Abstract
Cortical gray matter volume and resting state cortical electroencephalographic rhythms are typically abnormal in subjects with amnesic mild cognitive impairment (MCI) and Alzheimer's disease (AD). Here we tested the hypothesis that in amnesic MCI and AD subjects, abnormalities of EEG rhythms are a functional reflection of cortical atrophy across the disease. Eyes‐closed resting state EEG data were recorded in 57 healthy elderly (Nold), 102 amnesic MCI, and 108 AD patients. Cortical gray matter volume was indexed by magnetic resonance imaging recorded in the MCI and AD subjects according to Alzheimer's disease neuroimaging initiative project (http://www.adni-info.org/). EEG rhythms of interest were delta (2–4 Hz), theta (4–8 Hz), alpha1 (8–10.5 Hz), alpha2 (10.5–13 Hz), beta1 (13–20 Hz), beta2 (20–30 Hz), and gamma (30–40 Hz). These rhythms were indexed by LORETA. Compared with the Nold, the MCI showed a decrease in amplitude of alpha 1 sources. With respect to the Nold and MCI, the AD showed an amplitude increase of delta sources, along with a strong amplitude reduction of alpha 1 sources. In the MCI and AD subjects as a whole group, the lower the cortical gray matter volume, the higher the delta sources, the lower the alpha 1 sources. The better the score to cognitive tests the higher the gray matter volume, the lower the pathological delta sources, and the higher the alpha sources. These results suggest that in amnesic MCI and AD subjects, abnormalities of resting state cortical EEG rhythms are not epiphenomena but are strictly related to neurodegeneration (atrophy of cortical gray matter) and cognition. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: Alzheimer's disease neuroimaging initiative (ADNI), amnesic mild cognitive impairment (MCI), resting state electroencephalography (EEG), rhythms, magnetic resonance imaging (MRI), low resolution brain electromagnetic tomography (LORETA), gray matter volume
INTRODUCTION
Elderly subjects with amnesic mild cognitive impairment (MCI) are characterized by an objective impairment of memory, without (single‐domain) or with (multidomain) decline of other cognitive functions; furthermore, the impairment of cognitive and social skills do not yet fulfill the clinical picture of dementia [Petersen et al., 2001, 2004, 2008]. Noteworhty, there was a remarkably higher rate of AD conversion on annual basis in amnesic MCI patients [6–40%; Jack et al., 2005; Petersen et al., 2001] than in normal elderly subjects [0.17–3.86%; Frisoni et al., 2004; Petersen et al., 2001]. However, not all MCI patients progress to dementia, but they can even reverse back to complete normality [Jack et al., 2001; Petersen et al., 1995, 2001].
It is understandable that with such a scenario, a reliable diagnostic/prognostic marker of AD in amnesic MCI subjects would be extremely useful for screening large samples of elderly populations including subjects with subjective memory complaints and amnesic MCI, to immediately start medical and rehabilitation treatments. Because subjective memory complaints and amnesic MCI subjects are quite numerous, still falling within “normal condition,” screening methods should be noninvasive, widely available, and cheap. Along this line, a method based on the analysis of a routine electroencephalogram (EEG) would be an ideal candidate. Resting state eyes closed EEG rhythms probe general neurophysiological mechanisms of cortical neural synchronization, namely the global temporal summation of postsynaptic potentials at the basis of EEG activity. Therefore, quantitative EEG markers may contribute not only to a preliminary instrumental assessment of subjects with subjective memory complaints and amnesic MCI but also to a better understanding of neurophysiological mechanisms affected by neurodegeneration.
Previous studies in AD and MCI subjects have shown that resting state EEG rhythms may be promising markers of disease. When compared to normal elderly (Nold) subjects, AD patients have been characterized by high power of delta (0–4 Hz) and theta (4–7 Hz) rhythms, as well as by low power of posterior alpha (8–12 Hz) and/or beta (13–30 Hz) rhythms [Babiloni et al., 2004; Dierks et al., 1993, 2000; Huang et al., 2000; Jeong, 2004; Koenig et al., 2005; Ponomareva et al., 2003]. With reference to Nold subjects, MCI subjects have displayed increased theta power [Grunwald et al., 2001; Jelic et al., 1997; Zappoli et al., 1995] as well as decreased alpha power [Babiloni et al., 2006b; Elmstahl and Rosen, 1997; Frodl et al., 2002; Grunwald et al., 2001, 2002; Huang et al., 2000; Jelic et al., 1997, 2000; Koenig et al., 2005; Zappoli et al., 1995; for a review see Rossini et al., 2007]. In line with the “transition” hypothesis, power of resting state EEG alpha rhythms was high in Nold subjects, intermediate in MCI subjects, and low in AD patients [Elmstahl and Rosen, 1997; Huang et al., 2000; Jelic et al., 2000; Jeong, 2004].
Practical use of EEG markers for the assessment of amnesic MCI and mild AD subjects would require a preliminary validation study demonstrating that these markers specifically reflect neurodegenerative processes as revealed by brain atrophy. In this regard, structural magnetic resonance imaging (MRI) of the brain provides valid markers of AD state and progression. Brain tissue loss including hippocampal pathway and cerebral cortex correlates well with cognitive deficits in AD subjects both cross‐sectionally and longitudinally [Ezekiel et al., 2004; Frisoni et al., 2009; Jack et al., 2004; Ridha et al., 2006; Schott et al., 2005]. Furthermore, longitudinal pattern of regional brain volume changes has differentiated normal aging from MCI [Driscoll et al., 2009]. Finally, it has been reported evidence of an atrophy of cholinergic pathways and cortical white matter connectivity in MCI subjects when compared to healthy elderly subjects [Teipel et al., 2010] as well as a reduction of cortical gray matter volume [Prestia et al., 2010].
Here we tested the hypothesis that in amnesic MCI and AD subjects, abnormalities of resting state EEG rhythms correlate with cortical gray matter atrophy and cognitive functions. To this aim, eyes‐closed resting EEG rhythms were recorded in Nold, amnesic MCI, and AD subjects. Global cortical gray matter volume was indexed on the basis of the MRIs recorded in amnesic MCI and AD subjects. Power of cortical EEG rhythms was indexed by LORETA software, which has been repeatedly and successfully used to model these rhythms in MCI and AD subjects [Babiloni et al., 2004, 2006a, b, c, d, e].
METHODS
Subjects
In this study, 102 amnesic MCI subjects and 108 AD patients were recruited. Furthermore, 57 cognitively intact elderly (Nold) subjects were used as a control group (Table I). These Nold subjects had been recruited (Isola Tiberina Fatebenefratelli Hospital, Rome; IRCCS Fatebenefratelli Brescia; University “Campus Biomedico” Rome; IRCCS Oasi, Troina) in the framework of previous research projects, and their data were selected to globally match the personal variables of the MCI and AD subjects.
Table I.
Demographic and clinical data of healthy elderly (Nold), amnesic mild cognitive impairment (MCI), and Alzheimer (AD) subjects
| Subjects | Age (years) | IAF | MMSE | IADL | CDR | |
|---|---|---|---|---|---|---|
| Nold | 57 | 73.4 (± 0.9 SE) | 9.2 (± 0.2 SE) | 27.7 (± 0.2 SE) | — | — |
| MCI | 102 | 69.4 (± 1.0 SE) | 9.5 (± 0.1 SE) | 25.8 (± 0.3 SE) | 2.7 (± 0.2 SE) | 0.3 (± 0.08 SE) |
| AD | 108 | 71.0 (± 0.8 SE) | 8.8 (± 0.1 SE) | 19.8 (± 0.5 SE) | 4.2 (± 0.3 SE) | 1.0 (± 0.12 SE) |
The study was approved by the local Institutional ethics committee, and follows prescriptions of the Good Clinical Practice (GCP); informed and overt consent of subjects or subjects' legal representatives, in line with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and the standards established by the Author's Institutional Review Board.
Diagnostic Criteria
The present inclusion and exclusion criteria for amnesic MCI subjects were based on previous seminal reports [Albert et al., 1991; Devanand et al., 1997; Flicker et al., 1991; Petersen, 2004; Petersen et al., 1995, 1997, 2001; Portet et al., 2006; Rubin et al., 1989; Zaudig, 1992]. Summarizing, the inclusion criteria were as follows: (i) objective memory impairment on ADNI neuropsychological evaluation probing cognitive performance in the domains of memory, language, executive function/attention, etc; (ii) normal activities of daily living as documented by the history and evidence of independent living; and (iii) clinical dementia rating score of 0.5. The exclusion criteria included: (i) mild dementia of the AD type, as diagnosed by standard protocols including NINCDS‐ADRDA [McKhann et al., 1984] and DSM‐IV; (ii) evidence (including magnetic resonance imaging—MRI—procedures) of concomitant cerebral impairment such as frontotemporal degeneration, cerebrovascular disease as indicated by Hatchinski ischemic score [Rosen et al., 1980] and MRI vascular lesions, and reversible cognitive impairment (including pseudo‐depressive dementia); (iii) marked fluctuations in cognitive performance [Geser et al., 2005] compatible with Lewy body dementia and/or features of mixed cognitive impairment including cerebrovascular disease (particular attention was devoted to this point given the working hypothesis focused on cognitive stability in MCI subjects); (iv) evidence of concomitant extra‐pyramidal symptoms; (v) clinical and indirect evidence of depression as revealed by the geriatric depression scale [GDS; Yesavage et al., 1982] scores > 14; (vi) other psychiatric diseases, epilepsy, drug addiction, alcohol dependence (as revealed by a psychiatric interview) and use of psychoactive drugs including acetylcholinesterase inhibitors or other drugs enhancing brain cognitive functions; and (vii) current or previous uncontrolled or complicated systemic diseases (including diabetes mellitus) or traumatic brain injuries.
Probable AD was diagnosed according to NINCDS‐ADRDA [McKhann et al., 1984] and DSM IV criteria. Most of the AD patients (more than 95%) followed a long term treatment with standard daily doses of acetylcholinesterase inhibitors. The recruited AD patients underwent general medical, neurological, neuropsychological (ADNI battery), and psychiatric assessments. Patients were rated with a number of standardized diagnostic and severity instruments that included MMSE [Folstein et al., 1975], clinical dementia rating scale [CDR; Hughes et al., 1982], GDS [Yesavage et al., 1982], Hachinski ischemic score [HIS; Rosen et al., 1980], and instrumental activities of daily living scale [IADL; Lawton and Brodie, 1969]. Neuroimaging diagnostic procedures (MRI) and complete laboratory analyses were carried out to exclude other causes of progressive or reversible dementias, to have a clinically homogenous mild AD group. Exclusion criteria included any evidence of (i) frontotemporal dementia, diagnosed according to current criteria [Knopman, 2005], (ii) vascular dementia, diagnosed according to NINDS‐AIREN criteria [Roman et al., 1993], (iii) extrapyramidal syndromes, (iv) reversible dementias (including pseudodementia of depression); and (v) Lewy body dementia, according to the criteria by McKeith [ 2005].
A battery of neuropsychological tests was performed to assess cognitive performance in several domains including memory, language, executive function/attention, and visuo‐construction abilities. The tests to assess memory were the delayed recall of Rey figures [Rey, 1968] and the Prose Memory Test delayed recall of a story [Spinnler and Tognoni, 1987]. The tests to assess language were the 1‐min verbal fluency for letters [Novelli, 1986], and the 1‐min verbal fluency for fruits, animals, or car trades [Novelli, 1986]. The tests to assess executive function and attention were the Trail Making Test part A and B [Reitan, 1958]. Finally, the tests to assess visuo‐construction were the copy of Rey figures [Rey, 1968].
The Nold subjects were recruited mostly from nonconsanguineous relatives of AD patients. All Nold subjects underwent physical and neurological examinations as well as cognitive screening (including MMSE). Among them, those affected by chronic systemic illnesses (i.e., diabetes mellitus or organ failure) were excluded, as were subjects receiving psychoactive drugs. The Nold subjects with history of present or previous neurological or psychiatric disease were also excluded. All Nold subjects had a GDS score lower than 14 (no depression). Unfortunately, MRIs of the Nold subjects were not available for the present study, so we could not compare the MRI indexes between healthy controls and MCI subjects in the present study.
