example, have a corpus callosum with a reduced midsagittal area relative to more symmetrical ones [Witelson, 1985]. This might reflect fewer and/or thinner fibers connecting the two hemispheres [Galaburda et al., 1990]. The theory was confirmed by one recently fMRI‐DTI study, which revealed that atypical language lateralization (nonleftward dominance) was associated with high anisotropic diffusion through the corpus callosum [Haberling et al., 2011]. Another insight to these phenomena emerges from the split‐brain researches. Using patients with surgical disconnection of the cerebral hemispheres, previous studies confirmed that the two hemispheres relied on a common orienting system to maintain a single focus of attention, and the process of discriminating stimulus and making choice by one hemisphere will delay the other hemisphere in making a similar choice [Gazzaniga, 2000]. Therefore, we could speculate that the increased leftward asymmetry of inferior frontal ACR might diminish EC function by competitively suppressing the activity of right attention system or by decreasing the interactions between two hemispheres. Analogous to our speculation, one pioneering DTI study about language lateralization demonstrated that symmetry in direct connections between remote cortical territories, not extreme lateralization, might ultimately be advantageous for specific cognitive functions [Catani et al., 2007]. However, this study cannot expose the exact mechanisms for the resulting structure–behavior relationship. The combined fMRI‐DTI studies in the future are necessary to solve the conundrum.
The fact that there were no other significant correlations between WM integrity/asymmetries and attention subcomponents is surprising. Instead, Niogi et al. [ 2010] found the structure–function correlations between alerting and the anterior limb of the internal capsule. Besides, the splenium of the corpus callosum [Niogi et al., 2010], right SLF (II, III, and arcuate fascicle) [Umarova et al., 2010] and left cingulum [Nestor et al., 2007] have been reported to be linked with orienting function. Furthermore, adults with ADHD, which is characterized primarily by behavioral symptoms of inattention and impulsivity, showed significant FA abnormalities in the right cingulum and SLF II [Makris et al., 2008]. However, our voxel‐based and clusters of interest analyses did not found any significant relationships of alerting and orienting to the unilateral integrity or bilateral FA asymmetry indexes in these WM regions. It is conceivable that strong FDR correction in current voxel‐based analyses and differences in pre‐defined ROI regions could explain the incongruence with these previous findings. Taking together, we could discreetly suppose that alerting and orienting are irrelevant to the asymmetries of WM integrity.
Although these findings are robust, some limitations of the present study need to be addressed. First, given the relatively smaller sample size, we did not take account of sex and age effects on WM asymmetries. Pioneering VBA studies in large populations have showed that there were no significant relationships of sex [Jahanshad et al., 2010; Takao et al., 2011b] and age [Takao et al., 2010b] to WM FA lateralization. Secondly, we chose the FA skeleton, instead of the entire FA map, to test the relationships between WM asymmetries and attention function. In that case, our results will tend to be conservative because some WM regions would be omitted after being skeletonised. Meanwhile, the relatively smaller positive regions after statistical analyses would mismatch with actually large‐scale fiber tracts. Regardless of these drawbacks, our methods avoid the contaminations of standard registration algorithms and spatial smoothing in conventionally quantified DTI studies.
Besides, voxel‐based statistics are still difficult to estimate the FA changes at crossings or junctions. For instance, an apparent reduction in regional FA value might in fact be due to an increase in other tracts feeding into the junction [Smith et al., 2006]. In this case, the interpretations of FA asymmetries at crossings or junctions can be quietly complicated. Hence, a combination of different diffusion parameters (such as mean diffusivity, as well as axial and radial anisotropies) might be an alternative approach. At last, this study does not allow us to evaluate the direction of causality between WM structure and attention function. Although it is speculated that innate variations in brain structure influence subsequent cognition or behavioral levels, some studies also indicated that short‐term meditation training could improve EC performance on the ANT [Tang et al., 2007] and induce WM FA changes [Tang et al., 2010]. Furthermore, strong improvement of EC function after attention training was also shown in children [Rueda et al., 2005]. Future longitudinal studies using DTI should test whether alterations in attention load or training result in observable changes in WM integrity as well as asymmetries.