Magnetic Resonance Imaging (MRI)
The 3‐D T1‐weighted volumetric MRI data were recorded by the clinical units of the present Italian multicentric study (University of Foggia‐Ospedali Riuniti di Foggia; San Raffaele Cassino; Isola Tiberina Fatebenefratelli Hospital, Rome; IRCCS Fatebenefratelli Brescia; IRCCS Centro Neurolesi, Messina; Azienda Ospedaliera Sant'Andrea University of Rome “Sapienza”; University of Naples “Federico II”; Second University of Naples; University “Campus Biomedico” Rome; IRCCS and Fondazione SDN Naples; IRCCS Oasi, Troina). Some of these units (IRCCS Centro Neurolesi “Bonino‐Pulejo,” Messina; Azienda Ospedaliera Sant'Andrea University of Rome “Sapienza”; University of Naples “Federico II”; Second University of Naples; University “Campus Biomedico” Rome; IRCCS and Fondazione SDN Naples; IRCCS Oasi, Troina) collected the MRIs following the ADNI protocol (http://www.adni-info.org/). The MRI scans were visually inspected to verify the absence of structural abnormalities or technical artifacts. Centralized MRI data analysis was performed by MATLAB 7.0 (MathWorks, Natick, MA) and SPM2 (Wellcome Dept. Cogn. Neurol., London; http://www.fil.ion.ucl.ac.uk/spm). Specifically, the processing of the MRI data was as follows. First, a study‐specific customized anatomical template was created from the MRIs of all MCI and AD subjects, to reduce any potential bias for the spatial normalization [Good et al., 2001]: the native MRI data of each patient were registered to this specific template, by minimizing the residual sum of squared differences between them [Ashburner and Friston, 1997]. Second, the spatially normalized images were next partitioned into cortical gray matter, white matter, and cerebrospinal fluid compartments; this was done by using a modified mixture model based on cluster analysis technique with a correction for the nonuniformity of MRI intensity [Ashburner and Friston, 1997]. Third, the intensities of the partitioned MRIs were multiplied by the Jacobian determinants from spatial normalization (SPM2), to preserve the original volume of the three compartments. Fourthly, a home‐made MATLAB script was used to calculate the normalized volumes of cortical gray matter, white matter, and cerebrospinal fluid compartments. In this script, the absolute total volume of these compartments was calculated by summing the voxels in the modulated MRIs and multiplying by the voxel volume (i.e., product of voxel size). The normalized volumes were obtained dividing the absolute volume of each compartment (i.e., gray matter, white matter, and cerebrospinal fluid) by the total absolute volume. Of note, the above procedure seemed to be more appropriate than voxel‐based morphometry (VBM) for the analysis of the relationship between low‐resolution (LORETA) EEG source estimates and MRI markers. Indeed, VBM is based on an intrinsically high‐resolution voxel‐by‐voxel approach.
EEG Recordings
EEG data were recorded by the mentioned clinical units in resting state eyes‐closed subjects. These data were acquired (0.3–70 Hz bandpass) from 19 electrodes positioned according to the international 10–20 system (i.e., Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, O2; see Fig. 1). Linked earlobe reference electrode was appreciated but not mandatory, since all recorded EEG data were off‐line rereferenced to common average. To monitor eye movements, the horizontal and vertical electrooculogram (0.3–70 Hz bandpass) was simultaneously recorded. All data were digitalized in continuous recording mode (about 5 min of EEG; 128–256 Hz sampling rate, the sampling rate being fixed in each recording research unit of this multicentric study). In all subjects, EEG recordings were performed in the late morning. To keep constant the level of vigilance, an operator controlled on‐line the subject and the EEG traces, verbally alerting the subject any time there were signs of behavioral and/or EEG drowsiness.
Figure 1.
Recording sites of the 19 scalp electrodes positioned according to the International 10–20 System (i.e., Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2).
Preliminary Analysis of the EEG Data
The recorded EEG data were segmented and analyzed off‐line in consecutive 2 s epochs. We rejected the EEG segments associated with operator's markers indicating drowsiness, verbal warnings, eyes opening, arm/hand movements, or other events disturbing the EEG recordings. Furthermore, the EEG segments with ocular, muscular, and other types of artifacts were preliminarily identified by a computerized automatic procedure. EEG epochs with sporadic blinking artifacts (less than 15% of the total) were then corrected by an autoregressive method [Moretti et al., 2003]. Finally, two independent experimenters—blind to the diagnosis at the time of the EEG analysis—manually confirmed the EEG segments accepted for further analysis. The amount of segments rejected was lower than 20% of the recorded EEG data. Finally, we rereferenced artifact free EEG data to common average for further analysis.
Spectral Analysis of the EEG Data
The digital FFT‐based power spectrum analysis (Welch technique, Hanning windowing function, no phase shift) was evaluated to calculate the individual alpha frequency (IAF) peak, defined as the frequency associated to the strongest EEG power at the extended alpha range [Klimesch, 1999]. Mean IAF peak was 9.3 Hz (±0.2 standard error, SE) in the Nold subjects, 9.3 Hz (±0.1 SE) in the MCI subjects, and 8.6 Hz (±0.3 SE) in the AD subjects. No statistically significant ANOVA differences were found (P > 0.05). However, the IAF peak was used as a covariate (together with age, gender) in the statistics on the spectral coherence. Indeed, the IAF is a frequency of special importance, since it is associated with maximum power of resting eyes‐closed EEG rhythms [Klimesch, 1999]. The above procedure minimized the possibility that small differences in the IAF peak could confound the comparisons among the Nold, MCI, and AD groups. The standard frequency bands of interest were delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), beta 2 (20–30 Hz) and gamma (30–40 Hz), in continuity with a bulk of previous studies on the cortical sources of resting EEG rhythms in pathological aging [Babiloni et al., 2004, 2006a, b, c, d, e]. Choice of the fixed EEG bands did not account for IAF peak. However, this should not affect the results, since more than 90% of the subjects had the IAF peaks within the alpha 1 band (8–10.5 Hz) and the IAF was used as a covariate in the statistical analysis.
Cortical Source of EEG Rhythms as Computed by LORETA
Low resolution electromagnetic source tomography (LORETA) as provided at http://www.unizh.ch/keyinst/NewLORETA/LORETA01.htm was used for the estimation of cortical sources of EEG rhythms [Pascual‐Marqui and Michel, 1994; Pascual‐Marqui et al., 1999, 2002]. LORETA is a source reconstruction technique belonging to a family of linear inverse solution procedures modeling 3D distributions of EEG sources [Pascual‐Marqui et al., 2002]. It has been shown that LORETA was quite efficient when compared to other linear inverse algorithms like minimum norm solution, weighted minimum norm solution or weighted resolution optimization [Pascual‐Marqui et al., 1999; Phillips et al., 2002; Yao and He, 2001]. Furthermore, it has been successfully used by independent research groups in recent EEG studies on AD using the same experimental set up of the present study, including frequency sampling and 19‐electrodes 10–20 system montage [Babiloni et al., 2004, 2006a, b, c, d, e, 2007c; Dierks et al., 2000; Gianotti et al., 2007]. Noteworthy, this electrode montage is considered as an adequate EEG spatial sampling for the estimation of cortical sources of eyes closed resting state EEG rhythms, since these rhythms are widely represented across all human cerebral cortex in contrast to the circumscribed functional topography of event‐related EEG changes (especially at high frequencies) in response to specific sensory or motor events. Therefore, eyes closed resting state EEG rhythms can be properly sampled with a relatively low amount of electrodes, as opposed to the higher spatial sampling required to take into account to the detailed functional topography of event‐related EEG activity. This relatively low‐spatial sampling of EEG rhythms is in line with the fact that LORETA solutions are intrinsically maximally smoothed at source space, due to its regularization procedure [Pascual‐Marqui and Michel, 1994].
LORETA computes 3D linear solutions (LORETA solutions) for the EEG inverse problem within a three‐shell spherical head model including scalp, skull, and brain compartments. The brain compartment is restricted to the cortical gray matter/hippocampus of a head model coregistered to the Talairach probability brain atlas and digitized at the Brain Imaging Center of the Montreal Neurological Institute [Talairach and Tournoux, 1988]. This compartment includes 2,394 voxels (7‐mm resolution), each voxel containing an equivalent current dipole. LORETA computes relative currents for z, x, and y components of any dipole. As a methodological limitation of the present study, EEG electrode positions were not coregistered to individual brain source models. LORETA solutions consisted of voxel z‐current density values able to predict EEG spectral power density at scalp electrodes, being a reference‐free method of EEG analysis, in that one obtains the same LORETA source distribution for EEG data referenced to any reference electrode including common average. A normalization of the data was obtained by normalizing the LORETA current density at each voxel with the power density averaged across all frequencies (0.5–45 Hz) and across all 2,394 voxels of the brain volume. After the normalization, the solutions lost the original physical dimension and were represented by an arbitrary unit scale. As an advantage, this procedure reduced intersubject variability [Leuchter et al., 1993 and Nuwer, 1988]. Other methods of normalization using the principal component analysis are effective for estimating the subjective global factor scale of the EEG data [Hernández et al., 1994].
Solutions of the EEG inverse problem are under‐determined and ill conditioned when the number of spatial samples (electrodes) is lower than that of the unknown samples (current density at each voxel). To properly address this problem, the cortical LORETA solutions predicting scalp EEG spectral power density were regularized to estimate distributed rather than punctual EEG source patterns [Pascual‐Marqui and Michel, 1994; Pascual‐Marqui et al., 1999, 2002]. In line with the low spatial resolution of the adopted technique, we used our MATLAB software to average LORETA solutions across all voxels of a given cortical macroregion of interest (ROI) such frontal, central, parietal, occipital, temporal, and limbic regions of the brain model (Table II lists the ROIs in terms of Brodmann areas as defined within the LORETA source space). This methodological option may minimize the effects of poor LORETA estimates in deep voxels (i.e., including those of the limbic region) at which the estimation of EEG sources could be imprecise, especially using an EEG spatial sampling from 19 electrodes (10–20 system).
Table II.
Brodmann areas included in the cortical regions of interest (ROIs) of the present study. LORETA solutions were collapsed in frontal, central, parietal, occipital, temporal ROIs
| Loreta Brodmann areas into the regions of interest (ROIs) | |
|---|---|
| Frontal | 8, 9, 10, 11, 44, 45, 46, 47 |
| Central | 1, 2, 3, 4, 6 |
| Parietal | 5, 7, 30, 39, 40, 43 |
| Temporal | 20, 21, 22, 37, 38, 41, 42 |
| Occipital | 17, 18, 19 |
| Limbic | 31, 32, 33, 34, 35, 36 |
As a methodological remark, we emphasize that the use of the LORETA package using an average brain model and large macroregions of interest allowed the “confirmation” of the EEG markers extensively used in the our previous studies on MCI and AD subjects [Babiloni et al., 2004, 2006a, b, c, d, e]. This “confirmation” is represented by the present analysis of the relationship between the (LORETA) EEG markers and cortical atrophy as a standard marker of cortical neurodegeneration along the AD process. Another advantage using these procedures is the fact that all clinical units having a digital EEG system and access to Internet could download and use the LORETA freeware to replicate the present results and extend them to their clinical environment. However, LORETA cannot be considered as an international standard or the most scientifically appropriate approach for EEG source estimation. Future studies should validate the present results using more advanced methodological procedures to estimate EEG source of resting state EEG rhythms. Those procedures may include a higher number of electrodes, preprocessing using principal/independent component analysis, realistic subjects' brain models for LORETA source estimation, digitalization of the electrode position, and some mathematical threshold filtering out low‐amplitude LORETA solutions at deep voxels. Furthermore, LORETA solutions at macroregions of interest are not fully independent statistically, as a result of an inverse problem resolution based on EEG rhythms recorded from 19 electrodes. This is a potential source of bias in the data analysis, although previous studies have reported a good spatial correspondence between LORETA solutions and PET findings when the analysis is performed at cortical regions of interest rather than at single voxels [Dierks et al., 2000]. Furthermore, our previous studies using the present procedures of EEG data analysis unveiled that the differences among Nold, MCI, and AD groups were quite specific in frequency bands and regional spatial localization [Babiloni et al., 2004, 2006a, b, c, d, e].
We also used our MATLAB software to collapse all voxels of LORETA solutions across frontal, central, parietal, occipital, temporal, and limbic regions of the brain model to obtain the global LORETA.
Statistical Analysis of the LORETA Solutions
To map the spatial distribution of resting state EEG sources among the Nold, MCI, and AD subjects, the regional LORETA solutions were used as a dependent variable for an ANOVA design using subjects' age, gender, IAF peak, recording unit site, and use or not of the ADNI protocol as covariates. The ANOVA factors (levels) were Group (Nold, MCI, AD), Region of interest (frontal, central, limbic, temporal, parietal, and occipital), and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma). Mauchly's test evaluated the sphericity assumption. Correction of the degrees of freedom was made with the Greenhouse–Geisser procedure. Duncan test was used for post‐hoc comparisons (P < 0.05). The planned post‐hoc testing evaluated the prediction of regional changes in magnitude of the LORETA solutions across the Nold, MCI, and AD subjects. Specifically, we expected a statistical ANOVA effect including the factors Group and Region of interest (P < 0.05).
Statistical analysis aimed at evaluating five main working hypotheses. These hypotheses were the following: (1) LORETA solutions of resting state cortical EEG rhythms show difference in amplitude among the Nold, MCI, and AD subjects; (2) cortical gray matter volume is lower in the AD than MCI subjects; (3) LORETA solutions point to difference in amplitude across the MCI and AD subjects as a function of the normalized gray matter volume; (4) LORETA solutions point to difference in amplitude across the MCI and AD subjects as a function of the neuropsychological data; and (5) normalized gray matter volume points to difference across the MCI and AD subjects as a function of the neuropsychological data.
To test the first working hypothesis, the global LORETA solutions were used as a dependent variable for an ANOVA design using subjects' age, gender, IAF peak and recording unit site as covariates. The ANOVA factors (levels) were Group (Nold, MCI, AD) and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma). Mauchly's test evaluated the sphericity assumption. Correction of the degrees of freedom was made with the Greenhouse–Geisser procedure. Duncan test was used for post‐hoc comparisons (P < 0.05). The planned post‐hoc testing evaluated the prediction of progressive changes in magnitude of the LORETA solutions across the Nold, MCI, and AD subjects. Specifically, the working hypothesis would be confirmed by a statistical ANOVA effect including the factor Group (P < 0.05).
To test the second working hypothesis, the cortical gray matter volume was used as a dependent variable for an ANOVA design using subjects' age, gender, recording unit site, and use or not of the ADNI protocol as covariates (P < 0.05). The ANOVA factor (levels) was Group (MCI, AD). In the same line, the statistical analysis of the cerebrospinal fluid between the MCI and AD subjects was performed as a control of the measurement of the cortical gray matter, since an opposite trend is expected between cerebrospinal fluid and gray matter volume during the neurodegenerative process (i.e., the higher the neurodegeneration, the lower the cortical gray matter, the higher the cerebrospinal fluid). Finally, the same analysis for the white matter volume was reported to give a complete picture of the changes of the main MRI indexes in the AD patients compared to the amnesic MCI subjects.
To test the third working hypothesis, linear correlation was computed between the LORETA solutions and normalized cortical gray matter volume in all MCI and AD patients as a single group as well as in the single groups considered separately. Specifically, the linear correlation was computed with Pearson test (Bonferroni corrected, P < 0.05). As a control analysis, non‐linear correlations were computed evaluating the coefficient of determination r 2 for exponential, logarithmic, and power functions.
To test the fourth and fifth working hypotheses, a linear correlation was computed between the neuropsychological score and LORETA solutions or normalized gray matter volume in all MCI and AD patients as a single group (Pearson test, P < 0.05).
RESULTS
For illustrative purpose, Figure 2 maps the grand average of the normalized LORETA source solutions (i.e., relative power current density) modeling the distributed cortical EEG sources for delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma bands in the Nold, MCI, AD groups. Posterior alpha sources were generally higher in amplitude in the Nold than MCI and AD group, whereas the opposite is true for the posterior delta sources.
Figure 2.
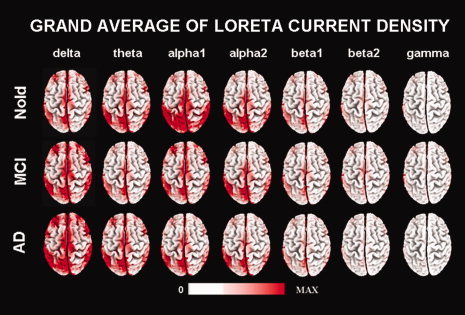
Grand average of LORETA solutions (i.e., normalized relative current density at the cortical voxels) modeling the distributed EEG sources for delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma bands in Nold, MCI, AD groups. The left side of the maps (top view) corresponds to the left hemisphere. Legend: LORETA, low resolution brain electromagnetic tomography. Color scale: all power density estimates were scaled based on the averaged maximum value (i.e., alpha 1 power value of occipital region in Nold).
Figure 3 shows the grand average of regional normalized LORETA source solutions (i.e., relative power current density averaged with each ROI) relative to an ANOVA interaction (F(60,7920) = 9.76; P < 0.0001) among the factors Group (Nold, MCI, AD), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and ROI (frontal, central, parietal, occipital, temporal, limbic). Subjects' age, gender, IAF peak, and recording unit site were used as covariates. Planned post‐hoc testing disclosed the pattern Nold>MCI>AD for the parietal, occipital, and limbic alpha 1 sources (P < 0.0001). Furthermore, central, frontal, parietal, occipital, temporal, and limbic delta sources were lower in amplitude in the Nold and MCI than in the AD groups (P < 0.00005). No statistically significant difference was observed in the delta sources between the Nold and MCI groups (P < 0.05). Table III reports the mean and standard error (SE) values of regional LORETA solutions for the Group (Nold, MCI, AD), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and ROI (frontal, central, parietal, occipital, temporal, limbic).
Figure 3.
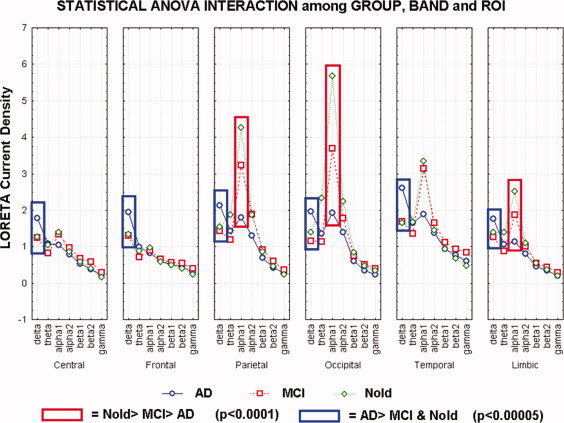
Statistical ANOVA interaction (F(60,7920) = 9.76; P < 0.0001) among the factors Group (Nold, MCI, AD), Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and ROI (frontal, central, parietal, occipital, temporal, limbic).
Table III.
Mean and standard error (SE) values of regional LORETA solutions for the group (Nold, MCI, AD), band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma), and ROI (frontal, central, parietal, occipital, temporal, limbic)
| Band | ROI | AD | MCI | Nold | |||
|---|---|---|---|---|---|---|---|
| Media | ±SE | Media | ±SE | Media | ±SE | ||
| Delta | Central | 1.78 | 0.11 | 1.24 | 0.09 | 1.27 | 0.06 |
| Frontal | 1.94 | 0.10 | 1.30 | 0.07 | 1.34 | 0.06 | |
| Parietal | 2.13 | 0.12 | 1.44 | 0.09 | 1.54 | 0.08 | |
| Occipital | 1.95 | 0.13 | 1.16 | 0.07 | 1.40 | 0.07 | |
| Temporal | 2.61 | 0.12 | 1.68 | 0.07 | 1.66 | 0.06 | |
| Limbic | 1.75 | 0.08 | 1.27 | 0.05 | 1.40 | 0.06 | |
| Theta | Central | 1.08 | 0.08 | 0.83 | 0.06 | 1.04 | 0.10 |
| Frontal | 0.98 | 0.05 | 0.71 | 0.04 | 0.88 | 0.07 | |
| Parietal | 1.44 | 0.11 | 1.20 | 0.10 | 1.88 | 0.26 | |
| Occipital | 1.35 | 0.10 | 1.14 | 0.10 | 2.34 | 0.41 | |
| Temporal | 1.64 | 0.09 | 1.36 | 0.11 | 1.67 | 0.19 | |
| Limbic | 1.06 | 0.06 | 0.88 | 0.06 | 1.39 | 0.18 | |
| Alpha1 | Central | 1.04 | 0.09 | 1.33 | 0.12 | 1.39 | 0.13 |
| Frontal | 0.82 | 0.06 | 0.89 | 0.06 | 0.97 | 0.08 | |
| Parietal | 1.79 | 0.16 | 3.22 | 0.30 | 4.25 | 0.47 | |
| Occipital | 1.92 | 0.18 | 3.69 | 0.43 | 5.68 | 0.75 | |
| Temporal | 1.89 | 0.13 | 3.15 | 0.28 | 3.34 | 0.33 | |
| Limbic | 1.14 | 0.08 | 1.86 | 0.18 | 2.51 | 0.26 | |
| Alpha2 | Central | 0.78 | 0.07 | 0.97 | 0.07 | 0.85 | 0.11 |
| Frontal | 0.60 | 0.04 | 0.65 | 0.03 | 0.58 | 0.05 | |
| Parietal | 1.30 | 0.13 | 1.89 | 0.14 | 1.87 | 0.19 | |
| Occipital | 1.40 | 0.20 | 1.78 | 0.14 | 2.23 | 0.32 | |
| Temporal | 1.38 | 0.16 | 1.64 | 0.08 | 1.44 | 0.12 | |
| Limbic | 0.81 | 0.08 | 1.01 | 0.06 | 1.10 | 0.12 | |
| Beta1 | Central | 0.53 | 0.04 | 0.68 | 0.04 | 0.59 | 0.05 |
| Frontal | 0.50 | 0.03 | 0.56 | 0.03 | 0.48 | 0.04 | |
| Parietal | 0.70 | 0.05 | 0.91 | 0.04 | 0.87 | 0.07 | |
| Occipital | 0.61 | 0.05 | 0.75 | 0.04 | 0.85 | 0.07 | |
| Temporal | 0.94 | 0.06 | 1.12 | 0.05 | 0.94 | 0.07 | |
| Limbic | 0.46 | 0.03 | 0.54 | 0.02 | 0.54 | 0.04 | |
| Beta2 | Central | 0.38 | 0.02 | 0.57 | 0.04 | 0.39 | 0.04 |
| Frontal | 0.46 | 0.03 | 0.54 | 0.04 | 0.40 | 0.04 | |
| Parietal | 0.43 | 0.03 | 0.60 | 0.03 | 0.46 | 0.04 | |
| Occipital | 0.35 | 0.02 | 0.52 | 0.04 | 0.47 | 0.06 | |
| Temporal | 0.77 | 0.06 | 0.94 | 0.06 | 0.68 | 0.08 | |
| Limbic | 0.34 | 0.02 | 0.44 | 0.02 | 0.36 | 0.03 | |
| Gamma | Central | 0.21 | 0.02 | 0.29 | 0.02 | 0.17 | 0.02 |
| Frontal | 0.29 | 0.02 | 0.39 | 0.03 | 0.23 | 0.02 | |
| Parietal | 0.26 | 0.02 | 0.36 | 0.03 | 0.23 | 0.03 | |
| Occipital | 0.23 | 0.02 | 0.41 | 0.04 | 0.34 | 0.06 | |
| Temporal | 0.61 | 0.06 | 0.85 | 0.07 | 0.48 | 0.07 | |
| Limbic | 0.20 | 0.01 | 0.30 | 0.02 | 0.20 | 0.02 | |
Figure 4 maps the grand average of the global LORETA source solutions (i.e., relative power current density averaged across all cortical voxels ± SE) modeling the distributed cortical EEG sources for delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma bands in the Nold, MCI, and AD groups. Subjects' age, gender, IAF peak, and recording unit site were used as covariates. In line with the above analysis for regions of interest, the Nold group presented alpha 1 sources with the maximal values of amplitude. Delta, theta, and alpha 2 sources had lower amplitude values when compared to alpha 1 sources. Finally, beta 1, beta 2 and gamma sources were characterized by lowest amplitude values. Compared to the Nold group, the MCI group showed a decrease in amplitude of alpha 1 sources. With respect to the Nold and MCI groups, the AD group showed an amplitude increase of widespread delta sources, along with a strong amplitude reduction of alpha 1 sources.