CONCLUSION
This study demonstrates that TBSS is a powerful unbiased technique for evaluating human brain WM asymmetries. Our data replicate many well established DTI findings of WM asymmetries, while expanding on the regional details, which are attractive for further studies. Specially, we identified, for the first time, the significant correlation between WM asymmetry in inferior frontal ACR and the independent EC function of attention. Our findings suggest that the WM asymmetries in ACR are heterogeneous and less asymmetry degree in inferior frontal ACR in the normal population might be advantageous for EC of attention. We speculate that WM anisotropy symmetry might be crucial for specific cognitive functions, especially the implement of which employs both hemispheres. Future studies with larger population sample and multi‐modal methods should be applied to further investigate the underlying explanations for the association between brain WM asymmetries and attention function.
Acknowledgements
The authors thank Dr. Lifei Ma (Engineer in GE Healthcare Company, Qingdao, China) for MR scanning support. They also thank Prof. Jin Fan (Department of Psychiatry, Mount Sinai School of Medicine, NY) for supplying and explicating the ANT program.
REFERENCES
- Adolfsdottir S, Sorensen L, Lundervold AJ ( 2008): The attention network test: A characteristic pattern of deficits in children with ADHD. Behav Brain Funct 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani S, Kumar A, Bartzokis G, Sinha U ( 2007): Exploratory voxel‐based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magn Reson Imaging 25: 154–167. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA ( 2004): Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Lawes IN, Mackay CE, Clark CA ( 2007): White matter pathway asymmetry underlies functional lateralization. Cereb Cortex 17: 591–598. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW ( 2007): Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34: 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen‐Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM ( 2003): Characterization and propagation of uncertainty in diffusion‐weighted MR imaging. Magn Reson Med 50: 1077–1088. [DOI] [PubMed] [Google Scholar]
- Bitan T, Lifshitz A, Breznitz Z, Booth JR ( 2010): Bidirectional connectivity between hemispheres occurs at multiple levels in language processing but depends on sex. J Neurosci 30: 11576–11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacz R, Wagenmakers EJ, Forstmann BU, Nieuwenhuis S ( 2010): The neural basis of the speed‐accuracy tradeoff. Trends Neurosci 33: 10–16. [DOI] [PubMed] [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horska A ( 2007): Diffusion tensor imaging in children and adolescents: Reproducibility, hemispheric, and age‐related differences. Neuroimage 34: 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD ( 1999): Conflict monitoring versus selection‐for‐action in anterior cingulate cortex. Nature 402: 179–181. [DOI] [PubMed] [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA ( 2004): White matter asymmetry in the human brain: A diffusion tensor MRI study. Cereb Cortex 14: 945–951. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Suckling J, Overmeyer S, Rabe‐Hesketh S, Taylor E, Brammer MJ ( 1999): Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18: 32–42. [DOI] [PubMed] [Google Scholar]
- Callejas A, Lupianez J, Tudela P ( 2004): The three attentional networks: On their independence and interactions. Brain Cogn 54: 225–227. [DOI] [PubMed] [Google Scholar]
- Cao Y, Whalen S, Huang J, Berger KL, DeLano MC ( 2003): Asymmetry of subinsular anisotropy by in vivo diffusion tensor imaging. Hum Brain Mapp 20: 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL ( 1994): Behavioral‐inhibition, behavioral activation, and affective responses to impending reward and punishment—The bis bas scales. J Personal Soc Psychol 67: 319–333. [Google Scholar]
- Catani M, Allin MP, Husain M, Pugliese L, Mesulam MM, Murray RM, Jones DK ( 2007): Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA 104: 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL ( 2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3: 201–215. [DOI] [PubMed] [Google Scholar]
- Dubois J, Hertz‐Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene‐Lambertz G ( 2009): Structural asymmetries in the infant language and sensori‐motor networks. Cereb Cortex 19: 414–423. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW ( 1974): Effects of noise letters upon identification of a target letter in a nonsearch task. Perception Psychophysics 16: 143–149. [Google Scholar]
- Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI ( 2007a): The relation of brain oscillations to attentional networks. J Neurosci 27: 6197–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu Y, Posner MI ( 2003): Mapping the genetic variation of executive attention onto brain activity. Proc Natl Acad Sci USA 100: 7406–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Kolster R, Ghajar J, Suh M, Knight RT, Sarkar R, McCandliss BD ( 2007b): Response anticipation and response conflict: An event‐related potential and functional magnetic resonance imaging study. J Neurosci 27: 2272–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI ( 2005): The activation of attentional networks. Neuroimage 26: 471–479. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI ( 2002): Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14: 340–347. [DOI] [PubMed] [Google Scholar]
- Fornito A, Wood SJ, Whittle S, Fuller J, Adamson C, Saling MM, Velakoulis D, Pantelis C, Yucel M ( 2008): Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: Associations with cortical thickness, surface area, volume, and sulcal depth. Hum Brain Mapp 29: 222–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yucel M, Wood S, Stuart GW, Buchanan JA, Proffitt T, Anderson V, Velakoulis D, Pantelis C ( 2004): Individual differences in anterior cingulate/paracingulate morphology are related to executive functions in healthy males. Cereb Cortex 14: 424–431. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR ( 2008): Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: A model‐based approach. J Neurosci 28: 9790–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes LJ, Campoy G ( 2008): The time course of alerting effect over orienting in the attention network test. Exp Brain Res 185: 667–672. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Rosen GD, Sherman GF ( 1990): Individual variability in cortical organization: Its relationship to brain laterality and implications to function. Neuropsychologia 28: 529–546. [DOI] [PubMed] [Google Scholar]
- Gamboz N, Zamarian S, Cavallero C ( 2010): Age‐related differences in the attention network test (ANT). Exp Aging Res 36: 287–305. [DOI] [PubMed] [Google Scholar]
- Gannon PJ, Holloway RL, Broadfield DC, Braun AR ( 1998): Asymmetry of chimpanzee planum temporale: Humanlike pattern of Wernicke's brain language area homolog. Science 279: 220–222. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA ( 2002): Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage 17: 1820–1829. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS ( 2000): Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain 123 ( Part 7): 1293–1326. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Rilling JK ( 2008): DTI tractography of the human brain's language pathways. Cereb Cortex 18: 2471–2482. [DOI] [PubMed] [Google Scholar]
- Gong G, Jiang T, Zhu C, Zang Y, Wang F, Xie S, Xiao J, Guo X ( 2005): Asymmetry analysis of cingulum based on scale‐invariant parameterization by diffusion tensor imaging. Hum Brain Mapp 24: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS ( 2001): Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel‐based morphometric analysis of 465 normal adult human brains. Neuroimage 14: 685–700. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Braun JG, Studer JA ( 2006): Attentional network task performance in patients with schizophrenia‐spectrum disorders: Evidence of a specific deficit. Schizophr Res 88( 1–3): 169–178. [DOI] [PubMed] [Google Scholar]
- Haberling IS, Badzakova‐Trajkov G, Corballis MC ( 2011): Callosal tracts and patterns of hemispheric dominance: A combined fMRI and DTI study. Neuroimage 54: 779–786. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, Papanicolaou AC, Fletcher JM, Ewing‐Cobbs L ( 2009): Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res 1276: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock M, Kinsbourne M ( 2011): Attention and the right‐ear advantage: What is the connection? Brain Cogn 76: 263–275. [DOI] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Kreuder F, Schweiger E, Wittling W ( 2009): Hemispheric and gender related differences in the midcingulum bundle: A DTI study. Hum Brain Mapp 30: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L ( 2009): White matter plasticity in the corticospinal tract of musicians: A diffusion tensor imaging study. Neuroimage 46: 600–607. [DOI] [PubMed] [Google Scholar]