Figure 4.
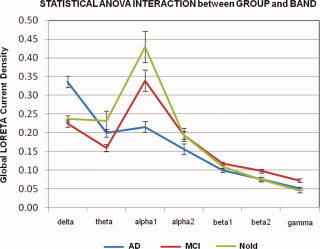
Grand average of Global low resolution brain electromagnetic tomography (LORETA) solutions (i.e., relative power current density averaged across all cortical voxels ± standard error, SE) modeling the distributed cortical EEG sources for delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma bands in normal elderly (Nold), amnesic mild cognitive impairment (MCI), and Alzheimer's disease (AD) subjects. The LORETA values refer to an ANOVA interaction (F(12,1542) = 13.87; P < 0.00001) between the factors Group (Nold, MCI, AD) and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Statistical analysis of the above global LORETA source solutions showed a statistically significant ANOVA interaction (F(12,1542) = 13.87; P < 0.00001) between the factors Group (AD, MCI, Nold) and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma). Subjects' age, gender, IAF peak and recording unit site were used as covariates. The planned post‐hoc testing indicated that the alpha 1 sources were higher in amplitude in the Nold than in the MCI group, and in the MCI than in the AD group (P < 0.00001); these results disclosed the pattern Nold>MCI>AD for the alpha sources. Furthermore, the delta sources were lower in amplitude in the Nold and MCI than in the AD groups (P < 0.00001), in line with the pattern Nold≠ AD. No statistically significant differences were observed in the delta sources between the Nold and MCI groups (P < 0.05).
Table IV reports the results of the analyses of the cortical gray matter, white matter, and cerebrospinal fluid volumes in the MCI vs. AD subjects. As expected, there were significantly higher values of gray and white matter volumes in the MCI than AD subjects (P < 0.005), whereas the opposite was true for the cerebrospinal fluid volume.
Table IV.
White matter, gray matter, and cerebrovascular fluid volumes (in ml) for the amnesic mild cognitive impairment (MCI), and Alzheimer (AD) subjects
| Subjects | Gray matter volume (ml) | White matter volume (ml) | Cerebrospinal fluid (ml) | |
|---|---|---|---|---|
| AD | 108 | 498.1 (± 6.0 SE) | 406.7 (± 6.0 SE) | 766.9 (± 13.2 SE) |
| MCI | 102 | 566.3 (± 7.5 SE) | 432.5 (± 5.8 SE) | 652.1 (± 11.2 SE) |
| MCI≠AD | P = 0.00001 | P = 0.005 | P = 0.00001 | |
The last line indicates the statistical significant trend of MCI values higher than AD ones.
As mentioned above, the power of delta and alpha 1 sources showed statistically significant differences in the Nold subjects when compared to the MCI and AD subjects (P < 0.05). Therefore, these sources were selected for the correlation analyses with the normalized cortical gray matter volume in the continuum of the MCI and AD subjects.
Concerning the linear correlation analysis between the LORETA source solutions and the normalized cortical gray matter volume, a negative correlation was observed between the central, frontal, parietal, occipital, temporal, and limbic delta sources and the normalized cortical gray matter volume (from r = ‐0.2173, P < 0.005, to r = ‐0.1362, P < 0.05). Furthermore, there was a positive correlation between the parietal, occipital, and limbic alpha 1 sources and the normalized gray matter volume (from r = 0.2039, P < 0.005, to r = ‐0.2702, P < 0.0001). The correlation values for any region of interest are reported in the Table V.
Table V.
P and r values of correlation analysis between individual regional LORETA solutions and normalized cortical gray matter volume in AD and MCI subjects as a single group
| Central delta | Frontal delta | Parietal delta | Occipital delta | Temporal delta | Limbic delta | Parietal alpha1 | Occipital alpha1 | Limbic alpha1 | |
|---|---|---|---|---|---|---|---|---|---|
| Normalized gray matter volume (N = 210) | r = −0.15; P < 0.05 | r = −0.16; P < 0.01 | r = −0.16; P < 0.01 | r = −0.14; P < 0.05 | r = −0.22; P < 0.001 | r = −0.28; P < 0.01 | r = 0.20; P < 0.005 | r = 0.21; P < 0.001 | r = 0.26; P < 0.0001 |
With regard to the linear correlation analysis between the global LORETA source solutions and the normalized cortical gray matter volume, a negative correlation was observed between the global delta sources and normalized cortical gray matter volume (r = ‐0.209, P = 0.005). Furthermore, there was a positive correlation between the global alpha 1 sources and the normalized gray matter volume in the MCI and AD subjects considered as a whole group (r = 0.26, P = 0.0001). Figure 5 plots the scatterplots of the above results on the global LORETA source solutions (MCI and AD groups are distinguished by different colors, while the regression line is in black). The lower the normalized cortical gray matter volume, the higher the delta sources, the lower the alpha 1 sources. Of note, the above linear correlations were statistically significant (P < 0.05) even if the r values were relatively low in amplitude, especially at global delta band. This may be due to the complexity of the relationships between the delta or alpha sources and the normalized cortical gray matter volume, clearly beyond the linearity. For example, the scatterplot of Figure 5 shows a relatively large variance of the global delta sources for low values of normalized global cortical gray matter. This suggests a nonlinearity of the relationships between the delta and the normalized cortical gray matter volume. Figure 5 also plots the regression lines for each group considered separately. Of note, the linear correlation for the single groups was statistically significant (P < 0.05) only for the MCI subjects at global alpha 1 band (r = 0.26, P = 0.01). The lower the normalized cortical gray matter volume, the lower the alpha 1 sources. No significant correlation (P > 0.05) was observed between the global delta/alpha 1 sources and the normalized cortical gray matter volume in the AD group.
Figure 5.
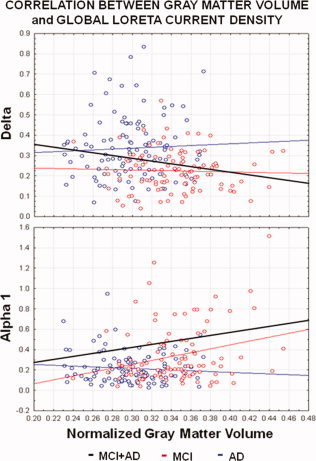
Scatterplots of LORETA solutions for the delta or alpha 1 rhythms and cortical gray matter volume values in the amnesic MCI (in red) and AD (in blue) subjects. For each group, the regression line is plotted in color. The regression line of the amnesic MCI and AD subjects considered as a whole group is plotted in black. Statistical values of linear correlation were obtained by Pearson test (P < 0.005). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Noteworthy, the neuropsychological score of several tests was correlated to the LORETA source solutions and to the normalized cortical gray matter volume in the continuum of the MCI and AD subjects as a single group (Pearson test, P < 0.05). The source solutions were those showing statistically significant differences in the Nold subjects when compared to the MCI and AD subjects (P < 0.05). Results showed that the score to MMSE, prose memory, figure Rey recall, verbal fluency for letter, and verbal fluency for category tests negatively correlated to the pathological delta sources, whereas the correlation was positive between the score to MMSE or verbal fluency for category tests and alpha 1 sources (Tables VI and VII). Furthermore, the score to MMSE, trail making (part A, B, B–A), figure Rey copy, verbal fluency for letter, and verbal fluency for category tests correlated to the normalized cortical gray matter volume, namely the higher this volume, the better the cognitive performance (Table VIII).
Table VI.
P and r values of correlation analysis between individual regional LORETA solutions and neuropsychological data in AD and MCI subjects as a single group
| Cognitive function | Neuropsychological test | Central delta | Frontal delta | Parietal delta | Occipital delta | Temporal delta | Limbic delta | Parietal alpha1 | Occipital alpha1 | Limbic alpha1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Global cognitive function | MMSE (N = 143) | r = −0.38; P < 0.0001 | r = −0.30; P < 0.0001 | r = −0.40; P < 0.0001 | r = −0.37; P < 0.0001 | r = −0.28; P < 0.001 | r = −0.26; P < 0.001 | r = 0.30; P < 0.0001 | r = 0.27; P < 0.001 | r = 0.33; P < 0.0001 |
| Structural verbal memory | Prose memory (N = 143) | r = −0.19; P < 0.01 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Executive function | Trail making test part A (N = 122) | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Executive function | Trail making test part B (N = 122) | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Executive function | Trail making test B‐A (N = 122) | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Visuospatial and visuoconstructive abilities | Figure Rey copy (N = 143) | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Visuospatial memory | Figure Rey recall (N = 143) | r = −0.19; P < 0.01 | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. | N.S. |
| Language production | Verbal fluency for letter (N = 143) | r = −0.25; P < 0.005 | r = −0.19; P < 0.05 | r = −0.21; P < 0.01 | r = −0.28; P < 0.001 | r = −0.18; P < 0.05 | r = −0.22; P < 0.05 | N.S. | N.S. | N.S. |
| Language production | Verbal fluency for category (N = 143) | r = −0.35; P < 0.0001 | r = −0.24; P < 0.005 | r = −0.30; P < 0.0001 | r = −0.29; P < 0.0001 | r = −0.18; P < 0.05 | r = −0.22; P < 0.005 | r = 0.22; P < 0.01 | r = 0.20; P < 0.01 | r = 0.29; P < 0.0001 |
Table VII.
P and r values of correlation analysis between global LORETA solutions and neuropsychological data in AD and MCI subjects as a single group
| Cognitive function | Neuropsychological test | Global delta | Global alpha1 |
|---|---|---|---|
| Global cognitive function | MMSE (N = 143) | r = −0.37; P <0.0001 | r = 0.33; P <0.0001 |
| Structural verbal memory | Prose memory (N = 143) | N.S. | N.S. |
| Executive function | Trail making test part A (N = 122) | N.S. | N.S. |
| Executive function | Trail making test part B (N = 122) | N.S. | N.S. |
| Executive function | Trail making test A–B (N = 122) | N.S. | N.S. |
| Visuospatial and visuoconstructive abilities | Figure Rey copy (N = 143) | N.S. | N.S. |
| Visuospatial memory | Figure Rey recall (N = 143) | N.S. | N.S. |
| Language production | Verbal fluency for letter (N = 143) | r = −0.23; P < 0.005 | N.S. |
| Language production | Verbal fluency for category (N = 143) | r = −0.29; P < 0.001 | r = 0.28; P < 0.001 |
Table VIII.
P and r values of correlation analysis between normalized cortical gray matter volume and neuropsychological data in AD and MCI subjects as a single group
| Cognitive function | Neuropsychological test | Normalized Gray matter volume |
|---|---|---|
| Global cognitive function | MMSE (N = 143) | r = 0.51; P < 0.0001 |
| Structural verbal memory | Prose memory (N = 143) | N.S. |
| Executive function | Trail making test part A (N = 122) | r = −0.20; p<0.01 |
| Executive function | Trail making test part B (N = 122) | r = −0.21; P < 0.01 |
| Executive function | Trail making test A–B (N = 122) | r = −0.18; P < 0.05 |
| Visuospatial and visuoconstructive abilities | Figure Rey copy (N = 143) | r = 0.24; P < 0.005 |
| Visuospatial memory | Figure Rey recall (N = 143) | N.S. |
| Language production | Verbal fluency for letter (N = 143) | r = 0.27; P < 0.001 |
| Language production | Verbal fluency for category (N = 143) | r = 0.38; P < 0.0001 |
Control Analyses
We performed a first control analysis to test whether the above statistical results were influenced by the presence of ADNI and non‐ADNI data sets in the MCI and AD groups. We divided the MCI and AD group in ADNI (15 MCI and 75 AD) and non‐ADNI (87 MCI and 33 AD) subgroups. Statistical analysis of the cortical gray matter volume showed no statistically significant difference (F(1.100) = 0.22; P < 0.6) between MCI ADNI and MCI non‐ADNI subgroups. The same was true in the AD patients, namely no statistically significant difference (F(1.106) = 3.03; P < 0.0844) between the AD ADNI and AD non‐ADNI subgroups.
A second control analysis was performed to ascertain if the results of the main statistical analysis was affected by the factor ROI. For each region of interest (frontal, central, limbic, temporal, parietal, occipital, and limbic), the LORETA solutions were used as a dependent variable for an ANOVA design using subjects' age, gender, IAF peak, and recording unit site as covariates. The ANOVA factors (levels) were Group (Nold, MCI, AD) and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, gamma). As expected, the control ANOVAs showed the well known abnormalities of delta and alpha sources. Specifically, the results disclosed the pattern Nold>MCI>AD for the alpha 1 sources in the parietal, occipital, and limbic regions of interest (P < 0.005). Furthermore, the delta sources were lower in amplitude in the Nold and MCI than in the AD groups in the central, frontal, parietal, temporal, and limbic regions of interest (P < 0.01). These results globally confirmed those of the main analysis.
A third control analysis was performed to test whether the correlation results on the global delta and alpha 1 sources were confirmed by the correlation analysis between the regional delta and alpha 1 sources and normalized cortical gray matter volume in the single groups (AD and MCI) considered separately. No correlation was observed in the AD group (P > 0.05). In the MCI group, we observed a negative correlation between central, frontal, parietal, occipital, temporal, and limbic delta sources and the normalized gray matter volume (from r = ‐0.270, P < 0.005, to r = ‐0.177, P < 0.05). Furthermore, there was a positive correlation between the central, parietal, occipital, temporal, and limbic alpha 1 sources and the normalized gray matter volume (from r = ‐0.294, P < 0.001, to r = ‐0.176, P < 0.05). The correlation values for any region of interest are reported in the Table IX.
Table IX.
P and r values of correlation analysis between individual regional LORETA solutions and normalized cortical gray matter volume in the amnesic MCI and AD groups considered separately
| Correlation between regional LORETA current density and normalized gray matter volume | ||
|---|---|---|
| AD (N=108) | MCI (N=108) | |
| Central delta loreta current density | r = 0.05 | r = −0.246 |
| P < 1 | P < 0.005 | |
| Frontal delta loreta current density | r = 0.08 | r = −0.27 |
| P < 0.5 | P < 0.005 | |
| Parietal delta loreta current density | r = 0.004 | r = −0.198 |
| P < 1 | P < 0.05 | |
| Occipital delta loreta current density | r = 0.0005 | r = −0.177 |
| P < 1 | P < 0.05 | |
| Temporal delta loreta current density | r = 0.03 | r = −0.270 |
| P < 1 | P < 0.05 | |
| Limbic delta loreta current density | r = 0.02 | r = −0.288 |
| P < 1 | P < 0.001 | |
| Central alpha 1 loreta current density | r =‐ −0.045 | r = 0.176 |
| P < 1 | P < 0.05 | |
| Frontal alpha 1 loreta current density | r = 0.074 | r = 0.162 |
| P < 0.5 | P < 0.1 | |
| Parietal alpha 1 loreta current density | r = −0.145 | r = 0.225 |
| P < 0.5 | P < 0.01 | |
| Occipital alpha 1 loreta current density | r = −0.156 | r = 0.243 |
| P < 0.5 | P < 0.005 | |
| Temporal alpha 1 loreta current density | r = −0.075 | r = 0.294 |
| P < 0.5 | P < 0.001 | |
| Limbic alpha 1 loreta current density | r = −0.098 | r = 0.291 |
| P < 0.5 | P < 0.001 | |
The main results suggest a not merely linear relationship between the MRI and EEG indexes used in the present study. To address this issue, the present control analyses computed the values of r 2 for some nonlinear correlations (logarithmic, exponential, power) between the normalized cortical gray matter volume and the above regional and global delta/alpha 1 sources in the MCI and AD subjects as a whole group, as well as in the single groups considered separately. Results showed that the values of r 2were quite similar in the linear and nonlinear correlations. Namely, (1) the values of r 2 for the nonlinear correlations ranged from r 2 = 0.01 to r 2 = 0.06, while the values of r 2 for the (Pearson) linear correlations ranged from r 2 = 0.02 to r 2 = 0.07, in the MCI and AD subjects as a whole group; (2) the values of r 2 for the nonlinear correlations ranged from r 2 = 0.0000005 to r 2 = 0.07, while the values of r 2 for the (Pearson) linear correlations ranged from r 2 = 0.001 to r 2 = 0.07 in the MCI group; and (3) the values of r 2 for the nonlinear correlations ranged from r 2 = 0.00000004 to r 2 = 0.02, while the values of r 2 for the (Pearson) linear correlations ranged from r 2 = 0.0000002 to r 2 = 0.02 in the AD group. These results are in line with the hypothesis of no statistical indication for a preponderant linear over nonlinear correlation between the MRI and EEG indexes used in the present study.
DISCUSSION
Here we tested the hypothesis that in amnesic MCI and AD subjects, abnormalities of the resting state EEG rhythms are statistically correlated to global cortical gray matter atrophy and cognitive functions. In this regard, one might claim that cortical gray matter volume as a whole represents a rough index of neurodegeration in AD. Indeed, AD is supposed to be a multifocal process that along its time evolution selectively impacts upon specific neuronal tracks rather than affecting the whole cortical mantle [Braak and Braak, 1996]. However, the global structural and functional indexes are quick to be automatically computed toward clinical applications. In other words, these global indexes can be computed by friendly and automated software toward desirable clinical applications, reducing the variance induced by the measurement of small brain regions from MRIs recorded by different scanners.
In the present study, cortical gray matter volume was basically indexed by MRIs recorded in amnesic MCI and AD subjects according to the ADNI project when possible (http://www.adni-info.org/). We followed the ADNI protocol, since it represents the most advanced attempt to standardize neuroimaging exam and neuropsychological assessment of aged people across different clinical units and equipments. This methodological attempt is the basis for the collection of biomarkers and neuroimaging data with standardized protocols that allow pooling data across wide multicentric studies (i.e., validation of new markers, clinical trials). As expected, the present results showed that cortical gray matter volume was lower in AD than amnesic MCI subjects. Unfortunately, the MRIs of age‐matched healthy elderly subjects were not available, so we could not perform the comparison of the mentioned MRI index between healthy and amnesic MCI subjects. This would have been a very useful control to characterize the features of the healthy and amnesic MCI subjects involved in this study, in line with previous evidence showing less cortical gray matter volume in MCI than healthy elderly subjects [Prestia et al., 2010]. In the present study, we only used the EEG indexes to preliminarily demonstrate the differences between healthy controls and amnesic MCI subjects. To this purpose, resting state cortical EEG rhythms were indexed by LORETA software averaging power density across all voxels of the cortical gray matter model. Compared with the Nold group, the amnesic MCI group showed a decrease in amplitude of low frequency alpha sources (8–10.5 Hz). With respect to the Nold and amnesic MCI groups, the AD group showed an amplitude increase of delta sources (2–4 Hz), along with a strong amplitude reduction of low frequency alpha sources. The present results are globally in line with previous evidence showing an enhancement of the delta rhythms in AD compared to Nold subjects [Babiloni et al., 2004a,b,c; Koenig et al., 2005, Prichep et al., 1994; Wolf et al., 2003], and a magnitude decrease of the alpha rhythms in AD and/or MCI compared to Nold subjects [Babiloni et al., 2004a,b,c; Dierks et al., 1993, 2000; Koenig et al., 2005; Moretti et al., 2004; Rodriguez et al., 1999a, b; for a review Rossini et al., 2007]. Of note, the present findings validated subjects' selection and EEG data analysis, thus corroborating the novel results of the present study, namely the relationship among cortical gray matter volume and EEG sources in the shadow zone between amnesic MCI and overt AD. From a physiological point of view, the present results extend previous evidence showing that amnesic MCI subjects with different degrees of hippocampal atrophy were characterized by a diverse power of resting state EEG rhythms, especially at dominant alpha frequencies [Babiloni et al., 2009; Moretti et al., 2007].
As a main result of the present study, we report the correlation between global cortical gray matter volume and delta/alpha sources in the amnesic MCI and AD groups. The magnitude of these sources correlated with the cortical gray matter volume in the MCI and AD subjects considered as a whole group, as well as in the separated single groups. In the amnesic MCI and AD subjects considered as a whole group, the lower the cortical gray matter volume, the higher the pathological delta sources, the lower the low frequency alpha sources. As reported in the Results section, these linear correlations were statistically significant (P < 0.05) but relatively low in amplitude, especially at global delta band. This may be due to the complex relationships occurring between EEG sources and global cortical atrophy. For example, we observed a relatively large variance of the global delta sources for high values of global cortical atrophy. Furthermore, there was no statistically significant correlation (P > 0.05) between global cortical gray matter volume and delta sources in the amnesic MCI and AD groups considered separately. Instead, we observed a statistically significant correlation (P < 0.005) between global cortical gray matter volume and low‐frequency alpha sources in the amnesic MCI group (but not in the AD group). Furthermore, we have to notice that in the AD group, the correlation values between the global cortical atrophy and global EEG sources considering the single groups separately did not fully confirm the correlation values found considering the groups together. Specifically, there was no statistically significant relationship between these two indexes in the AD group. When the regional EEG sources were used as an input for the correlation analysis, there were statistically significant correlations (P < 0.05) between cortical gray matter volume and several regional delta and low‐frequency alpha sources in the amnesic MCI group. These results suggest a variable and complex relationship between delta sources and cortical neurodegeneration in the shadow zone between amnesic MCI and overt AD subjects, which suggests the need of a more detailed spatial analysis of the delta sources (even if time consuming) for the correlation with cortical atrophy. It can be speculated that the poor correlation between the delta sources and cortical atrophy in AD subjects is due to the complexity of the parallel neurophysiopathological and neurovascular effects of advanced neurodegenerative processes on the mechanisms generating the resting state EEG rhythms. Furthermore, the present results suggest that low‐frequency alpha sources are quite sensitive to the early stages of global cortical neurodegeneration in amnesic MCI subjects and should be further considered for possible future clinical applications.
Why is global power of resting state delta and low frequency alpha rhythms related to the cortical gray matter volume in the shadow zone between amnesic MCI and AD subjects? The present results raise the issue of cerebral systems that produce and modulate delta and alpha rhythms in normal conditions. In the condition of slow‐wave sleep, corticofugal slow oscillations (<1 Hz) are effective in grouping thalamic‐generated delta rhythms (1–4 Hz) and spindling activity (7–14 Hz) rhythms (Steriade, 2003). In the condition of brain arousal, spindles as well as high and low components of the delta rhythms are blocked by the inhibition of oscillators within, respectively, reticulo‐thalamic (7–14 Hz), thalamo‐cortical (1–4 Hz), and intracortical (<1 Hz), neuronal circuits. These rhythms are replaced by fast (beta and gamma) cortical oscillations, which are mainly induced by forebrain (nucleus basalis) cholinergic inputs to hippocampus and cortex as well as by thalamocortical projections [Steriade, 2003 and Steriade et al., 1996]. In the condition of awake rest, low frequency (8–10.5 Hz) alpha would be mainly related to subject's global attentional readiness [Klimesch, 1996; Klimesch et al., 1997, 1998; Rossini et al., 1991; Steriade and Llinas, 1988]. Noteworthy, there is consensus that alpha rhythms represent the dominant resting oscillations of the adult, awake human brain [Rossini et al., 1991; Steriade and Llinas, 1988; Klimesch, 1996; Klimesch et al., 1997, 1998], and have been linked to intelligence quotient, memory, and cognition [Klimesch, 1999].