- Iturria‐Medina Y, Fernandez AP, Morris DM, Canales‐Rodriguez EJ, Haroon HA, Penton LG, Augath M, Garcia LG, Logothetis N, Parker GJM, et al. ( 2011): Brain hemispheric structural efficiency and interconnectivity rightward asymmetry in human and nonhuman primates. Cerebral Cortex 21: 56–67. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, Lee AD, Barysheva M, McMahon KL, de Zubicaray GI, Martin NG, Wright MJ, Toga AW, Thompson PM ( 2010): Genetic influences on brain asymmetry: A DTI study of 374 twins and siblings. Neuroimage 52: 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Floel A, Deppe M, van Randenborgh J, Drager B, Kanowski M, Knecht S ( 2004): Determining the hemispheric dominance of spatial attention: A comparison between fTCD and fMRI. Hum Brain Mapp 23: 168–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Smith S ( 2001): A global optimisation method for robust affine registration of brain images. Med Image Anal 5: 143–156. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Dagenbach D, Engle CM, Funke LJ ( 2007): Age‐related changes and the attention network task: An examination of alerting, orienting, and executive function. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 14: 353–369. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Della‐Maggiore V, Behrens TE, Smith SM, Paus T ( 2007): Integrity of white matter in the corpus callosum correlates with bimanual co‐ordination skills. Neuroimage 36 ( Suppl 2): T16–T21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X, Herron TJ, Woods DL ( 2011): Regional variation, hemispheric asymmetries and gender differences in pericortical white matter. Neuroimage 56: 2011–2023. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Takahashi T, Nohara S, McGuire PK, Seto H, Kurachi M ( 2008): Anomalous cerebral asymmetry in patients with schizophrenia demonstrated by voxel‐based morphometry. Biol Psychiatry 63: 793–800. [DOI] [PubMed] [Google Scholar]
- Keehn B, Lincoln AJ, Muller RA, Townsend J ( 2010): Attentional networks in children and adolescents with autism spectrum disorder. J Child Psychol Psychiatry 51: 1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmotsu N, Villalobos ME, Gaffrey MS, Courchesne E, Muller RA ( 2005): Activity and functional connectivity of inferior frontal cortex associated with response conflict. Brain Res Cogn Brain Res 24: 335–342. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M ( 1978): Asymmetry and the brain. Science 200: 651–652. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, Bartzokis G, Stanley J, Royall D, Schlosser AE, Null M, Fox PT ( 2010): Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage 49: 1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz‐Dahlmann B ( 2006): Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: Evidence from an event‐related functional magnetic resonance imaging study. Biol Psychiatry 59: 643–651. [DOI] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, Herpertz‐Dahlmann B, Fink GR ( 2005): Development of attentional networks: An fMRI study with children and adults. Neuroimage 28: 429–439. [DOI] [PubMed] [Google Scholar]
- Koziol JA, Wagner S, Sobel DF, Feng AC, Adams HP ( 2005): Asymmetries in the spatial distributions of enhancing lesions and black holes in relapsing‐remitting MS. J Clin Neurosci 12: 895–901. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. ( 2007): White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 130( Part 10): 2508–2519. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Kikinis R, Jolesz FA, McCarley RW, Shenton ME ( 2002): Uncinate fasciculus findings in schizophrenia: A magnetic resonance diffusion tensor imaging study. Am J Psychiatry 159: 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Nestor PG, Wible CG, Frumin M, Maier SE, Kikinis R, Jolesz FA, McCarley RW, Shenton ME ( 2003): Cingulate fasciculus integrity disruption in schizophrenia: A magnetic resonance diffusion tensor imaging study. Biol Psychiatry 54: 1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Dubray MB, Lee JE, Froimowitz MP, Froehlich A, Adluru N, Wright B, Ravichandran C, Fletcher PT, Bigler ED, Alexander AL, Lainhart JE ( 2010): Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res 3: 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C ( 2009): Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp 30: 3563–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Gaser C, Jancke L, Schlaug G ( 2004): A voxel‐based approach to gray matter asymmetries. Neuroimage 22: 656–664. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW ( 2006): Parasagittal asymmetries of the corpus callosum. Cereb Cortex 16: 346–354. [DOI] [PubMed] [Google Scholar]