An important neuroanatomical substrate of resting state alpha rhythms is the cholinergic innervation from basal forebrain to cerebral cortex, which may play an important role in preserving brain gray matter volume. This innervation would be targeted by neurodegenerative processes in AD [Helkala et al., 1996; Holschneider et al., 1999; Mesulam et al., 2004; Ricceri et al., 2004; Teipel et al., 2005], especially in AD patients not responding to long term cholinergic therapy [Tanaka et al., 2003]. Scopolamine—a cholinergic antagonist—has reproduced in the healthy the typical abnormal pattern of alpha and slow EEG rhythms observed in AD patients [Osipova et al., 2003], whereas posterior sources of resting alpha rhythms were especially impaired in AD patients not responding to cholinergic therapy [Babiloni et al., 2006f]. Finally, it has been shown that loss of cholinergic connections to cerebral cortex were related to the power reduction of resting posterior alpha sources in amnesic MCI subjects [Babiloni et al., 2010].
Cholinergic innervation to cerebral cortex might be also an important neuroanatomical substrate of resting state slow EEG rhythms including delta (2–4 Hz) oscillations. Several lines of evidence have shown that experimental lesions of the basal forebrain increased the amplitude of slow EEG rhythms and decreased the faster ones [Buzsaki et al., 1988, Ray and Jackson, 1991; Stewart et al., 1984]. The same was true for slow EEG rhythms in AD subjects supposed to have an impairment of cholinergic basal forebrain [Babiloni et al., 2004a; Dierks et al., 1993, 2000; Huang et al., 2000; Mesulam et al., 2004; Rodriguez et al., 1999a]. It has been reported in AD patients a clear‐cut correlation of delta rhythms with neuronal death in mesial‐temporal and posterior cortical areas [Fernandez et al., 2003]. Furthermore, there is a bulk of previous findings on the strict relationship between cerebral atrophy or lesion and generation of pathological delta rhythms [De Jongh et al., 2003; Harmony et al., 1993; Hensel et al., 2004; Murri et al., 1998].
Keeping in mind the present findings and mentioned data, changes of global cortical neural synchronization at the basis of resting state delta and alpha rhythms might be a marker of the impairment of cholinergic neuromodulation from basal forebrain to hippocampus and cerebral cortex, which could result in an impairment of cortical gray matter [Helkala et al., 1996, Holschneider et al., 1999; Mesulam et al., 2004]. This impairment would disinhibit cortical slow oscillators triggering resting delta rhythms [Steriade, 2003]. Furthermore, it would reduce cortico‐cortical functional coupling of EEG rhythms (which are also affected by the intrinsic degenerative mechanisms affecting synaptic transmission at the cortical level), which is the main generation mechanism of awake resting alpha rhythms in posterior cerebral cortex [Manshanden et al., 2002; Nunez et al., 2001]. In this view, cholinergic (and serotoninergic) neurons would subserve the replacement of spindles and delta rhythms by fast EEG rhythms during wakefulness [Dringenberg, 2000; Dringenberg et al., 2002]. However, this explanation should be considered as tentative at this early stage of research. The relationships between cholinergic tone and neurodegenerative processes in AD may be nonlinear. Indeed, it has been shown that cortical cholinergic deficits were characteristic of severely demented AD patients, but cholinergic deficits were not apparent in individuals with mild AD [Davis et al., 1999; DeKosky et al., 2002]. It has been also shown that the cholinergic system determined compensatory responses during the early stage of dementia [DeKosky et al., 2002]. This upregulation has been seen in frontal cortex and could be an important factor in preventing the transition of MCI subjects to AD [DeKosky et al., 2002]. Furthermore, it should be remarked that abnormal EEG rhythms can be observed not only in people with pathological aging but also in other kinds of neurologic disorders not clearly related to an impairment of cholinergic systems [Priori et al., 2004]. Together with neurodenegeration mechanisms and impairment of the cholinergic systems, unbalance of monoaminergic [Dringenberg, 2000] and glutamatergic pathways [Di Lazzaro et. al., 2004] might affect cortical excitability and EEG rhythms in MCI and AD subjects.
It should be remarked that we observed a correlation not only between the cortical gray matter atrophy and abnormalities of delta or alpha cortical sources in the MCI and AD subjects, but also between these structural/functional variables and cognitive functions as measured by standard neuropsychological tests. Specifically, the higher the pathological delta sources, the lower the alpha sources, and the less effective the global cognitive, verbal/visuospatial memory, and language production functions. In the same vein, the greater the cortical gray matter atrophy, the less effective the global cognitive, executive attentional, visuospatial/visuoconstructive memory, and language production functions. These findings clearly unveil the strict relationships among the derangement of cortical structure (gray matter atrophy), cortical synchronizing function generating the resting state EEG rhythms, and cognitive skills along different degrees of neurodegeneration in the present MCI and AD subjects (although not all amnesic MCI subjects suffer from preclinical AD). Noteworthy, the present methodological approach enlightened a novel peculiar aspect of the above complex relationship. According to the standard “double‐dissociation” approach, clinical neuropsychology typically tests the hypothesis that a focal lesion at brain region “X” impairs cognitive function “A” but not “B,” whereas a focal lesion at brain region “Y” impairs the cognitive function “B” but not “A” [Pisella et al., 2006]. However, such “double dissociation” approach is not able to capture some effects of AD neurodegenerative processes, since the disease often involves not only circumscribed brain regions (i.e., hippocampus, amigdala, temporo‐parietal junction) but also neural systems such as cholinergic ascending pathways subserving functional connectivity across a lot of brain regions [Helkala et al., 1996; Holschneider et al., 1999; Mesulam et al., 2004; Ricceri et al., 2004; Teipel et al., 2005]. The present methodological approach explored a peculiar aspect of brain research at the border between clinical neuropsychology and neurophysiology. This approach is able to probe “network” diseases affecting wide functional connectivity across the brain, by the evaluation of cortical gray matter, resting state EEG rhythms, and cognitive functions [Guerrini et al., 2003]. The present results suggest that diffuse neurodegenerative processes in the cerebral cortex prevent the distribution of wide synchronizing neural signals at the basis of the generation of ample resting state alpha rhythms in human brain, as a functional prerequisite of several cognitive functions such as attention [Babiloni et al., 2007a], primary consciousness [Babiloni et al., 2007b], visuo‐motor transformations [Del Percio et al., 2007], and memory [Klimesch, 1999].
CONCLUSIONS
Here we tested whether global neural synchronization mechanisms at the basis of resting cortical EEG rhythms and cognitive functions are progressively abnormal along brain aging leading to AD, as a function of global cortical atrophy. The main results showed that in the amnesic MCI and AD subjects, global power of cortical delta and low frequency alpha rhythms changed as a function of the global cortical atrophy. Furthermore, these cortical structural and functional abnormalities were correlated to the impairment of several cognitive functions in the amnesic MCI and AD subjects. In general the strength and linearity of such relationship was quite modest, especially for the delta sources. Specifically, there was no statistical relationship between the global cortical atrophy and global delta or alpha sources in the AD group. Instead, this relationship was statistically significant (P < 0.05) with the global alpha sources and regional delta and alpha sources in the amnesic MCI group. The present results need to be validated with a follow‐up study correlating in time these two indexes. In principle, they suggest that abnormalities of resting state cortical EEG rhythms are not mere epiphenomena, but are related to global cortical neurodegeneration in the shadow zone between amnesic MCI and AD status. Future studies should explore the clinical utility of this methodological approach at the light of the variability of the relationship between global cortical atrophy and delta/alpha sources in the amnesic MCI and AD subjects.
Acknowledgements
The authors thank Dr. Paul Suetens of Medical Image Computing Group at KU Leuven, for providing software EMS used for MRI data analysis. They also thank Prof/Drs. Carla Buttinelli, Brunello Lecce, Annamaria Papantonio, Paolo Tisei, Antonella De Carolis, Silvia Guidoni, Teresa Falco, Daniela Buonanno, Manuela De Stefano, Federica Scrascia, Livia Quintiliani, Simone Migliore, Ivano Triggiani, Daniela Cologno, Loreto Gesualdo, Elena Ranieri, Ivan Cincione, Antonello Bellomo, Annamaria Petito, Mario Altamura, Pietro Fiore, Andrea Santamato, Tommaso Cassano, Dario Colella, and Gaetano Serviddio for their excellent clinical, biometrics, technical, and/or data analysis work. It was conducted as part of the analysis of “historical” (archive) EEG and MRI data in control, amnesic MCI, and AD subjects performed by four units of the PharmaCog Consortium, namely the University of Foggia (Prof./Dr. Claudio Babiloni, Loreto Gesualdo, Elena Ranieri, Ivan Cincione, Antonello Bellomo, Annamaria Petito, Mario Altamura, Pietro Fiore, Andrea Santamato, Tommaso Cassano, Dario Colella, and Gaetano Serviddio), Lundbeck (Pedersen Torleif Jan) and AstraZeneca (Hardemark Hans‐Goran), and IRCCS Fatebenefratelli of Brescia (Dr. Giovanni B. Frisoni, Marina Boccardi, and Alberto Redolfi). PharmaCog funding was used to support the analysis but not the collection of the data reported in this manuscript. After a gentleman agreement among all participants to this research, only Prof./Dr. Claudio Babiloni, Giovanni B. Frisoni, Marina Boccardi, Pedersen Torleif Jan, Hardemark Hans‐Goran and Alberto Redolfi represented the PharmaCog Consortium in the Author list. The authors thank Prof./Drs. Gianluigi Forloni, Francesco Mattia Noe', Tilman Hensch, Oscar Dalla Pasqua, David Bartrés‐Faz, David Wille, Giuseppe Bertini, Paolo Fabene, and Elaine Irving for a fruitful scientific discussion of the results in the framework of the PharmaCog Consortium. For further information on the PharmaCog project please refer to http://www.alzheimer-europe.org.
REFERENCES
- Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH ( 1991): Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci 57: 167–178. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K ( 1997): Multimodal image coregistration and partitioning—A unified framework. Neuroimage 6: 209–217. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassetta E, Cerboneschi D, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Pascual‐Marqui RD, Rodriguez G, Romani GL, Salinari S, Tecchio F, Vitali P, Zanetti O, Zappasodi F, Rossini PM ( 2004): Mapping distributed sources of cortical rhythms in mild Alzheimers disease: A multi‐centric EEG study. NeuroImage 22: 57–67. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassarino A, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Galderisi S, Hirata K, Lanuzza B, Miniussi C, Mucci A, Nobili F, Rodriguez G, Romani GL, Rossini PM ( 2006a): Sources of cortical rhythms in adults during physiological aging: A multi‐centric EEG study. Hum Brain Mapp 27: 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Binetti G, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Hirata K, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Rodriguez G, Romani GL, Salinari S, Rossini PM ( 2006b): Sources of cortical rhythms change as a function of cognitive impairment in pathological aging: A multi‐centric study. Clin Neurophysiol 117: 252–268. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Benussi L, Binetti G, Bosco P, Busonero G, Cesaretti S, Dal Forno G, Del Percio C, Ferri R, Frisoni G, Ghidoni R, Rodriguez G, Squitti R, Rossini PM ( 2006c): Genotype (cystatin C) and EEG phenotype in Alzheimer disease and mild cognitive impairment: a multicentric study. Neuroimage 29: 948–964. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Benussi L, Binetti G, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Frisoni G, Ghidoni R, Miniussi C, Rodriguez G, Romani GL, Squitti R, Ventriglia MC, Rossini PM ( 2006d): Apolipoprotein E and alpha brain rhythms in mild cognitive impairment: A multicentric EEG study. Ann Neurol 59: 323–334. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Frisoni G, Steriade M, Bresciani L, Binetti G, Del Percio C, Geroldi C, Miniussi C, Nobili F, Rodriguez G, Zappasodi F, Carfagna T, Rossini PM ( 2006e): Frontal white matter volume and delta EEG sources negatively correlate in awake subjects with mild cognitive impairment and Alzheimer's disease. Clin Neurophysiol 117: 1113–1129. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Cassetta E, Dal Forno G, Del Percio C, Ferreri F, Ferri R, Lanuzza B, Miniussi C, Moretti DV, Nobili F, Pascual‐Marqui RD, Rodriguez G, Luca Romani G, Salinari S, Zanetti O, Rossini PM ( 2006f): Donepezil effects on sources of cortical rhythms in mild Alzheimer's disease: Responders vs. non‐responders. Neuroimage 31: 1650–1665. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Cassetta E, Binetti G, Tombini M, Del Percio C, Ferreri F, Ferri R, Frisoni G, Lanuzza B, Nobili F, Parisi L, Rodriguez G, Frigerio L, Gurzì M, Prestia A, Vernieri F, Eusebi F, Rossini PM ( 2007a): Resting EEG sources correlate with attentional span in mild cognitive impairment and Alzheimer's disease. Eur J Neurosci 25: 3742–3757. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Vecchio F, Rossi S, De Capua A, Bartalini S, Ulivelli M, Rossini PM ( 2007b): Human ventral parietal cortex plays a functional role on visuospatial attention and primary consciousness. A repetitive transcranial magnetic stimulation study. Cereb Cortex 17: 1486–1492. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Bosco P, Ghidoni R, Del Percio C, Squitti R, Binetti G, Benussi L, Ferri R, Frisoni G, Lanuzza B, Cassetta E, Anello G, Gurzì M, Bartesaghi S, Lizio R, Tombini M, Rossini PM ( 2007c): Homocysteine and electroencephalographic rhythms in Alzheimer disease: A multicentric study. Neuroscience 145: 942–954. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Frisoni GB, Pievani M, Vecchio F, Lizio R, Buttiglione M, Geroldi C, Fracassi C, Eusebi F, Ferri R, Rossini PM ( 2009): Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. Neuroimage 44: 123–135. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Frisoni GB, Vecchio F, Pievani M, Geroldi C, De Carli C, Ferri R, Vernieri F, Lizio R, Rossini PM ( 2010): Global functional coupling of resting EEG rhythms is related to white‐matter lesions along the cholinergic tracts in subjects with amnesic mild cognitive impairment. J Alzheimers Dis 19: 859–871. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1996): Evolution of the neuropathology of Alzheimer's disease. Acta Neurol Scand Suppl 165: 3–12 (Review). [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH ( 1988): Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci 8: 4007–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Mohs RC, Marin D, Purohit DP, Perl DP, Lantz M, Austin G, Haroutunian V ( 1999): Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA 281: 1401–1406. [DOI] [PubMed] [Google Scholar]
- De Jongh A, Baayen JC, De Munck JC, Heethaar RM, Vandertop WP, Stam CJ ( 2003): The influence of brain tumor treatment on pathological delta activity in MEG. Neuroimage 20: 2291–2301. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ ( 2002): Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol 51: 145–155. [DOI] [PubMed] [Google Scholar]
- Del Percio C, Marzano N, Tilgher S, Fiore A, Di Ciolo E, Aschieri P, Lino A, Toràn G, Babiloni C, Eusebi F ( 2007): Pre‐stimulus alpha rhythms are correlated with post‐stimulus sensorimotor performance in athletes and non‐athletes: A high‐resolution EEG study. Clin Neurophysiol 118: 1711–1720. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Folz M, Gorlyn M, Moeller JR, Stem J ( 1997): Questionable dementia: Clinical course and predictors of outcome. J Am Geriatr Soc 45: 321–328. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Daniele A, Ghirlanda S, Gainotti G, Tonali PA ( 2004): Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer's disease. J Neurol Neurosurg Psychiatry 75: 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks T, Ihl R, Frolich L, Maurer K ( 1993): Dementia of the Alzheimer type: Effects on the spontaneous EEG described by dipole sources. Psychiatry Res 50: 51–162. [DOI] [PubMed] [Google Scholar]
- Dierks T, Jelic V, Pascual‐Marqui RD, Wahlund LO, Julin P, Linden DEJ, Maurer K, Winblad B, Nordberg A ( 2000): Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intracerebral EEG‐generators in Alzheimer's disease. Clin Neurophysiol 111: 1817–1824. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC ( 2000): Alzheimer's disease: More than a “cholinergic disorder”—evidence that cholinergic‐monoaminergic interactions contribute to EEG slowing and dementia. Behav Brain Res 115: 235–249. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Rubenstein ML, Solty H, Tomaszek S, Bruce A ( 2002): Electroencephalographic activation by tacrine, deprenyl, and quipazine: Cholinergic vs. non‐cholinergic contributions. Eur J Pharmacol 447: 43–50. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Davatzikos C, An Y, Wu X, Shen D, Kraut M, Resnick SM ( 2009): Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology 72: 1906–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmstahl S, Rosen I ( 1997): Postural hypotension and EEG variables predict cognitive decline: Results from a 5‐year follow‐up of healthy elderly women. Dement Geriatr Cogn Disord 8: 180–187. [DOI] [PubMed] [Google Scholar]
- Ezekiel F, Chao L, Kornak J, Du AT, Cardenas V, Truran D, Jagust W, Chui H, Miller B, Yaffe K, Schuff N, Weiner M ( 2004): Comparisons between global and focal brain atrophy rates in normal aging and Alzheimer disease: Boundary shift integral versus tracing of the entorhinal cortex and hippocampus. Alzheimer Dis Assoc Disord 18: 196–201. [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Arrazola J, Maestu F, Amo C, Gil‐Gregorio P, Wienbruch C, Ortiz T ( 2003): Correlations of hippocampal atrophy and focal low‐frequency magnetic activity in Alzheimer disease: Volumetric MR imaging‐magnetoencephalographic study. AJNR Am J Neuroradiol 24: 481–487. [PMC free article] [PubMed] [Google Scholar]
- Flicker CS, Ferris H, Reisberg B ( 1991): Mild cognitive impairment in the elderly: Predictors of dementia. Neurology 41: 1006–1009. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): “Mini mental state”: A practical method for grading the cognitive state of patients for clinician. J Psychiat Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Padovani A, Wahlund LO ( 2004): The predementia diagnosis of Alzheimer disease. Alzheimer Dis Assoc Disord 18: 51–53. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Prestia A, Rasser PE, Bonetti M, Thompson PM ( 2009): In vivo mapping of incremental cortical atrophy from incipient to overt Alzheimer's disease. J Neurol 256: 916–924. [DOI] [PubMed] [Google Scholar]
- Geser F, Wenning GK, Poewe W, McKeith I ( 2005): How to diagnose dementia with Lewy bodies: State of the art. Mov Disord 20 ( Suppl 12): S11–S20. [DOI] [PubMed] [Google Scholar]
- Gianotti LR, Künig G, Lehmann D, Faber PL, Pascual‐Marqui RD, Kochi K, Schreiter‐Gasser U ( 2007): Correlation between disease severity and brain electric LORETA tomography in Alzheimer's disease. Clin Neurophysiol 118: 186–196. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS ( 2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14 ( 1, Part 1): 21–36. [DOI] [PubMed] [Google Scholar]
- Grunwald M, Busse F, Hensel A, Kruggel F, Riedel‐Heller S, Wolf H, Arendt T, Gertz HJ ( 2001): Correlation between cortical theta activity and hippocampal volumes in health, mild cognitive impairment, and mild dementia. J Clin Neurophysiol 18: 178–184. [DOI] [PubMed] [Google Scholar]
- Guerrini R, Sicca F, Parmeggiani L ( 2003): Epilepsy and malformations of the cerebral cortex. Epileptic Disord 5 ( Suppl 2): S9–S26. [PubMed] [Google Scholar]
- Harmony T, Fernández‐Bouzas A, Marosi E, Fernández T, Bernal J, Rodríguez M, Reyes A, Silva J, Alonso M, Casian G ( 1993): Correlation between computed tomography and voltage and current source density spectral EEG parameters in patients with brain lesions. Electroencephalogr Clin Neurophysiol 87: 196–205. [DOI] [PubMed] [Google Scholar]
- Helkala EL, Hanninen T, Hallikainen M, Kononen M, Laakso MP, Hartikainen P, Soininen H, Partanen J, Partanen K, Vainio P, Riekkinen P ( 1996): Sr. Slow‐wave activity in the spectral analysis of the electroencephalogram and volumes of hippocampus in subgroups of Alzheimer's disease patients. Behav Neurosci 110: 1235–1243. [DOI] [PubMed] [Google Scholar]
- Hensel S, Rockstroh B, Berg P, Elbert T, Schönle PW ( 2004): Left‐hemispheric abnormal EEG activity in relation to impairment and recovery in aphasic patients. Psychophysiology 41: 394–400. [DOI] [PubMed] [Google Scholar]
- Hernández JL, Valdés P, Biscay R, Virués T, Szava S, Bosch J, Riquenes A, Clark I ( 1994): A global scale factor in brain topography. Int J Neurosci 76: 267–278. [DOI] [PubMed] [Google Scholar]
- Holschneider DP, Waite JJ, Leuchter AF, Walton NY, Scremin OU ( 1999): Changes in electrocortical power and coherence in response to the selective cholinergic immunotoxin 192 IgG‐saporin. Exp Brain Res 126: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wahlund LO, Dierks T, Julin P, Winblad B, Jelic V ( 2000): Discrimination of Alzheimer's disease and mild cognitive impairment by equivalent EEG sources: A cross‐sectional and longitudinal study. Clin Neurophysiol 11: 1961–1967. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL ( 1982): A new clinical scale for the staging of dementia. Br J Psychiatry 140: 566–572. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr M, Petersen RC, Xu YC, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E ( 1999): Prediction of AD with MRI‐based hippocampal volume in mild cognitive impairment. Neurology 52: 1397–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Shiung MM, Gunter JL, O'Brien PC, Weigand SD, Knopman DS, Boeve BF, Ivnik RJ, Smith GE, Cha RH, Tangalos EG, Petersen RC ( 2004): Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology 62: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Shiung MM, Weigand SD, O'Brien PC, Gunter JL, Boeve BF, Knopman DS, Smith GE, Ivnik RJ, Tangalos EG, Petersen RC ( 2005): Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 65: 1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelic V, Julin P, Shigeta M, Nordberg A, Lannfelt L, Winblad B, Wahlund LO ( 1997): Apolipoprotein E epsilon4 allele decreases functional connectivity in Alzheimer's disease as measured by EEG coherence. J Neurol Neurosurg Psychiatry 63: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelic V, Johansson SE, Almkvist O, Shigeta M, Julin P, Nordberg A, Winblad B, Wahlund LO ( 2000): Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer's disease. Neurobiol Aging 21: 533–540. [DOI] [PubMed] [Google Scholar]