- Lutz J, Hemminger F, Stahl R, Dietrich O, Hempel M, Reiser M, Jager L ( 2007): Evidence of subcortical and cortical aging of the acoustic pathway: A diffusion tensor imaging (DTI) study. Acad Radiol 14: 692–700. [DOI] [PubMed] [Google Scholar]
- MacDonald AW III, Cohen JD, Stenger VA, Carter CS ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- MacLeod CM ( 1991): Half a century of research on the Stroop effect: An integrative review. Psychol Bull 109: 163–203. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Whiting WL, Bucur B, Provenzale JM, Cabeza R, White LE, Huettel SA ( 2007): Adult age differences in the functional neuroanatomy of visual attention: A combined fMRI and DTI study. Neurobiol Aging 28: 459–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JR, Verghese J, Goldin Y, Lipton R, Holtzer R ( 2010): Alerting, orienting, and executive attention in older adults. J Int Neuropsychol Soc 16: 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, Brown AB, Bush G, Monuteaux MC, Caviness VS, Kennedy DN, Seidman LJ ( 2008): Attention and executive systems abnormalities in adults with childhood ADHD: A DT‐MRI study of connections. Cereb Cortex 18: 1210–1220. [DOI] [PubMed] [Google Scholar]
- Malhotra P, Coulthard EJ, Husain M ( 2009): Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain 132( Part 3): 645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, Gado MH, Barch DM, Csernansky JG ( 2010): Anterior thalamic radiation integrity in schizophrenia: A diffusion‐tensor imaging study. Psychiatry Res 183: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund P, Fransson P, Cabeza R, Larsson A, Ingvar M, Nyberg L ( 2007): Unity and diversity of tonic and phasic executive control components in episodic and working memory. Neuroimage 36: 1361–1373. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H ( 2010): Anatomic dissection of the inferior fronto‐occipital fasciculus revisited in the lights of brain stimulation data. Cortex 46: 691–699. [DOI] [PubMed] [Google Scholar]
- Matchock RL, Toby Mordkoff J ( 2009): Chronotype and time‐of‐day influences on the alerting, orienting, and executive components of attention. Exp Brain Res 192: 189–198. [DOI] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PCM ( 2005): MRI Atlas of Human White Matter. Amsterdam, The Netherlands; San Diego, CA: Elsevier; pp 15–30. [Google Scholar]
- Mori S, Zhang J ( 2006): Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51: 527–539. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M ( 2003): The anatomy of visual neglect. Brain 126( Part 9): 1986–1997. [DOI] [PubMed] [Google Scholar]
- Muhlau M, Gaser C, Wohlschlager AM, Weindl A, Stadtler M, Valet M, Zimmer C, Kassubek J, Peinemann A ( 2007): Striatal gray matter loss in Huntington's disease is leftward biased. Mov Disord 22: 1169–1173. [DOI] [PubMed] [Google Scholar]
- Narr K, Thompson P, Sharma T, Moussai J, Zoumalan C, Rayman J, Toga A ( 2001): Three‐dimensional mapping of gyral shape and cortical surface asymmetries in schizophrenia: Gender effects. Am J Psychiatry 158: 244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME ( 2007): Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res 90( 1–3): 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus AH, Koehler S, Opgen‐Rhein C, Urbanek C, Hahn E, Dettling M ( 2007): Selective anterior cingulate cortex deficit during conflict solution in schizophrenia: An event‐related potential study. J Psychiatr Res 41: 635–644. [DOI] [PubMed] [Google Scholar]
- Niogi S, Mukherjee P, Ghajar J, Johnson CE, Kolster R, Lee H, Suh M, Zimmerman RD, Manley GT, McCandliss BD ( 2008): Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain 131( Part 12): 3209–3221. [DOI] [PubMed] [Google Scholar]
- Niogi S, Mukherjee P, Ghajar J, McCandliss BD ( 2010): Individual differences in distinct components of attention are linked to anatomical variations in distinct white matter tracts. Front Neuroanat 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC ( 2005): Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport 16: 791–794. [DOI] [PubMed] [Google Scholar]
- O'Donnell LJ, Westin CF, Norton I, Whalen S, Rigolo L, Propper R, Golby AJ ( 2010): The fiber laterality histogram: A new way to measure white matter asymmetry. Med Image Comput Comput Assist Interv 13( Part 2): 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechslin MS, Imfeld A, Loenneker T, Meyer M, Jancke L ( 2009): The plasticity of the superior longitudinal fasciculus as a function of musical expertise: A diffusion tensor imaging study. Front Hum Neurosci 3: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Opgen‐Rhein C, Neuhaus AH, Urbanek C, Hahn E, Sander T, Dettling M ( 2008): Executive attention in schizophrenic males and the impact of COMT Val(108/158)Met genotype on performance on the attention network test. Schizophrenia Bull 34: 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME ( 1991): Localization of a human system for sustained attention by positron emission tomography. Nature 349: 61–64. [DOI] [PubMed] [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, Frumin M, Kikinis R, Jolesz FA, McCarley RW, Shenton ME ( 2004): White matter hemisphere asymmetries in healthy subjects and in schizophrenia: A diffusion tensor MRI study. Neuroimage 23: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler‐Kingshott CA, Ciccarelli O, Lambon Ralph MA ( 2005): Lateralization of ventral and dorsal auditory‐language pathways in the human brain. Neuroimage 24: 656–666. [DOI] [PubMed] [Google Scholar]
- Peled S, Gudbjartsson H, Westin CF, Kikinis R, Jolesz FA ( 1998): Magnetic resonance imaging shows orientation and asymmetry of white matter fiber tracts. Brain Res 780: 27–33. [DOI] [PubMed] [Google Scholar]
- Perez‐Iglesias R, Tordesillas‐Gutierrez D, McGuire PK, Barker GJ, Roiz‐Santianez R, Mata I, de Lucas EM, Rodriguez‐Sanchez JM, Ayesa‐Arriola R, Vazquez‐Barquero JL, Crespo‐Facorro B ( 2010): White matter integrity and cognitive impairment in first‐episode psychosis. Am J Psychiatry 167: 451–458. [DOI] [PubMed] [Google Scholar]
- Pessoa L ( 2009): How do emotion and motivation direct executive control? Trends Cogn Sci 13: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI ( 2008): Measuring alertness. Ann NY Acad Sci 1129: 193–199. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE ( 1990): The attention system of the human brain. Annu Rev Neurosci 13: 25–42. [DOI] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler‐Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS ( 2006): Hemispheric asymmetries in language‐related pathways: A combined functional MRI and tractography study. Neuroimage 32: 388–399. [DOI] [PubMed] [Google Scholar]
- Prado J, Carp J, Weissman DH ( 2011): Variations of response time in a selective attention task are linked to variations of functional connectivity in the attentional network. Neuroimage 54: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper RE, O'Donnell LJ, Whalen S, Tie Y, Norton IH, Suarez RO, Zollei L, Radmanesh A, Golby AJ ( 2010): A combined fMRI and DTI examination of functional language lateralization and arcuate fasciculus structure: Effects of degree versus direction of hand preference. Brain Cogn 73: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam MC, Steven MS, Doron KW, Riggall AC, Gazzaniga MS ( 2010): Cortical projection topography of the human splenium: Hemispheric asymmetry and individual differences. J Cogn Neurosci 22: 1662–1669. [DOI] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Siok WT, Zhou K, Khong PL ( 2011): Lateralization of the arcuate fasciculus and its differential correlation with reading ability between young learners and experienced readers: A diffusion tensor tractography study in a chinese cohort. Hum Brain Mapp 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Tan LH, Siok WT, Zhou K, Khong PL. ( 2011): Lateralization of the arcuate fasciculus and its differential correlation with reading ability between young learners and experienced readers: A diffusion tensor tractography study in a chinese cohort. Hum Brain Mapp. doi: 10.1002/hbm.21168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Buhle J ( 2006): Typologies of attentional networks. Nat Rev Neurosci 7: 367–379. [DOI] [PubMed] [Google Scholar]
- Raz A, Fan J, Posner MI ( 2006): Neuroimaging and genetic associations of attentional and hypnotic processes. J Physiol Paris 99( 4–6): 483–491. [DOI] [PubMed] [Google Scholar]
- Reich DS, Smith SA, Jones CK, Zackowski KM, van Zijl PC, Calabresi PA, Mori S ( 2006): Quantitative characterization of the corticospinal tract at 3T. AJNR Am J Neuroradiol 27: 2168–2178. [PMC free article] [PubMed] [Google Scholar]
- Reite M, Teale P, Rojas DC, Arciniegas D, Sheeder J ( 1999): Bipolar disorder: Anomalous brain asymmetry associated with psychosis. Am J Psychiatry 156: 1159–1163. [DOI] [PubMed] [Google Scholar]