- Jeong J ( 2004): EEG dynamics in patients with Alzheimer's disease. Clin Neurophysiol 115: 1490–1505. [DOI] [PubMed] [Google Scholar]
- Klimesch W ( 1996): Memory processes, brain oscillations and EEG synchronization. Int J Psychophysiol 24: 61–100. [DOI] [PubMed] [Google Scholar]
- Klimesch W ( 1999): EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res Rev 29: 169–195. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Russegger H ( 1997): Event‐related desynchronization in the alpha band and the processing of semantic information. Brain Res Cogn Brain Res 6: 83–94. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Russegger H, Pachinger T, Schwaiger J ( 1998): Induced alpha band power changes in the human EEG and attention. Neurosci Lett 244: 73–76. [DOI] [PubMed] [Google Scholar]
- Knopman DS, Boeve BF, Parisi JE, Dickson DW, Smith GE, Ivnik RJ, Josephs KA, Petersen RC ( 2005): Antemortem diagnosis of frontotemporal lobar degeneration. Ann Neurol 57: 480–488. [DOI] [PubMed] [Google Scholar]
- Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, Jelic V ( 2005): Decreased EEG synchronization in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging 26: 165–171. [DOI] [PubMed] [Google Scholar]
- Lawton MP, Brodie EM ( 1969): Assessment of older people: Self maintaining and instrumental activity of daily living. J Gerontol 9: 179–186. [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Newton TF, Dunkin J, Walter DO, Rosenberg Tompson S, Lachenbruch PA, Weiner H ( 1993): Regional differences in brain electrical activity in dementia: Use of spectral power and spectral ratio measures. Electroenceph Clin Neurophysiol 87: 385–393. [DOI] [PubMed] [Google Scholar]
- Manshanden I, De Munck JC, Simon NR, Lopes da Silva FH ( 2002): Source localization of MEG sleep spindles and the relation to sources of alpha band rhythms. Clin Neurophysiol 113: 1937–1947. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez‐Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova‐Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M ( 2005): Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium. Neurology 65: 1863–1872. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM ( 1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology 34: 939–944. [DOI] [PubMed] [Google Scholar]
- Mesulam M, Shaw P, Mash D, Weintraub S ( 2004): Cholinergic nucleus basalis tauopathy emerges early in the aging‐MCI‐AD continuum. Ann Neurol 55: 815–828. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Babiloni F, Carducci F, Cincotti F, Remondini E, Rossini PM, Salinari S, Babiloni C ( 2003): Computerized processing of EEG‐EOG‐EMG artifacts for multicentirc studies in EEG oscillations and event‐related potentials. Int J Pshycophysiol 47: 199–216. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Babiloni C, Binetti G, Cassetta E, Dal Forno G, Ferreri F, Ferri R, Lanuzza B, Miniussi C, Nobili F, Rodriguez G, Salinari S, Rossini PM ( 2004): Individual analysis of EEG frequency and band power in mild Alzheimer's disease. Clin Neurophysiol 115: 299–308. [DOI] [PubMed] [Google Scholar]
- Moretti DV, Miniussi C, Frisoni GB, Geroldi C, Zanetti O, Binetti G, Rossini PM ( 2007): Hippocampal atrophy and EEG markers in subjects with mild cognitive impairment. Clin Neurophysiol 118: 2716–2729. [DOI] [PubMed] [Google Scholar]
- Murri L, Gori S, Massetani R, Bonanni E, Marcella F, Milani S ( 1998): Evaluation of acute ischemic stroke using quantitative EEG: A comparison with conventional EEG and CT scan. Neurophysiol Clin 28: 249–257. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Wingeier BM, Silberstein RB ( 2001): Spatial‐temporal structures of human alpha rhythms: Theory, microcurrent sources, multiscale measurements, and global binding of local networks. Hum Brain Mapp 13: 125–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuwer MR ( 1988): Quantitative EEG. I: Techniques and problems of frequency analysis and topographic mapping. J Clin Neurophysiol 5: 1–43. [PubMed] [Google Scholar]
- Osipova D, Ahveninen J, Kaakkola S, Jaaskelainen IP, Huttunen J, Pekkonen E ( 2003): Effects of scopolamine on MEG spectral power and coherence in elderly subjects. Clin Neurophysiol 114: 1902–1907. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD, Michel CM ( 1994): LORETA (low resolution brain electromagnetic tomography): New authentic 3D functional images of the brain. ISBET Newslett ISSN 5: 4–8. [Google Scholar]
- Pascual‐Marqui RD, Lehmann D, Koenig T, Kochi K, Merlo MC, Hell D, Koukkou M ( 1999): Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic‐naive, first‐episode, productive schizophrenia. Psychiatry Res 90: 169–179. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD, Esslen M, Kochi K, Lehmann D ( 2002): Functional imaging with low resolution brain electromagnetic tomography (LORETA): A review. Methods Findings Exp Clin Pharmacol 24: 91–95. [PubMed] [Google Scholar]
- Petersen RC ( 2004): Mild cognitive impairment as a diagnostic entity. J Intern Med 256: 183–194. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Negash S ( 2008): Mild cognitive impairment: An overview. CNS Spectr 13: 45–53. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid SN, Thibodeau SN, Kokmen E, Waring SC, Kurland LT ( 1995): Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory‐impaired individuals. JAMA 273: 1274–1278. [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG ( 1997): Aging, memory, and mild cognitive impairment. Int Psychogeriatr 9 ( Suppl 1): 65–69. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B ( 2001): Current concepts in mild cognitive impairment. Arch Neurol 58: 1985–1992. [DOI] [PubMed] [Google Scholar]
- Phillips C, Rugg MD, Friston KJ ( 2002): Systemic regularization of linear inverse solutions of the EEG source localization problem. Neuroimage 17: 287–301. [DOI] [PubMed] [Google Scholar]
- Pisella L, Binkofski F, Lasek K, Toni I, Rossetti Y ( 2006): No double‐dissociation between optic ataxia and visual agnosia: Multiple sub‐streams for multiple visuo‐manual integrations. Neuropsychologia 44: 2734–2748. [DOI] [PubMed] [Google Scholar]
- Ponomareva NV, Selesneva ND, Jarikov GA ( 2003): EEG alterations in subjects at high familial risk for Alzheimer's disease. Neuropsychobiology 48: 152–159. [DOI] [PubMed] [Google Scholar]
- Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, Scheltens P, Vellas B, Touchon J ( 2006): MCI Working Group of the European Consortium on Alzheimer's Disease Mild cognitive impairment (MCI) in medical practice: A critical review of the concept and new diagnostic procedure. Report of the MCI Working Group of the European Consortium on Alzheimer's Disease. J Neurol Neurosurg Psychiatry 77: 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestia A, Drago V, Rasser PE, Bonetti M, Thompson PM, Frisoni GB ( 2010): Cortical changes in incipient Alzheimer's disease. J Alzheimers Dis 22: 1339–1349. [DOI] [PubMed] [Google Scholar]
- Priori A, Foffani G, Pesenti A, Tamma F, Bianchi AM, Pellegrini M, Locatelli M, Moxon KA, Villani RM ( 2004): Rhythm‐specific pharmacological modulation of subthalamic activity in Parkinson's disease. Exp Neurol 189: 369–379. [DOI] [PubMed] [Google Scholar]
- Prichep LS, John ER, Ferris SH, Reisberg B, Almas M, Alper K, Cancro R ( 1994): Quantitative EEG correlates of cognitive deterioration in the elderly. Neurobiol Aging 15: 85–90. [DOI] [PubMed] [Google Scholar]
- Ray PG, Jackson WJ ( 1991): Lesions of nucleus basalis alter ChAT activity and EEG in rat frontal neocortex. Electroencephalogr Clin Neurophysiol 79: 62–68. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Minghetti L, Moles A, Popoli P, Confaloni A, De Simone R, Piscopo P, Scattoni ML, Di Luca M, Calamandrei G ( 2004): Cognitive and neurological deficits induced by early and prolonged basal forebrain cholinergic hypofunction in rats. Exp Neurol 189: 162–172. [DOI] [PubMed] [Google Scholar]
- Ridha BH, Barnes J, Bartlett JW, Godbolt A, Pepple T, Rossor MN, Fox NC ( 2006): Tracking atrophy progression in familial Alzheimer's disease: A serial MRI study. Lancet Neurol 5: 828–834. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Copello F, Vitali P, Perego G, Nobili F ( 1999a): EEG spectral profile to stage Alzheimer's disease. Clin Neurophysiol 110: 1831–1837. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Nobili F, Copello F, Vitali P, Gianelli MV, Taddei G, Catsafados E, Mariani G ( 1999b): 99mTc‐HMPAO regional cerebral blood flow and quantitative electroencephalography in Alzheimer's disease: A correlative study. J Nucl Med 40: 522–529. [PubMed] [Google Scholar]
- Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al ( 1993): Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS‐AIREN international workshop. Neurology 43: 250–260. [DOI] [PubMed] [Google Scholar]
- Rosen WG, Terry RD, Fuld PA, Katzman R, Peck A ( 1980): Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 7: 486–488. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, Lavaroni F, Caramia MD ( 1991): Brain excitability and electroencephalographic activation: Non‐invasive evaluation in healthy humans via transcranial magnetic stimulation. Brain Res 567: 111–119. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S, Babiloni C, Polich J ( 2007): Clinical neurophysiology of aging brain: From normal aging to neurodegeneration. Prog Neurobiol 83: 375–400. [DOI] [PubMed] [Google Scholar]
- Rubin EH, Morris JC, Grant EA, Vendegna T ( 1989): Very mild senile dementia of the Alzheimer type. I. Clinical assessment. Arch Neurol 46: 379–382. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Fox N, Barkhof F, De Carli C ( 2002): Structural magnetic resonance imaging in the practical assessment of dementia: Beyond exclusion. Lancet Neurol 1: 13–21. [DOI] [PubMed] [Google Scholar]
- Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC ( 2005): Measuring atrophy in Alzheimer disease: A serial MRI study over 6 and 12 months. Neurology 65: 119–124. [DOI] [PubMed] [Google Scholar]
- Steriade M ( 1996): Awakening the brain. Nature 383: 24–25. [DOI] [PubMed] [Google Scholar]
- Steriade M ( 2003): Cerebello‐cerebral interactions during states of vigilance. Cerebellum 2: 82–83. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás RR ( 1988): The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 68: 649–742. [DOI] [PubMed] [Google Scholar]
- Stewart GR, Frederickson CJ, Howell GA, Gage FH ( 1984): Cholinergic denervation‐induced increase of chelatable zinc in mossy‐fiber region of the hippocampal formation. Brain Res 290: 43–51. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. Stuttgart: Thieme. [Google Scholar]
- Tanaka Y, Hanyu H, Sakurai H, Takasaki M, Abe K ( 2003): Atrophy of the substantia innominata on magnetic resonance imaging predicts response to donepezil treatment in Alzheimer's disease patients. Dement Geriatr Cogn Disord 16: 119–125. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Flatz WH, Heinsen H, Bokde AL, Schoenberg SO, Stockel S, Dietrich O, Reiser MF, Moller HJ, Hampel H ( 2005): Measurement of basal forebrain atrophy in Alzheimer's disease using MRI. Brain 128 ( Part 11): 2626–2644. [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Grinberg L, Grothe M, Cantero JL, Reiser MF, Möller HJ, Heinsen H, Hampel H ( 2011): The cholinergic system in mild cognitive impairment and Alzheimer's disease: An in vivo MRI and DTI study. Hum Brain Mapp 32: 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdès P, Picton TW, Trujillo N, Bosch J, Aubert E, Riera J ( 1998): Constraining EEG‐MEG source imaging with statistical neuroanatomy. Neuroimage 4: 635. [Google Scholar]
- Wolf H, Jelic V, Gertz HJ, Nordberg A, Julin P, Wahlund LO ( 2003): A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand 107 ( Suppl 179): 52–76. [DOI] [PubMed] [Google Scholar]
- Yao D, He B ( 2001): A self‐coherence enhancement algorithm and its application to enhancing three‐dimensional source estimation from EEGs. Ann Biomed Eng 29: 1019–1027. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO( 1982–83): Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17: 37–49. [DOI] [PubMed] [Google Scholar]
- Zappoli R, Versari A, Paganini M, Arnetoli G, Muscas GC, Gangemi PF, Arneodo MG, Poggiolini D, Zappoli F, Battaglia A ( 1995): Brain electrical activity (quantitative EEG and bit‐mapping neurocognitive CNV components), psychometrics and clinical findings in presenile subjects with initial mild cognitive decline or probable Alzheimer‐type dementia. Ital J Neurol Sci 16: 341–376. [DOI] [PubMed] [Google Scholar]
- Zaudig M ( 1992): A new systematic method of measurement and diagnosis of “mild cognitive impairment” and dementia according to ICD‐10 and DSM‐III‐R criteria. Int Psychogeriatr 4 ( Suppl 2): 203–219. [DOI] [PubMed] [Google Scholar]