- Rodrigo S, Naggara O, Oppenheim C, Golestani N, Poupon C, Cointepas Y, Mangin JF, Le Bihan D, Meder JF ( 2007a): Human subinsular asymmetry studied by diffusion tensor imaging and fiber tracking. AJNR Am J Neuroradiol 28: 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo S, Oppenheim C, Chassoux F, Golestani N, Cointepas Y, Poupon C, Semah F, Mangin JF, Le Bihan D, Meder JF ( 2007b): Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur Radiol 17: 1663–1668. [DOI] [PubMed] [Google Scholar]
- Rudebeck SR, Scholz J, Millington R, Rohenkohl G, Johansen‐Berg H, Lee AC ( 2009): Fornix microstructure correlates with recollection but not familiarity memory. J Neurosci 29: 14987–14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI ( 2005): Training, maturation, and genetic influences on the development of executive attention. Proc Natl Acad Sci USA 102: 14931–14936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Muller‐Oehring EM, Rohlfing T, Pfefferbaum A, Sullivan EV ( 2010): White matter fiber degradation attenuates hemispheric asymmetry when integrating visuomotor information. J Neurosci 30: 12168–12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silton RL, Heller W, Towers DN, Engels AS, Spielberg JM, Edgar JC, Sass SM, Stewart JL, Sutton BP, Banich MT, Miller GA ( 2010): The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top‐down attentional control. Neuroimage 50: 1292–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Berbaum K ( 1990): Effect of conflicting cues on information processing: The 'Stroop effect' vs. the 'Simon effect'. Acta Psychol (Amst) 73: 159–170. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J ( 1999): Storage and executive processes in the frontal lobes. Science 283: 1657–1661. [DOI] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE ( 2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen‐Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM ( 2004): Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23 ( Suppl 1): S208–S219. [DOI] [PubMed] [Google Scholar]
- Snook L, Paulson LA, Roy D, Phillips L, Beaulieu C ( 2005): Diffusion tensor imaging of neurodevelopment in children and young adults. Neuroimage 26: 1164–1173. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V ( 2008): A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proc Natl Acad Sci USA 105: 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Vogel J, Ashtari M, Malhotra AK, Bates J, Kane JM, Bilder RM, Frevert T, Lim K ( 2003): Sex differences in frontal lobe white matter microstructure: A DTI study. Neuroreport 14: 2469–2473. [DOI] [PubMed] [Google Scholar]
- Takao H, Abe O, Yamasue H, Aoki S, Kasai K, Ohtomo K ( 2010a). Cerebral asymmetry in patients with schizophrenia: A voxel‐based morphometry (VBM) and diffusion tensor imaging (DTI) study. J Magn Reson Imaging 31: 221–226. [DOI] [PubMed] [Google Scholar]
- Takao H, Abe O, Yamasue H, Aoki S, Kasai K, Sasaki H, Ohtomo K ( 2010b): Aging effects on cerebral asymmetry: A voxel‐based morphometry and diffusion tensor imaging study. Magn Reson Imaging 28: 65–69. [DOI] [PubMed] [Google Scholar]
- Takao H, Hayashi N, Ohtomo K. 2011a): Effect of scanner in asymmetry studies using diffusion tensor imaging. Neuroimage 54: 1053–1062. [DOI] [PubMed] [Google Scholar]
- Takao H, Abe O, Yamasue H, Aoki S, Sasaki H, Kasai K, Yoshioka N, Ohtomo K. 2011b): Gray and white matter asymmetries in healthy individuals aged 21‐29 years: A voxel‐based morphometry and diffusion tensor imaging study. Hum Brain Mapp 32: 1762–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI ( 2010): Short‐term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci USA 107: 15649–15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, Yu Q, Sui D, Rothbart MK, Fan M, Posner MI ( 2007): Short‐term meditation training improves attention and self‐regulation. Proc Natl Acad Sci USA 104: 17152–17156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell'Acqua F, Allin M, Walshe M, Murray R, Williams SC, Murphy DG, Catani M ( 2011a): Atlasing location, asymmetry and inter‐subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage 54: 49–59. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Forkel S, Simmons A, Vergani F, Murphy Declan G.M, Catani M ( 2011b): A lateralized brain network for visuo‐spatial attention. nature precedings. Available at: < http://hdl.handle.net/10101/npre.2011.5549.1.> [DOI] [PubMed]
- Thiel CM, Zilles K, Fink GR ( 2004): Cerebral correlates of alerting, orienting and reorienting of visuospatial attention: An event‐related fMRI study. Neuroimage 21: 318–328. [DOI] [PubMed] [Google Scholar]
- Thimm M, Kircher T, Kellermann T, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, et al. ( 2010): Effects of a CACNA1C genotype on attention networks in healthy individuals. Psychol Med: 1–11. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM ( 2003): Mapping brain asymmetry. Nat Rev Neurosci 4: 37–48. [DOI] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y ( 1999): Top‐down signal from prefrontal cortex in executive control of memory retrieval. Nature 401: 699–703. [DOI] [PubMed] [Google Scholar]
- Trivedi R, Agarwal S, Rathore RK, Saksena S, Tripathi RP, Malik GK, Pandey CM, Gupta RK ( 2009): Understanding development and lateralization of major cerebral fiber bundles in pediatric population through quantitative diffusion tensor tractography. Pediatr Res 66: 636–641. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD ( 2005): Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci USA 102: 12212–12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umarova RM, Saur D, Schnell S, Kaller CP, Vry MS, Glauche V, Rijntjes M, Hennig J, Kiselev V, Weiller C ( 2010): Structural connectivity for visuospatial attention: Significance of ventral pathways. Cereb Cortex 20: 121–129. [DOI] [PubMed] [Google Scholar]
- Upadhyay J, Hallock K, Ducros M, Kim DS, Ronen I ( 2008): Diffusion tensor spectroscopy and imaging of the arcuate fasciculus. Neuroimage 39: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanek C, Neuhaus AHM, Opgen‐Rhein C, Strathmann S, Wieseke N, Schaub RT, Hahn E, Dettling M ( 2009): Attention network test (ANT) reveals gender‐specific alterations of executive function in schizophrenia. Biol Psychiatry 65: 102–109. [DOI] [PubMed] [Google Scholar]
- Van Hecke W, Leemans A, De Backer S, Jeurissen B, Parizel PM, Sijbers J ( 2010): Comparing isotropic and anisotropic smoothing for voxel‐based DTI analyses: A simulation study. Hum Brain Mapp 31: 98–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JS, Sage CA, Leemans A, Van Hecke W, Callaert D, Peeters R, De Cock P, Lagae L, Sunaert S ( 2010): Construction of a stereotaxic DTI atlas with full diffusion tensor information for studying white matter maturation from childhood to adolescence using tractography‐based segmentations. Hum Brain Mapp 31: 470–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A ( 2007): Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right‐ and left‐handed healthy subjects: A combined fMRI and DTI study. Neuroimage 35: 1064–1076. [DOI] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S ( 2007): Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 36: 630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae‐Poetscher LM, van Zijl PC, Mori S ( 2004): Fiber tract‐based atlas of human white matter anatomy. Radiology 230: 77–87. [DOI] [PubMed] [Google Scholar]
- Wang F, Sun Z, Cui L, Du X, Wang X, Zhang H, Cong Z, Hong N, Zhang D ( 2004): Anterior cingulum abnormalities in male patients with schizophrenia determined through diffusion tensor imaging. Am J Psychiatry 161: 573–575. [DOI] [PubMed] [Google Scholar]
- Wang K, Fan J, Dong Y, Wang CQ, Lee TM, Posner MI ( 2005): Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophr Res 78( 2–3): 235–241. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC ( 2001): Structural asymmetries in the human brain: A voxel‐based statistical analysis of 142 MRI scans. Cereb Cortex 11: 868–877. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Huster RJ, Kreuder F, Wittling W, Schweiger E ( 2007): Corticospinal tract asymmetries at the level of the internal capsule: Is there an association with handedness? Neuroimage 37: 379–386. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Grydeland H, Walhovd KB, Fjell AM ( 2011): Associations between regional cortical thickness and attentional networks as measured by the attention network test. Cereb Cortex 21: 345–356. [DOI] [PubMed] [Google Scholar]
- Witelson SF ( 1985): The brain connection: The corpus callosum is larger in left‐handers. Science 229: 665–668. [DOI] [PubMed] [Google Scholar]
- Yan H, Zuo XN, Wang D, Wang J, Zhu C, Milham MP, Zhang D, Zang Y ( 2009): Hemispheric asymmetry in cognitive division of anterior cingulate cortex: A resting‐state functional connectivity study. Neuroimage 47: 1579–1589. [DOI] [PubMed] [Google Scholar]
- Yu C, Li J, Liu Y, Qin W, Li Y, Shu N, Jiang T, Li K ( 2008): White matter tract integrity and intelligence in patients with mental retardation and healthy adults. Neuroimage 40: 1533–1541. [DOI] [PubMed] [Google Scholar]
- Zhou SY, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, Yamashita I, Seto H, Kurachi M ( 2003): Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol Psychiatry 54: 427–436. [DOI] [PubMed] [Google Scholar]


